Structured Abstract:
Purpose of review:
Modern innovations in cancer therapy have dramatically increased the number of cancer survivors. An unfortunately frequent side-effect of cancer treatment is enduring neurological impairment. Persistent deficits in attention, concentration, memory, and speed of information processing afflict a substantial fraction of cancer survivors following completion of these life-saving therapies. Here, we highlight chemotherapy-related cognitive impairment (CRCI) and discuss the current understanding of mechanisms underlying CRCI.
Recent findings:
New studies emphasize the deleterious impact of chemotherapeutic agents on glial-glial and neuron-glial interactions that shape the form, function and plasticity of the central nervous system. An emerging theme in cancer therapy-related cognitive impairment is therapy-induced microglial activation and consequent dysfunction of both neural precursor cells and mature neural cell types. Recent work has highlighted the complexity of dysregulated intercellular interactions involving oligodendrocyte lineage cells, microglia, astrocytes, and neurons following exposure to traditional cancer therapies such as methotrexate. This new understanding of the mechanistic underpinnings of CRCI has elucidated potential therapeutic interventions, including CSF1R inhibition, TrkB agonism, and aerobic exercise.
Summary:
Traditional cancer therapies induce lasting alterations to multiple neural cell types. Therapy-induced microglial activation is a critical component of the etiology of CRCI, contributing to dysregulation of numerous processes of neural plasticity. Therapeutic targeting of microglial activation or the consequent dysregulation of neural plasticity mechanisms are emerging.
Keywords: Cancer, Chemotherapy, CRCI, Cognition, Glia
Introduction
Advances in cancer treatment have considerably increased survivorship over the past 50 years, with the number of pediatric and adult cancer survivors in the United States projected to reach over 20 million in the next decade (1). Many of these survivors have been exposed to a combination of chemotherapeutic agents, radiation therapy, surgery, and, more recently, immunotherapy. Unfortunately, a majority of cancer survivors (2) exhibit a syndrome of long-term neurological impairments consisting of deficits in attention, speed of information processing, executive functioning, memory, and multi-tasking, with many individuals also exhibiting deficits in fine motor function and psychiatric symptoms such as anxiety (3). This syndrome of cognitive impairment following chemotherapy is similar to that observed after cranial radiation therapy, although cranial radiation-induced cognitive impairment is typically more severe. While the incidence and severity of this neurological syndrome varies amongst patient populations, cancer types, and treatment regimens used, a large proportion of the population will suffer from cancer therapy-induced neurological disruption. Chemotherapy-related cognitive impairment (CRCI), known colloquially as ‘chemobrain’ or ‘chemofog’, is a significant part of the cognitive consequences of cancer and its therapy, representing a serious health-care issue.
Cancer therapy-induced cognitive impairment is often, but not always, co-morbid with increased incidence of depression and anxiety (4). While some have suggested that CRCI is caused by cancer diagnosis or treatment stress, similar to post-traumatic stress disorder (PTSD; (5)), numerous groups have elegantly shown that the presence and location of cancer itself (6) and cancer treatments cause myriad neurobiological changes, arguing strongly against a purely psychological basis for the persistent neurological deficits many cancer survivors endure (2). CRCI is a multifaceted disorder, and pre-existing cognitive and psychiatric symptoms are exacerbated following cancer therapy (7, 8).
While the symptoms of cancer therapy-related cognitive impairment can be severe and lasting, overt neuropathological changes are often absent. Similarly, standard neuroimaging is frequently unremarkable, while advanced imaging techniques do reveal subtle changes in hippocampal volume and white matter structure (9, 10). Cognitive impairment following cancer therapy is therefore not explained by widespread neuronal loss or by frank demyelination in many cases, but rather must be due to subtle neurobiological changes. In this review, we discuss the salient pathophysiological basis of cancer therapy-related neurological impairment, focusing on chemotherapy-related cognitive impairment and also the mechanistic parallels between chemotherapy and radiation-induced cognitive impairment. We examine emerging molecular mechanisms underlying this complex neurological disorder, as well as potential therapeutic avenues and future directions for the field.
Neural precursor cell populations after cancer therapy
Neural precursor cells mediate ongoing generation of myelin-forming oligodendrocytes throughout the brain (11, 12) and ongoing neurogenesis in the hippocampus (13). New generation of oligodendrocytes contributes to both homeostatic and adaptive myelination, a recently recognized form of activity-dependent neural plasticity that contributes to learning and memory (11, 14, 15). Neurogenesis in the hippocampus contributes to hippocampal memory performance (16) and also represents a form of activity-dependent plasticity (17). As expected for cytotoxic drugs targeting proliferating cells, multiple chemotherapeutic agents are known to induce acute cytotoxicity of neural precursor cell populations, particularly oligodendrocyte precursor cells (18, 19). Neural precursor cells such as oligodendrocyte precursor cells rapidly repopulate in the healthy brain (20). However, examining the long-term effect of chemotherapy exposure reveal persistent depletion of OPCs in human and mouse white matter (21). This depletion of OPCs was found to be attributable to disruption in the gliogenic microenvironment following exposure to the anti-metabolite chemotherapeutic agent methotrexate (MTX), which in turn is due to a direct effect of methotrexate on microglial activation (21). MTX-activated microglia trigger a neurotoxic reactivity state in astrocytes and disrupt oligodendroglial lineage dynamics and myelin plasticity, ultimately resulting in dysmyelination and cognitive behavioral impairment. Microglial depletion rescues astrocyte reactivity, myelination and cognition in this MTX CRCI mouse model, suggesting that microglial reactivity is at the heart of the chronic OPC lineage dysfunction that follows MTX chemotherapy (15, 21).
Similar to chemotherapy, cranial irradiation results in an acute loss of neural precursor cells (22). In the hippocampus, the neural precursor cell population recovers (23) but hippocampal neurogenesis is persistently disrupted (23–25). This long-term radiation-induced decrease in hippocampal neurogenesis is similarly mediated by microglial activation and consequent perturbation of the neurogenic microenvironment (23–25).
Myelination in Cancer Therapy-Related Cognitive Impairment
Approximately half of the human brain is composed of white matter, predominately consisting of myelinated axons in which oligodendrocytes extend processes to ensheath axons and promote fast, saltatory neural conduction. Interestingly, when transcriptomic gene expression is assessed in non-human primates 1-year after fractionated whole brain radiation, alterations in transcription are most prominent in white matter and include gene expression changes relevant to neuroinflammation, neurotransmission and signal transduction (26), indicating long-term deficits in white matter structure and function. This is concordant with previous work demonstrating that white matter volume is decreased following cranial irradiation in both mice and humans (10).
Like cranial radiation, cancer survivors treated with chemotherapy regimens exhibit widespread abnormalities in white matter integrity as measured with diffusion tensor imaging (DTI) (9). The significant disruption of white matter following chemotherapy treatment, especially in survivors of pediatric cancer, is not surprising given the protracted nature of white matter maturation throughout the first three decades of life (27–29). Decreased myelin basic protein, a major constituent of myelin sheaths, in the corpus callosum of rats following CMF (cyclophosphamide, methotrexate, fluorouracil) chemotherapy treatment suggests deficits in myelin integrity (30). Myelination is also dysregulated following juvenile MTX exposure in a mouse model of CRCI. This dysmyelination in the form of thinner myelin sheaths is associated with lasting disruption of oligodendroglial lineage dynamics, astrocyte reactivity, and activation of microglia and can be rescued through microglial depletion with colony-stimulating factor 1 receptor (CSF1R) (21), suggesting complex glial-glial interactions underlie the myelin deficits associated with chemotherapy treatment.
Myelin formation can also be dynamic and can occur throughout life in order to modulate neural circuit function (11, 12). Neuronal activity-dependent myelin plasticity, also known as adaptive myelination, is thought to facilitate on-going circuit adaptation (11, 14, 31); it is important to recognize that activity-independent myelination also occurs as part of development and homeostasis (28, 29). The idea that neuronal activity may directly influence oligodendrocyte lineage cell dynamics was first suggested by Ben Barres and Martin Raff (32). Findings that in vitro neuronal activity can modulate OPC proliferation, oligodendrogenesis, and myelinogenesis (33) and in vivo experience can alter myelin microstructure all support the notion of adaptive myelination (34–36). Advances in technology, such as optogenetics and chemogenetics, that allow for specific temporal and spatial control of neural cells, have confirmed that neuronal activity can directly influence oligodendrocyte lineage cell dynamics and myelin, leading to circuit-specific functional changes (14, 31). Myelin plasticity has been shown to contribute to attention and memory function (21) as well as to certain forms of learning (37). In a mouse model of CRCI, MTX chemotherapy abrogates this neuronal-activity regulated OPC proliferation and myelination and contributes to cognitive behavioral dysfunction in a mouse model of CRCI, signifying that altered myelin plasticity could mediate some of the enduring white matter deficits associated with chemotherapy-related neurological dysfunction (15).
Aerobic Exercise: A neuroprotective intervention for CRCI?
Exercise is known to activate neural circuits and induce both neurogenesis (38) and gliogenesis (39). Aerobic fitness predicts increased white matter integrity and cognition in both healthy populations (40) and individuals with the demyelinating disorder multiple sclerosis (41). In pediatric brain tumor survivors, enhanced fitness level minimized radiation-induced cognitive decline (42). Aerobic exercise increased muscle coordination (43), reaction time, hippocampal volume, white matter integrity (44), cortical thickness and brain volume (45) in survivors of pediatric brain tumors treated with cranial irradiation. In a rat model of chemotherapy-induced cognitive impairment, doxorubicin chemotherapy exposure is associated with decreased short-term and spatial memory function, hippocampal neurogenesis, and mitochondrial functioning concomitant with increased apoptosis; all of which can be attenuated by aerobic exercise (46). These studies suggest that aerobic exercise promotes a level of neural plasticity that may prevent or reverse the harmful effects of cancer treatment (47, 48).
Putative Molecular Mechanisms of Cancer Therapy-Related Cognitive Impairment
Neurotransmission:
The mechanisms mediating cancer therapy-induced neurological disorder are complex and multifactorial (Figure 1). Previous and ongoing work link dysregulated neuronal signaling to the cognitive syndrome following cancer therapy. Cranial radiation in particular is associated with altered neural circuity, such as an initial, acute increase followed by a decrease in hippocampal spine density and excitatory synapses (49) and inhibition of long-term potentiation (LTP) (50). Subtle hippocampal structural changes and alterations in functional connectivity associated with chemotherapy-induced cognitive deficits are also found in human breast cancer survivors (51), indicating altered neuronal communication and possibly subcellular structural changes like decreased spine density. Chemotherapy exposure induced changes in dendritic architecture are associated with impaired spatial memory (52). Cisplatin, a commonly used agent in oncology, administered at low concentrations causes loss of hippocampal dendritic spines and synapses with higher concentrations leading to more rapid loss, as well as dendritic disintegration through decreased dendritic branching (53). These studies, along with many others, suggest that therapy-induced disruptions in synaptic form and function may contribute to the unremitting cognitive phenotype present after cancer treatment.
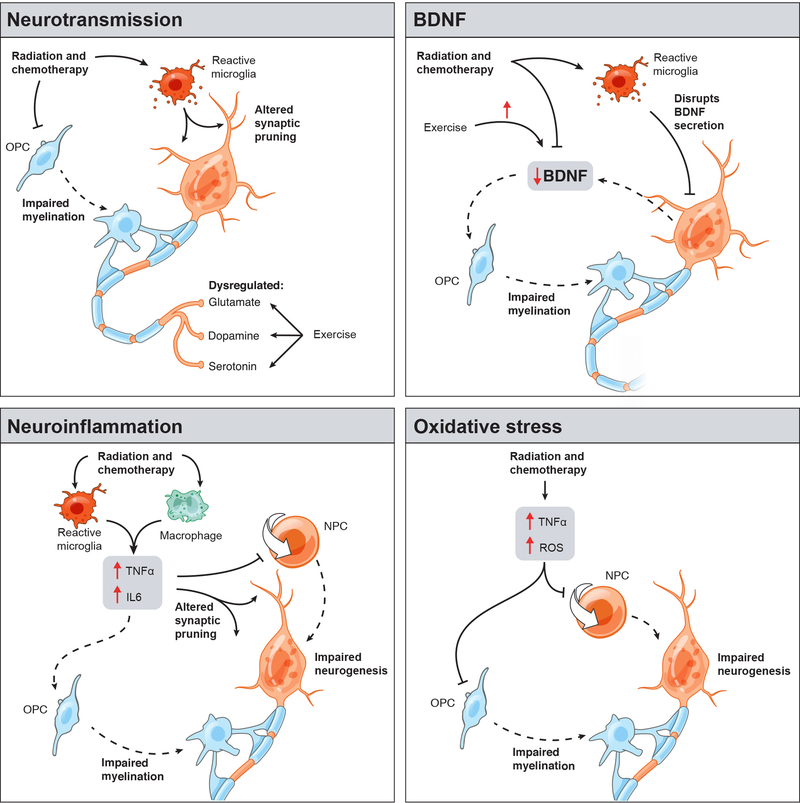
Figure 1: Putative mechanisms underlying cancer therapy-induced cognitive disorder.
Schematic illustration of core mechanisms mediating cancer therapy-induced cognitive impairment. While four distinct mechanisms (clockwise from top left: neurotransmission, BDNF signaling, oxidative stress, and neuroinflammation) have been identified as key modulators of the cognitive disorder associated with cancer therapy, many of these pathways share common regulatory mechanisms. Radiation and chemotherapy-induced reactive microglia (red cells) alter synaptic pruning and neurotransmission, BDNF signaling, and neuroinflammation via increased TNFα and IL6 levels centrally or peripherally by macrophages (green cells). This leads to subsequent impairment of oligodendrocyte precursor cell (OPC) proliferation and myelination by oligodendrocytes (blue cells) and neural precursor cell (NPC) proliferation and neurogenesis (orange cells). Radiation and chemotherapy also lead to increased reactive oxygen species (ROS) and TNFα signaling and consequent inhibition of neurogenesis and myelination via decrements in precursor cell proliferation. Many of these deficits can be rescued through aerobic exercise, including increases in glutamatergic, dopaminergic, serotonergic, and BDNF signaling.
Neural transmission is significantly compromised following cancer therapy. The chemotherapeutic agent 5-fluorouracil (5-FU) decreases striatal dopamine (DA) levels in rats (54); similarly, carboplatin, an alkylating chemotherapeutic agent, impairs DA reuptake and serotonin (5-HT) release (55). Concordantly, methylphenidate, a dopaminergic and noradrenergic agonist that affects the frontostriatal network and is commonly used to treat attention deficit/hyperactivity disorder, improves attention, cognitive flexibility, and information processing in childhood cancer survivors exposed to chemotherapy with or without cranial radiotherapy (56, 57). The evidence for methylphenidate in individuals treated with chemotherapy in adulthood is somewhat mixed, with some studies showing no improvement (58, 59) and one study reporting moderate improvement in neurocognitive deficits (60). Nicotinic signaling has also been implicated in chemotherapy-induced cognitive impairment; chemotherapy exposure concomitant with continine, the main derivative of nicotine, abrogates cognitive and depressive behaviors in a rat model of CRCI (61). In mice treated with doxorubicin, glutamate uptake is slower within the frontal cortex while clearance is slower in the dentate gyrus (62). Memantine, a commonly used drug in Alzheimer’s disease thought to prevent excess glutamate signaling, prevents cognitive impairment, altered dendritic spine dynamics (49) and LTP deficits following radiation (50). As noted above, cancer therapies disrupt numerous neurotransmitter signaling pathways. Dysregulation in neurotransmission represents a fundamental component in the pathophysiology of cancer therapy-related cognitive impairment, but a cohesive model coupling these findings with altered myelin integrity and disrupted hippocampal neurogenesis is lacking. One of the potential mechanisms by which exercise may abrogate the deleterious effects of cancer therapy on brain structure and function is through modification of neurotransmitters (63, 64) and their subsequent influences on neurogenesis and myelinogenesis. Future work focusing on deficits in neurotransmission at both neuronal and axo-glial synapses will lend insight into neural circuit dynamical disruption following cancer treatment.
BDNF:
Karadottir and colleagues have proposed that myelination occurs in two distinct modes: one which is independent of neuronal activity and glutamate and one that is dependent on glutamatergic signaling through NMDA receptors, with brain-derived neurotrophic factor (BDNF) as one of the possible mechanisms mediating this switch (65). Consistently, recent work demonstrates that a key mechanism mediating neuronal activity-regulated myelin plasticity in the healthy brain is neuronal BDNF signaling to its receptor TrkB on OPCs (15). In mice, MTX chemotherapy-induced microglial activation reduces frontal cortex neuronal BDNF levels and white matter TrkB signaling, and TrkB agonism can rescue chemotherapy-induced cognitive impairment in attention and short-term memory in a myelin-dependent manner (15). Concordantly, other groups have also reported that BDNF-TrkB signaling in the prefrontal cortex (PFC) and hippocampus is downregulated following exposure to multiple chemotherapeutic agents (46, 66). BDNF Val66Met polymorphisms that have been linked to sorting of BDNF into synaptic vesicles and to activity-dependent BDNF secretion appear protective against CRCI; humans carrying the mutation exhibit less impairment in memory, verbal ability and multi-tasking following cancer therapy (67). Similar to exercise-induced increases in neurotransmission, particularly in relation to glutamatergic signaling (64), aerobic activity may also mediate recovery from cancer therapy through increases in BDNF levels (68). These studies collectively suggest that dysregulation of BDNF-TrkB signaling plays a significant role in disrupted neuroplasticity, especially myelin plasticity, following cancer treatment.
Inflammation:
A common consequence of both irradiation and chemotherapy treatment is initiation of central nervous system (CNS) and non-CNS inflammatory responses. In mouse models of cranial irradiation in which hippocampal neurogenesis is reduced, microglial activation is elevated (23). IL-6, a cytokine secreted by activated microglia, inhibits hippocampal neurogenesis (24) and blockade of microglial inflammation following radiation by anti-inflammatory drugs (24) or microglial depletion through CSF1R inhibition (69) restores neurogenesis and improves cognition in rodent models. CSF1R-induced depletion of activated microglia following MTX chemotherapy exposure also normalizes oligodendrocyte lineage cell and myelin dynamics, astrocyte reactivity, and cognition in a juvenile mouse model of CRCI. Microglial depletion following chemotherapy restores BDNF expression levels as well, linking the neuroinflammation and BDNF models of cancer therapy-induced cognitive disorder (15, 21). How microglial activation results in a reduction of neuronal BDNF expression remains to be determined.
Activated microglia as well as peripheral macrophages produce the pro-inflammatory cytokines IL-6 and tumor necrosis factor alpha (TNFα). Breast cancer patients exhibit higher serum concentrations of TNFα and IL-6 and reduced hippocampal volume following chemotherapy (70). Along similar lines, soluble tumor necrosis factor receptor II (sTNFRII) was found to be elevated in breast cancer survivors and associated with increased self-reported memory dysfunction (71) and delayed matching to sample visual memory tests (72). Concordantly, mice exposed to the combinatorial chemotherapy regimen DAC (doxifluridine, adriamycin, cyclophosphamide) also exhibit increases in IL-6 and TNFα concurrent with reductions in the anti-inflammatory markers IL-4 and IL-10 and dendritic spine density in the prefrontal cortex; all of which is associated with cognitive deficits (73). Administering RGI, a ginseng-derived compound that inhibits neuroinflammation, prior to chemotherapy exposure normalized TNFα and IL-6 levels and reversed chemotherapy-induced decreases in PFC-hippocampal neuronal activity and dendritic spine elimination (74). Recent work has highlighted the important role of microglia in sculping neural circuits through pruning of dendritic spines in both health and disease (75). Increases in activated microglia following cancer treatment may thus account for the alterations in synaptic spine density described above and thus alter neural circuit dynamics through modulation of synaptic as well as myelin plasticity.
Adding an additional layer of complexity, bone marrow-derived macrophages and monocytes are fundamental to recovery of white matter and cognitive function after cranial irradiation (76). This finding underscores the principle that peripheral immunity is a central player in CNS recovery and cognitive and brain function (77), and indicates that disruption of immune cell function beneficial to brain function may contribute to cancer-related cognitive impairment as much as neurotoxic immune cell reactivity. Understanding the role of peripheral myeloid cells in reaction to cancer treatment is imperative to fully elucidating the brain parenchymal inflammatory response known to be a crucial player in CRCI.
Oxidative stress:
In addition to the well-documented benefits of physical activity on neurotransmission and BDNF levels, exercise is associated with the neuroprotective benefits related to changes in neuroinflammation and reactive oxygen species (for review see (78)). Free radical oxidative stress, or changes in redox state, is not only a hallmark of aging and neurodegenerative diseases, but also a known contributor to CRCI. The role of oxidative stress-derived reactive oxygen species (ROS) in initiating and sustaining brain damage is predominately through modulation of cell viability and differentiation in neurons (79) and glia (80). Cisplatin, a chemotherapeutic agent associated with deleterious effects on dendritic architecture, mitochondrial viability, and DNA damage, increases oxidative stress within the hippocampus of rats (81). Doxorubicin exposure in rodents results in decreased Ca2+ retention capacity in mitochondria and subsequent increases in Ca2+-induced mitochondrial H2o2 emissions, suggestive of increased oxidative stress in hippocampal mitochondria (46). Doxorubicin chemotherapy exposure also produces reactive oxygen species that cause oxidation of plasma proteins, such as ApoE, leading to TNFα-mediated oxidative stress in plasma and brain (82, 83). Doxorubicin is not known to cross the blood-brain-barrier (BBB) but induces oxidative stress in the brain via TNFα signaling (84). Co-administering MESNA, an antioxidant drug, during chemotherapy treatment blocks oxidative protein damage and rescues cognitive deficits in mice (83). Clinically, in patients with breast cancer and non-Hodgkin’s lymphoma, co-administration of MESNA with doxorubicin decreases TNFα and TNF receptor plasma levels (82). Chemotherapeutic activation of TNFα signaling is believed to be the fundamental event promoting oxidative injury in the brain and subsequent neural dysregulation.
While the majority of oxidation-based brain injury following cancer therapy is thought to be modulated by oxidative stress-TNFα signaling, chemotherapeutic agents may also directly alter the BBB leading to increases in CNS reactive oxygen species independent of TNFα signaling (85). Oxidative stress can be caused in the brain by the presence of cancer itself as well. When patient-derived breast cancer cells are grafted into the flanks of mice, oxidative stress levels elevate in the PFC irrespective of chemotherapy treatment (86). Taken together, the evidence demonstrates that both cancer and cancer therapy can directly or indirectly induce brain injury by elevated oxidative stress and contribute to cancer-related cognitive impairment.
Summary and Outlook
Coalescing the studies discussed above, cancer therapy-mediated dysregulation of intercellular interactions amongst neurons and glia lead to dysfunctional mechanisms of neural plasticity and neural circuit function in CRCI. Cancer therapy-related neurological impairment is, thus, a disorder of ‘aplasticity’ or the inability of the brain to maintain dynamic relationships between cells that are necessary for ongoing adaptive neural function required for attentional, learning and memory function. This concept is further supported by the observation that numerous genetic predispositions associated with poorer cognitive outcome following cancer therapy are important for cell signaling, oxidative stress, and neurotransmission within the brain, including mutations in APOE (87), NOS3 894T, SLCO2A1, GSTP1 (88), COMT (89), BDNF (67), and inflammatory markers (90).
One of the most consistent commonalities amongst cancer treatments is induction of an inflammatory response both within the CNS and periphery. Within the CNS, microglial activation by radiation therapy and chemotherapy suggests a common underlying etiology for cancer treatment-mediated cognitive impairment. Future work investigating the role of putatively altered microglial synaptic pruning will lend insights into dysregulation of this process in cognitive deficits following cancer treatment. Additionally, elucidating the molecular mechanisms by which activated microglia alter glial-glial interactions of both astrocyte and oligodendrocyte lineage cells is central to understanding CRCI, especially in relation to the myelin plasticity disorder evident following MTX chemotherapy exposure (15, 21). Demyelination and dysmyelination are often associated with aberrant neural transmission due to altered saltatory transduction; however, myelin also imparts an important metabolic role in supporting axon health (91, 92). The effect of cancer treatment on myelin-mediated metabolic upkeep of axons remains an open question, although studies implicating chemotherapy-induced changes to mitochondria directly or via oxidative stress (93) suggests maintenance of axonal health may be compromised (81). Another pressing question is the effect of new immunotherapeutic strategies on cognition in the long-term. While significant strides have been made towards understanding the multifactorial and synergistic causes of CRCI associated with traditional cancer therapies, the impact of novel immuno-oncology approaches on long-term cognition are largely unstudied. CAR T-cell immunotherapy is frequently associated with acute neurotoxicity, including mild confusion, aphasia, and seizures (94). Neurocognitive abilities immediately following CAR T-cell treatment were prospectively assessed in children and young adults with relapsed/refractory acute lymphoblastic leukemia. Neurocognitive function (i.e. cognitive flexibility, attention/inhibitory control, working memory, and processing speed) returned to pre-treatment baseline at one month post-treatment (95), although the long-term effects of immunotherapy on cognition remains unknown. Further basic, translational and clinical research is needed to improve the neurological outcomes for millions of cancer survivors.
Key Points:
Neural precursor cells that contribute to ongoing neurogenesis and production of myelin-forming oligodendrocytes are vulnerable to chemotherapeutic agents.
The persistent dysmyelination and cognitive deficits following methotrexate chemotherapy treatment are associated with astrocyte reactivity, ongoing depletion of the neural and oligodendrocyte precursor cell (OPC) populations, and altered adaptive myelination, all of which are mediated by chemotherapy-induced microglial activation.
Exercise, known to promote neurogenesis and gliogenesis, supports neural plasticity by attenuating cognitive and cellular dysregulation following cancer treatment.
Cancer therapy-related cognitive impairment is associated with disrupted neurotransmission, BDNF signaling, neuroinflammation, and oxidative stress.
Understanding how traditional cancer treatments, such as chemotherapy, and more recently developed immunotherapeutic strategies, alter neuroplasticity is imperative to developing regenerative or preventative strategies to ameliorate this neurological disorder afflicting millions of cancer survivors.
Acknowledgements:
Special thanks to Sigrid Knemeyer for illustrations.
Financial support and sponsorship: The authors gratefully acknowledge support from the National Institute of Neurological Disorders and Stroke (R01NS092597), NIH Director’s Pioneer Award (DP1NS111132), Unravel Pediatric Cancer, McKenna Claire Foundation, Virginia and D.K. Ludwig Fund for Cancer Research
Footnotes
Conflicts of interest: None
References:
- 1.American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2016–2017; 2016. [Google Scholar]
- 2.Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Current neurology and neuroscience reports. 2012;12(3):267–75. [DOI] [PubMed] [Google Scholar]
- 3.Bisen-Hersh EB, Hineline PN, Walker EA. Effects of early chemotherapeutic treatment on learning in adolescent mice: implications for cognitive impairment and remediation in childhood cancer survivors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(11):3008–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330(7493):702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hermelink K, Buhner M, Sckopke P, Neufeld F, Kaste J, Voigt V, et al. Chemotherapy and Post-traumatic Stress in the Causation of Cognitive Dysfunction in Breast Cancer Patients. J Natl Cancer Inst. 2017;109(10). [DOI] [PubMed] [Google Scholar]
- 6.Scheibel RS, Meyers CA, Levin VA. Cognitive dysfunction following surgery for intracerebral glioma: influence of histopathology, lesion location, and treatment. J Neurooncol. 1996;30(1):61–9. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Hendrix CC. Cancer-Related Cognitive Impairment in Breast Cancer Patients: Influences of Psychological Variables. Asia Pac J Oncol Nurs. 2018;5(3):296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lycke M, Pottel L, Pottel H, Ketelaars L, Stellamans K, Van Eygen K, et al. Predictors of baseline cancer-related cognitive impairment in cancer patients scheduled for a curative treatment. Psychooncology. 2017;26(5):632–9. [DOI] [PubMed] [Google Scholar]
- 9.Menning S, de Ruiter MB, Veltman DJ, Boogerd W, Oldenburg HSA, Reneman L, et al. Changes in brain white matter integrity after systemic treatment for breast cancer: a prospective longitudinal study. Brain imaging and behavior. 2018;12(2):324–34. [DOI] [PubMed] [Google Scholar]
- 10.Nieman BJ, de Guzman AE, Gazdzinski LM, Lerch JP, Chakravarty MM, Pipitone J, et al. White and Gray Matter Abnormalities After Cranial Radiation in Children and Mice. Int J Radiat Oncol Biol Phys. 2015;93(4):882–91. [DOI] [PubMed] [Google Scholar]
- 11.Hughes EG, Orthmann-Murphy JL, Langseth AJ, Bergles DE. Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat Neurosci. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill RA, Li AM, Grutzendler J. Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat Neurosci. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toda T, Parylak SL, Linker SB, Gage FH. The role of adult hippocampal neurogenesis in brain health and disease. Mol Psychiatry. 2019;24(1):67–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344(6183):1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geraghty AC, Gibson EM, Ghanem RA, Greene JJ, Ocampo A, Goldstein AK, et al. Loss of adaptive myelination contributes to methotrexate chemotherapy-related cognitive impairment. Neuron. in press.** This paper identified the role of neuronal BDNF to TrkB signaling on OPCs as a mechanism mediating myelin plasticity in the healthy brain and aberrant myelin plasticity in a mouse model of chemotherapy-induced cognitive impairment. This is the first report of a neurological disorder being associated with aberrant myelin plasticity and identify TrkB agonism as a potential therapeutic strategy to mitigate chemobrain.
- 16.Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–6. [DOI] [PubMed] [Google Scholar]
- 17.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–5. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5(7):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyrien O, Dietrich J, Noble M. Mathematical and experimental approaches to identify and predict the effects of chemotherapy on neuroglial precursors. Cancer Res. 2010;70(24):10051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16(6):668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN, et al. Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell. 2019;176(1–2):43–55 e13.**Using a mouse model of methotrexate (MTX)-induced CRCI, this paper demonstrates that a persistent disruption of glial cells, myelination, and cognition are mediated by MTX-induced microglial activity. Depletion of microglia normalize the cellular, myelin, and cognitive deficits, suggesting modulation of microglia as a potential therapeutic strategy to treat or prevent chemobrain.
- 22.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63(14):4021–7. [PubMed] [Google Scholar]
- 23.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–62. [DOI] [PubMed] [Google Scholar]
- 24.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–5. [DOI] [PubMed] [Google Scholar]
- 25.Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, Palmer TD. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62(5):515–20. [DOI] [PubMed] [Google Scholar]
- 26.Andrews RN, Dugan GO, Peiffer AM, Hawkins GA, Hanbury DB, Bourland JD, et al. White Matter is the Predilection Site of Late-Delayed Radiation-Induced Brain Injury in Non-Human Primates. Radiat Res. 2019;191(3):217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60(1):340–52. [DOI] [PubMed] [Google Scholar]
- 28.Lee S, Chong SY, Tuck SJ, Corey JM, Chan JR. A rapid and reproducible assay for modeling myelination by oligodendrocytes using engineered nanofibers. Nature protocols. 2013;8(4):771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bechler ME, Byrne L, Ffrench-Constant C. CNS Myelin Sheath Lengths Are an Intrinsic Property of Oligodendrocytes. Curr Biol. 2015;25(18):2411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Briones TL, Woods J. Dysregulation in myelination mediated by persistent neuroinflammation: possible mechanisms in chemotherapy-related cognitive impairment. Brain, behavior, and immunity. 2014;35:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitew S, Gobius I, Fenlon LR, McDougall SJ, Hawkes D, Xing YL, et al. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nature communications. 2018;9(1):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361(6409):258–60. [DOI] [PubMed] [Google Scholar]
- 33.Stevens B, Tanner S, Fields RD. Control of myelination by specific patterns of neural impulses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18(22):9303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 2012;15(12):1621–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337(6100):1357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12(11):1370–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, et al. Motor skill learning requires active central myelination. Science. 2014;346(6207):318–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17(10):525–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krityakiarana W, Espinosa-Jeffrey A, Ghiani CA, Zhao PM, Topaldjikian N, Gomez-Pinilla F, et al. Voluntary exercise increases oligodendrogenesis in spinal cord. Int J Neurosci. 2010;120(4):280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaddock L, Pontifex MB, Hillman CH, Kramer AF. A review of the relation of aerobic fitness and physical activity to brain structure and function in children. J Int Neuropsychol Soc. 2011;17(6):975–85. [DOI] [PubMed] [Google Scholar]
- 41.Prakash RS, Snook EM, Motl RW, Kramer AF. Aerobic fitness is associated with gray matter volume and white matter integrity in multiple sclerosis. Brain Res. 2010;1341:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolfe KR, Madan-Swain A, Hunter GR, Reddy AT, Banos J, Kana RK. An fMRI investigation of working memory and its relationship with cardiorespiratory fitness in pediatric posterior fossa tumor survivors who received cranial radiation therapy. Pediatric blood & cancer. 2013;60(4):669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piscione PJ, Bouffet E, Timmons B, Courneya KS, Tetzlaff D, Schneiderman JE, et al. Exercise training improves physical function and fitness in long-term paediatric brain tumour survivors treated with cranial irradiation. Eur J Cancer. 2017;80:63–72. [DOI] [PubMed] [Google Scholar]
- 44.Riggs L, Piscione J, Laughlin S, Cunningham T, Timmons BW, Courneya KS, et al. Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: a controlled clinical trial with crossover of training versus no training. Neuro Oncol. 2017;19(3):440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szulc-Lerch KU, Timmons BW, Bouffet E, Laughlin S, de Medeiros CB, Skocic J, et al. Repairing the brain with physical exercise: Cortical thickness and brain volume increases in long-term pediatric brain tumor survivors in response to a structured exercise intervention. Neuroimage Clin. 2018;18:972–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park HS, Kim CJ, Kwak HB, No MH, Heo JW, Kim TW. Physical exercise prevents cognitive impairment by enhancing hippocampal neuroplasticity and mitochondrial function in doxorubicin-induced chemobrain. Neuropharmacology. 2018;133:451–61. [DOI] [PubMed] [Google Scholar]
- 47.Mugele H, Freitag N, Wilhelmi J, Yang Y, Cheng S, Bloch W, et al. High-intensity interval training in the therapy and aftercare of cancer patients: a systematic review with meta-analysis. J Cancer Surviv. 2019. [DOI] [PubMed] [Google Scholar]
- 48.Zeng J, Wu J, Tang C, Xu N, Lu L. Effects of Exercise During or Postchemotherapy in Cancer Patients: A Systematic Review and Meta-Analysis. Worldviews Evid Based Nurs. 2019;16(2):92–101.* Systemic review and meta-analysis summarizing the effect of exercise during or post-chemotherapy treatment for cancer.
- 49.Duman JG, Dinh J, Zhou W, Cham H, Mavratsas VC, Paveskovic M, et al. Memantine prevents acute radiation-induced toxicities at hippocampal excitatory synapses. Neuro Oncol. 2018;20(5):655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang D, Zhou W, Lam TT, Weng C, Bronk L, Ma D, et al. Radiation induces age-dependent deficits in cortical synaptic plasticity. Neuro Oncol. 2018;20(9):1207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Apple AC, Schroeder MP, Ryals AJ, Wagner LI, Cella D, Shih PA, et al. Hippocampal functional connectivity is related to self-reported cognitive concerns in breast cancer patients undergoing adjuvant therapy. Neuroimage Clin. 2018;20:110–8.* A study using fMRI to investigate the functional connectivity of hippocampi in breast cancer survivors treated with chemotherapy compared to controls. Altered hippocampal connectivity is associated with cognitive impairment and elevated IL6 concentrations.
- 52.Alexander TC, Simecka CM, Kiffer F, Groves T, Anderson J, Carr H, et al. Changes in cognition and dendritic complexity following intrathecal methotrexate and cytarabine treatment in a juvenile murine model. Behavioural brain research. 2018;346:21–8.* Intrathecal chemotherapy (MTX/AraC) results in impaired spatial memory and dendritic architecture in a mouse model of chemobrain. The study lends insight into the effects of intrathecal chemotherapy on brain structure and function.
- 53.Andres AL, Gong X, Di K, Bota DA. Low-doses of cisplatin injure hippocampal synapses: a mechanism for ‘chemo’ brain? Exp Neurol. 2014;255:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jarmolowicz DP, Gehringer R, Lemley SM, Sofis MJ, Kaplan S, Johnson MA. 5-Fluorouracil impairs attention and dopamine release in rats. Behavioural brain research. 2019;362:319–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaplan SV, Limbocker RA, Gehringer RC, Divis JL, Osterhaus GL, Newby MD, et al. Impaired Brain Dopamine and Serotonin Release and Uptake in Wistar Rats Following Treatment with Carboplatin. ACS Chem Neurosci. 2016;7(6):689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conklin HM, Khan RB, Reddick WE, Helton S, Brown R, Howard SC, et al. Acute neurocognitive response to methylphenidate among survivors of childhood cancer: a randomized, double-blind, cross-over trial. J Pediatr Psychol. 2007;32(9):1127–39. [DOI] [PubMed] [Google Scholar]
- 57.Conklin HM, Reddick WE, Ashford J, Ogg S, Howard SC, Morris EB, et al. Long-term efficacy of methylphenidate in enhancing attention regulation, social skills, and academic abilities of childhood cancer survivors. J Clin Oncol. 2010;28(29):4465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mar Fan HG, Clemons M, Xu W, Chemerynsky I, Breunis H, Braganza S, et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer. 2008;16(6):577–83. [DOI] [PubMed] [Google Scholar]
- 59.Lower EE, Fleishman S, Cooper A, Zeldis J, Faleck H, Yu Z, et al. Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: a randomized clinical trial. J Pain Symptom Manage. 2009;38(5):650–62. [DOI] [PubMed] [Google Scholar]
- 60.Escalante CP, Meyers C, Reuben JM, Wang X, Qiao W, Manzullo E, et al. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J. 2014;20(1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iarkov A, Appunn D, Echeverria V. Post-treatment with cotinine improved memory and decreased depressive-like behavior after chemotherapy in rats. Cancer Chemother Pharmacol. 2016;78(5):1033–9. [DOI] [PubMed] [Google Scholar]
- 62.Thomas TC, Beitchman JA, Pomerleau F, Noel T, Jungsuwadee P, Butterfield DA, et al. Acute treatment with doxorubicin affects glutamate neurotransmission in the mouse frontal cortex and hippocampus. Brain Res. 2017;1672:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin TW, Kuo YM. Exercise benefits brain function: the monoamine connection. Brain Sci. 2013;3(1):39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maddock RJ, Casazza GA, Fernandez DH, Maddock MI. Acute Modulation of Cortical Glutamate and GABA Content by Physical Activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36(8):2449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lundgaard I, Luzhynskaya A, Stockley JH, Wang Z, Evans KA, Swire M, et al. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 2013;11(12):e1001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi DD, Dong CM, Ho LC, Lam CTW, Zhou XD, Wu EX, et al. Resveratrol, a natural polyphenol, prevents chemotherapy-induced cognitive impairment: Involvement of cytokine modulation and neuroprotection. Neurobiol Dis. 2018;114:164–73. [DOI] [PubMed] [Google Scholar]
- 67.Tan CJ, Lim SWT, Toh YL, Ng T, Yeo A, Shwe M, et al. Replication and Meta-analysis of the Association between BDNF Val66Met Polymorphism and Cognitive Impairment in Patients Receiving Chemotherapy. Mol Neurobiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mackay CP, Kuys SS, Brauer SG. The Effect of Aerobic Exercise on Brain-Derived Neurotrophic Factor in People with Neurological Disorders: A Systematic Review and Meta-Analysis. Neural Plast. 2017;2017:4716197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Acharya MM, Green KN, Allen BD, Najafi AR, Syage A, Minasyan H, et al. Elimination of microglia improves cognitive function following cranial irradiation. Sci Rep. 2016;6:31545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain, behavior, and immunity. 2013;30 Suppl:S109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ganz PA, Bower JE, Kwan L, Castellon SA, Silverman DH, Geist C, et al. Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction? Brain, behavior, and immunity. 2013;30 Suppl:S99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Williams AM, Shah R, Shayne M, Huston AJ, Krebs M, Murray N, et al. Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. J Neuroimmunol. 2018;314:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi DD, Huang YH, Lai CSW, Dong CM, Ho LC, Wu EX, et al. Chemotherapy-Induced Cognitive Impairment Is Associated with Cytokine Dysregulation and Disruptions in Neuroplasticity. Mol Neurobiol. 2019;56(3):2234–43. [DOI] [PubMed] [Google Scholar]
- 74.Shi DD, Huang YH, Lai CSW, Dong CM, Ho LC, Li XY, et al. Ginsenoside Rg1 Prevents Chemotherapy-Induced Cognitive Impairment: Associations with Microglia-Mediated Cytokines, Neuroinflammation, and Neuroplasticity. Mol Neurobiol. 2019. [DOI] [PubMed] [Google Scholar]
- 75.Hong S, Dissing-Olesen L, Stevens B. New insights on the role of microglia in synaptic pruning in health and disease. Curr Opin Neurobiol. 2016;36:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dietrich J, Baryawno N, Nayyar N, Valtis YK, Yang B, Ly I, et al. Bone marrow drives central nervous system regeneration after radiation injury. The Journal of clinical investigation. 2018;128(1):281–93.** A study demonstrating a link between bone marrow and brain in regeneration following radiation. Bone marrow derived monocytes and macrophages are essential in re-establishment of neural precursor populations and neurocognition following radiation.
- 77.Filiano AJ, Gadani SP, Kipnis J. How and why do T cells and their derived cytokines affect the injured and healthy brain? Nat Rev Neurosci. 2017;18(6):375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Radak Z, Suzuki K, Higuchi M, Balogh L, Boldogh I, Koltai E. Physical exercise, reactive oxygen species and neuroprotection. Free Radic Biol Med. 2016;98:187–96. [DOI] [PubMed] [Google Scholar]
- 79.Sayre LM, Perry G, Smith MA. Oxidative stress and neurotoxicity. Chem Res Toxicol. 2008;21(1):172–88. [DOI] [PubMed] [Google Scholar]
- 80.Smith J, Ladi E, Mayer-Proschel M, Noble M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(18):10032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lomeli N, Di K, Czerniawski J, Guzowski JF, Bota DA. Cisplatin-induced mitochondrial dysfunction is associated with impaired cognitive function in rats. Free Radic Biol Med. 2017;102:274–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hayslip J, Dressler EV, Weiss H, Taylor TJ, Chambers M, Noel T, et al. Plasma TNF-alpha and Soluble TNF Receptor Levels after Doxorubicin with or without Co-Administration of Mesna-A Randomized, Cross-Over Clinical Study. PloS one. 2015;10(4):e0124988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Keeney JTR, Ren X, Warrier G, Noel T, Powell DK, Brelsfoard JM, et al. Doxorubicin-induced elevated oxidative stress and neurochemical alterations in brain and cognitive decline: protection by MESNA and insights into mechanisms of chemotherapy-induced cognitive impairment (“chemobrain”). Oncotarget. 2018;9(54):30324–39.* A study illustrating the protective effects of the antioxidant drug MESNA (2-mercaptoethane sulfonate sodium) in prevention of chemotherapy-induced oxidative stress, neuroinflammation, and cognitive deficits following chemotherapy in mice.
- 84.Aluise CD, Miriyala S, Noel T, Sultana R, Jungsuwadee P, Taylor TJ, et al. 2-Mercaptoethane sulfonate prevents doxorubicin-induced plasma protein oxidation and TNF-alpha release: implications for the reactive oxygen species-mediated mechanisms of chemobrain. Free Radic Biol Med. 2011;50(11):1630–8. [DOI] [PubMed] [Google Scholar]
- 85.Branca JJV, Maresca M, Morucci G, Becatti M, Paternostro F, Gulisano M, et al. Oxaliplatin-induced blood brain barrier loosening: a new point of view on chemotherapy-induced neurotoxicity. Oncotarget. 2018;9(34):23426–38.* Oxaliplatin, a chemotherapeutic agent associated with peripheral neuropathy and chemobrain, is shown to compromise the blood-brain-barrier (BBB), identifying physical alterations to the BBB as an additional mechanism associated with chemotherapy-related neurological impairment.
- 86.Kovalchuk A, Ilnytskyy Y, Rodriguez-Juarez R, Shpyleva S, Melnyk S, Pogribny I, et al. Chemo brain or tumor brain - that is the question: the presence of extracranial tumors profoundly affects molecular processes in the prefrontal cortex of TumorGraft mice. Aging (Albany NY). 2017;9(7):1660–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahles TA, Li Y, McDonald BC, Schwartz GN, Kaufman PA, Tsongalis GJ, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: the impact of APOE and smoking. Psychooncology. 2014;23(12):1382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cole PD, Finkelstein Y, Stevenson KE, Blonquist TM, Vijayanathan V, Silverman LB, et al. Polymorphisms in Genes Related to Oxidative Stress Are Associated With Inferior Cognitive Function After Therapy for Childhood Acute Lymphoblastic Leukemia. J Clin Oncol. 2015;33(19):2205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Howarth RA, Adamson AM, Ashford JM, Merchant TE, Ogg RJ, Schulenberg SE, et al. Investigating the relationship between COMT polymorphisms and working memory performance among childhood brain tumor survivors. Pediatric blood & cancer. 2014;61(1):40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu Y, Zhou R, Sulman EP, Scheurer ME, Boehling N, Armstrong GN, et al. Genetic Modulation of Neurocognitive Function in Glioma Patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21(14):3340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485(7399):517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487(7408):443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ren X, Keeney JTR, Miriyala S, Noel T, Powell DK, Chaiswing L, et al. The triangle of death of neurons: Oxidative damage, mitochondrial dysfunction, and loss of choline-containing biomolecules in brains of mice treated with doxorubicin. Advanced insights into mechanisms of chemotherapy induced cognitive impairment (“chemobrain”) involving TNF-alpha. Free Radic Biol Med. 2018;134:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karschnia P, Jordan JT, Forst DA, Arrillaga-Romany IC, Batchelor TT, Baehring JM, et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood. 2019;133(20):2212–21. [DOI] [PubMed] [Google Scholar]
- 95.Shalabi H, Wolters PL, Martin S, Delbrook C, Yates B, Lee DW, et al. A Prospective Evaluation of Neurocognitive Function and Neurologic Symptoms in Pediatric and Young Adult Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia (ALL) Undergoing Anti-CD22 Chimeric Antigen Receptor Therapy. Blood. 2016;128(22):1625.27354722 [Google Scholar]