Abstract
Background
Even though the cause of autism spectrum disorders (ASD) remains unknown, the current understanding points towards complex interactions between environmental and genetic factors. One important environmental factor to consider is intake of toxic and essential elements, and their role in metabolism. Essential elements have received considerably less attention in the literature than the presence of toxins in urine.
Method
The purpose of this investigation is to comprehensively assess the association between urinary element compositions of 28 mothers who had young children with ASD and 29 mothers who had young typically developing (TD) children, and in a subset of their children (21 with ASD and 26 TD).
Results
The results show that there are significant differences between the ASD and TD children cohorts’ concentrations for four specific elements (sulfur, phosphorous, molybdenum, and tin). Utilizing multivariate statistical techniques (Fisher’s discriminant analysis and support vector machines), it was possible to distinguish the ASD from the TD children groups with an 81% accuracy after cross-validation utilizing the four significantly different elements. However, among the mother cohorts assessed, there were no significant differences between those that had children with ASD and those with TD children. There was a significant correlation of levels of phosphorus and sulfur in the children with ASD (r = 0.63, p = 3.0E-3) and in the TD children (r = 0.47, p = 0.02).
Conclusions
Notable differences were observed between the elemental concentration in urine of children with ASD and their TD peers. Analyzing cellular pathways related to these elements are promising areas of future research.
Keywords: ASD, Metabolism, Essential Elements, Urine Analysis
Introduction
Even though the most recent estimate of the prevalence of autism spectrum disorders (ASD) has increased to 1 in 59 (Baio et al., 2018), relatively little is known about the underlying pathophysiology of the disorder. The consensus is that ASD is caused by interactions between a genetic pre-disposition and environmental factors. One set of important environmental factors to consider is intake, absorption, and excretion of toxic and essential elements, since they can affect metabolic functions. While there is no clear definition of what constitutes a toxic or an essential element, this paper focuses on 20 elements with negative effects on the body and 18 elements that are essential for normal growth and development as they play important roles in many metabolic processes. For example, toxic elements such as lead can substitute for essential elements and disrupt normal metabolic processes and impair normal development. Assessing metabolic processes that are affected by environmental factors, as well as markers of their function, is a promising area of research. There have been many reports of abnormal levels of essential elements in children with ASD (Adams et al., 2011b; Yasuda et al., 2011; Hagmeyer et al., 2018). Specifically, the most prominent essential elements that have been hypothesized to have their metabolism perturbed in ASD are sulfur, zinc, and iron (Warring & Klovrza, 2000; Faber et al., 2009; Jory & McGinnis, 2008; Latif et al., 2002). However, several trace elements such as chromium and molybdenum have also been observed to be different in the urine of children with ASD (Blaurock-Busch et al., 2012; Yorbik et al., 2009).
Overall, there have been many studies involving toxic and essential elements in children with ASD. Most of the studies of essential elements have focused on blood levels, which are subject to homeostasis; for example, low intake of calcium can be compensated for by removing calcium from bone, to maintain constant blood levels at the expense of bone loss. Similarly, the body can alter absorption and excretion of most essential elements in effort to maintain constant levels in the blood. In contrast to this, urine measurements offer a different perspective as they indicate the amount of a component that is leaving the body through this route. A summary of previous studies examining the concentrations of essential elements in urine in children with ASD and typically developing (TD) peers is provided (see Table 1).
Table 1:
Urine Essential Elements Case–control Studies
| Study | Total Participants | ASD | Control | Age/Gender Matched | Co | Ca | Cr | Cu | Fe | I | Li | Mn | Mo | Sr | Se | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adams et al. 2011 | 83 | 44 | 39 | N/N | - | |||||||||||
| Blaurock-Busch et al. 2011 | 50 | 25 | 25 | Y/Y | - | - | - | ↑ | - | - | - | - | - | - | - | - |
| Błażewicz et al. 2016 | 80 | 40 | 40 | Y/Y | ↓ | |||||||||||
| Hewedi et al. 2015 | 100 | 50 | 50 | Y/Y | ↓ | |||||||||||
| Yorbik et al. 2010 | 50 | 30 | 20 | N/N | ↑ |
↑ indicates increased concentration observed in the urine of children with ASD relative to the control group, while ↓ indicates a decreased concentration. A “-” indicates that the element was included in the study but that the control group and ASD group were not statistically significantly different
As can be seen from Table 1, the extent to which urinary assessments have been conducted on individuals with ASD is somewhat limited, as only a single study has been conducted that has sought to examine a diverse range of distinct essential elements (Blaurock-Busch et al., 2012). This study examined the hair and urine of a cohort of 25 ASD and 25 age-matched TD children. Notably, this study observed that among the essential elements examined in urine, only copper was found to be distinct between the children with ASD and those that were TD. However, two other studies of urinary iodine found it was lower in ASD (Błażewicz et al., 2016; Hewedi et al., 2015). One study of urinary chromium found it was higher in ASD, in contradiction to the previously mentioned study (Blaurock-Busch et al., 2012). In general, these studies are limited by investigating only one or a few elements, small samples sizes and lack of correction for multiple-hypothesis testing.
For chromium, due to the very low amounts of this element within the human body, there are challenges for accurately assessing the levels of this element in blood and body fluids (Wilbur et al., 2012). It is possible that this challenge may account for some of the discrepancies observed for this element between studies. Even though chromium concentrations in the body are low, it is an essential element due to its role in stimulating fatty acid and cholesterol synthesis.
It has been found in several studies that sulfur metabolism is distinct between individuals with ASD and those that are TD (Midtvedt et al., 2012; Gevi et al., 2016; Page & Coleman, 2000). Subsequently, it has been observed that the urinary content of individuals with ASD shows higher concentrations of sulfate and sulfite (Warring & Klovrza, 2000). Physiological processes such as mucin formation, gastrointestinal hormones, catecholamine metabolism, and sulfur anion metabolism are highly reliant on plasma sulfur concentration, which underscores the fundamental role that this essential element plays in normal body function (Adams et al., 2011b; Hughes et al., 2018). The plasma levels of inorganic sulfate among children with ASD have been found to be significantly lower when compared to the average for TD cohorts (Midtvedt et al., 2012; Bowling et al., 2013; Yap et al., 2010).
It was observed in one study that children with ASD had a functional deficiency of molybdenum as evidenced by excess urinary sulfite, and that molybdenum supplementation was able to normalize sulfite levels in about 36% of children with ASD (Warring & Klovrza, 2000). This is possible because molybdenum is the enzymatic cofactor for sulfite oxidase, which is essential for breaking down amino acids that contain sulfur such as cysteine and methionine. For the most part, sulfite oxidase deficiency largely stems from underlying genetic causes, and there are only a few reported cases of molybdenum deficiency caused by a lack of dietary intake (Moessner et al., 2007). In another study, the Molybdenum Cofactor Sulfurase (MOCOS) enzyme has been found to be under-expressed in the stem cells of adults with ASD (Féron et al., 2015). This enzyme is known to play a role in purine metabolism and facilitates the synthesis of uric acid, which closely ties it to potential discrepancies that may be observed when comparing the urine of TD and ASD individuals. However, in the single assessment of urinary molybdenum content performed, no association was observed between ASD and this essential element (Blaurock-Busch et al., 2012).
As ASD is assumed to be caused by a combination of environmental and genetic factors, the role that toxic elements may play in the onset of this condition has been a frequent subject of investigation. Some prior work in this regard has indicated that metal toxicant uptake and essential element deficiency has been correlated with increased risk and severity of ASD pathology during early childhood development (Adams et al., 2017; Arora et al., 2017). Prior urine analysis studies have demonstrated significant differences in the content of toxic elements in children compared to age matched controls (Blaurock-Busch et al., 2012; Yorbik et al., 2009). Furthermore, utilizing nonlinear multivariate statistical analysis of toxic elements in urine has shown significant potential in distinguishing between ASD and TD cohorts. Prior work using significant toxic elements with kernel Fisher discriminant analysis to discriminate ASD vs. TD achieved sensitivity and specificity of 85% and 82%, respectively (Adams et al. 2017).
The purpose of this study is to focus on toxic and essential elements in the urine of young children with ASD compared to TD peers, and to conduct a similar evaluation for their mothers. Although it would be ideal to evaluate levels during pregnancy, this would require a prospective study which is challenging and expensive to implement as ASD is not diagnosed until years after birth. Instead, this study involves samples and data from mothers 2-5 years after birth, which may be somewhat reflective of prior conditions. This work investigates urine composition differences between ASD and TD cohorts which may provide insights into toxic and essential mineral intake, metabolism and regulation.
Materials and Methods:
IRB Approval and Consent:
This study was approved by the IRB of Mayo Clinic-Scottsdale and the IRB of Arizona State University (ASU). All mothers signed informed consents after the study was explained to them.
Advertising:
The study advertisement was emailed to several thousand autism families on the email lists of the ASU Autism/Asperger’s Research Program and the Zoowalk for Autism Research. Other local autism groups such as the Autism Society of Greater Phoenix also helped advertise the study. Finally, participants were invited to share the study advertisement with their network of friends.
Participants:
The inclusion criteria were:
Mother of a child ages 2-5 years
Child has ASD or has typical neurological and physical development (Typical Development, TD)
ASD diagnosis verified by the Autism Diagnostic Interview-Revised (ADI-R)
The exclusion criteria were:
Currently taking a vitamin/mineral supplement containing folic acid and/or vitamin B12
Pregnant or planning to become pregnant in the next six months
Using these criteria, a cohort of 57 mothers, 28 of whom had children with ASD (ASD-M) and 29 who were typically developing (TD-M), were recruited. Their children were included in the study if a urine collection was possible, yielding 21 children with ASD diagnosis (ASD) and 26 who were typically developing (TD). Evaluation of the children with the Aberrant Behavioral Checklist (ABC) was also performed. Demographic information on the mothers and children is shown in Table 2.
Table 2:
Study Participants Demographic information
| Variable | ASD Group | TD Group | Total | |
|---|---|---|---|---|
| Children | Number | 21 (45%) | 26 (55%) | 47 |
| Mean Age | 4.83 | 3.94 | 4.30 | |
| SD Age | 0.803 | 1.21 | 1.17 | |
| Gender of children | Male | 16 (76%) | 14 (54%) | 30 (64%) |
| Female | 5 (24%) | 12 (46%) | 17 (36%) | |
| Mothers | Number | 28 (49%) | 29 (51%) | 57 |
| Mean Age | 35.6 | 34.9 | 35.2 | |
| SD Age | 5.31 | 5.70 | 5.47 |
First-morning urine samples were collected from the 104 participants (57 mothers and 47 children) at their homes. Samples were immediately frozen and transported on dry ice to ASU, and they were stored at −80° C until being shipped on dry ice to a clinical laboratory operated by the commercial testing company Doctor’s Data. Samples were analyzed using inductively coupled plasma mass spectrometry for 18 essential and 20 toxic elements by Doctor’s Data.
Pre-processing:
Urine samples were normalized by creatinine to account for differences in dilution, and creatinine was measured using the Jaffe method (Slot, 1965). In cases in which the observed concentration for each element was below the detection limit, imputation was performed using the detection limit for each element and adjusted by the creatinine concentration. This was done by essentially normalizing the detection limit with respect to the creatine concentration for each element (Weaver et al., 2014).
Statistical Analysis:
Analysis was first performed using a univariate analysis for each element. The analysis was separately performed for the mother and child cohorts and the implementation of the analysis routines was done in MATLAB. The overarching goal of this analysis was to determine if there was a statistically significant difference between the TD and ASD groups. For the purposes of this analysis, each of the 38 elements examined were analyzed using the Anderson-Darling test for normality among both mother and child cohorts separately. Dependent on if the normality assumption was accepted or rejected, the samples were either subjected to an F-test or a Kolmogorov-Smirnov test. The purpose of the F-test was to determine if the samples had the same variance and the Kolmogorov-Smirnov test would ascertain if the samples had the same or different distributions (Velentgas et al., 2013). Depending on the circumstances, either a Mann-Whitney or t-test would ultimately be performed to determine whether the two sample sets from each cohort were likely derived from the same distribution (see Figure 1). The area under the receiver operating characteristic curve (AUROC) was also determined for each element. The receiver operator curve is determined by comparing the true positive and false positive rate at different discrimination thresholds for a binary decision. This metric gives an indication of how effectively a parameter can distinguish the outcome of a classification decision.
Figure 1:

Univariate Test Decision Tree. Protocol for determining univariate statistical test to perform for comparing each element of the ASD and TD cohorts.
Due to the investigation of multiple elements and the sample size, the false discovery rate (FDR) was also computed. The purpose of using FDR is to determine how robust the computed statistical tests are when leaving out samples (Benjamini & Hochberg, 2017). In this case, the FDR for each element was only determined if it had a p-value less than 0.05 as a p-value of 0.05 and above already resulted in an element not showing a statistically significant difference between the groups. In order to compute the FDR, all possible combinations of one, two and three excluded samples were calculated. Subsequently, the p-value was found for these truncated datasets utilizing the same protocol implemented for the complete univariate analysis. The FDR was determined by assessing the fraction of p-values that were determined to be more than 0.05. A test was considered significant if the p-value was less than 0.05 and the FDR value was less than 0.1.
Correlation Analysis:
Correlations between each element pair were computed to determine possible underlying relationships between elements. This analysis was performed for both the ASD and the TD datasets. Furthermore, using the elements deemed to be significant by the univariate analysis, the Pearson correlation coefficients, r, between these elements and the scores obtained from the ASD group for ABC and ADI-R, and its subscales, were calculated.
Multivariate Analysis:
The use of multivariate techniques was employed to further elucidate statistical differences and to determine if it was possible to correctly classify individuals into the ASD or TD groups (Howsmon et al. 2017; Howsmon et al. 2018). Fisher discriminant analysis (FDA) was utilized to ascertain the possibility to characterize individuals as belonging to the ASD group based solely on their urinary element composition. FDA seeks to maximize the between-group separation while also minimizing within-group scatter. Models for classification were derived using only the elements that had been deemed significant (p-value < 0.05) by the univariate analysis and that had a FDR < 0.1. Specifically, two models were determined using FDA: one model was using only the essential elements that met these statistical significance criteria and the other using both toxic and essential elements deemed significant.
In addition to FDA, a linear Support Vector Machines (SVM) approach also sought to determine a model that would distinguish between the two groups using all the significant elements. The primary goal of the SVM algorithm is to derive a separator between two classes of data samples. The algorithm seeks to maximize the margin that divides the groups, and it utilizes data points close to opposite groups known as support vectors. To determine the best possible separator, the SVM algorithm seeks to solve an optimization problem utilizing information about the support vectors (Wang, 2012). The main reason for using both FDA and SVM in this work is to highlight that the results are not just dependent on the approach being used. The results for both techniques were also separately visualized using a Probability Density Function (PDF), which was computed using kernel density estimation (Parzen, 1962).
Cross-validation:
As it is essential to show that results are statistically independent, cross-validation was incorporated into all the procedures. Cross validation was performed using the leave-one-out method (Hjorth, 2018). This was performed by removing a single sample from the data structure and replicating FDA and SVM on the abridged sample set and then testing the validity of the prediction of which group the left-out sample belonged to. This process was repeated removing different samples, until each sample was left out once. The main advantage of using this method is that it can be applied to reasonably small sample sizes, which is relevant for this study.
Results:
The univariate analysis of the essential and toxic element excretion data was performed for the TD and ASD groups for both the mother and the child cohorts. Additionally, the optimized parametric or non-parametric test utilized for each analysis was also recorded in addition to the AUROC values. The results for the essential elements are shown in Tables 3 (children: ASD vs TD) and 4 (mothers: ASD-M vs TD-M).
Table 3:
Univariate Essential Elements Analysis of the Child Cohorts
| Element | Univariate Test | ASD mean in μg/mL | TD mean in μg/mL | ASD SD | TD SD | % TD Below Detection limit | % ASD Below Detection limit | Ratio ASD/TD | P-value | AUROC | False Discovery Rate |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Boron | Mann-Whitney | 3.64 | 3.44 | 2.34 | 1.89 | 0.0 | 0 | 1.06 | 0.77 | 0.53 | |
| Calcium | Unequal Variance t-test | 1.57E+02 | 1.74E+02 | 9.81E+01 | 1.36E+02 | 0 | 0 | 9.02E-01 | 0.62 | 0.51 | |
| Chromium | Mann-Whitney | 4.41E-04 | 3.65E-04 | 3.12E-04 | 5.49E-04 | 81.5 | 54.5 | 1.21 | 5.3E-2 | 0.64 | |
| Cobalt | Mann-Whitney | 8.52E-04 | 9.28E-04 | 6.08E-04 | 6.35E-04 | 55.6 | 63.6 | 9.18E-01 | 0.66 | 0.54 | |
| Copper | Mann-Whitney | 2.09E-02 | 2.21E-02 | 9.29E-03 | 6.19E-03 | 0 | 0 | 9.45E-01 | 0.26 | 0.60 | |
| Iron | Unequal Variance t-test | 3.08E-01 | 3.21E-01 | 2.03E-01 | 2.53E-01 | 19.2 | 23.8 | 9.57E-01 | 0.84 | 0.51 | |
| Lithium | Unequal Variance t-test | 1.08E-01 | 1.33E-01 | 7.24E-02 | 6.25E-02 | 0 | 0 | 8.10E-01 | 0.21 | 0.66 | |
| Magnesium | Unequal Variance t-test | 2.02E+02 | 2.26E+02 | 8.61E+01 | 8.17E+01 | 0 | 0 | 8.97E-01 | 0.35 | 0.61 | |
| Manganese | Mann-Whitney | 3.45E-03 | 2.55E-03 | 2.57E-03 | 1.80E-03 | 0 | 0 | 1.35 | 0.34 | 0.58 | |
| Molybdenum | Unequal Variance t-test | 8.48E-02 | 1.27E-01 | 4.50E-02 | 5.90E-02 | 0 | 0 | 6.65E-01 | 8.6E-3 | 0.73 | 0 |
| Phosphorus | Equal Variance t-test | 9.14E+02 | 1.37E+03 | 4.59E+02 | 5.00E+02 | 0 | 0 | 6.65E-01 | 2.2E-03 | 0.75 | 0 |
| Potassium | Mann-Whitney | 7.76E+01 | 6.67E+01 | 4.59E+01 | 4.29E+01 | 0 | 0 | 1.16 | 0.44 | 0.57 | |
| Selenium | Equal Variance t-test | 9.60E-02 | 1.31E-01 | 4.06E-02 | 3.80E-02 | 0 | 0 | 7.36E-01 | 4E-03 | 0.74 | 0.18 |
| Sodium | Unequal Variance t-test | 1.92E+02 | 2.09E+02 | 9.79E+01 | 1.58E+02 | 0 | 0 | 9.21E-01 | 0.67 | 0.53 | |
| Strontium | Equal variance t-test | 2.26E-01 | 2.39E-01 | 1.12E-01 | 1.23E-01 | 0 | 0 | 9.47E-01 | 0.72 | 0.53 | |
| Sulfur | Mann-Whitney | 8.49E+02 | 1.32E+03 | 3.12E+02 | 3.80E+02 | 0 | 0 | 6.42E-01 | 7.9E-05 | 0.84 | 0 |
| Vanadium | Unequal Variance t-test | 4.53E-04 | 4.47E-04 | 2.14E-04 | 3.92E-04 | 26.9 | 19.0 | 1.01 | 0.95 | 0.59 | |
| Zinc | Equal Variance t-test | 5.70E-01 | 7.44E-01 | 2.34E-01 | 3.22E-01 | 0 | 0 | 7.66E-01 | 4.3E-2 | 0.64 | 0.54 |
The FDR is only computed for the five elements with p < 0.05. Only three of the elements were found to be statistically significant between the ASD and TD child cohorts. Results with p-values < 0.05 and FDR < 0.1 are highlighted in bold
Table 4:
Univariate Essential Elements Analysis of the Mother Cohorts
| Element | Univariate Test | ASD mean in μg/mL | TD mean in μg/mL | ASD SD | TD SD | % TD Below Detection limit | % ASD Below Detection limit | Ratio ASD/TD | P-value | AUROC | False Discovery Rate |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Boron | Mann-Whitney | 1.52 | 1.34 | 8.81E-01 | 7.29E-01 | 0 | 0 | 1.14 | 0.44 | 0.56 | |
| Calcium | Equal Variance t-test | 1.14E+02 | 91.5 | 72.9 | 59.6 | 0 | 0 | 1.24 | 0.22 | 0.58 | |
| Chromium | Mann-Whitney | 2.95E-04 | 3.43E-04 | 1.06E-04 | 1.16E-04 | 96.6 | 100 | 8.63E-01 | 0.13 | 0.60 | |
| Cobalt | Mann-Whitney | 8.77E-04 | 1.06E-03 | 3.45E-04 | 5.26E-04 | 69.0 | 82.1 | 8.31E-01 | 0.17 | 0.60 | |
| Copper | Mann-Whitney | 1.05E-02 | 9.86E-03 | 3.47E-03 | 6.92E-03 | 0 | 0 | 1.06 | 7.5E-02 | 0.64 | |
| Iron | Mann-Whitney | 2.22E-01 | 1.86E-01 | 1.30E-01 | 9.54E-02 | 31.0 | 25.0 | 1.20 | 0.31 | 0.58 | |
| Lithium | Mann-Whitney | 6.23E-02 | 6.97E-02 | 4.40E-02 | 5.35E-02 | 0 | 0 | 8.93E-01 | 0.60 | 0.54 | |
| Magnesium | Equal Variance t-test | 7.37E+01 | 6.63E+01 | 2.26E+01 | 2.66E+01 | 0 | 0 | 1.11 | 0.26 | 0.63 | |
| Manganese | Mann-Whitney | 1.02E-03 | 8.48E-04 | 5.00E-04 | 4.62E-04 | 6.9 | 0 | 1.20 | 0.14 | 0.61 | |
| Molybdenum | Mann-Whitney | 3.99E-02 | 5.01E-02 | 2.97E-02 | 2.58E-02 | 0 | 0 | 7.95E-01 | 6.5E-02 | 0.64 | |
| Phosphorus | Equal Variance t-test | 592 | 658 | 218 | 200 | 0 | 0 | 9.00E-01 | 0.24 | 0.60 | |
| Potassium | Mann-Whitney | 25.8 | 24.4 | 11.9 | 10.6 | 0 | 0 | 1.05 | 0.71 | 0.53 | |
| Selenium | Equal Variance t-test | 4.81E-02 | 4.86E-02 | 1.26E-02 | 1.64E-02 | 0 | 0 | 9.90E-01 | 0.90 | 0.52 | |
| Sodium | Mann-Whitney | 94.6 | 86.8 | 57.6 | 51.4 | 0 | 0 | 1.09 | 0.53 | 0.55 | |
| Strontium | Equal Variance t-test | 1.44E-01 | 1.24E-01 | 8.01E-02 | 5.91E-02 | 0 | 0 | 1.16 | 0.28 | 0.55 | |
| Sulfur | Mann-Whitney | 559 | 510 | 251 | 188 | 0 | 0 | 1.10 | 0.40 | 0.57 | |
| Vanadium | Mann-Whitney | 2.32E-04 | 2.45E-04 | 1.03E-04 | 6.92E-05 | 79.3 | 78.6 | 9.44E-01 | 0.26 | 0.58 | |
| Zinc | Mann-Whitney | 3.15E-01 | 3.01E-01 | 2.06E-01 | 1.60E-01 | 0 | 0 | 1.04 | 0.94 | 0.51 |
No FDR is computed as no element resulted in p < 0.05.
Taking into account a statistical significance limit of p < 0.05 and FDR < 0.1, three essential elements were significantly lower in the ASD child group compared to the TD child group as found by the univariate analysis. Specifically, these were molybdenum (−33.5%, p-value = 8.6E-3), phosphorous (−33.5%, p-value = 2.2E-03), and sulfur (−35.8%, p-value = 7.9E-05). Zinc and selenium were lower (p < 0.05, but FDR > 0.1), and chromium was higher (p < 0.05, but FDR > 0.1), however, these results were not deemed significant due to the FDR. Among the mothers, there were no statistically significant differences between groups. The elements with p < 0.05 and FDR > 0.1 for the children are recommended for further investigation in a larger cohort in the future. Note that most of the measurements for chromium and cobalt were below the detection limit for the children and mothers, and most of the measurements of vanadium were below the detection limit for the mothers, so our evaluations for those elements need to be interpreted cautiously, and more sensitive methods are needed for precise analysis of those trace elements.
The same type of procedure was used for the analysis of the toxic elements shown in Tables 5 (children: ASD vs TD) and 6 (mothers: ASD-M vs TD-M). The results from Table 5 show that tin (−48.05%, p-value = 3.03E-3, FDR = 0) is the only toxic element where there are statistically significant differences between the urinary concentration of the two child groups (ASD vs TD). For the mothers, no statistically significant differences were found between the two mothers cohorts for any of the measured toxic elements (ASD-M vs TD-M). Note that for 7 of the elements more than 95% of the measurements were below the detection limit, so those results need to be interpreted cautiously but they generally indicate very low exposure to those elements.
Table 5:
Univariate Toxic Elements Analysis of the Child Cohorts
| Element | Univariate Test | ASD mean in μg/mL | TD mean in μg/mL | ASD SD | TD SD | % TD Below Detection limit | % ASD Below Detection limit | Ratio ASD/TD | P-value | AUROC | False Discovery Rate |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aluminum | Mann-Whitney | 1.47E+01 | 1.66E+01 | 1.58E+01 | 1.38E+01 | 0 | 0 | 8.83E-01 | 0.39 | 0.58 | |
| Antimony | Mann-Whitney | 1.12E-01 | 1.30E-01 | 1.62E-01 | 1.87E-01 | 42.3 | 52.3 | 8.67E-01 | 0.47 | 0.56 | |
| Arsenic | Mann-Whitney | 1.71E+01 | 1.64E+01 | 2.48E+01 | 1.01E+01 | 3.84 | 9.52 | 1.04 | 0.27 | 0.59 | |
| Barium | Mann-Whitney | 4.38 | 4.07 | 3.31 | 2.68 | 0 | 0 | 1.08 | 0.67 | 0.54 | |
| Beryllium | Mann-Whitney | 3.79E-04 | 3.74E-04 | 1.16E-04 | 1.04E-04 | 100 | 90.5 | 1.01 | 0.94 | 0.50 | |
| Bismuth | Mann-Whitney | 1.43E-02 | 4.62E-02 | 4.78E-02 | 1.42E-01 | 88.4 | 81.0 | 0.31 | 0.87 | 0.51 | |
| Cadmium | Mann-Whitney | 2.86E-02 | 1.93E-02 | 6.43E-02 | 6.94E-02 | 92.3 | 77.3 | 1.48 | 0.39 | 0.56 | |
| Cesium | Unequal Variance t-test | 7.96 | 7.42 | 3.93 | 3.57 | 0 | 0 | 1.07 | 0.63 | 0.53 | |
| Gadolinium*** | Mann-Whitney | 5.07E-5*** | 5.24E-5 | 1.26 E-5*** | 1.46E-5 | 100 | 95.0*** | 0.97*** | 0.95*** | 0.51 | |
| Lead | Mann-Whitney | 6.05E-01 | 6.31E-01 | 5.73E-01 | 4.17E-01 | 0 | 0 | 9.59E-01 | 0.50 | 0.56 | |
| Mercury | Mann-Whitney | 1.24E-01 | 2.24E-04 | 2.84E-01 | 6.24E-05 | 100 | 77.3 | 5.53E+02 | 3.4E-02 | 0.63 | 0.68 |
| Nickel | Equal Variance t-test | 5.44 | 5.54 | 3.39 | 2.43 | 0 | 0 | 0.98 | 0.90 | 0.55 | |
| Palladium | Mann-Whitney | 4.55E-04 | 4.49E-04 | 1.39E-04 | 1.25E-04 | 100 | 100 | 1.01 | 0.94 | 0.50 | |
| Platinum | Mann-Whitney | 6.06E-05 | 5.98E-05 | 1.85E-05 | 1.66E-05 | 100 | 100 | 1.01 | 0.94 | 0.50 | |
| Tellurium | Mann-Whitney | 3.03E-04 | 2.99E-04 | 9.26E-05 | 8.32E-05 | 100 | 100 | 1.01 | 0.94 | 0.50 | |
| Thallium | Unequal Variance t-test | 2.99E-01 | 2.38E-01 | 1.54E-01 | 1.50E-01 | 3.84 | 0 | 1.25 | 0.18 | 0.63 | |
| Thorium | Mann-Whitney | 2.27E-05 | 2.24E-05 | 6.94E-06 | 6.24E-06 | 100 | 100 | 1.01 | 0.94 | 0.50 | |
| Tin | Mann-Whitney | 1.53 | 3.00 | 1.93 | 2.34 | 0 | 0 | 5.10E-01 | 3.2E-03 | 0.75 | 0 |
| Tungsten | Mann-Whitney | 2.57E-01 | 4.12E-01 | 2.51E-01 | 4.17E-01 | 3.84 | 0 | 6.25E-01 | 3.1E-02 | 0.68 | 0.30 |
| Uranium | Mann-Whitney | 1.81E-02 | 1.47E-02 | 4.97E-02 | 6.03E-02 | 92.3 | 85.7 | 1.24 | 0.56 | 0.54 |
The FDR is only computed for the three elements with p < 0.05. Only one of the elements (tin) was found to be statistically significant between the ASD and TD child cohorts. The FDR is only computed for the three elements with p < 0.05. Only one of the elements (tin) was found to be statistically significant between the ASD and TD child cohorts.
One of the children with ASD had an unusually high level of gadolinium, due to gadolinium being used as a contrast agent for their MRI a few weeks prior. However, the values for the gadolinium concentration for this one child have been removed while their values for all other toxic elements are still included. That being said, inclusion or exclusion of the data from this child does not change the result that tin is the only toxic element out of the 20 tested with statistically significant differences between the groups.
Table 6:
Univariate Toxic Elements Analysis of the Mother Cohorts
| Element | Univariate Test | ASD mean in μg/mL | TD mean in μg/mL | ASD SD | TD SD | % ASD Below Detection limit | % TD Below Detection limit | Ratio ASD/TD | P-value | AUROC | False Discovery Rate |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aluminum | Mann-Whitney | 4.81 | 3.69 | 7.82 | 2.40 | 3.57 | 3.44 | 1.30 | 0.90 | 0.51 | |
| Antimony | Mann-Whitney | 1.69E-02 | 8.97E-05 | 7.62E-02 | 2.84E-05 | 92.9 | 100 | 188 | 0.40 | 0.56 | |
| Arsenic | Mann-Whitney | 11.8 | 10.8 | 17.9 | 12.9 | 3.57 | 3.44 | 1.10 | 0.74 | 0.53 | |
| Barium | Mann-Whitney | 1.65 | 1.30 | 1.36 | 8.50E-01 | 0 | 0 | 1.27 | 0.51 | 0.55 | |
| Beryllium | Mann-Whitney | 4.92E-04 | 5.61E-04 | 1.76E-04 | 1.77E-04 | 100 | 100 | 0.88 | 0.15 | 0.60 | |
| Bismuth | Mann-Whitney | 3.68E-01 | 7.35E-01 | 1.66 | 2.58 | 89.3 | 89.7 | 5.00E-1 | 0.10 | 0.62 | |
| Cadmium | Mann-Whitney | 1.53E-01 | 1.13E-01 | 1.56E-01 | 1.22E-01 | 25.0 | 24.1 | 1.34 | 0.45 | 0.56 | |
| Cesium | Mann-Whitney | 3.69 | 3.19 | 1.36 | 1.18 | 0 | 0 | 1.16 | 0.17 | 0.61 | |
| Gadolinium | Mann-Whitney | 6.13E-03 | 7.85E-05 | 2.23E-02 | 2.48E-05 | 92.9 | 93.1 | 78.1 | 0.27 | 0.58 | |
| Lead | Mann-Whitney | 1.71E-01 | 1.69E-01 | 1.44E-01 | 9.30E-02 | 21.4 | 20.7 | 1.01 | 0.63 | 0.54 | |
| Mercury | Mann-Whitney | 1.54E-01 | 1.07E-01 | 3.29E-01 | 2.00E-01 | 75.0 | 72.4 | 1.44 | 0.48 | 0.55 | |
| Nickel | Mann-Whitney | 3.13 | 2.60 | 1.64 | 1.27 | 0 | 0 | 1.20 | 0.38 | 0.57 | |
| Palladium | Mann-Whitney | 5.91E-04 | 6.73E-04 | 2.11E-04 | 2.13E-04 | 100 | 100 | 8.78E-1 | 0.15 | 0.60 | |
| Platinum | Mann-Whitney | 7.88E-05 | 8.97E-05 | 2.81E-05 | 2.84E-05 | 100 | 100 | 8.78E-1 | 0.15 | 0.60 | |
| Tellurium | Mann-Whitney | 3.94E-04 | 4.49E-04 | 1.41E-04 | 1.42E-04 | 100 | 100 | 8.78E-1 | 0.15 | 0.60 | |
| Thallium | Mann-Whitney | 1.60E-01 | 1.28E-01 | 8.70E-02 | 7.96E-02 | 0 | 0 | 1.25 | 0.11 | 0.62 | |
| Thorium | Mann-Whitney | 2.95E-05 | 3.36E-05 | 1.06E-05 | 1.06E-05 | 100 | 100 | 8.78E-1 | 0.15 | 0.60 | |
| Tin | Mann-Whitney | 5.41E-01 | 5.28E-01 | 9.86E-01 | 4.82E-01 | 7.14 | 6.89 | 1.02 | 0.16 | 0.61 | |
| Tungsten | Mann-Whitney | 7.25E-02 | 1.14E-01 | 9.40E-02 | 1.11E-01 | 39.3 | 37.9 | 6.37E-1 | 6.4E-2 | 0.64 | |
| Uranium | Mann-Whitney | 6.48E-03 | 1.78E-03 | 2.37E-02 | 9.27E-03 | 92.9 | 93.1 | 3.64 | 0.50 | 0.55 |
No FDR is computed as no element resulted in p < 0.05. None of the elements was found to be statistically significant between the two cohorts of mothers.
As no significant differences were found for the mothers, correlation analysis between elements was only performed for the ASD and TD groups of children. For this analysis, all 38 elements included in this study were evaluated for their relationships with each other. The correlation pairs in the ASD group are summarized in Table 7 for high correlations (|r| > 0.7). Pairs that were shared by both the TD and ASD group are highlighted in bold. Approximately half of the element pairs determined to be highly correlated in the typically developing groups were also highly correlated in the ASD group.
Table 7:
ASD and TD Child Cohort Elemental Correlations
| Element Pair | ASD Correlation Coefficient | TD Correlation Coefficient |
|---|---|---|
| Iron x Calcium | 0.98 | 0.96 |
| Strontium x Calcium | 0.87 | 0.87 |
| Strontium x Iron | 0.87 | 0.81 |
| Tungsten x Gadolinium | 0.86 | (−0.09) |
| Boron x Lead | 0.82 | (0.24) |
| Strontium x Nickel | 0.80 | 0.73 |
| Potassium x Thallium | 0.79 | (0.63) |
| Aluminum x Lead | 0.77 | (0.62) |
| Manganese x Lead | 0.76 | (−0.06) |
| Copper x Uranium | 0.76 | (−0.17) |
| Barium x Lead | 0.76 | (0.44) |
| Sulfur x Lithium | 0.75 | (0.40) |
| Cobalt x Tin | 0.72 | (−0.35) |
| Manganese x Thallium | 0.72 | 0.72 |
| Sulfur x Selenium | 0.72 | 0.82 |
| Tin x Bismuth | 0.71 | (0.10) |
| Potassium x Manganese | 0.71 | 0.93 |
| Calcium x Nickel | (0.67) | 0.80 |
| Iron x Nickel | (0.67) | 0.77 |
| Strontium x Barium | (0.51) | 0.70 |
Only element pairs with coefficient greater than 0.7 or less than −0.7 are shown.
Element pairs that were significant in both groups are marked in bold.
(…) indicates that the correlation coefficient was not above the 0.7 or below the −0.7 threshold
Furthermore, correlation analysis was also carried out for the four elements where statistical differences were found between the child ASD and TD groups.The results are shown in Table 8. There was a significant correlation of sulfur with phosphorus in both the ASD and TD groups (r = 0.63, p = 3.0E-3 and r = 0.47, p = 0.02, respectively). No other correlation was observed which had a corresponding p-value less than 0.05.
Table 8:
Correlation between the four statistically significant elements for the child cohorts.
| Element | Sulfur | Molybdenum | Phosphorous | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | ASD | TD | ASD | TD | ASD | TD | ||||||
| Value | r | p-value | r | p-value | r | p-value | r | p-value | r | p-value | r | p-value |
| Molybdenum | 0.29 | n.s. | 0.26 | n.s. | - | - | - | - | 0.24 | n.s. | 0.04 | n.s. |
| Phosphorus | 0.63 | 0.003 | 0.47 | 0.02 | 0.24 | n.s. | 0.04 | n.s. | - | - | - | - |
| Tin | −0.12 | n.s. | −0.15 | n.s. | 0.40 | n.s. | −0.17 | n.s. | 0.15 | n.s. | −0.19 | n.s. |
The correlation coefficient is denoted by r for each element and group subset.
The correlation of the concentration of the four statistically significant elements with the ABC and the ADI-R subscales was also investigated. However, only one of these correlations was found to be significant, which was between phosphorus and the ADI-R B subscale on communication (r = 0.60, p = 0.01). This positive correlation is surprising, as it suggests that lower levels of phosphorus are associated with milder communication problems.
Using the most significant essential elements (molybdenum, phosphorous, and sulfur), Fisher discriminant analysis was used to further highlight differences between the ASD and TD groups in the child cohorts. Additionally, an analysis was performed that also included tin, which was the only toxic element that had been determined to be significantly different between the two groups. As no elements were significantly different between the two mother cohorts, this investigation only focused on the dataset involving children.
The results are shown in Figure 2. While the analysis using four elements performed slightly better than the one using only the essential elements, the difference is small. As can be seen from Figures 2(A) and 2(B), the two PDFs for the ASD cohort are different from the PDFs of their TD peers, but there is still substantial overlap between the groups. As such, this classification model will result in significant misclassification errors.
Figure 2:

ASD vs TD Probability Density Function. The figures show the probability density functions for the Fisher discriminant analysis models comparing ASD and TD children. (A) Using the essential elements phosphorous, sulfur and molybdenum and (B) Using all significant elements, i.e., phosphorous, sulfur, molybdenum, and tin. For both PDFs the two groups are distinct, but with a significant amount of overlap.
In addition to FDA, a support vector machine model was also developed to assess the differences between the ASD and TD child cohorts. This multivariate technique seeks to determine the optimal separation between two classes. For the child cohorts, the SVM method determined a hyperplane that was capable of distinguishing 85% of samples accurately. The results of the FDA and SVM analyses are shown in Figure 3.
Figure 3:
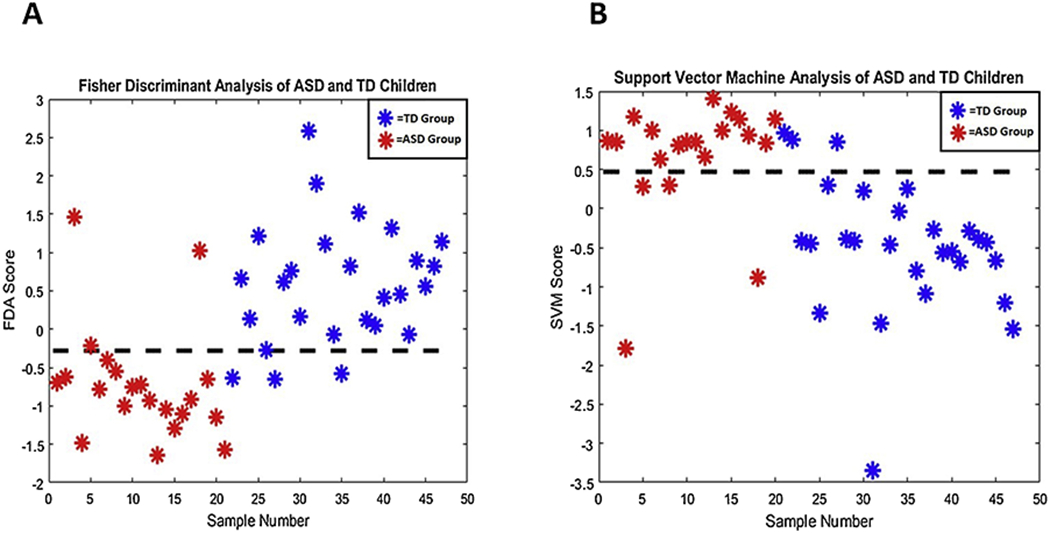
Results for FDA (A) and SVM (B) analysis. All four significantly different elements (phosphorus, molybdenum, sulfur and tin) were used for these classification tasks. The FDA model attained an AUROC value of 90%, while SVM resulted in 86%. It can be seen, that there is some separation, but also some overlap, between the data for the child ASD and TD groups.
Accuracy of the FDA and SVM models were determined using cross-validation. The confusion matrices for both methods are shown in Figure 4. The FDA model had an accuracy of 81% with a sensitivity of 86% and a specificity of 77%. In comparison, the SVM model had an accuracy of 81% with both an 81% specificity and sensitivity. As can be seen from these numbers there are only small differences in the results returned by the FDA and the SVM model, and as a result either multivariate approach is suitable for this investigation.
Figure 4:
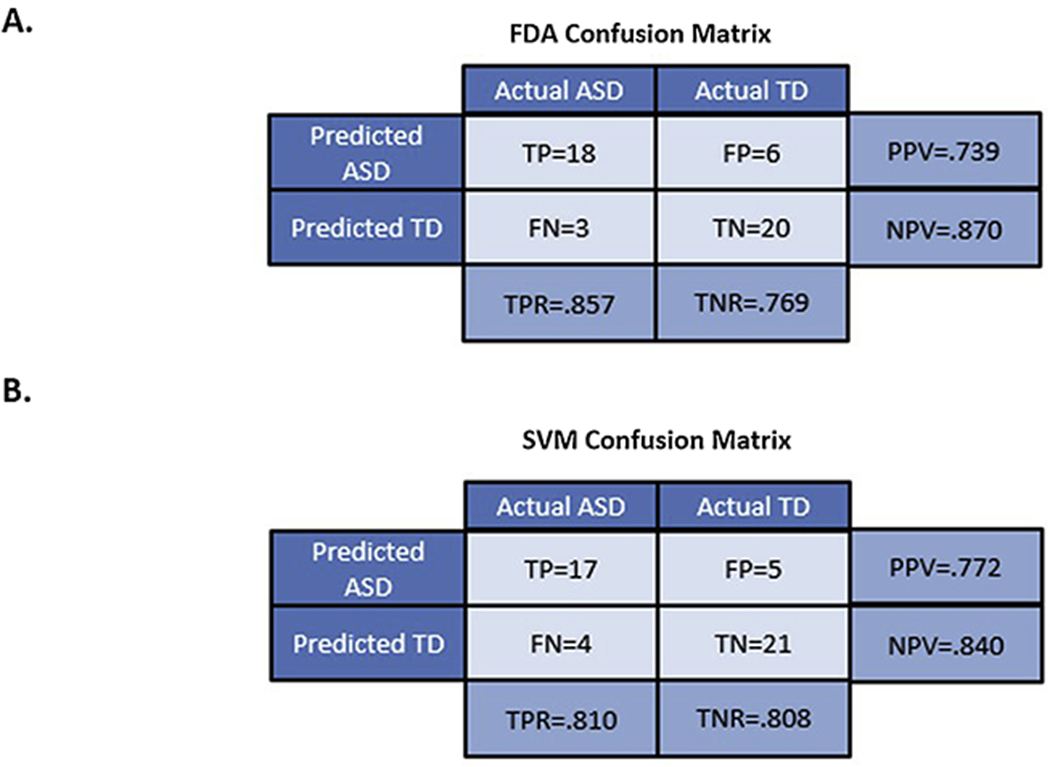
(A) FDA Confusion Matrix and (B) SVM Confusion Matrix. Both confusion matrices were computed using leave-one-out cross-validation with the four-element model utilizing the child cohorts. The accuracy of both FDA and SVM was determined to be 81%.
Discussion:
There have been few studies that have analyzed the concentrations of essential elements in urine between children with ASD and those that are TD (see Table 1). This study differs from these other studies in the literature in that results from both mothers and children were analyzed, and that a large number of elements were examined. It was found that there are statistically significant differences between the ASD and TD children cohorts for four specific elements, after correcting for false discovery rates. The elements identified were molybdenum, phosphorous, sulfur, and tin. The AUROC values for each of these elements were greater than 0.7 demonstrating the degree to which the ASD and TD subsets were distinct for these elements. In contrast to this, no elements were identified that were significantly different in terms of their concentration between the two mother cohorts.
Notably, the concentrations of three essential elements (molybdenum, phosphorous, sulfur) in the urine of children with ASD were significantly lower than for their TD peers. This is especially important as 48% of the children in the ASD cohort were taking multivitamins/mineral supplements while only 27% of the TD cohort were on such supplements, which might have resulted in higher levels for molybdenum than if no supplements had been used (phosphorus is not included in significant amounts in most supplements and sulfur is usually in the form of thiamine and biotin). To evaluate the effect of supplements, the same analysis was also conducted after excluding all individuals taking supplements; the same elements were found to be statistically significant for a comparison of the two subgroups. Thus, the findings suggest that there should be more investigation of mechanisms responsible for elemental uptake, absorption, usage, and excretion of those elements in children with ASD. It is recommended that future studies should include children not taking supplements or a detailed list of the supplement ingredients should be recorded. It should be noted that the differences in essential elements between the two child cohorts were deemed more significant than the differences of the toxic elements; this is important insofar as the focus in the existing literature has been more on toxic than on essential elements. The current results indicate that significant differences can be found by comparing certain essential elements.
For sulfur, this study found on average 35% lower levels in the urine of individuals with ASD. This is consistent with several studies that have reported decreased sulfation capacity for detoxification, and potentially consistent with several studies of lower levels of plasma sulfate (Midtvedt, 2012; Gevi et al., 2016). Urinary sulfur excretion has been of interest due to differences that have been consistently observed in the literature between TD and ASD cohorts. Children with ASD have been previously reported to have extremely high levels of urinary sulfite and higher levels of urinary sulfate (Adams et al., 2011a; Hughes et al., 2018). However, this study investigated sulfur, not sulfate, which is important since sulfur includes sulfate, sulfite, and all other molecules containing a sulfur atom. More research is needed to better understand sulfur/sulfate/sulfite metabolism in ASD.
Molybdenum was also found to have a significantly lower concentration (−33.5%) in the urine of the ASD group compared to the TD group of children, and 28.5% of ASD cohort had a concentration for this element below the measured level of any TD child. Molybdenum is closely tied to sulfate/sulfite metabolism due to its essential role as a co-factor for the sulfite oxidase enzyme. Additionally, the role that Molybdenum Cofactor Sulfurase plays in the purine pathway ties this element closely to urinary sulfur regulation. The interrelation between sulfate and sulfite concentrations is especially important for numerous physiological processes. For example, higher than average sulfite levels have been shown to inhibit the growth of certain beneficial gut bacteria (Irwin et al., 2017). Molybdenum supplementation has previously shown promising results in improving the urinary sulfur concentrations of about 36% of ASD children to more closely resemble those of their TD counterparts (Warring & Klovrza, 2000). However, as demonstrated by the results of the correlation analysis, urinary molybdenum levels were not shown to be significantly correlated with urinary sulfur concentration in this study.
One additional key finding of the univariate analysis was that the phosphorus content was observed to be significantly different between the two child cohorts, with the concentration of this essential element being 34% lower in the ASD group. Furthermore, 24% of the ASD cohort had lower phosphorus concentrations in their urine than even the TD child with the lowest measured concentration of this element. While there have not been any other studies that have examined the phosphorus content of urine of individuals with ASD, there was one study of phosphorus levels in blood. That study (Adams et al., 2011b) found slightly higher levels of phosphorus in red blood cells (RBC) (+5%, p = 0.004) but normal levels in plasma compared to controls. So, the interpretation of the current results for urinary levels of phosphorus are unclear. Given the significant effect of diet , and especially phosphate additives, on phosphorus levels (Calvo et al., 2014) and as diets of children with autism are often different from their typically developing peers, future work should include a dietary assessment
Zinc and selenium were both observed at lower levels in the ASD cohort, but the FDR rate for both these elements was above the FDR cutoff. The results of this investigation were consistent with prior zinc and selenium urine analysis studies examining children with ASD, as prior urinary analysis has not demonstrated significant differences. However, the role of zinc metabolism and interactions in ASD is an active area of research. It is suggested that normal neuronal development may be adversely affected by low levels of zinc and is compounded by a certain variant of the SHANK3 gene, which has been associated with ASD (Fourie et al., 2018). In one recent study, the association between deficits in prenatal developmental zinc levels and ASD pathology has been suggested for further investigation (Ha et al., 2018). Similarly, prior work has sought to elucidate the role of selenium in metabolic processes in children with ASD which has been inconclusive (Saldanha et al., 2018; Raymond et al., 2014).
The urinary content of toxic elements in children with an ASD diagnosis has commonly been the subject of investigation, and most studies have found higher levels of several toxic elements. In a comprehensive meta-analysis performed on studies that had examined toxicant biomarkers in ASD and TD cohorts, 71% of studies reported a relationship between toxicants and ASD (Rossignol et al., 2014). For the toxic metals mercury, cadmium, and aluminum, several studies indicated higher levels when comparing individuals in the ASD group to the TD group in hair, urine, and blood (Rossignol et al., 2014; Saghazadeh et al., 2017). Due to this, it is surprising that not many significant differences were found in this study. Tin was the only toxic element that was found to be significantly different between the ASD and TD groups, and it was found to be lower in children with ASD, which is surprising and inconsistent with other studies which found either higher urinary levels of tin in children with ASD (Adams et al., 2017) or no statistically different levels between groups (Blaurock-Busch et al., 2012).
Determining correlations among elements has the potential to provide insights into metabolism and shared interactions. As such, correlation analysis was performed in this work. Several of the elements were found to be strongly correlated with one another. Through correlation analysis, the relationship between calcium, iron and strontium were observed to be strongly correlated in both the ASD and TD groups. This relationship has been established in the literature, and it is known that strontium and calcium share certain metabolic properties (Blaschko et al., 2012; Samachson & Spencer-Laszlo, 1962). Prior work has also examined the relationship between iron and calcium concentrations, although their correlation in urine had not been examined (Otto-Duessel et al., 2011, Lönnerdal et al., 2010). It has been shown that interactions between these two elements may influence their respective uptake levels (Samachson et al., 1962).
Among the four significantly different elements identified for children, the strongest correlation observed for children with ASD was between the elements phosphorus and sulfur (r = 0.63, p-value = 0.003) which was also significant for the TD cohort (r = 0.47, p-value = 0.02). Other correlations were not significant between these four elements for both cohorts. The reason for the strong correlation between phosphorous and sulfur is unclear.
In addition to univariate analysis, this paper also employed multivariate approaches for the statistical analysis as they have the potential to further highlight differences in the data between ASD and TD cohorts. The maximal AUROC attained by any single univariate test for the child cohort was 0.84 which was attained for sulfur. The SVM and FDA models provided slightly greater AUROC values, however, they also used information about more elements to do so. FDA with four elements achieved an AUROC of 0.90, while the corresponding SVM analysis results in a value of 0.86. Both multivariate classification techniques for the child cohorts had an approximately 81% cross-validation accuracy which highlights the significant differences between the two groups. Although these elements are not sufficient for accurate classification of ASD by themselves, they may be useful when combined with other biomarkers not investigated in this study.
Unlike the child cohorts, the results attained for the mothers indicate that no differences can be found for essential and toxic element concentrations in urine between mothers who had children with ASD and those who did not. However, it is important to point out that these measurements were taken 2-5 years after birth, so they may not reflect levels that were present during pregnancy and lactation.
Vitamin/mineral supplementation (including sulfate and molybdenum) has been shown to ameliorate the severity of ASD symptoms in prior studies (Adams et al., 2011a; Adams, 2015). In another study, it was shown that supplementation with molybdenum was associated with urinary sulfite/sulfate profiles more similar to their TD counterparts in 36% of cases, without the need for sulfate supplementation (Warring & Klovrza, 2000). Generally, most vitamin/mineral supplements do not contain phosphorous in significant amounts. In the general US population, dietary intake of phosphorous is well above the RDA, and excess intake of phosphorous is a growing health concern, in part due to the increasing intake of phosphate additives in highly-processed foods (Calvo & Uribarri, 2013).
Limitations
One limitation of this pilot study is the small sample size. Another limitation was the lack of control over the diet of the participants. It is known that children with ASD are generally more selective in what foods they eat and beverages they drink, which would ultimately influence urine composition. Research into nutrition and ASD has determined that on average children with ASD would only eat about half the number of foods as their TD peers with the exception of starches, which they would eat two-thirds of the variety (Ranjan & Nasser, 2015). The assessment of how this behavior influences nutritional deficiency has been suggested as an area of further research (Cermak et al., 2010). So, it is unclear if the differences in urinary elements observed are due to diet and/or metabolism.
The study was performed exclusively using data from the ASU-Mayo Pilot Study of Young Children with ASD and their Mothers (AMPSYCAM), so some results may be specific to Arizona. The sample size of this pilot study is modest, and larger studies are needed to confirm the results.
Implications
This work highlighted that there is a significant difference in urine content in children with ASD and their TD peers, with significantly lower levels of phosphorus, molybdenum, and sulfur excretion for the children with ASD, and lower levels of tin. The usage of multivariate techniques such as FDA and SVM revealed a modest ability to differentiate the two groups (81% accuracy when using leave-one-out cross-validation). Prior published research has already suggested that supplementation with molybdenum and sulfur may be beneficial to children with ASD, so further investigation of supplementation with those elements and possibly with phosphorus is warranted.
Finally, this investigation is one of the first which examined the elemental urinary concentrations of mothers that had children with ASD. The results suggest that the mother cohorts revealed no significant difference between those that had children with ASD vs. TD children. While the eligibility requirements of the study focused on examining mothers of young children, future prospective studies are recommended for examining elemental urinary and blood concentrations of mothers during pregnancy and lactation.
Highlights:
Measured toxic and essential elements in mothers and their children with and without autism spectrum disorder
No significant differences for elements between the two cohorts of mothers was found
Four elements were distinct between child cohorts (sulfur, phosphorous, molybdenum, and tin)
Multivariate statistical techniques were utilized to distinguish the two groups of children
It was possible to distinguish children groups with an 81% accuracy after cross-validation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JB, Audhya T, McDonough-Means S, Rubin RA, Quig D, Geis E, … Lee W (2011a). Effect of a vitamin/mineral supplement on children and adults with autism. BMC Pediatrics, 11(1), 111 10.1186/1471-2431-11-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JB, Audhya T, McDonough-Means S, Rubin RA, Quig D, Geis E, … Lee W (2011b). Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutrition & Metabolism, 8(1), 34 10.1186/1743-7075-8-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J, Howsmon DP, Kruger U, Geis E, Gehn E, Fimbres V, … Hahn J (2017). Significant Association of Urinary Toxic Metals and Autism-Related Symptoms—A Nonlinear Statistical Analysis with Cross Validation. PLOS ONE, 12(1), e0169526 10.1371/journal.pone.0169526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora M, Reichenberg A, Willfors C, Austin C, Gennings C, Berggren S, … Bölte S (2017). Fetal and postnatal metal dysregulation in autism. Nature Communications, 8(1), 15493 10.1038/ncomms15493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baio J (2018). Correction and Republication: Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR. Morbidity and Mortality Weekly Report, 67(45), 1279 10.15585/mmwr.mm6745a7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Blaschko SD, Chi T, Miller J, Flechner L, Fakra S, Kapahi P, … Stoller ML (2013). Strontium Substitution for Calcium in Lithogenesis. Journal of Urology, 189(2), 735–739. 10.1016/j.juro.2012.08.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaurock-Busch E, Amin O, & Rabah T (2012). Heavy metals and trace elements in hair and urine of a sample of arab children with autistic spectrum disorder. European Psychiatry, 27, 1. [PMC free article] [PubMed] [Google Scholar]
- Błażewicz A, Makarewicz A, Korona-Glowniak I, Dolliver W, & Kocjan R (2016). Iodine in autism spectrum disorders. Journal of Trace Elements in Medicine and Biology, 34, 32–37. 10.1016/j.jtemb.2015.12.002 [DOI] [PubMed] [Google Scholar]
- Bowling FG, Heussler HS, McWhinney A, & Dawson PA (2013). Plasma and Urinary Sulfate Determination in a Cohort with Autism. Biochemical Genetics, 51(1–2), 147–153. 10.1007/s10528-012-9550-0 [DOI] [PubMed] [Google Scholar]
- Calvo MS, & Uribarri J (2013). Public health impact of dietary phosphorus excess on bone and cardiovascular health in the general population. The American Journal of Clinical Nutrition, 98(1), 6–15. 10.3945/ajcn.112.053934 [DOI] [PubMed] [Google Scholar]
- Calvo MS, Moshfegh AJ, & Tucker KL (2014). Assessing the Health Impact of Phosphorus in the Food Supply: Issues and Considerations. Advances in Nutrition, 5(1), 104–113. 10.3945/an.113.004861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak SA, Curtin C, & Bandini LG (2010). Food Selectivity and Sensory Sensitivity in Children with Autism Spectrum Disorders. Journal of the American Dietetic Association, 110(2), 238–246. 10.1016/j.jada.2009.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber S, Zinn GM, Kern JC II, & Skip Kingston HM (2009). The plasma zinc/serum copper ratio as a biomarker in children with autism spectrum disorders. Biomarkers, 14(3), 171–180. 10.1080/13547500902783747 [DOI] [PubMed] [Google Scholar]
- Féron F, Gepner B, Lacassagne E, Stephan D, Mesnage B, Blanchard M-P, … Erard-Garcia M (2016). Olfactory stem cells reveal MOCOS as a new player in autism spectrum disorders. Molecular Psychiatry, 21(9), 1215–1224. 10.1038/mp.2015.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourie C, Vyas Y, Lee K, Jung Y, Garner CC, & Montgomery JM (2018). Dietary Zinc Supplementation Prevents Autism Related Behaviors and Striatal Synaptic Dysfunction in Shank3 Exon 13–16 Mutant Mice. Frontiers in Cellular Neuroscience, 12 10.3389/fncel.2018.00374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevi F, Zolla L, Gabriele S, & Persico AM (2016). Urinary metabolomics of young Italian autistic children supports abnormal tryptophan and purine metabolism. Molecular Autism, 7(1), 47 10.1186/s13229-016-0109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha HTT, Leal-Ortiz S, Lalwani K, Kiyonaka S, Hamachi I, Mysore SP, … Kim SA (2018). Shank and Zinc Mediate an AMPA Receptor Subunit Switch in Developing Neurons. Frontiers in Molecular Neuroscience, 11 10.3389/fnmol.2018.00405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmeyer S, Sauer AK, & Grabrucker AM (2018). Prospects of Zinc Supplementation in Autism Spectrum Disorders and Shankopathies Such as Phelan McDermid Syndrome. Frontiers in Synaptic Neuroscience, 10 10.3389/fnsyn.2018.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza RT, Hewedi DH, & Sallam MT (2013). Iodine Deficiency in Egyptian Autistic Children and Their Mothers: Relation to Disease Severity. Archives of Medical Research, 44(7), 555–561. 10.1016/j.arcmed.2013.09.012 [DOI] [PubMed] [Google Scholar]
- Hjorth JSU (2017). Computer Intensive Statistical Methods. 10.1201/9781315140056 [DOI] [Google Scholar]
- Howsmon DP, Kruger U, Melnyk S, James SJ, & Hahn J (2017). Classification and adaptive behavior prediction of children with autism spectrum disorder based upon multivariate data analysis of markers of oxidative stress and DNA methylation. PLOS Computational Biology, 13(3), e1005385 10.1371/journal.pcbi.1005385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howsmon DP, Vargason T, Rubin RA, Delhey L, Tippett M, Rose S, … Hahn J (2018). Multivariate techniques enable a biochemical classification of children with autism spectrum disorder versus typically-developing peers: A comparison and validation study. Bioengineering & Translational Medicine, 3(2), 156–165. 10.1002/btm2.10095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes HK, Rose D, & Ashwood P (2018). The Gut Microbiota and Dysbiosis in Autism Spectrum Disorders. Current Neurology and Neuroscience Reports, 18(11), 81 10.1007/s11910-018-0887-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin SV, Fisher P, Graham E, Malek A, & Robidoux A (2017). Sulfites inhibit the growth of four species of beneficial gut bacteria at concentrations regarded as safe for food. PLOS ONE, 12(10), e0186629 10.1371/journal.pone.0186629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jory J, & MCGinnis W (2008). Red-Cell Trace Minerals in Children with Autism. American Journal of Biochemistry and Biotechnology, 4(2), 201–204. [Google Scholar]
- Lönnerdal B (2010). Calcium and Iron Absorption - Mechanisms and Public Health Relevance. International Journal for Vitamin and Nutrition Research, 80(45), 293–299. 10.1024/0300-9831/a000036 [DOI] [PubMed] [Google Scholar]
- Midtvedt T (2012). The gut: a triggering place for autism – possibilities and challenges. Microbial Ecology in Health & Disease, 23(0). 10.3402/mehd.v23i0.18982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moessner R, Marshall CR, Sutcliffe JS, Skaug J, Pinto D, Vincent J, … Scherer SW (2007). Contribution of SHANK3 Mutations to Autism Spectrum Disorder. The American Journal of Human Genetics, 81(6), 1289–1297. 10.1086/522590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra JH, Zhuowen Tu, Apostolova LG, Green AE, Toga AW, & Thompson PM. (2010). Comparison of AdaBoost and Support Vector Machines for Detecting Alzheimer’s Disease Through Automated Hippocampal Segmentation. IEEE Transactions on Medical Imaging, 29(1), 30–43. 10.1109/TMI.2009.2021941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto-Duessel M, Brewer C, & Wood JC (2011). Interdependence of cardiac iron and calcium in a murine model of iron overload. Translational Research, 157(2), 92–99. 10.1016/j.trsl.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page T, & Coleman M (2000). Purine metabolism abnormalities in a hyperuricosuric subclass of autism. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1500(3), 291–296. 10.1016/S0925-4439(99)00113-1 [DOI] [PubMed] [Google Scholar]
- Parzen E (1962). On Estimation of a Probability Density Function and Mode. The Annals of Mathematical Statistics, 33(3), 1065–1076. 10.1214/aoms/1177704472 [DOI] [Google Scholar]
- Ranjan S, & Nasser JA (2015). Nutritional Status of Individuals with Autism Spectrum Disorders: Do We Know Enough? Advances in Nutrition, 6(4), 397–407. 10.3945/an.114.007914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond LJ, Deth RC, & Ralston NVC (2014). Potential Role of Selenoenzymes and Antioxidant Metabolism in relation to Autism Etiology and Pathology. Autism Research and Treatment, 2014, 1–15. 10.1155/2014/164938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol DA, Genuis SJ, & Frye RE (2014). Environmental toxicants and autism spectrum disorders: a systematic review. Translational Psychiatry, 4(2), e360–e360. 10.1038/tp.2014.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghazadeh A, Ahangari N, Hendi K, Saleh F, & Rezaei N (2017). Status of essential elements in autism spectrum disorder: systematic review and meta-analysis. Reviews in the Neurosciences, 28(7). 10.1515/revneuro-2017-0015 [DOI] [PubMed] [Google Scholar]
- Saldanha Tschinkel PF, Bjørklund G, Conón LZZ, Chirumbolo S, & Nascimento VA (2018). Plasma concentrations of the trace elements copper, zinc and selenium in Brazilian children with autism spectrum disorder. Biomedicine & Pharmacotherapy, 106, 605–609. 10.1016/j.biopha.2018.06.174 [DOI] [PubMed] [Google Scholar]
- Samachson J, & Spencer-Laszlo H (1962). Urinary excretion of calcium and strontium 85 in man. Journal of Applied Physiology, 17(3), 525–530. 10.1152/jappl.1962.17.3.525 [DOI] [PubMed] [Google Scholar]
- Slot C (1965). Plasma Creatinine Determination A New and Specific Jaffe Reaction Method. Scandinavian Journal of Clinical and Laboratory Investigation, 17(4), 381–387. 10.3109/00365516509077065 [DOI] [PubMed] [Google Scholar]
- Velentgas P, Dreyer N, Nourjah P, Smith S, & Torchia M (2013). Developing a Protocol for Observational Comparative Effectiveness Research (1st ed.). Rockville, MD: Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- Warring R, & Klovrza L (2000). Sulphur Metabolism in Autism. Journal of Nutritional & Environmental Medicine, 10(1), 25–32. [Google Scholar]
- Weaver VM, Vargas GG, Silbergeld EK, Rothenberg SJ, Fadrowski JJ, Rubio-Andrade M, … Guallar E (2014). Impact of urine concentration adjustment method on associations between urine metals and estimated glomerular filtration rates (eGFR) in adolescents. Environmental Research, 132, 226–232. 10.1016/j.envres.2014.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur S, Abadin H, Fay M, & Yu D (2012). Toxicological Profile for Chromium (1st ed.). Atlanta: Agency for Toxic Substances and Disease Registry; . [PubMed] [Google Scholar]
- Yap IKS, Angley M, Veselkov KA, Holmes E, Lindon JC, & Nicholson JK (2010). Urinary Metabolic Phenotyping Differentiates Children with Autism from Their Unaffected Siblings and Age-Matched Controls. Journal of Proteome Research, 9(6), 2996–3004. 10.1021/pr901188e [DOI] [PubMed] [Google Scholar]
- Yasuda H, Yoshida K, Yasuda Y, & Tsutsui T (2011). Infantile zinc deficiency: Association with autism spectrum disorders. Scientific Reports, 1(1), 129 10.1038/srep00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorbik Ö, Kurt İ, Haşimi A, & Öztürk Ö (2010). Chromium, Cadmium, and Lead Levels in Urine of Children with Autism and Typically Developing Controls. Biological Trace Element Research, 135(1–3), 10–15. 10.1007/s12011-009-8494-7 [DOI] [PubMed] [Google Scholar]


