Abstract
Smoking is a potentially causal behavioral risk factor for type 2 diabetes (T2D), but not all smokers develop T2D. It is unknown whether genetic factors partially explain this variation. We performed genome-environment-wide interaction studies to identify loci exhibiting potential interaction with baseline smoking status (ever vs. never) on incident T2D and fasting glucose (FG). Analyses were performed in participants of European (EA) and African ancestry (AA) separately. Discovery analyses were conducted using genotype data from the 50,000-single-nucleotide polymorphism (SNP) ITMAT-Broad-CARe (IBC) array in 5 cohorts from from the Candidate Gene Association Resource Consortium (n = 23,189). Replication was performed in up to 16 studies from the Cohorts for Heart Aging Research in Genomic Epidemiology Consortium (n = 74,584). In meta-analysis of discovery and replication estimates, 5 SNPs met at least one criterion for potential interaction with smoking on incident T2D at p<1x10-7 (adjusted for multiple hypothesis-testing with the IBC array). Two SNPs had significant joint effects in the overall model and significant main effects only in one smoking stratum: rs140637 (FBN1) in AA individuals had a significant main effect only among smokers, and rs1444261 (closest gene C2orf63) in EA individuals had a significant main effect only among nonsmokers. Three additional SNPs were identified as having potential interaction by exhibiting a significant main effects only in smokers: rs1801232 (CUBN) in AA individuals, rs12243326 (TCF7L2) in EA individuals, and rs4132670 (TCF7L2) in EA individuals. No SNP met significance for potential interaction with smoking on baseline FG. The identification of these loci provides evidence for genetic interactions with smoking exposure that may explain some of the heterogeneity in the association between smoking and T2D.
Introduction
Cigarette smoking and type 2 diabetes (T2D) are both costly burdens on human health in the United States and worldwide [1–4]. These public health threats are interrelated: smoking is a dose-dependent risk factor for incident T2D, independent of potential confounders including physical activity and body-mass index (BMI) [5]. Moreover, smoking raises fasting glucose (FG) [6, 7] itself a predictor of incident T2D [8–10]. Experimental studies point to plausible biologic mechanisms through which smoking may directly cause T2D, such as the impairment of insulin-mediated glucose transport [11], insulin sensitivity [12–18], and insulin secretion [19–21].
Not every individual who smokes develops T2D, and the relationship between smoking and T2D has considerable heterogeneity. This variation suggests the possibility of genetic modifiers of the effect of smoking on T2D risk. Genetic studies of smoking behavior [22–27] and T2D and FG [28–36] have separately uncovered hundreds of loci associated with these traits, but no genome-wide association study to date has sought genetic loci that modify the relationships among them. We conducted gene-environment-wide interaction studies (GEWIS) to identify potential gene-by-smoking interactions for both T2D risk and FG among 97,773 cohort study participants of European (EA) and African ancestry (AA).
Materials and methods
Study design overview
We conducted two-stage GEWIS analyses to identify potential genotype-smoking interactions for two related traits: incident T2D and baseline FG. Smoking status was dichotomized as individuals who were current or former smokers at baseline (ever smokers) and individuals with no current or past smoking history (never smokers). The discovery stage analyses leveraged data from 5 cohort studies from the Candidate Gene Association Resource (CARe) Consortium. Single-nucleotide polymorphisms (SNPs) that had significant association with a trait in meta-analysis of the discovery cohort data were carried forward for replication in up to 16 cohorts from the Cohorts for Heart & Aging Research in Genomic Epidemiology (CHARGE) Consortium Gene-Lifestyle Interactions Working Group and combined discovery plus replication meta-analysis. The Partners Human Research Committee approved this study.
Cohort descriptions and sample sizes
In the discovery stage, we analyzed data from five cohorts from the CARe Consortium [37]: The Atherosclerosis Risk in Communities Study (ARIC), the Coronary Artery Risk Development in Young Adults Study (CARDIA), the Cardiovascular Health Study (CHS), the Framingham Heart Study (FHS), and the Multi-Ethnic Study of Atherosclerosis (MESA) (S1 Table) [37]. The total sample size of these five discovery stage cohorts was 23,189, including 18,365 European American (EA) and 4,824 African American (AA). Among 23,189 CARe participants, 10,120 were never smokers and 13,069 were ever smokers, as assessed at their baseline study examinations. In the replication stage, 74,584 individuals from up to 16 cohorts in the Cohorts for Heart & Aging Research in Genomic Epidemiology (CHARGE) Consortium Gene-Lifestyle Interactions Working Group were included, comprised of 61,397 EA participants and 13,187 AA participants. A total of 40,819 and 33,765 were never and ever smokers, respectively (S1 Table) [38]. All five discovery cohorts contributed data for both traits of interest: incident T2D and baseline glucose. Eight replication cohorts contributed data for the incident T2D analyses, and 15 replication cohorts contributed data for the fasting glucose analyses (S1 Table). Across the discovery and replication cohorts, there were 4,040 T2D cases and 48,521 controls among EA participants and 717 cases and 7,180 controls among AA participants.
Description of phenotype and covariates
We considered two traits: incident T2D and baseline FG. Presence of T2D was defined by any one of the following criteria: 1) FG ≥ 7 mmol/L; 2) on diabetes treatment or HbA1c ≥ 6.5%; 3) 2-hr oral glucose tolerance test ≥11.1 mmol/L; 4) random/non-fasting glucose ≥ 11.1 mmol/L; 5) physician diagnosis of diabetes; or 6) self-reported diabetes (S1 Table). For the analysis of incident T2D, participants meeting the T2D definition at baseline were excluded. For the remaining participants, time-to-T2D was defined as the time from the date of the baseline examination to the date the T2D case definition was met or, for controls, to the last date of follow-up. For the FG analyses, participants with T2D were excluded, and FG was identified from the baseline measurement taken after a fast of 8 hours or more (S1 Table).
Genotyping
Participants in the CARe Consortium were genotyped with the custom ITMAT-Broad-CARe (IBC) genotyping array (IBC v2 chip), which contains around 50,000 SNPs across 2,000 loci selected for their relationship to cardiovascular disease and its risk factors. Details about SNP selection criteria and genotyping quality control (QC) procedures have been described [39]. Details of the genotyping methods used in the individual CHARGE replication cohorts are presented in S1 Table.
Cohort-level statistical analysis
We performed ancestry-stratified analyses for the two traits within each discovery and replication cohort. Smoking-stratified analyses were also conducted separately in each of the four trait-ancestry combinations. In total, we performed four models for each of four trait-ancestry combinations: an interaction model regressing the trait (incident T2D or FG) on the genetic variant, smoking status, and their interaction term (Model 1); a main effect-only model (Model 2); and two smoking-stratified models, regressing incident T2D or FG on the genetic variant predictor in smokers (Model 3) and nonsmokers (Model 4) separately. All models were covariate-adjusted as described below.
We analyzed incident T2D using Cox proportional hazards models and robust sandwich variance estimators. For cohorts with related individuals, each family was treated as a cluster. Models were adjusted for age, BMI, and the genetic principal components associated with incident T2D at p<0.05. Models were not adjusted for sex in the discovery cohorts due to insufficient numbers of incident T2D cases in all sex/ancestry categories; models were conducted with or without sex adjustment in the replication analyses, depending on the sample size of stratified samples.
For baseline FG, we used linear regression for cohorts with independent samples. For cohorts with family structures, we used generalized estimating equations (GEE) to obtain estimates for Model 1, assuming an exchangeable working correlation matrix, since the GEE model with an interaction term provides robust standard error estimates. Linear mixed effects models were used to evaluate Models 2–4, with random effects to account for family structures. All FG analyses were adjusted for age, sex, BMI and the genetic principal components associated with FG at p<0.05.
Meta-analysis
For both traits, we obtained summary statistics of association from each cohort and then conducted fixed-effect meta-analysis to combine the results. For each trait (incident T2D and FG), we meta-analyzed the results across the cohorts using inverse variance weighting, in EA and AA separately. We defined a potential interaction effect between a locus and smoking if at least one of the following criteria was met: 1) significant SNP-by-smoking interaction; 2) significant joint 2-degree-of-freedom test of interaction and main effect, excluding SNPs with significant main effects; or 3) significant SNP effect in only one smoking stratum (never or ever smokers). In the discovery stage, significance was defined as p<10−3; we selected all SNPs significant for at least one of these 3 criteria as candidate SNPs. Candidate SNPs were then carried forward for replication in the cohorts of the CHARGE Consortium. We performed meta-analyses with summary statistics from the discovery and replication stages, defining significance as p < 1×10−7 for at least one of the 3 criteria above. We selected this significance threshold to conservatively account for multiple hypothesis-testing, since p < 2×10−6 is commonly used for studies with the 50,000-SNP IBC genotyping array [40, 41] and we performed a total of 20 tests (5×2×2), comprised of 5 models (main effect, interaction effect, joint effect, and 2 smoking stratified analyses) for 2 traits in 2 ancestry groups for each variant.
Power calculations
Power analyses were performed for a significance level of α = 1x10-7 to detect a potential interaction effect on both T2D and FG. For T2D, we approximated the power analysis to detect potential interaction with logistic regression. Under the assumption that the effect size for interaction is similar to the effect size of the main SNP effect, the sample sizes of 4,040 EA cases and 717 AA cases enabled 80% power to detect an odds ratio (OR) of 1.39 in EA and 1.76 in AA, using an unmatched population-based case-control design under an additive genetic model and assuming MAF = 0.3 with 10% T2D prevalence and 30% smoking prevalence. For FG, the sample sizes of 58,783 EA and 17,675 AA enabled 80% power to detect SNPs with R2GE ≥ 0.06% EA and ≥ 0.2% AA for SNP*interaction effect in interaction testing, using an additive genetic model and assuming variants with R2G = 0.1%
Conditional analysis
We performed conditional analyses for the two significant variants identified in TCF7L2 in the T2D analysis. In each corhort, we ran the joint (Model 1) and main effect only models (Model 2) described above for rs4132670 conditioned on the most significant variant, rs12243326. The cohort-level conditional analyses were meta-analyzed to obtain overall summary statistics.
Locus characterization
We queried the National Human Genome Research Institute (NHGR)–European Bioinformatics Institute (EBI) GWAS Catalog for any published trait associations with SNPs achieveing GEWIS significance in this study [42]. We also examined the overlap between these SNPs and genomic annotation using HaploReg [43], which collects information from multiple functional annotation resources and reports information about queried SNPs such as genomic position, protein-coding impact, available expression quantitative trait locus (eQTL) data, overlap with known transcription factor binding sites or predicted transcription factor binding motifs, and overlap with DNAse hypersensitivity sites or histone marks associated with promoters and enhancers. In addition, we queried each GEWIS-significant SNP in RegulomeDB [44], a database of known and predicted regulatory elements in human intergenic regions, and in the Genotype-Tissue Expression project (GTEx) portal to obtain additional eQTL data [45].
Results
Incident T2D
A total of 371 SNPs met the p<10−3 threshold for incident T2D in discovery stage analyses and were carried forward to the replication stage. Of these, 171 were identified among EA individuals and 200 were identified in AA individuals; no SNP was identified in both subgroups (S2 Table).
In meta-analysis of discovery and replication estimates, five SNPs were significant for potential interaction at p<1×10−7 by at least one criterion, and two of these were significant by two criteria (Table 1). Two SNPs had significant joint effects in the overall model and significant main effects in only one smoking stratum in stratified analyses: rs140637 (FBN1 on chromosome 15, MAF = 0.13) among AA smokers and rs1444261 (closest gene C2orf63 on chromosome 2, MAF = 0.05) among EA nonsmokers. Among AA participants, rs140637 in FBN1 was consistently associated with lower T2D risk among smokers only. In the discovery, replication, and combined stage meta-analyses, the per-allele HR for T2D was 0.34 (95% CI = 0.23, 0.51, p = 8.8 x 10−8), 0.39 (95% CI = 0.20, 0.76, p = 5.3 x 10−3), and 0.34 (95% CI = 0.24, 0.49, p = 2.9 x 10−9), respectively. For rs1444261 near C2orf63, in the discovery stage, the per-allele hazard ratio (HR) for T2D was 0.64 (95% CI = 0.51, 0.82, p = 3.7 x 10−4) among never smokers, but the direction of effect reversed in the replication stage (HR 1.24, 95% CI = 1.18, 1.29, p = 3.1 x 10−21) and overall meta-analysis (HR 1.21, 95% CI = 1.16, 1.26, p = 5.1 x 10−18).
Table 1. Results of discovery (D), replication (R), and combined (D+R) stage meta-analyses of genotype-by-ever smoking for incident type 2 diabetes (T2D).
Bold text indicates a significant potential interaction effect between a SNP and smoking by at least one of the following criteria: (1) significant SNP-by-smoking interaction (p_int); (2) significant joint 2 degree of freedom test of interaction and main effect, excluding SNPs with significant main effects (p_joint); or (3) significant SNP effect in only one smoking stratum (ever or never smokers, p_ever or p_never). No locus met D+R significance at p<10−7 for association with baseline fasting glucose.
| Trait | Race | SNP | CHr | Position | A1 | A2 | Freq1 | Closest gene | Stage | beta_main | p_main | beta_int | p_int | p_joint | beta_ever | p_ever | beta_never | p_never |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T2D | EA | rs1444261 | 2 | 55207970 | T | C | C2orf63 | D | -2.00E-01 | 1.8E-02 | 3.30E-01 | 5.3E-02 | 2.8E-03 | 8.00E-02 | 4.8E-01 | -4.40E-01 | 3.7E-04 | |
| 0.95 | R | 3.90E-03 | 8.4E-01 | -1.87E-01 | 1.6E-04 | 1.6E-23 | -1.64E-02 | 7.1E-01 | 2.11E-01 | 3.1E-21 | ||||||||
| D+R | -6.80E-03 | 7.3E-01 | -1.47E-01 | 2.0E-03 | 2.5E-20 | -2.44E-02 | 5.5E-01 | 1.90E-01 | 5.1E-18 | |||||||||
| T2D | EA | rs4132670 | 10 | 114757761 | A | G | TCF7L2 | D | 2.30E-01 | 4.0E-07 | 6.90E-03 | 9.4E-01 | 2.8E-06 | 2.41E-01 | 1.5E-05 | 2.23E-01 | 3.5E-03 | |
| 0.30 | R | 5.30E-02 | 5.4E-09 | 2.27E-02 | 3.1E-01 | 2.7E-09 | 9.89E-02 | 9.9E-07 | 4.15E-02 | 4.5E-05 | ||||||||
| D+R | 6.00E-02 | 1.7E-11 | 2.18E-02 | 3.2E-01 | 1.3E-12 | 1.16E-01 | 9.6E-10 | 4.48E-02 | 8.9E-06 | |||||||||
| T2D | EA | rs12243326 | 10 | 114778805 | T | C | TCF7L2 | D | -2.54E-01 | 3.7E-08 | -1.24E-02 | 8.9E-01 | 2.3E-07 | -2.71E-01 | 1.5E-06 | -2.21E-01 | 4.9E-03 | |
| 0.74 | R | -4.84E-02 | 2.6E-07 | -1.50E-02 | 5.1E-01 | 3.5E-07 | -8.45E-02 | 4.4E-05 | -3.80E-02 | 3.1E-04 | ||||||||
| D+R | -5.67E-02 | 7.1E-10 | -1.48E-02 | 5.0E-01 | 1.3E-10 | -1.07E-01 | 3.2E-08 | -4.13E-02 | 7.5E-05 | |||||||||
| T2D | AA | rs1801232 | 10 | 16910918 | T | G | CUBN | D | 7.77E-01 | 8.2E-06 | 6.95E-01 | 1.1E-01 | 4.7E-07 | 9.67E-01 | 5.0E-07 | 2.72E-01 | 4.9E-01 | |
| 0.12 | R | 1.20E-03 | 9.9E-01 | 1.39E+00 | 6.4E-02 | 1.7E-01 | 1.29E+00 | 4.2E-02 | -3.12E-01 | 4.6E-01 | ||||||||
| D+R | 6.24E-01 | 6.4E-05 | 8.64E-01 | 2.0E-02 | 1.3E-07 | 1.02E+00 | 5.5E-08 | 9.70E-03 | 9.7E-01 | |||||||||
| T2D | AA | rs140637 | 15 | 46554147 | A | G | FBN1 | D | -6.38E-01 | 1.4E-03 | -1.27E+00 | 6.0E-03 | 2.8E-06 | -1.07E+00 | 8.8E-08 | 1.25E-01 | 7.6E-01 | |
| 0.87 | R | -5.27E-01 | 1.6E-02 | -7.25E-01 | 1.3E-01 | 6.9E-03 | -9.41E-01 | 5.3E-03 | 5.40E-03 | 9.9E-01 | ||||||||
| D+R | -5.88E-01 | 6.7E-05 | -1.01E+00 | 2.2E-03 | 2.2E-08 | -1.07E+00 | 2.9E-09 | 5.49E-02 | 8.3E-01 |
Abbreviations: A: allele, AA: African-American, Chr: chromosome, EA: European-American. Freq1: allele frequency of the coded effect allele (A1).
Three additional SNPs were significant by one criterion only, namely, significant main effect only among smokers in stratified analyses. Among EA smokers, these included rs4132670 (MAF = 0.30) and rs12243326 (MAF = 0.26), both in the well-described T2D-associated gene TCF7L2. Among AA smokers, rs1801232, a missense SNP in CUBN on chromosome 10 (MAF = 0.12), exhibited a significant main effect (S1 Fig). We observed the largest effect size for potential interaction at this CUBN missense variant, where the per-allele hazard ratio for T2D was 2.78 (95% CI = 1.92, 4.03, p = 5.5 x 10−8) among smokers and 1.01 (95% CI = 0.58, 1.77, p = 0.97) among non-smokers (pjoint = 1.3 x 10−7).
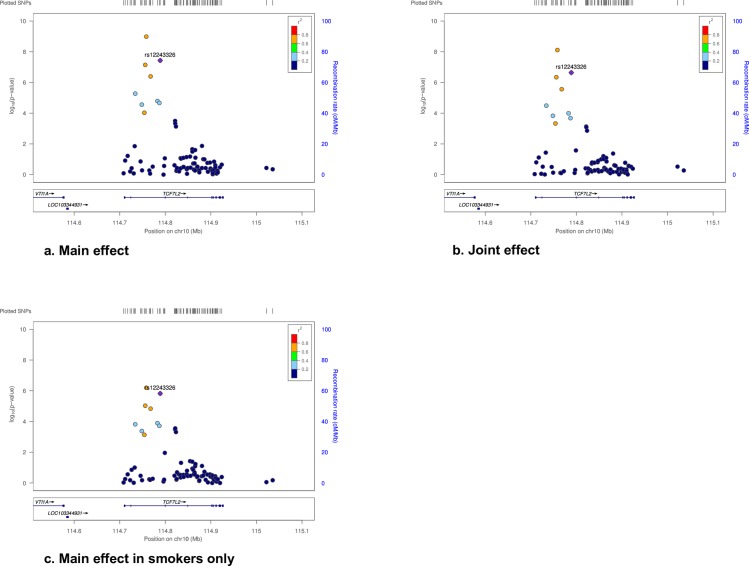
We provide regional plots for rs1224336 in TCF7L2 in Fig 1 because the discovery stage, replication stage, and combined meta-analysis showed chip-wide significance for joint effect and main effect in smokers among EA participants. Among smokers and non-smokers, the per-allele HR for T2D in the discovery plus replication meta-analysis was 0.90 (95% CI = 0.86, 0.93, p = 3.2 x 10−8) and 0.96 (95% CI = 0.94, 0.98, p = 7.5 x 10−5), respectively. In analyses conditioned on rs12243326, rs4132670 (r2 = 0.72 and D' = 0.95) was no longer significantly associated with main effect with T2D (all p>0.4).
Fig 1. Regional plots for the association of rs1224336 in TCF7L2 with T2D.
Fasting glucose
In the discovery stage analysis for baseline FG among 23,189 participants, we observed 343 SNPs meeting the significance threshold of p<10−3 in at least one of the three planned strategies for potential interaction: 175 among EA participants and 168 among AA participants. Again, no locus was identified in both ancestral subgroups (S3 Table). Meta-analysis identified rs4132670 in TCF7L2 (MAF = 0.30) as the most significant variant for the joint effect analysis in EA participants only (p = 4.6 x10-8), but it did not meet the criteria for potential interaction because its main effect association was also significant (p = 2.8 ×10−10)
Locus characterization
Of the five SNPs at four loci achieving statistical significance in the GEWIS analyses (TCF7L2, CUBN, FBN1, and near C2orf63), only rs12243326, an intronic variant in TCF7L2, has trait associations in the NHGRI-EBI GWAS Catalog, with the glycemic traits of 2-hour glucose challenge, fasting insulin, FG, and BMI interaction on FG. Of the five SNPs at four loci achieving statistical significance in the GEWIS analyses (TCF7L2, CUBN, FBN1, and near C2orf63), only the missense CUBN SNP is a nonsynonymous variant. All five GEWIS-significant SNPs overlap with at least one promoter or enhancer regulatory mark in at least one tissue with relevance to diabetes, including brain, muscle, gastrointestinal tract, pancreas, adipose, and liver (S4 Table). SNPs at three of the four loci (C2orf63, TCF7L2, and CUBN) had eQTL associations, and SNPs at all four loci overlap with either a DNA-binding site or alter a predicted DNA-binding motif (S4 Table).
Discussion
Using data from 61,164 participants from 19 cohort studies, we performed two GEWIS to identify potential SNP-by-smoking interactions in the risk of T2D and baseline FG. We identified potential interactions between smoking status and five SNPs at or near four genes (TCF7L2, CUBN, C2orf63 (closest gene), and FBN1) on the risk of incident T2D in EA or AA participants. We identified no significant SNP-smoking interactions for FG.
The relationship between smoking and T2D is complex and likely results from both confounding and true causal relationships [46]. Smokers are less likely to be physically active [47] and more likely to have unhealthier dietary intake [48, 49]. Still, a meta-analysis of 25 prospective studies by Willi found that smokers had a risk ratio for incident T2D of 1.44 (95% CI 1.31, 1.58) over 5 to 30 years of follow-up after adjustment, when possible, for BMI, physical activity, and other potential confounders. Individuals with the greatest smoking exposure had the greatest T2D risk [5]. Moreover, experimental data suggest plausible causal pathways between smoking and T2D. First, smoking generates reactive oxygen species (ROS) [50], which decrease in vitro insulin-mediated glucose transport [11]. Second, smoking stimulates the sympathetic system and cortisol release, increasing central obesity and insulin resistance [12–14]. Nicotine may mediate these pathways, as it increases insulin resistance [15–18], possibly through increased ROS production and TNF-α expression [18]. Nicotine also decreases insulin secretion from pancreatic β-cells [19], and fetal and neonatal exposure to nicotine results in β-cell dysfunction and apoptosis [20,21]. GEWIS might help elucidate additional biological pathways to explain the relationship between smoking and T2D. A linkage disequilibrium regression score study of 276 genetic correlations among 24 traits found no genetic correlation between smoking status and either T2D or FG [51], but one small study has reported that smoking status accounted for 22% of the gene-environment variance in β-cell function, as measured by the homeostatic model assessment (HOMA-β) [52].
We observed the largest potential interaction effect size at the missense SNP rs1801232 in the CUBN gene in individuals of African ancestry, where the per-allele hazard ratio for T2D was 2.78 (95% CI = 1.92, 4.03, p = 5.5 x 10−8) among smokers and 1.01 (95% CI = 0.58, 1.77, p = 0.97) among non-smokers (pjoint = 1.3 x 10−7). Cubilin is a component of the vitamin B12-intrinsic factor complex receptor in the ileal mucosa [53], and it is expressed in the apical brush border of the renal proximal tubule, where it participates in receptor-mediated endocytosis of low-molecular-weight proteins [54]. Defects in the CUBN gene have been associated with both vitamin B12 deficiency and proteinuria, and the absence of cubilin results in the autosomal recessive condition Imerslund-Gräsbeck syndrome, characterized by B12 malabsorption and variable levels of proteinuria from impaired renal protein reabsorption [55]. Mice heterozygous for CUBN deletion have increased albuminuria and decreased levels of blood albumin and high-density lipoprotein (HDL) cholesterol [56]. The CKDGen consortium meta-analysis identified a missense SNP in CUBN (rs18801239) associated with urinary albumin/creatinine ratio and clinical microalbuminuria in the general population, an association replicated in an AA cohort with type 1 diabetes [57] and later in the Framingham Offspring Study [58]. This SNP appears independent from the CUBN SNP identified in the present analysis: in conditional analyses on rs18801239 in the discovery cohort, we found that rs18801232 remained significantly associated with incident T2D among AA smokers only. These CUBN observations point to plausible mechanisms, namely depressed levels of vitamin B12 and HDL cholesterol, through which smoking might interact with cubilin to cause T2D. Cigarette smoking impairs cubilin-mediated renal protein reabsorption through cadmium and other contaminants, which form complexes with proteins that have high affinity for cubilin and accumulate in the proximal tubule [59]. A mendelian randomization study found an association between a genetic instrument for low vitamin B12 levels (including one CUBN variant) and higher fasting glucose levels and lower pancreatic beta-cell secretory function, as measured by HOMA-β, but not with higher odds of T2D [60]. Mendelian randomization studies have been inconsistent in whether genetic instruments for low HDL are associated with increased T2D risk [61–64]. Whether CUBN defects and smoking interact to cause T2D through these or other mechanisms merits further investigation.
We observed more modest potential interaction effects at four other SNPs. Among AA participants, one SNP in FBN1 was associated with T2D only in smokers. The glycoprotein fibrillin-1 is a component of microfibrils in the extracellular matrix, which contribute to the elasticity of skin, blood vessels, and other tissues. Variants in FBN1 are associated with Marfan syndrome, an autosomal dominant connective tissue disorder characterized by ocular, skeletal, and cardiovascular abnormalities, including aortic dilatation and cardiac valve regurgitation [65]. Among EA participants, one locus near C2orf63, which encodes a neurite outgrowth inhibitor, was associated with T2D only in never smokers. This observation may suggest either a protective role of smoking in the association of C2orf63 and T2D or an C2orf63-T2D association otherwise obscured by the association between smoking and T2D. The two remaining loci we identified were in TCF7L2, a gene whose well-established association with T2D was first identified in 2006 and which remains the locus with the largest effect on T2D risk [66–68]. Variants in TCF7L2 are associated with decreased pancreatic beta-cell function [69,70] and incretin sensitivity [71], and their association with increased proinsulin levels suggest defects in insulin processing and secretion [72]. Experimental models support the role of TCF7L2 variants in developmental beta cell proliferation, proinsulin processing, and insulin vesicle docking [73].
Examination of the functional genomic annotation of the GEWIS-significant SNPs generates novel biological hypotheses. For example, allele-specific differential gene expression impacting glucose homeostasis in smokers versus non-smokers could explain the observed potential gene-smoking interaction. A mechanism of interaction involving gene expression would be consistent with all five statistically-significant SNPs being associated with regulatory histone marks. Even the missense variant in the CUBN gene overlaps with regulatory annotation in numerous tissues, including active enhancer histone marks in muscle, adipose, pancreas, and liver, and tags multiple DNA-binding protein sites. Similarly, the intergenic SNP at the C2orf63 locus overlaps with both active enhancer and promoter histone marks from brain/neural tissues. The intronic variant in the FBN1 gene overlaps with promoter and/or active enhancer marks in brain, muscle, adipose, gastrointestinal tract, or pancreatic tissues. Finally, each of the two intronic SNPs at the TCF7L2 locus has a slightly different pattern of regulatory annotation. In addition, the pattern of regulatory marks overlapping the two TCF7L2 SNPs identified in this study differs from the regulatory annotation related to the lead TCF7L2 SNP associated in T2D case-control GWAS, suggesting multiple, potentially distinct regulatory mechanisms underlying T2D in smokers and non-smokers. Further work is required to illuminate how smoking might modify biologic pathways, including gene regulation, and may suggest novel targets for diabetes therapy.
Prior studies of gene-smoking interaction for T2D risk have used a candidate gene approach, focusing on loci associated either with smoking behavior, such as CYP2A6 [74] or the nicotinic acetylcholine receptor gene (CHRNA4) [75], or with T2D and other metabolic traits [76], including HNF1A [77] and APOC3 [78]. Our analyses did not replicate the findings of these small candidate-gene studies at our predefined genome-wide significance thresholds, highlighting unique contributions using unbiased GEWIS approaches. Limitations of our study include the dichotomous categorization of the smoking exposure (ever vs. never), which likely masks some of the effect of smoking dose and duration on our outcomes of interest. Nonetheless, similar approaches have successfully identified gene-smoking interactions for traits such as blood pressure [79], pulmonary function [80], and BMI [81]. Second, a locus identified by the inclusion of a significant joint test as one criterion for potential locus-smoking interaction may actually have a significant main effect, not a significant interaction with smoking, if the inclusion of smoking in the model explained residual variability in the outcome and increased power to detect main effects. To limit the impact of this misclassification, we excluded SNPs with significant main effects from eligibility for this criterion. Third, although we used data from about 75,000 individuals across the CHARGE Consortium Gene-Lifestyle Interactions Working Group to replicate our discovery analyses, data from larger cohorts such as the UK Biobank and Million Veteran Program now exist and might provide future opportunity for additional replication. Fourth, our discovery analyses only leveraged genotype data from the IBC array available from the CARe Consortium; the use of increasingly available sequencing data from large cohort studies might enable the detection of rare variants that mediate the relationship between smoking and glycemic traits. Fifth, the lack of adequate numbers of T2D cases in all sex/ancestry groups impeded adjustment for sex in some models. It is unknown whether this lack of sex adjustment biased the results and, if so, the direction and magnitude of effect. Larger studies in individuals of non-European ancestry are needed to address this limitation.
Conclusions
We have demonstrated the feasibility and utility of GEWIS to identify potential gene-smoking interactions in T2D risk. Future mechanistic study of the loci identified may help untangle the complex relationship between the dual public health threats of T2D and smoking.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(PDF)
Acknowledgments
A portion of this research utilized the Linux Cluster for Genetic Analysis (LinGA-II) funded by the Robert Dawson Evans Endowment of the Department of Medicine at Boston University School of Medicine and Boston Medical Center.
Genotyping of GENOA was performed at the Mayo Clinic (Stephen T. Turner, MD, Mariza de Andrade PhD, Julie Cunningham, PhD). We thank Eric Boerwinkle, PhD and Megan L. Grove from the Human Genetics Center and Institute of Molecular Medicine and Division of Epidemiology, University of Texas Health Science Center, Houston, Texas, USA for their help with genotyping. We would also like to thank the families that participated in the GENOA study.
The Mount Sinai IPM Biobank Program is supported by The Andrea and Charles Bronfman Philanthropies.
The Rotterdam Study is supported by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam.The authors are grateful to the study participants, the staff from the Rotterdam Study and the participating general practitioners and pharmacists. The generation and management of GWAS genotype data for the Rotterdam Study (RS I, RS II, RS III) was executed by the Human Genotyping Facility of the Genetic 20 Laboratory of the Department of Internal Medicine, Erasmus MC, Rotterdam, The Netherlands. The GWAS datasets are additionally supported by the Genetic Laboratory of the Department of Internal Medicine, Erasmus MC. We thank Pascal Arp, Mila Jhamai, Marijn Verkerk, Lizbeth Herrera and Marjolein Peters, MSc, and Carolina Medina-Gomez, MSc, for their help in creating the GWAS database, and Karol Estrada, PhD, Yurii Aulchenko, PhD, and Carolina Medina-Gomez, MSc, for the creation and analysis of imputed data.
The ERF study as a part of EUROSPAN (European Special Populations Research Network) was additionally supported by ENGAGE consortium and CMSB. We are grateful to all study participants and their relatives, general practitioners and neurologists for their contributions and to P. Veraart for her help in genealogy, J. Vergeer for the supervision of the laboratory work, P. Snijders for his help in data collection and E.M. van Leeuwen for genetic imputation.
This research was conducted in part using data and resources from the Framingham Heart Study of the National Heart Lung and Blood Institute of the National Institutes of Health and Boston University School of Medicine. The analyses reflect intellectual input and resource development from the Framingham Heart Study investigators participating in the SNP Health Association Resource (SHARe) project.
The HyperGEN (Hypertension Genetic Epidemiology Network) study involves University of Utah: (Network Coordinating Center, Field Center, and Molecular Genetics Lab); Univ. of Alabama at Birmingham: (Field Center and Echo Coordinating and Analysis Center); Medical College of Wisconsin: (Echo Genotyping Lab); Boston University: (Field Center); University of Minnesota: (Field Center and Biochemistry Lab); University of North Carolina: (Field Center); Washington University: (Data Coordinating Center); Weill Cornell Medical College: (Echo Reading Center); National Heart, Lung, & Blood Institute. For a complete list of HyperGEN Investigators:http://www.biostat.wustl.edu/hypergen/Acknowledge.html.
The AGES study is additionally supported by the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart 21 Association), and the Althingi (the Icelandic Parliament).
Data Availability
Our study data are now available at the following URL on the AMP T2D Knowledge Portal: http://www.kp4cd.org/dataset_downloads/t2d.
Funding Statement
WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. The grant funding of WHI are R21 HL123677, R56 DK104806 and R01 MD012765 to NF. The FamHS was funded by R01HL118305 and R01HL117078 NHLBI grants, and 5R01DK07568102 and 5R01DK089256 NIDDK grant." and "The Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health (project # Z01-AG000513 and human subjects protocol number 09-AGN248). Support for GENOA was provided by the National Heart, Lung and Blood Institute (HL119443, HL087660, HL054464, HL054457, and HL054481) of the National Institutes of Health. Ruth loos is supported by the NIH (R01DK110113, U01HG007417, R01DK101855, R01DK107786). The Rotterdam Study GWAS datasets are supported by the Netherlands Organisation of Scientific Research NWO Investments (nr. 175.010.2005.011, 911-03-012), the Research Institute for Diseases in the Elderly (014-93-015; RIDE2), and the Netherlands Genomics Initiative (NGI)/Netherlands Organisation for Scientific Research (NWO) Netherlands Consortium for Healthy Aging (NCHA), project nr. 050-060-810. The ERF study as a part of EUROSPAN (European Special Populations Research Network) was supported by European Commission FP6 STRP grant number 018947 (LSHG-CT-2006- 01947) and also received funding from the European Community's Seventh Framework Programme (FP7/2007-2013)/grant agreement HEALTH-F4-2007-201413 by the European Commission under the programme "Quality of Life and Management of the Living Resources" of 5th Framework Programme (no. QLG2-CT-2002- 01254). The ERF study was further supported by ENGAGE consortium and CMSB. Highthroughput analysis of the ERF data was supported by joint grant from Netherlands Organisation for Scientific Research and the Russian Foundation for Basic Research (NWORFBR 047.017.043).ERF was further supported by the ZonMw grant (project 91111025), and this work was partially supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No. N01-HC25195) and its contract with Affymetrix, Inc for genotyping services (Contract No. N02-HL-6- 4278). This study is also supported by National Institute for Diabetes and Digestive and Kidney Diseases (NIDDK) R01 DK078616 to Drs. Meigs, Dupuis and Florez, NIDDK K24 DK080140 to Dr. Meigs, and a Doris Duke Charitable Foundation Clinical Scientist Development Award to Dr. Florez. The HERITAGE Family Study was supported by National Heart, Lung, and Blood Institute grant HL-45670. The Women's Genome Health Study is supported by the National Heart, Lung, and Blood Instutute (HL043851 and HL080467) and the National Cancer Institute (CA047988 and UM1CA182913). Additional support for endpoint collection was provided by the National Heart, Lung, and Blood Institute under ARRA funding (HL099355). HyperGEN (Hypertension Genetic Epidemiology Network): The hypertension network is funded by cooperative agreements (U10) with NHLBI: HL54471, HL54472, HL54473, HL54495, HL54496, HL54497, HL54509, HL54515, and 2 R01 HL55673- 12. The AGES study has been funded by NIH contracts N01-AG-1-2100 and 271201200022C. Caroline Hayward is supported by an MRC University Unit Programme Grant MC_UU_00007/10 (QTL in Health and Disease)”and “Generation Scotland received core funding from the Chief Scientist Office of the Scottish Government Health Directorate CZD/16/6, the Scottish Funding Council HR03006 and the Wellcome Trust through a Strategic Award (reference 104036/Z/14/Z) for Stratifying Resilience and Depression Longitudinally (STRADL). Genotyping was funded by the UK's Medical Research Council. Jose C. Florez, NIDDK K24 DK110550 The MESA project is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators. Support for MESA is provided by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-000040, UL1-TR-001079, UL1-TR-001420, UL1-TR-001881, and DK063491. Additionally, one or more authors are affiliated with the following commercial entities: Interleukin Genetics, GlaxoSmithKline, Daiichi-Sankyo, AstraZeneca, Data Tecnica International LLC, Illumina Inc., University of California Healthcare, Janssen Pharmaceuticals, Goldfinch Bio, and Novo Nordisk. Please see the Competing Interests Statement for additional details. The funders provided support in the form of salaries for authors but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 2014. [cited 2019 12 June]. [Google Scholar]
- 2.Global Report on Diabetes Geneva, Switzerland: World Health Organzation; 2016 [cited 2019 12 June]. Available from: (https://www.who.int/diabetes/global-report/en/).
- 3.WHO report on the global tobacco epidemic 2017. 2017 [cited 2019 12 June]. Available from: (https://www.who.int/tobacco/global_report/2017/en/).
- 4.National Diabetes Statistics Report Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017 [cited 2019 12 June].
- 5.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298:2654–64. 10.1001/jama.298.22.2654 . [DOI] [PubMed] [Google Scholar]
- 6.Nakanishi N, Nakamura K, Matsuo Y, Suzuki K, Tatara K. Cigarette smoking and risk for impaired fasting glucose and type 2 diabetes in middle-aged Japanese men. Annals of Internal Medicine. 2000;133:183–91. 10.7326/0003-4819-133-3-200008010-00009 . [DOI] [PubMed] [Google Scholar]
- 7.Rafalson L, Donahue RP, Dmochowski J, Rejman K, Dorn J, Trevisan M. Cigarette Smoking Is Associated with Conversion from Normoglycemia to Impaired Fasting Glucose: The Western New York Health Study. Annals of Epidemiology. 2009;19:365–71. 10.1016/j.annepidem.2009.01.013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, et al. Normal fasting plasma glucose levels and type 2 diabetes in young men. New England Journal of Medicine. 2005;353:1454–62. 10.1056/NEJMoa050080 . [DOI] [PubMed] [Google Scholar]
- 9.Gerstein HC, Santaguida P, Raina P, Morrison KM, Bailon C, Hunt D, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: A systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract 2007;78(3):305–12. 10.1016/j.diabres.2007.05.004 [DOI] [PubMed] [Google Scholar]
- 10.Meigs JB, Nathan DM, Wilson PWF, Cupples LA, Singer DE. Metabolic risk factors worsen continuously across the spectrum of nondiabetic glucose tolerance: The Framingham Offspring Study. Annals of Internal Medicine. 1998;128:524–33. 10.7326/0003-4819-128-7-199804010-00002 . [DOI] [PubMed] [Google Scholar]
- 11.Dokken BB, Saengsirisuwan V, Kim JS, Teachey MK, Henriksen EJ. Oxidative stress-induced insulin resistance in rat skeletal muscle: role of glycogen synthase kinase-3. Am J Physiol Endocrinol Metab. 2008;294:E615–21. 10.1152/ajpendo.00578.2007 . [DOI] [PubMed] [Google Scholar]
- 12.Friedman AJ, Ravnikar VA, Barbieri RL. Serum steroid hormone profiles in postmenopausal smokers and nonsmokers. Fertility and Sterility. 1987;47:398–401. 10.1016/S0015-0282(16)59044-X [DOI] [PubMed] [Google Scholar]
- 13.Hofstetter A, Schutz Y, Jéquier E, Wahren J. Increased 24-Hour Energy Expenditure in Cigarette Smokers. New England Journal of Medicine. 1986;314:79–82. 10.1056/NEJM198601093140204 . [DOI] [PubMed] [Google Scholar]
- 14.Cryer P HM, Santiago J, & Shah S. Norepinephrine and Epinephrine release and Adrenergic Mediation od Smoking-Associated Hemodynamicand Metabolic Events. New England Journal of Medicine. 1976;295:573–7. 10.1056/NEJM197609092951101 . [DOI] [PubMed] [Google Scholar]
- 15.Assali AR, Beigel Y, Schreibman R, Shafer Z, Fainaru M. Weight gain and insulin resistance during nicotine replacement therapy. Clinical Cardiology. 1999;22:357–60. 10.1002/clc.4960220512 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Attvall S, Fowelin J, Lager I, Von Schenck H, Smith U. Smoking induces insulin resistance—a potential link with the insulin resistance syndrome. Journal of Internal Medicine. 1993;233:327–32. 10.1111/j.1365-2796.1993.tb00680.x . [DOI] [PubMed] [Google Scholar]
- 17.Axelsson T, Jansson PA, Smith U, Eliasson B. Nicotine infusion acutely impairs insulin sensitivity in type 2 diabetic patients but not in healthy subjects. Journal of Internal Medicine. 2001;249:539–44. 10.1046/j.1365-2796.2001.00840.x . [DOI] [PubMed] [Google Scholar]
- 18.Tatebe J, Morita T. Enhancement of TNF-α expression and inhibition of glucose uptake by nicotine in the presence of a free fatty acid in C2C12 skeletal myocytes. Hormone and Metabolic Research. 2011;43:11–6. 10.1055/s-0030-1267996 . [DOI] [PubMed] [Google Scholar]
- 19.Yoshikawa H, Hellström-Lindahl E, Grill V. Evidence for functional nicotinic receptors on pancreatic beta cells. Metabolism. 2005;54:257–54. 10.1016/j.metabol.2004.08.020 . [DOI] [PubMed] [Google Scholar]
- 20.Bruin JE, Gerstein HC, Morrison KM, Holloway AC. Increased pancreatic beta-cell apoptosis following fetal and neonatal exposure to nicotine is mediated via the mitochondria. Toxicological Sciences. 2008;103:362–70. 10.1093/toxsci/kfn012 . [DOI] [PubMed] [Google Scholar]
- 21.Bruin JE, Petre MA, Raha S, Morrison KM, Gerstein HC, Holloway AC. Fetal and neonatal nicotine exposure in wistar rats causes progressive pancreatic mitochondrial damage and beta cell dysfunction. PLoS ONE. 2008:e3371 10.1371/journal.pone.0003371 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.David SP, Hamidovic A, Chen GK, Bergen AW, Wessel J, Kasberger JL, et al. Genome-wide meta-analyses of smoking behaviors in African Americans. Transl Psychiatry. 2012;2:e119 Epub 2012/07/27. 10.1038/tp.2012.41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon D, Kim YJ, Cui WY, Van der Vaart A, Cho YS, Lee JY, et al. Large-scale genome-wide association study of Asian population reveals genetic factors in FRMD4A and other loci influencing smoking initiation and nicotine dependence. Hum Genet. 2012;131(6):1009–21. Epub 2011/10/19. 10.1007/s00439-011-1102-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nature Genetics. 2010;42(5):448–53. Epub 2010/04/27. 10.1038/ng.573 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nature Genetics. 2010;42(5):441–7. Epub 2010/04/27. 10.1038/ng.571 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nature Genetics. 2019;51(2):237–44. Epub 2019/01/14. 10.1038/s41588-018-0307-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erzurumluoglu AM, Liu M, Jackson VE, Barnes DR, Datta G, Melbourne CA, et al. Meta-analysis of up to 622,409 individuals identifies 40 novel smoking behaviour associated genetic loci. Mol Psychiatry. 2019:10.1038/s41380-018-0313-0. 10.1038/s41380-018-0313-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris AP, Voight BF, Teslovich TM, Ferreira T, Segre AV, Steinthorsdottir V, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nature Genetics. 2012;44(9):981–90. Epub 2012/08/14. 10.1038/ng.2383 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nature Genetics. 2010;42(2):105–16. 10.1038/ng.520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu CT, Ng MC, Rybin D, Adeyemo A, Bielinski SJ, Boerwinkle E, et al. Transferability and fine-mapping of glucose and insulin quantitative trait loci across populations: CARe, the Candidate Gene Association Resource. Diabetologia. 2012;55(11):2970–84. Epub 2012/08/16. 10.1007/s00125-012-2656-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooke JN, Ng MC, Palmer ND, An SS, Hester JM, Freedman BI, et al. Genetic risk assessment of type 2 diabetes-associated polymorphisms in African Americans. Diabetes Care. 2012;35(2):287–92. Epub 2012/01/26. 10.2337/dc11-0957 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manning AK, Hivert MF, Scott RA, Grimsby JL, Bouatia-Naji N, Chen H, et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nature Genetics. 2012;44(6):659–69. Epub 2012/05/15. 10.1038/ng.2274 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott RA, Scott LJ, Magi R, Marullo L, Gaulton KJ, Kaakinen M, et al. An Expanded Genome-Wide Association Study of Type 2 Diabetes in Europeans. Diabetes. 2017;66(11):2888–902. Epub 2017/06/02. 10.2337/db16-1253 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahajan A, Taliun D, Thurner M, Robertson NR, Torres JM, Rayner NW, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nature Genetics. 2018:(epub ahead of print). Epub 2018/10/10. 10.1038/s41588-018-0241-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuchsberger C, Flannick J, Teslovich TM, Mahajan A, Agarwala V, Gaulton KJ, et al. The genetic architecture of type 2 diabetes. Nature. 2016;536(7614):41–7. Epub 2016/07/12. 10.1038/nature18642 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flannick J, Mercader JM, Fuchsberger C, Udler MS, Mahajan A, Wessel J, et al. Exome sequencing of 20,791 cases of type 2 diabetes and 24,440 controls. Nature. 2019;570(7759):71–6. Epub 2019/05/22. 10.1038/s41586-019-1231-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musunuru K, Lettre G, Young T, Farlow DN, Pirruccello JP, Ejebe KG, et al. Candidate gene association resource (CARe): design, methods, and proof of concept. Circ Cardiovasc Genet. 2010;3(3):267–75. Epub 2010/04/20. 10.1161/CIRCGENETICS.109.882696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao DC, Sung YJ, Winkler TW, Schwander K, Borecki I, Cupples LA, et al. Multiancestry Study of Gene-Lifestyle Interactions for Cardiovascular Traits in 610 475 Individuals From 124 Cohorts: Design and Rationale. Circ Cardiovasc Genet. 2017;10(3). Epub 2017/06/18. 10.1161/circgenetics.116.001649 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keating BJ, Tischfield S, Murray SS, Bhangale T, Price TS, Glessner JT, et al. Concept, design and implementation of a cardiovascular gene-centric 50 K SNP array for large-scale genomic association studies. PLoS ONE. 2008;3:e3583 10.1371/journal.pone.0003583 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lo KS, Wilson JG, Lange LA, Folsom AR, Galarneau G, Ganesh SK, et al. Genetic association analysis highlights new loci that modulate hematological trait variation in Caucasians and African Americans. Human Genetics. 2011;129:307–17. 10.1007/s00439-010-0925-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu C-T, Garnaas MK, Tin A, Kottgen A, Franceschini N, Peralta CA, et al. Genetic association for renal traits among participants of African ancestry reveals new loci for renal function. PLoS Genetics. 2011;79:e1002264 10.1371/journal.pgen.1002264 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47(D1):D1005–d12. Epub 2018/11/18. 10.1093/nar/gky1120 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward LD, Kellis M. HaploReg: A resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Research. 2012;40D1:D930–D4. 10.1093/nar/gkr917 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;22(9):1790–7. Epub 2012/09/08. 10.1101/gr.137323.112 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lonsdale J, Thomas J, Salvatore M, Phillips R, Lo E, Saboor S, et al. The Genotype-Tissue Expression (GTEx) project. Nature Genetics. 2013;45:580–585. 10.1038/ng.2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. American Journal of Clinical Nutrition. 2008;87:801–9. 87/4/801 [pii]. 10.1093/ajcn/87.4.801 . [DOI] [PubMed] [Google Scholar]
- 47.Klesges RC, Eck LH, Isbell TR, Fulliton W, Hanson CL. Smoking status: effects on the dietary intake, physical activity, and body fat of adult men. American Journal of Clinical Nutrition. 1990;51:784–9. 10.1093/ajcn/51.5.784 . [DOI] [PubMed] [Google Scholar]
- 48.Thompson RL, Margetts BM, Wood DA, Jackson AA. Cigarette smoking and food and nutrient intakes in relation to coronary heart disease. Nutr Res Rev. 1992;5(1):131–52. 10.1079/NRR19920011 . [DOI] [PubMed] [Google Scholar]
- 49.Serdula MK, Byers T, Mokdad AH, Simoes E, Mendlein JM, Coates RJ. The association between fruit and vegetable intake and chronic disease risk factors. Epidemiology. 1996;7(2):161–5. 10.1097/00001648-199603000-00010 . [DOI] [PubMed] [Google Scholar]
- 50.Nowak D, Antczak A, Krol M, Pietras T, Shariati B, Bialasiewicz P, et al. Increased content of hydrogen peroxide in the expired breath of cigarette smokers. European Respiratory Journal. 1996;9:652–7. 10.1183/09031936.96.09040652 . [DOI] [PubMed] [Google Scholar]
- 51.Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, et al. An atlas of genetic correlations across human diseases and traits. Nature Genetics. 2015;47(11):1236–41. Epub 2015/09/29. 10.1038/ng.3406 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng JS, Arnett DK, Lee YC, Shen J, Parnell LD, Smith CE, et al. Genome-Wide Contribution of Genotype by Environment Interaction to Variation of Diabetes-Related Traits. PLoS ONE. 2013;8:e77442 10.1371/journal.pone.0077442 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gräsbeck R. Imerslund-Gräsbeck syndrome (selective vitamin B12 malabsorption with proteinuria). Orphanet Journal of Rare Diseases. 2006;1(17) 10.1186/1750-1172-1-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ca Böger, Heid IM. Chronic kidney disease: novel insights from genome-wide association studies. Kidney & Blood Pressure Research. 2011;34:225–34. 10.1159/000326901 . [DOI] [PubMed] [Google Scholar]
- 55.Christensen EI, Verroust PJ, Nielsen R. Receptor-mediated endocytosis in renal proximal tubule. Pflugers Arch. 2009. October;458(6):1039–48. 10.1007/s00424-009-0685-8 [DOI] [PubMed] [Google Scholar]
- 56.Aseem O, Smith BT, Cooley MA, Wilkerson BA, Argraves KM, Remaley AT, et al. Cubilin maintains blood levels of HDL and albumin. J Am Soc Nephrol. 2014;25(5):1028–36. Epub 2013/12/21. 10.1681/ASN.2013060671 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boger CA, Chen M-H, Tin A, Olden M, Kottgen A, de Boer IH, et al. CUBN Is a Gene Locus for Albuminuria. Journal of the American Society of Nephrology. 2011;22:555–70. 10.1681/ASN.2010060598 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McMahon GM, O'Seaghdha CM, Hwang SJ, Meigs JB, Fox CS. The association of a single-nucleotide polymorphism in CUBN and the risk of albuminuria and cardiovascular disease. Nephrology Dialysis Transplantation. 2014;29:342–7. 10.1093/ndt/gft386 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fels J, Scharner B, Zarbock R, Zavala Guevara IP, Lee WK, Barbier OC, et al. Cadmium complexed with beta2-microglubulin, albumin and lipocalin-2 rather than metallothionein cause megalin:cubilin dependent toxicity of the renal proximal tubule. Int J Mol Sci. 2019;20(10). Epub 2019/05/17. 10.3390/ijms20102379 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moen GH, Qvigstad E, Birkeland KI, Evans DM, Sommer C. Are serum concentrations of vitamin B-12 causally related to cardiometabolic risk factors and disease? A Mendelian randomization study. Am J Clin Nutr. 2018;108(2):398–404. Epub 2018/07/10. 10.1093/ajcn/nqy101 . [DOI] [PubMed] [Google Scholar]
- 61.White J, Swerdlow DI, Preiss D, Fairhurst-Hunter Z, Keating BJ, Asselbergs FW, et al. Association of Lipid Fractions With Risks for Coronary Artery Disease and Diabetes. JAMA Cardiol. 2016;1(6):692–9. Epub 2016/08/04. 10.1001/jamacardio.2016.1884 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marott SC, Nordestgaard BG, Tybjaerg-Hansen A, Benn M. Components of the Metabolic Syndrome and Risk of Type 2 Diabetes. J Clin Endocrinol Metab. 2016;101(8):3212–21. Epub 2016/06/11. 10.1210/jc.2015-3777 . [DOI] [PubMed] [Google Scholar]
- 63.Haase CL, Tybjaerg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. HDL Cholesterol and Risk of Type 2 Diabetes: A Mendelian Randomization Study. Diabetes. 2015;64(9):3328–33. Epub 2015/05/15. 10.2337/db14-1603 . [DOI] [PubMed] [Google Scholar]
- 64.Fall T, Xie W, Poon W, Yaghootkar H, Magi R, Knowles JW, et al. Using Genetic Variants to Assess the Relationship Between Circulating Lipids and Type 2 Diabetes. Diabetes. 2015;64(7):2676–84. Epub 2015/05/08. 10.2337/db14-1710 . [DOI] [PubMed] [Google Scholar]
- 65.Dietz HC. Marfan Syndrome. GeneReviews(®). 2017. .20301510 [Google Scholar]
- 66.Scott RA, Scott LJ, Mägi R, Marullo L, Gaulton KJ, Kaakinen M, et al. An Expanded Genome-Wide Association Study of Type 2 Diabetes in Europeans. Diabetes. 2017;66:2888–902. 10.2337/db16-1253 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grant SFA, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nature Genetics. 2006;38:320–3. 10.1038/ng1732 . [DOI] [PubMed] [Google Scholar]
- 68.Flannick J, Mercader JM, Fuchsberger C, Udler MS, Mahajan A, Wessel J, et al. Exome sequencing of 20,791 cases of type 2 diabetes and 24,440 controls. Nature. 2019;570(7759):71–6. Epub 2019/05/24. 10.1038/s41586-019-1231-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Florez JC, Jablonski KA, Bayley N, Pollin TI, de Bakker PIW, Shuldiner AR, et al. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. New England Journal of Medicine. 2006;355:241–50. 10.1056/NEJMoa062418 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lyssenko V, Lupi R, Marchetti P, Del Guerra S, Orho-Melander M, Almgren P, et al. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest. 2007;117:2155–63. 10.1172/JCI30706 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schäfer SA, Tschritter O, Machicao F, Thamer C, Stefan N, Gallwitz B, et al. Impaired glucagon-like peptide-1-induced insulin secretion in carriers of transcription factor 7-like 2 (TCF7L2) gene polymorphisms. Diabetologia. 2007;50:2443–50. 10.1007/s00125-007-0753-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strawbridge RJ, Dupuis J, Prokopenko I, Barker A, Ahlqvist E, Rybin D, et al. Genome-wide association identifies nine common variants associated with fasting proinsulin levels and provides new insights into the pathophysiology of type 2 diabetes. Diabetes. 2011;60:2624–34. 10.2337/db11-0415 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Florez JC. Pharmacogenetics in type 2 diabetes: precision medicine or discovery tool? Diabetologia. 2017. May;60(5):800–807. 10.1007/s00125-017-4227-1 Epub 2017 Mar 10. [DOI] [PubMed] [Google Scholar]
- 74.Liu T, Chen WQ, David SP, Tyndale RF, Wang H, Chen YM, et al. Interaction between heavy smoking and CYP2A6 genotypes on type 2 diabetes and its possible pathways. European Journal of Endocrinology. 2011;165:961–7. 10.1530/EJE-11-0596 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang J, Zhu Y, Cole SA, Haack K, Zhang Y, Beebe LA, et al. A gene-family analysis of 61 genetic variants in the nicotinic acetylcholine receptor genes for insulin resistance and type 2 diabetes in American Indians. Diabetes. 2012;61:1888–94. 10.2337/db11-1393 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Uma Jyothi K, Reddy BM. Gene-gene and gene-environment interactions in the etiology of type 2 diabetes mellitus in the population of Hyderabad, India. Meta Gene. 2015;5:9–20. 10.1016/j.mgene.2015.05.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ley SH, Hegele RA, Harris SB, Mamakeesick M, Cao H, Connelly PW, et al. HNF1A G319S variant, active cigarette smoking and incident type 2 diabetes in Aboriginal Canadians: A population-based epidemiological study. BMC Medical Genetics. 2011;12:1 10.1186/1471-2350-12-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Onat A, Erginel-Unaltuna N, Çoban N, Çiçek G, Yüksel H. APOC3 -482C>T polymorphism, circulating apolipoprotein C-III and smoking: Interrelation and roles in predicting type-2 diabetes and coronary disease. Clinical Biochemistry. 2011;44:391–6. 10.1016/j.clinbiochem.2010.12.009 . [DOI] [PubMed] [Google Scholar]
- 79.Sung YJ, De Las Fuentes L, Schwander KL, Simino J, Rao DC. Gene-smoking interactions identify several novel blood pressure loci in the framingham heart study. American Journal of Hypertension. 2015;28:343–54. 10.1093/ajh/hpu149 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hancock DB, Artigas MS, Gharib SA, Henry A, Manichaikul A, Ramasamy A, et al. Genome-Wide Joint Meta-Analysis of SNP and SNP-by-Smoking Interaction Identifies Novel Loci for Pulmonary Function. PLoS Genetics. 2012;8:e1003098 10.1371/journal.pgen.1003098 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Justice AE, Winkler TW, Feitosa MF, Graff M, Fisher VA, Young K, et al. Genome-wide meta-analysis of 241,258 adults accounting for smoking behaviour identifies novel loci for obesity traits. Nature Communications. 2017;8:14977 10.1038/ncomms14977 . [DOI] [PMC free article] [PubMed] [Google Scholar]