Abstract
The Immune Deficiency (IMD) pathway in Drosophila melanogaster is activated upon microbial challenge with Gram-negative bacteria to trigger the innate immune response. In order to decipher this nuclear factor κB (NF-κB) signaling pathway, we undertook an in vitro RNAi screen targeting E3 ubiquitin ligases specifically and identified the HECT-type E3 ubiquitin ligase Hyperplastic discs (Hyd) as a new actor in the IMD pathway. Hyd mediated Lys63 (K63)-linked polyubiquitination of the NF-κB cofactor Akirin was required for efficient binding of Akirin to the NF-κB transcription factor Relish. We showed that this Hyd-dependent interaction was required for the transcription of immunity-related genes that are activated by both Relish and Akirin but was dispensable for the transcription of genes that depend solely on Relish. Therefore Hyd is key in NF-κB transcriptional selectivity downstream of the IMD pathway. Drosophila depleted of Akirin or Hyd failed to express the full set of genes encoding immune-induced anti-microbial peptides and succumbed to immune challenges. We showed further that UBR5, the mammalian homolog of Hyd, was also required downstream of the NF-κB pathway for the activation of Interleukin 6 (IL6) transcription by LPS or IL-1β in cultured human cells. Our findings link the action of an E3 ubiquitin ligase to the activation of immune effector genes, deepening our understanding of the involvement of ubiquitination in inflammation and identifying a potential target for the control of inflammatory diseases.
Author summary
Ubiquitination has been recently identified in pathogenesis and progression of various diseases where inflammation is critical. NF-κB transcription factors are key actors in the transcriptional cascade leading to inflammation as they activate genes with pro- or anti-inflammatory activities. The similarity between the immune pathways in flies and mammals makes Drosophila melanogaster an excellent model to study the innate response. Accordingly, we decided to identify E3 ubiquitin-ligases involved in the regulation of NF-κB pathway, using Drosophila as a model system. A RNAi based screen in immortalized embryonic macrophage-like Drosophila cells points to the HECT-E3 ubiquitin ligase Hyd as a new regulator of the Immune-deficiency (IMD) NF-κB pathway, activated after Gram-negative immune challenge.
More precisely, we showed that Hyd acts at the level of Akirin, an evolutionarily conserved player in the NF-κB pathway, required for the transcription of pro-inflammatory genes, but not for the NF-κB-dependent genes contributing to the down-regulation of inflammation.
In addition, we could show that the human homologue of Hyd (UBR5) acts genetically at the level of human AKIRIN2, pointing to a unique dichotomy between Hyd/Akirin-dependent and -independent gene activation, allowing for the decoupling activation and resolution of inflammation. These results identified UBR5 as a putative target for anti-inflammatory compounds.
Introduction
During evolution, metazoans developed strategies to effectively protect themselves from microbial threats. Since the molecular pathways mediating the innate immune response in insects and mammals are conserved, the fruit fly Drosophila melanogaster is a relevant model to explore the immune response [1, 2]. In Drosophila [3], the defense against microbes is executed mainly through the production of antimicrobial peptides (AMPs) under the control of two NF-κB transcription factors: Dorsal-related Immunity Factor (DIF) and Relish, respectively acting downstream of Toll and IMD pathways and homologues of mammalian RelB and p50 transcription factors.
Posttranslational regulation of proteins by the ubiquitin pathway is key for proper immune response [4]. The conjugation of ubiquitin polymers to target proteins by an ubiquitin ligase is a key mechanism for controlling the activity, localization, or stability of the targets. Lysine (Lys) residues of proteins can be modified by a polymer of ubiquitin (polyubiquitin) linked through Lys48 (K48) or Lys63 (K63) of ubiquitin molecules. Whereas K48-linked polyubiquitin mainly triggers degradation of proteins by the proteasome, K63-linked polyubiquitin mainly regulates the activity and the subcellular localization of proteins by modifying their protein-protein interactions [5]. In both mammals and Drosophila, ubiquitination is involved at various levels of the NF-κB pathways [6]. Furthermore, deregulation of ubiquitin ligases is implicated in inflammatory pathologies [7, 8] and tumor progression [9]. HECT-domain E3 ligases directly attach ubiquitin to a substrate, conversely to RING domain E3 ubiquitin ligases [10]. To achieve selectivity and specificity toward their substrates, HECT ubiquitin E3 ligases are tightly regulated, thus the identification of their substrates and regulators is critical in developing targets for drug discovery in the treatment of HECT E3 ubiquitin ligases related diseases [11, 12].
In Drosophila, Death-associated inhibitor of apoptosis 2 (Diap2) is the only E3 ubiquitin ligase identified thus far as a positive regulator of the IMD pathway [13, 14]. IMD protein functions as an adaptor protein in the IMD signaling pathway. The activation of pathogen recognition receptors (PRRs) by bacterial infection initiates the IMD cascade that transduces immunity signals notably through Diap2 [15, 16]. This protein is involved in the formation and activation of protein complexes organized around the IMD protein. To deepen our understanding of NF-κB pathway regulation by the ubiquitin system, we focused on identifying Drosophila ubiquitin ligases that are required for the activity of the IMD pathway through a RNAi-based screen in cultured Drosophila S2 cells.
Several E3 ubiquitin ligases emerged from our screen as positive or negative regulators of the IMD pathway. We focused on Hyperplastic discs (Hyd) because it was the only HECT-type E3 ubiquitin ligase identified in this screen and it had also emerged as a potential IMD pathway regulator in a parallel pilot screen [17]. Our results showed that Hyd was required in vivo for flies to survive an immune challenge with Gram-negative bacteria. Genetic epistasis analysis revealed that Hyd acted at the level of the NF-κB co-factor Akirin, which orchestrates the activation of a subset of NF-κB target genes in combination with the SWI/SNF chromatin remodeling complex [18–20].
We showed that Hyd decorated Akirin with K63-polyubiquitin chains that were required for Akirin binding to the NF-κB homolog Relish. Furthermore, we observed that the human ortholog of Hyd, UBR5 (also known as EDD1) [21], played a conserved role in NF-κB signaling in human cell lines. Similarly to human-AKIRIN2 (AKIRIN2), UBR5 was required for the activation of only a subset of NF-κB target genes after stimulation by LPS or IL-1β in cultured human cells. Thus, upon immune challenge, ubiquitin chains are instrumental to bridge NF-κB and its co-factor Akirin to activate an effective immune response.
Results
The E3 ubiquitin ligase Hyd is required for activation of the IMD pathway
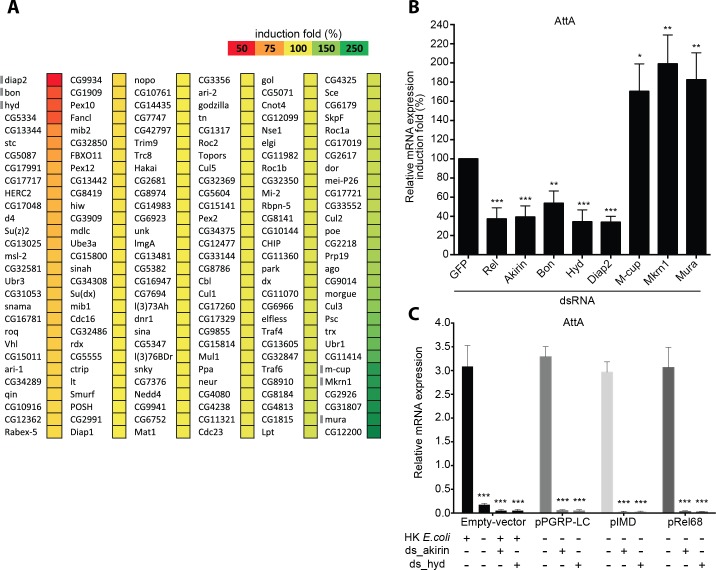
To identify E3 ubiquitin ligases that modulate the IMD pathway, we screened a library of 174 double-strand RNAs (dsRNAs) targeting Drosophila proteins classified as putative E3 ubiquitin ligases in Flybase [22]. We used stably transfected Drosophila S2 cells expressing the Attacin-A-luciferase gene, a reporter of activation of the IMD pathway upon immune challenge with Gram-negative bacteria [23]. We evaluated the ability of dsRNA targeting each putative E3 ubiquitin ligase to interfere with the luciferase reporter upon stimulation of cells with heat-killed Escherichia coli (HKE), a general IMD pathway agonist (S1 Table).
Diap2 is an E3 ubiquitin ligase that positively regulates the pathway by binding, polyubiquitinating and activating Death-related ced-3/Nedd2-like caspase (Dredd) which is required for Relish-mediated induction of antimicrobial peptides [24, 25]. Knockdown of Diap2 resulted in a strong decrease of the luciferase reporter induction upon immune stimulation relative to the control GFP-targeting dsRNA (dsGFP) that does not target any transcripts present in the cells (Fig 1A), providing proof of concept for the screen. Knockdown of six E3-ubiquitin ligases (M-cup, Mkrn1, CG2926, CG31807, Mura, and CG12200) resulted in a strong increase in reporter activity upon immune stimulation. Therefore these E3 ubiquitin ligases behave as negative regulators of the IMD pathway. Conversely, knockdown of two Really Interesting New Gene (RING) domain E3-ubiquitin ligases, Bon and CG5334, or of the homologous to the E6-AP carboxyl terminus (HECT) domain E3 ubiquitin ligase Hyd resulted in an important decrease in reporter activity (Fig 1A). This suggests that Bon, CG5334, and Hyd are positive regulators of the IMD pathway. We decided to focus on Hyd because it was the only HECT domain E3-ubiquitin ligase we identified.
Fig 1. E3-ubiquitin ligases screen identified in vitro Hyd as involved in IMD pathway.
(A) Induction of the IMD pathway measured by luciferase activity. 174 Drosophila E3 ubiquitin ligases were knocked down in S2 cells harboring the Attacin-A–luciferase reporter gene. Cells were transfected with individual dsRNAs targeting each E3 ligase before the IMD pathway was induced by stimulating the cells with heat-killed E. coli (HKE). Luciferase activity is expressed as an induction percentage compared to control cells treated with dsRNA targeting GFP. Three independent experiments were performed. Genes indicated by a gray bar were analyzed in Fig 1B. (B) Quantitative RT-PCR of Attacin-A mRNA from HKE-stimulated S2 cells transfected with dsRNA targeting GFP (negative control), Relish or Akirin (positive controls), and a subset of E3 ubiquitin ligases. After the ratio of stimulated over unstimulated values for each condition was determined, statistical significance was calculated by comparing genes knockdown to GFP dsRNA control. (C) Genetic epistasis experiment to place Hyd within the IMD pathway in S2 cells. The IMD pathway was induced by HKE stimulation or by transfecting the cells with Pgrp-LC, Imd-V5, or Rel-HA expressing plasmids. Cells were also transfected with dsRNA targeting Akirin or Hyd. Statistical significance was established by comparing values from the different conditions with cells treated with empty vector alone. Data are represented as mean ± standard deviation of three independent experiments (B-C). *P-value < 0.05; **P-value < 0.01; ***P-value < 0.001.
To validate the reporter assay, we transfected Drosophila S2 cells with dsRNA targeting either the NF-κB factor Relish, the Relish cofactor Akirin, Hyd, or E3 ubiquitin ligases chosen randomly among those that presented in the screen the strongest increase or decrease of Attacin-A induction (Bon, Diap2, M-cup, Mkrn1 and Mura). Then, we stimulated the cells with HKE and monitored endogenous Attacin-A mRNA by quantitative reverse transcription PCR (RT-qPCR). Reducing the abundance of Relish, Akirin, or Hyd significantly decreased HKE-mediated Attacin-A induction, compared to cells treated with dsGFP (Fig 1B and S1 Fig). We observed that the RING-domain E3 ubiquitin ligases Bon, M-cup, Mkrn1, and Mura were required for the normal activation of Attacin-A expression and that the HECT E3 ubiquitin ligase Hyd acted as a positive regulator of Attacin-A expression in Drosophila S2 cells (Fig 1B).
In order to identify at which level of the IMD pathway Hyd is required, we undertook an epistasis analysis. Drosophila S2 cells were treated by dsRNA targeting Hyd or Akirin as a control and the IMD pathway was activated at different levels by transfecting either a truncated form of PeptidoGlycan Receptor Protein-Long Chain a (Pgrp-LCa), Imd or the 68kD active-form of Relish (Rel68) [18]. Measurement of Attacin-A expression by RT-qPCR assessed activation of the IMD pathway. We show that Hyd was required at the same level of Relish (Fig 1C) to exert its positive regulation on IMD pathway activation.
Hyd acts at the level of Akirin to trigger full activation of the IMD pathway
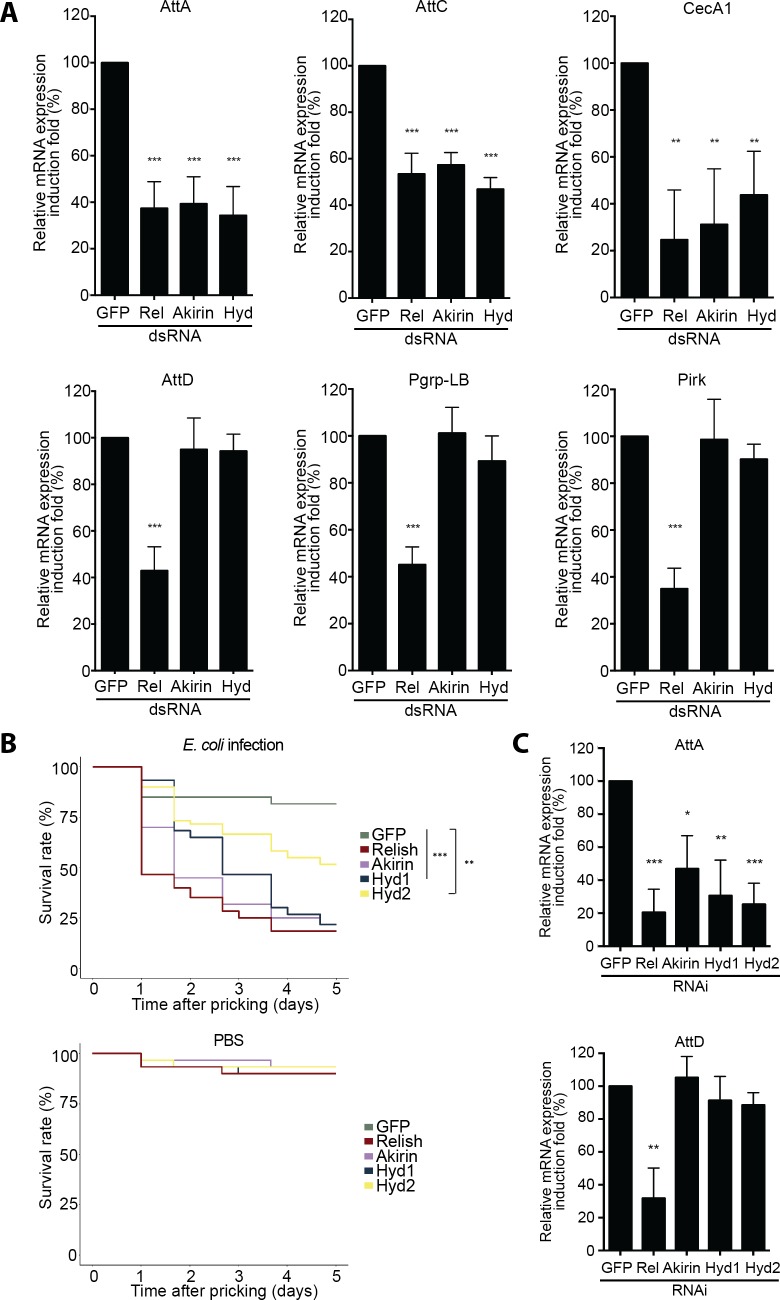
Downstream of the IMD pathway, Relish target genes are divided into two subsets: genes that depend only on Relish for their expression (including Attacin-D, Pgrp-LB, Pirk and the majority of negative regulators) and ones requiring Akirin, and the deposition of an acetyl group on the lysine 4 of the histone 3 (H3K4ac), in addition to Relish (including Attacin-A, Attacin-C, CecA1 and the majority of effectors) [19]. Upon immune challenge in S2 cells, using RT-qPCR, we observed that Hyd depletion recapitulated the immune phenotype of cells depleted for Akirin (Fig 2A). Consequently, Hyd is acting on Akirin-dependent NF-κB transcriptional selectivity in vitro.
Fig 2. Hyd is required for the full activation of IMD response.
(A) Quantitative RT-PCR of Attacin-A, Attacin-C, Cecropin-A1, Attacin-D, Pgrp-LB and Pirk mRNA from HKE-stimulated S2 cells transfected with dsRNA targeting GFP (negative control), Relish or Akirin (positive controls), and Hyd. (B) In vivo survival experiments performed on C564-Gal4/UAS-RNAi females Drosophila. They were infected with E. coli by septic injury (with PBS pricking as control). (C) Quantitative RT-PCR of Attacin-A and Attacin-D mRNA from C564-Gal4/UAS-RNAi Drosophila males infected with E. coli by septic injury. Data are represented as mean ± standard deviation of three independent experiments (A-C). After the ratio of stimulated over unstimulated values for each condition was determined, statistical significance was established by comparing genes knockdown with GFP dsRNA or RNAi control (B-C). *P-value < 0.05; **P-value < 0.01; ***P-value < 0.001.
We next investigated if Akirin and Hyd were similarly required for NF-κB transcriptional selectivity in vivo, using RNAi. As Drosophila embryonic development is impaired in absence of Akirin, we used the C564-Gal4 transgene [26] to express RNAi constructs targeting Akirin, Hyd and Relish in the adult fat body, the main immune organ of Drosophila [3]. Flies depleted of Akirin (C564-Gal4/UAS-RNAi-akirin), Relish (C564-Gal4/UAS-RNAi-relish) or Hyd (C564-Gal4/UAS-RNAi-hyd1 or C564-Gal4/UAS-RNAi-hyd2) displayed an impaired survival following E. coli infection when compared to control flies (C564-Gal4/UAS-RNAi-GFP) or to PBS pricking (Fig 2B and S2 Fig).
Following immune challenge by E. coli, expression of Attacin-A, but not of Attacin-D, was reduced in the absence of Akirin or Hyd, when compared to control flies (C564-Gal4/UAS-RNAi-GFP) (Fig 2C).
Our results suggest that Hyd is required at the level of Relish to activate the Akirin-dependent subset of Relish target genes during the immune response, allowing Drosophila to survive a Gram-negative bacterial challenge.
Hyd-mediated K63-polyubiquitination of Akirin is critical for Akirin binding to Relish
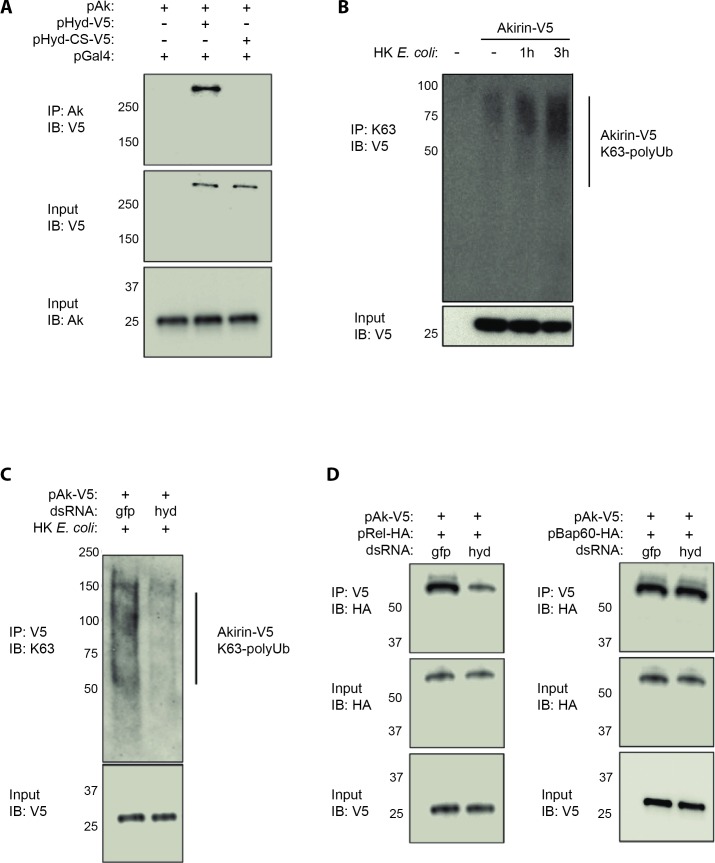
We next investigated if Akirin could be a bona fide target for the E3 ubiquitin-ligase Hyd. Co-immunoprecipitation assay in S2 cells showed that V5-tagged Hyd (Hyd-V5) [27] binds to endogenous Akirin (Fig 3A). By contrast, V5-tagged Hyd-CS (Hyd-CS-V5), which displays a mutated HECT domain by conversion of the catalytic cysteine at position 2854 to serine [27], is unable to bind to Akirin (Fig 3A). As a control we confirmed that Diap2, the E3-ubiquitin ligase acting upstream of Akirin in the IMD signaling cascade [13, 14], does not interact with Akirin (S3 Fig).
Fig 3. Hyd mediated-ubiquitination of Akirin is necessary for interaction with Relish.
(A) Co-immunoprecipitation assay between over-expressed Akirin and Hyd in S2 cells. The cells were transiently transfected with Akirin, Gal4, Hyd-V5 and/or Hyd-CS-V5 expressing plasmids. Cell lysates were immunoprecipitated with anti-Akirin coupled agarose beads. Immunoprecipitates were analyzed by Western blotting with anti-V5 or anti-Akirin antibodies. (B) Immunoprecipitation assay of K63-polyUb chains on Akirin before and after immune challenge. S2 cells were transiently transfected with Akirin-V5 expressing plasmid. Cell lysates were immunoprecipitated with anti-K63-polyUb coupled agarose beads. Immunoprecipitates were analyzed by Western blotting with anti-V5 antibodies. (C) Immunoprecipitation assay of Akirin after immune challenge. S2 cells were transiently transfected with Akirin-V5 expressing plasmid and dsRNA targeting GFP or Hyd. Cell lysates were immunoprecipitated with anti-V5 coupled agarose beads. Immunoprecipitates were analyzed by Western blotting with anti-K63-polyUb and anti-V5 antibodies. (D) Co-immunoprecipitation assay between over-expressed Akirin and Relish or Bap60 in S2 cells. The cells were transiently transfected with Akirin-V5 and Rel-HA or Bap60-HA expressing plasmids and dsRNA targeting GFP or Hyd. Cell lysates were immunoprecipitated with anti-V5 coupled agarose beads. Immunoprecipitates were analyzed by Western blotting with anti-HA or anti-V5 antibodies. Data are representative of 2 independent experiments.
Protein extracts from cells transfected with a tagged version of Akirin (Akirin-V5) were immunoprecipitated with an anti-V5 antibody. Using cellular fractioning, we showed that upon immune challenge, polyubiquitinated Akirin-V5 accumulated in the nucleus of Drosophila S2 cells (S4 Fig). Western-blot experiments with antibodies targeting K63-polyUb chains showed that Akirin was K63-polyubiquitinated 1h and 3h after immune challenge with HKE (Fig 3B). This immune-induced post-translational modification of Akirin was attenuated upon knockdown of Hyd (Fig 3C).
According to literature, Hyd is suspected to also deposit K48 polyubiquitin chains on its target proteins [28]. Here we observed that Akirin-V5 was also decorated by K48-polyubiquitin chains, but independently of Hyd (S5 Fig).
Collectively, these data suggest that upon immune challenge, Hyd physically interacts with Akirin through its catalytic HECT domain to decorate Akirin with K63-polyUb chains. We previously published that Akirin physically bridges the NF-κB factor Relish and Bap60, a core member of the SWI/SNF chromatin-remodeling complex [19]. To understand whether Akirin K63-polyubiquitination is instrumental for the interaction of Akirin with Relish or Bap60, we performed co-immunoprecipitation experiments in S2 cells depleted for Hyd and transfected with Akirin-V5 and Rel68-HA or BAP60-HA (Fig 3D). As previously reported [19], Akirin-V5 co-precipitated either with the active form of the NF-κB homolog Relish (Rel68-HA) or with Bap60 (Bap60-HA) (Fig 3D). However, in the absence of Hyd, the interaction between Akirin-V5 and Rel68-HA was weakened (Fig 3D). Of note the interaction between Akirin-V5 and Bap60-HA is independent of Hyd (Fig 3D). Upon immune challenge, the transcriptional kinetic of Akirin-dependent and Akirin-independent genes was different. Akirin-independent genes (AttD, Pgrp-LB) were strongly transcribed after 1 hour, whereas Akirin-dependent genes (AttA, AttC) were strongly detectable after 3 hours (S6 Fig). Interestingly, the delayed activation of Akirin-dependent genes correlated with the robust K63-polyUb chains detection on Akirin at 3 hours after immune challenge (Fig 3B).
These results suggest that, upon immune challenge, Hyd is required to deposit K63-polyUb chains on Akirin for subsequent binding to the NF-κB factor Relish, and efficient transcription of Akirin-dependent genes.
UBR5, the human ortholog of Hyd, is required for NF-κB transcriptional selectivity during the inflammatory response
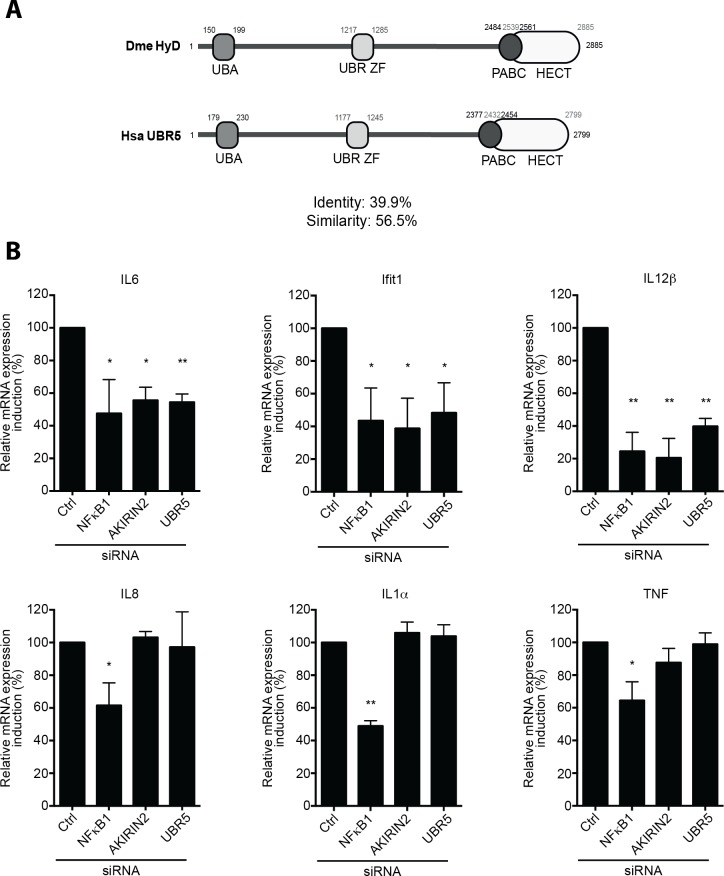
The Akirin-dependent molecular mechanism underlying the selective activation of NF-κB target genes is well conserved from Drosophila to mammals [18–20]. It is established that i) in human, AKIRIN2 is the functional homologue of Drosophila Akirin [18, 20], and ii) downstream of NF-κB pathways, AKIRIN2 operates a dichotomy between the AKIRIN2-dependent and -independent genes to be transcribed [20]. UBR5 is the human ortholog of the Drosophila E3-ubiquitin ligase Hyd [21, 28]. Comparison between UBR5 and Hyd revealed the presence of similar functional domains, as well as 39,9% of sequence identity and 56,5% of sequence similarity (Fig 4A and S7 Fig). Therefore, we addressed the potential requirement of UBR5 in NF-κB selective transcriptional response mediated by AKIRIN2 during the human inflammatory response. A previous study using human HeLa cells and mouse macrophages has identified AKIRIN2-dependent genes (such as IL6, Ifit1, and, IL12β), and AKIRIN2-independent genes (such as IL8, IL1α and TNF) [20]. Here, we depleted THP1 (a human monocytic cell line) or HeLa cell lines for either NF-κB1, AKIRIN2 or UBR5 by siRNA (using scrambled siRNA as controls). We first confirmed that, as in HeLA cells, the AKIRIN2-dependent genes (IL6, Ifit1, and IL12β) and AKIRIN2-independent genes (IL8, IL1α and TNF) conserved their transcriptional activation dichotomies in THP1 cells (Fig 4B, S8 Fig and S9 Fig). Lacking NF-κB1 in these human cell lines impaired NF-κB target genes activation upon LPS or IL-1β stimulation (Fig 4B, S8 Fig and S9 Fig). THP1 and HeLa cells depleted for AKIRIN2 or UBR5 showed a decreased level of AKIRIN2-dependent target gene (IL6, Ifit1, and, IL12β) activation when compared to control (Fig 4B, S8 Fig and S9 Fig). This result suggests a conserved function of UBR5 in the selective transcription of NF-κB target genes mediated by AKIRIN2. However, the precise mechanisms by which UBR5 impacts the transcription of AKIRIN2-dependent target genes remains to be explored.
Fig 4. Hyd/UBR5 is necessary for NF-κB target genes activation.
(A) Graphical representation of the predicted functional domains of Drosophila melanogaster Hyd and Homo sapiens UBR5. This representation highlights a conserved Ubiquitin-associated (UBA) domain, poly(A)-binding protein C-terminal (PABC) domain, Homologous to the E6-AP Carboxyl Terminus (HECT) domain and UBR type Zinc Finger (UBR ZF) domain. Annotation based InterPro software. Sequence similarity percentages calculated using the EMBOSS NEEDLE tool (default settings). (B) Quantitative RT-PCR of IL6, Ifit1, IL12β, IL8, Il1α and TNF mRNA from LPS-stimulated THP1 cells. They were transfected with scrambled siRNA (negative control) or siRNA targeting NF-κB1, AKIRIN2 (positive controls) or UBR5. Data are represented as mean ± standard deviation of three independent experiments. After the ratio of stimulated over unstimulated values for each condition was determined, statistical significance was established by comparing genes knockdown with scrambled siRNA control. *P-value < 0.05; **P-value < 0.01; ***P-value < 0.001.
Taken altogether, our results show that the HECT-type E3 ubiquitin ligase Hyd/UBR5 is involved in NF-κB pathway regulation in Drosophila and mammals. In fruit fly, Hyd deposits K63-polyUb chains on Akirin and is required to bridge Akirin and the NF-κB factor Relish. This interaction is necessary for the transcription of an essential subset of NF-κB target genes, downstream of the IMD pathway (Fig 5).
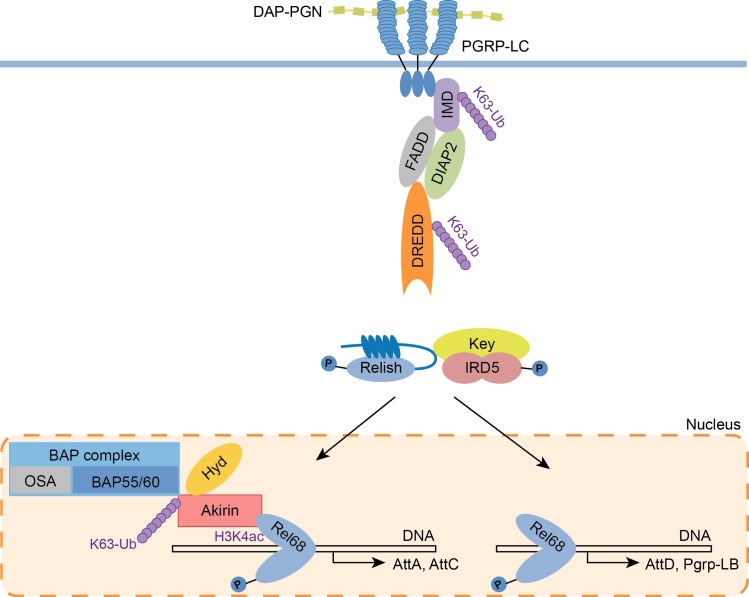
Fig 5. Schematic model of Hyd involvement in the IMD signaling pathway in Drosophila.
Model showing the role of Hyd in the expression of the Akirin-dependent genes in the IMD pathway. After activation of the pathway, allowed by the K63-polyUb chains deposition on the complexes IMD and DREDD by the E3-ubiquitin ligase Diap2, Relish is translocated. Hyd is necessary for the K63-polyUb of the NF-κB co-factor Akirin and its interaction with the NF-κB factor Relish. This interaction is crucial for the expression of Akirin-dependent genes (like Attacin-A and Attacin-C), necessary for an adequate innate immune response. DAP-PGN: meso-diaminopimelic acid-type peptidoglycan of Gram-negative bacteria, P-tag: phosphorylation marks.
Discussion
Using Drosophila genetics, we describe here a function for the HECT E3 ubiquitin ligase Hyd in the innate immune response. Using human cell lines (HeLa and THP1), we could also show that this function of Hyd downstream of the NF-κB pathway is conserved in humans.
In both Drosophila and humans, NF-κB dependent signaling pathways are among the best-known examples of the role of ubiquitin linkage to target proteins in signal transduction [4, 29], ubiquitination being involved at every level of the NF-κB pathway, from membrane receptors to chromatin-associated proteins. In order to identify new E3-ubiquitin ligases involved in the Drosophila innate immune response, we conducted a RNAi-based screen. We showed, in addition to Diap2 already known to be a bona fide member of the IMD pathway [13, 14], that other RING-domain E3 ubiquitin ligases (CG5334, bon) were involved in the activation of the IMD pathway in Drosophila S2 cells after immune challenge. In addition, our results suggested that other RING-domain E3 ubiquitin ligases such as M-cup, Mkrin1 and Mura down-regulate IMD pathway target genes activation. This screen also suggested that a HECT E3-ubiquitin ligase, namely Hyd, is involved in the innate immune response.
In Drosophila, Hyd is reported to be located in the nuclear and in the cytoplasmic fraction of cells to participate in various phenomena during development such as cellular proliferation [30, 31]. More precisely, Hyd shapes hedgehog signaling by differentially restraining the transcriptional activity of Cubitus interuptus via selective association with respective promoters [27]. More recently, Hyd and its orthologue UBR5 were reported to act at the level of Wnt signaling target gene promoter to enable gene transcription [28]. Here we identified the HECT E3 ubiquitin-ligase Hyd in Drosophila as responsible for the ubiquitination of Akirin and its subsequent binding to the NF-κB transcription factor Relish. In addition, identification of protein interactors for AKIR-1, the Caenorhabditis elegans homologue of Drosophila Akirin, also revealed Hyd/UBR5 [32]. Altogether these results point to a conserved function of the HECT E3 ubiquitin ligase Hyd/UBR5 as nuclear selector for gene activation.
Downstream of the IMD pathway, the NF-κB transcription factor Relish target genes could be divided into two subgroups, Akirin-dependent and Akirin-independent genes [19]. Targeting Hyd by RNAi in Drosophila S2 cells dampened the activation of Akirin-dependent genes upon immune stimulation. Depleting Hyd from Drosophila fat-body prevents fly survival to immune challenge with the Gram-negative bacteria E. coli, demonstrating the biological relevancy of its function. Co-immunoprecipitation experiments showed that Hyd interacts with Akirin through its catalytic domain to deposit K63-polyUb chains. Of note, the K63-polyubiquitination of Akirin by Hyd is performed only after immune challenge, suggesting that an immune-triggered signal governs this event and remains to be explored. Because our observations are based on overexpressed proteins, it would be of interest to evaluate if endogenous Akirins are K63-polyubiquitinated by Hyd upon immune challenge. Additionally, it is still unclear how the K63-polyubiquitin chains on Akirin physically interact with Relish to set a bridge, as no Ubiquitin Binding Domain (UBD) have been described for Relish. During development, Akirin is also known to be able to link another transcription factor, Twist [33]. It remains to be investigated if Hyd affects Akirin-Twist dependent transcriptional response.
The HECT Ubiquitin ligase family is known in mammals and Drosophila to regulate biological phenomena [11]. We found that the mammalian ortholog of Hyd, UBR5 [21] is involved in NF-κB transcriptional selective response in human cell lines as well. This suggests a conserved role for Hyd/UBR5 on AKIRIN2, even though we do not know if AKIRIN2 is ultimately ubiquitinated. A dedicated study of UBR5 role in NF-κB pathways is needed to completely assess it. It is known that UBR5 inhibits the TNF receptor associated factor 3 (Traf3) [34], an inhibitor of the NF-κB pathway [35]. Thus, the role of UBR5 might be indirect.
When Hyd or UBR5 was attenuated, only a subset of NF-κB target genes is expressed, diminishing the intensity of the innate immune response in Drosophila and inflammatory response in mammals, similarly to the inactivation of Akirin [19, 20]. The link between excessive activation of NF-κB signaling pathways during e.g chronic inflammation and cancer progression or appearance is now on the spotlight [36]. Uncontrolled activation of NF-κB due to deregulation of ubiquitin-ligases has been reported in many diseases [37] and UBR5 has been described to be involved in several types of cancer in humans [38]. Our findings point to the HECT E3-ubiquitin ligase UBR5 as an interesting drug target to modulate NF-κB signaling, control the development of inflammatory diseases and potentially improve treatments for cancer.
Material and methods
Cell culture
S2 cells were cultured at 25°C in Schneider's medium (Biowest) supplemented with 10% fetal (vol/vol) calf serum (FCS), penicillin/streptomycin (50 μg/ml of each) and 2 mM glutamax. HeLa cell line (gift from IGBMC, Illkirch, France) was cultured in DMEM containing 10% (vol/vol) FCS, 40 μg/mL gentamycin. THP1 cell line (ATCC-TIB-202) was cultured in RPMI containing 10% (vol/vol) FCS and penicillin/streptomycin (50 μg/ml of each).
dsRNA E3 ubiquitin ligases screen
A list containing 174 E3 ubiquitin ligases in the Drosophila genome, consisting predominantly of HECT, RING, and U-box proteins was curated manually by GO- and protein domain-term search in Flybase FB2012_06 Dmel Release 5.48 [22]. Based on this list, a Drosophila E3 ligase dsRNA library was generated in Michael Boutros’s laboratory [39], listed in S2 Table. The screen experiments were performed using 1F3 cells stably expressing AttA firefly luciferase [17]. Two days after transfection with an Actin renilla luciferase construct, cells were collected and distributed into 96-well screening plates at a density of 4.5 x 104 cells per well. Cells were transfected with 3 μg of each dsRNA in the Drosophila E3-ubiquitin ligase dsRNA library in triplicate by bathing method as previously described [19]. At day 5 post-transfection, cells were stimulated for 48 h with heat-killed E. coli (40:1) before being lysed. Luciferase activity was quantified in a luminometer (Mithras LB940, Berthold) after addition of the substrate (Dual luciferase assay kit, Promega).
RNA interference
The double-strand RNAs for the knockdown experiments in Drosophila cells were prepared according to [19]. Fragments for the different genes were generated from genomic DNA templates using oligonucleotides designed for use with Genome-RNAi libraries [40] and are listed in S3 Table. The small interfering RNAs used for the knockdown experiment in mammalian cell lines cells were purchased from Ambion (S4 Table).
Plasmid constructs
pAC-Akirin, pAC-Akirin-V5, pAC-Pgrp-LC, pAC-Imd, pMT-Rel-HA, pMT-Bap60-HA and pAC-Gal4 constructs were described previously [18, 19]. pUAS-Hyd-V5 and pUAS-Hyd-CS-V5 were kindly provided by Xinhua Lin laboratory [27].
Cell transfection
Drosophila S2 cells were transfected with double-strand RNAs using the bathing method described in [19] or with plasmids using the Effectene transfection kit (Qiagen). HeLa cells were transfected with siRNA using Lipofectamine RNAiMax Transfection Reagent (Invitrogen). THP1 cells were transfected with siRNA using Lipofectamine 3000 Transfection Reagent (Invitrogen).
RNA extraction and quantification
For the in vitro experiments, RNA was extracted from cells and treated with DNAse, using RNA Spin kit (Macherey Nagel). For the in vivo experiments, the procedure was done accordingly to [19]. Similarly, reverse-transcription and quantitative RT-PCR were performed as indicated in [19]. The levels of expression of genes of interest were normalized against the measured level of the RNA coding determined in each sample for ribosomal protein-49 in the case of Drosophila experiments and for GAPDH for HeLa and THP1 cells. Primers used for quantitative RT-PCR are listed in S5 Table.
Isolation of nuclear and cytoplasmic cell fractions
Nuclear/cytoplasmic fractionation was performed using the NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Thermo Scientific), following the manufacturer’s instructions. The cell pellet was suspended in 500 μl of cytoplasmic extraction reagent I. After a 10 min incubation on ice, 11 μl of cytoplasmic extraction reagent II was added. The supernatant fraction was then collected after a 5 min centrifugation at 16 000 g. The nuclear fraction was collected following the suspension of the nuclear pellet in 250 μl of Nuclear extraction reagent, incubation for 40min on ice and centrifugation for 10 min at 16 000g.
Immunoprecipitation and Western blot
Cells were treated for the indicated times with heat-killed E. coli (40:1) at 25°C. The cells were harvested, washed in PBS and lysed in 200 μl of Pierce IP Lysis Buffer (Thermo Scientific), with protease inhibitor cocktail (Roche). Immunoprecipitations were performed overnight at 4°C based on [19, 41], with either rabbit polyclonal anti-Akirin [19] or anti-ubiquitin Lys63 specific antibodies (Millipore 05–1308), or anti-V5 (Merck V8137) coupled with Dynabeads Protein G (Invitrogen) and anti-V5 or anti-HA antibodies coupled to agarose beads (Sigma). Proteins were detected by Western blotting using anti-Akirin, anti-ubiquitin Lys63 specific, anti-ubiquitin Lys48 specific (Merck 05–1307), anti-Ubiquitin (SantaCruz Biotechnology P4D1), anti-H3 histone (Abcam ab1791), anti-RpS15 (Abcam ab157193), anti-V5 (Invitrogen r96025) and anti-HA (Abcam ab9110) antibodies.
Fly strains
Stocks were raised on standard cornmeal-yeast-agar medium at 25°C with 60% humidity. To generate conditional knockdown in adult flies, we used the GAL4-GAL80ts system [26]. Fly lines carrying a UAS-RNAi transgene targeting relish (108469), akirin (109671), and hyd (44675, named here Hyd1; 44676, named here Hyd2) were obtained from the Vienna Drosophila RNAi Center (http://stockcenter.vdrc.at/control/main). Fly line carrying a UAS-RNAi transgene against GFP (397–05) was obtained from the Drosophila Genetic Resource Center (Kyoto, Japan; http://www.dgrc.kit.ac.jp/index.html). UAS-RNAi flies were crossed with Actin-GAL4/CyO; Tub-GAL80ts flies at 18°C. Emerged adult flies were then transferred to 29°C to activate the UAS-GAL4 system for 6–7 days. Nine-day-old flies were used—three batches of twenty females for survival assays and ten males for quantitative RT-PCR experiments.
Immune challenge
Drosophila S2 cells were stimulated with heat-killed E. coli (40:1) [42]. HeLa cells were stimulated with recombinant human IL-1β (10 ng/ml) for 4h. THP1 cells were stimulated with Lipopolysaccharide (LPS) Solution (1 μg/ml) for 4h. IL-1β and LPS were purchased from Invitrogen. Microbial challenges were performed by pricking adult flies with a sharpened tungsten needle dipped into PBS or concentrated E. coli strain DH5aGFP bacteria solution at 25°C, for either several days (for survival assays) or 6h (for quantitative RT-PCR experiments) [19, 42]. Bacteria were grown in Luria broth (LB) at 37°C.
Bioinformatic analysis of HyD and UBR5
Amino acid sequences of Drosophila melanogaster HyD (ID: P51592) and Homo sapiens UBR5 (ID: O95071) were retrieved from UniProt (uniport.org). Predicted functional domains were annotated using the InterPro database (v77.0) from the European Molecular Biology Laboratory [43]. In order to calculate identity and similarity percentages, sequences were aligned to each other using the EMBOSS NEEDLE tool [44], also from the European Molecular Biology Laboratory, under default settings. Graphical visualization of the similarity percentage between HyD and UBR5 alongside the amino acid sequence was performed with the LALNVIEW software [45] after sequence alignment with the SIM–Local similarity program [46] under default settings.
Statistical analysis
P values for quantitative RT-PCR were calculated using the two-tailed unpaired Student t test using GraphPad Prism version 6.0c for Mac, GraphPad Software, San Diego, California USA. Log-rank analyses of survival assay were performed using RStudio version 1.1.463 and the function survdiff of the survival package (version 2.43–3; Terry M Therneau, 2018). RStudio Team (2016). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA USA.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors thanks Karine Fels for technical assistance and Dr. Izabela Sumara (IGBMC, Illkirch-Graffenstaden, France) for sharing reagents and expertise. We are grateful to the Drosophila Genomics Resource Center at Indiana University, the Drosophila Genetic Resource Center at the Kyoto Institute of Technology and the Vienna Drosophila RNAi Center for fly stocks.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the Centre National de la Recherche Scientifique (http://www.cnrs.fr/fr/page-daccueil) in the frame of the LIA «REL2 and resistance to malaria». This work was performed under the framework of the LABEX: ANR-10- LABX-0036_NETRNA and ANR-17-EURE-0023, through a funding of the state managed by the French National Research Agency (https://anr.fr/) as part of the Investments for the future program and also from a European Research Council (https://erc.europa.eu/) Advanced Grant (AdG_20090506 ‘‘Immudroso,’’ to J.-M.R.). Generation of RNAi reagents by M.B. was supported by DFG DRiC (https://www.dfg.de/). N.M. is a Fellow at the University of Strasbourg Institute for Advanced Study (USIAS-2018-073; http://www.usias.fr/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vidal M, Cagan RL. Drosophila models for cancer research. Current opinion in genetics & development. 2006;16(1):10–6. Epub 2005/12/20. 10.1016/j.gde.2005.12.004 . [DOI] [PubMed] [Google Scholar]
- 2.Maeda S, Omata M. Inflammation and cancer: role of nuclear factor-kappaB activation. Cancer Sci. 2008;99(5):836–42. Epub 2008/02/26. 10.1111/j.1349-7006.2008.00763.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nature reviews Immunology. 2007;7(11):862–74. Epub 2007/10/20. 10.1038/nri2194 . [DOI] [PubMed] [Google Scholar]
- 4.Park Y, Jin HS, Aki D, Lee J, Liu YC. The ubiquitin system in immune regulation. Advances in immunology. 2014;124:17–66. 10.1016/B978-0-12-800147-9.00002-9 . [DOI] [PubMed] [Google Scholar]
- 5.Swatek KN, Komander D. Ubiquitin modifications. Cell research. 2016;26(4):399–422. 10.1038/cr.2016.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thevenon D, Engel E, Avet-Rochex A, Gottar M, Bergeret E, Tricoire H, et al. The Drosophila ubiquitin-specific protease dUSP36/Scny targets IMD to prevent constitutive immune signaling. Cell host & microbe. 2009;6(4):309–20. 10.1016/j.chom.2009.09.007 . [DOI] [PubMed] [Google Scholar]
- 7.Aksentijevich I, Zhou Q. NF-kappaB Pathway in Autoinflammatory Diseases: Dysregulation of Protein Modifications by Ubiquitin Defines a New Category of Autoinflammatory Diseases. Frontiers in immunology. 2017;8:399 10.3389/fimmu.2017.00399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kattah MG, Malynn BA, Ma A. Ubiquitin-Modifying Enzymes and Regulation of the Inflammasome. Journal of molecular biology. 2017;429(22):3471–85. 10.1016/j.jmb.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallo LH, Ko J, Donoghue DJ. The importance of regulatory ubiquitination in cancer and metastasis. Cell Cycle. 2017;16(7):634–48. 10.1080/15384101.2017.1288326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metzger MB, Hristova VA, Weissman AM. HECT and RING finger families of E3 ubiquitin ligases at a glance. J Cell Sci. 2012;125(Pt 3):531–7. 10.1242/jcs.091777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheffner M, Kumar S. Mammalian HECT ubiquitin-protein ligases: biological and pathophysiological aspects. Biochimica et biophysica acta. 2014;1843(1):61–74. 10.1016/j.bbamcr.2013.03.024 . [DOI] [PubMed] [Google Scholar]
- 12.Weber J, Polo S, Maspero E. HECT E3 Ligases: A Tale With Multiple Facets. Front Physiol. 2019;10:370 10.3389/fphys.2019.00370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleino A, Valanne S, Ulvila J, Kallio J, Myllymaki H, Enwald H, et al. Inhibitor of apoptosis 2 and TAK1-binding protein are components of the Drosophila Imd pathway. EMBO J. 2005;24(19):3423–34. 10.1038/sj.emboj.7600807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gesellchen V, Kuttenkeuler D, Steckel M, Pelte N, Boutros M. An RNA interference screen identifies Inhibitor of Apoptosis Protein 2 as a regulator of innate immune signalling in Drosophila. EMBO Rep. 2005;6(10):979–84. 10.1038/sj.embor.7400530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemaitre B, Kromer-Metzger E, Michaut L, Nicolas E, Meister M, Georgel P, et al. A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc Natl Acad Sci U S A. 1995;92(21):9465–9. 10.1073/pnas.92.21.9465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paquette N, Broemer M, Aggarwal K, Chen L, Husson M, Erturk-Hasdemir D, et al. Caspase-mediated cleavage, IAP binding, and ubiquitination: linking three mechanisms crucial for Drosophila NF-kappaB signaling. Molecular cell. 2010;37(2):172–82. 10.1016/j.molcel.2009.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuyama H, Verdier Y, Guan Y, Makino-Okamura C, Shilova V, Liu X, et al. Landscape of protein-protein interactions in Drosophila immune deficiency signaling during bacterial challenge. Proc Natl Acad Sci U S A. 2013;110(26):10717–22. Epub 2013/06/12. 10.1073/pnas.1304380110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goto A, Matsushita K, Gesellchen V, El Chamy L, Kuttenkeuler D, Takeuchi O, et al. Akirins are highly conserved nuclear proteins required for NF-kappaB-dependent gene expression in drosophila and mice. Nature immunology. 2008;9(1):97–104. Epub 2007/12/11. 10.1038/ni1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonnay F, Nguyen XH, Cohen-Berros E, Troxler L, Batsche E, Camonis J, et al. Akirin specifies NF-kappaB selectivity of Drosophila innate immune response via chromatin remodeling. EMBO J. 2014. Epub 2014/09/03. 10.15252/embj.201488456 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tartey S, Matsushita K, Vandenbon A, Ori D, Imamura T, Mino T, et al. Akirin2 is critical for inducing inflammatory genes by bridging IkappaB-zeta and the SWI/SNF complex. EMBO J. 2014. Epub 2014/08/12. 10.15252/embj.201488447 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callaghan MJ, Russell AJ, Woollatt E, Sutherland GR, Sutherland RL, Watts CK. Identification of a human HECT family protein with homology to the Drosophila tumor suppressor gene hyperplastic discs. Oncogene. 1998;17(26):3479–91. 10.1038/sj.onc.1202249 . [DOI] [PubMed] [Google Scholar]
- 22.Gramates LS, Marygold SJ, Santos GD, Urbano JM, Antonazzo G, Matthews BB, et al. FlyBase at 25: looking to the future. Nucleic Acids Res. 2017;45(D1):D663–D71. 10.1093/nar/gkw1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tauszig S, Jouanguy E, Hoffmann JA, Imler JL. Toll-related receptors and the control of antimicrobial peptide expression in Drosophila. Proc Natl Acad Sci U S A. 2000;97(19):10520–5. Epub 2000/09/06. 10.1073/pnas.180130797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleino A, Silverman N. The Drosophila IMD pathway in the activation of the humoral immune response. Developmental and comparative immunology. 2014;42(1):25–35. 10.1016/j.dci.2013.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meinander A, Runchel C, Tenev T, Chen L, Kim CH, Ribeiro PS, et al. Ubiquitylation of the initiator caspase DREDD is required for innate immune signalling. EMBO J. 2012;31(12):2770–83. 10.1038/emboj.2012.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGuire SE, Roman G, Davis RL. Gene expression systems in Drosophila: a synthesis of time and space. Trends Genet. 2004;20(8):384–91. 10.1016/j.tig.2004.06.012 . [DOI] [PubMed] [Google Scholar]
- 27.Wang G, Tang X, Chen Y, Cao J, Huang Q, Ling X, et al. Hyperplastic discs differentially regulates the transcriptional outputs of hedgehog signaling. Mech Dev. 2014;133:117–25. 10.1016/j.mod.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flack JE, Mieszczanek J, Novcic N, Bienz M. Wnt-Dependent Inactivation of the Groucho/TLE Co-repressor by the HECT E3 Ubiquitin Ligase Hyd/UBR5. Molecular cell. 2017;67(2):181–93 e5. 10.1016/j.molcel.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh S, Dass JF. Study of pathway cross-talk interactions with NF-kappaB leading to its activation via ubiquitination or phosphorylation: A brief review. Gene. 2016;584(1):97–109. 10.1016/j.gene.2016.03.008 . [DOI] [PubMed] [Google Scholar]
- 30.Lee JD, Amanai K, Shearn A, Treisman JE. The ubiquitin ligase Hyperplastic discs negatively regulates hedgehog and decapentaplegic expression by independent mechanisms. Development. 2002;129(24):5697–706. 10.1242/dev.00159 . [DOI] [PubMed] [Google Scholar]
- 31.Mansfield E, Hersperger E, Biggs J, Shearn A. Genetic and molecular analysis of hyperplastic discs, a gene whose product is required for regulation of cell proliferation in Drosophila melanogaster imaginal discs and germ cells. Developmental biology. 1994;165(2):507–26. 10.1006/dbio.1994.1271 . [DOI] [PubMed] [Google Scholar]
- 32.Polanowska J, Chen JX, Soule J, Omi S, Belougne J, Taffoni C, et al. Evolutionary plasticity in the innate immune function of Akirin. PLoS genetics. 2018;14(7):e1007494 10.1371/journal.pgen.1007494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nowak SJ, Aihara H, Gonzalez K, Nibu Y, Baylies MK. Akirin links twist-regulated transcription with the Brahma chromatin remodeling complex during embryogenesis. PLoS genetics. 2012;8(3):e1002547 Epub 2012/03/08. 10.1371/journal.pgen.1002547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho JH, Kim SA, Seo YS, Park SG, Park BC, Kim JH, et al. The p90 ribosomal S6 kinase-UBR5 pathway controls Toll-like receptor signaling via miRNA-induced translational inhibition of tumor necrosis factor receptor-associated factor 3. J Biol Chem. 2017;292(28):11804–14. 10.1074/jbc.M117.785170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He JQ, Oganesyan G, Saha SK, Zarnegar B, Cheng G. TRAF3 and its biological function. Adv Exp Med Biol. 2007;597:48–59. 10.1007/978-0-387-70630-6_4 . [DOI] [PubMed] [Google Scholar]
- 36.Taniguchi K, Karin M. NF-kappaB, inflammation, immunity and cancer: coming of age. Nature reviews Immunology. 2018;18(5):309–24. 10.1038/nri.2017.142 . [DOI] [PubMed] [Google Scholar]
- 37.Iwai K. Diverse roles of the ubiquitin system in NF-kappaB activation. Biochimica et biophysica acta. 2014;1843(1):129–36. 10.1016/j.bbamcr.2013.03.011 . [DOI] [PubMed] [Google Scholar]
- 38.Shearer RF, Iconomou M, Watts CK, Saunders DN. Functional Roles of the E3 Ubiquitin Ligase UBR5 in Cancer. Mol Cancer Res. 2015;13(12):1523–32. 10.1158/1541-7786.MCR-15-0383 . [DOI] [PubMed] [Google Scholar]
- 39.Horn T, Sandmann T, Boutros M. Design and evaluation of genome-wide libraries for RNA interference screens. Genome Biol. 2010;11(6):R61 10.1186/gb-2010-11-6-r61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt EE, Pelz O, Buhlmann S, Kerr G, Horn T, Boutros M. GenomeRNAi: a database for cell-based and in vivo RNAi phenotypes, 2013 update. Nucleic Acids Res. 2013;41(Database issue):D1021–6. Epub 2012/11/30. 10.1093/nar/gks1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emmerich CH, Cohen P. Optimising methods for the preservation, capture and identification of ubiquitin chains and ubiquitylated proteins by immunoblotting. Biochem Biophys Res Commun. 2015;466(1):1–14. 10.1016/j.bbrc.2015.08.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reichhart JM, Gubb D, Leclerc V. The Drosophila serpins: multiple functions in immunity and morphogenesis. Methods in enzymology. 2011;499:205–25. Epub 2011/06/21. 10.1016/B978-0-12-386471-0.00011-0 . [DOI] [PubMed] [Google Scholar]
- 43.Mitchell AL, Attwood TK, Babbitt PC, Blum M, Bork P, Bridge A, et al. InterPro in 2019: improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res. 2019;47(D1):D351–D60. 10.1093/nar/gky1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Needleman SB, Wunsch CD. A general method applicable to the search for similarities in the amino acid sequence of two proteins. Journal of molecular biology. 1970;48(3):443–53. 10.1016/0022-2836(70)90057-4 . [DOI] [PubMed] [Google Scholar]
- 45.Duret L, Gasteiger E, Perriere G. LALNVIEW: a graphical viewer for pairwise sequence alignments. Comput Appl Biosci. 1996;12(6):507–10. 10.1093/bioinformatics/12.6.507 . [DOI] [PubMed] [Google Scholar]
- 46.Huang XM, W. A time-efficient, linear-space local similarity algorithm,. Advances in Applied Mathematics,. 1991;12(3):Pages 337–57. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.