Abstract
Background
The Zika virus (ZIKV) has been associated with Guillain-Barré syndrome (GBS) in epidemiological studies. Whether ZIKV-associated GBS is related to a specific clinical or electrophysiological phenotype has not been established. To this end, we performed a systematic review and meta-analysis of all published studies on ZIKV-related GBS.
Methods
We searched Pubmed, EMBASE and LILACS, and included all papers, reports or bulletins with full text in English, Spanish or Portuguese, reporting original data of patients with GBS and a suspected, probable or confirmed recent ZIKV infection. Data were extracted according to a predefined protocol, and pooled proportions were calculated.
Results
Thirty-five studies were included (13 single case reports and 22 case series, case-control or cohort studies), reporting on a total of 601 GBS patients with a suspected, probable or confirmed ZIKV infection. Data from 21 studies and 587 cases were available to be summarized. ZIKV infection was confirmed in 21%, probable in 22% and suspected in 57% of cases. ZIKV PCR was positive in 30% (95%CI 15–47) of tested patients. The most common clinical features were: limb weakness 97% (95%CI 93–99), diminished/absent reflexes 96% (95%CI 88–100), sensory symptoms 82% (95%CI 76–88), and facial palsy 51% (95%CI 44–58). Median time between infectious and neurological symptoms was 5–12 days. Most cases had a demyelinating electrophysiological subtype and half of cases were admitted to the Intensive Care Unit (ICU). Heterogeneity between studies was moderate to substantial for most variables.
Conclusions
The clinical phenotype of GBS associated with ZIKV infection reported in literature is generally a sensorimotor demyelinating GBS with frequent facial palsy and a severe disease course often necessitating ICU admittance. Time between infectious and neurological symptoms and negative PCR in most cases suggests a post-infectious disease mechanism. Heterogeneity between studies was considerable and results may be subject to reporting bias. This study was registered on the international Prospective Register of Systematic Reviews (CRD42018081959).
Author summary
Guillain-Barré syndrome (GBS) is a rare but severe neurological disease, characterized by an acute onset flaccid paralysis. GBS is thought to be caused by an exaggerated immune response to common infections that damages the peripheral nerves. The Zika virus (ZIKV) is the most recent pathogen to be connected to GBS, when large outbreaks of ZIKV infection in French Polynesia and Latin America were followed by an increased incidence of GBS patients. To better understand the clinical features and outcome of ZIKV-related GBS, we have performed a systematic review and meta-analysis of all published studies on GBS related to ZIKV. We identified 35 studies, reporting on a total of 601 patients with GBS and a suspected, probable or confirmed Zika virus infection, and were able to summarize data of 587 patients from 21 studies in a pooled analysis. Our study shows that published cases with ZIKV-related GBS generally have both sensory and motor symptoms, facial palsy, demyelination on electrophysiological examination, and a severe disease course that often necessitates ICU admittance. The relatively long time between infectious and neurologic symptoms and the lack of detection of viral particles in bodily fluids in most patients suggest a post-infectious rather than an infectious pathogenesis. However, these results should be interpreted taking into account the heterogeneity between studies, which was considerable for many variables, and a possible reporting bias of more severe cases. Outbreaks of ZIKV and GBS may appear in the future and our study can help clinicians in diagnosing and managing GBS patients in ZIKV endemic areas, and increases our understanding of the neuropathology of ZIKV.
Introduction
Guillain-Barré syndrome (GBS) is the most common cause of acute flaccid paralysis worldwide, with an incidence rate of approximately 1 per 100,000 person-years.[1] GBS is an acute immune-mediated polyradiculoneuropathy, and is presumed to be triggered by preceding infections with specific pathogens, such as Campylobacter jejuni, cytomegalovirus (CMV), and Epstein-Barr virus (EBV).[2] Recently, the incidence of GBS increased during Zika virus (ZIKV) epidemics in French Polynesia (2013) and Latin America (2015–2016) and an association between GBS and ZIKV was established through epidemiological studies.[3, 4]
The classic form of GBS is characterized by a rapidly progressive and symmetrical weakness of the limbs, with sensory symptoms and reduced or absent tendon reflexes.[4] Cranial nerve involvement is frequent, with facial and bulbar muscles most often affected.[5] Electrophysiological studies help to confirm the diagnosis of GBS, and can indicate different subtypes, including acute inflammatory demyelinating polyradiculoneuropathy (AIDP), acute motor axonal neuropathy (AMAN), and acute motor and sensory axonal neuropathy (AMSAN).[4] The majority of patients will lose the ability to walk during the acute phase of the disease and about 25% of patients need to be mechanically ventilated at the Intensive Care Unit (ICU).[6] Clinical presentation and severity of GBS can vary extensively between patients. This variability is thought to be, in part, caused by differences in the type of preceding infections. For instance, C. jejuni has been associated with a pure motor axonal form of GBS with a severe disease course, while CMV has been linked to a sensorimotor GBS with pronounced respiratory insufficiency.[6–8]
Since the ZIKV epidemics, numerous studies have been published on ZIKV-related GBS, but it has not been established if there is a specific clinical and electrophysiological phenotype of GBS after ZIKV, and whether this differs from GBS triggered by other pathogens.[3, 4] Therefore, we have performed a systematic review and meta-analysis of all published studies on ZIKV-related GBS, and give a comprehensive overview of demographic characteristics, clinical features, diagnostic investigations, and outcome of ZIKV-related GBS patients.
Methods
This systematic literature review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and was registered on the international Prospective Register of Systematic Reviews (PROSPERO) with number CRD42018081959.[9]
Information sources and search strategy
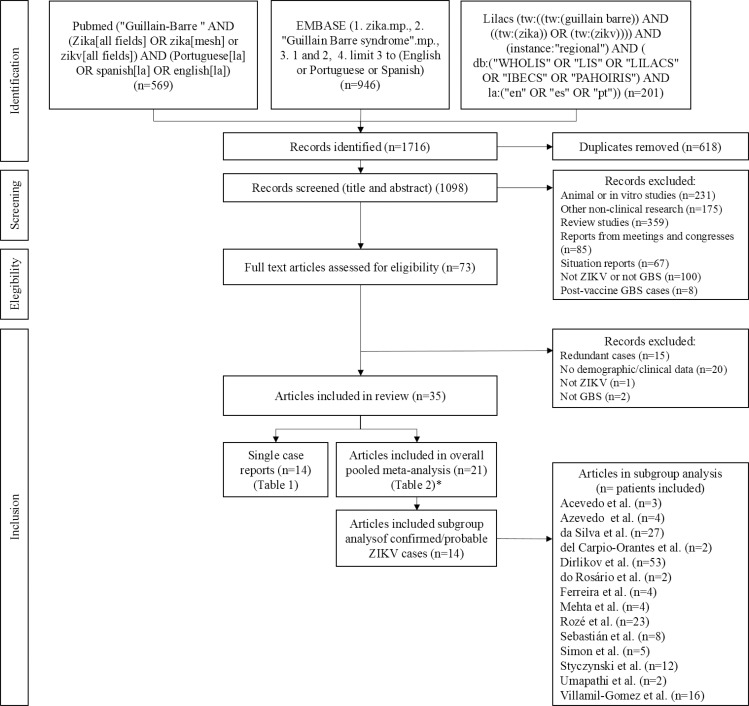
First, by selecting key words from relevant articles, search strategies were constructed for the Pubmed, EMBASE and LILACS databases (Fig 1), which were searched on 24 November 2017 and on 24 January 2019. Second, the titles and abstracts were screened by two researchers (JDLB and SC) to identify the key words (‘Guillain-Barre Syndrome', ‘viruses’, ‘virus’, ‘Zika virus’ and ‘Zika’), and to exclude in vitro or in animal studies and reports from meetings or congresses. The selected papers were read in full by two independent reviewers (CCBS, MFPMA) and a third reviewer (SEL) was consulted in case of disagreement.
Fig 1. PRISMA Flowchart of search and selection of studies on GBS associated with recent ZIKV infection.
*excluding Geurtsvankessel et al. (only one GBS case associated with a recent ZIKV infection).
We included all papers, reports or bulletins with available full text in English, Spanish or Portuguese, without restriction in year of publication, reporting original data of patients with GBS and a suspected, probable or confirmed recent ZIKV infection, of any age, gender and in any setting. Predefined exclusion criteria were: GBS within 3 months after a vaccination or other proven triggering infection (e.g. C. jejuni), and studies with no information on age, residence, and at least one clinical variable of interest. When the study population of reported cases overlapped with cases published in other papers, the paper reporting the highest amount of cases was included. When only part of the cases in a study fulfilled our inclusion criteria, only these cases were included, but if separate data of these cases were not available after contacting the corresponding author, the article was excluded.
Data extraction and management
Data were extracted independently by one of three reviewers (CCBS, MFPMA, SEL) according to a predefined protocol. The data extraction was then checked by one of the other two reviewers, and discrepancies were solved by discussion among all of them. Variables of interest comprised demographics, clinical characteristics (symptoms and signs of arbovirus infection and GBS), ancillary diagnostic investigations (electrophysiology and CSF), treatment, clinical course, and outcome of GBS. The corresponding authors were requested to share data on variables of interest that were not reported.
Cases were classified according to the reported diagnostic certainty levels for GBS and ZIKV infection. To classify the diagnosis GBS we employed the Brighton Collaboration Criteria (2011).[10] If the Brighton Criteria were not reported, these were defined based on available reported data, and if the clinical description did not correspond to the reported Brighton level, cases were reclassified after clarification was sought with the corresponding author. The diagnostic certainty of ZIKV infection was classified as confirmed, probable or suspected, according to the Centers for Disease Control and Prevention (CDC) criteria[11] (Table 1), based on the results of laboratory tests: case-by-case in case reports and series, and all cases combined in larger studies.
Table 1. Zika virus disease case definition.
| Suspected | Acute onset of fever (measured or reported), OR maculopapular rash, OR arthralgia, OR conjunctivitis; OR Guillain-Barré syndrome (not explained by another etiology)* |
| Probable | Suspected ZIKV disease AND Epidemiologic linkage AND Laboratory evidence of recent ZIKV or flavivirus infection by: • Positive ZIKV IgM (serum/CSF) with: ° Positive neutralizing antibody titers against ZIKV and DENV (or other flaviviruses endemic to region of exposure) OR ° Negative DENV IgM and no neutralizing antibody testing performed. |
| Confirmed | Suspected ZIKV disease AND Laboratory evidence of recent ZIKV infection by: • Positive ZIKV culture, viral antigen or RNA (serum, CSF, tissue, or other specimen) OR • Positive ZIKV IgM (serum/CSF) with positive ZIKV and negative DENV (or other flaviviruses endemic to region of exposure) neutralizing antibody titers |
Zika virus case definition according to the Centers for Disease Control (CDC).[11] ZIKV = Zika virus | DENV = Dengue virus | CSF = cerebrospinal fluid | RNA = Ribonucleic acid
*During a ZIKV epidemic
Clinical characteristics were retrieved as the number of patients in whom the variable was present in the numerator, and the total number of reported cases in the denominator: n/N (%). For arbovirus symptoms, we assumed symptoms were absent rather than missing if they were not cited in the manuscript, to account for the reporting bias, and therefore described as zero (n) out of the total number of reported cases (N). For the neurologic findings, variables not cited were considered missing data, because a risk of measurement bias was deemed higher than a risk of a reporting bias for these variables. If clinical characteristics were reported at multiple time points, data representing the full disease course were presented. Continuous variables (age, time between infectious and neurologic symptoms, duration of progression and plateau phase of GBS, duration hospital admission) were extracted as medians and or means, depending on how they were presented in the original article.
Statistical analysis
First, we calculated the proportions per study of each variable of interest, and then the pooled proportions with data from all included studies reporting more than one GBS case. We were unable to summarize continuous variables, as in most studies these were reported as medians without availability of individual data or means. To address the possibility of an ascertainment bias of ZIKV infection among study populations, we then performed a subgroup sensitivity analysis, repeating the pooled analysis with grouped data of only probable or confirmed ZIKV cases (overall study populations comprising only probable/confirmed ZIKV cases, and subsamples of probable/confirmed cases from studies that also included suspected ZIKV cases, when available). We also performed sensitivity analyses by excluding papers that recruited only ICU patients, to account for selection bias in the pooled proportion of mechanical ventilation and ICU assistance.
The pooled proportions and the 95% confidence intervals (CI) were estimated using the random effects model and the Freedman Tukey double arcsine transformation, to account for proportions near 0 and 1. Heterogeneity between studies was calculated using the Chi-square test and I2 statistics, which was interpreted as follows: not important (I2 = 0–40%); moderate (I2 = 30–60%); substantial (I2 = 50–90%); considerable (I2 = 75–100%).[12] The meta-analysis was done using the metaprop command in STATA 15.1.[13]
Results
Study selection
We identified 1716 articles in the databases researched, of which 35 studies were included in our systematic review. The 35 selected studies reported on a total of 601 GBS cases with a suspected, probable or confirmed ZIKV infection with data of at least one variable of interest, and consisted of 13 single case reports and one cohort in which only one case fulfilled our inclusion criteria (n = 14, Table 2), and 14 case series and seven case-control studies (n = 587, Table 3). For the pooled analysis of the studies, we were only able to use the studies that reported on more than one case. (Table 3). For the subgroup meta-analysis of probable/confirmed ZIKV cases, data of 165 GBS cases with probable or confirmed ZIKV infection, from 14 studies, could be pooled (Fig 1).
Table 2. Single case reports of Guillain-Barré syndrome with recent ZIKV infection.
| First author | Journal, year | City, country | Period | Clinical description | ZIKV diagnosis |
|---|---|---|---|---|---|
| Beattie[34] | Infect Dis Clin Pract 2018 |
Dominican Republic (DR) | 2016 |
64 y/o woman returning from DR to USA. Paresthesias, sensory signs, tetraparesis, areflexia, difficulty walking, facial palsy. Preceding (10d) fever, rash, malaise, arthralgia, conjunctivitis, headache, cough, rhinorrhea. CSF: ACD. EMG: AIDP with axonal damage. Brighton level 1. Treatment: IVIg. ICU and MV. Discharge at 35d (tetraparesis). |
ZIKV PCR+ (S,U) IgM: ZIKV- (S,CSF), DENV/CHIKV- (S) VNT ZIKV< DENV (S) |
| Brasil[35] | Lancet 2016 |
Rio de Janeiro, Brazil | Jun 2014 |
24 y/o woman. Paresthesias, tetraparesis, areflexia, difficulty walking. Concurrent fever, rash, headache, ocular pain, conjunctivitis, edema. Normal CSF and EMG. Brighton level 3. No treatment. Discharge at 13d (recovered). |
PCR: ZIKV+ (S,CSF,U,Sa)PCR: DENV/CHIKV-(S, CSF) |
| Fabrizius[36] | Am J Trop Med Hyg 2016 |
Guyana | Mar 2016 |
44 y/o man. Paresthesias, sensory signs, ataxia, LL paresis, areflexia. Preceding (8d) fever, headache, rash, arthralgia, arthritis, conjunctivitis. CSF: ACD. EMG: sensorimotor peripheral neuropathy. Brighton 1. Treatment: IVIg. Discharge at 15d (walking with aid). |
PCR: ZIKV/DENV/CHIKV- (S), ZIKV+ (U) IgM: ZIKV+ (S,CSF); DENV/CHIKV- (S) VNT ZIKV = DENV |
| Fontes[37] | Neuroradiol 2016 |
Rio de Janeiro, Brazil | 2016 |
51 y/o woman. LL paresis, difficulty walking, facial palsy. Preceding (?d) rash, myalgia, arthralgia, conjunctivitis. CSF: ACD. EMG: AIDP. Treatment: IVIg. Clinical improvement. Discharge NR. |
ZIKV+ (S,U–type tests NR) |
| Gonzalez-Escobar[38] | Rev Panam Salud Publica 2016 |
Tunapuna, Trinidad Tobago | Aug 2016 |
29 y/o man. Paresthesias, ataxia, LL paresis and progressing to tetraparesis. Preceding (7d) fever, rash, headache, malaise. Treatment: IVIg. Mild weakness at 10m. |
PCR: ZIKV+(S), DENV/CHIKV-(S) IgM: ZIKV/DENV/CHIKV- (S) |
| Geurtsvankessela[39] | Ann Clin Transl Neur, 2018 | Dhaka, Bangladesh | Nov 2013-Dec 2015 |
58 y/o woman. Distal hypesthesia, tetraparesis, facial and bulbar palsy, autonomic symptoms (constipation). Treatment: IVIg. Independent walking at 3m. |
IgM, IgG, VNT: ZIKV+ (S) PCR: ZIKV- (S) |
| Hamer[40]b | Ann Intern Med 2017 |
Suriname | May 2015-Feb 2016 | 60 y/o woman Returning from Surinam to the Netherlands. Tetraparesis, bulbar and bilateral facial palsy, areflexia, sensory signs. Preceding (?d) fever, myalgia, diarrhea, vomiting. CSF: ACD. EMG: AIDP. Brighton 1. Hospitalized 15d. | PCR: ZIKV+(U, S) IgM: ZIKV+(CSF). |
| Kassavetis[41] | Neurology 2016 |
Haiti | Jan 2016 | 35 y/o man. Paresthesias, sensory signs, bulbar and bilateral facial palsy, ophthalmoplegia, ataxia, areflexia. Preceding (1d) fever, headache, ocular pain, nasal congestion. CSF: ACD. Brighton 2 (MFS-GBS overlap). Treatment: IVIg. Discharge at 5d, walking with aid at 3w. | IgM&VNT: ZIKV+(S,CSF) |
| Miller[42] | J Neurol Sci 2017 |
Dominican Republic | May 2016 |
55 y/o woman. Paresthesias, LL paresis progressing to tetraparesis, bulbar and sensory signs, ataxia, areflexia. Concurrent asthenia, malaise, myalgia. CSF: ACD. EMG: AIDP. Brighton 1. Treatment: IVIg. Discharge at 22d (walking with aid). |
PCR: ZIKV-(S,CSF,U) IgM: ZIKV+ (S,CSF), DENV+(S),CHIKV-(S) VNT ZIKV< DENV (S) |
| Rabelo[43] | Front Microbiol 2018 |
Rio de Janeiro, Brazil | Jun 2016 | 28 y/o pregnant woman (stillbirth). LL paresis progressing to tetraparesis, unable to walk, areflexia, paresthesias, sensory, autonomic, and respiratory signs. Preceding (20d) rash, vomiting. CSF: normal. EMG: AMSAN. Treatment: IVIg. Discharge at 28d, walking with aid at 40d. | ZIKV confirmed in placental and fetal tissues IgM: ZIKV/DENV/CHIKV- (S) |
| Raboni[44] | Transpl Infect Dis 2017 |
Maranhão, Brazil | Jun 2015 |
9 y/o girl. LL paresthesia, paresis, unable to walk, progressing to respiratory dysfunction. Preceding (90d) hematopoietic stem cell transplant. CSF: raised cell count and protein level. EMG: AIDP. Treatment: IVIg and PE. ICU, MV. Hospitalized (?d). Recovered at 4m. |
PCR: ZIKV/DENV-(S), DENV NS1- IgM: ZIKV/DENV+(S) VNT ZIKV< DENV |
| Reyna-Villasmil[45] | Med Clin 2016 |
Zulia, Venezuela | 2016 |
28 y/o pregnant woman (normal birth). Tetraparesis, bulbar palsy, areflexia, progressing to respiratory dysfunction. Preceding (10d) fever, rash, myalgia, conjunctivitis. CSF: ACD. EMG: AIDP. Brighton 1. Treatment: IVIg. ICU and MV. Discharge at 21d (recovered). |
Serology for ZIKV+ (type tests NR) |
| Siu[46] | Neurology 2016 |
Tonga, Polynesia | 2016 | 47 y/o man. Returning from Tonga to New Zealand. Paresthesias, progressive tetraparesis, areflexia, sensory and respiratory signs. Preceding (6d) edematous leg with pustular lesions. CSF: ACD. EMG: AIDP. Brighton 1. Treatment: IVIg and PE. ICU and MV. Discharge at 33d (bedbound). | PCR: ZIKV/DENV/CHIKV-(CSF)ZIKV+/DENV/CHIKV-(S), DENV NS1- IgM: ZIKV/DENV+(S) |
| Zambrano[47] | Am J Trop Med Hyg 2016 |
Guayaquil, Ecuador | Mar 2016 |
57 y/o woman. Paresthesia, facial palsy, tetraparesis, areflexia. Preceding (5d) headache, fever, lumbar back pain. CSF: ACD. Treatment: PE. ICU. Discharge at 10d. |
PCR: ZIKV/CHIKV+/DENV- (S,CSF,U) |
NR = Not Reported | y/o = year-old | USA = United States of America | LL = lower limbs | UL = upper limbs | ICU = Intensive Care Unit | MV = mechanical ventilation | CSF = cerebrospinal fluid | ACD = albuminocytological dissociation | EMG = electromyography/nerve conduction studies | IVIg = intravenous immunoglobulin | PE = plasma exchange | Brighton = Brighton Collaboration Criteria level | MFS = Miller Fisher Syndrome | ZIKV = Zika virus | CHIKV = chikungunya virus | DENV = dengue virus | PCR = polymerase chain reaction | VNT = virus neutralization test | DENV NS1 = NS1 antigen of DENV | S = serum | Sa = saliva | CSF = cerebrospinal fluid | U = urine.
aNot published as a case report but only one case fulfilling our criteria for suspected/probable/confirmed ZIKV in larger cohort of 418 cases.
bReturning travelers with suspected, probable or confirmed ZIKV infection reported to the GeoSentinel Surveillence Network. 93 cases reported, 2 GBS cases, one is already described in the Kassavetis’ paper, the other is described here.
Table 3. Demographic characteristics, case selection and ascertainment in studies reporting more than one Guillain-Barré syndrome case with a recent Zika virus infection.
| First author | Journal, year |
Provenance (city, country) |
ZIKV outbreak | Study design | Incidence period | Study population | Ascertainment GBSa | Ascertainment ZIKV | N cases in analysis (mal:fem) |
Median age (IQR) or [range] |
|---|---|---|---|---|---|---|---|---|---|---|
| Cao-Lormeaub[4] | Lancet 2016 | Papeete, Tahiti,French- Polynesia |
Oct 2013-Apr 2014 | Prospective case-control | Oct 2013 - Mar 2014 |
All GBS inpatients in French Polynesia during ZIKV outbreak | Brighton 1–3 by neurologist or intensivist | PCR: ZIKV(S) VNT,IgM&IgG: ZIKV,DENV(S) |
42 (11:31) |
42 (36–56) |
| Simon[23] | J Neurovirol 2018 |
Noumea, New Caledonia, Melanesia | Jan-Dec 2014 |
Prospective case-control |
Jan—Dec 2014 | All GBS adult patients in New Caledonia during ZIKV outbreak | Brighton 1–2 | PCR, IgM&IgG: ZIKV, DENV(S) VNT: ZIKV(S) |
5c (3:2) |
52 (mean) [29–75] |
| Ferreira[16] | Am J Trop Med Hyg 2016 |
Recife, Brazil | Nov 2014–2015 | Case series | 15 Dec 2014–30 Jun 2015 | First six adults with acute neurological illness and ZIKV PCR+, in reference neurology hospital | Criteria NR, data compatible with Brighton 1 and 4 (2:2) | PCR: ZIKV, DENV(S) IgM&IgG: ZIKV, DENV(S) |
4 (1:3) |
33.5 [25–48] |
| Nóbrega[29] | Epidemiol Serv Saude 2018 |
Recife, Brazil | Nov 2014–2015 | Case series | 23 Dec 2014–19 Jun 2015 |
All GBS inpatients in metropolitan region identified in the Hospital Information System, with arboviral symptoms (<60d) and/or laboratory positivity | Brighton 1–4 by medical records review | ZIKV PCR tested in 1 case (S) DENV IgM tested in1 case (S) |
18 (9:9) |
44 [14–62] |
| Styczynski[27] | PLoS Negl Trop Dis 2017 |
Salvador, Brazil | Jan 2015-May 2016 | Retrospective case-control | 1 Jan 2015–31 Aug 2015 | All GBS cases (≥ 12y/o) reported to the Bahia Epidemiologic Surveillance Center | Brighton 1–3 by medical records review | IgM: ZIKV, DENV(S) VNT: ZIKV, DENV(S) |
50d (19:22) |
44 [32–54] |
| do Rosário[17] | Am J Trop Med Hyg 2016 | Salvador, Brazil | Jan 2015-May 2016 | Case series | 15 May - 30 Jul 2015 |
Adult patients admitted to ICU with ascending paresis, preceding exanthema, ZIKV IgM+ | Wakerley Criteria, 2014 | PCR: ZIKV, DENV, CHIKV(S) IgM&IgG: ZIKV, DENV, CHIKV (18 arboviruses panel in S) VNT:ZIKV, DENV, CHIKV, YFV(S) |
2 (1:1) |
46,5 [22 and 49] |
| Keesen[30] | Lancet 2017 | João Pessoa, Brazil |
2016 | Case series | 2016 | GBS cases in Paraiba province admitted to neurology reference hospital during the ZIKV epidemic in 2016 | NR | PCR: ZIKV IgM&IgG: DENV,CHIKV (type biosample NR) |
12 (8:4) |
35,5 [7–73] |
| da Silva[18] | JAMA Neurol 2017 |
Rio de Janeiro, Niteroi and São Gonçalo, Brazil |
May 2015-Nov 2016 | Cohort | 5 Dec 2015- 10 May 2016 |
All adults with <60d onset of transverse myelitis, meningo-encephalitis or GBS admitted to neuromuscular expertise center | Brighton | ZIKV PCR if -: ZIKV IgM(S,CSF) ZIKV IgM if+: DENV IgM |
28e (9:19) |
42 (22–67) |
| Azevedo[19] | Rev Soc Bras Med Trop 2018 | Rio de Janeiro, Brazil | May 2015-Nov 2016 |
Case series | Jun 2015 - Dec 2016 |
All non-congenital neurologic disorders reported to Information System for Notifiable Diseases and Arboviral Neurologic Manifestation Report | PAHO criteria | PCR: ZIKV,CHIKV(S) IgM: DENV,CHIKV(S) IgG: CHIKV(S) |
72 (NR) |
45 |
| Mehta[21] | PLoS Negl Trop Dis 2018 |
Rio de Janeiro, Brazil |
May 2015- Nov 2016 | Case series | 1 Nov 2015–1 Jun 2016 | Patients ≥12 y/o admitted to one of 11 participating hospitals, with acute neurologic disease, suspected and tested for ZIKV | Brighton by medical records review | PCR:ZIKV,DENV,CHIKV(S,CSF,U) IgM&IgG: ZIKV(S), DENV,CHIKV (S,CSF) |
7f (4:3) |
41 [19–67] |
| Sebastián[22] | J Crit Care 2017 |
7 Latin American countriesg |
2015–2016 | Case series | 1 Dec 2015- 2 Apr 2016 |
Adults with a confirmed ZIKV infection in one of 24 ICUs of the Latin America Surveillance Network | Brighton by intensivist or neurologist (results NR) | PCR: ZIKV(S) | 8 (2:6) |
38 [18–67] |
| Salinas[24] | J Neurol Sci 2017 |
Barranquilla, Colombia |
Oct 2015-Apr 2016 | Retrospective case-control |
1 Oct 2015- 2 Apr 2016 |
All GBS cases in Barranquilla reported to the national and the local surveillance systemh | Brighton 1–3 by medical records review | IgM&VNT: ZIKV, DENV(S) | 47 (25:22) |
49 [10–83] |
| Parra[32] | NEJM 2016 | Cucuta, Medellin, Cali, Barranquilla, Neiva, Colombia | Oct 2015-Apr 2016 | Prospective case-control |
Jan—Mar 2016 | All patients with GBS at six university-based hospitals | Brighton by neurologist or internist | PCR: ZIKV(S,CSF,U), DENV(S,CSF) IgM&IgG: DENV(S,CSF) |
68i (30:38) |
47 (35–57) |
| Villamil-Gomez[28] | Travel Med Infect Dis 2017 |
Sucre, Colombia |
Oct 2015-Apr 2016 | Case series | 2016 | Adults with confirmed ZIKV infection and GBS, admitted to ICU of two major clinical reference centers in Sincelejo-Sucre | NR | PCR: ZIKV; DENV NS1 IgM&IgG: DENV,CHIKV (samples NR) |
16 (4:12) |
53 (47–68) |
| Acevedo[20] | Front Microbiol 2017 |
Guayaquil, Ecuador |
Jan-Oct 2016 | Case series | 1 Feb—31 Aug 2016 | 16 adult patients with neurological symptoms and PCR+ ZIKV, DENV or CHIKV in CSF, admitted to ER or ICU of largest hospital of Guayaquil | Criteria NR, data compatible with Brighton 1, 2, 4 | PCR:ZIKV,DENV,CHIKV(CSF) | 3 (1:2) |
54 [18–62] |
| Langerak[31] | Front Neurol 2016 |
Paramaribo, Suriname | Oct 2015–2016 | Cases series | Jan—Mar 2016 | Consecutive adult patients diagnosed with GBS and preceding ZIKV infection | Criteria NR, data compatible with Brighton 1 | PCR: ZIKV(S,CSF,U); IgM&IgG: ZIKV,DENV(S) VNT: ZIKV(S), DENV NS1(S) |
3 (0:3) |
50 [40–60] |
| Dirlikov-a[33] | MMWR 2016 | Puerto Rico | Dec 2015-Dec 2016 | Case series | 1 Jan—31 Jul 2016 | GBS cases admitted at 13 hospitals, identified by the GBS Passive Surveillance System-Puerto Rico Department of Health | Brighton by medical records review | PCR: ZIKV,DENV,CHIKV(S,CSF) IgM: ZIKV,DENV,CHIKV(S,CSF) |
34 (20:14) |
55 [21–88] |
| Dirlikov-b[25] | JAMA Neurology 2018 | Puerto Rico | Dec 2015-Dec 2016 | Case series | Jan—Dec 2016 | All GBS cases admitted at all the 57 general hospitals of Puerto Rico and identified by the GBS Passive Surveillance System. | Brighton1-3 by medical records review | PCR: ZIKV,DENV,CHIKV (S,CSF,U,Sa) IgM: ZIKV,DENV,CHIKV(S,CSF) |
107j (47:60) |
54 [4–88] |
| Rozé[26] | Clin Infect Dis 2017 | Martinique, French Caribbean |
Jan-Oct 2016 | Case series | Jan—Oct 2016 | All GBS inpatients at only specialized center in the country | Brighton 1–2 by neurologist |
PCR:ZIKV,DENV,CHIKV (S,CSF,U) IgM&IgG: ZIKV,DENV,CHIKV(S) VNT ZIKV (if ZIKV PCR-&IgM-or ZIKV&DENV IgM+) |
30k (8:15) |
61 (56–71) |
| del Carpio-Orantes[14] | Neurología 2018 | Veracruz, Mexico |
2016 | Case series | 2016–2017 | All GBS cases documented by Instituto Mexicano del Seguro Social with GBS and tested for arboviruses | Brighton 1–3 by medical records review |
PCR: ZIKV,DENV,CHIKV(S) IgM&IgG: ZIKV(S) IgM: DENV/CHIKV(S) |
18 | 47 [19–70] |
| Umapathi[15] | J Peripher Nerv Syst 2018 |
Singapore, Singapore | Aug-Nov 2016 | Prospective case-control | May—Dec 2016 | All GBS cases from all public and private hospitals in Singapore before and during ZIKV outbreak | ICD10 G61.0 records in electronic databases | PCR: ZIKV,DENV(S,U) VNT, IgM&IgG: ZIKV,DENV(S) |
12m (7:5) |
55,5 [25–81] |
| Total | 587 |
Age as median and IQR (interquartile range) or [range] unless indicated otherwise. mal = male | fem = female | NA = not applicable | Brighton = Brighton Collaboration Criteria levels[10] | EMG = electromyography/nerve conduction studies | y/o = years old | ICU = Intensive Care Unit | ZIKV = Zika virus | CHIKV = Chikungunya virus | DENV = Dengue virus | PCR = polymerase chain reaction | VNT = virus neutralization test | DENV NS1 = DENV NS1 antigen | NR = Not Reported | S = serum | CSF = cerebrospinal fluid | U = urine | Sa = saliva ER = emergency room | ICD10 = 10th revision of the International Statistical Classification of Diseases and Related Health Problems).
aPatients not fulfilling the Brighton Criteria were included: da Silva (n = 3), Mehta (n = 1), Parra (n = 6).
bAdditional data retrieved from previous publication by Watrin et al, 2016[48].
cClinical data available for 5 cases with laboratory evidence of ZIKV infection (IgM & IgG positive).
dAge, infectious symptoms and laboratory data available for 41 cases included in case-control study, neurologic signs and symptoms available for all 50 reported cases.
eOne post-vaccine case was excluded from data extraction, data on CSF examination were available for all 29 cases, age and clinical data were available for 27 ZIKV positive cases.
fA total of 13 GBS cases with suspected/probable/confirmed ZIKV were reported but data were available for only 7 cases with positive arbovirus tests.
gColombia, Venezuela, Salvador, Guatemala, Puerto Rico, Ecuador, Perú and Chile.
h Colombia National Surveillance System (Sivigila) and Secretaria de Salud de Barranquilla.
iFive cases from Barranquilla may overlap with cases reported by Salinas et al.
jfA total of 123 GBS cases with suspected/probable/confirmed ZIKV were reported but clinical and laboratory data were available for 107 cases tested for ZIKV.
kLaboratory data available for all cases and clinical data for 23 cases with laboratory evidence of ZIKV.
lClinical data of 8 cases additionally retrieved from previous publication by del Carpio-Orantes et al, 2017.[49]
mA total of 14 cases were reported, data were extracted from 11 cases collected during the ZIKV outbreak plus one case with laboratory evidence of recent ZIKV infection before the outbreak.
Study characteristics: case selection, case ascertainment and risk of bias
In Table 2, the single case reports are presented alphabetically with a brief clinical description per case. Eleven cases were from ZIKV epidemic or endemic regions and three were travelers returning from epidemic regions. Eight cases were positive for ZIKV PCR, four for IgM and plaque-reduction neutralization test (PRNT), and two were reported to be ZIKV positive with no further information provided. Six of eight cases of whom the Brighton classification was reported, fulfilled level 1. The most frequent clinical phenotype was a demyelinating sensorimotor GBS with facial and/or bulbar palsy.
In Table 3, the 21 studies reporting more than one patient are displayed according to the location and time-period of cases, in line with the global spread of the ZIKV epidemics on the Pacific islands (Oct 2013-Dec 2014) and Latin America (Dec 2014–2017). The first study was from French Polynesia in 2013–2014,[4] and the last was from Mexico in 2016–2017.[14] One study reported cases during and outside of a ZIKV outbreak period in Singapore[15]
Inclusion criteria, case selection and setting differed between studies. A diagnosis of GBS was the inclusion criterion in 14 studies, and seven studies also included other acute neurologic illnesses besides GBS.[16–22] Six studies included all GBS patients in their reference population,[4, 15, 23–26] one study included all GBS patients >12 years old,[27] and one study included all arbovirus-related neurologic manifestations.[19] All other studies included a convenience sample of patients seen at one or more health-care centres. Three studies only included patients admitted to the ICU,[17, 22, 28] and nine studies only included GBS patients with a clinical suspicion or laboratory evidence of a ZIKV infection.[14, 17, 20–22, 28–31] Seven studies were set in a specialized hospital (academic or reference centre),[4, 16, 18, 23, 26, 31, 32] and two multi-centre studies were set in both specialized and non-specialized hospitals.[21, 28] These differences are potential sources of selection bias within studies and heterogeneity across studies.
Sixteen studies reported the criteria that were applied for diagnostic certainty of GBS, and 13 used the Brighton Criteria. In four studies the Brighton Criteria were prospectively applied by a physician; in seven, retrospectively through records review; two studies gave no information on how the Brighton level was assessed; and three employed other criteria. The risk of ascertainment bias of GBS is likely to be low or very low, as the vast majority of all cases with this data available in this review fulfilled Brighton levels 1–3 (396/407, 97%).
Regarding the ascertainment of ZIKV infection, 13 studies tested their cases for both PCR and IgM,[4, 14–18, 21, 25, 26, 28, 30, 31, 33] five only for PCR,[19, 20, 22, 29, 32] and three only for IgM.[23, 24, 27] Based on the CDC ZIKV case definition, more than a half of all GBS cases with this data available had a suspected ZIKV infection (324/570, 57%), which gives a high risk for ascertainment bias within studies and heterogeneity across studies.
Patient characteristics
Demographics
The median age of the study populations varied between 34 and 61 years, and only 11 pediatric patients were included in four studies.[19, 24, 27, 30] The majority of patients was male (62%) and the male:female ratio of all studies combined was 1.63. In multicenter studies or those including all GBS cases in the reference population, the male:female ratio was 1:1, with the exception of studies from French Polynesia[4] and Martinique[26], which had ratios of 3:1 and 2:1, respectively (Table 3).
Certainty levels of GBS diagnosis and ZIKV infection
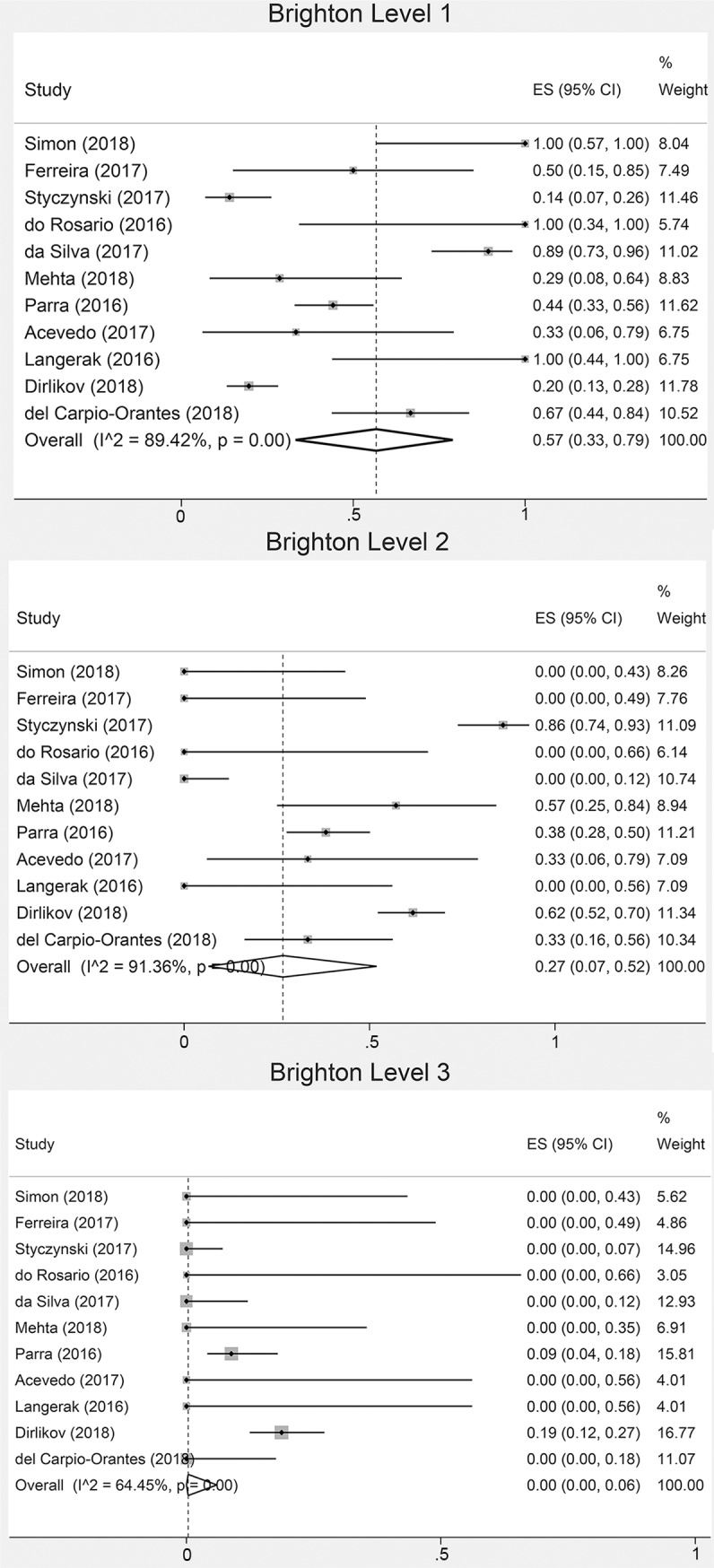
Separate proportions of each Brighton level (1–4) were available in ten studies[14, 16–18, 20, 27, 31–33] (295 cases): 110 cases fulfilling level 1; 146 level 2; 26 level 3 and 13 level 4. Miller Fisher Syndrome (MFS) was reported in only four studies: one study from Singapore (five cases),[15] and three studies from Latin America (six cases).[14, 18, 32] ZIKV infection was confirmed in 118 (21%), probable in 128 (22%) and suspected in 324 (57%) of all cases with reported separate proportions of each ZIKV certainty level. In the overall pooled estimates of study populations with available proportions of at least the Brighton level 1 and a suspected ZIKV infection, 57% of cases had Brighton level 1 and 44% had a suspected ZIKV infection (Fig 2, S2 Fig). We re-calculated these pooled frequencies after excluding two studies that only included cases with Brighton levels 1–2,[23, 26] finding 51% (95%CI: 28–74; I2 89.2%) with Brighton 1 (105/290), and re-calculated pooled frequencies after excluding eight studies that only included cases with probable/confirmed ZIKV,[16, 17, 20–23, 28, 31] finding 65% (95%CI: 47–80; I2 93.2%) with a suspected ZIKV infection (319/522).
Fig 2. Overall pooled proportions (forest plots) of Brighton classification of GBS cases during ZIKV epidemics.
Clinical characteristics
All but one study reported the presence of clinical symptoms of infection.[19] Two or more symptoms were present in 91% of cases (378/444; 95%CI 84–96, I2 61.2%). The most common symptoms were rash, fever and arthralgia, with similar pooled frequencies between overall estimates and the probable/confirmed subgroup (Table 4). The median time between the start of infectious symptoms and neurologic symptoms ranged from -1 to 12 days in the 16 studies reporting on this (Fig 3). For arbovirus symptoms the heterogeneity ranged from considerable (I2 = 75–100%), in the overall analysis, to substantial (I2 = 50–90%), in the probable/confirmed subgroup.
Table 4. Demographics and clinical characteristics of GBS cases associated with ZIKV reported in 21 case series.
| All cases (N 587) | Probable/confirmed ZIKV infection (N 165) | ||||
|---|---|---|---|---|---|
| Demographics | |||||
| Adults % (n/N) | 98% (550/563) | 100% (165/165) | |||
| Female % (n/N) | 38% (216/570) | 41% (67/165) | |||
| Symptoms | |||||
| Infectious symptoms | n/N | Pooled proportion (95%CI; I2) | n/N | Pooled Proportion (95%CI; I2) | |
| Arboviral symptoms | |||||
| Rash | 253/544 | 56% (43–69; 83%) | 86/149 | 61% (37–82; 78%) | |
| Fever | 228/539 | 45% (33–57; 77%) | 66/149 | 42% (21–64; 75%) | |
| Arthralgia | 150/539 | 35% (21–49; 86%) | 50/149 | 31% (15–50; 64%) | |
| Myalgia | 126/550 | 25% (12–41; 89%) | 40/149 | 29% (7–55; 83%) | |
| Headache | 106/550 | 22% (8–38; 91%) | 32/149 | 25% (5–50; 83%) | |
| Conjunctivitis | 98/539 | 17% (8–28; 80%) | 30/149 | 15% (7–24; 14%) | |
| Ocular pain | 24/550 | 1% (0–6; 74%) | 3/149 | 0% (0–3; 41%) | |
| Gastrointestinala | 59/550 | 8% (3–14; 66%) | 15/149 | 6% (0–21; 67%) | |
| Rhinorrhea | 12/550 | 0% (0–1; 0%) | 1/149 | 0% (0–0; 0%) | |
| Cough or chest pain | 28/550 | 2% (0–7; 71%) | 9/149 | 2% (0–13; 61%) | |
| Neurologic symptoms | n/N | Pooled proportion (95%CI; I2) | n/N | Pooled proportion (95%CI; I2) | |
| Sensory symptoms | 333/421 | 82% (76–88; 30%) | 97/119 | 86% (73–96; 34%) | |
| Dysphagia | 133/351 | 30% (17–45; 90%) | 49/112 | 34% (7–67; 85%) | |
| Dysarthria | 64/281 | 11% (1–25; 78%) | 3/13 | 17% (0–60; 48%) | |
| Diplopia | 11/234 | 0% (0–4; 33%) | 1/13 | 2% (0–25; 0%) | |
| Neurologic signs | n/N | Pooled proportion (95%CI; I2) | n/N | Pooled proportion (95%CI; I2) | |
| Facial palsy | 246/486 | 51% (44–58; 36%) | 75/139 | 56% (42–71; 38%) | |
| Bulbar palsy | 60/182 | 25% (10–42; 70%) | 4/11 | 32% (0–76; 33%) | |
| Ocular palsy | 22/232 | 5% (0–12; 46%) | 0/11 | 0% (0–19; 0%) | |
| Any limb paresis | 544/582 | 97% (93–99; 49%) | 153/165 | 98% (93–100; 17%) | |
| Tetraparesis | 153/251 | 64% (51–77; 53%) | 79/110 | 74% (61–87; 25%) | |
| Paraparesis | 69/251 | 24% (18–31; 0%) | 21/110 | 15% (7–24; 0%) | |
| Sensory deficits | 155/317 | 49% (29–68; 86%) | 59/104 | 59% (39–78; 48%) | |
| Areflexia or hyporeflexia | 400/435 | 96% (88–100; 79%) | 131/142 | 97% (86–100, 56%) | |
| Ataxia | 76/317 | 17% (4–35; 87%) | 34/91 | 29% (4–61; 74%) | |
| Respiratory dysfunctionb | 124/369 | 23% (13–35; 77%) | 37/104 | 24% (10–41; 38%) | |
| Dysautonomia | 73/359 | 13% (5–24; 71%) | 21/102 | 16% (8–26; 0%) | |
| GBS classification | n/N | Pooled proportion (95%CI; I2) | n/N | Pooled proportion (95%CI; I2) | |
| Brighton criteria | |||||
| Level 1–3 | 396/407 | 100% (97–100; 56%) | 128/135 | 99% (93–100; 49%) | |
| Level 4 | 13/407 | 0% (3–100; 62%) | 7/135 | 1% (0–11; 54%) | |
| Miller Fisher Syndrome | 11/419 | 0% (0–2; 53%) | 1/137 | 0% (0–0; 0%) | |
| Other variants | 3/419 | 0% (0–0; 0%) | 0/137 | 0% (0–0; 0%) | |
Brighton level = Brighton Collaboration Criteria[10] levels.
aNausea, vomiting or diarrhea.
bReported as ‘trouble breathing’, ‘difficulty breathing’ or ‘respiratory dysfunction’
Fig 3. Per study medians and ranges of days of time between onset of infectious and neurologic symptoms, and the progressive and plateau phase of GBS cases.
() = inter quartile range, [] = range.
Among neurologic findings, paresis was reported in all studies, and almost all studies reported on sensory symptoms, tendon reflexes, and facial palsy, while other symptoms were reported less frequently. The most frequent neurological findings were limb paresis, sensory symptoms, and hypo/areflexia. Other frequent symptoms were facial palsy in about half, and bulbar palsy and respiratory dysfunction in about a quarter of cases. Frequencies of tetraparesis, sensory deficits, bulbar palsy and ataxia were higher in the probable/confirmed cases compared to overall proportions (Table 4). Separate data on tetraparesis vs paraparesis were reported in ten studies.[4, 16–18, 20, 21, 23, 25, 27, 31] Paraparesis was present in 69 of 251 reported cases (24% 95%CI 18–31). This included reports of cases with only lower limb weakness at nadir (30/251), cases with only lower limb weakness at an unclear time point in the disease (33/251), and cases that were reported as having a paraparetic variant of GBS (6/251). Heterogeneity in the analysis of all cases combined was substantial (I2 = 50–90%) for dysarthria, dysphagia, bulbar palsy, sensory deficits, areflexia/hyporeflexia, ataxia, respiratory dysfunction, and dysautonomia. In the probable/confirmed subgroup analysis this was substantial only for dysphagia and ataxia.
Diagnostic investigations
PCR, principally in serum, was the most frequently performed test for ZIKV diagnosis, although anti-ZIKV IgM was positive twice more often (Table 5). In the CSF, ZIKV PCR was positive in only 10 of 244 tested cases. Presence of neutralizing antibodies against ZIKV in the serum was tested in eight studies.[4, 15, 17, 23, 24, 26, 27, 31] To differentiate ZIKV from DENV, IgM antibodies against DENV were tested in 18 studies (426 cases), and were positive in 70 patients.[4, 14–18, 21, 23–33] Of these patients, 54 were also positive for ZIKV PCR, IgM and/or ZIKV neutralizing antibodies, and in 16 cases no separate information on ZIKV test results was available. Infection with CHIKV was investigated in nine studies and 187 cases, of which 16 were PCR or IgM positive.[14, 17, 19–21, 25, 28, 30]
Table 5. Ancillary investigations, treatment and disease progression of GBS cases associated with ZIKV reported in 21 case series.
| Ancillary investigations | All cases (N 587) | Cases with probable/confirmed ZIKV infection (N 165) | ||
|---|---|---|---|---|
| n/N | Pooled proportion (CI; I2) | n/N | Pooled proportion (CI; I2) | |
| Zika virus certainty level | ||||
| Confirmed | 118/570 | 24% (11–40; 92%) | 88/165 | 63% (32–90; 90%) |
| Probable | 128/570 | 14% (3–30; 93%) | 75/165 | 36% (9–67; 90%) |
| Suspected | 324/570 | 44% (28–62; 92%) | ----- | ----- |
| Arboviral tests | ||||
| ZIKV infectiona | ||||
| PCR (any sample) | 118/470 | 30% (15–47; 90%) | 88/153 | 71% (40–95; 88%) |
| PCR Serum | 43/409 | 10% (1–24; 87%) | 42/134 | 32% (5–66; 89%) |
| PCR CSF | 10/244 | 3% (0–16; 74%) | 6/78 | 11% (0–38; 80%) |
| PCR Urine | 48/253 | 28% (7–54; 90%) | 31/69 | 63% (21–97; 81%) |
| IgM (any sample) | 254/375 | 68% (49–85; 90%) | 126/137 | 97% (87–100; 52%) |
| IgM Serum | 228/374 | 67% (45–85; 91%) | 124/137 | 94% (81–100; 66%) |
| IgM CSF | 36/111 | 60% (7–100; 95%) | 33/50 | 77% (23–100; 91%) |
| PRNT ZIKV | 121/154 | 86% (62–100; 86%) | 23/23 | 100% (94–100; 0%) |
| PRNT ZIKV>DENV | 20/105 | 16% (7–26; 14%) | 11/18 | 67% (20–100; 52%) |
| DENV infection (PCR) | 3/235 | 0% (0–1; 0%) | 2/75 | 0% (0–10; 35%) |
| CHIKV infection (PCR or IgM) | 16/187 | 1% (0–8; 56%) | 4/88 | 0% (0–10; 29%) |
| DENV and CHIKV co-infection | 6/165 | 1% (0–14; 71%) | 2/84 | 0% (0–8; 42%) |
| CSF analysis | 425/537 | 92% (79–100; 92%) | 122/139 | 99% (87–100; 65%) |
| Increased protein levelb | 253/289 | 94% (89–98; 19%) | 64/70 | 97% (89–100; 0%) |
| ACD | 276/335 | 89% (80–96; 64%) | 91/99 | 98% (92–100; 0%) |
| Electrophysiological exam | 245/477 | 68% (49–85; 93%) | 86/145 | 77% (46–98; 88%) |
| AIDP | 143/244 | 62% (38–83; 89%) | 62/86 | 68% (44–88; 59%) |
| AMAN | 58/244 | 16% (0–41; 92%) | 11/85 | 13% (1–33; 56%) |
| AMSAN | 13/244 | 1% (0–6; 51%) | 9/85 | 3% (0–11; 8%) |
| Equivocal | 9/240 | 0% (0–2; 0%) | 0/86 | 0% (0–0; 0%) |
| Unexcitable | 4/240 | 0% (0–0; 0%) | 1/86 | 0% (0–1; 0%) |
| Normal | 11/245 | 0% (0–4; 26%) | 2/86 | 0% (0–1; 0%) |
| Immunomodulatory treatment | 458/555 | 92% (81–99; 88%) | 153/160 | 100% (97–100; 8%) |
| IVIg | 441/555 | 89% (77–97; 90%) | 152/160 | 99% (94–100; 27%) |
| Plasma exchange | 6/555 | 0% (0–0; 0%) | 1/160 | 0% (0–0; 0%) |
| IVIg and plasma exchange | 11/555 | 0% (0–1; 25%) | 0/160 | 0% (0–0; 0%) |
| Disease progression | ||||
| Admission to ICU | 287/544 | 49% (35–62; 86%) | 82/146 | 57% (29–84; 86%) |
| Mechanical ventilation | 118/567 | 21% (15–28; 44%) | 35/140 | 19% (7–34; 57%) |
| Died | 23/485 | 1% (0–3; 0%) | 4/133 | 0% (0–2; 0%) |
Abbreviations: ZIKV = Zika virus | CHIKV = Chikungunya virus | DENV = Dengue virus | PCR = polymerase chain reaction | CSF = cerebrospinal fluid | ACD = albuminocytological dissociation | AIDP = acute inflammatory demyelinating polyradiculoneuropathy | AMAN = acute motor axonal neuropathy | AMSAN = acute motor sensory axonal neuropathy | IVIg = intravenous immunoglobulin | ICU = Intensive Care Unit
aProportions calculated per case, not per biological sample.
bDefinition of increased protein level in CSF differed per study (>45 mg/dL, >51mg/dL or no cut-off reported).
Only five studies tested all ZIKV suspected cases for other infections that have been associated with GBS (C.jejuni, CMV, EBV, Hepatitis E virus, Mycoplasma pneumoniae).[4, 17, 23, 26, 31] And all tested cases (80/587; 14%) were negative for recent infection. None of the studies tested for all of these pathogens. Heterogeneity was considerable for all ZIKV laboratory tests (I2 = 75–100%).
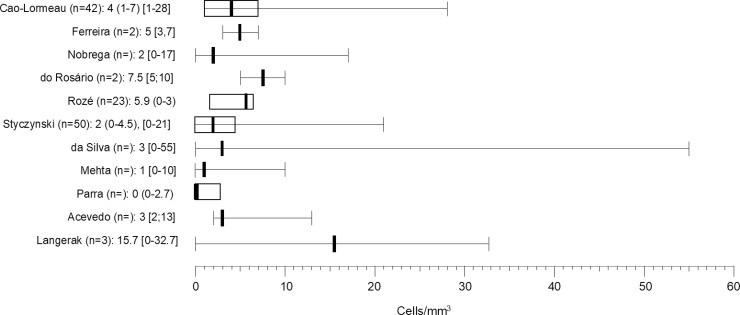
CSF was examined in most studies, and information on protein level and cell count was provided by about half of these. Increased protein level and albuminocytological dissociation were present in the vast majority of cases and results were similar between all studies combined and the probable/confirmed subgroup. Eleven studies reported the CSF cell count, which did not exceed 55 cells/mm3, and medians were below 5 cells/mm3 (Fig 4).[4, 16–18, 20, 21, 26, 27, 29, 31, 32] Heterogeneity was limited for increased protein level and albuminocytological dissociation in all studies combined and the probable/confirmed subgroup.
Fig 4. Overview of cell count in the CSF in reported studies.
Cell count in medians, () = inter quartile range, [] = range.
Electrophysiological studies were done in about half of reported cases. In five studies no information on electrophysiological examination was reported.[16, 19, 28–30] Criteria used to classify cases into the different electrophysiological subtypes were reported in only five studies,[18, 23, 26, 31, 32] and included criteria by Hadden et al, Ho et al, and Rajabally et al.[50–52]. The most frequent electrophysiological subtype was AIDP in 62% (95%CI 38–83), followed by AMAN in 16% (95%CI 0–41), with both similar pooled proportions in the probable/confirmed ZIKV subgroup. In most studies, the majority of cases had an AIDP subtype, except for the study from French-Polynesia[4] where all cases were classified as AMAN, three studies with similar percentages of AMAN and AIDP,[20, 22, 27] a study from Singapore[15] with similar frequencies of AIDP and a normal EMG (in patients with MFS), and a Brazilian case series[21] reporting only a normal EMG and AMAN or AMSAN subtypes.
Treatment and disease progression
All but three studies[15, 20, 30] provided information on treatment, and in most studies almost all cases were treated with IVIg, except for three large studies, from Colombia[24, 32] and Brazil[19], where only 55–70% of patients were treated with immunomodulating therapy. Three studies provided no information on ICU admission,[23, 29, 30] which was necessary in about 50% of all reported cases, and even more frequent in the probable/confirmed subgroup (57%, 95%CI 29–84). Mechanical ventilation (MV) was necessary in about 20% of all cases and of the probable/confirmed subgroup. Death was infrequent in all cases combined and the probable/confirmed subgroup. Heterogeneity was substantial for immunomodulatory treatment and ICU admission, and moderate for MV (Table 5). We recalculated the pooled proportions of ICU, MV and death after excluding three studies that only selected cases admitted to the ICU,[17, 22, 28] and found that ICU admissions (261/518) were lower although still frequent (40%, 95%CI: 28–52), frequency of MV (111/441) was unchanged (22%, 95%CI: 16–28), and frequency of death (22/475) was similar (2%, 95%CI 0–4%), with comparable frequencies in the probable/confirmed subgroup analysis.
Eight studies informed about the time between onset and nadir of neurologic deficits (progressive phase), and only three studies reported the duration of the plateau phase (Fig 3). Only one large study from French-Polynesia informed about the functional evaluation of mobility of patients at nadir, showing incapacity to walk in 27/42 and difficulty to walk in 3/42.[4] The mobility of patients at 6 months after onset of disease was described in a study from Brazil[27] (33/50 walking without aid, 17/50 incapacity to walk) and a study from Puerto Rico[25] (48/79 able to walk 10 meters without aid, 39/79 any difficulty walking, and 12/79 incapacity to walk).
Discussion
Our systematic review and meta-analysis show that published studies on ZIKV-related GBS typically report a classic sensorimotor type of GBS often with a facial palsy and a demyelinating electrophysiological subtype. The disease course is frequently severe with high rates of respiratory dysfunction and ICU admission. The time between onset of infectious and neurologic symptoms and negative PCR in most patients suggests a post-infectious rather than a direct infectious disease mechanism. These results should however be interpreted with caution as the studies included in this systematic review are variable in study design and setting, selection criteria, diagnostic ascertainment, and reporting of variables, which are potential sources of bias.
The combination of sensorimotor signs with facial palsy and respiratory insufficiency and a demyelinating electrophysiological subtype has previously been described in GBS patients with other preceding virus infections, such as CMV, indicating that such a clinical and electrophysiological profile may be related to preceding virus infections in general, in contrast to a bacterial infection with C.jejuni, that is associated with a pure motor axonal type of GBS.[7, 8, 53, 54] Additionally, although GBS is generally more common in men than in women, we found equal distributions of male and female frequencies in larger studies, similar to previous reports on GBS after other virus infections, suggesting that females may be more prone to virus-related GBS.[7, 53] This finding could however also be due to a higher incidence of ZIKV disease in females compared to males as has been shown in some studies.[55, 56] Another interesting finding was the high frequency of paraparesis (24%) compared to previous literature on GBS (1–11%), indicating that this may be a GBS variant related to ZIKV, although a lower percentage of paraparesis in the subgroup of patients with probable/confirmed ZIKV makes this feature less specific.[5, 57, 58] Furthermore, in some studies it is not clear if the paraparesis evolved to tetraparesis at a later time point, and whether myelitis, which has been linked to ZIKV in other studies, was excluded.[5, 57–59]
Some included studies diverged from the generally reported phenotype. Most importantly, the study from French Polynesia[4], in which all 42 patients had an AMAN electrophysiological subtype, 17 (40%) had a paraparesis and only 26 (62%) had hypo- or areflexia; and the study from Singapore[15], in which 4 out of 12 patients (33%) had MFS and one (8%) had MFS-GBS overlap syndrome. The high percentage of MFS in Singapore is in line with other publications that show high prevalence of MFS in Asian countries, but whether an AMAN subtype is typical for the Pacific region has not been studied.[5] As most of the other studies described cases from Latin America and the Caribbean, these discrepancies may be due to regional differences in host and/or environmental factors, including differences in the ZIKV strains.[5, 60] However, some dissimilarities could also be due to differences in diagnostic and electrophysiological accuracy between studies. For instance, the interpretation of electrophysiological data in the study from French Polynesia[4] has previously been questioned, as the prolonged distal motor latencies, found at first examination and persisting after 4 months, would be more consistent with the AIDP subtype.[61]
The median time between the onset of infectious symptoms and the start of neurologic symptoms varied between 5 and 12 days, which is similar to other infections preceding GBS.[7, 62, 63] Considering that the incubation period of ZIKV infection is estimated at 1–2 weeks, the latency between ZIKV infection and GBS was more than a week for most cases, suggesting a post-infectious immunopathogenesis, rather than direct neuronal damage or a para-infectious mechanism, as has been suggested in previous publications.[64, 65] A low frequency of ZIKV PCR positivity in blood and CSF, and a low cell count in the CSF in the majority of cases, further argues against a direct infection. These findings are in line with an in vivo study that showed resistance of peripheral nerve cells to infection by ZIKV.[66]
Remarkably, half of all cases combined and more than a half of probable/confirmed cases were admitted to the ICU. This proportion is higher than expected based on other literature (15–30%)[67, 68], and remained higher (40%) after we excluded papers that only included patients admitted to the ICU. These data may indicate that GBS following ZIKV infection is often severe enough to necessitate ICU admission. However, the percentage of mechanically ventilated patients (20%) is similar to most other publications.[5, 58, 69, 70] It is not clear what causes this discrepancy. A possible explanation is that presence of autonomic symptoms, rapid progression, severe weakness, or respiratory problems that did not evolve into respiratory insufficiency, were reasons to admit to the ICU, especially during the ZIKV epidemic when an increased vigilance for GBS may have lowered the threshold for intensive care monitoring. Furthermore, many studies were done in specialized centres that may receive more severely affected patients referred from other centres, or may more easily admit patients to the ICU for monitoring compared to non-specialized centres.
The large variability of study designs and settings, selection criteria, diagnostic ascertainment and citation of variables were important sources of bias within studies and heterogeneity across studies, which is a critical limitation of our meta-analysis. Most importantly, diagnostic ascertainment of GBS and ZIKV differed, and electrophysiological criteria were not reported in most studies. Diagnostic certainty of ZIKV infection was limited in most studies, and other preceding infections in GBS were often not excluded. Furthermore, the type of hospital may have biased the inclusion of severe cases, causing heterogeneity in both clinical signs and disease progression. We calculated the I2 to quantify this heterogeneity between studies, and have performed a sensitivity analysis to estimate the pooled frequencies among a subgroup of cases with only probable/confirmed ZIKV to analyse the clinical picture of GBS among cases with a higher ascertainment of ZIK infection.
The I2 was considerable for most infectious symptoms, which is likely due to recall and reporting bias, and as we assumed infectious symptoms were absent, rather than missing, if not reported, we may have increased this heterogeneity. Heterogeneity in neurologic symptoms and signs was considerable for some variables, which may be due to differences in study design and methodology and geographical location. Heterogeneity of arboviral test results was also considerable, which may be due to differences between timing of sample collection and variation in incubation and viremia periods. In general, the variables with considerable heterogeneity are difficult to interpret and preclude any firm conclusions to be drawn from these data. However, the I2 in the probable/confirmed ZIKV subgroup was generally lower than in all cases combined, indicating that the heterogeneity was partly caused by differences in the diagnostic certainty of ZIKV infection, providing more evidence for a specific clinical and electrophysiological phenotype of ZIKV-related GBS.
Conclusion
Published studies on ZIKV-related GBS generally report a sensorimotor demyelinating GBS with a frequent facial palsy and a severe disease course that often necessitates ICU admittance. The paraparetic variant of GBS is also common, which should caution clinicians to exclude myelitis in ZIKV-related cases. The time between onset of infectious and neurologic symptoms and absence of viral genome detected by PCR in most cases suggest a post-infectious, rather than a direct infectious or para-infectious mechanism.
Supporting information
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.
(DOC)
Protocol used for data extraction of the selected papers.
(DOCX)
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart (idem to Fig 1).
(TIF)
(TIF)
Excel sheet showing the data as extracted from the selected papers. All cases combined and the cases with probable or confirmed Zika virus infection as displayed separately.
(XLSX)
Acknowledgments
We gratefully thank Dr. J. Anaya, Dr. Yhojan Rodríguez, Dr. G. Castellanos, Dr. V. Saraceni, Dr. P. Brasil, Dr. Guilherme Calvet, Prof. Dr. Arnaud Fontanet, Dr. E. Dirlikov, Dr. C. Major, Dr. Tyler Sharp, Dr. I.C. Siqueira, Dr. Gonzalez-Escobar, Dr. D. Hamer, Dr. P. J.J. van Genderen, Dr. A. Berkowitz, Dr. M. Perloff, Dr T. Langerak, Dr K. Thakur, Dr. C. Geurtsvankessel, Dr. L. del Carpio-Orantes, Dr. S.M. Raboni, Dr. Rozé, Dr. J.L. Salinas, Dr. J.J. Sejvar, Dr. J. Soares, Dr. A.V.A. Ricardo (on behalf of the Latin American Critical Care Investigators Network (LACCTIN)), Dr. P. Timmings, and Dr. A. Styczynski for answering our queries regarding their publications, and for sending us individual data of patients or of subgroups of patients for our analysis.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
SEL, CCBS, MLBF, MFPMA and BCJ are supported by ZikaPLAN, a global research consortium funded by the European Union within the Horizon2020 program (grant agreement number: 734584). The funding body had no role in the design, acquisition or interpretation of the data.
References
- 1.Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology. 2011;36(2):123–33. Epub 2011/03/23. 000324710 [pii] 10.1159/000324710 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobs BC, Rothbarth PH, van der Meche FG, Herbrink P, Schmitz PI, de Klerk MA, et al. The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurology. 1998;51(4):1110–5. Epub 1998/10/22. 10.1212/wnl.51.4.1110 . [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Zika situation report 5 February 2016. World Health Organization; 2016:Available from: https://www.who.int/emergencies/zika-virus/situation-report/5-february-2016/en/. [Google Scholar]
- 4.Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387(10027):1531–9. Epub 2016/03/08. 10.1016/S0140-6736(16)00562-6 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doets AY, Verboon C, van den Berg B, Harbo T, Cornblath DR, Willison HJ, et al. Regional variation of Guillain-Barré syndrome. Brain. 2018;141(10):2866–77. 10.1093/brain/awy232 [DOI] [PubMed] [Google Scholar]
- 6.van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014;10(8):469–82. 10.1038/nrneurol.2014.121 . [DOI] [PubMed] [Google Scholar]
- 7.Orlikowski D, Porcher R, Sivadon-Tardy V, Quincampoix JC, Raphael JC, Durand MC, et al. Guillain-Barre syndrome following primary cytomegalovirus infection: a prospective cohort study. Clin Infect Dis. 2011;52(7):837–44. Epub 2011/03/24. cir074 [pii] 10.1093/cid/cir074 . [DOI] [PubMed] [Google Scholar]
- 8.Jacobs BC, van Doorn PA, Groeneveld JH, Tio-Gillen AP, van der Meche FG. Cytomegalovirus infections and anti-GM2 antibodies in Guillain-Barre syndrome. J Neurol Neurosurg Psychiatry. 1997;62(6):641–3. Epub 1997/06/01. 10.1136/jnnp.62.6.641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1 10.1186/2046-4053-4-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sejvar JJ, Kohl KS, Gidudu J, Amato A, Bakshi N, Baxter R, et al. Guillain-Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29(3):599–612. Epub 2010/07/06. S0264-410X(10)00798-X [pii] 10.1016/j.vaccine.2010.06.003 . [DOI] [PubMed] [Google Scholar]
- 11.Prevention CfDCa. Zika Virus Disease and Zika Virus Infection 2016 Case Definition. 2016.
- 12.Higgins JPT GS. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011:12. [Google Scholar]
- 13.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39 10.1186/2049-3258-72-39 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Carpio-Orantes L, Peniche Moguel KG, Sanchez Diaz JS, Pola-Ramirez MDR, Mata Miranda MDP, Garcia-Mendez S, et al. Guillain-Barre syndrome associated with Zika virus infection: Analysis of a cohort from the region of northern Veracruz in 2016–2017. Neurologia. 2018. Epub 2018/08/04. S0213-4853(18)30173-7 [pii] 10.1016/j.nrl.2018.05.002 . [DOI] [PubMed] [Google Scholar]
- 15.Umapathi T, Kam YW, Ohnmar O, Ng BCJ, Ng Y, Premikha M, et al. The 2016 Singapore Zika virus outbreak did not cause a surge in Guillain-Barre syndrome. J Peripher Nerv Syst. 2018;23(3):197–201. Epub 2018/08/03. 10.1111/jns.12284 . [DOI] [PubMed] [Google Scholar]
- 16.Brito Ferreira ML, Antunes de Brito CA, Moreira AJP, de Morais Machado MI, Henriques-Souza A, Cordeiro MT, et al. Guillain-Barre Syndrome, Acute Disseminated Encephalomyelitis and Encephalitis Associated with Zika Virus Infection in Brazil: Detection of Viral RNA and Isolation of Virus during Late Infection. Am J Trop Med Hyg. 2017;97(5):1405–9. 10.4269/ajtmh.17-0106 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.do Rosario MS, de Jesus PA, Vasilakis N, Farias DS, Novaes MA, Rodrigues SG, et al. Guillain-Barre Syndrome After Zika Virus Infection in Brazil. Am J Trop Med Hyg. 2016;95(5):1157–60. Epub 2016/11/04. ajtmh.16-0306 [pii] 10.4269/ajtmh.16-0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Silva IRF, Frontera JA, Bispo de Filippis AM, Nascimento O, Group R-G-ZR. Neurologic Complications Associated With the Zika Virus in Brazilian Adults. JAMA Neurol. 2017;74(10):1190–8. Epub 2017/08/15. 2647256 [pii] 10.1001/jamaneurol.2017.1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azevedo MB, Coutinho MSC, Silva MAD, Arduini DB, Lima JDV, Monteiro R, et al. Neurologic manifestations in emerging arboviral diseases in Rio de Janeiro City, Brazil, 2015–2016. Rev Soc Bras Med Trop. 2018;51(3):347–51. Epub 2018/07/05. S0037-86822018000300347 [pii] 10.1590/0037-8682-0327-2017 . [DOI] [PubMed] [Google Scholar]
- 20.Acevedo N, Waggoner J, Rodriguez M, Rivera L, Landivar J, Pinsky B, et al. Zika Virus, Chikungunya Virus, and Dengue Virus in Cerebrospinal Fluid from Adults with Neurological Manifestations, Guayaquil, Ecuador. Front Microbiol. 2017;8:42 Epub 2017/02/09. 10.3389/fmicb.2017.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta R, Soares CN, Medialdea-Carrera R, Ellul M, da Silva MTT, Rosala-Hallas A, et al. The spectrum of neurological disease associated with Zika and chikungunya viruses in adults in Rio de Janeiro, Brazil: A case series. PLoS Negl Trop Dis. 2018;12(2):e0006212 Epub 2018/02/13. 10.1371/journal.pntd.0006212 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sebastian UU, Ricardo AVA, Alvarez BC, Cubides A, Luna AF, Arroyo-Parejo M, et al. Zika virus-induced neurological critical illness in Latin America: Severe Guillain-Barre Syndrome and encephalitis. J Crit Care. 2017;42:275–81. Epub 2017/08/15. S0883-9441(17)30267-8 [pii] 10.1016/j.jcrc.2017.07.038 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon O, Acket B, Forfait C, Girault D, Gourinat AC, Millon P, et al. Zika virus outbreak in New Caledonia and Guillain-Barré syndrome: a case-control study. J Neurovirol. 2018;24(3):362–8. Epub 2018/03/30. 10.1007/s13365-018-0621-9 [pii]. . [DOI] [PubMed] [Google Scholar]
- 24.Salinas JL, Walteros DM, Styczynski A, Garzon F, Quijada H, Bravo E, et al. Zika virus disease-associated Guillain-Barré syndrome-Barranquilla, Colombia 2015–2016. J Neurol Sci. 2017;381:272–7. Epub 2017/10/11. S0022-510X(17)33767-X [pii] 10.1016/j.jns.2017.09.001 . [DOI] [PubMed] [Google Scholar]
- 25.Dirlikov E, Major CG, Medina NA, Lugo-Robles R, Matos D, Munoz-Jordan JL, et al. Clinical Features of Guillain-Barre Syndrome With vs Without Zika Virus Infection, Puerto Rico, 2016. JAMA Neurol. 2018;75(9):1089–97. Epub 2018/05/26. 2680895 [pii] 10.1001/jamaneurol.2018.1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roze B, Najioullah F, Ferge JL, Dorleans F, Apetse K, Barnay JL, et al. Guillain-Barré Syndrome Associated With Zika Virus Infection in Martinique in 2016: A Prospective Study. Clin Infect Dis. 2017;65(9):1462–8. Epub 2017/10/12. 3979682 [pii] 10.1093/cid/cix588 . [DOI] [PubMed] [Google Scholar]
- 27.Styczynski AR, Malta J, Krow-Lucal ER, Percio J, Nobrega ME, Vargas A, et al. Increased rates of Guillain-Barré syndrome associated with Zika virus outbreak in the Salvador metropolitan area, Brazil. PLoS Negl Trop Dis. 2017;11(8):e0005869 Epub 2017/08/31. 10.1371/journal.pntd.0005869 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villamil-Gomez WE, Sanchez-Herrera AR, Hernandez H, Hernandez-Iriarte J, Diaz-Ricardo K, Castellanos J, et al. Guillain-Barré syndrome during the Zika virus outbreak in Sucre, Colombia, 2016. Travel Med Infect Dis. 2017;16:62–3. Epub 2017/03/30. S1477-8939(17)30043-1 [pii] 10.1016/j.tmaid.2017.03.012 . [DOI] [PubMed] [Google Scholar]
- 29.Nobrega M, Araujo ELL, Wada MY, Leite PLE, Dimech GS, Percio J. Outbreak of Guillain-Barre syndrome possibly related to prior Zika virus infection, Metropolitan Region of Recife, Pernambuco, Brazil, 2015. Epidemiol Serv Saude. 2018;27(2):e2017039 Epub 2018/07/12. S2237-96222018000200309 [pii] 10.5123/S1679-49742018000200016 . [DOI] [PubMed] [Google Scholar]
- 30.Keesen TSL, de Almeida RP, Gois BM, Peixoto RF, Pacha ASC, Vieira FCF, et al. Guillain-Barre syndrome and arboviral infection in Brazil. Lancet Infect Dis. 2017;17(7):693–4. Epub 2017/06/28. S1473-3099(17)30333-X [pii] 10.1016/S1473-3099(17)30333-X . [DOI] [PubMed] [Google Scholar]
- 31.Langerak T, Yang H, Baptista M, Doornekamp L, Kerkman T, Codrington J, et al. Zika Virus Infection and Guillain-Barre Syndrome in Three Patients from Suriname. Front Neurol. 2016;7:233 Epub 2017/01/10. 10.3389/fneur.2016.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parra B, Lizarazo J, Jimenez-Arango JA, Zea-Vera AF, Gonzalez-Manrique G, Vargas J, et al. Guillain-Barré syndrome associated with Zika virus infection in Colombia. N Engl J Med. 2016;375(16):1513–23. Epub 2016/11/01. 10.1056/NEJMoa1605564 . [DOI] [PubMed] [Google Scholar]
- 33.Dirlikov E, Major CG, Mayshack M, Medina N, Matos D, Ryff KR, et al. Guillain-Barre Syndrome During Ongoing Zika Virus Transmission—Puerto Rico, January 1-July 31, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(34):910–4. Epub 2016/09/02. 10.15585/mmwr.mm6534e1 . [DOI] [PubMed] [Google Scholar]
- 34.Beattie J, Parajuli S, Sanger M, Lee G, Pleninger P, Crowley G, et al. Zika Virus-Associated Guillain-Barre Syndrome in a Returning US Traveler. Infect Dis Clin Pract (Baltim Md). 2018;26(6):e80–e4. Epub 2019/03/30. 10.1097/IPC.0000000000000654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brasil P, Sequeira PC, Freitas AD, Zogbi HE, Calvet GA, de Souza RV, et al. Guillain-Barre syndrome associated with Zika virus infection. Lancet. 2016;387(10026):1482 Epub 2016/04/27. S0140-6736(16)30058-7 [pii] 10.1016/S0140-6736(16)30058-7 . [DOI] [PubMed] [Google Scholar]
- 36.Fabrizius RG, Anderson K, Hendel-Paterson B, Kaiser RM, Maalim S, Walker PF. Guillain-Barre Syndrome Associated with Zika Virus Infection in a Traveler Returning from Guyana. Am J Trop Med Hyg. 2016;95(5):1161–5. Epub 2016/11/04. ajtmh.16-0397 [pii] 10.4269/ajtmh.16-0397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fontes CA, Dos Santos AA, Marchiori E. Magnetic resonance imaging findings in Guillain-Barre syndrome caused by Zika virus infection. Neuroradiology. 2016;58(8):837–8. Epub 2016/04/14. 10.1007/s00234-016-1687-9 [pii]. . [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Escobar G, Valadere AM, Adams R, Polson-Edwards K, Hinds AQJ, Misir A, et al. Prolonged Zika virus viremia in a patient with Guillain-Barre syndrome in Trinidad and Tobago. Rev Panam Salud Publica. 2018;41:e136 10.26633/RPSP.2017.136 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.GeurtsvanKessel CH, Islam Z, Islam MB, Kamga S, Papri N, van de Vijver D, et al. Zika virus and Guillain-Barre syndrome in Bangladesh. Ann Clin Transl Neurol. 2018;5(5):606–15. Epub 2018/05/16. 10.1002/acn3.556 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamer DH, Barbre KA, Chen LH, Grobusch MP, Schlagenhauf P, Goorhuis A, et al. Travel-Associated Zika Virus Disease Acquired in the Americas Through February 2016: A GeoSentinel Analysis. Ann Intern Med. 2017;166(2):99–108. Epub 2016/11/29. 2587368 [pii] 10.7326/M16-1842 . [DOI] [PubMed] [Google Scholar]
- 41.Kassavetis P, Joseph JM, Francois R, Perloff MD, Berkowitz AL. Zika virus-associated Guillain-Barre syndrome variant in Haiti. Neurology. 2016;87(3):336–7. Epub 2016/05/11. WNL.0000000000002759 [pii] 10.1212/WNL.0000000000002759 . [DOI] [PubMed] [Google Scholar]
- 42.Miller E, Becker Z, Shalev D, Lee CT, Cioroiu C, Thakur K. Probable Zika virus-associated Guillain-Barre syndrome: Challenges with clinico-laboratory diagnosis. J Neurol Sci. 2017;375:367–70. Epub 2017/03/23. S0022-510X(17)30126-0 [pii] 10.1016/j.jns.2017.02.029 . [DOI] [PubMed] [Google Scholar]
- 43.Rabelo K, Souza LJ, Salomao NG, Oliveira ERA, Sentinelli LP, Lacerda MS, et al. Placental Inflammation and Fetal Injury in a Rare Zika Case Associated With Guillain-Barre Syndrome and Abortion. Front Microbiol. 2018;9:1018 Epub 2018/06/06. 10.3389/fmicb.2018.01018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raboni SM, Bonfim C, Almeida BM, Zanluca C, Koishi AC, Rodrigues P, et al. Flavivirus cross-reactivity in serological tests and Guillain-Barré syndrome in a hematopoietic stem cell transplant patient: A case report. Transpl Infect Dis. 2017;19(4). 10.1111/tid.12700 . [DOI] [PubMed] [Google Scholar]
- 45.Reyna-Villasmil E, Lopez-Sanchez G, Santos-Bolivar J. Guillain-Barré syndrome due to Zika virus during pregnancy. Med Clin (Barc). 2016;146(7):331–2. Epub 2016/03/08. S0025-7753(16)00083-X [pii] 10.1016/j.medcli.2016.02.002 . [DOI] [PubMed] [Google Scholar]
- 46.Siu R, Bukhari W, Todd A, Gunn W, Huang QS, Timmings P. Acute Zika infection with concurrent onset of Guillain-Barré Syndrome. Neurology. 2016;87(15):1623–4. Epub 2016/10/22. WNL.0000000000003038 [pii] 10.1212/WNL.0000000000003038 . [DOI] [PubMed] [Google Scholar]
- 47.Zambrano H, Waggoner JJ, Almeida C, Rivera L, Benjamin JQ, Pinsky BA. Zika Virus and Chikungunya Virus CoInfections: A Series of Three Cases from a Single Center in Ecuador. Am J Trop Med Hyg. 2016;95(4):894–6. Epub 2016/07/13. ajtmh.16-0323 [pii] 10.4269/ajtmh.16-0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watrin L, Ghawche F, Larre P, Neau JP, Mathis S, Fournier E. Guillain-Barre Syndrome (42 Cases) Occurring During a Zika Virus Outbreak in French Polynesia. Medicine (Baltimore). 2016;95(14):e3257 Epub 2016/04/09. doi: 10.1097/MD.0000000000003257 00005792-201604050-00050 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Del Carpio Orantes L, Juarez Rangel FJ, Garcia-Mendez S. Incidence of Guillain-Barre syndrome at a secondary centre during the 2016 zika outbreak. Neurologia. 2017. Epub 2017/09/30. S0213-4853(17)30279-7 [pii] 10.1016/j.nrl.2017.07.019 . [DOI] [PubMed] [Google Scholar]
- 50.Hadden RD, Cornblath DR, Hughes RA, Zielasek J, Hartung HP, Toyka KV, et al. Electrophysiological classification of Guillain-Barre syndrome: clinical associations and outcome. Plasma Exchange/Sandoglobulin Guillain-Barre Syndrome Trial Group. Ann Neurol. 1998;44(5):780–8. Epub 1998/11/18. 10.1002/ana.410440512. 10.1002/ana.410440512 . [DOI] [PubMed] [Google Scholar]
- 51.Ho TW, Mishu B, Li CY, Gao CY, Cornblath DR, Griffin JW, et al. Guillain-Barre syndrome in northern China. Relationship to Campylobacter jejuni infection and anti-glycolipid antibodies. Brain. 1995;118 (Pt 3):597–605. Epub 1995/06/01. 10.1093/brain/118.3.597 . [DOI] [PubMed] [Google Scholar]
- 52.Rajabally YA, Durand MC, Mitchell J, Orlikowski D, Nicolas G. Electrophysiological diagnosis of Guillain-Barré syndrome subtype: could a single study suffice? J Neurol Neurosurg Psychiatry. 2015;86(1):115–9. Epub 2014/05/13. jnnp-2014-307815 [pii] 10.1136/jnnp-2014-307815 . [DOI] [PubMed] [Google Scholar]
- 53.Caudie C, Quittard Pinon A, Taravel D, Sivadon-Tardy V, Orlikowski D, Rozenberg F, et al. Preceding infections and anti-ganglioside antibody profiles assessed by a dot immunoassay in 306 French Guillain-Barre syndrome patients. J Neurol. 2011;258(11):1958–64. 10.1007/s00415-011-6042-9 . [DOI] [PubMed] [Google Scholar]
- 54.Rees JH, Hughes RA. Campylobacter jejuni and Guillain-Barré syndrome. Ann Neurol. 1994;35(2):248–9. Epub 1994/02/01. 10.1002/ana.410350228 . [DOI] [PubMed] [Google Scholar]
- 55.Coelho FC, Durovni B, Saraceni V, Lemos C, Codeco CT, Camargo S, et al. Higher incidence of Zika in adult women than adult men in Rio de Janeiro suggests a significant contribution of sexual transmission from men to women. Int J Infect Dis. 2016;51:128–32. 10.1016/j.ijid.2016.08.023 . [DOI] [PubMed] [Google Scholar]
- 56.Lozier M, Adams L, Febo MF, Torres-Aponte J, Bello-Pagan M, Ryff KR, et al. Incidence of Zika Virus Disease by Age and Sex—Puerto Rico, November 1, 2015-October 20, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(44):1219–23. 10.15585/mmwr.mm6544a4 . [DOI] [PubMed] [Google Scholar]
- 57.Wakerley BR, Kokubun N, Funakoshi K, Nagashima T, Hirata K, Yuki N. Clinical classification of 103 Japanese patients with Guillain-Barre syndrome. J Neurol Sci. 2016;369:43–7. 10.1016/j.jns.2016.08.002 . [DOI] [PubMed] [Google Scholar]
- 58.Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain-Barré syndrome and validation of Brighton criteria. Brain. 2014;137(Pt 1):33–43. Epub 2013/10/29. awt285 [pii] 10.1093/brain/awt285 . [DOI] [PubMed] [Google Scholar]
- 59.Hiew FL, Ramlan R, Viswanathan S, Puvanarajah S. Guillain-Barré Syndrome, variants & forms fruste: Reclassification with new criteria. Clin Neurol Neurosurg. 2017;158:114–8. 10.1016/j.clineuro.2017.05.006 . [DOI] [PubMed] [Google Scholar]
- 60.Beaver JT, Lelutiu N, Habib R, Skountzou I. Evolution of Two Major Zika Virus Lineages: Implications for Pathology, Immune Response, and Vaccine Development. Front Immunol. 2018;9:1640–. 10.3389/fimmu.2018.01640 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uncini A, Shahrizaila N, Kuwabara S. Zika virus infection and Guillain-Barré syndrome: a review focused on clinical and electrophysiological subtypes. J Neurol Neurosurg Psychiatry. 2017;88(3):266–71. Epub 2016/11/02. jnnp-2016-314310 [pii] 10.1136/jnnp-2016-314310 . [DOI] [PubMed] [Google Scholar]
- 62.Rees JH, Soudain SE, Gregson NA, Hughes RA. Campylobacter jejuni infection and Guillain-Barré syndrome. N Engl J Med. 1995;333(21):1374–9. 10.1056/NEJM199511233332102 . [DOI] [PubMed] [Google Scholar]
- 63.Takahashi M, Koga M, Yokoyama K, Yuki N. Epidemiology of Campylobacter jejuni isolated from patients with Guillain-Barré and Fisher syndromes in Japan. J Clin Microbiol. 2005;43(1):335–9. 10.1128/JCM.43.1.335-339.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muñoz LS, Parra B, Pardo CA, Neuroviruses Emerging in the Americas S. Neurological Implications of Zika Virus Infection in Adults. J Infect Dis. 2017;216(suppl_10):S897–S905. 10.1093/infdis/jix511 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fourié T, Grard G, Leparc-Goffart I, Briolant S, Fontaine A. Variability of Zika Virus Incubation Period in Humans. Open Forum Infect Dis. 2018;5(11):ofy261–ofy. 10.1093/ofid/ofy261 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cumberworth SL, Barrie JA, Cunningham ME, de Figueiredo DPG, Schultz V, Wilder-Smith AJ, et al. Zika virus tropism and interactions in myelinating neural cell cultures: CNS cells and myelin are preferentially affected. Acta Neuropathol Commun. 2017;5(1):50 Epub 2017/06/25. 10.1186/s40478-017-0450-8 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gracey DR, McMichan JC, Divertie MB, Howard FM Jr. Respiratory failure in Guillain-Barre syndrome: a 6-year experience. Mayo Clin Proc. 1982;57(12):742–6. . [PubMed] [Google Scholar]
- 68.van Leeuwen N, Lingsma HF, Vanrolleghem AM, Sturkenboom MCJM, van Doorn PA, Steyerberg EW, et al. Hospital Admissions, Transfers and Costs of Guillain-Barré Syndrome. PLOS ONE. 2016;11(2):e0143837 10.1371/journal.pone.0143837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Durand MC, Porcher R, Orlikowski D, Aboab J, Devaux C, Clair B, et al. Clinical and electrophysiological predictors of respiratory failure in Guillain-Barre syndrome: a prospective study. Lancet Neurol. 2006;5(12):1021–8. 10.1016/S1474-4422(06)70603-2 . [DOI] [PubMed] [Google Scholar]
- 70.Ruts L, Drenthen J, Jongen JL, Hop WC, Visser GH, Jacobs BC, et al. Pain in Guillain-Barré syndrome: a long-term follow-up study. Neurology. 2010;75(16):1439–47. 10.1212/WNL.0b013e3181f88345 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.
(DOC)
Protocol used for data extraction of the selected papers.
(DOCX)
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart (idem to Fig 1).
(TIF)
(TIF)
Excel sheet showing the data as extracted from the selected papers. All cases combined and the cases with probable or confirmed Zika virus infection as displayed separately.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.