Abstract
Improving insulin sensitivity may reduce impacts of heat stress (HS) in pigs by facilitating heat dissipation. Chromium (Cr) has been reported to improve insulin sensitivity in pigs. Therefore, the aim of this experiment was to investigate whether Cr supplementation can mitigate HS in growing pigs. Thirty-six gilts were randomly assigned to 2 diets containing 0 (control) or 400 ppb Cr. After 14 d the supplemented pigs were allocated to either 8 d thermoneutral (20°C constant; TN) or cyclic HS (35°C, 0900 h to 1700 h) conditions and continued their respective diet (n = 9 per group). Growth performance was recorded during the 14-d supplementation period. The physiological responses to HS were monitored by measuring respiration rate, rectal temperature, blood gas chemistry, and feed intake during thermal exposure. Kinetics of plasma glucose, insulin and NEFA were studied by intravenous glucose tolerance test (IVGTT) on d 8 of thermal treatment. Results showed Cr alleviated the HS-increased rectal temperature (P < 0.05) and respiration rate (P < 0.01) at 1300 h and 1600 h during thermal exposure. However, Cr did not mitigate the reduction in average daily feed intake which was reduced by 35% during HS or the HS-induced respiratory alkalosis. Chromium tended to increase average daily gain (0.86 vs. 0.95 kg, P = 0.070) during the 14-d supplementation under TN conditions before thermal exposure, which might be associated with the potential of Cr in improving overall insulin sensitivity, as evidenced by a reduced insulin resistance index calculated by Homeostatic Model Assessment (HOMA-IR; 0.65 vs. 0.51, P = 0.013) and a tendency of reduced fasting plasma insulin concentration (1.97 vs. 1.67 μU/mL, P = 0.094). Heat stress decreased the acute insulin releasing rate (P = 0.012) and consequently slowed glucose clearance rate (P = 0.035) during IVGTT. Besides, HS enlarged the values of area under the curve of NEFA during IVGTT (P < 0.01), indicating a reduced lipid mobilization. In conclusion, HS reduced insulin response to IVGTT. Chromium supplementation exhibited a potential in improving insulin sensitivity and mitigating HS symptoms in growing pigs.
Keywords: chromium, heat stress, insulin, physiology, pig
INTRODUCTION
Heat stress (HS) reduces feed intake and alters physiology, endocrine status and metabolism in pigs (Baumgard and Rhoads, 2013; Cottrell et al., 2015; Gabler and Pearce, 2015). Chromium, an insulin sensitizer (Davis et al., 1997; Vincent, 2000), may aid in alleviating these negative impacts of HS. Chromium has been used as a feed additive to improve growth rate and carcass leanness in pigs (Wang et al., 2009; Kim et al., 2010; Sales and Jancik, 2011). While a quantitative Cr requirement has not been established in pigs (NRC 2012), chromium tri-picolinate is widely used as a feed additive due to its stability and bioavailability.
By administering Cr, the negative impacts of HS may be alleviated through increasing insulin sensitivity. First, improving insulin sensitivity can enhance skin micro-circulation (Serné et al., 1999; Forst et al., 2006) and Cr can facilitate vasodilation (Abebe et al., 2010). Therefore, Cr may have an ability to facilitate radiant heat dissipation and thus etiologically alleviate HS. Second, fat deposition is increased in heat-stressed pigs (Christon, 1988; Kouba et al., 2001; Wu et al., 2016) due to reduced lipid mobilization (Pearce et al., 2013a; Sanz Fernandez et al., 2015a), which may be caused by hyperinsulinemia as reported in heat-stressed ruminants (O'Brien et al., 2010; Wheelock et al., 2010; Baumgard et al., 2011). Chromium supplementation has been shown to improve lipid metabolism in hyperinsulinemic rats (Cefalu et al., 2002) and reduces body fat composition in pigs (Lindemann et al., 1995; Hung et al., 2015), therefore Cr may normalize lipid mobilization in heatstressed pigs if hyperinsulinemia is the reason for the reduced lipolysis in the heat-stressed pigs. However, the reports on the effects of Cr supplementation in mitigating HS impacts in pigs are limited and vary; for example, Cr did not affect growth performance or blood cortisol in heat-stressed weaning pigs (Kim et al., 2009), but Cr increased feed intake and reduced blood cortisol in heatstressed finisher pigs (Hung et al., 2014). Therefore, the aim of the study was to systematically investigate the effects of Cr on physiology, feed intake, and insulin related metabolism in growing pigs during HS. The hypothesis being tested is that Cr supplementation can alleviate physiological symptoms and reduction of feed intake by improving insulin sensitivity in heat-stressed pigs.
MATERIAL AND METHODS
Animal and Experimental Design
All experimental procedures involving animals in this study were approved by the Faculty of Veterinary and Animal Ethics Committee of the University of Melbourne (Protocol:1413128). The protocols adhered to the Australian Code for the Care and Use of Animals for Scientific Purposes (8th edition).
The experiment was conducted using a 2 × 2 factorial design with 2 levels of dietary Cr and 2 environmental conditions. The experiment was divided into a 14-d supplementation period and an 8-d thermal exposure period. A total of 36 female pigs (Large White × Landrace, body weight 29 ± 4 kg, mean ± SD) were selected and randomly allocated to a control diet containing no supplemental Cr or a diet supplemented with 400 ppb Cr in the form of Cr picolinate (Feedworks Pty Ltd, Romsey, VIC, Australia) for 14-d. The dose of Cr used in the current experiment followed a previous study (Hung et al., 2014). The control diet was formulated to meet or exceed NRC (2012) recommend nutrient requirements (Table 1). After the 14-d initial dietary supplementation period 9 pigs from each dietary treatment were exposed to either thermoneutral (TN, 20°C, 35–45% relative humidity) or “heat stress” conditions (HS; 35°C 0900–1700 h; 28°C 1700–0900 h, 35–45% relative humidity) for 8 d with pigs remaining on their assigned diet during this period. All pigs were fed ad libitum at 0900 h and 1700 h daily, and individual feed refusals were recorded at 0900 h daily. Water was supplied via nipple drinker ad libitum. Body weights were recorded on a weekly basis.
Table 1.
Composition of control diet
| Ingredient | % of fed basis |
|---|---|
| Wheat | 57.0 |
| Barley | 12.2 |
| Peas | 11.7 |
| Canola meal | 12.0 |
| Meat meal | 2.00 |
| Blood meal | 0.50 |
| Tallow | 0.60 |
| Salt | 0.20 |
| Limestone | 1.10 |
| Monocalcium phosphates | 0.30 |
| Lysine-HCl | 0.39 |
| Methionine | 0.11 |
| Threonine | 0.15 |
| Tryptophan | 0.02 |
| Enzymes | 0.04 |
| Premix1 | 0.20 |
| Calculated Composition | |
| DE, MJ/kg | 14.0 |
| CP, % | 16.6 |
| Fat, % | 3.53 |
| Fiber, % | 4.25 |
| Ash, % | 4.42 |
| Lysine, % | 1.06 |
| Calcium, % | 0.91 |
| phosphorus, % | 0.53 |
Supplied per kg of diet: vitamin A, 1000 IU; vitamin D3, 175 IU; vitamin E, 60 IU; vitamin K, 2 mg; vitamin B-1, 2 mg; vitamin B-2, 5 mg; vitamin B-6 3 mg; vitamin B-12 20 mg; Niacin, 25 mg; pantothenic acid, 20 mg; biotin, 150 mg; folic acid, 20 mg; copper, 12 mg; cobalt, 0.5mg; manganese, 40 mg; zinc, 120 mg; iron, 100 mg; iodine, 0.5 mg; selenium; 0.3 mg; chromium, 0 mg.
Physiological Monitoring
Respiration rate and rectal temperatures were monitored 3 times daily at 0900 h, 1300 h, and 1600 h during the 8-d thermal exposure period. Respiration rate (breaths/min) was counted visually within 20 s, and rectal temperatures were measured with a digital thermometer (Fast-Read, Livingstone Pty Ltd., NSW, Australia). As a precaution pigs were removed from the climatic rooms if their rectal temperature exceeded 41°C until their rectal temperature returned to below 40°C. One pig was removed for 1 h due to hyperthermia and was returned to the rooms after cooling without incident.
Blood Sampling for Blood Gas Measurement
Blood samples (5 mL) were collected in 10 mL vacutainers (sodium heparin coated, BD vacutainer, BD, North Ryde, NSW, Australia) at 1300 h on d 6 of the thermal exposure period via venepuncture from the jugular vein. Fresh blood was immediately loaded into an automatic blood gas analyzer (EPOC, Alere, Waltham, MA) for determination of blood gas variables such as partial pressure of CO2 (pCO2) and O2 (pO2), pH, and bicarbonate.
Intravenous Glucose Tolerant
Test and Metabolite Assays
All pigs were fasted for 15 h starting at 1800 h of d 7 during a thermal exposure period and then received an intravenous glucose tolerance test (IVGTT) at 0900 h of d 8. Each pig was catheterized via an ear vein and then rested for 40 min in their respective environment. For the IVGTT, basal blood metabolites and insulin concentrations were determined by taking small samples (4 mL) at −30, −15, −1 min in relative to glucose administration via the ear vein catheter. At 0 min, a bolus of glucose (40% dextrose solution, Baxter Healthcare, NSW, Australia) was administered intravenously at a dose rate of 0.3 g/kg live weight. Repeated blood samples (4 mL) were taken at 2, 3, 4, 5, 6, 8, 10, 12, 15, 18, 20, 22, 25, 30, 35, 40, 45, 50, 55, 60, 75, 90, 120, 150, 180, 210, and 240 min postglucose administration. In between samples, the catheter was flushed with sterile saline and heparin (diluted to 10 IU per mL). Plasma glucose was assayed by using glucose oxidase kits (Infinity, Thermo Fisher Scientific, Waltham, MA) with intra- and inter- assay coefficients of variability (CV) of 3.7% and 6.6%, respectively. Non-esterified fatty acids concentration was quantified using NEFA C kits (Wako Chemicals, Kawagoe, Japan), and the interand intra-variance of NEFA measurement was 4.3 and 7.0%, respectively. Insulin concentration was quantified by a double anti-body radioimmunoassay (Tindal et al., 1978; Zhang et al., 2004) with all samples processed in a single assay and limit of detection was 0.39 μU/mL. Six replicates of 3 control samples contained 2.36 μU/ml, 4.32 μU/ml, and 8.97 μU/ml were included in the assay to calculate the CV of 3.97%.
Glucose, Insulin, and NEFA Kinetics during IVGTT
The kinetics of glucose, insulin, and NEFA during IVGTT were separately described by indices such as the fasting plasma concentrations, maximum or minimum concentrations, increase or clearance rate, and area under the curve (AUC). The kinetics analyses were limited to the samples obtained before 60 min, because plasma glucose and insulin concentrations had returned to baseline by 60 min after glucose infusion. The increase or clearance rate was calculated from the slope of plasma variables plotted against time in Excel software. The insulin resistance index of Homeostatic Model Assessment (HOMA-IR) was calculated using fasting glucose and insulin concentrations (Matthews et al., 1985). Values of AUC were calculated using the trapezoidal rule with fasting concentrations used as the baseline for subtraction.
Statistical Analysis
Data were analyzed in ANOVA model in GenStat, 15th edition, (VSN international, Hemel Hempstead, UK). For growth performance and feed intake, temperature and diet were set as fixed effects, and initial body weight was used as a covariate. For analyzing the glucose, insulin and NEFA kinetics in response to IVGTT, temperature, diet, and the time relative to glucose intake were set as fixed effects. For physiological variables, temperature, diet, day, and time were set as fixed effects. For blood gas variables, temperature, and diet were used as fixed effects. Individual pig was used as a random effect in all statistical analysis. Duncan's multiple range test was used for the post-hoc multiple comparisons. Values of physiological variables and metabolites from IVGTT were plotted against time points in line charts. Results of the other variables were shown in tables using mean and standard error of difference (SED) of the interactions between temperature and diet. Results were considered to differ significantly when P ≤ 0.05, and a trend was identified when P ≤ 0.10.
RESULTS
Growth Performance before Heat Event
Pigs were allocated into control and Cr dietary treatment with similar body weights (P = 0.57). Average daily feed intake remained similar between the control and Cr fed pigs. Average daily gain tended to be improved by Cr diet (P = 0.070), whereas, the improvement on gain:feed was not significant (P = 0.11; Table 2).
Table 2.
Growth performance of growing pigs during 2-wk chromium supplementation before heat stress
| Variables | Control (n = 18) | Cr (n = 18) | SED | P-value |
|---|---|---|---|---|
| Body weight, initial, kg | 29.2 | 28.9 | 0.62 | 0.57 |
| Average daily feed intake, kg | 1.77 | 1.83 | 0.054 | 0.27 |
| Average daily gain, kg | 0.86 | 0.95 | 0.047 | 0.070 |
| Gain:feed | 0.49 | 0.52 | 0.020 | 0.11 |
| Body weight, 2-wk, kg | 40.3 | 41.4 | 0.76 | 0.16 |
Physiology and Feed Intake
Heat stress increased rectal temperature from 38.8°C to 40.4°C (P < 0.001). While rectal temperature was constant across the day under TN conditions, during HS conditions rectal temperature increased from 0900 h until 1600 h (39.1°C, 40.3°C, 40.7°C for 0900 h, 1100 h, and 1600 h) as evidenced by an interaction between temperature and time (P < 0.001). While there was no main dietary effect on rectal temperature (P = 0.17), there tended to be an interaction between temperature and diet (P = 0.065) and there was a threeway interaction between temperature, diet and time (P = 0.013), such that pigs fed with Cr had reduced rectal temperatures than those on control diet at 1300 h (40.5°C vs. 40.2°C for control vs. Cr diet) and at 1600 h (40.9°C vs. 40.6°C for control vs. Cr diet) under HS conditions but not at other times (Fig. 1 A).
Figure 1.
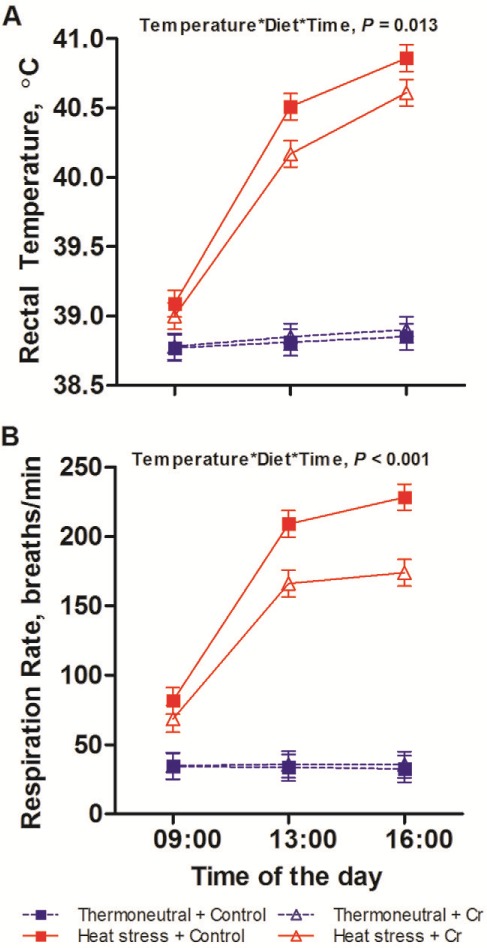
Physiology of pigs fed control or chromium diet exposed to thermoneutral or heat stress conditions. Rectal temperature (A), respiration rate (B) of growing pigs which fed on control (non-chromium) diet or 400 ppb chromium (Cr) diet when being subjected to 8-d thermoneutral condition (20°C) or heat stress condition (35°C from 0900 h to 1700 h, and 28°C from 1700 h to 0900 h; n = 9 per group). The error bars are the SED for the interaction of temperature × diet × time. The P-values for the effects diet, temperature, time, diet × temperature, diet × time, temperature × time, and temperature × diet × time were 0.17, < 0.001, < 0.001, 0.065, 0.080, < 0.001, and 0.013 for rectal temperature; 0.016, < 0.001, < 0.001, 0.008, < 0.001, < 0.001, and < 0.001 for respiration rate.
Heat stress increased respiration rate from 34 to 154 breaths/min (P < 0.001). While respiration rate was constant across the day under TN conditions, during the HS conditions respiration rate increased from 0900 h until 1600 h (75, 187 to 201 breaths/min for 0900 h, 1100 h and 1600 h) as evidenced by an interaction between temperature and time (P < 0.001). Chromium had a main effect in reducing respiration rate from 103 to 86 breaths/min (P = 0.016) and there was an interaction between temperature and diet (P = 0.008) and a three-way interaction between temperature, diet, and time (P < 0.001), such that the pigs fed the Cr diet had reduced respiration rate than those on the control diet at 1300 h (209 vs. 166 breaths/min for control vs. Cr diet) and 1600 h (208 vs. 174 breaths/min for control vs. Cr diet) under HS conditions but not at other times (Fig. 1 B).
During the 8-d thermal exposure period, HS reduced average daily feed intake by 35% (2.20 vs. 1.39 kg/d, P < 0.001), whereas there was no effect of dietary Cr on average daily feed intake (P = 0.11; Fig. 2).
Figure 2.
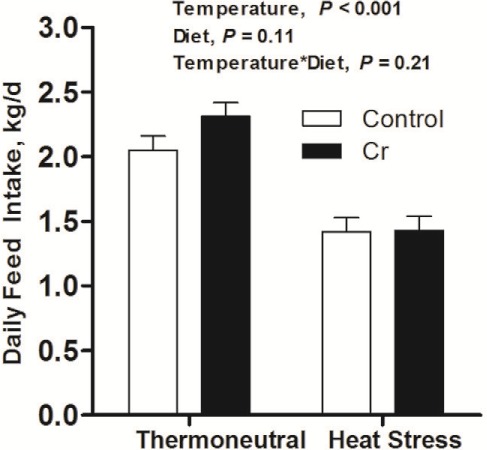
Average daily feed intake. Feed intake of growing pigs which fed on control diet (non-chromium) or 400 ppb chromium (Cr) diet when being subjected to 8-d 35°C or 20°C ambient environment (n = 9 per group).
Blood Gas Variables
Heat stress reduced blood pCO2 (60.4 vs. 53.1 mmHg for TN vs. HS, P = 0.002), bicarbonate (38.4 vs. 35.0 mM for TN vs. HS, P < 0.001). Blood pH remained constant across treatments. Heat stress tended to increase pO2 pressure (34.9 vs. 35.8 mmHg for TN vs. HS, P = 0.083). Chromium supplementation did not affect the above blood gas variables. (Table 3)
Table 3.
Blood gas variables of the pigs fed control or chromium diet and subjected to thermoneutral or heat stress conditions
| Variables | 20°C | 35°C | SED | P-values | ||||
|---|---|---|---|---|---|---|---|---|
| Control (n = 9) | Cr (n = 9) | Control (n = 9) | Cr (n = 9) | Temperature | Diet | Interaction | ||
| pCO2, mmHg | 59.5 | 61.3 | 53.0 | 53.2 | 3.07 | 0.002 | 0.65 | 0.71 |
| pO2, mmHg | 34.2 | 35.5 | 38.0 | 39.0 | 2.71 | 0.083 | 0.54 | 0.94 |
| Bicarbonate, mM | 38.3 | 38.5 | 35.4 | 34.6 | 0.92 | <0.001 | 0.62 | 0.48 |
| pH | 7.42 | 7.41 | 7.43 | 7.42 | 0.021 | 0.34 | 0.54 | 0.98 |
Basal Fasting Glucose, Insulin, NEFA, and HOMA-IR
There was no effect of HS on fasting plasma glucose concentrations. Fasting plasma insulin concentrations tended to be reduced by HS (1.96 vs. 1.68 μU/mL, P = 0.10) and dietary Cr (1.97 vs. 1.67 μU/mL, P = 0.094) and these effects were additive. Similarly, fasting HOMA-IR was reduced by HS (0.66 vs. 0.51 for TN vs. HS, P = 0.007) and dietary Cr (0.65 vs. 0.51 for TN vs. HS, P = 0.013). Fasting NEFA concentrations were greater in the heat-stressed pigs (491 vs. 784 μM for TN vs. HS, P = 0.015) whereas there was no effect of dietary Cr. (Table 4).
Table 4.
Glucose, insulin, NEFA kinetics during IVGTT
| Variables | 20°C | 35°C | SED | P-values | ||||
|---|---|---|---|---|---|---|---|---|
| Control (n = 9) | Cr (n = 9) | Control (n = 9) | Cr (n = 9) | Temperature | Diet | Interaction | ||
| Glucose fasting, mM | 7.89 | 7.94 | 7.67 | 7.50 | 0.489 | 0.29 | 0.89 | 0.79 |
| Glucose peak, mM | 12.4 | 11.6 | 11.1 | 11.2 | 0.70 | 0.10 | 0.52 | 0.35 |
| Glucose AUC60min, mM min | 39.4 | 44.0 | 55.7 | 77.3 | 20.36 | 0.094 | 0.37 | 0.56 |
| Glucose clearance rate (slope2–60min), mM min−1 | 0.21 | 0.17 | 0.13 | 0.13 | 0.039 | 0.035 | 0.36 | 0.38 |
| Insulin fasting, μU/mL | 2.15 | 1.77 | 1.78 | 1.58 | 0.0240 | 0.10 | 0.094 | 0.61 |
| Insulin peak, μU/mL | 16.9 | 16.6 | 14.6 | 12.9 | 1.60 | 0.012 | 0.38 | 0.55 |
| Insulin AUC10 min, μU mL−1min | 109 | 109 | 90 | 80 | 11.7 | 0.006 | 0.56 | 0.58 |
| Insulin AUC60 min, μU mL−1min | 144 | 170 | 177 | 176 | 20.4 | 0.19 | 0.39 | 0.36 |
| Insulin acute release rate (slope0–10min), μU mL−1min−1 | 0.73 | 0.77 | 0.49 | 0.64 | 0.122 | 0.045 | 0.27 | 0.53 |
| Insulin clearance rate (slope0–60min), μU mL−1min−1 | 0.15 | 0.17 | 0.15 | 0.15 | 0.030 | 0.65 | 0.60 | 0.52 |
| HOMA-Insulin Resistance | 0.76 | 0.56 | 0.54 | 0.47 | 0.073 | 0.007 | 0.013 | 0.21 |
| NEFA fasting, μM | 463 | 520 | 853 | 716 | 155.7 | 0.015 | 0.72 | 0.39 |
| NEFA minimum, μM | 167 | 203 | 231 | 186 | 47.7 | 0.50 | 0.89 | 0.24 |
| NEFA AUC30min, μM min | -3987 | -3356 | -9239 | -8352 | 2355 | 0.006 | 0.70 | 0.95 |
| NEFA AUC60min, μM min | -2223 | -1743 | -22882 | -17512 | 5639 | < 0.001 | 0.47 | 0.55 |
| NEFA decrease rate (slope2–30min), μM min−1 | 12.0 | 14.8 | 23.6 | 19.2 | 4.14 | 0.013 | 0.80 | 0.20 |
| NEFA decrease rate (slope30–60min), μM min−1 | 6.5 | 8.7 | 1.6 | 4.4 | 1.85 | 0.002 | 0.068 | 0.84 |
Kinetics of Glucose, Insulin, and NEFA during IVGTT
Plasma glucose concentrations peaked immediately after glucose infusion before decreasing and returning to baseline within 60 min (Time, P < 0.001; Fig. 3). Heat stress tended to reduce peak plasma glucose concentration (12.0 vs. 11.2 mM for TN vs. HS, P = 0.10) and decreased plasma glucose clearance rate (slope2–60 min; 0.19 vs. 0.13 mM min−1 for TN vs. HS, P = 0.035). Heat stress tended to increase glucose AUC60min (41.7 vs. 66.5 mM min for TN vs. HS, P = 0.094). Chromium diet did not affect any plasma glucose variables (Table 4).
Figure 3.
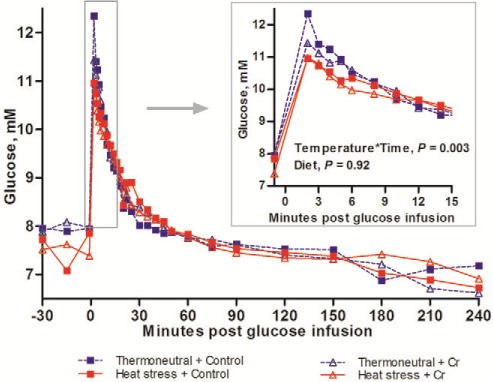
Glucose concentrations during IVGTT. Glucose concentrations during IVGTT of growing pigs which fed on control diet vs. 400 ppb Chromium (Cr) when being subjected to 8-d thermoneutral condition (20°C) or heat stress condition (35°C from 0900 h to 1700 h, and 28°C from 1700 h to 0900 h; n = 9 per group). Standard error of difference (SED) for glucose is 0.516 mM. The P-values for the effects of temperature, diet, time, temperature × diet, temperature × time, diet × time, temperature × diet × time are 0.76, 0.92, < 0.001, 0.94; 0.003, 0.95, and 0.89.
Plasma insulin concentrations increased immediately in response to glucose infusion peaking at approximately 10 min, before declining back to baseline within 60 min (Time, P < 0.001; Fig. 4). Heat stress decreased the insulin acute release rate (slope0–10 min; 0.75 vs. 0.57 μU mL−1 min−1 for TN vs. HS, P = 0.045), the peak plasma insulin concentration (16.8 vs. 13.8 μU/mL, P = 0.012) and the insulin AUC10 min (109.3 vs. 84.9 μU mL−1 min for TN vs. HS, P = 0.006). However, neither insulin disposal rate (slope10–60 min) nor AUC60min were affected by HS. Dietary Cr but did not affect any other insulin indices (Table 4).
Figure 4.
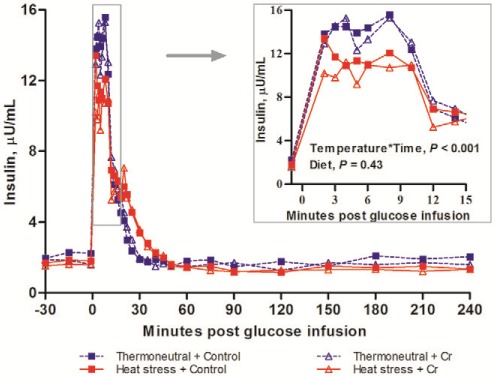
Insulin concentrations during IVGTT. Insulin concentrations during IVGTT of growing pigs which fed on control diet vs. 400 ppb Chromium (Cr) when being subjected to 8 d thermoneutral conditions (20°C) or heat stress conditions (35°C from 0900 h to 1700 h, and 28°C from 1700 h to 0900 h; n = 9 per group). Standard error of difference (SED) for insulin is 0.962 µU/mL. The P-values for the effects of temperature, diet, time, temperature × diet, temperature × time, diet × time, temperature × diet × time are 0.03, 0.43, < 0.001, 0.65, < 0.001, 0.65, and 0.98.
Plasma NEFA concentrations decreased after glucose infusion reaching a nadir concentration around 30 min. Plasma NEFA concentrations then gradually increased to a recovery state (time, P < 0.001; Fig. 5). Heat stress increased the rate of the decrease in plasma NEFA (slope2–30 min; −13.4 vs. −21.4 μM min−1 for TN vs. HS, P = 0.013) and reduced the rate of recovery of plasma NEFA (slope30–60 min; 7.57 vs. 3.00 μM min−1 for TN vs. HS, P = 0.002). The reduction of NEFA in response to IVGTT, as expressed as NEFA AUC, was increased by HS at 30 min (AUC30min; −3672 vs. −8795 μM min for TN vs. HS, P = 0.006) and at 60 min (AUC60min; −1984 vs. −20197 μM min for TN vs. HS, P < 0.001). Dietary Cr tended to increase the recovery rate of plasma NEFA (slope30–60 min; 4.02 vs. 6.55 μM min−1, P = 0.068). Heat stress did not affect the nadir in plasma NEFA concentrations. Dietary Cr diet did not affect any other plasma NEFA variables (Table 4).
Figure 5.
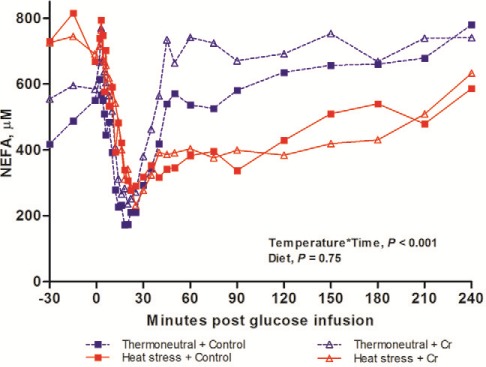
NEFA concentrations during IVGTT. NEFA concentrations during IVGTT of growing pigs which fed on control diet vs. 400 ppb Chromium (Cr) when being subjected to 8 d thermoneutral conditions (20°C) or heat stress conditions (35°C from 0900 h to 1700 h, and 28°C from 1700 h to 0900 h; n = 9 per group). Standard error of difference (SED) for NEFA is 119 µM. The P-values for the effects of temperature, diet, time, temperature × diet, temperature × time, diet × time, temperature × diet × time are 0.89, 0.75, < 0.001, 0.30, < 0.001, 0.30, and 0.70.
Discussion
In this study, it was hypothesized that Cr supplementation may improve insulin sensitivity and mitigate the impacts of HS in pigs (physiological responses, reduction in feed intake, and reduced lipolysis). These data demonstrated that dietary Cr partially mitigated physiological responses to HS, as evidenced by reduced respiration rate and rectal temperature during HS. However, the reduction in feed intake caused by HS was not alleviated by dietary Cr possibly because the impacts of the HS conditions on feed intake was so pronounced. Chromium supplementation reduced fasting insulin concentration and HOMA-IR index suggesting its potential in increasing insulin sensitivity. The responses to the IVGTT suggest HS decreased insulin release and therefore slowed the glucose disposal rate. Heat stress also increased magnitude of the reduction of NEFA during IVGTT, as evidenced by greater NEFA AUC, indicating that HS inhibited lipid mobilization. In summary, Cr improved thermoregulation in growing pigs subjected to chronic HS, and the mechanism may be associated with the improved insulin sensitivity.
The alleviation of the physiological responses to HS by dietary Cr supplementation may be associated with its ability to improve insulin sensitivity. To maintain the core temperature during hot conditions, pigs reduce feed intake to limit the thermal effects of feed and heat production, increase their respiration rate to aid evaporative heat dissipation (Huynh et al., 2005), and increase skin blood flow for radiant heat dissipation (Collin et al., 2001). In the current study, dietary Cr supplementation facilitated greater thermoregulation during HS conditions, and these effects may be associated with the improved radiant heat dissipation from skin. Insulin sensitivity is positively related with capillary microcirculatory function of skin (Serné et al., 1999; Forst et al., 2006) which regulates radiant heat dissipation from the skin. The Cr diet increased insulin sensitivity in this experiment, and thus theoretically, blood flow and radiant heat loss from the skin should have been improved; however, the microcirculatory function of pigs was not assessed in the current study due to the technical difficulty. The facilitated thermoregulation by Cr spared the heat dissipation that happened in the respiratory route and ameliorated the increase in rectal temperature observed under HS conditions. Although respiration rate during HS was decreased (−24%) by dietary Cr, the rate was still 4 times that of the pigs under TN conditions, and thus the loss of blood CO2 was still substantial. Consistent with previous report (Liu et al., 2016), the loss of blood CO2 triggered respiratory alkalosis that was compensated by reduced blood bicarbonate concentration to stabilize blood pH during HS.
Heat stress markedly reduced feed intake (−35%) in growing pigs in the current study. However, Cr supplementation did not mitigate the reduction in feed intake, suggesting that the Cr-facilitated heat dissipation was insufficient to enable the pigs to tolerate the thermic effects and heat production from extra feed intake. The effects of Cr supplementation on feed intake may depend on magnitude of HS. For example, supplementing 1,000 or 2,000 ppb Cr in the form of Cr picolinate did not alleviate the reduction of feed intake in the piglets (10 to 20 kg body weight) which were subjected to 40.5°C room temperature (Kim et al., 2009). However, under a lower level of HS observed during an Australian summer (29.7°C), Cr supplementation improved feed intake by 8% in growing pigs (Hung et al., 2014).
In this study, dietary Cr improved average daily gain in pigs housed under TN conditions which is consistent with the meta-analyses of Sales and Jancik (2011). The improved growth performance might be also associated with the main effect of Cr in improving insulin sensitivity. Chromium supplementation reduced HOMA-IR index and tended to decrease fasting insulin concentration and in current study, which is similar as a previous study where pigs were supplemented with Cr-picolinate (Amoikon et al., 1995; Hung et al., 2015). Also, Hung (2014) found that dietary nano Cr improved the insulinsignaling pathway in porcine peripheral tissue possibly via increased AKT and GLUT-4 mRNA abundance in skeletal muscle, as well as increased adiponectin in subcutaneous adipose tissue. Chromium enhances insulin sensitivity via chromodulin, and the mode of action was proposed by Vincent (2000). Briefly, in response to glucose ingestion, blood insulin increases and binds to its receptor in insulin-sensitive cells, Cr is then moved from the blood into the cells and binds to apo-chromodulins which exist in cells to convert the molecule to their functional form, Cr4–chromodulin. Cr4–chromodulin can bind to insulin-stimulated receptors and mimic the stimulation of insulin on tyrosine kinase thus amplifying the insulin signaling. The Cr-improved insulin sensitivity requires less amount of circulating insulin to maintain glucose homeostasis (Amoikon et al., 1995) or increases glucose disposal at the same concentration of exogenous insulin in pigs (Matthews et al., 2001), the former effect being demonstrated by this study in the pigs fed Cr diet.
Heat stress reduced insulin acute release rate, slowed glucose clearance rate, and tended to increase glucose AUC in the current study, which is in an agreement of a previous study (Sanz Fernandez et al., 2015a). In their study, pigs had reduced insulinogenic index (ratio of insulin AUC20min: glucose AUC20min) and greater glucose AUC after being exposed to 32°C for 2 d. In addition, there was reduced pancreatic insulin staining found in the same study after 7 d of HS (Sanz Fernandez et al., 2015a). Taken together, these results suggest that pigs produce less insulin after being heat-stressed, although the exact mechanism remains unknown. It is possible that islet insulin synthesis is impaired by HS, because islet is susceptible to oxidative damage due to a weak antioxidant defense system (Grankvist et al., 1981). Oxidative stress occurs in the gastrointestinal tract in heat-stressed pigs (Pearce et al., 2013b; Liu et al., 2016), so it is likely this also occurs in the pancreas. Further studies are required to quantify oxidative stress biomarkers in the islet of heat-stressed pigs.
Admittedly, the experimental design in the current study does not allow us to make a valid interpretation on the effect of heat stress on the whole-body insulin sensitivity because the measurements on whole-body insulin sensitivity (IVGTT) and resistance (HOMA-IR) could be confounded by the dissimilar feed intake between thermoneutral and heat-stressed pigs. The 35% reduction in the plane of nutrition of heat-stressed pigs should decrease peripheral insulin sensitivity, because the glucose usages in brain and other vital tissues need to be prioritized (Bauman and Currie, 1980). For example, a restricted feed intake (60% vs. ad libitum) decreased the overall insulin sensitivity by 36% in growing pigs housed under TN conditions (Sanz Fernandez et al., 2015b). The direct effect of HS on insulin sensitivity in pigs was investigated in the pair-fed study (Sanz Fernandez et al., 2015b), in which HS increased insulin-stimulated glucose uptake and muscular protein abundance of insulin receptor substrate- 1 growing pigs, suggesting an increased wholebody insulin sensivity. Similarly, the increased insulin sensitivity was found in rodents (Gupte et al., 2009; Gupte et al., 2011) and sheep (Hung, 2014) subjected to HS. By contrast, heat-stressed dairy cows exhibited greater concentrations of basal and hyperglycemic stimulated insulin as well as greater blood glucose concentrations than the pair-fed TN dairy cows (Wheelock et al., 2010; Baumgard et al., 2011), which may indicate HS triggered hyperinsulinemia. So far the species differences or physiological state on the insulin response to HS remain unclear. The improved insulin sensitivity in the heat-stressed pigs, rodents and dry sheep could be associated with cross-talks between heat shock protein 70 and the insulin cascade which has been discussed in previous reviews (McCarty, 2006; Henstridge et al., 2010; Kondo et al., 2011). From the point of view of the adaption to hot environment, the improved insulin sensitivity in heatstressed pigs may be an attempt to facilitate skin microcirculation and radiant heat dissipation from surface.
Heat stress reduced lipid mobilization in pigs in the current study as evidenced by a more rapid decrease in plasma NEFA in response to IVGTT, and a slower return to basal. The reduced lipid mobilization is in accordance with the previous reports in both pigs and ruminants; for example, pigs subjected to 7-d HS had reduced basal NEFA concentration (Pearce et al., 2013a) and reduced NEFA concentration in response to epinephrine challenge (Sanz Fernandez et al., 2015a). Similar findings were also reported in heat-stressed lactating cows (Wheelock et al., 2010; Baumgard et al., 2011) and dry sheep (Hung et al., 2014). Although, the exact mechanism remains unknown, hyperinsulinemia seems the reason in lactating cows (Baumgard et al., 2011; Baumgard and Rhoads, 2012), as insulin is a potent anti-lipolytic hormone. However, the reduced lipid mobilization in the heat-stressed pigs was not due to increased insulin concentration because the HS did not cause hyperinsulinemia in the current study. More likely, the attenuated lipolysis in heat-stressed pigs was independent of insulin actions, as demonstrated by the recent study (Sanz Fernandez et al., 2015a), in which a reduced NEFA and reduced insulin response to glucose tolerance test occurred simultaneously in the heat-stressed pigs. At the end of the thermal exposure period, the heat-stressed pigs had greater fasting NEFA concentrations which was due to the lower plane of nutrition (feed intake reduced by 35%) and increased lipolysis. In response to glucose-stimulated insulin, the heat-stressed pigs exhibited a faster reduction and slower recovery of NEFA, which consequently resulted in a greater reduction in plasma NEFA. Dietary Cr supplementation tended to increase NEFA recovery rate during IVGTT in the heat-stressed pigs. The plasma NEFA recovery phase during IVGTT is associated with the return of plasma insulin concentrations to basal. As dietary Cr did not affect the insulin AUC during IVGTT, it is more likely that Cr had a direct effect in facilitating lipolysis, which needs to be further explored.
In conclusions, 400 ppb Cr ameliorated the increase in respiration rate and rectal temperature in the pigs subjected to HS, and improved growth performance under TN conditions. The beneficial effects of dietary Cr may be associated with its potential in improving insulin sensitivity. Heat stress decreased insulin release, reduced glucose disposal rate, and attenuated lipid mobilization in pigs whereas dietary Cr may potentially normalize NEFA metabolism. Therefore, dietary Cr may be a useful supplement to include in a summer ration or when a HS event is anticipated.
Acknowlegements
The authors express appreciations to Maree Cox, Evan Bittner, Paul Eason, Pablo Alvarez Hess and Juan Pablo Villanueva Cabezas for their technical assistance.
Literature Cited
- Abebe W., Liu J. Y., Wimborne H., and Mozaffari M. S.. 2010. Effects of chromium picolinate on vascular reactivity and cardiac ischemia reperfusion injury in spontaneously hypertensive rats. Pharmacol. Rep. 62:674–682. doi: 10.1016/S1734-1140(10)70324-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoikon E. K., Fernandez J. M., Southern L. L., Thompson D. L., Ward T. L., and Olcott B. M.. 1995. Effect of chromium tripicolinate on growth, glucose tolerance, insulin sensitivity, plasma metabolites, and growth hormone in pigs. J. Anim. Sci. 73:1123–1130. doi: 10.2527/1995.7341123x [DOI] [PubMed] [Google Scholar]
- Bauman D. E., and Currie W. B.. 1980. Partitioning of nutrients during pregnancy and lactation: A review of mechanisms involving homeostasis and homeorhesis. J. Dairy Sci. 63:1514–1529. doi: 10.3168/jds.S0022-0302(80)83111-0 [DOI] [PubMed] [Google Scholar]
- Baumgard L. H., and Rhoads R. P.. 2012. Ruminant Nutrition Symposium: Ruminant production and metabolic responses to heat stress. J. Anim. Sci. 90:1855–1865. doi: 10.2527/jas.2011-4675 [DOI] [PubMed] [Google Scholar]
- Baumgard L. H., and Rhoads R. P. Jr.. 2013. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 1:311–337. doi: 10.1146/annurev-animal-031412-103644 [DOI] [PubMed] [Google Scholar]
- Baumgard L. H., Wheelock J. B., Sanders S. R., Moore C. E., Green H. B., Waldron M. R., and Rhoads R. P.. 2011. Postabsorptive carbohydrate adaptations to heat stress and monensin supplementation in lactating Holstein cows. J. Dairy Sci. 94:5620–5633. doi: 10.3168/jds.2011-4462 [DOI] [PubMed] [Google Scholar]
- Cefalu W. T., Wang Z. Q., Zhang X. H., Baldor L. C., and Russell J. C.. 2002. Oral chromium picolinate improves carbohydrate and lipid metabolism and enhances skeletal muscle Glut-4 translocation in obese, hyperinsulinemic (JCR-LA Corpulent) rats. J. Nutr. 132:1107–1114. [DOI] [PubMed] [Google Scholar]
- Christon R. 1988. The effect of tropical ambient temperature on growth and metabolism in pigs. J. Anim. Sci. 66:3112–3123. doi: 10.2527/jas1988.66123112x [DOI] [PubMed] [Google Scholar]
- Collin A., Lebreton Y., Fillaut M., Vincent A., Thomas F., and Herpin P... 2001. Effects of exposure to high temperature and feeding level on regional blood flow and oxidative capacity of tissues in piglets. Exp. Physiol. 86:83–91. doi: 10.1113/eph8602102 [DOI] [PubMed] [Google Scholar]
- Cottrell J. J., Liu F., Hung A. T., DiGiacomo K., Chauhan S. S., Leury B. J., Furness J. B., Celi P., and Dunshea F. R... 2015. Nutritional strategies to alleviate heat stress in pigs. Anim. Prod. Sci. 55:1391–1402. 10.1071/an15255 [DOI] [Google Scholar]
- Davis C. M., Royer A., and Vincent J.. 1997. Synthetic multinuclear chromium assembly activates insulin receptor kinase activity: Functional model for low-molecular-weight chromium-binding substance. Inorg. Chem. 36:5316–5320. doi: 10.1021/ic970568h [DOI] [Google Scholar]
- Forst, Caduff A., Talary M., Weder M., Braendle M., Brändle M., Kann P., Flacke F., Friedrich C., and fützner A.. 2006. Impact of environmental temperature on skin thickness and microvascular blood flow in subjects with and without diabetes. Diabetes Technol. Ther. 8:94–101. doi: 10.1089/dia.2006.8.94 [DOI] [PubMed] [Google Scholar]
- Gabler N. K., and Pearce S. C... 2015. The impact of heat stress on intestinal function and productivity in grow-finish pigs. Anim. Prod. Sci. 55:1403–1410. 10.1071/an15280 [DOI] [Google Scholar]
- Grankvist K., Marklund S. L., and Täljedal I. B.. 1981. CuZnsuperoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem. J. 199:393–398. doi: 10.1042/bj1990393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte A. A., Bomhoff G. L., Swerdlow R. H., and Geiger P. C.. 2009. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes 58:567–578. doi: 10.2337/db08-1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte A. A., Bomhoff G. L., Touchberry C. D., and Geiger P. C... 2011. Acute heat treatment improves insulin-stimulated glucose uptake in aged skeletal muscle. J. Appl. Physiol. 110:451–457. doi: 10.1152/japplphysiol.00849.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henstridge D., Forbes J., Penfold S., Formosa M., Dougherty S., Gasser A., de Courten M., Cooper M., Kingwell B., and de Courten B.. 2010. The relationship between heat shock protein 72 expression in skeletal muscle and insulin sensitivity is dependent on adiposity. Metabolism 59:1556–1561. doi: 10.1016/j.metabol.2010.01.027 [DOI] [PubMed] [Google Scholar]
- Hung A. T., Leury B. J., Sabin M. A., Collins C. L., and Dunshea F. R.. 2014. Dietary nano-chromium tripicolinate increases feed intake and decreases plasma cortisol in finisher gilts during summer. Trop. Anim. Health Prod. 46:1483–1489. doi: 10.1007/s11250-014-0673-7 [DOI] [PubMed] [Google Scholar]
- Hung A. T., Leury B. J., Sabin M. A., Lien T. F., and F. R. Dunshea. 2015. Dietary chromium picolinate of varying particle size improves carcass characteristics and insulin sensitivity in finishing pigs fed low- and high-fat diets. Anim. Prod. Sci. 55:454–460. doi: 10.1071/AN12255 [DOI] [Google Scholar]
- Hung T. Y. 2014. The effects of nano-chromium on growth performance and metabolism of pigs and sheep. PhD Diss, The University of Melbourne, Australia. [Google Scholar]
- Huynh T. T. T., Aarnink A. J. A., Verstegen M. W. A., Gerrits W. J. J., Heetkamp M. J. W., Kemp B., and Canh T. T.. 2005. Effects of increasing temperatures on physiological changes in pigs at different relative humidities. J. Anim. Sci. 83:1385–1396. doi: 10.2527/2005.8361385x [DOI] [PubMed] [Google Scholar]
- Kim B., Lindemann M., and Cromwell G.. 2010. Effects of dietary chromium (III) picolinate on growth performance, respiratory rate, plasma variables, and carcass traits of pigs fed high-fat diets. Biol. Trace Elem. Res. 133:181–196. doi: 10.1007/s12011-009-8417-7 [DOI] [PubMed] [Google Scholar]
- Kim B. G., Lindemann M. D., and Cromwell G. L.. 2009. The effects of dietary chromium(III) picolinate on growth performance, blood measurements, and respiratory rate in pigs kept in high and low ambient temperature. J. Anim. Sci. 87:1695–1704. doi: 10.2527/jas.2008-1218 [DOI] [PubMed] [Google Scholar]
- Kondo T., Koga S., Matsuyama R., Miyagawa K., Goto R., Kai H., and Araki E.. 2011. Heat shock response regulates insulin sensitivity and glucose homeostasis: Pathophysiological impact and therapeutic potential. Curr. Diabetes Rev. 7:264–269. doi: 10.2174/157339911796397811 [DOI] [PubMed] [Google Scholar]
- Kouba M., Hermier D., and Le Dividich J.. 2001. Influence of a high ambient temperature on lipid metabolism in the growing pig.J. Anim. Sci. 79:81–87. doi: 10.2527/2001.79181x [DOI] [PubMed] [Google Scholar]
- Lindemann M. D., Wood C. M., Harper A. F., Kornegay E. T., and Anderson R. A.. 1995. Dietary chromium picolinate additions improve gain:feed and carcass characteristics in growingfinishing pigs and increase litter size in reproducing sows. J. Anim. Sci. 73: 457–465. doi: 10.2527/1995.732457x [DOI] [PubMed] [Google Scholar]
- Liu F., Cottrell J. J., Furness J. B., Rivera L. R., Kelly F. W., Wijesiriwardana U., Pustovit R. V., Fothergill L. J., Bravo D. M., Celi P., Leury B. J., Gabler N. K., and Dunshea F. R.. 2016. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Exp. Physiol. 101:801–810. doi: 10.1113/EP085746 [DOI] [PubMed] [Google Scholar]
- Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., and Turner R. C.. 1985. Homeostasis model assessment: Insulin resistance and ?-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- Matthews J. O., Southern L. L., Fernandez J. M., Pontif J. E., Bidner T. D., and Odgaard R. L.. 2001. Effect of chromium picolinate and chromium propionate on glucose and insulin kinetics of growing barrows and on growth and carcass traits of growing-finishing barrows. J. Anim. Sci. 79: 2172-2178. doi: 10.2527/2001.7982172x [DOI] [PubMed] [Google Scholar]
- McCarty M. F. 2006. Induction of heat shock proteins may combat insulin resistance. Med. Hypotheses 66:527–534. doi: 10.1016/j.mehy.2004.08.033 [DOI] [PubMed] [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. 11th rev. ed. Natl Acad. Press, Washington, DC. [Google Scholar]
- O'Brien M. D., Rhoads R. P., Sanders S. R., Duff G. C., and Baumgard L. H.. 2010. Metabolic adaptations to heat stress in growing cattle. Domest. Anim. Endocrinol. 38:86–94. doi: 10.1016/j.domaniend.2009.08.005 [DOI] [PubMed] [Google Scholar]
- Pearce S. C., Gabler N. K., Ross J. W., Escobar J., Patience J. F., Rhoads R. P., and Baumgard L. H.. 2013a. The effects of heat stress and plane of nutrition on metabolism in growing pigs. J. Anim. Sci. 91:2108–2118. doi: 10.2527/jas.2012-5738 [DOI] [PubMed] [Google Scholar]
- Pearce S. C., Mani V., Weber T. E., Rhoads R. P., Patience J. F., Baumgard L. H., and Gabler N. K.. 2013b. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs. J. Anim. Sci. 91:5183–5193. doi: 10.2527/jas.2013-6759 [DOI] [PubMed] [Google Scholar]
- Sales J., and Jancik F.. 2011. Effects of dietary chromium supplementation on performance, carcass characteristics, and meat quality of growing-finishing swine: A meta-analysis. J. Anim. Sci. 89:4054–4067. doi: 10.2527/jas.2010-3495 [DOI] [PubMed] [Google Scholar]
- Sanz Fernandez M. V., Johnson J. S., Abuajamieh M., Stoakes S. K., Seibert J. T., Cox L., Kahl S., Elsasser T. H., Ross J. W., Isom S. C., Rhoads R. P., and Baumgard L. H.. 2015a. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol. Rep. 3:e12315. doi: 10.14814/phy2.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz Fernandez M. V., Stoakes S. K., Abuajamieh M., Seibert J. T., Johnson J. S., Horst E. A., Rhoads R. P., and Baumgard L. H.. 2015b. Heat stress increases insulin sensitivity in pigs. Physiol. Rep. 3: (in press). doi: 10.14814/phy2.12478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serné E. H., Stehouwer C. D. A., Maaten J. C. ter, ter Wee P. M., Rauwerda J. A., Donker A. J. M., and Gans R. O. B.. 1999. Microvascular function relates to insulin sensitivity and blood pressure in normal subjects. Circulation 99:896–902. doi: 10.1161/01.CIR.99.7.896 [DOI] [PubMed] [Google Scholar]
- Tindal J. S., Knaggs G. S., Hart I. C., and Blake L. A.. 1978. Release of growth hormone in lactating and non-lactating goats in relation to behaviour, stages of sleep, electroencephalograms, environmental stimuli and levels of prolactin, insulin, glucose and free fatty acids in the circulation. J. Endocrinol. 76: 333–346. doi: 10.1677/joe.0.0760333 [DOI] [PubMed] [Google Scholar]
- Vincent J. B. 2000. The Biochemistry of Chromium. J. Nutr. 130:715–718. 10.1002/jtra.10038 [DOI] [PubMed] [Google Scholar]
- Wang M. Q., He Y. D., Lindemann M. D., and Jiang Z. G.. 2009. Efficacy of Cr (III) supplementation on growth, carcass composition, blood metabolites, and endocrine variables in finishing pigs. Asian-australas. J. Anim. Sci. 22:1414–1419. doi: 10.5713/ajas.2009.90111 [DOI] [Google Scholar]
- Wheelock J. B., Rhoads R. P., VanBaale M. J., Sanders S. R., and Baumgard L. H.. 2010. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 93:644–655. doi: 10.3168/jds.2009-2295 [DOI] [PubMed] [Google Scholar]
- Wu X., Li Z., Jia A., Su H., Hu C., Zhang M., and Feng J.. 2016. Effects of high ambient temperature on lipid metabolism in finishing pigs. J. Integr. Agric. 15:391–396. doi: 10.1016/S2095-3119(15)61061-9 [DOI] [Google Scholar]
- Zhang S., Blache D., Blackberry M. A., and Martin G. B.. 2004. Dynamics of the responses in secretion of luteinising hormone, leptin and insulin following an acute increase in nutrition in mature male sheep. Reprod. Fertil. Dev. 16: 823–829. doi: 10.1071/RD04086 [DOI] [PubMed] [Google Scholar]


