Abstract
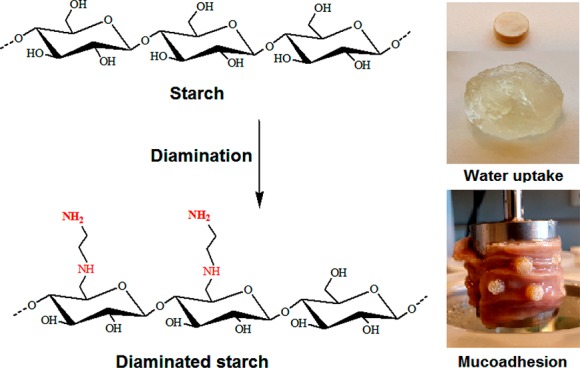
The purpose of this study was to synthesize diaminated starch as a novel mucoadhesive polymer. Starch was tosylated and then reacted with ethylenediamine. The degree of amination was determined by 2,4,6-trinitrobenzene sulfonic acid assay. Properties of diaminated starch including solubility, cytotoxicity, swelling behavior, and mucoadhesion were compared to chitosan. Diaminated starch displayed 2083 ± 121.6 μmol of diamine substructures/g of polymer. At pH 6, diaminated starch exhibited a ζ potential of 6 mV, whereas it was close to zero in the case of unmodified starch. In addition, diaminated starch displayed water solubility over the entire pH range and minor cytotoxicity. The novel polymer showed pronounced swelling behavior in water increasing its initial weight 18- and 6-fold at pH 5 and 6, respectively. Moreover, diaminated starch exhibited 92-fold higher-mucoadhesivity properties than those of chitosan. According to these results, diaminated starch might be a promising novel excipient for the design of mucoadhesive formulations.
1. Introduction
Due to mucoadhesive polymers, the residence time of most drug delivery systems on mucosal surfaces such as the intraoral, ocular, or vaginal mucosa can be substantially prolonged. Consequently, a more sustained therapeutic effect of the incorporated drug can be provided.1 Among noncovalently binding mucoadhesive polymers, cationic polymers such as chitosan exhibit comparatively highly mucoadhesive properties as these polymers can form ionic bonds with anionic substructures such as sialic acid and sulfonic acid moieties of mucus glycoproteins.2,3 So far, established mucoadhesive cationic polymers, however, have various drawbacks strongly limiting their application. Chitosan, for instance, precipitates at pH > 6, quaternary ammonium chitosans exhibit poor mucoadhesive properties, and polyethyleneimines and poly(amidoamine) dendrimers are less biodegradable and comparatively toxic.4−8
To overcome these shortcomings, recently, starch was aminated with primary amine groups resulting in high solubility and improved mucoadhesivity. In comparison to chitosan, this novel polymer provided almost 10-fold longer residence time on intestinal mucosa and showed even less cell-toxic effects.9
Encouraged by these results, it was the aim of this study to design an improved aminated starch derivative exhibiting a comparatively higher cationic charge density. To increase the positive charge density and hydrogen-bonding sites, a diaminated starch with primary and secondary amine groups using ethylenediamine as a ligand was generated. The introduction of vicinal cationic substructures on the polymer should additionally improve the stability of ionic bonds with anionic substructures of the mucus as when the ion pair between one of these cationic moieties with an anionic moiety of mucus dissociates, there is still another cationic moiety in close proximity available in order to maintain the electrostatic attraction. Generally, it is well known that the stability of ionic complexes increases with the increasing number of either neighboring anionic or neighboring cationic charges on each component. Zhou et al., for instance, could achieve tremendous improvement in the adsorptive binding of anionic dyes exhibiting the same sulfonic acid substructure as found in mucus due to the covalent attachment of ethylenediamine to chitosan.10 As aminated starch was previously synthesized via oxidative ring opening of glucose units resulting in partial destruction of the polysaccharide,9 it was an additional aim of this study to introduce these diamine substructures without ring opening. First, starch was tosylated through toluene sulfonyl chloride, and subsequently, tosylated starch was aminated by ethylenediamine. In the following, the new polymer was characterized regarding solubility, cytotoxicity, swelling behavior, and mucoadhesive properties.
2. Materials and Methods
2.1. Materials
Starch Hylon VII was donated by Ingredion, Germany. Fresh human blood was donated by Central Institute of Blood Transfusion in Innsbruck, Austria. The use of this human biomaterial was approved by the local ethics committee (Medical University of Innsbruck). Toluenesulfonyl chloride, triethylamine, ethylenediamine, medium-molecular weight chitosan (75–85% deacetylated), 2,4,6-trinitrobenzene sulfonic acid solution (TNBS), Triton X-100, and sterile Dulbecco’s phosphate-buffered saline were purchased from Sigma-Aldrich, Germany. All other components were of analytical grade and used without further purification.
2.2. Methods
2.2.1. Tosylation of Starch in Aqueous Solution
Tosylation of starch was achieved according to a method having been described for tosylation of cyclodextrin previously.11 Briefly, 4.0 g of starch was dissolved in 100 mL of 0.4 M NaOH solution. TsCl (24 g) was slurred in 30 mL of 0.4 M NaOH in an ice bath. Triethylamine (14 mL) was added dropwise to the starch solution under stirring for 20 min in an ice bath. The starch mixture was added to the TsCl slurry and stirred for 12 h at 0–5 °C. The reaction mixture was neutralized with 1 M HCl solution, and the precipitate was filtered and washed three times with 400 mL of ethanol followed by 300 mL of water. Tosylated starch was dried at 40 °C for 24 h.
2.2.2. Amination of Tosylated Starch
Diaminated starch was obtained by the reaction of tosylated starch with ethylenediamine.12 Briefly, 3.0 g of tosylated starch was dissolved in 50 mL of dimethyl sulfoxide (DMSO) and heated up to 80 °C. Then, 25 mL of ethylenediamine was added, and the reaction mixture was heated up to 100 °C and stirred for 6 h at this temperature. The color of the reaction mixture changed to reddish brown. After cooling down to room temperature, the reaction mixture was added dropwise to 1300 mL of cold isopropanol in order to precipitate the targeted compound. After centrifugation at 12,300 rpm at 2 °C for 15 min, the precipitate was washed with 400 mL of isopropanol and two times with 400 mL of ethanol. It was then dissolved in 4 mL of demineralized water, dialyzed for three days against demineralized water, and finally freeze-dried.
Starch having been treated in exactly the same way as described above but omitting TsCl served as the negative control. The purification of synthesized diaminated starch was evaluated by a thin-layer chromatography (TLC) technique and TNBS assay. n-Butanol:acetic acid:water (3:1:4) was used for TLC as the mobile phase. Plates were sprayed with 0.3 m/v ninhydrin in ethanol used for detection of primary amines and heated up to 100 °C.
2.3. Characterization
2.3.1. Structural Analyses
CHNS elemental analysis of tosylated starch and diaminated starch was performed with a Thermo Finnigan EA1112 series elemental analyzer. The degree of tosylation was calculated according to the following equation (eq 1):13
| 1 |
where 161 is the molecular weight of the starch repeating unit without one hydrogen, 155 is the molecular weight of the tosylate group, and S is the sulfur content. The degree of amination was calculated using the following equation (eq 2):
| 2 |
where 59 is the molecular weight of the diamine group and N is the nitrogen content in diaminated starch.
A thermo alpha FT-IR spectrophotometer (Bruker, Billerica, U.S.A.) in arrangement with its associated software Opus Version 7.0 (Bruker) was used to study unmodified starch, tosylated starch, and diaminated starch. The FT-IR spectra of the compounds were recorded with 24 scans at 22 °C in a wavenumber range from 4000 to 400 cm–1 at a resolution of 1 cm–1. 1H NMR and 13C NMR spectra of tosylated starch and diaminated starch were recorded by a Bruker 500 MHz NMR spectrometer using DMSO-d6 as the solvent at 25 °C. UV–vis spectra were obtained using a Tecan infinite M200 spectrophotometer (Grödig, Austria).
2.3.2. Determination of the Degree of Amination with TNBS
Primary amine groups of unmodified starch, diaminated starch, and chitosan were quantified by reactions with TNBS.14 Briefly, 1.0 mg of each sample was dissolved in 1 mL of 0.01 M HCl, and then 200 μL of each solution was mixed with 300 μL of 8% (m/v) NaHCO3. Thereafter, 500 μL of 0.1% TNBS solution was added, and the reaction mixture was incubated in the dark for 2 h at 37 °C. The absorbance of 100 μL of each sample was measured at 335 nm. The standard curve was obtained by a series of solutions with increasing concentrations of cysteine hydrochloride in 0.5% NaCl and a cysteine hydrochloride-free solution as reference. Each sample was measured three times.
2.3.3. Determination of the Degree of Modification via Titration
Titration is a common approach to determine the degree of deacetylation of chitosan based on the quantity of NaOH required to neutralize H+ on protonated amine groups.15 Briefly, 10 mg of diaminated starch, chitosan, and unmodified starch was dissolved in 10 mL of 0.01 M HCl and titrated with 0.01 M NaOH. Each sample was titrated three times separately.
The degree of deacetylation (DD) of chitosan was calculated using the following equation (eq 3):
| 3 |
where 203 is the molecular weight of the chitin repeating unit, C1 and C2 are the concentrations of NaOH solution in molar at two inflexion points, V1 and V2 are the volumes of NaOH solution added up to two inflexion points in liters, m is the sample weight, and 42 is the molecular weight difference between repeating units of chitin and acetylated forms.
The degree of amination (DAm) of starch was calculated by the following equation (eq 4):
| 4 |
where 1/2 is due to having two amine groups per repeating unit, 162 is the molecular weight of the unmodified starch repeating unit, 42 is the difference between molecular weights of unmodified starch and diaminated starch repeating units, and the other parameters have the same meaning as described above.
2.4. ζ Potential Measurements
The ζ potential showing the surface electric charges of polymers in solution was used to assess the effect of diamine substructures on the surface electric charge of starch under acidic conditions.16 The ζ potential of diaminated starch in comparison to chitosan and unmodified starch was determined in a concentration of 1 mg/mL in a 0.1 mM citric acid–sodium phosphate dibasic buffer at pH 3–7 at room temperature. ζ potential measurements were performed by Zetasizer Nano ZSP from Malvern Instruments.
2.5. Isoelectric Point Predictions
Chemicalize software in connection with ChemSpider software from ChemAxon (Cambridge, MA, U.S.A.) was utilized for structure-based predictions of isoelectric points of test compounds.
2.6. Solubility Studies
In order to compare the solubility of diaminated starch with that of chitosan, each polymer was dispersed in a series of buffer solutions including the citric acid–Na2HPO4 buffer (0.1 M, pH 3), acetate buffer (0.1 M, pH 4, 5), phosphate buffer (0.1 M, pH 6 and 7), trizma buffer (0.1 M, pH 8 and 9), and carbonate–bicarbonate buffer (0.1 M, pH 10) in a final concentration of 0.5% (m/v). In another experimental setup, 5 mg of diaminated starch and chitosan was dissolved in 1 mL of 0.001 M hydrochloric solution. Polymer precipitation was triggered through gradually increasing the pH by adding 0.1 M NaOH to the polymer solution.17
2.7. In Vitro Cytotoxicity Studies
2.7.1. Hemolysis Assay
Sterile 0.1 mM PBS, pH 6, was prepared by addition of potassium dihydrogen phosphate to sterile Dulbecco’s PBS and sterilized through a sterile vacuum filter with a 0.22 μm pore size. Red blood cells (200 μL) were added to 1800 μL of unmodified starch, diaminated starch, and chitosan having been dissolved/dispersed in 0.1 M sterile Dulbecco’s PBS at pH 6 and 7.4 in a final concentration of 0.1, 0.2, 0.5, 1, 2, 3, and 5 mg/mL. Sterile 0.1 M PBS, pH 6 and 7.4, was used as a negative control, and 1% (v/v) Triton X-100 solution was used as a positive control. The mixtures were incubated at 300 rpm and 37 °C for 4 h and then mixed by inversion every 15 min. At predetermined time points, mixtures were centrifuged at 13,400 rpm at 4 °C for 5 min, and the absorbance of the supernatant was measured using a M200 multimode microplate reader (Tecan, Grödig, Austria).18 Each concentration was evaluated in triplicate, and the percentage of hemolysis was calculated according to the following equation (eq 5):
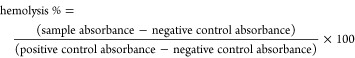 |
5 |
2.7.2. Resazurin Assay
Unmodified starch, diaminated starch, and chitosan were evaluated for in vitro cell viability via resazurin assay as described elsewhere.19 In this test, a 24-well plate containing 25,000 Caco-2 cells per well in 500 μL of minimum essential medium (MEM) containing 10% (v/v) fetal calf serum (FCS) and penicillin/streptomycin solution (100 units/0.1 mg/mL) was incubated at 37 °C under a 5% CO2 environment. The cell culture medium was replaced every 48 h for 14 days to obtain a monolayer. Cells were washed twice with preheated MEM without serum. Test solutions including 0.05, 0.1, and 0.2 mg/mL unmodified starch, diaminated starch, and chitosan were prepared in MEM. Since chitosan is not soluble at neutral pH, it was pre-dissolved in 0.01 M HCl followed by dilution with MEM until the final pH was 7.2. For the negative control, MEM and MEM containing 10% (v/v) HCl were used, whereas 1% (v/v) Triton X-100 served as a positive control. Prewarmed 500 μL of each solution was added in triplicate to the cells. Cells were incubated at 37 °C in the 5% CO2 environment for 4 h. Test solutions were removed from the wells, and cells were washed twice with preheated MEM. After that, 250 μL of resazurin solution (2.2 μM) was added to each well and incubated for 3 h at 37 °C in the 5% CO2 environment. Fluorescence of the supernatant of each well was measured at an excitation wavelength of 540 nm and emission wavelength at 590 nm. Cell viability of each sample was calculated by the following equation (eq 6):
| 6 |
2.8. Evaluation of the Swelling Behavior
The swelling behavior of unmodified starch, diaminated starch, and chitosan was evaluated at predefined pH values by a gravimetric method.20 First, 30 mg of each polymer was compressed with a constant compaction pressure of 11 kN applied for 1 min into 5.0 mm in diameter flat-faced tablets. Each tablet was fixed on a needle and incubated at 37 °C in 5 mL of a 0.1 M hydrochloric acid–potassium chloride buffer (pH 1.2), 0.1 M glycine buffer (pH 3), 0.1 M acetate buffer (pH 5), and 0.1 M phosphate buffer (pH 6.8 and 7.4). At predetermined time points, tablets were removed from the aqueous medium and weighed after having removed unbound water from tablets with filter paper. Each experiment was performed three times, and the amount of water uptake was calculated using the following equation (eq 7):
| 7 |
where Wt is the weight of the swollen tablet at time t and W0 is the initial weight of the tablet. Data are reported as mean ± SD.
2.9. Mucoadhesion Studies
2.9.1. Rotating Cylinder Method
In order to compare the mucoadhesive properties of the unmodified and diaminated starch with chitosan, the rotating cylinder method was used.21 Freshly excised intestinal porcine mucosa (4 × 5 cm) was fixed on a stainless-steel cylinder (apparatus: 6 rotating cylinders, 2 in.) using cyanoacrylate glue, and test tablets of unmodified starch, diaminated starch, and chitosan were attached on the mucosa. Thereafter, the cylinder was placed in the dissolution apparatus (Erweka) according to the European Pharmacopoeia, containing 900 mL of 0.1 M phosphate buffer (pH 6.8) at 37 ± 0.2 °C, and rotated with 50 rpm. The detachment times were observed over 5 days, and the average times were reported.
2.9.2. Half-Pipe Method
Unmodified starch, diaminated starch, and chitosan were fluorescence-labeled by incorporation of fluorescence diacetate (FDA) for assessment of mucoadhesive properties.22 Briefly, 20 mg of each polymer was hydrated in 20 mL of demineralized water, and the pH was adjusted to 6.5 with 0.1 M HCl, except with chitosan that was hydrated in 0.01 M HCl and then diluted with demineralized water. To each polymer solution, 1 mL of 0.1% (m/v) ethanolic FDA solution was added. After 24 h of stirring at room temperature, the solutions were freeze-dried.
For evaluation of mucoadhesive properties, half-cut 50 mL falcon tubes were placed at a 45° angle on a ramp-shaped device in an incubation chamber at 37 °C and 100% relative humidity. Then, freshly excised porcine small intestine was cleaned, cut into pieces of 4 × 2 cm, and fixed on the falcon tubes. The mucosa was continuously rinsed with 2 M phosphate buffer pH 6.8 for 10 min at a flow rate of 1 mL/min through a pump (multichannel peristaltic pump, Ismatec, Germany). Thereafter, 10 mg of FDA-labeled samples were placed on the intestinal mucosa. Mucosa and test samples were continuously rinsed with a flow rate of 1 mL/min with phosphate buffer. After 30, 60, 120, and 180 min, the phosphate buffer having been rinsed from the mucosa was quantified.
Phosphate buffer of pH 6.8 containing 0.2% (m/v) FDA and pure phosphate buffer of pH 6.8 served as positive and negative controls. NaOH (2 mL, 5 M) was added to 1 mL of each test sample in order to hydrolyze FDA to sodium fluorescein. After incubation under shaking at 37 °C for 30 min, reaction mixtures were centrifuged at 13,400 rpm for 5 min. Fluorescence intensity of 100 μL of each supernatant was measured at an excitation wavelength of 485 nm and emission wavelength of 535 nm. All experiments were performed in triplicate.
2.9.3. Tensile Studies
Tensile studies were performed according to a method having been described previously.23 Unmodified starch, diaminated starch, and chitosan tablets were attached to a stainless-steel flat disc with a cyanoacrylate adhesive. The porcine intestinal mucosa was attached on the bottom of a beaker utilizing a cyanoacrylate adhesive then 500 mL of 0.1 M phosphate buffer of pH 6.8 was added to the beaker at 37 °C and placed on a balance, which was linked to a personal computer. The steel disc with the attached tablet was hung from a laboratory stand and brought into contact with the mucosa for 30 min at 37 °C. After this incubation, the balance was pulled down at a rate of 0.1 mm/s. Obtained data were automatically transferred to Sarta Collect software (Sartorius AG, Austria). Maximum detachment force (MDF) and total work of adhesion (TWA) were calculated by this software for each tablet.
2.10. Statistical Data Analysis
GraphPad Prism 7 software was applied to analyze statistical data. All data were analyzed by one-way ANOVA and t-test with p < 0.05 as a minimal level of significance, and the results are expressed as means ± SD of at least three experiments.
3. Results and Discussion
3.1. Characterization of Diaminated Starch
3.1.1. Structural Characterization
Native starches are a blend of two polyglycans, amylose and amylopectin. Amylose is the linear fraction consisting of α-d-glucopyranose linked through α(1→4) linkages with an average molecular mass of 105–106 g/mol, whereas amylopectin with a molecular mass of 106–107 g/mol is the branched fraction containing short chains linking linear chains via α(1→6) linkages.24,25 As the interpenetration of branched high-molecular mass polymers into the mucus gel layer essential for highly mucoadhesive properties is lower than that of linear polymers of comparatively lower molecular mass, a starch of high amylose content was chosen. According to the specification of the provider, the amylose content of the starch used in this study was at least 68%. Diamination of this starch was achieved in aqueous solution by tosylation and amination. Tosylated starch was synthesized in 0.4 M sodium hydroxide in the presence of triethylamine as a weak base without any organic solvents. Tosylated starch was aminated in ethylenediamine at 100 °C. The synthetic pathway is illustrated in Figure 1. Thin-layer chromatography (TLC) was used to confirm the successful purification of diaminated starch in comparison to control the sample and ethylenediamine as a reference.
Figure 1.
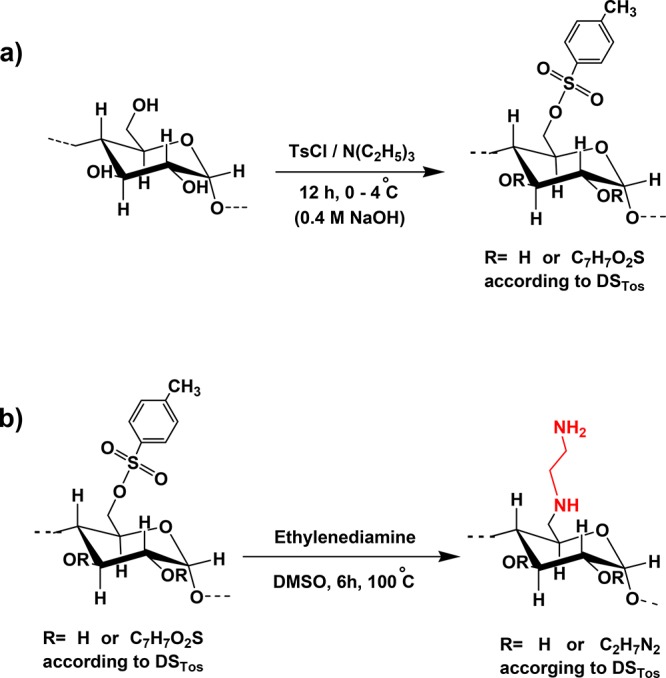
Amination of starch in two steps: (a) tosylation of starch and (b) amination of tosylated starch with ethylenediamine.
As shown in Table 1, elemental analysis of tosylated starch revealed sulfur content of 10.54%, and based on which, the degree of tosylation was calculated to be 1.08. Diaminated starch showed nitrogen content of 10.03% corresponding to a degree of substitution of 0.73.
Table 1. CHNS Elemental Analysis of Tosylated Starch and Diaminated Starch.
| polymer | carbon (%) | hydrogen (%) | nitrogen (%) | sulfur (%) | degree of substitution |
|---|---|---|---|---|---|
| tosylated starch | 49.47 | 5.18 | 0.39 | 10.54 | 1.08 |
| diaminated starch | 47.41 | 6.85 | 10.03 | 0.89 | 0.73 |
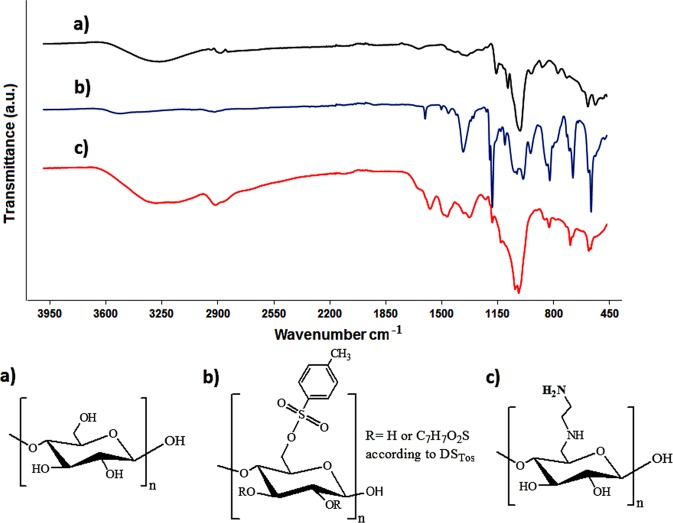
The formation of tosylated and diaminated starch was also confirmed via FT-IR spectroscopy. Figure 2 shows FT-IR spectra of unmodified starch, tosylated starch, and diaminated starch. In the spectrum of starch, the broad band at 3271 cm–1 is attributed to ν O–H, the band at 2923.12 cm–1 is related to ν C–H of the glucose ring, and the bands at 1148, 1076, and 995 cm–1 are ascribed to δ C–O–C, δ C–OH, and C–H (δ C–H) of the glucose ring, respectively.26 In the FT-IR spectrum of tosylated starch, the peak at 3510 cm–1 corresponds to ν O–H, whereas the bands at 3062 and 2931 cm–1 are related to ν C–H of the aromatic ring and glucose ring, respectively. The bands at 1597, 1495, 1450 (ν C–C aromatic), and 812 cm–1 (ν C–H aromatic) are assigned to the aromatic ring of the tosyl group. The bands at 1365 and 1173 cm–1 can be attributed to the antisymmetric and symmetric stretching vibrations of SO2.13 The characteristic peaks of diaminated starch are a broad peak at 3100–3400 cm–1 (ν NH and ν OH) and peaks at 2915 (ν C–H), 1567 (δ N–H), 1174 (δ C–O–C), 1006 (ν C–N), and 681 cm–1 (δ NH2).
Figure 2.
FT-IR results of (a) starch, (b) tosylated starch, and (c) diaminated starch.
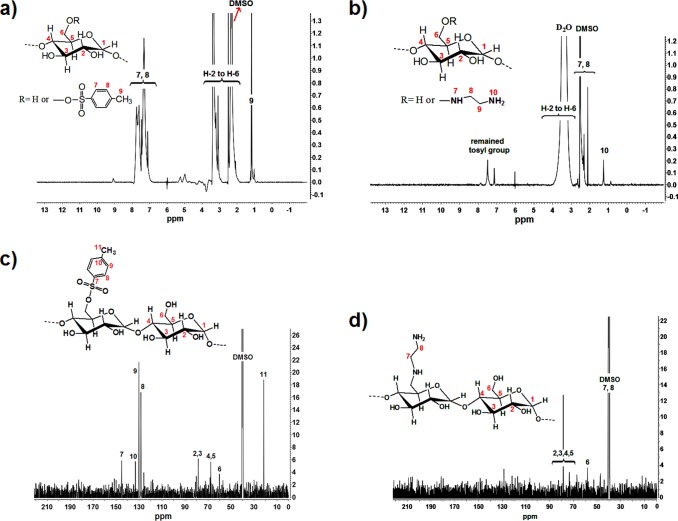
1H NMR and 13C NMR spectra of tosylated starch and diaminated starch are shown in Figure 3. The proton resonance peaks for tosylated starch are 1.15 (CH2 attached to toluene sulfonyl), 2.32 (CH3), 3–4.1 (starch backbone), and 7.1–7.7 (CH aromatic),27 and those of diaminated starch are 1.23 (NH2 primary), 2–2.3 (CH2 of ethylenediamine and attached to amine), and 3–4 ppm (starch backbone).28 The carbon resonance peaks at 145, 130, 129, and 125 ppm are ascribed to the tosyl group moiety, which disappeared in diaminated starch by replacing with ethylenediamine.
Figure 3.
1H NMR spectra of (a) tosylated starch and (b) diaminated starch in DMSO-d6 and 13C NMR spectra of (c) tosylated starch and (d) diaminated starch in DMSO-d6.
In contrast to previous studies focusing on the generation of cationic starch derivatives such as starch methylene dimethylamine,29 starch amino acid conjugates,30 or starch exhibiting quaternary ammonium substructures,31 the cationic starch described in this study is, to our knowledge, the first exhibiting vicinal amino groups as ligands.
3.1.2. Degree of Amination
The amount of primary amine groups on the polymer was determined via TNBS assay. The results of diaminated starch, the negative control sample of diaminated starch, and chitosan were 2083 ± 121.6, 33 ± 4.5, and 3068 ± 157.3 μmol of primary amine groups/g, respectively. According to these results, a significant amount of amine groups is present on diaminated starch. Furthermore, the marginal amount of the remaining primary amine groups in the negative control sample provided evidence for the efficacy of the applied purification method for diaminated starch.
Potentiometric titration revealed one inflexion point for unmodified starch and two inflexion points for chitosan and diaminated starch as illustrated in Figure 4. The degree of deacetylation of chitosan was determined to be 83% being in accordance with the specification of the provider. For diaminated starch, a higher amount of NaOH was required to reach the second inflexion point as it contains more protonated amines in comparison to chitosan. The degree of amination of diaminated starch was determined to be 63.5% of repeating units, which was in agreement with the result of TNBS assay and close to the result of elemental analysis. However, diaminated starch exhibited more cationic amines per repeating unit in comparison to chitosan. The second inflexion point of diaminated starch was at pH ≥ 8, whereas that of chitosan was at pH 7. This shows that diaminated starch can maintain its cationic character at a higher pH because of the comparatively higher acidic pK of the secondary amine.
Figure 4.
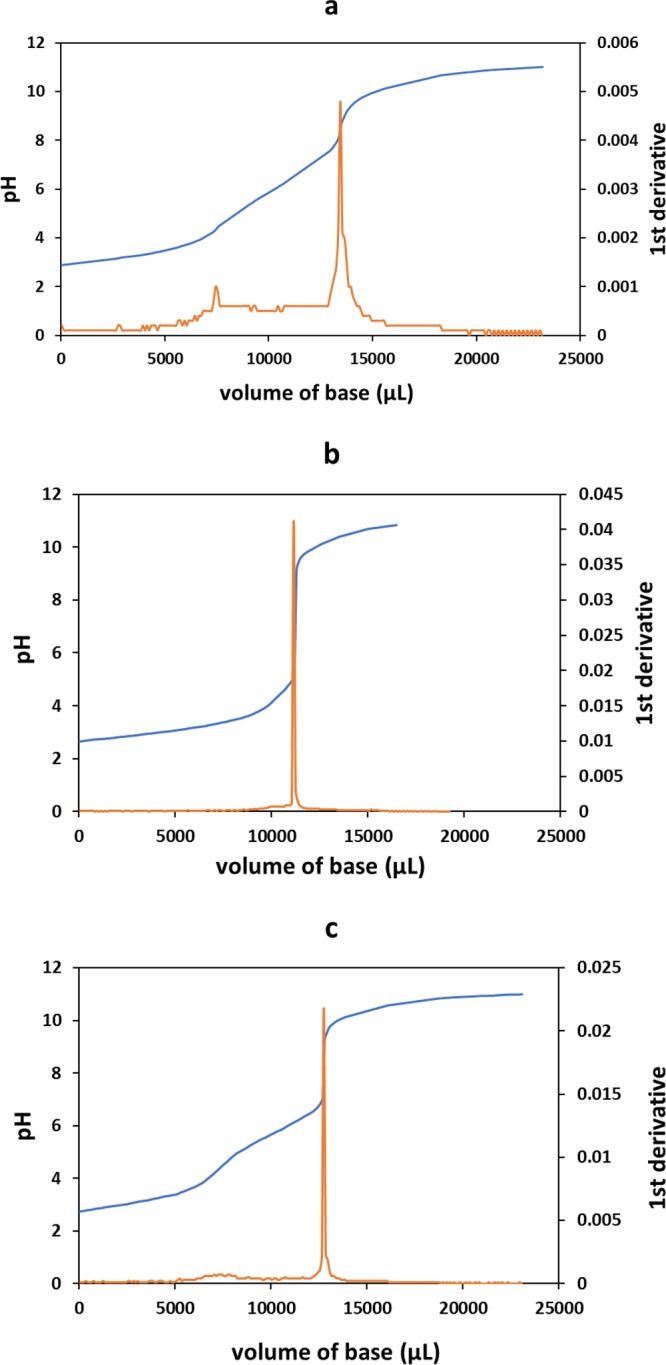
Titration curves of (a) diaminated starch, (b) unmodified starch, and (c) chitosan and their first derivatives.
3.2. ζ Potential
Due to protonation of the amine groups, diaminated starch showed a pronounced positive ζ potential at an acidic pH, whereas the ζ potential of unmodified starch was close to zero. The ζ potential of diaminated starch and chitosan decreased with increasing pH. In contrast, unmodified starch did not show any pH-dependent changes in its ζ potential. Results of this study are illustrated in Figure 5. The ζ potentials of tested polymers at increasing pH are listed in detail in Table 2.
Figure 5.
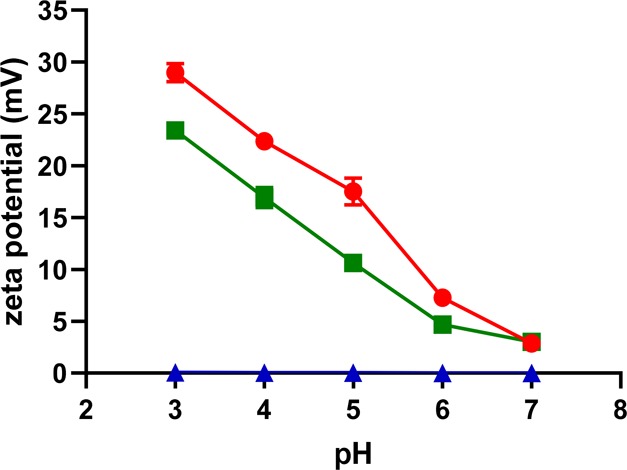
ζ potentials of diaminated starch (green solid square), unmodified starch (blue solid triangle), and chitosan (red solid circle) at indicated pH values; values are the mean of three experiments ± SD.
Table 2. ζ potential of Diaminated Starch, Unmodified Starch, and Chitosan at Indicated pH Valuesa.
| ζ potential (mV) |
|||
|---|---|---|---|
| pH | diaminated starch | unmodified starch | chitosan |
| 3 | 23.40 ± 0.38 | 0.06 ± 0.008 | 28.96 ± 0.56 |
| 4 | 16.93 ± 0.37 | 0.04 ± 0.005 | 22.36 ± 0.48 |
| 5 | 10.62 ± 0.28 | 0.04 ± 0.003 | 17.50 ± 0.37 |
| 6 | 4.69 ± 0.22 | 0.02 ± 0.003 | 7.28 ± 0.21 |
| 7 | 3.43 ± 0.08 | 0.002 ± 0.001 | 2.87 ± 0.11 |
Values are means of three independent measurements ± standard deviation (mean ± SD).
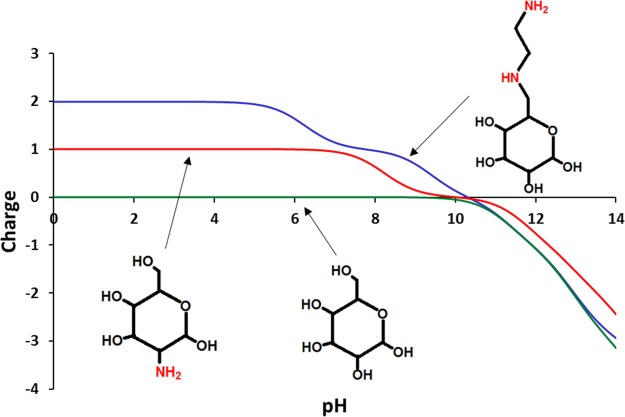
3.3. Isoelectric Points
Isoelectric points of diaminated starch, chitosan, and unmodified starch are illustrated in Figure 6. Diaminated starch shows +2 positive charges at pH ≤ 6 because of the primary and secondary amine groups. At pH 6 to 8, the charge of diaminated starch is +1. In contrast, chitosan bears a charge of +1 at pH ≤ 6.2. Due to the lack of amine groups, unmodified starch does not show any charge over the aforementioned pH range. The surface charge of diaminated starch that can be tuned by adjusting pH makes it a pH-responsive smart material for a wide variety of applications including stimuli-responsive drug delivery systems.
Figure 6.
Isoelectric points of diaminated starch, unmodified starch, and chitosan repeating units at indicated pH values according to the Chemicalize platform developed by ChemAxon.
3.4. pH-Dependent Solubility
According to solubility studies, diaminated starch was entirely soluble at acidic, neutral, and basic pH, whereas chitosan showed solubility just at acidic pH as the protonation of its amine groups triggers electrostatic repulsion between the polymer chains. In the case of diaminated starch, this additional solubility improving effect does not seem to be necessary.
3.5. Cytotoxicity Studies
3.5.1. Hemolysis Study
Blood biocompatibility of diaminated starch was evaluated by hemolysis assay. Toxic compounds such as Triton X-100 having been used as a positive control cause hemolysis of red blood cells and complete hemoglobin release. In contrast, unmodified starch was completely biocompatible and nontoxic. As shown in Figure 7, even in the highest applied concentration, the hemolytic activity of diaminated starch was less than 10%. At pH 6, the hemolytic activity of diaminated starch was in the same range as that of chitosan. Chitosan is well known for its hemostatic properties that are primarily based on electrostatic interactions between the positive charge of the surface of chitosan and the negative charge on the surface of red blood cells.32 These ionic interactions of chitosan with red blood cells are likely also responsible for minor hemolytic activity of this polymer as already shown by Wang et al.33 Our results showed strongly pH-dependent hemolytic activity of chitosan. Being dissolved at pH 6, its hemolytic activity was at least 10-fold higher at this pH than at pH 7.4. As the cationic surface charge of chitosan is more pronounced at pH 6 than at pH 7.4, more intensive interactions with the anionic surface of red blood cells can be assumed.
Figure 7.
Hemolysis rate of diaminated starch, unmodified starch, and chitosan in different concentrations of 0.1, 0.2, 0.5, 1, 2, 3, and 5 mg/mL (from the left to right) in two different pH values, 6 and 7.4. (Data shown are the mean ± SD, n = 3.)
3.5.2. Cell Viability
Cytotoxicity studies of diaminated starch, unmodified starch, and chitosan were carried on Caco-2 cells by resazurin colorimetric assay. Because of the ability of viable cells to reduce the resazurin to the highly fluorescent resorufin dye, the impact of the polymer on cell viability can be quantified. As depicted in Figure 8, more than 95% of the cells were viable and metabolically active after incubation with unmodified starch. Cell viability was above 90% in the case of chitosan and above 80% in the case of diaminated starch up to a concentration of 0.2 mg/mL.
Figure 8.
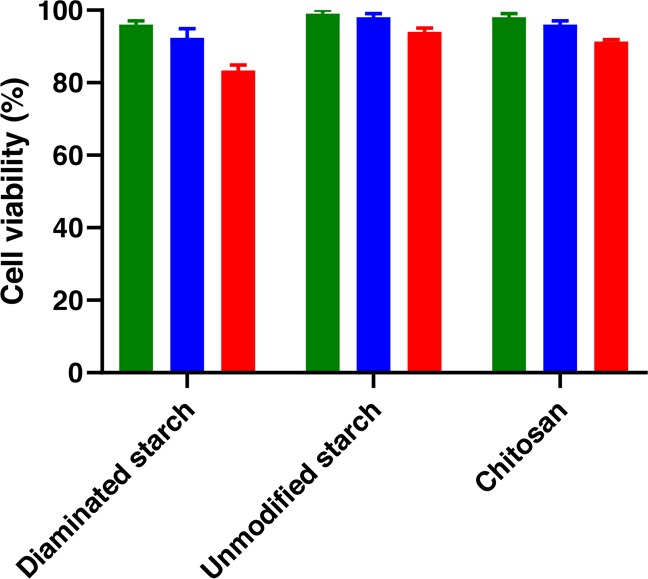
Viability of Caco-2 cells was determined by resazurin assay after 4 h of incubation. Three concentrations including 0.05 (black bars), 0.1 (gray bars), and 0.2 mg/mL (white bars) were applied. Each value represents the mean ± SD of the three experiments.
Although cationic polymers, especially those containing amine groups like polyethyleneimine, show a high cytotoxic potential, diaminated starch demonstrated just minor toxicity to normal cells. In the resazurin assay, chitosan was less harmful to cells than diaminated starch, which may be explained by the precipitation of chitosan at neutral pH. In the hemolysis assay, chitosan showed slightly higher toxicity at pH 6 than at pH 7.4, whereas diaminated starch showed similar toxicity at both pH values. Polymers exhibiting a high density of cationic charges such as polyethyleneimine or polyallylamine are obviously cytotoxic.34 However, toxicological concerns are minor when the density of cationic charges on polymers is lower. Aminated polymethacrylates such as Eudragit RL, RS, and E for instance are generally regarded as safe and used as coating materials in numerous pharmaceutical products. The same counts for chitosan and likely also for diaminated starch as the density of cationic charges is also comparatively low. In contrast to the abovementioned synthetic polymers, polysaccharides offer the additional advantage of biodegradability. As not all enzyme recognition sites on starch such as those for amylase were modified, diaminated starch is likely still biodegradable. According to these considerations, the minor cytotoxic effect of diaminated starch shown in Figure 8 does not seem to be a hindrance for further pharmaceutical developments.
3.6. Swelling Behavior
As shown in Figure 9, diaminated starch tablets exhibited significant swelling behavior due to water penetrability and protonation of amine groups at pH 1.2. After 120 min, their weight was even 53-fold higher. They appeared as transparent jelly balls. After 3 h, erosion intensified so that they dissolved completely. At pH 3 and 5, diaminated starch tablets swelled more slowly and reached up to 30- and 18-fold of their initial weights, respectively. At pH 6.8 and 7.4, diaminated starch tablets exhibited just minor swelling as illustrated in Figure 10. Unmodified starch showed generally poor water uptake properties, and tablets disintegrated completely and dispersed within 5 min. Chitosan tablets exhibited minor swelling at pH 1.2 and 3. They disintegrated after 15 min and finally dissolved completely by creating dense and viscous solutions. Chitosan tablets did not show any swelling at pH ≥ 5. They disintegrated and dispersed within 15 min at pH 5 and after 25 min at pH 6.8 and 7.4.
Figure 9.
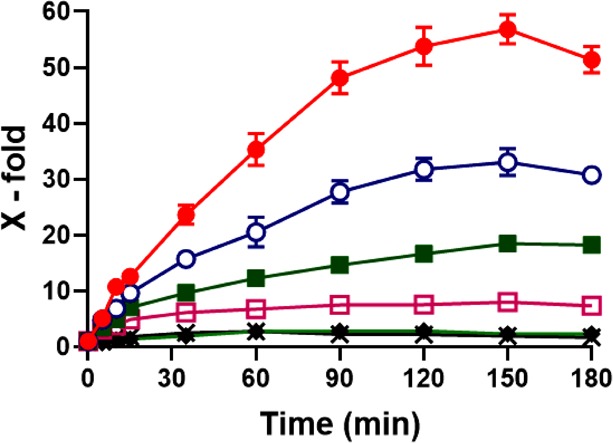
Swelling behavior of diaminated starch tablets at pH 1.2 (solid circle), 3 (open circle), 5 (solid square), 6 (open square), 6.8 (diamond), and 7.4 (cross). Indicated values are means ± SD (n = 3); chitosan tablets did not show swelling behavior and disintegrated within 15 min.
Figure 10.
Images of diaminated starch tablets after swelling in different buffers of pH 1.2, 3, 5, 6.8, and 7.4 after 90 min.
3.7. Mucoadhesive Properties
Mucoadhesion studies of diaminated starch, unmodified starch, and chitosan were performed by the rotating cylinder method. Diaminated starch showed significant mucoadhesive properties as tablets detached after 5 days and 3 h in average. In contrast, unmodified starch tablets started to disintegrate already after 10 min. As depicted in Figure 11, on average, after 1 h and 20 min, chitosan tablets detached and dispersed into the buffer solution.
Figure 11.
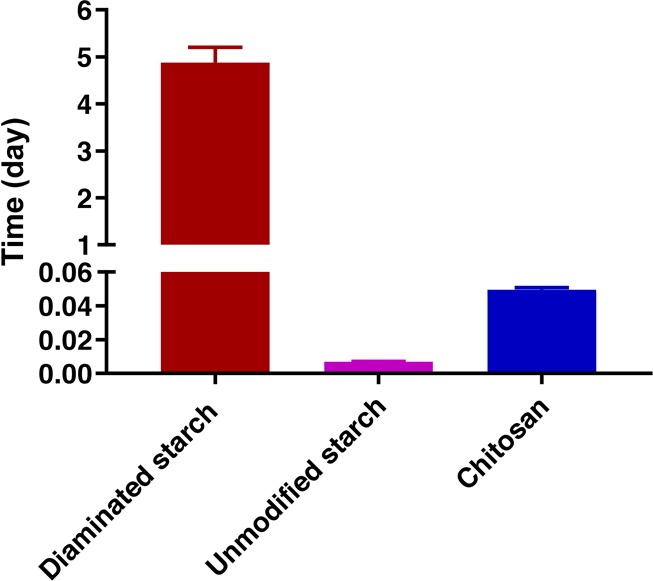
Mucoadhesion results of diaminated starch, unmodified starch, and chitosan by the rotatory cylinder method. Indicated values are means of three experiments (±SD).
Furthermore, the mucoadhesion properties of polymers were characterized by their residence time on the mucosa via the half-pipe method.35 Diaminated starch and chitosan showed mucoadhesive properties for 3 h. Diaminated starch displayed significantly higher-mucoadhesivity properties than those of chitosan. Unmodified starch displayed no mucoadhesive properties being in agreement with previous studies. Results of this study are illustrated in Figure 12. Additional tensile studies confirmed the superior mucoadhesive properties of diaminated starch. The TWA and MDF of diaminated starch were 7.6- and 10.9-fold higher than those of chitosan, respectively. Unmodified starch tablets disintegrated and did not show adhesivity to the mucosa at all.
Figure 12.
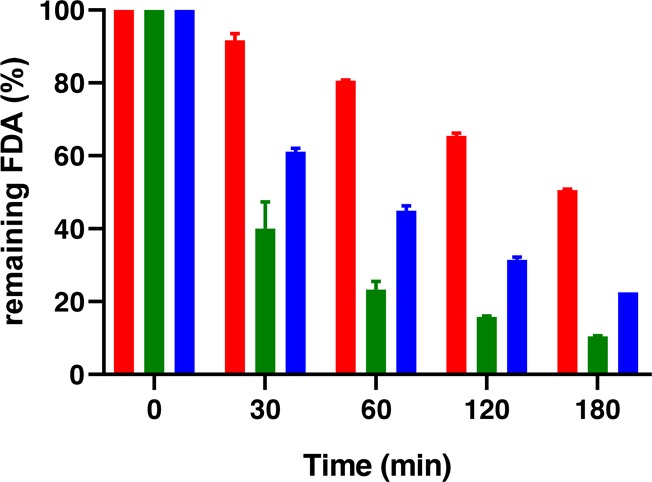
Mucoadhesion study with determining the amount of fluorescein diacetate (FDA) remaining on the mucosa. FDA-labeled diaminated starch (red bars), unmodified starch (green bars), and chitosan (blue bars). Indicated values are means ± standard deviation.
Because of two amine groups in repeating units, diaminated starch exhibited higher-mucoadhesivity properties than those of aminated starch as described previously,9 which can be attributed to two main reasons: (i) an increased local cationic charge density of diaminated starch providing stronger ionic interactions and (ii) the existence of two hydrogen-bonding sites on each repeating unit. Diaminated starch exhibits a higher level of tensile strength than that of chitosan due to elastic properties. In addition, the highly swelling properties of diaminated starch make it a good choice for drug delivery systems.
4. Conclusions
In this study, highly substituted diaminated starch (degree of substitution, DS, 0.65) was synthesized in an aqueous solution. Due to the presence of primary and secondary amines providing a higher local cationic charge density and more hydrogen-bonding sites, diaminated starch showed comparatively highly mucoadhesive properties. Good solubility, superior mucoadhesion properties, and minor cytotoxicity of diaminated starch seem to make it a useful cationic polysaccharide for drug delivery systems.
Acknowledgments
We would like to thank the Iranian Ministry of Science and Technology for the grant of scholarship and Ingredion for donating starch Hylon VII.
The authors declare no competing financial interest.
References
- Bonferoni M. C.; Sandri G.; Rossi S.; Ferrari F.; Caramella C. Chitosan and its salts for mucosal and transmucosal delivery. Expert Opin. Drug Delivery 2009, 6, 923–939. 10.1517/17425240903114142. [DOI] [PubMed] [Google Scholar]
- Sogias I. A.; Williams A. C.; Khutoryanskiy V. V. Why is chitosan mucoadhesive?. Biomacromolecules 2008, 9, 1837–1842. 10.1021/bm800276d. [DOI] [PubMed] [Google Scholar]
- Arca H. Ç.; Günbeyaz M.; Şenel S. Chitosan-based systems for the delivery of vaccine antigens. Expert Rev. Vaccines 2009, 8, 937–953. 10.1586/erv.09.47. [DOI] [PubMed] [Google Scholar]
- Ways T. M. M.; Lau W. M.; Khutoryanskiy V. V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. 10.3390/polym10030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourya V. K.; Inamdar N. N. Trimethyl chitosan and its applications in drug delivery. J. Mater. Sci. Mater. Med. 2009, 20, 1057. 10.1007/s10856-008-3659-z. [DOI] [PubMed] [Google Scholar]
- Godbey W. T.; Wu K. K.; Mikos A. G. Size matters: molecular weight affects the efficiency of poly (ethylenimine) as a gene delivery vehicle. J. Biomed. Mater. Res. 1999, 45, 268–275. . [DOI] [PubMed] [Google Scholar]
- Calixto G. M. F.; Victorelli F. D.; Dovigo L. N.; Chorilli M. Polyethyleneimine and chitosan polymer-based mucoadhesive liquid crystalline systems intended for buccal drug delivery. AAPS PharmSciTech 2018, 19, 820–836. 10.1208/s12249-017-0890-2. [DOI] [PubMed] [Google Scholar]
- Khutoryanskiy V. V. Advances in Mucoadhesion and Mucoadhesive Polymers. Macromol. Biosci. 2011, 11, 748–764. 10.1002/mabi.201000388. [DOI] [PubMed] [Google Scholar]
- Jelkmann M.; Leichner C.; Menzel C.; Kreb V.; Bernkop-Schnürch A. Cationic starch derivatives as mucoadhesive and soluble excipients in drug delivery. Int. J. Pharm. 2019, 570, 118664. 10.1016/j.ijpharm.2019.118664. [DOI] [PubMed] [Google Scholar]
- Zhou L.; Jin J.; Liu Z.; Liang X.; Shang C. Adsorption of acid dyes from aqueous solutions by the ethylenediamine-modified magnetic chitosan nanoparticles. J. Hazard. Mater. 2011, 185, 1045–1052. 10.1016/j.jhazmat.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Onozuka S.; Kojima M.; Hattori K.; Toda F. The Regiospecific Mono Tosylation of Cyclodextrins. Bull. Chem. Soc. Jpn. 1980, 53, 3221–3224. 10.1246/bcsj.53.3221. [DOI] [Google Scholar]
- Schmidt S.; Liebert T.; Heinze T. Synthesis of soluble cellulose tosylates in an eco-friendly medium. Green Chem. 2014, 16, 1941–1946. 10.1039/C3GC41994K. [DOI] [Google Scholar]
- Heinze T.; Talaba P.; Heinze U. Starch derivatives of high degree of functionalization. 1. Effective, homogeneous synthesis of p-toluenesulfonyl (tosyl) starch with a new functionalization pattern. Carbohydr. Polym. 2000, 42, 411–420. 10.1016/S0144-8617(99)00182-4. [DOI] [Google Scholar]
- Malhotra M.; Tomaro-Duchesneau C.; Saha S.; Kahouli I.; Prakash S. Development and characterization of chitosan-PEG-TAT nanoparticles for the intracellular delivery of siRNA. Int J Nanomedicine 2013, 8, 2041–2052. 10.2147/IJN.S43683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X.; Chen L.; Zhong W. A new linear potentiometric titration method for the determination of deacetylation degree of chitosan. Carbohydr. Polym. 2003, 54, 457–463. 10.1016/j.carbpol.2003.05.004. [DOI] [Google Scholar]
- Chang S.-H.; Lin H.-T. V.; Wu G.-J.; Tsai G. J. pH Effects on solubility, zeta potential, and correlation between antibacterial activity and molecular weight of chitosan. Carbohydr. Polym. 2015, 134, 74–81. 10.1016/j.carbpol.2015.07.072. [DOI] [PubMed] [Google Scholar]
- Hauptstein S.; Bonengel S.; Griessinger J.; Bernkop-Schnürch A. Synthesis and Characterization of pH Tolerant and Mucoadhesive (Thiol–Polyethylene Glycol) Chitosan Graft Polymer for Drug Delivery. J. Pharm. Sci. 2014, 103, 594–601. 10.1002/jps.23832. [DOI] [PubMed] [Google Scholar]
- Qu N.; Lee R. J.; Sun Y.; Cai G.; Wang J.; Wang M.; Lu J.; Meng Q.; Teng L.; Wang D.; Teng L. Cabazitaxel-loaded human serum albumin nanoparticles as a therapeutic agent against prostate cancer. Int J Nanomedicine 2016, 11, 3451–3459. 10.2147/IJN.S105420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J.; Shahnaz G.; Dünnhaupt S.; Müller C.; Hintzen F.; Bernkop-Schnürch A. Preactivated thiomers as mucoadhesive polymers for drug delivery. Biomaterials 2012, 33, 1528–1535. 10.1016/j.biomaterials.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast C. E.; Bernkop-Schnürch A. Thiolated polymers — thiomers: development and in vitro evaluation of chitosan–thioglycolic acid conjugates. Biomaterials 2001, 22, 2345–2352. 10.1016/S0142-9612(00)00421-X. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnürch A.; Steininger S. Synthesis and characterisation of mucoadhesive thiolated polymers. Int. J. Pharm. 2000, 194, 239–247. 10.1016/S0378-5173(99)00387-7. [DOI] [PubMed] [Google Scholar]
- Asim M. H.; Moghadam A.; Ijaz M.; Mahmood A.; Götz R. X.; Matuszczak B.; Bernkop-Schnürch A. S-protected thiolated cyclodextrins as mucoadhesive oligomers for drug delivery. J. Colloid Interface Sci. 2018, 531, 261–268. 10.1016/j.jcis.2018.07.062. [DOI] [PubMed] [Google Scholar]
- Mortazavi S. A.; Smart J. D. An investigation of some factors influencing the in vitro assessment of mucoadhesion. Int. J. Pharm. 1995, 116, 223–230. 10.1016/0378-5173(94)00299-K. [DOI] [Google Scholar]
- Namazi H.; Dadkhah A. Convenient method for preparation of hydrophobically modified starch nanocrystals with using fatty acids. Carbohydr. Polym. 2010, 79, 731–737. 10.1016/j.carbpol.2009.09.033. [DOI] [Google Scholar]
- Namazi H.; Mosadegh M.. Bio-nanocomposites based on naturally occurring common polysaccharides chitosan, cellulose and starch with their biomedical applications. In book:Recent Dev Bio-nanocomposites Biomed Appl; Publisher: Nova Publisher: Editor: Tiwari A.2011, 379–397. [Google Scholar]
- Namazi H.; Dadkhah A.; Mosadegh M. New Biopolymer Nanocomposite of Starch-Graft Polystyrene/Montmorillonite Clay Prepared Through Emulsion Polymerization Method. J. Polym. Environ. 2012, 20, 794–800. 10.1007/s10924-012-0496-4. [DOI] [Google Scholar]
- Tripodo G.; Wischke C.; Neffe A. T.; Lendlein A. Efficient synthesis of pure monotosylated beta-cyclodextrin and its dimers. Carbohydr. Res. 2013, 381, 59–63. 10.1016/j.carres.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Namazi H.; Heydari A. Synthesis of β-cyclodextrin-based dendrimer as a novel encapsulation agent. Polym. Int. 2014, 63, 1447–1455. 10.1002/pi.4637. [DOI] [Google Scholar]
- Jiang Y.; Ju B.; Zhang S.; Yang J. Preparation and application of a new cationic starch ether–Starch–methylene dimethylamine hydrochloride. Carbohydr. Polym. 2010, 80, 467–473. 10.1016/j.carbpol.2009.12.002. [DOI] [Google Scholar]
- Namazi H.; Abdollahzadeh E. Drug nanocarrier agents based on starch-g-amino acids. BioImpacts: BI 2018, 8, 99–106. 10.15171/bi.2018.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Xie W. Synthesis of cationic starch with a high degree of substitution in an ionic liquid. Carbohydr. Polym. 2010, 80, 1172–1177. 10.1016/j.carbpol.2010.01.042. [DOI] [Google Scholar]
- Pusateri A. E.; Holcomb J. B.; Kheirabadi B. S.; Alam H. B.; Wade C. E.; Ryan K. L. Making sense of the preclinical literature on advanced hemostatic products. J. Trauma Acute Care Surg. 2006, 60, 674–682. 10.1097/01.ta.0000196672.47783.fd. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Fu Y.; Li J.; Mu Y.; Zhang X.; Zhang K.; Liang M.; Feng C.; Chen X. Multifunctional chitosan/dopamine/diatom-biosilica composite beads for rapid blood coagulation. Carbohydr. Polym. 2018, 200, 6–14. 10.1016/j.carbpol.2018.07.065. [DOI] [PubMed] [Google Scholar]
- Pathak A.; Patnaik S.; Gupta K. C. Recent trends in non-viral vector-mediated gene delivery. Biotechnol. J. 2009, 4, 1559–1572. 10.1002/biot.200900161. [DOI] [PubMed] [Google Scholar]
- Elieh-Ali-Komi D.; Hamblin M. R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Curr. Adv. Res. 2016, 4, 411–427. [PMC free article] [PubMed] [Google Scholar]