Abstract
Activation of the transient receptor potential (TRP) channel TRPA1 by cinnamaldehyde has been shown to stimulate serotonin release in enterochromaffin QGP-1 cells. However, the impact of cinnamaldehyde on serotonin release in enterocytes is less well understood. In addition, since the neurotransmitter serotonin plays a regulatory role in a large variety of gastrointestinal and metabolic functions, it is of interest to study which structural characteristics determine cinnamaldehyde-induced serotonin release by enterocytes. Thus, the present study analyzed serotonin release in differentiated Caco-2 cells as a model for enterocytes in comparison to enterochromaffin QGP-1 cells after stimulation with cinnamaldehyde and 17 naturally occurring structurally related compounds by means of a serotonin ELISA. Stimulation with cinnamaldehyde induced a dose-dependent increase in serotonin release starting from 0.5 mM in both cell lines, with a larger effect size in Caco-2 enterocytes compared to that in QGP-1 enterochromaffin cells. Serotonin release in Caco-2 cells induced by additional 17 structurally related compounds correlated with serotonin release in QGP-1 cells, showing the highest effects for coniferylaldehyde with a 15.84 ± 3.23-fold increase in Caco-2 cells, followed by the parent compound cinnamaldehyde (13.45 ± 2.15), cinnamyl alcohol (6.68 ± 1.08), and α-methyl-cinnamaldehyde (6.59 ± 0.93). Analysis of structural and molecular characteristics that modulate serotonin release in Caco-2 enterocytes revealed that the ability of a compound to activate TRPA1, demonstrated by means of HEK293 cells transiently expressing hTRPA1, is a decisive factor to stimulate serotonin release in Caco-2 enterocytes, preferring small, electrophilic compounds with a lower polar surface area. In addition, blocking of TRPA1 using 30 μM AP-18 significantly reduced the cinnamaldehyde-induced serotonin release by 30.0 ± 5.24%, confirming a TRPA1-dependent component in serotonin release by Caco-2 cells.
Keywords: cinnamaldehyde, coniferylaldehyde, Caco-2, QGP-1, serotonin, TRPA1
Introduction
Activation of the transient receptor potential (TRP) nonselective cation channel TRPA1 has been previously demonstrated to potently stimulate serotonin release in the enterochromaffin cell model QGP-1.1 TRPA1 has been shown to be not only activated by noxious cold (<17 °C)2 but also by a number of naturally occurring bioactive aroma compounds present in the human diet. For example, compounds present in ginger, clove oil, mustard oil as well as in cinnamon oil have been shown to be potent ligands for the TRPA1 channel.3 Only recently, cinnamaldehyde, the main ingredient and key aroma compound of cinnamon oil, has been demonstrated to effectively increase serotonin release and block serotonin reuptake more potently than the other tested cinnamon-derived compounds cinnamic acid, cinnamyl alcohol, and cinnamyl isobutyrate in differentiated Caco-2 cells.4 The effects of cinnamaldehyde on serotonin release have been demonstrated to be at least partly allocated to the activation of TRPA1.4
Serotonin, a neurotransmitter and hormone, plays a regulatory role in a large variety of gastrointestinal and metabolic functions. Earlier studies focused on the role of central serotonin in the brain and largely ignored the peripheral occurring serotonin, although about 95% of the total serotonin is located in the gut.5 Therefore, several functions of peripheral serotonin have been unraveled only in the past years. For example, serotonin is nowadays recognized to be a hormone, neurotransmitter, and growth factor that is involved in the regulation of gastrointestinal motility during digestion, liver regeneration, bone formation, intestinal mucosal growth, and intestinal inflammation.6 Serotonin is biosynthesized by a multistep pathway. First, 5-hydroxytryptophan (5-HTP) is generated in the gut from the amino acid tryptophan by the enzyme tryptophan hydroxylase (TPH-1), whereas enteric and central neurons express TPH-2. Serotonin is then synthesized from its precursor 5-HTP by the aromatic L-amino acid decarboxylase (AAAD).7 The vast majority of peripheral serotonin is produced by enterochromaffin cells8 and may be released in response to nutrients, tastants, and olfactants, which in turn stimulate contraction via vagal afferent fibers to facilitate digestion.9−11 Inactivation of 5-HT in the mucosa of the bowel is executed by enterocytes that express serotonin reuptake transporters (SERT).12 Dysregulation of this process is associated with gastrointestinal disorders like irritable bowel syndrome.7 Thus, understanding the regulation of serotonin release by gastrointestinal cells in response to compounds present in our diet is of interest.
In this context, also cell types other than enterochromaffin cells have been shown to be able to synthesize and release serotonin upon stimulation, e.g., parietal cells of the stomach.13 Also, Nakamura et al.14 showed TPH expression not only in enterochromaffin cells but also in brush border cells of adult rat intestines. In addition, they demonstrated that the widely used intestinal cell model Caco-2 has the enzymes to synthesize and degrade serotonin, and also detected smaller amounts of serotonin in the cells,14 although data from human enterocytes are lacking so far. In a previous study by our group, we confirmed the usage of Caco-2 cells as a model for peripheral serotonin release by intestinal cells as well.15
However, since serotonin is involved in the regulation of various gastrointestinal and metabolic functions,5 it is of interest to understand which structural characteristics of cinnamaldehyde are important for the stimulation of serotonin release in intestinal cells. Cinnamaldehyde is the key aroma compound of cinnamon oil, reaching concentrations of around 8 mg/g in commercially available cinnamon.16 Thus, following the ingestion of 6 g of cinnamon powder, a concentration of around 400 μM could theoretically reach enterocytes in the gastrointestinal tract,4 demonstrating the nutritional relevance of higher concentrations of cinnamaldehyde.
In the present study, we aimed to investigate which molecular structural characteristics of cinnamaldehyde and related compounds are advantageous for stimulating or inhibiting serotonin release by enterocytes. We analyzed serotonin release in two intestinal cell models, QGP-1 cells as a model for human enterochromaffin cells and differentiated Caco-2 cells as a model for human enterocytes, after incubation with 500 μM cinnamaldehyde and additional 17 naturally occurring compounds that are structurally related to cinnamaldehyde. The selected concentration was chosen based on dose–response experiments carried out in the cell models.
Material and Methods
Chemicals
All chemicals (purity ≥ 95%), reagents, media, and media supplements were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany), unless stated otherwise.
Cell Culture
QBP-1 cells were a kind gift from Prof. Dr. Massimo Donadelli (University of Verona, Italy) and cultivated as described before17 in RPMI medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS) (Thermo Fisher Scientific, Austria), 1% (v/v) penicillin/streptomycin at 37 °C at 5% CO2 in a humidified atmosphere. Caco-2 and HEK293 cells were obtained from CLS Cell Lines Service GmbH, Eppelheim, Germany and cultivated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) heat-inactivated FBS (Thermo Fisher Scientific, Austria), 1% (v/v) penicillin/streptomycin at 37 °C at 5% CO2 in a humidified atmosphere. Caco-2 cells were differentiated to an enterocyte-like phenotype within 21 days as described before.18,19 Previous studies by our group showed that this method results in an intact monolayer, confirmed by transepithelial electrical resistance (TEER) values >350 Ω*cm2.18,19
Cellular Vitality
Negative effects of treatment with the test compounds were excluded using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromid) assays as a measure of cellular proliferation. MTT assays were performed as described in detail before.19 In brief, the different cell lines were treated according to the respective assay conditions before the MTT reagent (5 mg/mL in PBS), diluted in 5 parts serum-free DMEM, was added to the cells. After 15–20 min incubation at 37 °C, the MTT solution was aspirated and the formed purple formazan salt was dissolved in dimethyl sulfoxide (DMSO) before measuring the absorption at 570 nm by a Tecan infinite M200 device (Tecan, Männedorf, Switzerland).
Serotonin Release Assay
Serotonin release assays are based on the serotonin levels in the cell supernatant and were basically performed as described previously.15 Confluent Caco-2 cells were differentiated for 21 days in 12-well plates, whereas 250 000 cells per well of QGP-1 cells were seeded in 24-well plates, and the serotonin release assay was performed after 72 h.17 The test compounds were dissolved in DMSO as stock solutions (1000×) and applied to the cells diluted in Krebs–Ringer–HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer supplemented with 0.1% (v/v) ascorbic acid (incubation solution) for 5 min. The final concentration of DMSO in the incubation solution was 0.1% (v/v), or 0.2% (v/v) in the co-incubation assays with AP-18. The incubation solutions (cellular supernatants) were then collected in tubes and briefly centrifuged for 5 min at 1000 rpm and 4 °C to remove cellular debris. The supernatants were stored at −80 °C for a maximum of two weeks until final analysis using a Serotonin High Sensitive ELISA kit (DLD Diagnostica, Hamburg, Germany) according to the manufacturer’s protocol with a test volume of 50 μL. The sensitivity of the used test kit is 0.39 pg per sample and the intra-assay variation is <9% according to the manufacturer.
Transfection of HEK293 Cells and Analysis of Intracellular Calcium Levels
HEK293 cells were seeded in poly-l-ornithine-coated 96-wells plates (50 μL/well of a 20 μg/mL solution) to a density of 12 500 cells per well. Transfection was started 24 h after plating by the addition of Viromer One Red (Bio-Rad, Austria) with 10 ng/well pCDN3 vector (Thermo Fisher Scientific, Germany) with human TRPA1 (kindly provided by Prof. H. Hatt, Ruhr University Bochum, Germany). The transfection rate was typically between 80 and 95%, which was monitored by a GFP control using a pCDNA3 backbone (kindly provided by Prof. H. Hatt) at 10 ng/well, and analyzed on an EVOS FL digital microscope (Thermo Fisher Scientific, Austria) with 10× magnification.
Intracellular calcium mobilization in transfected HEK293 cells was carried out according to the protocol of Luo et al.20 using a Fluo-4 dye on a Flex-Station III equipped with SoftMax Pro 7.0.2 software (Molecular Devices, Biberach, Germany). In brief, transfected HEK293 cells were loaded for 1 h at 37 °C with 50 μL of loading dye solution (2 μM Fluo-4, 0.04% (w/v) pluronic acid) and 0.1% (w/v) bovine serum albumin dissolved in Hank’s balanced salt solution (HBSS #H6648, Sigma-Aldrich, Austria), containing 10 mM HEPES, with the pH adjusted to 7.4 using NaOH. After washing with assay buffer (2 mM probenecid in HBSS), cells with 80 μL of assay buffer/well were transferred to a Flex-Station instrument and the Ca2+ signal was monitored in Flex mode every 1.5 s for a total of 240 s at 494 nm excitation and 525 nm emission after stimulation with 40 μL of 3× concentrated test compound dissolved in assay buffer. A second application with a final concentration of 10 μM ionomycin was used to assess cell vitality. For analysis, the data range (maximum–baseline) for each compound was calculated after the subtraction of the blank and subsequently treated over its solvent control.
RT-qPCR Detection of TRPA1
To confirm the expression of TRPA1 in the cell lines, a RT-qPCR experiment was performed. RNA was isolated from fully differentiated Caco-2 cells and QGP-1 cells using the Epicentre Masterpure complete DNA and RNA purification kit (Lucigen, Madison, WI, USA) and reverse-transcribed to cDNA using the High-Capacity RNA-to-cDNA kit (Applied Biosystems, Thermo Fisher Scientific, Austria). PCR was subsequently performed using Fast Master Mix (Applied Biosystems via Thermo Fisher Scientific, Austria) on a Step-One Plus Device (Applied Biosystems via Thermo Fisher Scientific, Austria). The primer pairs used during the reaction can be found in Table 1.
Table 1. Primer Pairs Used for the qPCR Analysis of TRPA1 Gene Expression in QGP-1 and Caco-2 Cells.
Statistical and Computational Analyses
MS Excel 2013 and SigmaPlot 13 were used for statistical analysis of the data. All data are shown as the mean fold change ± standard error of mean (SEM) calculated from at least three independent experiments with multiple technical replicates each. Outliers (P < 0.001) were identified with the Nalimov outlier test and excluded from the final calculation. Data were tested for normality using the Shapiro–Wilk test. Differences between two groups were tested using Student’s t-test. Two-way analysis of variance (ANOVA) with the Holm–Sidak posthoc test was used to compare treatments and dose-dependent effects. Significant differences were assumed at P values < 0.05.
The calculation of physicochemical descriptors of each test molecule was carried out using RDKit node for the KNIME Analytics Platform 3.7.0. A number of topological descriptors were calculated, but only those displaying values with considerable difference between the tested molecules and less than 5% zero-values were used for further correlation analysis as indicated in the Results section. Electrostatic potential maps of selected compounds were drawn using the software ‘Flare for academics’ (Cresset, U.K.). Matched-molecular pair analysis was carried out using Vortex (Version 2019.04, Dotmatics Ltd., U.K.) including single-atom changes and nonring fragmentations (with a maximum fragment size of eight and a minimum core size of eight atoms), using both experimental readouts of TRPA1 activation and serotonin secretion by Caco-2 enterocytes as parameters of interest.
Results
Cellular Proliferation Using the MTT Assay
All test compounds were tested for their impact on cellular proliferation as a measure for toxicity in QGP-1 and Caco-2 cells at the concentrations and incubation times applied at the serotonin release experiments (Figure S1). Cinnamaldehyde applied at the highest concentration of 5 mM reduced the cellular proliferation to 69.8 ± 5.34% in QGP-1 cells (Figure S2). Since this value is below the cutoff level of 70% according to ISO 10993:5,21 2.5 mM was chosen as the highest test concentration in QGP-1 cells. In Caco-2 cells, no negative effects on cellular proliferation were detected after applying the compounds in the described assay conditions (Figure S1 and S2).
Dose-Dependent Serotonin Release by QGP-1 Cells and Caco-2 Induced by Cinnamaldehyde
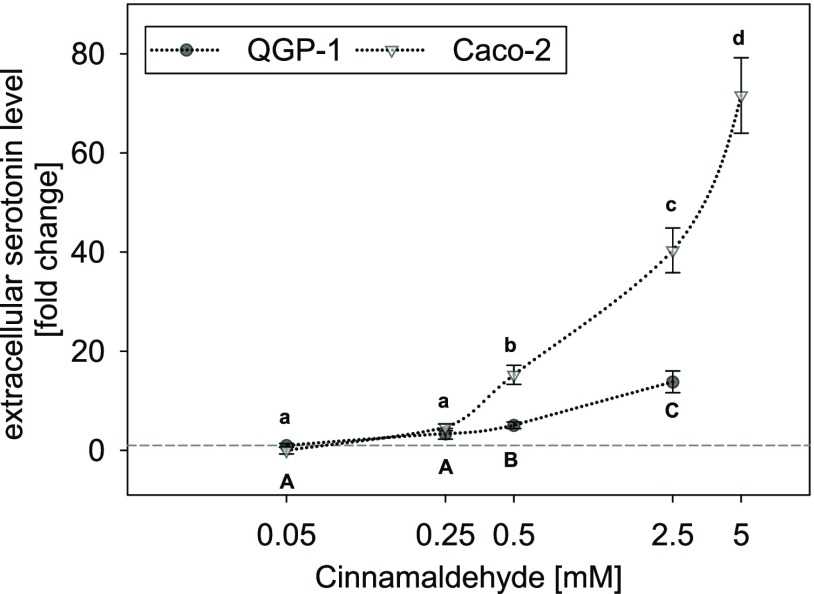
Treatment of QGP-1 cells with final concentrations of 0.05–2.5 mM cinnamaldehyde dose-dependently stimulated serotonin release, starting with a 5.10 ± 0.71-fold increase at a concentration of 0.5 mM and up to 13.8 ± 2.20-fold increase at 2.5 mM (Figure 1). Due to the negative effects on cellular toxicity as described above, higher test concentrations were not applied and no saturation of serotonin release was reached. An EC50 value is therefore not presented.
Figure 1.
Concentration-dependent serotonin release in QGP-1 enterochromaffin cells (gray circles) and Caco-2 enterocytes (white triangles) after stimulation with 0.05–2.5 or 5 mM cinnamaldehyde, respectively. Data are presented as mean fold change ± SEM calculated from four independent experiments with two technical replicates each. Significant differences between the treatments and the cell models were tested by two-way ANOVA with the Holm–Sidak posthoc test; the concentration-dependent effects are marked by distinct letters in the figure.
Similarly, Caco-2 cells were treated with final concentrations ranging from 0.05 up to 5 mM cinnamaldehyde, which led to a dose-dependent stimulation of serotonin release as well. Also here, a significant stimulation of serotonin release started at 0.5 mM with a fold change of 15.2 ± 1.92, reaching up to 71.6 ± 7.62-fold change at a test concentration of 5 mM (Figure 1). Due to its limited solubility in aqueous solutions, cinnamaldehyde was not tested in concentrations exceeding 5 mM. An EC50 value for the effects in Caco-2 cells is therefore not presented as well.
A comparison of the stimulating effect of cinnamaldehyde in the two cell models revealed a more pronounced effect in Caco-2 enterocytes than in QGP-1 cells (Two-way ANOVA, P < 0.001).
Serotonin Release by Structural Analogues of Cinnamaldehyde
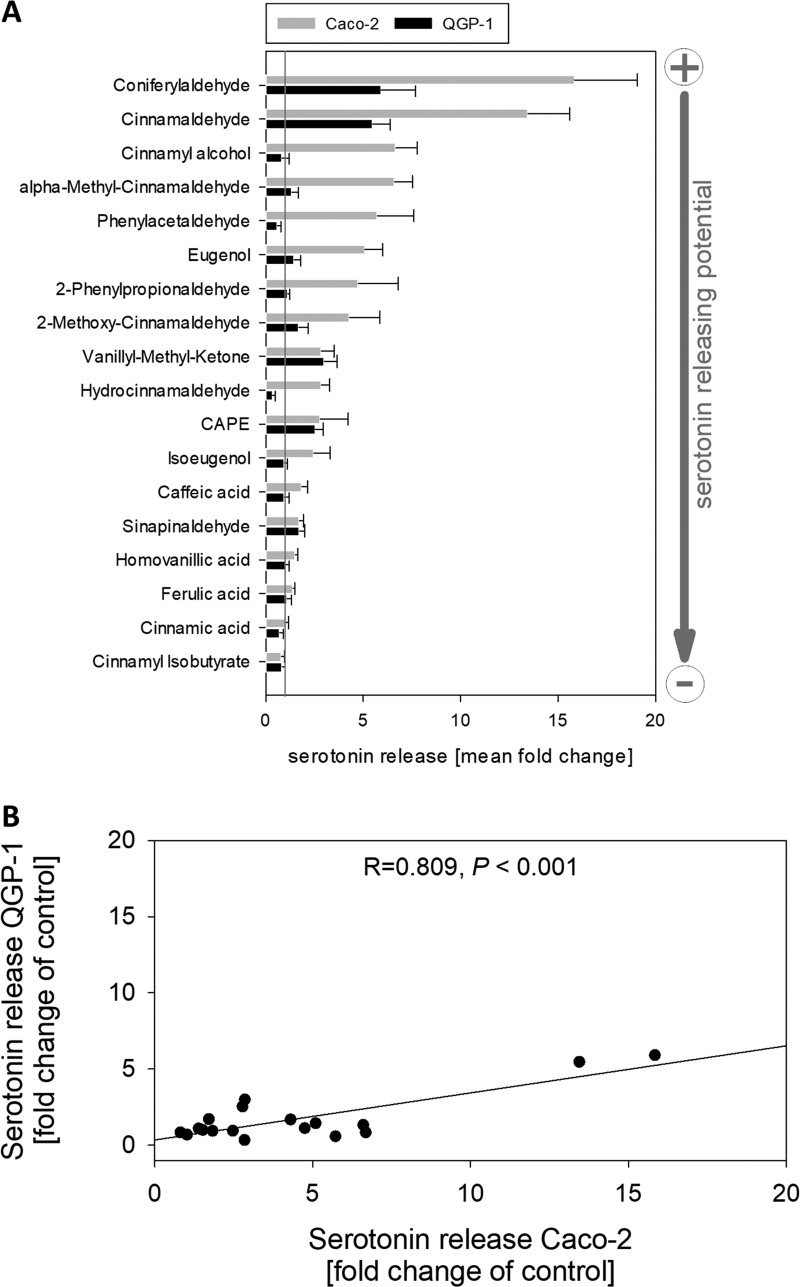
To determine which structural characteristics are important for the serotonin-releasing potential of a compound, additional 17 naturally occurring compounds that are structurally related to cinnamaldehyde were selected and tested in Caco-2 and QGP-1 cells at a concentration of 0.5 mM, namely, coniferylaldehyde, cinnamyl alcohol, vanillyl methyl ketone, sinapinaldehyde, caffeic acid phenethylester (CAPE), eugenol, 2-methoxy-cinnamaldehyde, α-methyl-cinnamaldehyde, hydrocinnamaldehyde, caffeic acid, isoeugenol, homovanillic acid, ferulic acid, cinnamic acid, cinnamyl isobutyrate, 2-phenylpropionaldehyde, and phenylacetaldehyde (see Figure 2 for an overview and the corresponding structures). The concentration of 0.5 mM was chosen as the lowest concentration that significantly increased serotonin release after stimulation with cinnamaldehyde in both tested cell models. The results of the comparison are displayed in Figure 3A. In both cell lines, coniferylaldehyde was the most potent compound, followed by the lead aroma compound cinnamaldehyde. Moreover, the serotonin-releasing potential of the test compounds in Caco-2 cells correlated with the serotonin-releasing potential of the test compounds in QGP-1 cells (Pearson’s product-moment correlation, R = 0.809, P < 0.001, n = 18, see Figure 3B).
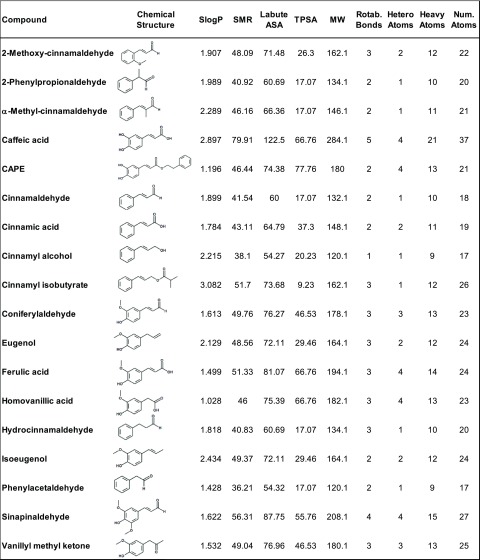
Figure 2.
Overview of the tested compounds in the alphabetical order, showing their chemical structure as well as the calculated molecular descriptors: Log of the octanol/water partition coefficient (SlogP); molecular refractivity (SMR); accessible surface area (Labute ASA); topological polar surface area (Å2) (TPSA); molecular weight (MW); and the number of rotable bonds (Rotab. Bonds), atoms, heteroatoms, and heavy atoms.
Figure 3.
(A) Serotonin release in QGP-1 enterochromaffin cells (black bars) and Caco-2 enterocytes (light gray bars) induced by cinnamaldehyde and the 17 tested structural related compounds at a concentration of 0.5 mM. Data are presented as mean fold change ± SEM calculated from three to six independent experiments with two technical replicates each. Serotonin release induced by buffer with or without 0.1% DMSO is set to 1 and represented as a gray line. The SEM values for the controls are 0.11 (control) and 0.13 (DMSO control) for QGP-1 cells and 0.25 (control) and 0.06 (DMSO control) for differentiated Caco-2 cells, respectively. (B) Correlation analysis (Pearson’s product-moment correlation) of serotonin release induced by 0.5 mM cinnamaldehyde or its structural analogues in QGP-1 enterochromaffin cells and Caco-2 enterocytes. Correlation coefficient (R) = 0.809, P < 0.001, n = 18.
Structural Determinants of Serotonin Release in Caco-2 Enterocytes
Since structural characteristics that determine serotonin release by enterocytes are largely unknown, the following matched pair analysis (see also Figure S1) focused on serotonin-releasing potential by cinnamaldehyde derivatives in Caco-2 enterocytes.
Aldehydes were more active than the corresponding acids: Stimulation with coniferylaldehyde induced the highest serotonin release (15.84 ± 3.23), in comparison to ferulic acid with a fold change of 1.39 ± 0.09 (P < 0.01). Similar to that, the second most potent compound cinnamaldehyde is more potent than cinnamic acid, with a fold change of 13.45 ± 2.15 vs 1.02 ± 0.15 (P < 0.001). In addition, exchanging the α,β,-unsaturated carbonyl group of coniferylaldehyde with an allyl group, leading to the aroma compound eugenol, reduced the serotonin-releasing potential of the phenylpropanoids by 10.7. Out of the eight tested aldehydes, sinapinaldehyde, which has two methoxy and one hydroxyl group at the phenyl ring, was the least potent aldehyde with an effect size of 1.71 ± 0.22, which is in the range of the tested acids. The test compound 2-methoxy-cinnamaldehyde with one methoxy group at the phenyl ring was also less potent than cinnamaldehyde (4.30 ± 1.55, P < 0.01) and equally potent as sinapinaldehyde (P > 0.05).
Moreover, the four most potent compounds coniferylaldehyde, cinnamaldehyde, cinnamyl alcohol, and α-methyl-cinnamaldehyde, inducing a fold change of 5 or higher, are phenylpropanoids with an unsaturated side chain. A saturated side chain, as shown by stimulation with hydrocinnamaldehyde, led to a reduced serotonin release (P < 0.05) compared to the direct unsaturated analogue, cinnamaldehyde, with a 2.84 ± 0.44 fold increase for hydrocinnamaldehyde compared to a fold change of 13.45 ± 2.15 for cinnamaldehyde.
TRPA1 Dependency of Serotonin Release in Caco-2 Cells
The identified beneficial structural characteristics to induce serotonin release in Caco-2 cells have similarities to those described for potent TRPA1 activators.22 In addition, for QGP-1 and Caco-2 cells, a stimulating effect of cinnamaldehyde on serotonin release was demonstrated to depend at least partly on TRPA1 stimulation.1,4 Thus, a potential TRPA1-dependent component for serotonin release in Caco-2 cells was analyzed here as well.
Using RT-qPCR, we confirmed the gene expression of TRPA1 in QGP-1 cells and differentiated Caco-2 cells that were used for the experiments. In relation to the reference gene HPRT1, Caco-2 enterocytes expressed with 0.55 ± 0.28% significantly higher levels of TRPA1 than QGP-1 cells (0.06 ± 0.02%, P < 0.01, data not shown in a figure). Moreover, pre-incubation for 20 min with 30 μM of the selective TRPA1 inhibitor AP-1823 reduced the response of Caco-2 cells to cinnamaldehyde by 30 ± 13.9% (Figure 4A).
Figure 4.
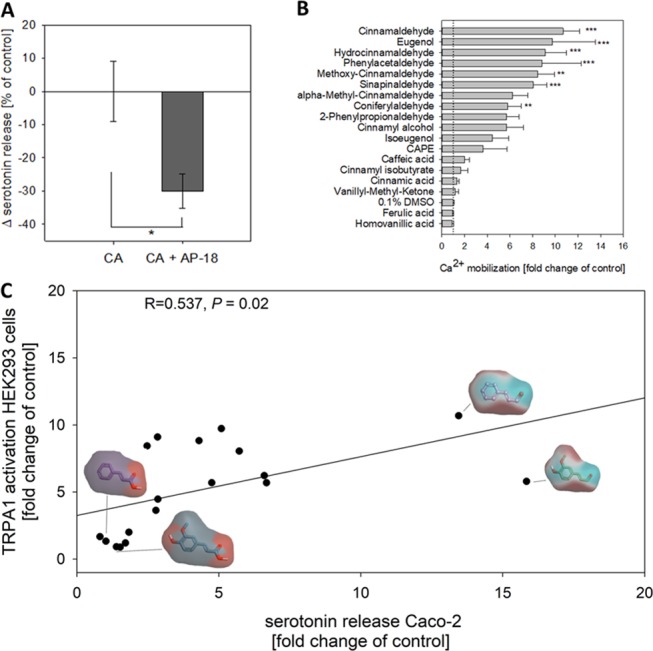
(A) Difference in serotonin release in Caco-2 enterocytes induced by 0.5 mM cinnamaldehyde (CA) solely and after 20 min pre-incubation with 30 μM of the specific TRPA1 inhibitor AP-18. Data are presented as mean fold change ± SEM calculated from three independent experiments with two technical replicates each. The significant difference (*P < 0.05) was tested using Student’s t-test. (B) Maximum Ca2+ response after stimulation with cinnamaldehyde and the 17 tested structural related compounds at a concentration of 0.5 mM in transiently hTRPA1-expressing HEK293 cells. Data are presented as mean fold change of the control ± SEM calculated from four to six independent experiments with four technical replicates each. Significant differences were analyzed using one-way ANOVA and are marked by **P < 0.01, ***P < 0.001. Mock-transfected cells showed no increase in Ca2+ after stimulation with 0.5 mM cinnamaldehyde (data not shown in the figure). (C) Correlation analysis (Pearson’s product-moment correlation) of serotonin release in Caco-2 enterocytes and the maximum Ca2+ response in transiently hTRPA1-expressing HEK293 cells induced by 0.5 mM cinnamaldehyde or its structural analogues. Correlation coefficient (R) = 0.537, P = 0.02, n = 18. Electrostatic potential maps visualizing areas of high (cyan) and low (red) electron densities are exemplarily shown for cinnamaldehyde and coniferylaldehyde as high-potency and cinnamic acid and ferulic acid as low-potency compounds.
Next, we aimed to compare the potency of the test compounds to activate TRPA1 with serotonin release in Caco-2 cells. For this purpose, HEK293 cells that transiently expressed hTRPA1 were stimulated with 0.5 mM of the test compound and the maximum Ca2+ mobilization was evaluated using Fluo-4. Mock-transfected cells were stimulated with 0.5 mM as a control and showed no increase in Ca2+ levels (data not shown). Cinnamaldehdyde, eugenol, hydrocinnamaldehyde, phenylacetaldehyde, and methoxy-cinnamaldehyde had the most pronounced effects, followed by other aldehydes (Figure 4B). The tested organic acids (caffeic acid, cinnamic acid, ferulic acid, and homovanillic acids) did not induce Ca2+ mobilization in TRPA1-expressing HEK293 cells (Figure 4B). Moreover, matched-molecular pair analysis of TRPA1 activation by the test compounds demonstrated that an α,β-unsaturated aldehyde or an allyl group is more active than the corresponding organic acid or ketone (Figure S3). The maximum Ca2+ response of hTRPA1-transfected HEK293 cells induced by stimulation with the structural analogues of cinnamaldehyde significantly correlated with the serotonin release in Caco-2 cells (Pearson’s product-moment correlation, R = 0.537, P = 0.02, Figure 4C). An electrostatic potential map exemplarily shown for the most potent compounds cinnamaldehyde and coniferylaldehyde and their respective nonpotent acids cinnamic acid and ferulic acid demonstrates the areas of high (cyan) or low (red) electron densities as a marker for their electrophilic and nucleophilic reaction sites.
Since serotonin release in Caco-2 cells is modulated by TRPA1 activation, molecular characteristics of the test compounds that are beneficial for TRPA1 stimulation were analyzed. For that purpose, several physical and surface descriptors, as well as atom and bond counts, were calculated using RDKit node for the KNIME Analytics Platform, using the R server as a backend. Using Pearson’s product-moment correlation, the relevant descriptors (>95% nonzero values, differences in values between molecules) were correlated to their potential to stimulate intracellular Ca2+ mobilization in hTRPA1-transfected HEK293 cells. The descriptors tested were log of the octanol/water partition coefficient (SlogP); molecular refractivity (SMR); accessible surface area (Labute ASA); topological polar surface area (Å2) (TPSA); molecular weight (MW); and the number of rotable bonds, atoms, heteroatoms, and heavy atoms. An overview of the compounds, structures, and descriptors can be found in Figure 2. The descriptors TPSA (−0.526, P < 0.05) and the number of heteroatoms (−0.495, P < 0.05) negatively correlated with the intracellular Ca2+ mobilization in hTRPA1-transfected HEK293 cells. In addition, there was a trend for a negative correlation of the MW (−0.477, P = 0.06) and TRPA1 activation.
Discussion
The present study aimed to clarify which structural characteristics determine serotonin release induced by the cinnamon-derived aroma compound cinnamaldehyde in intestinal cells. Therefore, serotonin release induced by cinnamaldehyde and additional 17 structurally related compounds was analyzed in QGP-1 enterochromaffin cells and Caco-2 enterocytes.
As a first step, we compared dose-dependent serotonin release induced by cinnamaldehyde in QGP-1 cells as a model for human enterochromaffin cells and differentiated Caco-2 cells as a model for human enterocytes. In both cell models, cinnamaldehyde induced a dose-dependent increase in serotonin release starting from a concentration of 0.5 mM, although the effect size in Caco-2 cells was significantly higher than that in QGP-1 cells. Based on the limited solubility of cinnamaldehyde in water, the highest test concentration applied was 5 mM for Caco-2 cells. Due to negative effects on cellular viability, concentrations up to 2.5 mM of cinnamaldehyde were tested in QGP-1 cells. With the here-tested concentrations, no saturation point was reached and, thus, no EC50 values were calculated.
As a next step, we investigated which molecular structural characteristics are advantageous for stimulating or inhibiting serotonin release by analyzing the serotonin release in the two intestinal cell models after incubation with further 17 naturally occurring compounds that are structurally related to the lead aroma compound cinnamaldehyde. Based on the dose dependency of cinnamaldehyde, a concentration of 0.5 mM was chosen to investigate serotonin release after stimulation with aroma compounds. The concentrations chosen for the present study are, although relatively high, in the typically used range of concentrations for TRPA1-based mechanistic studies.3 These concentrations do not necessarily reflect dietary doses of cinnamaldehyde, although a concentration of around 400 μM could theoretically reach enterocytes in the gastrointestinal tract following ingestion of 6 g of cinnamon powder.4 The concentrations were selected to induce a strong serotonin response to reach higher effect levels, and consequently, larger differences to analyze characteristics that will lead to an increased serotonin release in gastrointestinal cell models.
The serotonin release induced by the test compounds in Caco-2 significantly correlated with the serotonin release in QGP-1, which points to a common mechanism for structure-dependent serotonin release in the two cell models. More specifically, the matched pair analysis used in the present study revealed the following structural characteristics to be advantageous to stimulate serotonin release by enterochromaffin cells and enterocytes: In both cell models, unsaturated phenylpropanoids led to the highest effect levels. An aldehyde group was more effective than a hydroxyl group and a ketone group, which was advantageous over a carboxyl group. Due to the electronic and steric profile of the compounds, aldehydes are generally more reactive toward nucleophilic substitutions than ketones.24 This suggests electrophilic compounds with electron-withdrawing properties to be beneficial to stimulate serotonin release from intestinal cells, which is additionally suggested by the fact that the four most potent compounds are α,β-unsaturated aldehydes. Direct comparison of the efficacy of hydrocinnamaldehyde and cinnamaldehyde also confirms the importance of the double bond in the C3 side chains of phenylpropanoids. Due to the electronic profile, α,β-unsaturated aldehydes are especially prone to nucleophiles such as the −SH group in cysteine residues25 However, also, phenylacetaldehyde with a shorter side of C2 was among the most potent compounds. The substitution of the phenylethyl group with one methoxy and one hydroxyl group was beneficial, as shown for coniferylaldehyde. However, the introduction of an additional methoxy group, as demonstrated using sinapinaldehyde, largely reduced the serotonin-releasing potential. A reason for this could lie in the change in the steric profile of the compound.26
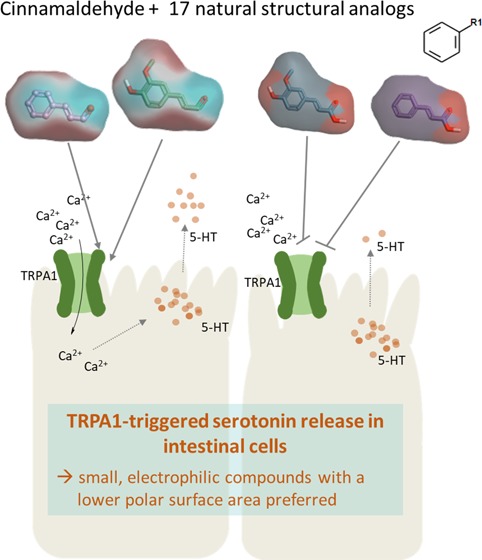
The here-identified structural characteristics are in accordance with characteristics of known TRPA1 ligands: Electrophysiological studies showed that several TRPA1 agonists are electrophilic molecules, for example, cinnamaldehyde, that activate the ion channel via covalent modification of conserved cysteine or lysine residues within the cytoplasmic N terminus.22 In that context, aldehydes are more reactive than ketones due to the more pronounced polar nature of the carbonyl group.27 Esters and carboxylic acids bear an additional oxygen that reduces the electrophilic reactivity of the respective carbonyl carbon,26 which may explain the reduced serotonin-releasing potential of the tested acids and esters in comparison to aldehydes in Caco-2 cells. This hypothesis is illustrated by a graphical representation of the electrostatic potential map of the most potent compounds, namely, cinnamaldehyde, visualizing the electrophilic reactive site (red) of the molecules at the ß-carbonyl carbon, which is lacking at the respective acids. Likewise, we detected reduced TRPA1-dependent Ca2+-mobilization after stimulation with the tested organic acids, namely, cinnamic acid, caffeic acid, homovanillic acid, and ferulic acid, and the esters, namely, CAPE and cinnamyl isobutyrate, in comparison to aldehydes using a transiently hTRPA1-transfected HEK293 cell model. This is also reflected by the finding that the compound’s potential to induce serotonin release correlates with the potential to activate TRPA1 in transiently transfected HEK293 cells. In addition, using AP-18 as a specific TRPA1 inhibitor,23 our results verify a TRPA1-dependent component in serotonin release, confirming the results from Doihara et al.1 in QGP-1 cells and Hoi et al.4 in Caco-2 cells. However, since only about 30% of the signal was blocked by AP-18, it cannot be excluded that also other pathways might play a role. These unknown additional pathways could also explain why, although there is a correlation between serotonin release in Caco-2 cells and the TRPA1 activation in a single-receptor model, there are differences in the order of the most effective compounds.
Moreover, serotonin release after stimulation with cinnamaldehyde was more pronounced in Caco-2 cells than in QGP-1 cells. The exact reason for that remains unknown and needs to be addressed in future studies. However, since serotonin release in the gastrointestinal tract is regulated by an extracellular Ca2+ influx mediated by voltage-gated Ca2+-channels,28 an influx of Ca2+ via TRPA1 may be responsible for the TRAP1-dependent component in serotonin release in Caco-2 cells. Thus, we hypothesize here that the higher effect size in Caco-2 cells compared to QGP-1 cells could be due to the higher TRPA1/HPRT ratio found in the fully differentiated Caco-2 enterocytes used in the present study. In contrast to our finding, Doihara et al.1 showed a higher TRPA1 gene expression in QGP-1 than in undifferentiated Caco-2 cells. Thus, an increase of TRPA1 gene expression upon differentiation to an enterocyte model is assumed, which might largely influence serotonin release and reuptake behavior of the cells.
To further examine the underlying structural characteristics, several molecular descriptors, representing numerical properties of the molecules, were computed independently from the molecule’s conformation based on their chemical 2D structure and compared to TRPA1 activation in transfected HEK293 cells. The descriptors’ topological polar surface area, molecular weight, and the number of heteroatoms were shown to be negatively correlated with TRPA1 activation. This points to smaller molecules with a lower polar surface area to be beneficial. As a rule of thumb, molecules with a polar surface area higher than 140 Å2 tend to have a lower cell membrane permeability.27 Since electrophilic molecules such as cinnamaldehyde and related structures target intracellularly located cysteine residues of the TRPA1 channels, the tested compounds’ ability to permeate the cell membrane is associated with the ability to activate the TRPA1 channel. Although the tested acids have a polar surface area below 140 Å2, the carboxyl group leads to a higher polarity of the molecule,26 which may reduce their potency to activate TRPA1. This is also in accordance with the observation that the tested organic acids did not induce a noteworthy response in hTRPA1-expressing HEK293 cells. However, also nonelectrophilic compounds such as menthol or carvacrol can activate the TRPA1 cation channel without modifying cysteine residues,2,29 opening the possibility for alternative pathways as well.
In transfected HEK293 cells, EC50 values for cinnamaldehyde of 61 ± 9 μM at 23 °C and 84 ± 9 μM at 35 °C for TRPA1 activation have been reported previously,3 demonstrating also the temperature sensitivity of TRPA1 channels. The here-presented results were recorded at room temperature, which might also have an impact on the results. In addition to temperature sensitivity, in respect of concentration-dependent TRPA1 activation by the test compounds, it has to be taken into account that Caco-2 and QGP-1 models are native cells, which might react differently as a single-receptor model such as transfected HEK293 cells due to the possible synergistic or agonistic pathways.
It has to be noticed that, with the present study design, it cannot be fully distinguished between an increased serotonin release and a decreased activity of the serotonin reuptake transporter (SERT). Reduced activity of SERT would lead to a decreased reuptake of serotonin and thus to increased serotonin levels in the cellular supernatant. This might also play a role in data obtained with Caco-2 cells in the present study, as a recent study by Hoi et al.4 showed that the application of cinnamaldehyde reduced the SERT activity while increasing serotonin levels in the cellular supernatant of differentiated Caco-2 cells.
In conclusion, the present study showed that the ability of a compound to activate TRPA1 is a decisive factor to stimulate serotonin release in Caco-2 enterocytes, preferring small, electrophilic compounds with a lower polar surface area. The results of the present study may serve as an important base for studying the prediction of peripheral serotonin release. Future studies are needed to show whether these results may be transferred to other compound classes as well and to confirm the results in vivo.
Acknowledgments
The financial support by the Austrian Ministry for Digital and Economic Affairs and the Symrise AG is highly acknowledged.
Glossary
Abbreviations
- ANOVA
analysis of variance
- CAPE
caffeic acid phenethylester
- TRP
transient receptor potential
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jafc.9b08163.
Cell viability of Caco-2 and QGP-1 cells after treatment with 0.5 mM test compounds used in the present study assessed via the MTT assay (Figure S1); cell viability of Caco-2 and QGP-1 cells after treatment with 0.5–5 mM cinnamaldehyde assessed via the MTT assay (Figure S2); and graphical representation of the matched-molecular pair analysis carried out with Vortex software (version 2019.04, Dotmatics Ltd., U.K.) including single atom changes and nonring fragmentations (with a maximum fragment size of 8 and a minimum core size of 8 atoms), using both experimental readouts of TRPA1 activation and serotonin secretion by Caco-2 enterocytes as parameters of interest (Figure S3) (PDF)
The authors declare the following competing financial interest(s): The authors JH and JL are employees of the company Symrise AG.
Supplementary Material
References
- Doihara H.; Nozawa K.; Kojima R.; Kawabata-Shoda E.; Yokoyama T.; Ito H. QGP-1 cells release 5-HT via TRPA1 activation; a model of human enterochromaffin cells. Mol. Cell. Biochem. 2009, 331, 239–245. 10.1007/s11010-009-0165-7. [DOI] [PubMed] [Google Scholar]
- Karashima Y.; Talavera K.; Everaerts W.; Janssens A.; Kwan K. Y.; Vennekens R.; Nilius B.; Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 1273–1278. 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M.; Story G. M.; Hwang S. W.; Viswanath V.; Eid S. R.; Petrus M. J.; Earley T. J.; Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. 10.1016/S0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Hoi J. K.; Lieder B.; Pignitter M.; Hans J.; Ley J. P.; Lietard J.; Hoelz K.; Somoza M.; Somoza V. Identification of Cinnamaldehyde as Most Effective Fatty Acid Uptake Reducing Cinnamon-Derived Compound in Differentiated Caco-2 Cells Compared to Its Structural Analogues Cinnamyl Alcohol, Cinnamic Acid, and Cinnamyl Isobutyrate. J. Agric. Food Chem. 2019, 67, 11638–11649. 10.1021/acs.jafc.9b04274. [DOI] [PubMed] [Google Scholar]
- Gershon M. D. Serotonin and its implication for the management of irritable bowel syndrome. Rev. Gastenterol. Dis. 2003, 3, S25–S34. [PubMed] [Google Scholar]
- Gershon M. D. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 14–21. 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikander A.; Rana S. V.; Prasad K. K. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin. Chim. Acta 2009, 403, 47–55. 10.1016/j.cca.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Racké K.; Reimann A.; Schwörer H.; Kilbinger H. Regulation of 5-HT release from enterochromaffin cells. Behav. Brain Res. 1995, 73, 83–87. 10.1016/0166-4328(96)00075-7. [DOI] [PubMed] [Google Scholar]
- Zhu J. X.; Wu X.; Owyang C.; Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J. Physiol. 2001, 530, 431–442. 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kidd M.; Modlin I. M.; Gustafsson B. I.; Drozdov I.; Hauso O.; Pfragner R. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G260–72. 10.1152/ajpgi.00056.2008. [DOI] [PubMed] [Google Scholar]
- Braun T.; Voland P.; Kunz L.; Prinz C.; Gratzl M. Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology 2007, 132, 1890–1901. 10.1053/j.gastro.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Wade P.; Chen J.; Jaffe B.; Kassem I.; Blakely R.; Gershon M. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J. Neurosci. 1996, 16, 2352–2364. 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zopun M.; Lieder B.; Holik A. K.; Ley J. P.; Hans J.; Somoza V. Noncaloric Sweeteners Induce Peripheral Serotonin Secretion via the T1R3-Dependent Pathway in Human Gastric Parietal Tumor Cells (HGT-1). J. Agric. Food Chem. 2018, 66, 7044–7053. 10.1021/acs.jafc.8b02071. [DOI] [PubMed] [Google Scholar]
- Nakamura K.; Sato T.; Ohashi A.; Tsurui H.; Hasegawa H. Role of a serotonin precursor in development of gut microvilli. Am. J. Pathol. 2008, 172, 333–344. 10.2353/ajpath.2008.070358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieder B.; Hoi J. K.; Holik A. K.; Geissler K.; Hans J.; Friedl B.; Liszt K.; Krammer G. E.; Ley J. P.; Somoza V. The flavanone homoeriodictyol increases SGLT-1-mediated glucose uptake but decreases serotonin release in differentiated Caco-2 cells. PLoS One 2017, 12, e0171580 10.1371/journal.pone.0171580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M.; Kozukue N.; Harden L. A. Cinnamaldehyde content in foods determined by gas chromatography-mass spectrometry. J. Agric. Food Chem. 2000, 48, 5702–5709. 10.1021/jf000585g. [DOI] [PubMed] [Google Scholar]
- Kalbe B.; Schlimm M.; Mohrhardt J.; Scholz P.; Jansen F.; Hatt H.; Osterloh S. Helional induces Ca2 + decrease and serotonin secretion of QGP-1 cells via a PKG-mediated pathway. J. Mol. Endocrinol. 2016, 57, 201–210. 10.1530/JME-16-0063. [DOI] [PubMed] [Google Scholar]
- Riedel A.; Lang R.; Rohm B.; Rubach M.; Hofmann T.; Somoza V. Structure-dependent effects of pyridine derivatives on mechanisms of intestinal fatty acid uptake: regulation of nicotinic acid receptor and fatty acid transporter expression. J. Nutr. Biochem. 2014, 25, 750–757. 10.1016/j.jnutbio.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Rohm B.; Riedel A.; Ley J. P.; Widder S.; Krammer G. E.; Somoza V. Capsaicin, nonivamide and trans-pellitorine decrease free fatty acid uptake without TRPV1 activation and increase acetyl-coenzyme A synthetase activity in Caco-2 cells. Food Funct. 2015, 6, 172–185. 10.1039/C4FO00435C. [DOI] [PubMed] [Google Scholar]
- Luo J.; Zhu Y.; Zhu M. X.; Hu H. Cell-based calcium assay for medium to high throughput screening of TRP channel functions using FlexStation 3. J. Visualized Exp. 2011, e3149 10.3791/3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Organization for Standardization . 10993–5: 2009 Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, 2009. [Google Scholar]
- Macpherson L. J.; Dubin A. E.; Evans M. J.; Marr F.; Schultz P. G.; Cravatt B. F.; Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 2007, 445, 541–545. 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- Petrus M.; Peier A. M.; Bandell M.; Hwang S. W.; Huynh T.; Olney N.; Jegla T.; Patapoutian A. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol. Pain 2007, 3, 40 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollhardt K.; Schore N.. Aldehydes and Ketones: The Carbonyl Group, In Organic Chemistry: Structure and Function; WH Freeman, 2018, Chapter 17. [Google Scholar]
- Esterbauer H.; Ertl A.; Scholz N. The reaction of cysteine with α, β-unsaturated aldehydes. Tetrahedron 1976, 32, 285–289. 10.1016/0040-4020(76)87015-9. [DOI] [Google Scholar]
- Harrold M. W.; Zavod R. M.. Basic Concepts in Medicinal Chemistry.; Taylor & Francis, 2014. [Google Scholar]
- Pajouhesh H.; Lenz G. R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. 10.1602/neurorx.2.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand P. P.; Bertrand R. L. Serotonin release and uptake in the gastrointestinal tract. Auton. Neurosci. 2010, 153, 47–57. 10.1016/j.autneu.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Lee S. P.; Buber M. T.; Yang Q.; Cerne R.; Cortes R. Y.; Sprous D. G.; Bryant R. W. Thymol and related alkyl phenols activate the hTRPA1 channel. Br. J. Pharmacol. 2008, 153, 1739–1749. 10.1038/bjp.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohm B.; Zaunschirm M.; Widder S.; Ley J.; Krammer G.; Somoza V. In Neurotransmitter-Releasing Potency of Structural Capsaicin-analogs in SH-SY5Y Cells, Hofmann T.; M W.; Schieberle P., Eds.; Proceedings of the 10th Wartburg Symposium. Advances and Challenges in Flavor Chemistry & Biology, Eisenach, 2014; Verlag Deutsche Forschungsanstalt für Lebensmittelchemie: Eisenach, 2014; pp 43–50.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.