Abstract
Two experiments were conducted to investigate the effects of exogenous catalase (CAT) in the diet of weaned piglets on growth performance, oxidative capacity, and hepatic apoptosis after challenge with lipopolysaccharide (LPS). In experiment 1, 72 weaned piglets [Duroc × Landrace × Yorkshire, 6.90 ± 0.01 kg body weight (BW), 21 d of age] were randomly assigned to be fed either a basal diet (CON group) or a basal diet supplemented with 2,000 mg/kg CAT (CAT group; dietary CAT activity, 120 U/kg) for 35 d. Blood samples were collected on day 21 and day 35. At the end of this experiment, 12 pigs were selected from each of the CON and CAT groups, and six pigs were injected with LPS (50 μg/kg BW), while the remaining six pigs were injected with an equal amount of sterile saline, resulting in a 2 × 2 factorial arrangement of treatments (experiment 2). Blood samples and rectal temperature data were collected 0 and 4 h after challenge, and liver samples were obtained after evisceration. The gain-to-feed ratio was higher (P < 0.05) in piglets in the CAT group than in those in the CON group from day 1 to 35. Catalase and total superoxide dismutase (T-SOD) activities were higher (P < 0.05), whereas malondialdehyde (MDA) concentrations were lower (P < 0.05), in piglets in the CAT group than in those in the CON group at day 35. During challenge, rectal temperature and liver MDA and H2O2 concentrations increased significantly (P < 0.05), whereas plasma CAT and glutathione peroxidase (GSH-Px) activities and liver CAT activity decreased markedly (P < 0.05), in LPS-challenged piglets 4 h post-challenge. Increased CAT activity and decreased MDA concentration were observed in the plasma and liver of piglets in the CAT group 4 h post-challenge (P < 0.05). Dietary CAT supplementation markedly suppressed the LPS-induced decrease in plasma GSH-Px activity and liver CAT activity to levels observed in the CON group (P < 0.05) as well as significantly decreasing the concentration and mRNA expression of caspase-3 and caspase-9 (P < 0.05). LPS-induced liver injury was also attenuated by dietary CAT supplementation, as demonstrated by a decrease in liver caspase-3 mRNA expression (P < 0.05). Overall, dietary supplementation with 2,000 mg/kg exogenous CAT (dietary CAT activity, 120 U/kg) improves growth performance and has a beneficial effect on antioxidant capacity in weaned piglets; alleviates oxidative stress and reduces liver damage by suppressing hepatic apoptosis in LPS-challenged piglets.
Keywords: apoptosis, catalase, growth performance, lipopolysaccharide, oxidative stress, weaned piglets
Introduction
Oxidative stress is an imbalance between the generation of reactive oxygen species (ROS) and the antioxidant defense capacity of the body (Zheng et al., 2013). Reactive oxygen species, such as superoxide and H2O2, are constantly generated from oxygen in all aerobic metabolism and pathogenic processes (Nappi and Vass, 1998). These can damage cell membranes via peroxidation, the formation of malondialdehyde (MDA), and the opening of tight junctions between enterocytes, which leads to increased intestinal permeability to endotoxins and local or systemic inflammatory reactions (Hall et al., 2001; Muccioli et al., 2010). When the balance between ROS generation and antioxidant defense mechanisms is disrupted, oxidative stress causes a cascade of reactions that may result in damage of lipids, proteins, and/or DNA (Skrzydlewska et al., 2005; Rezaie et al., 2007; Hamer et al., 2009). In piglets, multiple factors such as the environment, weaning, and infection can lead to oxidative stress, which may result in growth restriction, disease, and even death (Chen et al., 2018a, 2018c). The liver is an important metabolic organ and plays an important role in protecting against bacterial entry and bacterial products (Yi et al., 2014). Luo et al. (2016) reported that weaning increased hepatic oxidative stress and aminotransferases and initiated hepatic apoptosis in piglets. Therefore, alleviating the negative effects of oxidative stress on weaned piglets is crucial to the development of the pig industry.
Catalase (CAT) is an important antioxidative enzyme in the body that can break down H2O2 into oxygen and H2O and reduces damage to the body caused by ROS (Schrader and Fahimi, 2006). Currently, exogenous CAT extracted from animal tissues or microorganisms (Reubsaet et al., 1991; Nakamura et al., 2000; Yumoto et al., 2000) is used in the pharmaceutical, food, environmental protection, paper, textile, and other industries (Jackson et al., 1992; Zamocky et al., 2008). It has been reported that CAT activity is inhibited by ROS (Kono and Fridovich, 1982). Wang (2016) studied the ability of dietary supplementation with 0.09%, 0.12%, and 0.15% exogenous CAT to alleviate intestinal oxidative stress and improve the intestinal flora structure of rats fed a high-fat diet, and found higher CAT, total antioxidative capacity (T-AOC), total superoxide dismutase (T-SOD), and glutathione peroxidase (GSH-Px) activities in rats whose diets were supplemented with exogenous CAT. However, little information is available in the scientific literature on the effects of dietary supplementation with exogenous CAT on the growth performance, antioxidative capacity, and liver protection of weaned piglets. Models of lipopolysaccharide (LPS) immune and oxidative challenge have been demonstrated to mimic bacterial infections in weaned piglets in vivo (Zhu et al., 2015; Chen et al., 2018b). Therefore, the objective of this study was to investigate the effects of adding CAT from microbial cultures to the diet of weaned piglets challenged with LPS on growth performance, antioxidative capacity, and hepatic apoptosis.
Materials and Methods
The current study was conducted at the Research Farm of the Animal Nutrition Institute, Sichuan Agricultural University, Ya’an, China. The experimental protocols used in the present study were approved by the Animal Care and Use Committee of Sichuan Agricultural University and followed the current laws of animal protection (ethics approval code: SCAUAC201408-3).
Exogenous CAT production
Exogenous CAT was produced via fermentation by Penicillium notatum (collection number: ACCC 30443) and shown to have an activity of 60 U/g. Catalase was provided by Liaoning Vetland Bio-Technology Co., Ltd (Liaoning, China).
Experiment 1: feeding period
Animals and treatments
A total of 72 Duroc, Landrace, and Yorkshire crossbred weaned piglets (21 d of age, 36 females and 36 males) with an initial average body weight (BW) of 6.90 ± 0.01 kg were used in a two-treatment study for 35 d. Animals were housed in 12 pens containing three females and three males per pen and were randomly assigned to one of two dietary treatments (six pens per treatment). The treatments consisted of a basal diet (CON group) or a basal diet supplemented with exogenous CAT (CAT group).
Diets and management
The basal diets were formulated to meet or exceed the nutrient requirements recommended by the NRC (2012). The ingredient composition and nutrient levels of the diets are shown in Table 1. The basal diets were based on corn and soybean meal and offered to piglets according to a two-phase feeding program (day 1−21 and day 22−35). A total of 2,000 mg/kg exogenous CAT was added to the basal diets at the expense of corn, and the CAT activity of the diet was found to be 120 U/kg. Pigs were housed in a completely enclosed, temperature-controlled room containing 12 pens (1.5 × 2.5 m). Each pen was equipped with a stainless-steel nipple drinking fountain and a one-sided feeding hopper. The room temperature was set to 28 ± 1 °C for the first week and was gradually decreased to 25 °C by the end of the experiment. Pigs were fed four times daily at 0800, 1400, 1800, and 2000 h. Water and diets were provided ad libitum throughout the experimental period. Plenty of feed was placed in the hoppers to ensure feed was always available, and hoppers were checked daily to ensure ad libitum access and to minimize feed wastage (Li et al., 2015). No vaccines or antibiotics were administered to the pigs during the experimental period. Feed intake per pen was recorded daily to calculate average daily feed intake (ADFI), and pigs were weighed individually after fasting for 12 h on the mornings of day 21 and day 35 of the feeding trial to calculate average daily gain (ADG). The gain-to-feed ratio (G:F) was calculated based on ADFI and ADG.
Table 1.
Ingredients composition and nutrient levels of basal diets for Exp. 1 (as-fed basis)
| Items | Phases | |
|---|---|---|
| 1–21 d | 22–35 d | |
| Ingredients, % | ||
| Corn | 37.55 | 48.09 |
| Extruded corn | 18.00 | 15.00 |
| Soybean meal | 13.00 | 18.50 |
| Extruded soybean | 10.00 | 6.00 |
| Fish meal | 4.00 | 3.00 |
| Spray-dried plasma protein | 3.00 | 0.00 |
| Whey powder | 10.00 | 5.00 |
| Soy oil | 1.03 | 1.08 |
| Monocalcium phosphate | 0.78 | 0.66 |
| Limestone | 0.95 | 0.90 |
| Salt | 0.30 | 0.30 |
| L-Lysine HCl | 0.32 | 0.39 |
| DL-Methionine | 0.16 | 0.20 |
| L-Threonine | 0.11 | 0.16 |
| L-Tryptophan | 0.00 | 0.02 |
| Corn starch | 0.30 | 0.20 |
| Vitamin-mineral premix1 | 0.50 | 0.00 |
| Vitamin-mineral premix2 | 0.00 | 0.50 |
| Total | 100.00 | 100.00 |
| Nutrient composition3 | ||
| Digestible energy, Mcal/kg | 3.54 | 3.49 |
| Crude protein, % | 20.56 | 18.88 |
| Ca, % | 0.80 | 0.70 |
| Digestible P, % | 0.40 | 0.34 |
| Lysine, % | 1.35 | 1.24 |
| Methionine, % | 0.39 | 0.36 |
| Threonine, % | 0.79 | 0.73 |
| Tryptophan, % | 0.23 | 0.20 |
1The premix provided for per kg of feed: Zn, 100 mg; Mn, 4 mg; Fe, 100 mg; Cu, 6 mg; I, 0.14 mg; Se, 0.3 mg; choline chloride, 500 mg; vitamin A, 10,500 IU; vitamin D3, 3,300 IU; vitamin E, 22.5 IU; vitamin K3, 3 mg; vitamin B1, 3 mg; vitamin B2, 7.5 mg; vitamin B6, 4.5 mg; vitamin B12, 0.03 mg; niacin, 30 mg; pantothenate, 15 mg; folic acid, 1.5 mg; biotin, 0.12 mg.
2The premix provided for per kg of feed: Zn, 80 mg; Mn, 3 mg; Fe, 100 mg; Cu, 5 mg; I, 0.14 mg; Se, 0.25 mg; choline chloride, 400 mg; vitamin A, 10,500 IU; vitamin D3, 3,300 IU; vitamin E, 22.5 IU; vitamin K3, 3 mg; vitamin B1, 3 mg; vitamin B2, 7.5 mg; vitamin B6, 4.5 mg; vitamin B12, 0.03 mg; niacin, 30 mg; pantothenate, 15 mg; folic acid, 1.5 mg; biotin, 0.12 mg.
3All data were calculated according to the tables of Feed Composition and Nutrient Values in China (2016) in two diets.
Sampling
On the mornings of day 21 and day 35 of the experiment, fasting blood samples (approximately 10 mL per piglet) were collected via jugular venipuncture from a total of 12 piglets, including one piglet from each replicate and six replicates per treatment, into 5-mL heparinized vacutainer tubes. Plasma was obtained by centrifugation at 3,000 × g at 4 °C for 15 min and was transferred to 200-μL microcentrifuge tubes and stored at −35 °C until analysis of antioxidant enzymes was performed.
Antioxidative capacity analysis
The concentrations of oxidants and antioxidants, including T-AOC, CAT, T-SOD, GSH-Px, and MDA, were measured using specific assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the protocol described in a previous study (Chen et al., 2018c).
Statistical analyses
For all indices, data were analyzed for main effect of treatment (CON or CAT) using Student’s t-test with pen as the experimental unit using SAS version 9.0 (SAS Institute Inc., Cary, NC). Results are expressed as mean ± standard error. Normality of the data was assessed using the Shapiro–Wilk’s statistic (W > 0.05). Differences between treatments were considered significant at P < 0.05, and a tendency toward difference was considered at 0.05 < P < 0.10.
Experiment 2: challenge period
General procedures
After the feeding trial, 24 pigs (14.63 ± 0.14 kg) were selected from the CON and CAT treatment groups (12 pigs per treatment including two pigs from each replicate pen with similar BW to the pen average) to conduct subsequent challenge trials. On day 36 of the experiment, one of the two pigs in each pen was intraperitoneally injected with sterile LPS (50 μg/kg BW), while the other pig was intraperitoneally injected with an equal amount of sterile saline solution as previously described (Chen et al., 2018b). The LPS (Escherichia coli L2880; Sigma-Aldrich, St. Louis, MO) was dissolved in sterile saline (9 g/L) to obtain a 400 mg/L LPS solution. Treatments were arranged into a 2 × 2 factorial design with the main effects being CAT (0 or 2,000 mg/kg) and LPS challenge (0 or 50 μg/kg BW). The four treatment groups (six replicates per treatment with one piglet in each replicate) were as follows: 1) CON group (piglets fed the basal diet and administered sterile saline intraperitoneally); 2) CAT group (piglets fed the basal diet supplemented with 2,000 mg/kg exogenous CAT and administered sterile saline intraperitoneally); 3) CON + LPS group (piglets fed the basal diet and administered LPS intraperitoneally); and 4) CAT + LPS group (piglets fed the basal diet supplemented with 2,000 mg/kg exogenous CAT and administered LPS intraperitoneally). All chosen piglets were housed individually in metabolism cages (1.8 × 0.7 m) in a temperature-controlled room set at 25 ± 1 °C and given free access to feed and water. The rectal temperature of each piglet was recorded 0 and 4 h after LPS or saline injection.
Sampling
Blood samples were collected 0 and 4 h after LPS or saline injection, and plasma was obtained as described in experiment 1. On day 36, 4 h after administration of LPS, all 24 piglets were injected intramuscularly with 0.1 mg/kg BW Zoletile 50 Vet (Virbac, Carros, France) and immediately slaughtered for sampling (Wang et al., 2011). Approximately 2 g liver samples were obtained from each pig and stored at −80 °C until analysis.
Determination of H2O2 in liver tissue
H2O2 concentrations in liver tissues were determined using kits purchased from Beyotime Biotech (Shanghai, China) according to the manufacturer’s instructions. Briefly, liver tissues were weighed and homogenized in H2O2 lysis buffer (1:20 w/v), and supernatants were collected by centrifugation at 12,000 × g for 10 min. After the sample solution (50 μL) was incubated with reaction solution (100 μL) at room temperature for 30 min, the absorbance was read at 560 nm. H2O2 concentrations were calculated using a standard curve made using standard solutions (Luo et al., 2016).
Antioxidant enzyme activity analysis
All oxidant and antioxidant indicators in plasma and liver samples were measured following the protocol described in experiment 1.
Enzyme activities of hepatic function in plasma
The activities of alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were determined 4 h after challenge using standard spectrophotometric methods with an Autolab-PM4000 Automatic Analyzer (AMS Co., Rome, Italy). Kits were purchased from Biosino Bio-Technology and Science Inc. (Beijing, China).
Hepatic caspase activity analysis
The activities of caspase-3, caspase-8, and caspase-9 were determined using commercially available enzyme-linked immunosorbent assay kits (Nanjing Jiancheng Bioengineering Institute). Briefly, liver tissues were homogenized in 0.9% saline solution followed by centrifugation at 12,000 × g for 15 min to release the enzymes into solution. The microplates were then coated with caspase-3, caspase-8, or caspase-9. After incubation for 10 min at 37 °C, absorbance values were obtained at 450 nm using a spectrophotometer.
Gene expression
The mRNA expression levels of Fas, Bax, Bcl-2, caspase-3, caspase-8, and caspase-9 in liver samples were assessed using a CFX-96 real-time PCR detection system (Bio-Rad, Hercules, CA). Frozen liver tissue samples (50 to 100 mg) were ground to a powder in a mortar to which liquid nitrogen was continually added. Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA). RNA quality was determined by 1.0% agarose gel electrophoresis at 80 V for 25 min at a low temperature. Gels were observed under ultraviolet light for clear bands with no smearing. The absorbance of RNA solutions was measured at wavelengths of 260 nm and 280 nm using a Beckman DU-800 scanning spectrophotometer (Beckman Coulter Inc., Brea, CA). RNA concentrations were confirmed using a Beckman DU-800 nucleic-acid/protein analyzer (Beckman Coulter Inc.). cDNA was then synthesized using a commercial reverse transcription kit (TaKaRa Biotechnology, Tokyo, Japan) according to the manufacturer’s instructions and stored at −20 °C for relative quantification by polymerase chain reaction (PCR). Primer sequences used for real-time PCR were described by Chen et al. (2018c) and are shown in Table 2. The cDNA was amplified using an ABI 7900HT instrument (Applied Biosystems, Foster City, CA). The mixture (10 mL) contained 5 mL SYBR Green Supermix (TaKaRa Biotechnology), 1 mL cDNA, 0.4 mL each primer (10 mM), 0.2 mL ROX Reference Dye, and 3 mL ddH2O. The cycling conditions were as follows: predenaturation at 95 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 5 s and annealing at 60 °C for 34 s. To confirm the specificity of each product, melting curve analysis (50 °C increased to 95 °C at a rate of 0.1 °C/s with continuous fluorescence measurements) was performed. GAPDH was amplified as an internal control in parallel with the target gene, which allowed for gene normalization and quantification. All samples were measured in duplicate, and product sizes were determined by agarose gel electrophoresis. Relative mRNA abundances of the detected genes in liver samples were calculated using the 2−ΔΔ Ct method.
Table 2.
Primer sequences used for quantitative real-time PCR
| Genes | GenBank | Primer sequences, 5′-3′ | Size, bp |
|---|---|---|---|
| GAPDH | NM_001206359.1 | F: TCGGAGTGAACGGATTTGGC | 147 |
| R: TGCCGTGGGTGGAATCATAC | |||
| Fas | NM_213839 | F: TGATGCCCAAGTGACTGACC | 103 |
| R: GCAGAATTGACCCTCACGAT | |||
| Bax | XM_013998624.2 | F: GACGCTGGACTTCCTTCGAG | 334 |
| R: GTGGCCCGAGAGAGGTTTATT | |||
| Blc-2 | XM_021099593.1 | F: GCTACTTACTGCCAAAGGGA | 161 |
| R: TTCAGGCGGAGCTGTAAGAG | |||
| Caspase-3 | NM_214131.1 | F: GGAATGGCATGTCGATCTGGT | 351 |
| R: ACTGTCCGTCTCAATCCCAC | |||
| Caspase-8 | XM_011074714.1 | F: TCTGCGGACTGGATGTGATT | 165 |
| R: TCTGAGGTTGCTGGTCACAC | |||
| Caspase-9 | XM_013998997.2 | F: AATGCCGATTTGGCTTACGT | 195 |
| R: CATTTGCTTGGCAGTCAGGTT |
Statistical analyses
Individual piglet was considered the experimental unit. For all indices, data were analyzed as a 2 × 2 factorial arrangement of treatments (CAT and LPS) using the repeated statement of the MIXED procedure of SAS according to the following model:
where Yijk is the analyzed variable, μ is the mean, α i is the effect of CAT (i = 0 or 2,000 mg/kg), β j is the effect of LPS (j = sterile saline or LPS), γ k is the effect of time (k = 0 or 4 h), (αβ)ij is the interaction between CAT and LPS, (αγ)ik is the interaction between CAT and time, (βγ)jk is the interaction between LPS and time, (αβγ)ijk is the interaction between CAT, LPS, and time, and ε ijk is the residual error. When time was not included in the model, the generalized linear modeling (GLM) procedure of SAS was used with main effects of dietary CAT treatment (0 or 2,000 mg/kg) and LPS challenge (sterile saline or LPS). When CAT and LPS interactions were detected, mean separations by least square differences were used to assess treatment differences. Normality of the data was assessed using the Shapiro–Wilk’s statistic (W > 0.05). Results are expressed as means ± standard error. Differences between treatments were considered significant at P < 0.05, and a tendency toward difference was considered at 0.05 < P < 0.10.
Results
Experiment 1
Growth performance
The effects of dietary supplementation with exogenous CAT on the growth performance of weaned piglets are presented in Table 3. There were no significant differences in BW, ADG, or ADFI between piglets fed the CON diet and the CAT diet throughout the experiment (P > 0.05). However, dietary supplementation with 2,000 mg/kg CAT significantly increased the G:F of piglets compared with the CON diet from day 1 to 35 (P = 0.015).
Table 3.
Effects of dietary supplemented with exogenous CAT on growth performance of weaned piglets (Exp. 1)
| Items | CON1 | CAT1 | P-value |
|---|---|---|---|
| BW, kg | |||
| Day 0 | 6.90 ± 0.01 | 6.90 ± 0.01 | 0.977 |
| Day 21 | 8.54 ± 0.22 | 8.86 ± 0.34 | 0.425 |
| Day 35 | 14.72 ± 0.36 | 15.56 ± 0.32 | 0.120 |
| ADG, kg | |||
| Day 1–21 | 0.08 ± 0.01 | 0.09 ± 0.02 | 0.427 |
| Day 22–35 | 0.44 ± 0.01 | 0.48 ± 0.02 | 0.211 |
| Day 1–35 | 0.22 ± 0.01 | 0.25 ± 0.01 | 0.121 |
| ADFI, kg | |||
| Day 1–21 | 0.19 ± 0.01 | 0.20 ± 0.02 | 0.837 |
| Day 22–35 | 0.70 ± 0.02 | 0.73 ± 0.02 | 0.315 |
| Day 1–35 | 0.39 ± 0.01 | 0.41 ± 0.01 | 0.448 |
| G:F | |||
| Day 1–21 | 0.40 ± 0.04 | 0.46 ± 0.05 | 0.350 |
| Day 22–35 | 0.64 ± 0.01 | 0.65 ± 0.02 | 0.525 |
| Day 1–35 | 0.57 ± 0.01 | 0.60 ± 0.01 | 0.016 |
1CON, piglets fed basal diet; CAT, piglets fed basal diet supplemented with 2,000 mg/kg exogenous CAT production.
Values are mean ± standard error (n = 6). Differences between treatments were considered significant at P < 0.05.
Antioxidant capacity
As shown in Table 4, piglets fed the CAT diet had lower plasma MDA concentrations (P = 0.047) at day 21 and higher activities of CAT and T-SOD and lower MDA concentrations at day 35 than piglets fed the CON diet (P < 0.05). In addition, the activities of CAT and T-SOD tended to increase in the plasma of piglets fed the CAT diet compared with those fed the CON diet at day 21 (P < 0.10). No significant differences were observed in the activities of T-SOD or GSH-Px in plasma (P > 0.05).
Table 4.
Effects of dietary supplemented with exogenous CAT on plasma antioxidant capacity of weaned piglets (Exp. 1)
| Items | CON1 | CAT1 | P-value |
|---|---|---|---|
| CAT, U/mL | |||
| Day 21 | 8.68 ± 1.09 | 14.34 ± 2.32 | 0.091 |
| Day 35 | 9.09 ± 0.61 | 12.06 ± 0.95 | 0.039 |
| T-SOD, U/mL | |||
| Day 21 | 33.69 ± 1.21 | 36.75 ± 0.93 | 0.073 |
| Day 35 | 38.79 ± 0.29 | 40.22 ± 0.22 | 0.007 |
| GSH-Px, U/mL | |||
| Day 21 | 1,086.47 ± 36.72 | 1,099.85 ± 6.83 | 0.728 |
| Day 35 | 1,125.67 ± 33.84 | 1,138.33 ± 21.61 | 0.759 |
| T-AOC, U/mL | |||
| Day 21 | 0.56 ± 0.02 | 0.52 ± 0.05 | 0.491 |
| Day 35 | 0.59 ± 0.01 | 0.59 ± 0.01 | 0.840 |
| MDA, mmol/mL | |||
| Day 21 | 5.73 ± 0.96 | 2.78 ± 0.36 | 0.047 |
| Day 35 | 8.56 ± 2.16 | 2.15 ± 0.21 | 0.041 |
1CON, piglets fed basal diet; CAT, piglets fed basal diet supplemented with 2,000 mg/kg exogenous CAT production.
Values are mean ± standard error (n = 6). Differences between treatments were considered significant at P < 0.05.
Experiment 2
Rectal temperature
Rectal temperature data of weaned piglets challenged or not challenged with LPS are shown in Table 5. The rectal temperature of piglets increased markedly 4 h post-challenge (P < 0.05), and the rectal temperature of LPS-challenged piglets was significantly higher than that of nonchallenged piglets at 4 h (P < 0.05).
Table 5.
Changes of rectal temperature of weaned piglets challenged or not challenged with LPS (Exp. 2)1
| Items, °C | CON | CAT | P-values | ||||
|---|---|---|---|---|---|---|---|
| −LPS | +LPS | −LPS | +LPS | CAT | LPS | CAT × LPS | |
| 0 h2 | 39.33 ± 0.05 | 39.45 ± 0.06 | 39.38 ± 0.07 | 39.33 ± 0.05 | 0.565 | 0.565 | 0.159 |
| 4 h2 | 39.38 ± 0. 05 | 40.87 ± 0.07 | 39.43 ± 0.04 | 40.98 ± 0.06 | 0.146 | <0.01 | 0.551 |
1CON, piglets fed basal diet; CAT, piglets fed basal diet supplemented with 2,000 mg/kg exogenous CAT production; −LPS, piglets not challenged with LPS; +LPS, piglets challenged with LPS; CAT × LPS, interaction between CAT and LPS.
20 h and 4 h, 0 and 4 h after injecting LPS or saline. Time effect, P < 0.05.
Values are mean ± standard error (n = 6). Differences between treatments were considered significant at P < 0.05.
Antioxidant capacity in plasma
The effects of dietary supplementation with exogenous CAT on the plasma antioxidant capacity of weaned piglets challenged with LPS are shown in Table 6. At 0 and 4 h post-challenge, the activities of CAT and T-SOD increased markedly, whereas the concentration of MDA decreased, in the plasma of piglets fed the CAT diet compared with piglets fed the CON diet (P < 0.05). Moreover, LPS administration significantly decreased the plasma activities of CAT and GSH-Px and increased the activity of T-AOC and the concentration of MDA (P < 0.05) 4 h post-challenge. An interaction between CAT treatment and LPS administration was observed for plasma GSH-Px activity 4 h after challenge (P = 0.045), and GSH-Px activity was significantly lower in the CON + LPS group than in other groups (P < 0.05).
Table 6.
Effects of dietary supplemented with exogenous CAT on plasma antioxidant capacity of weaned piglets challenged with LPS (Exp. 2)1
| Items2 | CON | CAT | P-values | ||||
|---|---|---|---|---|---|---|---|
| −LPS | +LPS | −LPS | +LPS | CAT | LPS | CAT × LPS | |
| CAT, U/mL | |||||||
| 0 h | 16.38 ± 0.68 | 15.72 ± 0.74 | 19.29 ± 0.78 | 18.19 ± 0.72 | 0.002 | 0.242 | 0.762 |
| 4 h | 17.50 ± 0.64 | 11.61 ± 0.65 | 20.72 ± 1.70 | 14.27 ± 0.57 | 0.009 | <0.001 | 0.860 |
| T-SOD, U/mL | |||||||
| 0 h | 29.03 ± 1.09 | 30.75 ± 0.74 | 32.77 ± 1.15 | 31.76 ± 1.04 | 0.030 | 0.731 | 0.195 |
| 4 h | 27.37 ± 0.93 | 31.00 ± 0.80 | 32.40 ± 0.71 | 35.21 ± 1.01 | <0.001 | 0.001 | 0.644 |
| GSH-Px, U/mL | |||||||
| 0 h | 905.99 ± 23.10 | 945.10 ± 13.34 | 902.25 ± 21.62 | 954.31 ± 28.72 | 0.978 | 0.087 | 0.866 |
| 4 h | 1,074.70 ± 14.92a | 1,017.46 ± 24.76b | 1,086.03 ± 8.92a | 1,110.59 ± 11.44a | 0.398 | 0.013 | 0.045 |
| T-AOC, U/mL × 10 | |||||||
| 0 h | 5.66 ± 0.09 | 5.80 ± 0.05 | 5.71 ± 0.05 | 5.74 ± 0.05 | 0.988 | 0.232 | 0.403 |
| 4 h | 5.77 ± 0.04 | 5.88 ± 0.02 | 5.60 ± 0.13 | 5.82 ± 0.04 | 0.117 | 0.030 | 0.491 |
| MDA, mmol/mL | |||||||
| 0 h | 11.05 ± 1.05 | 12.27 ± 1.06 | 9.42 ± 1.46 | 8.82 ± 1.09 | 0.043 | 0.791 | 0.447 |
| 4 h | 15.38 ± 1.56 | 21.37 ± 2.17 | 10.51 ± 1.07 | 13.83 ± 1.49 | 0.001 | 0.009 | 0.419 |
1CON, piglets fed basal diet; CAT, piglets fed basal diet supplemented with 2,000 mg/kg exogenous CAT production; −LPS, piglets not challenged with LPS; +LPS, piglets challenged with LPS; CAT × LPS, interaction between CAT and LPS.
20 h and 4 h, 0 and 4 h after injecting LPS or saline.
a,bMeans with different superscripts differ (P < 0.05).
Values are mean ± standard error (n = 6). Differences between treatments were considered significant at P < 0.05.
Antioxidative enzyme and related product activities in the liver
The effects of dietary supplementation with exogenous CAT on the liver antioxidant capacity of weaned piglets challenged with LPS are presented in Table 7. Piglets fed the CAT diet had higher (P < 0.05) CAT activity and lower (P < 0.05) MDA and H2O2 concentrations in the liver than those fed the CON diet. LPS administration decreased (P < 0.05) liver CAT activity and increased (P < 0.05) liver H2O2 concentrations. An interaction between CAT treatment and LPS administration was detected for liver CAT activity. Liver CAT activity was significantly lower (P < 0.05) and H2O2 concentrations were significantly higher (P < 0.05) in the CON + LPS group than in the CON, CAT, and CAT + LPS groups. No significant differences were observed between the CON group and the CAT + LPS group (P > 0.05).
Table 7.
Effects of dietary supplemented with exogenous CAT on liver antioxidant capacity of weaned piglets challenged with LPS (Exp. 2)1
| Items | CON | CAT | P-values | ||||
|---|---|---|---|---|---|---|---|
| −LPS | +LPS | −LPS | +LPS | CAT | LPS | CAT × LPS | |
| CAT, U/mg protein | 95.19 ± 8.25a | 50.57 ± 7.22b | 107.26 ± 8.92a | 96.79 ± 3.25a | 0.003 | 0.002 | 0.047 |
| T-SOD, U/mg protein | 13.03 ± 2.36 | 9.54 ± 0.89 | 8.68 ± 1.77 | 21.33 ± 6.21 | 0.199 | 0.118 | 0.010 |
| GSH-Px, U/mg protein | 903.85 ± 227.40 | 1,140.68 ± 104.64 | 982.78 ± 128.88 | 1,190.85 ± 223.56 | 0.747 | 0.274 | 0.943 |
| T-AOC, U/mg protein | 0.27 ± 0.02 | 0.27 ± 0.01 | 0.24 ± 0.02 | 0.28 ± 0.01 | 0.368 | 0.123 | 0.263 |
| MDA, mmol/mg protein | 10.85 ± 2.46 | 11.16 ± 0.57 | 5.44 ± 1.07 | 7.48 ± 0.67 | 0.019 | 0.508 | 0.627 |
| H2O2, μmol/g protein | 16.34 ± 0.79b | 20.85 ± 1.29a | 15.65 ± 0.91b | 15.73 ± 0.69b | 0.006 | 0.025 | 0.030 |
1CON, piglets fed basal diet; CAT, piglets fed basal diet supplemented with 2,000 mg/kg exogenous CAT production; −LPS, piglets not challenged with LPS; +LPS, piglets challenged with LPS; CAT × LPS, interaction between CAT and LPS.
a,bMeans with different superscripts differ (P < 0.05).
Values are mean ± standard error (n = 6). Differences between treatments were considered significant at P < 0.05.
Enzyme activities of hepatic function in plasma
Enzyme activities of ALT and ALP in the plasma 4 h after challenge are shown in Table 8. The activities of plasma ALT and ALP were increased (P < 0.05) in LPS-challenged piglets compared with nonchallenged piglets. In contrast, dietary CAT supplementation attenuated increases in the activities of ALT and ALP to levels observed in the absence of dietary CAT supplementation (P < 0.05). Dietary CAT supplementation also tended to attenuate LPS-induced increases in ALT activity (P = 0.086).
Table 8.
Effects of dietary supplemented with exogenous CAT on the activities of ALT and ALP in the plasma of weaned piglets at 4 h after challenged with LPS (Exp. 2)1
| Items, U/L | CON | CAT | P-values | ||||
|---|---|---|---|---|---|---|---|
| −LPS | +LPS | −LPS | +LPS | CAT | LPS | CAT × LPS | |
| ALT | 57.83 ± 4.61 | 81.33 ± 7.26 | 53.33 ± 3.67 | 57.50 ± 5.19 | 0.015 | 0.018 | 0.086 |
| ALP | 178.50 ± 10.55 | 219.83 ± 8.57 | 171.33 ± 12.32 | 180.17 ± 10.64 | 0.039 | 0.028 | 0.141 |
1CON, piglets fed basal diet; CAT, piglets fed basal diet supplemented with 2,000 mg/kg exogenous CAT production; −LPS, piglets not challenged with LPS; +LPS, piglets challenged with LPS; CAT × LPS, interaction between CAT and LPS.
Values are mean ± standard error (n = 6). Differences between treatments were considered significant at P < 0.05.
Hepatic caspase activity
The effects of dietary supplementation with exogenous CAT on the activities of caspase-3, caspase-8, and caspase-9 in the liver of weaned piglets challenged with LPS are shown in Table 9. A significant decrease (P < 0.05) in caspase-3 and caspase-9 activities was observed in the livers of piglets fed the CAT diet compared with those fed the CON diet, whereas piglets challenged with LPS had higher (P < 0.05) caspase-3 and caspase-8 activities in the liver. In addition, dietary CAT supplementation tended to decrease caspase-8 activity in the liver (P = 0.059) and suppress the activation of LPS-induced caspase-3 (P = 0.078).
Table 9.
Effects of dietary supplemented with exogenous CAT on the activities of caspase-3, caspase-8, caspase-9 in the liver of weaned piglets challenged with LPS (Exp. 2)1
| Items, pmol/g protein | CON | CAT | P-values | ||||
|---|---|---|---|---|---|---|---|
| −LPS | +LPS | −LPS | +LPS | CAT | LPS | CAT × LPS | |
| Caspase-3 | 4.04 ± 0.66 | 5.87 ± 0.53 | 3.27 ± 0.21 | 3.38 ± 0.32 | 0.002 | 0.049 | 0.078 |
| Caspase-8 | 15.62 ± 0.99 | 18.73 ± 0.81 | 14.29 ± 0.56 | 16.81 ± 0.83 | 0.059 | 0.002 | 0.722 |
| Caspase-9 | 5.82 ± 0.43 | 5.88 ± 0.79 | 3.68 ± 0.42 | 4.97 ± 0.48 | 0.012 | 0.236 | 0.274 |
1CON, piglets fed basal diet; CAT, piglets fed basal diet supplemented with 2,000 mg/kg exogenous CAT production; −LPS, piglets not challenged with LPS; +LPS, piglets challenged with LPS; CAT × LPS, interaction between CAT and LPS.
Values are mean ± standard error (n = 6). Differences between treatments were considered significant at P < 0.05.
The mRNA expression of apoptosis regulators
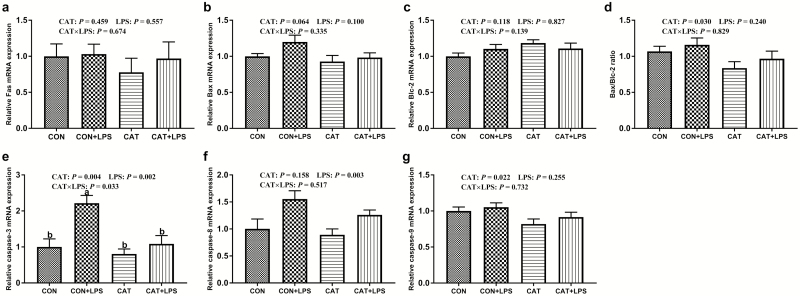
As shown in Figure 1, Bax/Bcl-2 ratios and mRNA expression of caspase-3 and caspase-9 were significantly lower (P < 0.05) in the livers of piglets fed the diet supplemented with CAT compared with those fed the diet without CAT. Liver Bax mRNA expression tended to decrease in piglets fed the diet supplemented with CAT (P = 0.064). LPS challenge markedly increased (P < 0.05) caspase-3 and caspase-8 mRNA expression in the liver, and dietary CAT supplementation suppressed the LPS-induced increase in caspase-3 mRNA expression to levels observed in piglets fed the CON diet (P < 0.05).
Figure 1.
Effects of dietary supplemented with exogenous CAT on the mRNA expression of apoptosis regulator (Exp. 2). CON, piglets fed basal diet and receiving administration of sterile saline intraperitoneally; CAT, piglets fed basal diet supplemented with 2,000 mg/kg exogenous CAT production and receiving administration of sterile saline intraperitoneally; CON + LPS, piglets fed basal diet and receiving administration of LPS intraperitoneally; CAT + LPS, piglets fed basal diet supplemented with 2,000 mg/kg exogenous CAT production and receiving administration of LPS intraperitoneally. CAT × LPS, interaction between exogenous CAT and LPS. Values are mean ± standard error (n = 6). Differences between treatments were considered significant at P < 0.05. a,bMeans with different superscripts differ (P < 0.05).
Discussion
Weaning pigs from sows is one of the most stressful events in a pig’s life and can result in postweaning stress syndrome (Campbell et al., 2013). It was previously reported that weaning can disrupt the oxidant/antioxidant equilibrium and lead to oxidative stress (Zhu et al., 2012). When this equilibrium is destroyed, cellular macromolecules are damaged and irreparable oxidative injury and cell death result (Yin et al., 2013). Oxidative stress can also weaken intestinal digestion and absorption functions and decrease the growth performance of piglets (Chen et al., 2018a). Therefore, decreasing postweaning oxidative stress in weaned piglets is crucial. Oxidative damage is usually caused by high ROS concentrations in the body (Nappi and Vass, 1998). Because CAT is an important enzyme against oxidative stress that can be inactivated by ROS-derived free radicals (Pigeolet et al., 1990), exogenous CAT has been used in the feed industry (Wang, 2016).
In the current study, the G:F during the whole period was higher in piglets fed a diet supplemented with exogenous CAT, and dietary CAT supplementation improved antioxidant enzyme (CAT and T-SOD) activity in the plasma of weaned piglets 21 and 35 d postweaning. Catalase and superoxide dismutase (SOD) play vital roles in protecting the body from oxidative damage; SOD converts ROS into H2O2, and CAT is responsible for the detoxification of H2O2 to oxygen and H2O (Finkel and Holbrook, 2000). Wang (2016) also found that serum CAT and T-SOD activities were increased in mice fed a diet supplemented with exogenous CAT. Besides, we also found that CAT supplementation decreased MDA concentrations in the plasma of weaned piglets 21 and 35 d postweaning. Because MDA is one of the major secondary decomposition products of lipid oxidation and is closely associated with cell damage, it is widely considered to be a biomarker for assessing the degree of lipid peroxidation (Bucala et al., 1993). Previously, it was reported that oxidative stress decreases ADFI, nutrient digestibility, and ADG in piglets (Toescu et al., 2002), while enhancing antioxidant capacity could improve the growth performance of weaned piglets (Chen et al., 2018a, 2018c). Therefore, it appears that exogenous CAT supplementation can improve growth performance by enhancing the antioxidative capacity of piglets. In addition, poor growth performance was observed on day 1 to 21 of the experiment in the present study, which might be related to the low feed intake caused from weaning stress induced by weaning from its sow, environmental change, and dietary shift from sow milk to solid feed (Campbell et al., 2013). Previous study has also reported that metabolizable energy (ME) intake will drop to about 60% to 70% of preweaning milk intake by the end of the first week postweaning, and it takes about 2 wk to achieve full recovery to the preweaning ME intake level (Sève, 2000). Therefore, negative growth performance often happens during the first week postweaning (Björk et al., 1988). Besides, previous studies have reported that weaning stress could damage the small intestinal barrier function (Spreeuwenberg et al., 2001) and cause higher diarrhea score in the first 3 wk postweaning (Chen et al., 2018b), which might be another reason for the poor growth performance on day 1 to 21 of the experiment.
Weaned piglets are susceptible to invasion by pathogenic bacteria, which is one of the major causes of the stress response (Sugiharto et al., 2014). To investigate whether exogenous CAT can resist oxidative stress caused by pathogenic bacteria, we used an LPS challenge model. LPS is a major component of the outer membranes of Gram-negative bacteria, and LPS-induced effects are mediated by the release of inflammatory mediators and radical oxygen intermediates in vivo (Su, 2002; Masaki et al., 2004). Therefore, LPS-challenged animals are often used as immune and oxidative stress models (Zhu et al., 2015; Chen et al., 2018b). To the best of our knowledge, this is the first study to explore the potential of exogenous CAT to modulate oxidative status and hepatic apoptosis after an LPS challenge in weaned piglets. In the present study, LPS administration significantly altered the rectal temperature, MDA concentration, and CAT, GSH-Px, and T-AOC activities of plasma in piglets 4 h post-challenge, demonstrating that an LPS-induced oxidative stress animal model was successfully established (Yi et al., 2014; Chen et al., 2018b). In addition, dietary CAT supplementation attenuated the LPS-induced decrease in GSH-Px activity to levels observed in piglets fed a diet without CAT supplementation, suggesting that piglets fed the CAT diet were better able to resist oxidative stress induced by LPS.
The liver is an important metabolic organ that plays a vital role in the metabolism and transformation of nutrients and defense against bacterial invasion and bacterial products (Yi et al., 2014). The liver is located between the absorptive surfaces of the gastrointestinal tract, and its blood supply originates mainly from the intestine via the portal vein (Luo et al., 2016). When intestinal function is disrupted, intestinal permeability increases, which leads to the translocation of metabolites to the liver and the impairment of liver function (Michalopoulos, 1990; Seki and Schnabl, 2012). Moreover, the mammalian liver contains a large number of mitochondria and is a regulator of energy homeostasis and a location of high oxygen consumption and ROS formation (Turrens, 2003; Assaad et al., 2014). Therefore, the liver is more vulnerable to oxidative stress damage (Milagro et al., 2012). In the present study, piglets fed a diet supplemented with exogenous CAT had higher CAT activity and lower MDA and H2O2 concentrations in the liver than those fed the CON diet, and piglets fed the CON diet were much more susceptible to LPS-induced oxidative stress than those fed the CAT diet, suggesting that dietary CAT supplementation improved the antioxidant capacity of the liver. The high T-SOD activity observed in the liver of piglets in the CAT + LPS group may indicate an increase in the antioxidant response of the body in order to balance liver redox homeostasis in piglets fed a diet supplemented with CAT (Droge, 2002), which is consistent with the results of Wang (2016). Oxidative stress is often associated with liver damage (Yi et al., 2014). Furthermore, it was reported that weaning induces hepatic injuries such as apoptosis (Luo et al., 2016), and that this damage could be exacerbated by LPS challenge (Masaki et al., 2004). Similarly, in the present study, higher plasma ALT and ALP activities were observed in piglets challenged with LPS, suggesting that LPS-challenged piglets may have suffered from liver injury, as ALP and ALT are released into the blood when the liver is damaged (Li et al., 2015). However, dietary CAT supplementation decreased the activities of ALT and ALP in plasma, suggesting a beneficial effect of exogenous CAT on the liver during LPS challenge.
To further explore the mechanism underlying the suppression of hepatic injury by exogenous CAT in weaned pigs, hepatic caspase activity and mRNA expression levels of apoptotic and antiapoptotic genes were evaluated. In general, the apoptosis response in cells is regulated by either the intrinsic pathway or the extrinsic pathway depending on the stimuli (Riedl and Shi, 2004; Zhu et al., 2013). The intrinsic pathway is mediated by mitochondria and is represented mainly by the activation of caspase-9 (Wang, 2001), whereas the extrinsic pathway is mediated by the activation of membrane death receptors (especially Fas) and is characterized mainly by the activation of caspase-8 (Budihardjo et al., 1999). Caspase-8 and caspase-9 are initiator caspases that can activate caspase-3, which is an effector caspase that initiates the process of apoptosis in mammals (Riedl and Shi, 2004). A previous study demonstrated that early weaning increased the activities of caspase-3, caspase-8, and caspase-9 and induced hepatic injury, which manifested as increased concentrations of ALT and glutamic-oxalacetic aminotransferase in the liver (Luo et al., 2016). LPS also stimulates caspase-3 activation through the action of caspase-8, but not caspase-9, in vivo (Alikhani et al., 2003). In the present study, we found that LPS administration increased the activities of caspase-3 and caspase-8. Moreover, lower caspase-3 and caspase-9 activities were found in the livers of piglets fed the CAT diet compared with those fed the CON diet, demonstrating that exogenous CAT can inhibit hepatic apoptosis by suppressing activation of caspase-9, thereby decreasing the activity of caspase-3. Consistent results were found for the relative mRNA expression of caspase-3, caspase-8, and caspase-9. Pro-apoptotic Bax and antiapoptotic Bcl-2, which belongs to the Bcl-2 family, are the main regulatory proteins of cell death (Reed et al., 1996). A high Bax/Bcl-2 ratio was confirmed to increase activation of caspases such as caspase-9, which is involved in the intrinsic pathway (Korsmeyer, 1999). Luo et al. (2016) reported that the relative expression ratio of Bax/Bcl-2 and the activities of caspase-3, caspase-8, and caspase-9 significantly increased 1 d postweaning. In the present study, dietary CAT supplementation decreased the Bax/Bcl-2 ratio, which further explained the beneficial effect of CAT on resistance to weaning-induced hepatic apoptosis. Overall, the present study provided evidence from a weaned pig model that CAT suppressed excessive hepatic apoptosis and limited the liver damage induced by weaning and LPS.
In conclusion, dietary supplementation with 2,000 mg/kg exogenous CAT (dietary CAT activity, 120 U/kg) improved growth performance by increasing G:F and enhancing antioxidative capacity in weaned piglets and alleviated oxidative stress and liver injury by suppressing hepatic apoptosis in LPS-challenged piglets. LPS initiated hepatic apoptosis by stimulating caspase-3 activation via the action of caspase-8, whereas exogenous CAT supplementation inhibited LPS-induced hepatic apoptosis by suppressing caspase-3 activation via decreasing the ratio of Bax/Bcl-2 and activating caspase-9. The current study provides the first evidence that exogenous CAT is potentially an effective feed additive for protecting weaned piglets from weaning stress-induced growth retardation.
Acknowledgments
The present study was supported by Sichuan Province’s 13th Five-Year Plan: Breeding Tackle Project (no. 2016NYZ0052).
Glossary
Abbreviations
- ADFI
average daily feed intake
- ADG
average daily gain
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- BW
body weight
- CAT
catalase
- G:F
gain-to-feed ratio
- GSH-Px
glutathione peroxidase
- LPS
lipopolysaccharide
- MDA
malondialdehyde
- ME
metabolizable energy
- PCR
polymerase chain reaction
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- T-AOC
total antioxidative capacity
- T-SOD
total superoxide dismutase
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Alikhani M., Alikhani Z., He H., Liu R., Popek B. I., and Graves D. T.. . 2003. Lipopolysaccharides indirectly stimulate apoptosis and global induction of apoptotic genes in fibroblasts. J. Biol. Chem. 278:52901–52908. doi: 10.1074/jbc.M307638200 [DOI] [PubMed] [Google Scholar]
- Assaad H., Yao K., Tekwe C. D., Feng S., Bazer F. W., Zhou L., Carroll R. J., Meininger C. J., and Wu G.. . 2014. Analysis of energy expenditure in diet-induced obese rats. Front. Biosci. (Landmark Ed). 19:967–985. doi: 10.2741/4261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk A., Olsson N. G., Christensson E., Martinsson K., and Olsson O.. . 1988. Effects of amperozide on biting behavior and performance in restricted-fed pigs following regrouping. J. Anim. Sci. 66:669–675. doi: 10.2527/jas1988.663669x [DOI] [PubMed] [Google Scholar]
- Bucala R., Makita Z., Koschinsky T., Cerami A., and Vlassara H.. . 1993. Lipid advanced glycosylation: pathway for lipid oxidation in vivo. Proc. Natl. Acad. Sci. USA 90:6434–6438. doi: 10.1073/pnas.90.14.6434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budihardjo I., Oliver H., Lutter M., Luo X., and Wang X.. . 1999. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 15:269–290. doi: 10.1146/annurev.cellbio.15.1.269 [DOI] [PubMed] [Google Scholar]
- Campbell J. M., Crenshaw J. D., and Polo J.. . 2013. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 4:19. doi: 10.1186/2049-1891-4-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Li Y., Yu B., Chen D., Mao X., Zheng P., Luo J., and He J.. . 2018a. Dietary chlorogenic acid improves growth performance of weaned pigs through maintaining antioxidant capacity and intestinal digestion and absorption function. J. Anim. Sci. 96:1108–1118. doi: 10.1093/jas/skx078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Li S., Zheng J., Li W., Jiang X., Zhao X., Li J., Che L., Lin Y., Xu S., . et al. 2018b. Effects of dietary Clostridium butyricum supplementation on growth performance, intestinal development, and immune response of weaned piglets challenged with lipopolysaccharide. J. Anim. Sci. Biotechnol. 9:62. doi: 10.1186/s40104-018-0275-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Yu B., Chen D., Huang Z., Mao X., Zheng P., Yu J., Luo J., and He J.. . 2018c. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 59:84–92. doi: 10.1016/j.jnutbio.2018.06.005 [DOI] [PubMed] [Google Scholar]
- Droge W. 2002. Free radicals in the physiological control of cell function. Physiol. Rev. 82:47–95. doi: 10.1152/physrev.00018.2001 [DOI] [PubMed] [Google Scholar]
- Finkel T., and Holbrook N. J.. . 2000. Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247. doi: 10.1038/35041687 [DOI] [PubMed] [Google Scholar]
- Hall D. M., Buettner G. R., Oberley L. W., Xu L., Matthes R. D., and Gisolfi C. V.. . 2001. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol. Heart Circ. Physiol. 280:H509–H521. doi: 10.1152/ajpheart.2001.280.2.H509 [DOI] [PubMed] [Google Scholar]
- Hamer H. M., Jonkers D. M., Bast A., Vanhoutvin S. A., Fischer M. A., Kodde A., Troost F. J., Venema K., and Brummer R. J.. . 2009. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin. Nutr. 28:88–93. doi: 10.1016/j.clnu.2008.11.002 [DOI] [PubMed] [Google Scholar]
- Jackson R. M., Russell W. J., and Veal C. F.. . 1992. Endogenous and exogenous catalase in reoxygenation lung injury. J. Appl. Physiol. (1985). 72:858–864. doi: 10.1152/jappl.1992.72.3.858 [DOI] [PubMed] [Google Scholar]
- Kono Y., and Fridovich I.. . 1982. Superoxide radical inhibits catalase. J. Biol. Chem. 257:5751–5754. [PubMed] [Google Scholar]
- Korsmeyer S. J. 1999. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 59(7 Suppl.): 1693s–1700s. [PubMed] [Google Scholar]
- Li Y., Chen L., Lin Y., Fang Z. F., Che L. Q., Xu S. Y., and Wu D.. . 2015. Effects of replacing soybean meal with detoxified Jatropha curcas kernel meal in the diet on growth performance and histopathological parameters of growing pigs. Anim. Feed Sci. Tech. 204:18–27. doi: 10.1016/j.anifeedsci.2015.02.002 [DOI] [Google Scholar]
- Luo Z., Zhu W., Guo Q., Luo W., Zhang J., Xu W., and Xu J.. . 2016. Weaning induced hepatic oxidative stress, apoptosis, and aminotransferases through MAPK signaling pathways in piglets. Oxid. Med. Cell. Longev. 2016:1–10. doi: 10.1155/2016/4768541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki T., Chiba S., Tatsukawa H., Yasuda T., Noguchi H., Seike M., and Yoshimatsu H.. . 2004. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology 40:177–184. doi: 10.1002/hep.20282 [DOI] [PubMed] [Google Scholar]
- Michalopoulos G. K. 1990. Liver regeneration: molecular mechanisms of growth control. FASEB J. 213:286–300. doi: 10.1096/fasebj.4.2.2404819 [DOI] [PubMed] [Google Scholar]
- Milagro F. I., Campión J., and Martínez J. A.. . 2012. Weight gain induced by high-fat feeding involves increased liver oxidative stress. Obesity 14:1118–1123. doi: 10.1038/oby.2006.128 [DOI] [PubMed] [Google Scholar]
- Muccioli G. G., Naslain D., Bäckhed F., Reigstad C. S., Lambert D. M., Delzenne N. M., and Cani P. D.. . 2010. The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 6:392. doi: 10.1038/msb.2010.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Watanabe M., Sasaki Y., and Ikeda T.. . 2000. Purification and characterization of liver catalase in acatalasemic beagle dog: comparison with normal dog liver catalase. Int. J. Biochem. Cell Biol. 32:89–98. doi: 10.1016/s1357-2725(99)00110-7 [DOI] [PubMed] [Google Scholar]
- Nappi A. J., and Vass E.. . 1998. Hydroxyl radical formation via iron-mediated Fenton chemistry is inhibited by methylated catechols. Biochim. Biophys. Acta 1425:159–167. doi: 10.1016/s0304-4165(98)00062-2 [DOI] [PubMed] [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. 11th rev. ed. Natl. Acad. Press, Washington, DC. [Google Scholar]
- Pigeolet E., Corbisier P., Houbion A., Lambert D., Michiels C., Raes M., Zachary M. D., and Remacle J.. . 1990. Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech. Ageing Dev. 51:283–297. doi: 10.1016/0047-6374(90)90078-t [DOI] [PubMed] [Google Scholar]
- Reed J. C., Miyashita T., Takayama S., Wang H. G., Sato T., Krajewski S., Aimé-Sempé C., Bodrug S., Kitada S., and Hanada M.. . 1996. BCL-2 family proteins: regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J. Cell. Biochem. 60:23–32. doi: [DOI] [PubMed] [Google Scholar]
- Reubsaet F. A., Veerkamp J. H., Brückwilder M. L., Trijbels J. M., and Monnens L. A.. . 1991. Peroxisomal oxidases and catalase in liver and kidney homogenates of normal and di(ethylhexyl)phthalate-fed rats. Int. J. Biochem. 23:961–967. doi: 10.1016/0020-711x(91)90086-3 [DOI] [PubMed] [Google Scholar]
- Rezaie A., Parker R. D., Abdollahi M.. . 2007. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Digest. Dis. Sci. 52:2015. doi: 10.1007/s10620-006-9622-2 [DOI] [PubMed] [Google Scholar]
- Riedl S. J., and Shi Y.. . 2004. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 5:897–907. doi: 10.1038/nrm1496 [DOI] [PubMed] [Google Scholar]
- Schrader M., and Fahimi H. D.. . 2006. Peroxisomes and oxidative stress. Biochim. Biophys. Acta 1763:1755–1766. doi: 10.1016/j.bbamcr.2006.09.006 [DOI] [PubMed] [Google Scholar]
- Seki E., and Schnabl B.. . 2012. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J. Physiol. 590:447–458. doi: 10.1113/jphysiol.2011.219691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sève B. 2000. Effects of underfeeding during the weaning period on growth, metabolism, and hormonal adjustments in the piglet. Domest. Anim. Endocrine. 19:63–74. doi: 10.1016/s0739-7240(00)00067-9 [DOI] [PubMed] [Google Scholar]
- Skrzydlewska E., Sulkowski S., Koda M., Zalewski B., Kanczuga-Koda L., and Sulkowska M.. . 2005. Lipid peroxidation and antioxidant status in colorectal cancer. World J. Gastroenterol. 11:403–406. doi: 10.3748/wjg.v11.i3.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreeuwenberg M. A., Verdonk J. M., Gaskins H. R., and Verstegen M. W.. . 2001. Small intestine epithelial barrier function is compromised in pigs with low feed intake at weaning. J. Nutr. 131:1520–1527. doi: 10.1093/jn/131.5.1520 [DOI] [PubMed] [Google Scholar]
- Su G. L. 2002. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am. J. Physiol. Gastrointest. Liver Physiol. 283:G256–G265. doi: 10.1152/ajpgi.00550.2001 [DOI] [PubMed] [Google Scholar]
- Sugiharto S., Hedemann M. S., and Lauridsen C.. . 2014. Plasma metabolomic profiles and immune responses of piglets after weaning and challenge with E. coli. J. Anim. Sci. Biotechnol. 5:17. doi: 10.1186/2049-1891-5-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu V., Nuttall S. L., Martin U., Kendall M. J., and Dunne F.. . 2002. Oxidative stress and normal pregnancy. Clin. Endocrinol. 57:609–613. doi: 10.1046/j.1365-2265.2002.01638.x [DOI] [PubMed] [Google Scholar]
- Turrens J. F. 2003. Mitochondrial formation of reactive oxygen species. J. Physiol. 552(Pt 2):335–344. doi: 10.1113/jphysiol.2003.049478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. 2001. The expanding role of mitochondria in apoptosis. Genes Dev. 15:2922–2933. [PubMed] [Google Scholar]
- Wang H. 2016. Effects of exogenous catalase on tissue antioxidant capacity and intestinal microflora. Doctoral dissertation, Jiangnan University. [Google Scholar]
- Wang J. P., Yoo J. S., Jang H. D., Lee J. H., Cho J. H., and Kim I. H.. . 2011. Effect of dietary fermented garlic by Weissella koreensis powder on growth performance, blood characteristics, and immune response of growing pigs challenged with Escherichia coli lipopolysaccharide. J. Anim. Sci. 89:2123–2131. doi: 10.2527/jas.2010-3186 [DOI] [PubMed] [Google Scholar]
- Yi D., Hou Y., Wang L., Ding B., Yang Z., Li J., Long M., Liu Y., and Wu G.. . 2014. Dietary N-acetylcysteine supplementation alleviates liver injury in lipopolysaccharide-challenged piglets. Br. J. Nutr. 111:46–54. doi: 10.1017/S0007114513002171 [DOI] [PubMed] [Google Scholar]
- Yin J., Ren W., Wu X., Yang G., Wang J., Li T., Ding J., Cai L., Su D.. . 2013. Oxidative stress-mediated signaling pathways: a review. J. Food Agric. Environ. 11:132–139. [Google Scholar]
- Yumoto I., Ichihashi D., Iwata H., Istokovics A., Ichise N., Matsuyama H., Okuyama H., and Kawasaki K.. . 2000. Purification and characterization of a catalase from the facultatively psychrophilic bacterium Vibrio rumoiensis S-1(T) exhibiting high catalase activity. J. Bacteriol. 182:1903–1909. doi: 10.1128/jb.182.7.1903-1909.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamocky M., Furtmüller P. G., and Obinger C.. . 2008. Evolution of catalases from bacteria to humans. Antioxid. Redox Signal. 10:1527–1548. doi: 10.1089/ars.2008.2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P., Yu B., He J., Tian G., Luo Y., Mao X., Zhang K., Che L., and Chen D.. . 2013. Protective effects of dietary arginine supplementation against oxidative stress in weaned piglets. Br. J. Nutr. 109:2253–2260. doi: 10.1017/S0007114512004321 [DOI] [PubMed] [Google Scholar]
- Zhu L., Cai X., Guo Q., Chen X., Zhu S., and Xu J.. . 2013. Effect of N-acetyl cysteine on enterocyte apoptosis and intracellular signalling pathways’ response to oxidative stress in weaned piglets. Br. J. Nutr. 110:1938–1947. doi: 10.1017/S0007114513001608 [DOI] [PubMed] [Google Scholar]
- Zhu C., Wu Y., Jiang Z., Zheng C., Wang L., Yang X., Ma X., Gao K., and Hu Y.. . 2015. Dietary soy isoflavone attenuated growth performance and intestinal barrier functions in weaned piglets challenged with lipopolysaccharide. Int. Immunopharmacol. 28:288–294. doi: 10.1016/j.intimp.2015.04.054 [DOI] [PubMed] [Google Scholar]
- Zhu L. H., Zhao K. L., Chen X. L., and Xu J. X.. . 2012. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 90:2581–2589. doi: 10.2527/jas.2012-4444 [DOI] [PubMed] [Google Scholar]