Abstract
Background
Microcytosis (smaller than normal red blood cells) has previously been identified as a possible early risk marker for some cancers. However, the role of microcytosis across all cancers has not been fully investigated.
Aim
To examine cancer incidence in a cohort of patients with microcytosis, with and without accompanying anaemia.
Design and setting
Cohort study of patients aged ≥40 years using UK primary care electronic patient records.
Method
The 1-year cancer incidence was compared between cohorts of patients with a mean red cell volume of <85 femtolitres (fL) (low) or 85–101 fL (normal). Further analyses examined sex, age group, cancer site, and haemoglobin values.
Results
Of 12 289 patients with microcytosis, 497 had a new cancer diagnosis within 1 year (4.0%, 95% confidence interval [CI] = 3.7 to 4.4), compared with 1465 of 73 150 without microcytosis (2.0%, CI = 1.9 to 2.1). In males, 298 out of 4800 with microcytosis were diagnosed with cancer (6.2%, CI = 5.5 to 6.9), compared with 940 out of 34 653 without (2.7%, CI = 2.5 to 2.9). In females with microcytosis, 199 out of 7489 were diagnosed with cancer (2.7%, CI = 2.3 to 3.1), compared with 525 out of 38 497 without (1.4%, CI = 1.3 to 1.5). In patients with microcytosis but normal haemoglobin, 86 out of 2637 males (3.3%, CI = 2.6 to 4.0) and 101 out of 5055 females (2.0%, CI = 1.6 to 2.4) were diagnosed with cancer.
Conclusion
Microcytosis is a predictor of underlying cancer even if haemoglobin is normal. Although a benign explanation is more likely, clinicians in primary care should consider simple testing for cancer on encountering unexplained microcytosis, particularly in males.
Keywords: cancer, diagnosis, early detection of cancer, general practice, microcytosis, primary health care
INTRODUCTION
There were 163 444 deaths from cancer in the UK in 2016,1 accounting for more than one-quarter (28%) of all UK deaths.2 Although cancer survival rates are improving, the UK still lags behind many other economically developed countries3,4 and has generally lower survival rates than comparable European countries.5 These differences are due in part to delays in diagnosis,3 with cancers in the UK diagnosed at a later stage compared with other European countries.6 The target of the NHS Long Term Plan, released in 2019, is that by 2028 the proportion of cancers diagnosed at stages 1 and 2 will rise from the current figure of around one-half to three-quarters.7 It is important for primary care clinicians to recognise features of possible cancer in order to investigate appropriately.8
Some previous primary care research studies have found a number of blood-test features to be associated with cancer, which could act as early risk markers. These include thrombocytosis,9 raised inflammatory markers,10 hypoalbuminaemia,11 and hypercalcaemia.12 Thrombocytosis was more commonly associated with patients who had lung and colorectal cancers, and one-third of the patients with lung or colorectal cancer and thrombocytosis had no other symptoms indicating malignancy.9 A study into early detection of multiple myeloma in primary care found an association between myeloma and macrocytosis.13
Previous studies have recently identified microcytosis (smaller than normal red blood cells) as a potential early risk marker for certain cancers including: lymphoma,14 oesophago-gastric,15 colorectal,16 and kidney cancer.17 These risks were independent of any anaemia. The precise role of microcytosis in primary care across all cancers is not currently known, particularly in patients without anaemia. This study aims to investigate the role of microcytosis as a risk marker for all cancers.
METHOD
Data sources
This cohort study used electronic patient records from the Clinical Practice Research Datalink (CPRD), which holds anonymised primary care records from a network of over 1400 UK practices. It includes information on symptoms, referrals, and laboratory tests.18 The cases for this study were derived from the control sample of previously published CPRD studies.12,19 CPRD cases were patients aged ≥40 years with a record of cancer at 1 of 13 cancer sites between 2000 and 2009. Each case was matched to five controls with no record of the cancer of interest at the diagnosis date of the case, but controls could have had any other cancer. Matching was done by sex, practice, and year of birth.12,19
Patient sample
In total, 108 993 patients with a mean cell volume (MCV) result between 2006 and 2008, and who were aged ≥40 years at the time of testing, were studied. A starting point of 2006 was chosen to account for the introduction of the Quality and Outcomes Framework, and the introduction in 2005 of the National Institute for Health and Care Excellence guideline on referral for suspected cancer.20 A cut-off of 2008 allowed for 1 year of follow-up for looking at cancer diagnoses. Patients were grouped according to whether they had microcytosis or a normal MCV. An upper boundary for microcytosis was chosen as 85 femtolitres (fL) due to the common use of that value as a threshold in UK practice, though 80 fL is commonly used in North America.21 Patients with MCV values <50 fL were excluded for two reasons: the result could have been erroneous, and, even if not, clinically such patients are likely to warrant investigation on such an extreme finding alone. The index date was defined as the date of the first MCV result. A comparison subcohort of patients with a normal MCV, defined as 85–101 fL, was used with the same age and date criteria. Values >101 fL were defined as macrocytosis and were therefore excluded. Haemoglobin values reported on the same day as the MCV were also identified. Patients diagnosed with cancer (other than non-melanoma skin cancer) before the index date were excluded from both study and comparison groups.
How this fits in
| Microcytosis has long been recognised as being comorbid with iron deficiency and with haemoglobinopathies. Similarly, iron deficiency has been identified as a feature of some cancers, particularly colorectal. However, the relationship between microcytosis and other cancers is largely unknown, including the importance of microcytosis without anaemia. This study found an overall cancer risk of 6.2% in males aged ≥40 years, and 2.7% in females aged ≥40 years, with colorectal and lung cancer being the most common. Furthermore, even with a normal haemoglobin count, microcytosis represents a small but significant risk of underlying cancer. |
Cancer outcomes
New diagnoses of cancer (other than non-melanoma skin cancer) within 1 year of the index date were found by searching the patient records, using a previously published list of cancer codes (available from the authors on request).
Statistical methods and analysis
The primary analysis was the 1-year cancer incidence, expressed as a percentage (with 95% confidence intervals [CI]) for patients in the microcytosis group and for the normal MCV group. This 1-year incidence could be regarded as a positive predictive value for microcytosis.
Further subanalyses were performed by sex, age group, and cancer site. Additional analyses examined: 1) the incidence in patients with a second MCV test result within 3 months and 6 months of the index test; and 2) the incidence in the microcytosis group if the upper threshold were lowered to 80 fL. Cancer incidences in patients with microcytosis with and without anaemia are also reported. Anaemia was defined as <13.0 grams per decilitre (g/dL) for males and <11.5 g/dL for females. Analyses were performed using Stata (version 15).
RESULTS
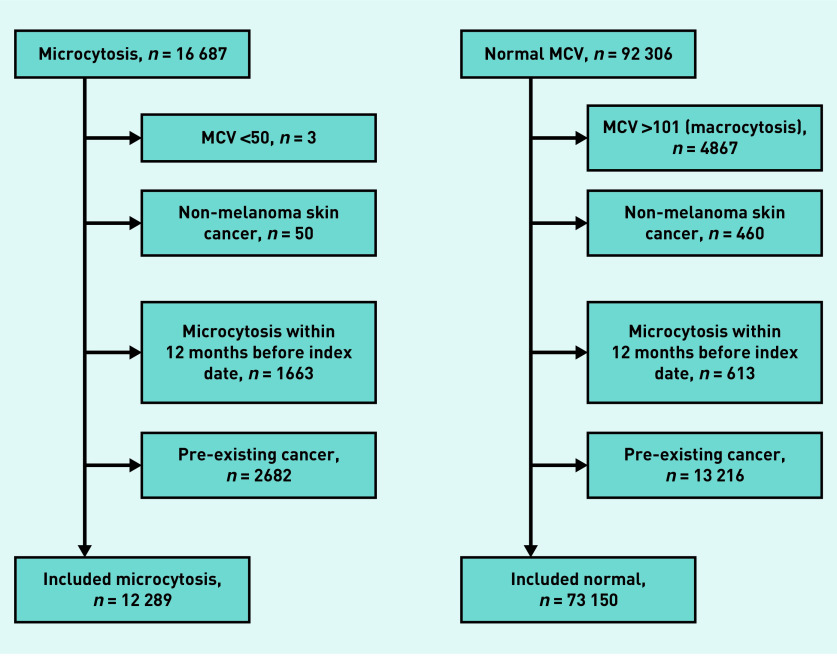
After all exclusions, there were 85 439 participants: 12 289 with microcytosis and 73 150 with a normal MCV (Figure 1). In the microcytosis cohort, the median age was 73 years (interquartile range [IQR] = 64 to 81) and 4800 (39.1%) were male. In the normal cohort, the median age was 71 years (IQR = 63 to 79) and 34 653 (47.4%) being male.
Figure 1.
Exclusions flow diagram.
MCV = mean cell volume.
Cancer diagnoses
Of the patients in the microcytosis group, 497 had a cancer diagnosis, representing a 1-year cancer incidence of 4.0% (CI = 3.7 to 4.4). In the normal group, 1465 patients were diagnosed with cancer, a 1-year cancer incidence of 2.0% (CI = 1.9 to 2.1). The median age at cancer diagnosis in the microcytosis group was 76 years (IQR = 70 to 83) and for the normal group it was 75 years (IQR = 68 to 81).
Sex
The 1-year cancer incidence was higher in males for both groups with 298 of 4800 males having microcytosis (6.2%, CI = 5.5 to 6.9), and 199 of 7489 females (2.7%, CI = 2.3 to 3.1). In males with a normal MCV, 940 of 34 653 were diagnosed with cancer (2.7%, CI = 2.5 to 2.9), as were 525 of 38 497 females (1.4%, CI = 1.3 to 1.5) (Table 1). The cancer incidence with microcytosis was higher than that with a normal MCV across both age groups: the highest cancer incidence being in males aged ≥70 years with microcytosis, with 225 out of 3008 developing cancer (7.5%, CI = 6.6 to 8.5) (data not shown).
Table 1.
The number of patients in the cohort, the number with cancer, and the cancer incidence for males, females, 40–69 year olds, and ≥70 year olds, in the microcytosis and normal MCV cohorts
| Microcytosis | Normal MCV | |||||
|---|---|---|---|---|---|---|
| N | With cancer | Cancer incidence, % (95% CI) | N | With cancer | Cancer incidence, % (95% CI) | |
| Males | 4800 | 298 | 6.21 (5.54 to 6.93)a | 34 653 | 940 | 2.71 (2.54 to 2.89) |
| Females | 7489 | 199 | 2.66 (2.30 to 3.05) | 38 497 | 525 | 1.36 (1.25 to 1.48) |
| Aged 40–69 | 4647 | 125 | 2.69 (2.24 to 3.20) | 32 631 | 437 | 1.34 (1.22 to 1.47) |
| Aged 70 | 7642 | 372 | 4.87 (4.40 to 5.37)a | 40 519 | 1028 | 2.54 (2.39 to 2.69) |
Incidence >3% NICE threshold for referral. MCV = mean cell volume. NICE = National Institute for Health and Care Excellence.
Cancer sites
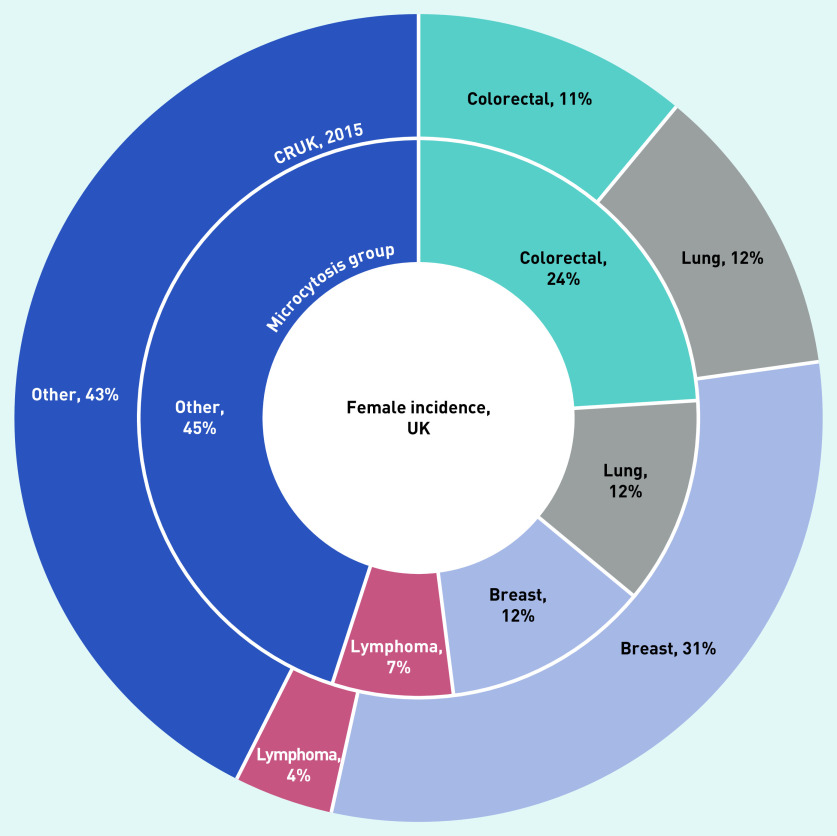
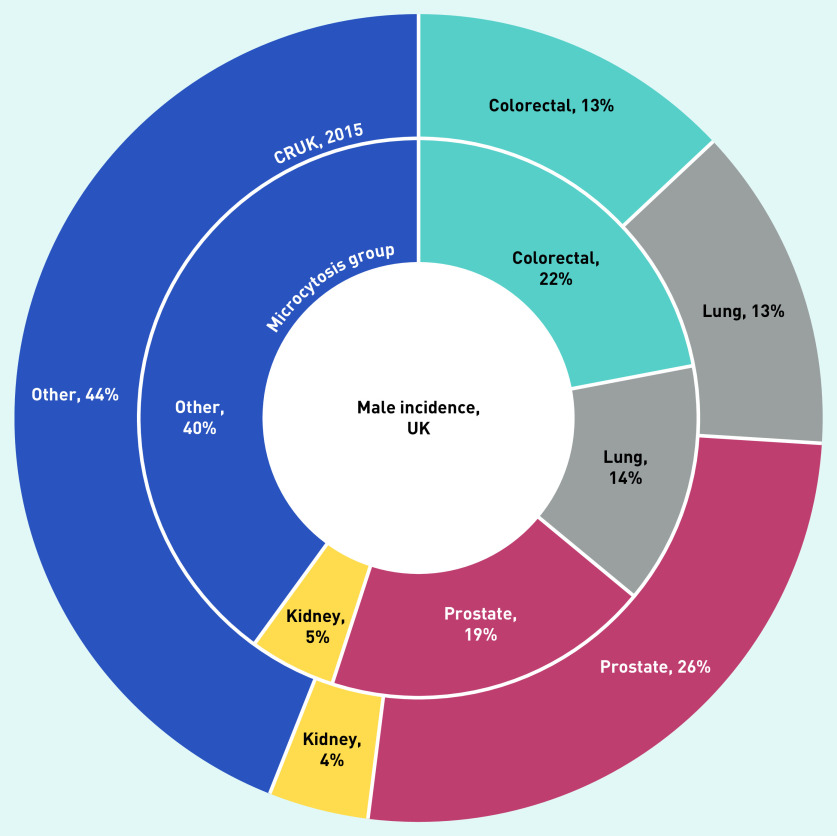
The cancer sites for the two sexes are shown in Figures 2 and 3. Cancer sites that made up a greater proportion of cancers diagnosed in the microcytosis cohort than the normal cohort were: colorectal (113, 23%), lung (67, 13%), lymphoma (24, 5%), kidney (22, 4%), and stomach (15, 3%) (data not shown).
Figure 2.
The most commonly diagnosed cancer types in females with microcytosis compared with the general UK female population (from CRUK).a
aThe inner ring shows the proportions of the most common cancer types in the microcytosis cohort. The outer ring shows the proportions of incidences of these types in the general population in 2015. CRUK = Cancer Research UK.
Figure 3.
The most commonly diagnosed cancer types in males with microcytosis compared with the general UK male population (from CRUK).a
aThe inner ring shows the proportions of the most common cancer types in the microcytosis cohort. The outer ring shows the proportions of incidences of these types in the general population in 2015. CRUK = Cancer Research UK.
In the microcytosis cohort, 3187 participants had a second MCV result within 3 months of the index date (74.6% of these also showed microcytosis). In those who remained microcytic, 175 out of 2377 were diagnosed with cancer (7.4%, CI = 6.3 to 8.5) compared with 27 of 809 (3.3%, CI = 2.2 to 4.8) in those whose second MCV was within the normal range. Similar figures were found for repeat blood tests within 6 months (data not shown).
Re-analysis using 80 fL as the upper limit for the microcytosis group increased the cancer incidence in those with an ‘abnormal’ result to 190 out of 2940 overall (6.5%, CI = 5.6 to 7.4), with 120 of 1101 males (10.9%, CI = 9.1 to 12.9) and 70 of 1839 females (3.8%, CI = 3.0 to 4.8). In the microcytosis group, the median period between the index date and cancer diagnosis was 80 days, whereas in the normal MCV group the median period to cancer diagnosis was 113 days (data not shown).
Concomitant anaemia
In the microcytosis group, 2162 of 4799 (45.1%) males and 2433 of 7488 (32.5%) females also had anaemia at the index date. Two cases apparently had a second blood test on the index date yielding a discordant result. These two were omitted from this subanalysis. In those patients with microcytosis and anaemia, 212 of 2162 males (9.8%, CI = 8.6 to 11.1) and 98 of 2433 females (4.0%, CI = 3.3 to 4.9) were diagnosed with cancer. In those patients with microcytosis and normal haemoglobin, 86 of 2637 males (3.3%, CI = 2.6 to 4.0) and 101 of 5055 females (2.0%, CI = 1.6 to 2.4) were diagnosed with cancer within 1 year (data not shown). Colorectal cancer was the most common cancer in all females and males with both microcytosis and anaemia, whereas prostate cancer was the most common in males with microcytosis only (Table 2).
Table 2.
The three most common cancer sites in males and females with both microcytosis and anaemia and microcytosis only, the proportion of the group without cancer, and the proportion with cancer at each site
| Male | Female | |||
|---|---|---|---|---|
| Microcytosis and anaemia, N = 2162, n (%) | Microcytosis only, N = 2637, n (%) | Microcytosis and anaemia, N = 2433, n (%) | Microcytosis only, N = 5055, n (%) | |
| No cancer, 1950 (90.2) | No cancer, 2551 (96.7) | No cancer, 2335 (96.0) | No cancer, 4954 (98.0) | |
| 1 | Colorectal, 56 (2.6) | Prostate, 27 (1.0) | Colorectal, 34 (1.4) | Colorectal, 14 (0.3) |
| 2 | Prostate, 28 (1.3) | Lung, 15 (0.6) | Breast, 10 (0.4) | Lung, 14 (0.3) |
| 3 | Lung, 28 (1.3) | Colorectal, 9 (0.3) | Lung, 10 (0.4) | Breast, 13 (0.3) |
DISCUSSION
Summary
This study is the first to report the incidence of cancer in patients with microcytosis compared with those with a normal MCV in primary care across all cancer types. The overall 1-year cancer incidence in those patients with microcytosis was 4.0% (CI = 3.7 to 4.4), compared with 2.0% (CI = 1.9 to 2.1) in those with a normal MCV. The difference was more marked in males, with 6.2% (CI = 5.5 to 6.9) of microcytic patients developing cancer, but only 2.7% (CI = 2.3 to 3.1) of females doing so. Individual cancers that were disproportionately more common with microcytosis were colorectal, lung, lymphoma, kidney, and stomach. In patients who had microcytosis but normal haemoglobin, 3.3% (CI = 2.6 to 4.0) of males and 2.0% (CI = 1.6 to 2.4) of females had a diagnosis of cancer within a year.
Strengths and limitations
The large size of this study is a key strength, as well as the setting in primary care, as this is where patients often present with symptoms that could trigger cancer investigation. The study is largely representative of the UK population, other than the matching to a previous cancer case population. This may have increased the cancer risk in the population, but should have done so equally for those patients with microcytosis and their comparison group. The study is reliant on the quality of CPRD data; however, since 2000, laboratory test data have been automatically transmitted to most GP practices,16 which considerably reduces the chance of transcription error. The authors do not know the reason for the blood test being performed. Blood tests are commonly performed in primary care for many different reasons; around one-quarter of the adult UK population have a full blood count in any given year.16 As such this population is expected to be somewhat more ill than the untested population. An upper threshold of 85 fL was used to define ‘microcytosis’. Although this was a conservative choice, it nonetheless reflects common UK practice.
Comparison with existing literature
The results of this study largely agree with previous CPRD studies that found that microcytosis was associated with non-Hodgkin lymphoma,14 oesophago-gastric cancer,15 and kidney cancer.17 The association between microcytosis and colorectal cancer reported from another case–control study16 also supports these findings. No primary care study has reported cancer incidence with microcytosis across all cancers, or in patients with normal haemoglobin. Secondary care studies of microcytosis concentrate on iron-deficiency anaemia and possible causes of the anaemia, and no reports on microcytosis unaccompanied by anaemia could be found.
Implications for practice
Although the risk of cancer with microcytosis is above the 3% figure that the National Institute for Health and Care Excellence recommends for urgent cancer investigation, GPs have in-house tests to help in this situation. This increased risk is made up of a small number of cancers, particularly colorectal (as shown in Figures 2 and 3). There seems to be no effect for some other cancers, for example, breast. For GPs, an MCV is only reported alongside the haemoglobin value. Anaemia accompanied by microcytosis strongly suggests iron deficiency, and therefore measurement of iron stores (which were too few in this study for reliable analysis) would be the usual next step. If iron deficiency is identified, its cause will be sought, which would generally involve testing for gastrointestinal blood loss. This diagnostic pathway does not remove the need to enquire about other symptoms suggestive of the malignancies reported here, particularly lung cancer. Who this study affects, however, is patients with microcytosis but without anaemia. Some may be iron deficient, simplifying the investigation strategy. It seems sensible for all these patients to be also offered faecal immunochemical testing for hidden gastrointestinal blood loss, and a chest X-ray if respiratory symptoms suggest lung cancer is possible. In this way, the small number of patients whose microcytosis has been caused by cancer could receive a more timely diagnosis, without exposing the majority to unnecessary referral and invasive testing.
Patients in primary care with microcytosis may harbour cancer, with colorectal and lung cancers being the most probable. Most of the relevant initial investigations are available in primary care, allowing initial assessment of possible cancer to be performed rapidly.
Funding
This research arises from the CanTest Collaborative, which is funded by Cancer Research UK (ref: C8640/A23385). Funding was provided by the National Institute for Health Research (NIHR), through the NIHR Policy Research Unit in Cancer Awareness, Screening, and Early Diagnosis, and NIHR Programme Grants for Applied Research (Grant ref: RP-PG-0608-10045). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health and Social Care, other government departments, or arm’s length bodies.
Ethical approval
Independent Scientific Advisory Committee — protocol 09-110.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
William Hamilton was clinical lead on the 2015 revision of the National Institute for Health and Care Excellence (NICE) guidance on investigation of suspected cancer. His contribution to this article is in a personal capacity, and does not represent the view of the Guideline Development Group, or of NICE itself. Rhian Hopkins, Sarah Bailey, and Elizabeth Shephard have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Cancer Research UK Cancer statistics for the UK: CRUK. 2019 https://www.cancerresearchuk.org/health-professional/cancer-statistics-for-the-uk#heading-One (accessed 22 Apr 2020).
- 2.Cancer Research UK Cancer mortality statistics: CRUK. 2019 https://www.cancerresearchuk.org/health-professional/cancer-statistics/mortality#heading-Zero (accessed 22 Apr 2020).
- 3.Foot C, Harrison T. How to improve cancer survival: explaining England’s relatively poor rates. 2011 https://www.kingsfund.org.uk/publications/how-improve-cancer-survival (accessed 22 Apr 2020). [Google Scholar]
- 4.Hamilton W, Walter FM, Rubin G, Neal RD. Improving early diagnosis of symptomatic cancer. Nat Rev Clin Oncol. 2016;13(12):740–749. doi: 10.1038/nrclinonc.2016.109. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Rahman M, Stockton D, Rachet B, et al. What if cancer survival in Britain were the same as in Europe: how many deaths are avoidable? Br J Cancer. 2009;101(Suppl 2):S115–S124. doi: 10.1038/sj.bjc.6605401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allgar VL, Neal RD. Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS Patients: Cancer. Br J Cancer. 2005;92(11):1959–1970. doi: 10.1038/sj.bjc.6602587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NHS NHS Long Term Plan. 2019 https://www.longtermplan.nhs.uk (accessed 22 Apr 2020). [Google Scholar]
- 8.Green T, Atkin K, Macleod U. Cancer detection in primary care: insights from general practitioners. Br J Cancer. 2015;112(Suppl 1):S41–S49. doi: 10.1038/bjc.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey SE, Ukoumunne OC, Shephard EA, Hamilton W. Clinical relevance of thrombocytosis in primary care: a prospective cohort study of cancer incidence using English electronic medical records and cancer registry data. Br J Gen Pract. 2017 doi: 10.3399/bjgp17X691109. [DOI] [PMC free article] [PubMed]
- 10.Watson J, Salisbury C, Banks J, et al. Predictive value of inflammatory markers for cancer diagnosis in primary care: a prospective cohort study using electronic health records. Br J Cancer. 2019;120(11):1045–1051. doi: 10.1038/s41416-019-0458-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merriel SW, Carroll R, Hamilton F, Hamilton W. Association between unexplained hypoalbuminaemia and new cancer diagnoses in UK primary care patients. Fam Pract. 2016;33(5):449–452. doi: 10.1093/fampra/cmw051. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton F, Carroll R, Hamilton W, Salisbury C. The risk of cancer in primary care patients with hypercalcaemia: a cohort study using electronic records. Br J Cancer. 2014;111(7):1410–1412. doi: 10.1038/bjc.2014.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koshiaris C, Van den Bruel A, Oke JL, et al. Early detection of multiple myeloma in primary care using blood tests: a case–control study in primary care. Br J Gen Pract. 2018 doi: 10.3399/bjgp18X698357. [DOI] [PMC free article] [PubMed]
- 14.Shephard EA, Neal RD, Rose PW, et al. Quantifying the risk of non-Hodgkin lymphoma in symptomatic primary care patients aged ≥40 years: a large case-control study using electronic records. Br J Gen Pract. 2015 doi: 10.3399/bjgp15X684793. [DOI] [PMC free article] [PubMed]
- 15.Stapley S, Peters TJ, Neal RD, et al. The risk of oesophago-gastric cancer in symptomatic patients in primary care: a large case-control study using electronic records. Br J Cancer. 2013;108(1):25–31. doi: 10.1038/bjc.2012.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton W, Lancashire R, Sharp D, et al. The importance of anaemia in diagnosing colorectal cancer: a case-control study using electronic primary care records. Br J Cancer. 2008;8(2):323–327. doi: 10.1038/sj.bjc.6604165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shephard E, Neal R, Rose P, et al. Clinical features of kidney cancer in primary care: a case-control study using primary care records. Br J Gen Pract. 2013 doi: 10.3399/bjgp13X665215. [DOI] [PMC free article] [PubMed]
- 18.Clinical Practice Research Datalink (CPRD) CPRD data. 2019 https://cprd.com/Data. (accessed 22 Apr 2020).
- 19.Taylor A, Stapley S, Hamilton W. Jaundice in primary care: a cohort study of adults aged >45 years using electronic medical records. Fam Pract. 2012;29(4):416–420. doi: 10.1093/fampra/cmr118. [DOI] [PubMed] [Google Scholar]
- 20.National Institute for Health and Care Excellence Suspected cancer: recognition and referral NG12. 2017 https://www.nice.org.uk/guidance/NG12 (accessed 22 Apr 2020). [Google Scholar]
- 21.DeLoughery TG. Iron deficiency anemia. Med Clin North Am. 2017;101(2):319–322. doi: 10.1016/j.mcna.2016.09.004. [DOI] [PubMed] [Google Scholar]