Abstract
Objective:
To compare the effectiveness of bucindolol and metoprolol succinate for the maintenance of sinus rhythm in a genetically defined heart failure (HF) population with atrial fibrillation (AF).
Background:
Bucindolol is a beta-blocker whose unique pharmacologic properties provide greater benefit in HF patients with reduced ejection fraction (HFrEF) who have the beta1-adrenergic receptor (ADRB1) Arg389Arg genotype.
Methods:
267 HFrEF patients with a left ventricular ejection fraction (LVEF) < 0.50, symptomatic AF, and the ADRB1 Arg389Arg genotype were randomized 1:1 to bucindolol or metoprolol and up-titrated to target doses. The primary endpoint of AF/atrial flutter (AFL) or all-cause mortality (ACM) was evaluated by electrocardiogram (ECG) during a 24-week period.
Results:
The hazard ratio (HR) for the primary endpoint was 1.01 (95% CI: 0.71, 1.42) but trends for bucindolol benefit were observed in several subgroups. Precision therapeutic phenotyping revealed that a differential response to bucindolol was associated with: 1) the interval of time from the initial diagnosis of HF and AF to randomization, and; 2) the onset of AF relative to initial HF diagnosis. In a cohort whose first HF and AF diagnoses were < 12 years prior to randomization, in which AF onset did not precede HF by more than 2 years (N=196) the HR was 0.54 (95% CI: 0.33, 0.87; p=0.011).
Conclusion:
Pharmacogenetic-guided bucindolol therapy did not reduce the recurrence of AF/AFL/ACM compared to metoprolol in HFrEF patients, but populations were identified that merit further investigation in future Phase 3 trials.
Keywords: atrial fibrillation, bucindolol, heart failure, beta-blocker, pharmacogenetics, precision medicine
Introduction
Atrial fibrillation (AF) is a common and serious medical problem associated with significant morbidity and mortality, especially in patients with heart failure (HF) (1). Development of AF is associated with increased risk of adverse cardiovascular outcomes, and when AF occurs in patients with HF these adverse effects are accentuated (2,3). AF and HF often co-exist and have common risk factors, as well as overlapping pathophysiologies (3). Therefore, there is a strong rationale to minimize the occurrence of AF in patients with HF. Antiarrhythmic drugs can reduce AF burden but have many side effects including proarrhythmia, with many agents being contraindicated in HF patients (1). Although catheter ablation shows promise for preventing recurrent AF in HF patients with reduced ejection fraction (HFrEF) (4,5), it may not be suitable or practical for many patients. Thus, there is an unmet need for safe and effective drugs to reduce AF in patients with HF. Beta-blockers are first-line therapy for HFrEF due to their benefits in reducing morbidity and mortality and are widely used in HF patients with AF to control ventricular response rate. In addition, beta-blockers have modest AF prevention effects in HFrEF patients (6).
Bucindolol is a non-selective beta-blocker with mild vasodilator properties and two unique antiadrenergic properties; a moderate sympatholytic effect (7) and inverse agonism for the ADRB1 Arg389 major allele gene product (8), a property which promotes inactivation of constitutively active beta1-adrenergic receptors. The treatment effects of bucindolol appear to be enhanced in patients homozygous for ADRB1 Arg389 (ADRB1 Arg389Arg) (8,9). In advanced HFrEF patients with this genotype, a 74% reduction in the development of AF was observed for patients in sinus rhythm at baseline who received bucindolol compared to placebo (10). Metoprolol and carvedilol do not appear to confer similar clinical benefits in patients with an ADRB1 Arg389Arg genotype (11,12). Therefore, the GENETIC-AF trial (i.e., Genotype-Directed Comparative Effectiveness Trial of Bucindolol and Toprol-XL for the Prevention of Symptomatic Atrial Fibrillation/Atrial Flutter in Patients with Heart Failure) was designed to evaluate the efficacy of a pharmacogenetically-guided rhythm control intervention with bucindolol compared to metoprolol for the prevention of AF/AFL in an ADRB1 Arg389Arg HFrEF population at risk of AF/AFL recurrence.
Methods
Study Design
GENETIC-AF was a multicenter, randomized, double-blind, comparative efficacy trial in a genotype-defined population with HFrEF, defined as a left ventricular ejection fraction (LVEF) < 0.50 and AF (Online Supplement). The trial had an adaptive design allowing for seamless transition from Phase 2B to Phase 3 based on review of interim data. The rationale and design of the trial have been previously reported (13).
Patients were randomly assigned to receive bucindolol or metoprolol and were up-titrated to target doses (Online Table 1). Following up-titration, electrical cardioversion (ECV) was performed if needed to establish sinus rhythm prior to the start of follow-up. During the 24-week follow-up period, heart rhythm was monitored by 12-lead electrocardiogram (ECG) every 4 weeks (Online Figure 1). A prospectively defined device substudy permitted continuous heart rhythm monitoring to assess AF burden. Substudy participants had a pre-existing Medtronic pacemaker or defibrillator with an atrial lead or were implanted with a Medtronic Reveal LINQ insertable cardiac monitor (ICM) prior to the start of follow-up. After week 24, patients continued to receive blinded study drug and had clinic visits every 12 weeks for assessments of efficacy and safety.
Patients had HFrEF with a LVEF < 0.50 assessed in the past 12 months, symptomatic paroxysmal or persistent AF in the past 180 days and were receiving optimal anticoagulation therapy for stroke prevention. Patients were genotyped at screening and those who were ADRB1 Arg389Arg were eligible for randomization.
Exclusion criteria included New York Heart Association (NYHA) Class IV symptoms, clinically significant fluid overload, permanent AF (ongoing AF event >1 year), antiarrhythmic therapies in past 7 days, prior atrioventricular node ablation, high-grade atrioventricular block, catheter ablation for AF or atrial flutter (AFL) in past 30 days, and prior intolerance or contraindication to beta-blocker therapy. Details of the trial entry criteria have been previously reported (13).
The active comparator, metoprolol succinate (Toprol-XL), is a selective beta1-adrenergic receptor blocker indicated for the treatment of HF. Metoprolol was selected as the active comparator to ensure continuity with previous HF trials and because it has demonstrated effectiveness in preventing AF in HFrEF patients (14,15), but does not appear to confer enhanced benefits in patients with an ADRB1 Arg389Arg genotype (11,12).
Patients were randomized (1:1) to treatment with bucindolol or metoprolol, which was over-encapsulated to maintain blinding. Since bucindolol is administered twice-daily (bid), and metoprolol is given once-daily (qd), a placebo dose was included for the metoprolol arm and all study drugs were administered twice-daily. Randomization was centralized and stratified by HF etiology (ischemic, non-ischemic), LVEF (< 0.35, ≥ 0.35), device type (ICM, pacemaker/defibrillator, no device), and rhythm at randomization (sinus rhythm, AF/AFL), using 16,000 randomly generated numbers and a block size of four. Study drug was titrated weekly to obtain a target dose of 100 mg bid (50 mg bid if < 75 kg) for bucindolol (16) and 200 mg qd for metoprolol (17). For more details see Online Table 1. Patients experiencing AF/AFL during follow-up remained on blinded study drug and could undergo ECV, ablation, or initiate therapy with amiodarone or dofetilide.
ADRB1 Arg389Gly genotype was determined by RT-PCR in DNA extracted from whole blood. Systemic venous plasma norepinephrine was assayed by high-pressure liquid chromatography with electrochemical detection and venous plasma NT-proBNP was measured by electrochemiluminescence immunoassay.
Study design, conduct, and performance were overseen by a 11-member Steering Committee and was monitored by a 3-member Data and Safety Monitoring Committee (DSMB) who also performed the interim efficacy analysis (committee composition in Online Supplement). The protocol was approved by the Institutional Review Board/Ethics Committee and all patients provided written informed consent.
Statistical Analyses
For the interim analysis, the endpoint of interest was time to first event of AF/AFL or all-cause mortality (ACM) during a 24-week follow-up period. The primary endpoint for the planned Phase 3 study was time to symptomatic AF/AFL or ACM, with symptoms captured by a study-specific questionnaire (Online Supplement). A clinical events committee, blinded to treatment assignment, adjudicated the first occurrence of the AF/AFL endpoint, including the association of new or worsening symptoms. Sample size for Phase 3 assumed a 60% event rate in the metoprolol arm, a 25% relative risk reduction with bucindolol, and accrual of 330 primary events in approximately 620 patients for 90% power at alpha=0.01.
The efficacy analysis was conducted according to intention-to-treat with censoring at 24 weeks for patients not experiencing an event. Hazard ratio (HR) and 95% confidence interval (CI) values were determined by Cox proportional hazards models with adjustment for the four randomization strata, and treatment as a covariate. Testing for superiority was performed using a 2-sided significance level of 0.05. Patients who died prior to start of follow-up and patients who failed to establish sinus rhythm post-ECV were assigned an event on day 1. Patients were censored on day 1 if they were in AF/AFL and the ECV procedure was not performed, or if they withdrew from the study prior to start of follow-up.
Variables identified in the GENETIC-AF Statistical Analysis Plan (SAP, Online Supplement) that were potential predictors of the primary endpoint were investigated by precision therapeutic phenotyping. Hypothesis-based (e.g., AF duration, AF type, LVEF, NYHA Class, NT-proBNP, norepinephrine) and hypothesis-free (e.g. HF duration, initial study dose) elements were included in the multivariate methodology, which was applied to both obvious and non-obvious data to identify a therapeutic phenotype appropriate for investigating in Phase 3. To examine the relationship between HF duration and bucindolol effectiveness for reducing HF events, we analyzed data from the BEST trial (16) and pharmacogenetic substudy (8) for the endpoint of time to all-cause mortality or first HF hospitalization (ACM/HFH).
Time to first event of AF/AFL or ACM was assessed in the device substudy following similar methodology for the primary endpoint, with an AF/AFL event prospectively-defined as AF burden ≥ 6 hours per day as recorded by continuous monitoring. Six hours of AF burden has previously been shown to be associated with an increased rate of hospitalization for HF (18). Due to the smaller sample size in the substudy, treatment effect estimates were determined based on Cox proportional hazards models with no adjustment for randomization strata.
Normally distributed continuous variables were analyzed by t-tests or ANOVA where appropriate. Neurohormonal changes from baseline and DTRI data were analyzed by the Wilcoxon signed rank test, and between group differences by the Wilcoxon rank sum test. Categorical variable differences were assessed by Chi square or Fisher’s exact test.
An interim analysis examined data from the initial Phase 2B population. If the DSMB determined that the data were consistent with pre-trial assumptions, the trial was to seamlessly proceed to Phase 3 (see Online Supplement for SAP). To aid in signal detection, Bayesian predictive probability of success estimates (19,20) were generated and compared to prespecified thresholds for each potential outcome (i.e., Phase 3 transition, Phase 2B completion, or futility). Based on the interim analysis the DSMB recommended completion of Phase 2B, and the data from this population are presented below.
Results
Population and Baseline Characteristics
The trial was conducted in 92 centers in 6 countries (Canada, Hungary, The Netherlands, Poland, Serbia, and the United States) between April 2014 and December 2017. A total of 760 patients were screened (Figure 1); 362 (48%) failed screening due to genotype, 73 (9.6%) did not meet other eligibility criteria, and 58 (7.6%) failed due to other reasons (e.g., withdrawal of consent, lost to follow-up). The remaining 267 patients were randomized to study drug and up-titrated to target doses. Compliance was >90% in both groups, with a higher proportion of patients attaining target dose for bucindolol compared to metoprolol (84% and 72%, respectively; p = 0.035).
FIGURE 1. Consort Diagram.
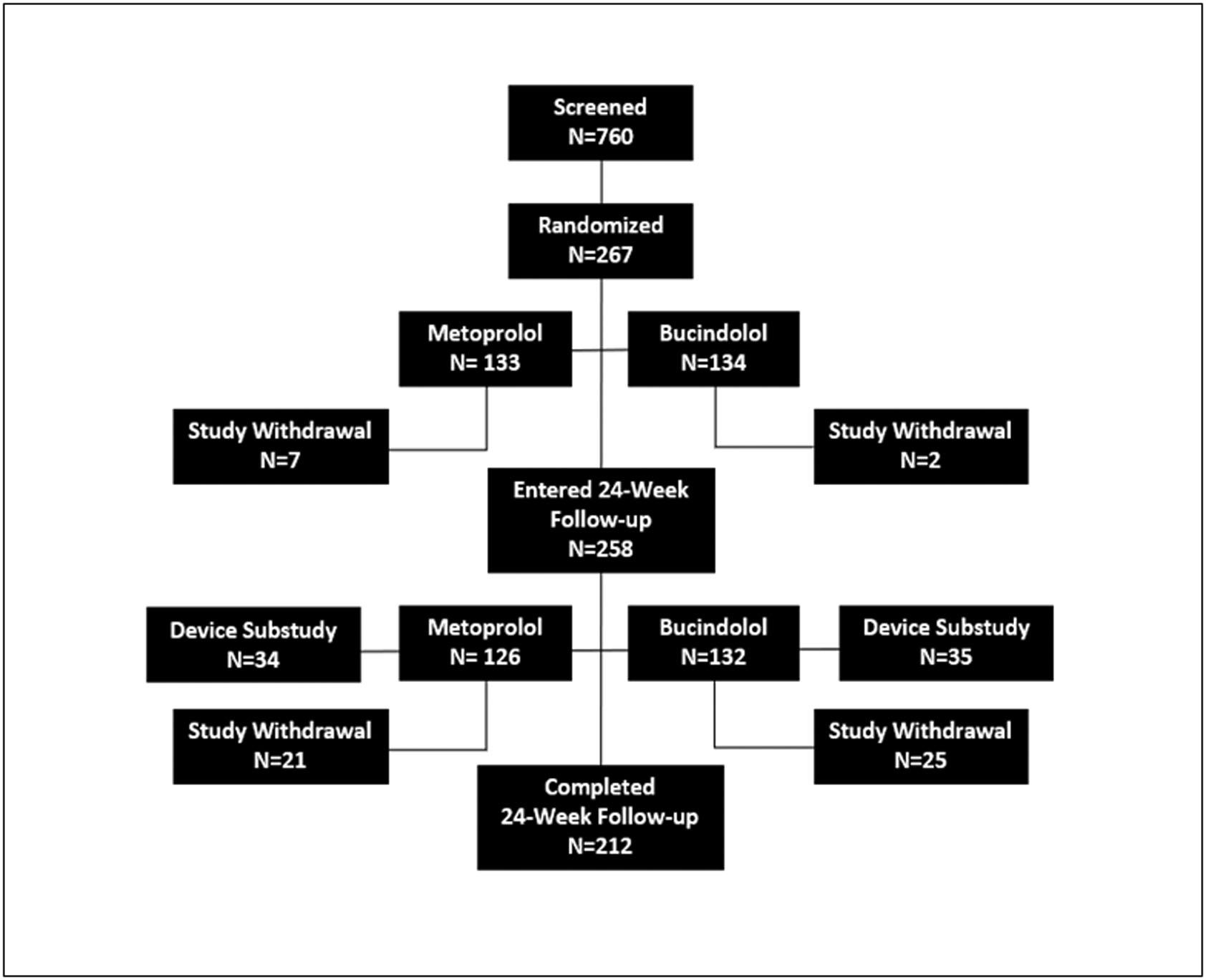
Proportion of patients with the ADRB1 Arg389Arg genotype was consistent with previous findings (8–11)
Baseline characteristics were well-balanced between treatment groups (Table 1). Mean LVEF was 0.36±0.10, 72% had NYHA II or III symptoms at baseline, 51% had persistent AF, and plasma NT-proBNP were elevated at baseline (median = 801 pg/ml; inter quartile range (IQR): 384, 1420). ECV was required in 46% of patients to establish sinus rhythm prior to follow-up start. About half (48%) of all patients had implanted monitoring devices, which included ICMs inserted for the trial (16%) and pre-existing pacemakers or defibrillators (32%).
TABLE 1.
BASELINE CHARACTERISTICS
| Entire Study | Device Substudy | |||||
|---|---|---|---|---|---|---|
| Parameter | All Patients N = 267 |
Bucindolol N = 134 |
Metoprolol N = 133 |
All Patients N = 69 |
Bucindolol N = 35 |
Metoprolol N = 34 |
| Age, years | 65.6 ± 10.1 | 65.8 ± 10.3 | 65.5 ± 10.0 | 66.1 ± 10.7 | 65.5 ± 11.5 | 66.8 ± 9.9 |
| Male/Female, % | 82/18 | 83/17 | 81/19 | 93/7 | 94/6 | 91/9 |
| Race: W/B/A/O, % | 96/2/1/1 | 96/1/1/2 | 96/2/1/1 | 96/1/1/2 | 94/0/3/3 | 97/3/0/0 |
| LVEF | 0.36 ± 0.10 | 0.36 ± 0.10 | 0.36 ± 0.10 | 0.34 ± 0.08 | 0.33 ± 0.08 | 0.36 ± 0.09 |
| NYHA I/II/III, % | 28/57/15 | 30/60/10 | 26/54/20 | 23/57/20 | 29/49/23 | 18/65/18 |
| Ischemic/Non-Ischemic HF, % | 32/68 | 31/69 | 33/67 | 28/72 | 29/71 | 26/74 |
| Randomized in AF/Not in AF, % | 51/49 | 49/51 | 52/48 | 65/35 | 63/37 | 68/32 |
| Persistent/Paroxysmal AF, % | 51/49 | 51/49 | 51/49 | 64/36 | 63/37 | 65/35 |
| HF DxT Duration, days | 1153 ± 1909 | 1252 ± 2070 | 1054 ± 1733 | 1168 ± 1723 | 1208 ± 1880 | 1126 ± 1572 |
| AF DxT Duration, days | 1306 ± 2240 | 1431 ± 2271 | 1180 ± 2209 | 1355 ± 1984 | 1444 ± 1997 | 1263 ± 1995 |
| Systolic blood pressure, mm Hg | 123.3 ± 15.3 | 124.7 ± 14.9 | 121.8 ± 15.7 | 123.3 ± 15.1 | 122.4 ± 15.7 | 124.2 ± 14.5 |
| Diastolic blood pressure, mmHg | 75.3 ± 10.8 | 75.8 ± 11.0 | 74.8 ± 10.6 | 75.0 ± 10.1 | 73.7 ± 9.9 | 76.3 ± 10.3 |
| Heart Rate, bpm | 76.3 ± 17.8 | 76.5 ± 17.9 | 76.0 ± 17.7 | 78.4 ± 17.2 | 76.8 ± 16.4 | 80.1 ± 18.1 |
| Previous ECV/AF Ablation/Type III AAD, % | 49/21/48 | 49/21/50 | 50/20/46 | 55/13/54 | 57/17/57 | 53/9/50 |
| Device Type: ICM/PM/ICD, % | 16/17/15 | 17/15/18 | 15/20/12 | 62/22/16 | 66/20/14 | 59/24/18 |
| Norepinephrine, pg/ml | 673 ± 353 | 682 ± 348 | 664 ± 359 | 706 ± 368 | 710 ± 398 | 702 ± 339 |
| NT-proBNP, pg/ml, median (IQR) | 801 (384, 1420) | 777 (355, 1326) | 861 (420,1607) | 996 (457, 1645) | 923 (365, 1506) | 1013 (537, 1806) |
W/B/A/O=White/Black/Asian/Other. HF DxT Duration=time from HF diagnosis to randomization. AF DxT Duration=time from AF diagnosis to randomization. ECV=electrical cardioversion. AAD=antiarrhythmic drug. ICM=insertable cardiac monitor. ICD=implanted cardiac defibrillator. PM=pacemaker. IQR=interquartile range. Note: mean ± standard deviations are presented unless otherwise specified.
Efficacy Outcomes
A total of 143 events were observed for the efficacy endpoint, including 121 AF/AFL events, 19 ECV failures, and 3 deaths. Nearly all AF/AFL events were adjudicated as symptomatic by a blinded clinical events committee (114/121; 94%). Event rates were similar for the bucindolol and metoprolol groups (54% and 53%, respectively), with a HR of 1.01 (95% CI: 0.71, 1.42) for the covariate-adjusted Cox proportional hazards model (Figure 2). In a prespecified analysis (Online Supplement, Statistical Analysis Plan and Phase 2B Amendment) of regional subgroups (Table 2, Online Figure 3), a trend for bucindolol benefit compared to metoprolol was observed in the U.S. subgroup (HR = 0.70; 95% CI: 0.41, 1.19), which was not seen in Canada (HR = 1.52; 95% CI: 0.68, 3.43) or in Europe (HR = 1.01; 95% CI: 0.48, 0.48, 2.14).
FIGURE 2. Time to First AF/AFL/ACM Event.
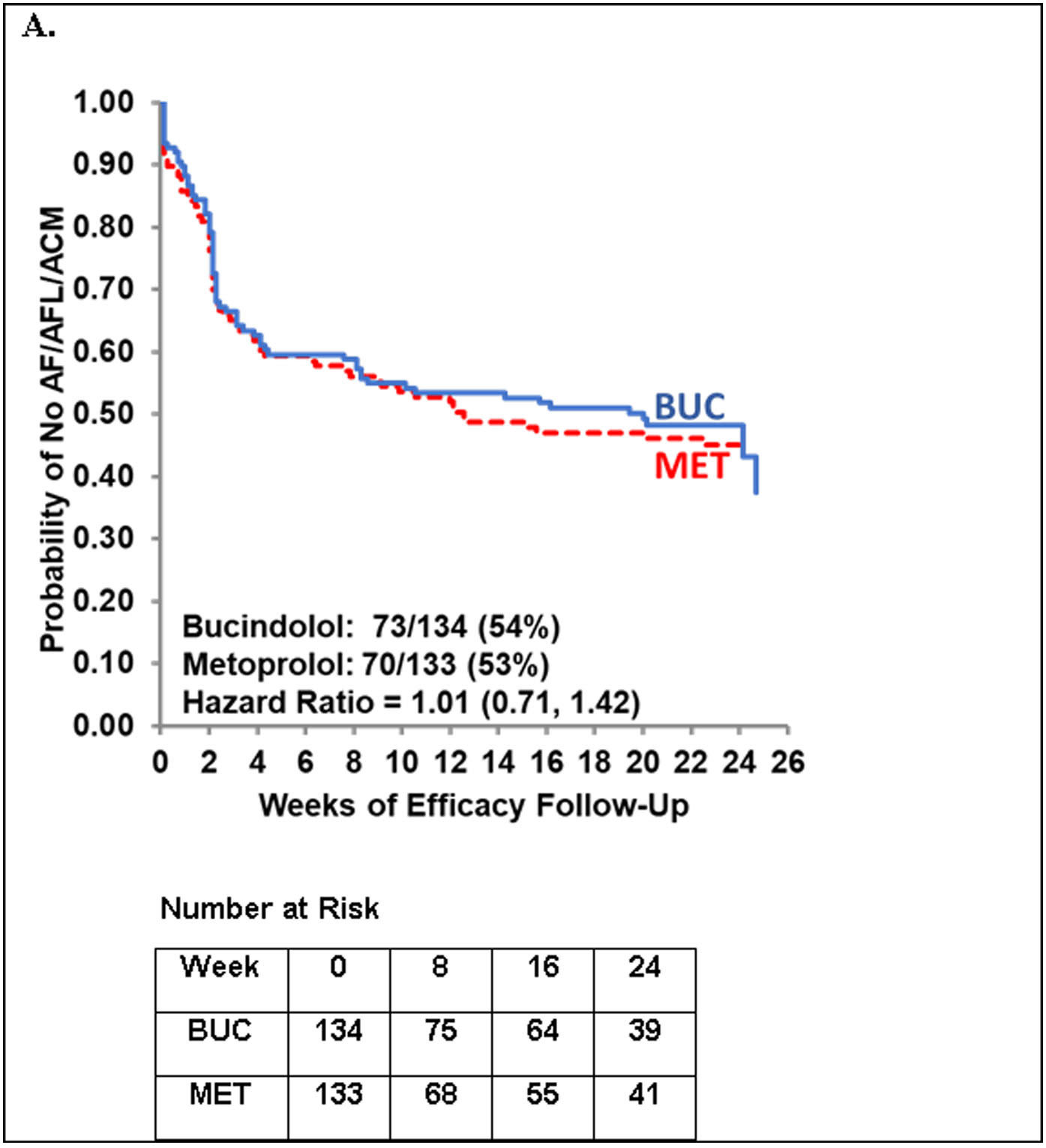
Cox proportional hazards model adjusted for the four randomization strata. Non-stratified hazard ratio = 0.96 (95% CI: 0.69, 1.33). Stratified analysis including adjustment for previous use of class III anti-arrhythmic drugs (yes/no): HR = 0.92 (95% CI: 0.63, 1.33).
TABLE 2.
Timing of HF and AF Onset Relative to Randomization
| Cohort | HF DxT (years) | AF DxT (years) | DTRI (years) | Time to AF/AFL/ACM | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Mean | Median | Mean | Median | P value* | Stratified HR (95% CI) | Non-stratified HR (95% CI) | |
| U.S. (N=127) | 4.5 | 1.5 | 3.4 | 1.0 | 1.1 | 0.0 | – | 0.70 (0.41, 1.19) | 0.77 (0.48, 1.22) |
| Non-U.S. (N=140) | 2.0 | 0.4 | 3.8 | 0.9 | −1.8 | 0.0 | 0.0005 | 1.34 (0.79, 2.28) | 1.22 (0.76, 1.96) |
| Canada (N=59) | 2.5 | 0.5 | 3.4 | 0.6 | −0.9 | 0.0 | 0.024 | 1.52 (0.68, 3.43) | 1.42 (0.72, 2.79) |
| Europe (N=81) | 1.6 | 0.4 | 4.0 | 1.7 | −2.4 | 0.0 | 0.0009 | 1.01 (0.48, 2.14) | 1.06 (0.55, 2.07) |
| Hungary (N=33) | 1.5 | 0.3 | 7.5 | 4.1 | −5.9 | −2.8 | <0.0001 | 2.90 (0.71, 11.8) | 3.57 (0.99, 12.9) |
| Poland (N=23) | 1.6 | 0.9 | 1.4 | 0.7 | 0.3 | 0.0 | 0.590 | 0.25 (0.03, 2.22) | 0.28 (0.07, 1.14) |
| Serbia (N=21) | 0.4 | 0.3 | 0.9 | 0.4 | −0.5 | 0.0 | 0.175 | 0.42 (0.08, 2.18) | 0.59 (0.15, 2.36) |
| Netherlands (N=4) | 8.0 | 7.1 | 6.4 | 3.8 | 1.6 | −0.1 | ND | ND | ND |
AF DxT=time from AF diagnosis to randomization. HF DxT=time from HF diagnosis to randomization. DTRI=diagnosis to randomization index; DTRI=HF DxT − AF DxT.
Wilcoxon rank sum test for comparison to U.S. Cohort.
Device Substudy
The device substudy included 69 patients from the U.S. (N=42), Canada (N=21), and Europe (n=6) who underwent continuous atrial rhythm monitoring. Cardiac monitors were inserted in 43 patients for the trial, whereas, 26 patients had pre-existing pacemakers or implantable cardioverter defibrillators (ICDs). The baseline characteristics of the substudy were well-balanced between the two groups and were generally similar to the overall population (Table 1); however, the substudy had a higher proportion of males (93% vs. 82%), persistent AF (64% vs. 51%), and AF at the time of randomization (65% vs. 51%), compared to the overall population.
An analysis of time to first event of AF/AFL or ACM was conducted in the device substudy following similar methodology for the primary endpoint. As shown in the Figure 3, a trend for bucindolol benefit compared to metoprolol was observed by device-based detection (HR = 0.75; 95% CI: 0.43, 1.32). Similar results were observed when the substudy population was assessed by intermittent, clinic-based 12-lead ECGs (HR = 0.69; 95% CI: 0.38, 1.23); however, the device-detected endpoint generally occurred earlier than the ECG-based endpoint (median = 6.5 days; p < 0.0001). For detection of subsequent ECG-determined AF, AF burden ≥ 6 hours had a sensitivity of 100%, a specificity of 87% and an accuracy of 96%.
FIGURE 3. Time to First Event of AF/AFL/ACM in the Device Substudy.
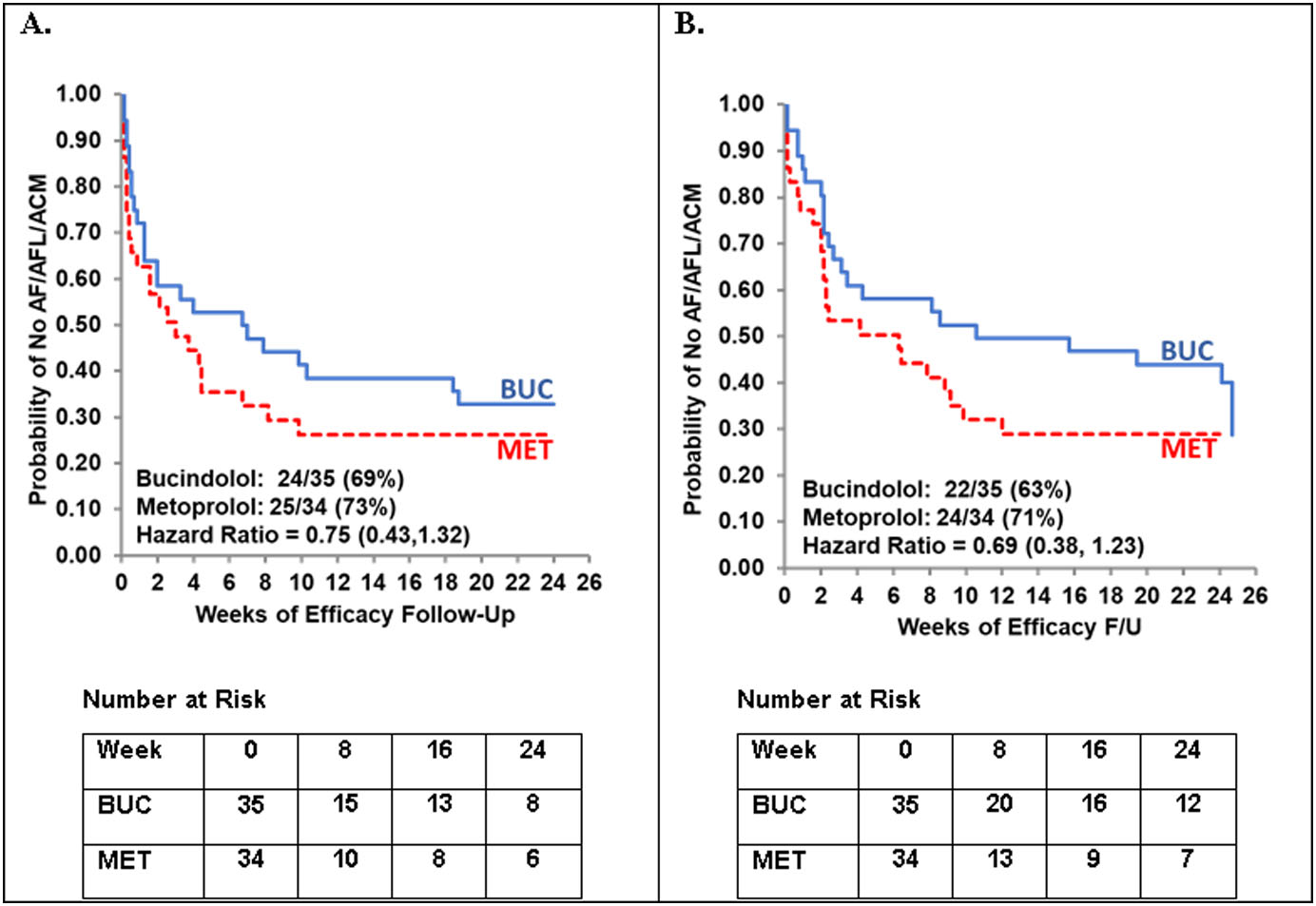
A. Device-based detection. B. ECG-based detection. For device-based detection an AF/AFL event was defined as AF burden ≥ 6 hours per day. Non-stratified Cox proportional hazards model.
Patient Characteristics and Treatment Response by Region
The differences in treatment response observed in the U.S. and non-U.S. cohorts prompted examination of baseline characteristics by region (Online Table 2). In general, the non-U.S. cohort had less severe HF compared to the U.S. cohort, as demonstrated by significantly higher LVEF (0.39 vs. 0.33), systolic blood pressure (126 v. 120 mmHg), and NYHA class I symptoms (39% vs. 17%), as well as significantly lower plasma NT-proBNP (1135 vs. 1380 pg/mL) and NYHA class III symptoms (5% vs. 26%). Notably, patients in the non-U.S. cohort had a more recent diagnosis of HF (Table 2, Online Table 2), with a mean time from HF diagnosis to randomization that was less than half of that in the U.S. group (2.0 vs. 4.5 years); whereas, mean time from AF diagnosis to randomization was similar between the two groups (3.8 vs. 3.4 years).
To quantify the relationship between the initial development of HF and AF, an index termed the diagnosis to randomization index (DTRI) was derived from information provided in case report forms. This index represents the differences between the HF duration (i.e., the time of HF diagnosis to randomization) and the AF duration (i.e., the time of AF diagnosis to randomization), with positive values representing HF onset prior to AF and negative values representing AF onset prior to HF. As shown in Table 2, the U.S. and non-U.S. cohorts had significant differences in the relative timing of HF and AF onset as measured by mean DTRI (p < 0.0005). The U.S. cohort, on average, had HF for more than a year prior to developing AF; whereas, the non-U.S. cohort had a diagnosis of AF for nearly 2 years prior to developing HF. Interestingly, bucindolol response for the primary endpoint correlated with mean DTRI (ρ = −0.93, p = 0.020), with poor response seen in populations having long-standing AF prior to the development of HF (i.e., Hungary and Canada) and good response in populations with concurrent or previous onset of HF prior to the development of AF (i.e., U.S., Poland, and Serbia).
Baseline Characteristics Predicting Endpoint Frequency and/or Interaction with Treatment
Cox proportional hazards regression modeling was performed to explore prespecified variables (SAP, Online Supplement) that were potential predictors of the primary endpoint (Online Table 3). Three variables violated the Cox model proportionality of hazards assumption. Of these, atrial rhythm at randomization was previously addressed by randomization stratification, as was heart rate, which generally correlates with atrial rhythm. The third variable, prior treatment with class III anti-arrhythmic drugs, was not previously identified and was included as a covariate in all subsequent analyses to account for non-proportional influence on baseline hazard.
On multivariate analysis, ten variables predicted the occurrence of the primary endpoint. In addition to the initial dose of study drug, which was based on beta blocker therapy prior to enrollment, the two-predictor model identified five variables related to the degree or duration of HF (i.e., systolic blood pressure, HF duration, HF etiology, NT-proBNP, and NYHA Class) and four variables related to heart rhythm (i.e., rhythm at randomization, baseline heart rate, AF type, and the number of prior ECVs). The only predictor by treatment interaction variable having a p-value < 0.05 was duration of time from initial AF diagnosis to randomization (i.e., AF DxT).
The time from initial HF diagnosis to randomization (i.e., HF DxT) was a significant predictor for the occurrence of primary endpoint but did not predict treatment or treatment by predictor interactions in Cox modeling of the primary endpoint (Online Table 3). However, since AF DxT predicted bucindolol response for the prevention of AF recurrence, we examined data from the placebo-controlled BEST HF trial (16) to determine whether HF DxT had a similar relationship to bucindolol response for the HF endpoint, ACM or first HF hospitalization (HFH). As shown in Online Figure 3, an attenuation of treatment response for the BEST ACM/HFH endpoint is observed in cohorts with greater values of HF DxT upper bound (i.e., inclusion of long-standing HF prior to randomization). This strong, negative correlation was observed in both the entire cohort (N = 2708; r = −0.82; 95% CI: −0.92, −0.59) and for the ADRB1 Arg389Arg subgroup (N = 493; r = −0.79; 95% CI: −0.91, −0.54).
Effect of Duration and Relative Onset of AF and HF on Treatment Effect
To further examine the effects of AF and HF duration identified in the above analyses, a 3-dimensional plot was constructed with treatment effect (i.e., 1-hazard ratio) for the GENETIC-AF primary endpoint as the dependent variable (z-axis), and HF DxT (x-axis) and AF DxT (y-axis) as independent variables. As shown in the Central Illustration (A), an attenuation of treatment effect was associated with increasing values of both AF and HF DxT. When equivalent DxT values (both HF and AF DxT values had to be < the timepoint duration on the x axis) were used to examine the combined effects of AF and HF duration (Online Figure 4), a strong negative correlation was observed (r = −0.94; 95% CI: −0.97, −0.89), with substantial attenuation of treatment effect seen with the inclusion of a small proportion of patients with both AF and HF durations greater than 12–15 years.
CENTRAL ILLUSTRATION. Treatment Effect by Duration and Relative Onset of AF and HF prior to Randomization.
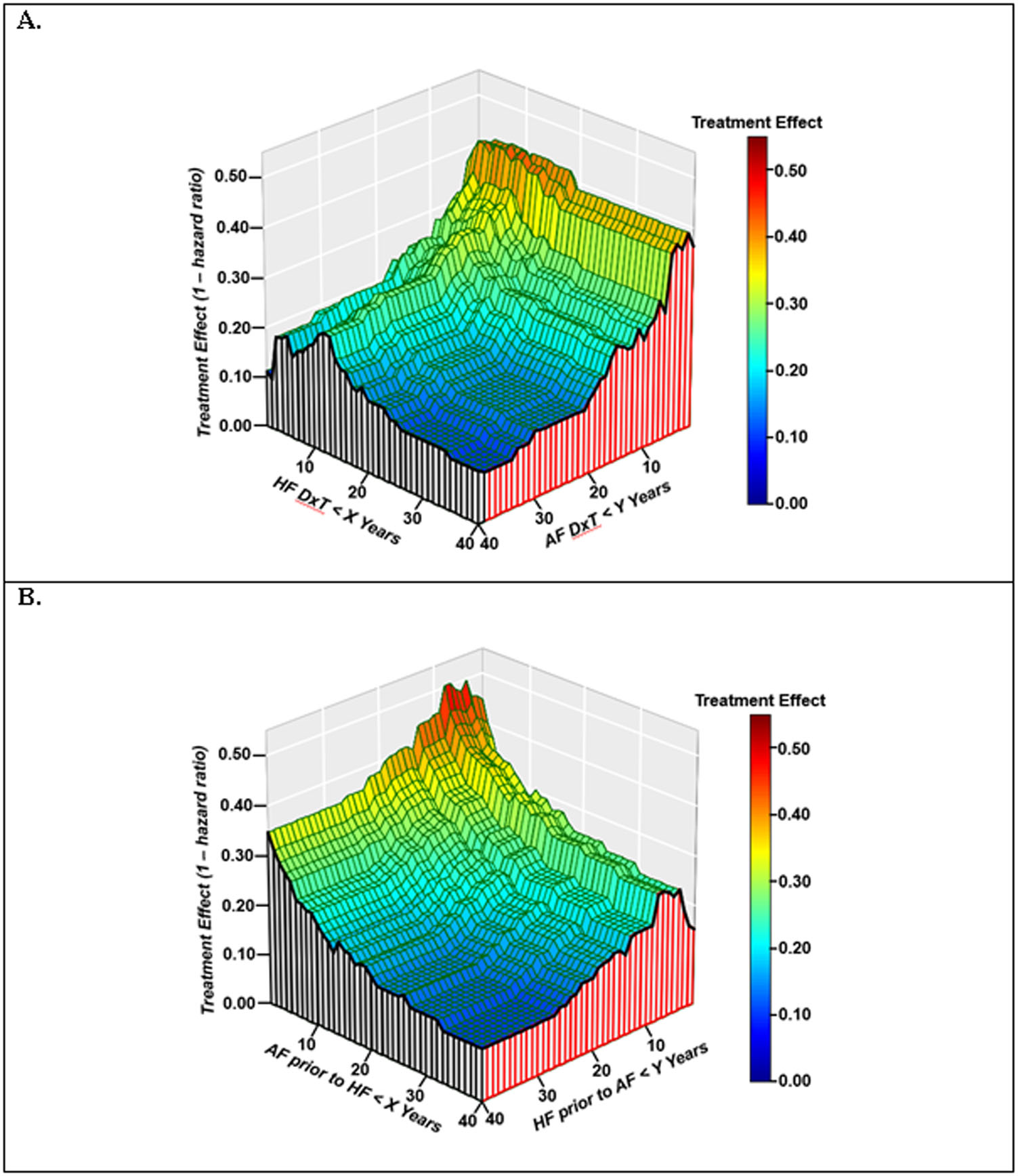
A. 3-dimensional plot of HF DxT (x-axis) and AF DxT (y-axis) versus treatment effect (z-axis).
B. 3-dimensional plot of AF onset prior to HF (x-axis) and HF onset prior to AF (y-axis) versus treatment effect (z-axis). Hazard ratio is for time to AF/AFL/ACM endpoint. HF DxT=time from initial HF diagnosis to randomization. AF DxT=time from initial AF diagnosis to randomization. DTRI (Diagnosis to Randomization Index) = HF DxT − AF DxT. AF onset prior to HF = absolute value of DTRI lower bound. HF onset prior to AF = DTRI upper bound.
To examine the effects of the relative onset of AF and HF on treatment effect, a 3-dimensional plot was constructed with treatment effect as the dependent variable (z-axis), and the absolute value of DTRI lower bound (i.e., years of AF prior to HF) and DTRI upper bound (i.e., years of HF prior to AF) and as independent variables. As shown in Central Illustration (B), there is an attenuation of treatment effect associated with increasing absolute values of DTRI lower and upper bound (i.e., increasing time between the initial presentations of AF and HF). When equivalent absolute values for DTRI lower and upper bounds were used to examine the concept of contemporaneous AF and HF development (Online Figure 5A), there was a nearly linear, negative correlation with treatment effect (r = −0.96; 95% CI: −0.98, −0.92).
Prevention of AF Recurrence in the Precision Therapeutic Selected Phenotype
Duration and relative onset of AF and HF are indirectly related characteristics that may have additive and/or overlapping effects. Therefore, we examined their use in combination to identify a precision therapeutic phenotype appropriate for further study. Details of the precision therapeutic phenotype analyses are presented in the Online Supplement.
In the example presented below, we selected a population with an AF and HF DxT < 12 years (i.e., DxT12 cohort), as this cutoff retained a high proportion (86%) of the overall population while minimizing attenuation of the observed treatment effect. We then applied a DTRI lower bound of −2 years (i.e., AF not preceding HF by more than 2 years; DxT12/DTRI-2 cohort), as this cutoff retained 85% of the DxT12 cohort. As shown in Online Figure 6, restriction of DTRI upper bound (i.e., years of HF prior to AF) was not required when examined in a DxT12 background.
Patient characteristics of the DxT12 and DxT12/DTRI-2 cohorts are shown in Online Table 4. Patients excluded by the DxT12 criteria had characteristics consistent with longstanding AF and HF; whereas the population excluded by the DTRI > −2 years criteria had characteristics consistent with longstanding AF as primary diagnosis and treatment history, with primarily mild left ventricular dysfunction. Of note, patients who had contemporaneous development of both AF and HF (i.e., DTRI values within 2 years of zero) are the majority of those included in the 230 patient DxT12 cohort (“DTRI included”); whereas DTRI patients with values ±2 years are conspicuously absent from the 37 patient cohort excluded by the DxT12 criteria, i.e. those with the first diagnosis of both AF and HF ≥12 years prior to randomization (Online Figure 5B). The accumulation of a substantial number (> 10) of patients with DTRI values ±2 years does not occur until the DxT cutoff is restricted to < 6 years (data not shown).
The primary endpoint of time to first event of AF/AFL/ACM for the DxT12/DTRI-2 cohort (N=196) is shown in Figure 4. In HFrEF patients (LVEF < 0.50) the HR was 0.54 (95% CI: 0.33, 0.87) by ECG-based detection, with similar results observed by device-based detection (HR = 0.59; 95% CI: 0.30, 1.19; N=49). In HF patients with mid-range ejection fraction (HFmrEF; LVEF ≥ 0.40 and < 0.50) the HR was 0.42 (95% CI: 0.21, 0.86; p = 0.017) and in HF patients with lower-range ejection fraction (HFlrEF; LVEF < 0.40) the HR was 0.69 (95% CI: 0.33, 1.43; p = 0.32). Device-based estimate for HFmrEF and HFlrEF are not presented due to the small sample size. See Online Table 5 for more details.
FIGURE 4. Time to First Event of AF/AFL/ACM in the DxT12/DTRI-2 Cohort.
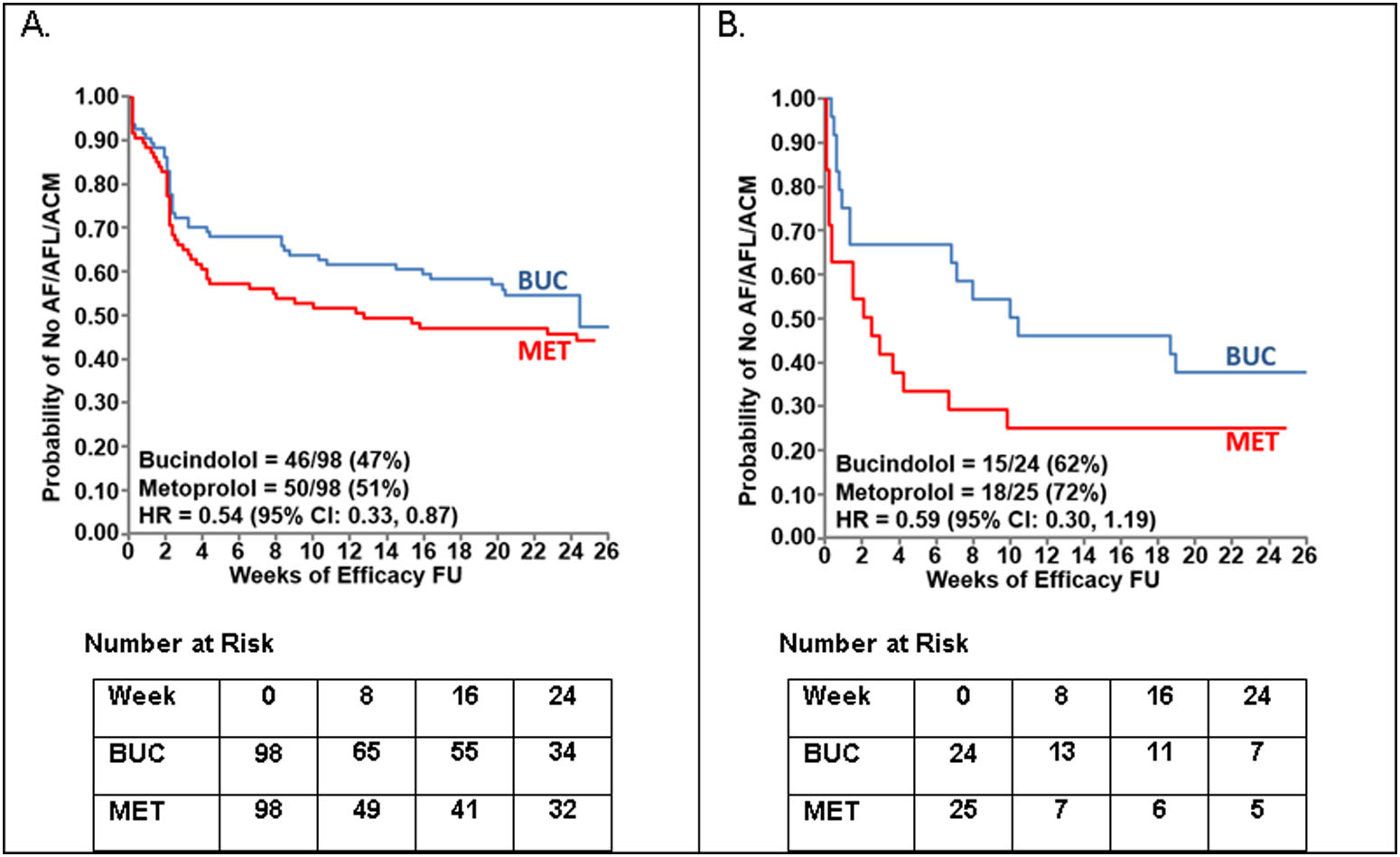
A. ECG-based detection in the entire cohort. B. Device-based detection in the substudy cohort. For device-based detection an AF/AFL event=AF burden ≥6 hours per day. HR=hazard ratio. FU=follow-up.
Effects on Norepinephrine and NT-proBNP
Plasma norepinephrine at baseline was similar in the bucindolol (682 ± 348 pg/ml, n=128) and metoprolol (664 ± 359 pg/ml, n=134) groups. At 4 weeks, there was a significant decrease from baseline in the bucindolol group (−124 ± 26 pg/ml; p < 0.001) that was not observed in the metoprolol group (−36 ± 32 pg/ml; p = 0.30). The change from baseline at 4 weeks was significantly different between the two groups (p = 0.012).
Plasma NT-proBNP was non-normally distributed in both groups, and median values at baseline were similar (777 and 861 pg/ml, p = 0.38; Online Table 6). There was a significant decrease from baseline in the bucindolol group at week 4 (−96 pg/ml; p = 0.003) and week 12 (−96 pg/ml; p =0.002) that was not observed in the metoprolol group. At week 24, significant decreases relative to baseline values were observed in both the bucindolol (−197 pg/ml; p = 0.005) and metoprolol (−100 pg/ml; p = 0.014) groups, but the change from baseline was not significantly different between the two groups (p = 0.220).
Safety
The proportion of patients experiencing adverse events (AEs) was similar in the two groups (Table 3). More patients in the metoprolol group had symptomatic bradycardia or bradycardia leading to dose reduction or discontinuation of study drug compared to the bucindolol group (9.0% vs. 3.0%; p=0.042). Three (2.3%) patients in each group died while receiving study drug or within 30 days of their last dose. All deaths in the metoprolol group occurred during the primary endpoint period (worsening HF – day 25; sudden cardiac death – day 43; motor vehicle accident – day 77). All deaths in the bucindolol group occurred during the long-term extension period (respiratory failure – day 385; sudden death – day 535; cardiac tamponade – day 779). Rates of HF hospitalization (7.5% vs. 8.3%) and ACM/HF hospitalization (8.2% vs. 9.0%) were similar for the bucindolol and metoprolol groups, respectively. There were no strokes in either treatment group, with 93% of patients receiving oral anticoagulants prior to randomization.
TABLE 3.
Treatment Emergent Adverse Events
| Endpoint | Bucindolol (N=134) |
Metoprolol (N=133) |
|---|---|---|
| Any adverse event (AE) | 100 (74.6%) | 95 (71.4%) |
| AE possible/probably related to study drug | 32 (23.9%) | 40 (30.1%) |
| AE leading to permanent study drug discontinuation | 11 (8.2%) | 11 (8.3%) |
| AE leading to study withdrawal (excluding death) | 2 (1.5%) | 2 (1.5%) |
| AE of symptomatic bradycardia or bradycardia leading to dose reduction or discontinuation of study drug | 4 (3.0%) | 12 (9.0%) |
| Any serious adverse event | 34 (25.4%) | 27 (20.3%) |
| AE leading to death | 3 (2.3%) | 3 (2.3%) |
Data presented from randomization through 30 days after last dose of study drug.
Discussion
The GENETIC-AF trial was designed as an adaptive, randomized, controlled trial that was powered for a full Phase 3 investigational comparison if evidence from the Phase 2B study suggested efficacy was likely on expansion to the Phase 3 sample size (9). In the Phase 2B analysis, pharmacogenetic-guided bucindolol did not reduce the recurrence of AF/AFL/ACM compared to metoprolol in the overall population. However, trends for bucindolol benefit were observed in key subgroups, particularly in those without long-standing and heavily treated AF prior to the development of HF. A lower proportion of patients with longstanding AF diagnosed prior to the development of HF likely contributed to the favorable bucindolol treatment effect in U.S. and device substudy patients, who were majority U.S. enrolled. In addition to the findings relevant to the investigational drug, this study also has several important findings relative to detection of AF in clinical trials.
GENETIC-AF also represents several firsts in the conduct of pharmacogenetic studies in cardiovascular disease and AF in particular. It is the first pharmacogenetically-targeted, randomized, controlled trial of rhythm control therapy in AF. Moreover, it is the first pharmacogenetic trial for prevention of recurrent AF in HFrEF, defined as HF with any decrease in LVEF (23). It is also the first study to compare AF burden to symptomatic AF/AFL as determined by adjudication of symptoms and ECG data. Finally, it represents the first comparative beta-blocker trial to include HF patients with mid-range ejection fraction (HFmrEF), defined as a LVEF ≥ 0.40 and < 0.50 (24).
There are several important findings from GENETIC-AF regarding AF in this HFrEF population. For example, nearly all patients who experienced AF recurrence had symptomatic AF, defined as new or worsening symptoms as adjudicated by a blinded clinical events committee. Recently, there has also been considerable interest in methods of AF diagnosis in clinical practice, including telemetry and device-based technologies (21,22). Our device substudy defined an AF/AFL event as AF burden ≥ 6 hours per day because this amount of burden had previously been shown to be associated with an increased rate of hospitalization for HF (18). We found that AF burden ≥ 6 hours per day as recorded by continuous monitoring exhibited high predictive accuracy for clinically symptomatic AF/AFL and tended to identify these events earlier than intermittent ECG monitoring.
Approximately half of patients screened for this trial had the ADRB1 Arg389Arg genotype, consistent with previous findings (8–11). In this genotype only norepinephrine high affinity beta1 Arg389 receptors are present, providing a substrate for the favorable effect of sympatholysis (9) that was again observed for bucindolol. Bucindolol lowered plasma norepinephrine levels after 4 weeks of treatment, which was not observed for metoprolol. Plasma NT-proBNP levels also decreased significantly with bucindolol treatment but not with metoprolol. These data indicate that the pharmacodynamic profile that contributes to the pharmacogenetic differentiation of bucindolol was operative in the trial.
It is also notable there were no safety concerns identified with bucindolol. Similar rates of death and hospitalization were observed in both treatment arms, though power was limited for detection of uncommon events. Interestingly, bradycardia was significantly lower in the bucindolol arm, suggesting that bucindolol may lead to less bradycardia than metoprolol in patients with the ADRB1 Arg389Arg genotype.
A major goal of a Phase 2 clinical trial is to further refine the study population that will be investigated in Phase 3. To this end we conducted an exercise in precision therapeutic phenotyping, or “individual treatment effect modeling” (23), designed to identify both prespecified obvious as well as nonobvious variables associated with a beneficial treatment effect of bucindolol. Exploration of factors contributing to the heterogeneity in response observed for regional subgroups led us to examine the timing of AF and HF onset prior to randomization and relative to one another. This led us to identify two variables that were strongly associated with an attenuation of bucindolol response: 1) the interval of time from the initial diagnosis of HF and AF to randomization (i.e., DxT), and; 2) the onset of AF relative to initial HF diagnosis (i.e., DTRI). AF duration has previously been reported to modulate response for other drug therapies post-ECV (24) and for catheter ablation (25). Less well appreciated is how the HF duration may impact medical therapy, and how these two variables interact in HF patients with concomitant AF. It should also be noted that GENETIC-AF compared two members of a drug class that had been administered chronically to this population, in some cases for years, prior to randomization. As such, a survivor effect due to loss of patients who develop AF and HF within a few years of each other, potentially due to adverse effects on mortality with the combination (26), may be responsible for altering the composition of certain subpopulations (i.e., those with longstanding AF/HF DxT, Online Figure 5B) in a manner that influences treatment response (Online Figure 6). If a contemporaneous relationship between the onset of AF and HF is optimal for bucindolol to maintain sinus rhythm, potentially related to higher levels of adrenergic activity when both conditions manifest in some proximity (10, 26), then this would explain the phenotype identified in our analysis. Alternatively, or in addition, it is also possible that the DTRI effect has a biological origin based on differences in atrial and ventricular pathophysiology when AF precedes or dominates over HF, the major difference residing in chamber interstitial fibrosis being a more prominent feature in AF (27, 28).
For comparative efficacy studies that seek to observe a differential response between two drugs in the same drug class it is critical to identify a study population with high potential for overall response to the drug class. This is necessary because a differential response is, by definition, a fraction of the overall response to a specific drug and, therefore, is more difficult to observe in a given study population. In this exploratory Phase 2 trial with limited sample size and statistical power, we identified HF populations who respond differentially to two beta-blockers based on genetic targeting. This approach circumvents potential issues associated with conventional subset analyses by evaluating monotonicity and consistency of trends across the full continuum of candidate variables such that the classifiers are readily conducive to numerical calibration (examples provided in Online Supplement). We propose that increasing the permissible limits of variation (i.e., tolerance) for the phenotype selection criteria increases the likelihood of reproducibility of these results in future studies.
Limitations
The results of this Phase 2B trial are best considered in light of its limitations. Given the conclusion of the study at Phase 2B, there was not adequate power to definitively test superiority. Although AF DxT and HF DxT were prespecified in the SAP prior to unblinding as potential predictors of treatment response, the onset relationship derived from these variables (i.e., DTRI) was retrospectively defined. Multiplicity via subgroup analysis can lead to false discovery, although this was tempered by examination for consistent trends across the entire dataset and other comparable datasets (i.e. BEST). Lastly, the selection of the precision therapeutic phenotype was based on response, but also considered the sample size needed to maintain feasibility for enrollment in future trials. As such, the treatment effect estimates derived from these analyses are hypothesis generating only and will need to be evaluated in a subsequent, prospectively-designed trial.
Conclusion
In the first trial of a pharmacogenetic-guided rhythm control intervention, bucindolol did not reduce the recurrence of AF/AFL or ACM compared to metoprolol in the overall population. However, precision therapeutic phenotyping identified a large population of HF patients with an ADRB1 Arg389Arg genotype who display a differential response to bucindolol compared to metoprolol for the prevention of AF/AFL. This experience underscores the utility of performing relatively large Phase 2 studies comprised of heterogeneous populations in order to generate the data necessary to identify appropriate therapeutic phenotypes suitable for Phase 3 investigation.
Supplementary Material
Competency in Medical Knowledge.
The intersection of atrial fibrillation (AF) and heart failure (HF) is common, worsens the prognosis of each disorder and lacks effective, easily administered and safe drug therapy. In the BEST trial pharmacogenetic substudy, against placebo in patients with an ADRB1 Arg389Arg genotype the 4th generation beta-blocker bucindolol reduced the risk of developing AF by 74%, leading to design and performance of the Phase 2 trial GENETIC-AF where 267 high AF risk HFrEF patients were randomized to bucindolol vs. the conventional, 2nd generation compound metoprolol succinate. Overall there was no difference in effectiveness (hazard ratio (HR) 1.01; 95% CI: 0.71, 1.42), but a trend for benefit with bucindolol was observed in the U.S. subgroup (N=127; HR=0.70; 95% CI: 0.41, 1.19) and in patients with implanted devices (N=69; HR=0.75; 95% CI: 0.43, 1.32). The trial exhibited marked regional heterogeneity, which was attributed to 2 countries predominately enrolling patients whose AF diagnosis preceded HF by many years; in countries that enrolled patients with a more contemporaneous presentation of AF and HF bucindolol was associated with a positive efficacy signal.
Translational Outlook.
The theoretical basis for bucindolol’s advantage over conventional beta-blockers for preventing AF and reducing HF events in HFrEF patients who are genotype ADRB1 Arg389Arg is its more powerful inhibition of the higher functioning Arg389 polymorphic variant of the beta1-adrenergic receptor. The ADRB1 Arg389Gly polymorphism is not present in other species but can be and has been investigated by transgenic overexpression in mice. In terms of the potential for reverse translation, precision therapeutic phenotyping in GENETIC-AF identified a group of patients in whom AF developed many years prior to HF who did not respond favorably to bucindolol, suggesting different pathophysiology compared to patients who develop AF and HF contemporaneously. This putative pathophysiologic difference and its impact on therapy, potentially related to a greater burden of atrial and ventricular fibrosis associated with longstanding AF, could be translationally investigated in animal models of AF and HF.
Funding:
ARCA biopharma
Disclosure Information
GENETIC-AF was sponsored by ARCA biopharma. JPP receives research funding from ARCA, Boston Scientific, Gilead, Janssen Pharmaceuticals, Spectranetics, and St Jude Medical and serves as consultant to Allergan, Amgen, GlaxoSmithKline, Johnson & Johnson, Medtronic, and Spectranetics. SBW reports research support from Medtronic, Abbott, and Boston Scientific and serves as consultant to ARCA. WTA receives consulting fees from ARCA. PDZ is an employee of Medtronic. MRB is an officer and director of ARCA. CD, DAM, IAC, LLE and GWD are employees of ARCA.
List of Abbreviations
- ADRB1
beta1-adrenergic receptor gene
- AF
atrial fibrillation
- AFL
atrial flutter
- Arg
arginine
- DTRI
diagnosis to randomization index
- DxT
Time fror initial diagnosis to randomization
- HF
heart failure
- HFlrEF
HF with lower-range ejection fraction (LVEF < 0.40)
- HFmrEF
HF with mid-range ejection fraction (0.40 ≤ LVEF < 0.50)
- HFrEF
HF with reduced ejection fraction (LVEF < 0.50)
- ICM
insertable cardiac monitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Trulock KM, Narayan SM, Piccini JP. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol 2014;64:710–21. [DOI] [PubMed] [Google Scholar]
- 2.Olsson LG, Swedberg K, Ducharme A et al. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol 2006;47:1997–2004. [DOI] [PubMed] [Google Scholar]
- 3.Wang TJ, Larson MG, Levy D et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920–5. [DOI] [PubMed] [Google Scholar]
- 4.Turagam MK, Garg J, Whang W et al. Catheter Ablation of Atrial Fibrillation in Patients With Heart Failure: A Meta-analysis of Randomized Controlled Trials. Ann Intern Med. 2019;170(1):41–50.. [DOI] [PubMed] [Google Scholar]
- 5.Marrouche NF, Brachmann J, Andresen D et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N Engl J Med 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 6.Nasr IA, Bouzamondo A, Hulot JS, Dubourg O, Le Heuzey JY, Lechat P. Prevention of atrial fibrillation onset by beta-blocker treatment in heart failure: a meta-analysis. Eur Heart J 2007;28:457–62. [DOI] [PubMed] [Google Scholar]
- 7.Bristow MR, Krause-Steinrauf H, Nuzzo R et al. Effect of Baseline or changes in adrenergic activity on clinical outcomes in the beta-blocker evaluation of survival trial (BEST). Circulation 2004;110:1437–42. [DOI] [PubMed] [Google Scholar]
- 8.Liggett SB, Mialet-Perez J, Thaneemit-Chen S et al. A polymorphism within a highly conserved β1-adrenergic receptor motif alters beta-blocker response in multiple models and human heart failure. Proc Natl Acad Sci 2006;103:11288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connor CM, Fiuzat M, Carson PE et al. Combinatorial pharmacogenetic interactions of bucindolol and beta1, alpha2C adrenergic receptor polymorphisms. PLoS One 2012;7:e44324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aleong RG, Sauer WH, Davis G, et al. Prevention of atrial fibrillation by bucindolol is dependent on the beta-1 389 Arg/Gly adrenergic receptor polymorphism. JACC Heart Fail 2013;1:338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sehnert AJ, Daniels SE, Elashoff M et al. Lack of association between adrenergic receptor genotypes and survival in heart failure patients treated with carvedilol or metoprolol. J Am Coll Cardiol 2008;52:644–51. [DOI] [PubMed] [Google Scholar]
- 12.White HL, de Boer RA, Maqbool A et al. An evaluation of the beta-1 adrenergic receptor Arg389Gly polymorphism in individuals with heart failure: a MERIT-HF sub-study. Eur J Heart Fail 2003;5:463–8. [DOI] [PubMed] [Google Scholar]
- 13.Piccini JP, Connolly SJ, Abraham WT et al. A genotype-directed comparative effectiveness trial of Bucindolol and metoprolol succinate for prevention of symptomatic atrial fibrillation/atrial flutter in patients with heart failure: Rationale and design of the GENETIC-AF trial. Am Heart J 2018;199:51–58. [DOI] [PubMed] [Google Scholar]
- 14., van Veldhuisen DJ, Aass H, El Allaf D, Dunselman PH, Gullestad L, Halinen M, Kjekshus J, Ohlsson L, Wedel H, Wikstrand J and Group M-HS. Presence and development of atrial fibrillation in chronic heart failure. Experiences from the MERIT-HF Study. Eur J Heart Fail 2006;8:539–46. [DOI] [PubMed] [Google Scholar]
- 15.Nergårdh AK, Rosenqvist M, Nordlander R, Frick M. Maintenance of sinus rhythm with metoprolol CR initiated before cardioversion and repeated cardioversion of atrial fibrillation: a randomized double-blind placebo-controlled study. Eur Heart J 2007;28:1351–7. [DOI] [PubMed] [Google Scholar]
- 16.BEST Investigators. Beta-Blocker Evaluation of Survival Trial I. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001;344:1659–67. [DOI] [PubMed] [Google Scholar]
- 17.MERIT-HF Investigators. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001–7. [PubMed] [Google Scholar]
- 18.Sarkar S, Koehler J, Crossley GH et al. Burden of atrial fibrillation and poor rate control detected by continuous monitoring and the risk for heart failure hospitalization. Am Heart J 2012;164:616–24. [DOI] [PubMed] [Google Scholar]
- 19.Spiegelhalter DJ, Freedman LS, Blackburn PR. Monitoring clinical trials: conditional or predictive power? Control Clin Trials. 1986;7(1):8–17. [DOI] [PubMed] [Google Scholar]
- 20.Berry SM, Spinelli W, Littman GS, Liang JZ, Fardipour P, Berry DA, Lewis RJ, Krams M. A Bayesian dose-finding trial with adaptive dose expansion to flexibly assess efficacy and safety of an investigational drug. Clin Trials. 2010;7:121–35. [DOI] [PubMed] [Google Scholar]
- 21.Steinhubl SR, Waalen J, Edwards AM et al. Effect of a Home-Based Wearable Continuous ECG Monitoring Patch on Detection of Undiagnosed Atrial Fibrillation: The mSToPS Randomized Clinical Trial. JAMA 2018;320:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turakhia MP, Desai M, Hedlin H et al. Rationale and design of a large-scale, app-based study to identify cardiac arrhythmias using a smartwatch: The Apple Heart Study. Am Heart J 2019;207:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23., Wilson FP, Parikh CR. Translational Methods in Nephrology: Individual Treatment Effect Modeling. Am Soc Nephrol. 2018;29:2615–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toso E, Blandino A, Sardi D, Battaglia A, Garberoglio L, Miceli S, Azzaro G, Capello AL, Gaita F. Electrical cardioversion of persistent atrial fibrillation: acute and long-term results stratified according to arrhythmia duration. Pacing Clin Electrophysiol 2012;35:1126–34. [DOI] [PubMed] [Google Scholar]
- 25.Bunch TJ, May HT, Bair TL, et al. Increasing time between first diagnosis of atrial fibrillation and catheter ablation adversely affects long-term outcomes. Heart Rhythm 2013;10:1257–62. [DOI] [PubMed] [Google Scholar]
- 26.Aleong RG, Sauer WH, MD, Davis G, Bristow MR. New onset atrial fibrillation predicts heart failure progression. Am J Med. 2014;127:963–71. [DOI] [PubMed] [Google Scholar]
- 27.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation 1999;100:87–95. [DOI] [PubMed] [Google Scholar]
- 28.Dzeshka MS, Lip GY, Snezhitskiy V, Shantsila E. Cardiac Fibrosis in Patients With Atrial Fibrillation: Mechanisms and Clinical Implications. J Am Coll Cardiol 2015;66:943–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


