Abstract
When switching languages, bilinguals recruit a ‘language control network’ that overlaps with brain regions known to support general cognitive control, but it is unclear whether these same regions are recruited in passive comprehension of language switches. Using fMRI with a blocked design, 24 Spanish-English bilinguals silently read 36 paragraphs in which the default language was Spanish or English, and that had either 1) no switches, 2) function word switches or 3) content word switches. Relative to no switches, function switches activated the right IFG, bilateral MFG, and left IPL/ SMG. In contrast, switching on content words produced limited neural switching costs, and language dominance effects were also small. Finally, neural switching costs in silent reading were correlated with switching costs in cued picture-naming. Seemingly passive reading comprehension involves brain regions known to support cognitive control in active switching during production, possibly reflecting the operation of a modality-general switch mechanism.
Keywords: bilingualism, language switching, fMRI, cognitive control, neural switch costs
An important human ability known as cognitive control enables flexibly changing between tasks, maintenance of multiple concepts active simultaneously, and inhibition of irrelevant responses in the service of goal-directed behavior. A related form of control is exercised by bilinguals, who must be able to manage dual-language activation to enable switching between languages when needed, while also avoiding switching by mistake. In the spotlight of current research on bilingualism is the extent to which mechanisms responsible for selecting which language to speak overlap with non-linguistic cognitive control processes (Abutalebi et al., 2012; for reviews see Abutalebi & Green, 2008; Bialystok, Craik, & Luk, 2012; Declerck & Philipp, 2015). Consistent with the possibility of shared mechanisms are studies in which bilinguals outperformed monolinguals in nonlinguistic task-switching (Prior & MacWhinney, 2010; Prior & Gollan, 2011; Stasenko, Matt, & Gollan, 2017), and inhibitory control (Bialystok, 2011; but see Hilchey & Klein, 2011; Paap et al., 2017). Further support for a cognitive control advantage for bilinguals are findings that lifelong bilingualism is associated with a delayed onset of cognitive decline due to Alzheimer’s disease (for review see Bak & Alladi, 2014; Guzmán-Vélez and Tranel, 2015). However, this proposal remains controversial, as others have suggested that bilingual advantages are difficult to replicate and are highly variable (Lehtonen et al., 2018; Paap, Johnson, & Sawi, 2015; Paap & Sawi, 2014).
Neuroimaging studies also suggest overlap in mechanisms involved in language control and cognitive control (Abutalebi et al., 2012; Abutalebi & Green, 2007, 2008; Blanco-Elorrieta & Pylkkänen, 2016; de Baene, Duyck, Brass, & Carreiras, 2015; Garbin et al., 2010; Ma et al., 2014; Wang et al., 2007; Wang, Kuhl, Chen, and Dong, 2009). For instance, bilinguals activated regions of the brain hypothesized to support language control during nonlinguistic task switching (Branzi, Della Rosa, Canini, Costa, & Abutalebi; 2016; de Baene et al., 2015; Garbin et al., 2010), and switch trials in linguistic and nonlinguistic tasks elicited considerable overlap in activated brain regions, suggesting a shared “switch mechanism” (Weissberger et al., 2015). Abutalebi and Green (2007; 2008; 2016; see also Green & Abutalebi, 2013) defined a “Language Control Network” which includes the dorsal anterior cingulate cortex (dACC), left caudate nucleus (CN), supramarginal gyrus (SMG), dorsolateral prefrontal cortex (dlPFC), and inferior frontal gyrus (IFG), regions also recruited for nonlinguistic control tasks (for a meta-analysis of fMRI studies of language switching see Luk et al., 2011).
A surprising gap in research on bilingual language switching is that the vast majority of studies focused on switches in speech production—even though language switches must be both produced and comprehended. Presumably, people spend more time comprehending than producing language and thus, the extent to which bilingualism entails an exercise in cognitive control might be much broader if the same cognitive control regions that support switching in production also support comprehension. A widely cited model of bilingual language processing assumes that similar brain mechanisms support production and comprehension of switches (e.g., van Heuven & Dijkstra, 2010; Grainger, Midgley, & Holcomb, 2010). While behavioral switching costs (i.e., the increase in reaction time when bilinguals need to switch languages compared to when they use the same language as the previous response) are less robust in comprehension than production (Declerck, Koch, Duñabeitia, Grainger, & Stephan, 2019; Declerck & Philipp, 2015), event-related potential (ERP) studies found that switches elicited an increased N400 component in sentence comprehension (e.g., Jackson et al., 2004; Moreno et al., 2002; Proverbio et al., 2004;). Switching may require more cognitive control in production than comprehension, because only in production do bilinguals need to choose between translation-equivalent alternatives, suppressing dual-language activation, whereas in comprehension whatever meanings and representations become active can remain active so long as they fit the intended meaning, without requiring inhibition.
The debate on whether language switches elicit processing costs in comprehension has been extended to neuroimaging studies; and here too, the findings are mixed. Abutalebi et al., (2007) reported significant activation in the caudate and the ACC for auditory perception of language switches, in particular for switching into the less-exposed language. They interpreted this pattern of activation as a switch cost at the neural level, similar to what is reported in production. One MEG study directly compared language switches in both production and comprehension, and to a nonlinguistic switching task, and found that switches in speech activated the DLPFC, a region that was also active in nonlinguistic switching, whereas switches in comprehension instead activated the left ACC, a region not associated with nonlinguistic switching in this study (Blanco-Elorrieta & Pylkkänen, 2016). In a follow-up study, both the DLPFC and ACC were implicated for language switches in comprehension, but no costs were found in these regions when switches were supported by more ecologically valid context (described in detail in the Discussion; Blanco-Elorietta & Pylkkänen, 2017). Finally, Hut, Helenius, Leminen, Mäkelä, and Lehtonen (2017) used MEG to examine trilingual language switching in auditory comprehension in early Finnish-Swedish bilinguals who learned English later in life. In this study, switching from English to either of the two native languages elicited a neural cost in the superior temporal gyrus (a region identified in the meta-analysis by Luk et al., 2011), but switching between the two native languages elicited no switch cost.
Given relatively few and mixed findings, it remains an open question if bilinguals rely on cognitive control regions when comprehending mixed-language speech. The majority of studies to date have focused on out-of-context speech in behavioral and neuroimaging studies, often requiring an explicit decision task such as semantic categorization, which likely misses critical aspects of bilingual language control as it occurs in more naturalistic use. Discrepant findings between ERP and behavioral studies could arise if behavioral measures are not as sensitive.
To that end, we used functional MRI to elucidate the neural regions involved in switching during silent reading by presenting full paragraphs written mostly in one language but with a handful of language switches, using a blocked design. We hypothesized that if switching during silent reading recruits the same language control network as observed in production, the same regions observed during production (e.g., DLPFC, ACC/SMA, IFG, caudate, SMG) will be recruited during switching in silent reading (as compared to non-switching). In previous work, when bilinguals read aloud mixed-language paragraphs, they produced intrusion errors (i.e., failures to switch) mostly on language-switched function words, and relatively infrequently on switched content word targets—a highly robust part-of-speech effect (Gollan & Goldrick, 2016; 2018; Gollan, Schotter, Gomez, Murillo, & Rayner, 2014;). These results suggest that, at least under some circumstances, controlling or monitoring selection of function words is more demanding for cognitive control mechanisms. As such, we hypothesize that conditions with function words (vs. content) switches should elicit more activation when compared against no switch conditions. However, because the requirement to produce switches is removed in silent reading, language control demands may change. For instance, it is possible that we would observe more robust neural costs for content than function words, since content words are prominent when prioritizing meaning during reading comprehension and are less likely to be skipped. Further, a prominent theory posits that unbalanced bilinguals use inhibition to switch between their languages, possibly inhibiting the dominant language to a greater degree (Inhibitory Control Model; Green, 1998). If inhibitory control also supports language switching in comprehension (e.g., Hut et al., 2017), we could observe greater activation of control regions for silent reading of paragraphs written primarily in the non-dominant language, i.e., Spanish-default paragraphs that required switching into English than vice versa. Finally, to provide further evidence on the nature of control mechanisms recruited during silent reading, after the scanning session we tested participants on two production-based language switching tasks (reading aloud of mixed-language paragraphs, picture naming with cued language switches), and examined correlations between neural costs of switching in silent reading and behavioral measures of switching in speech.
Methods
Participants
Participants were 24 right-handed (18 female) English-dominant Spanish-English bilinguals between 18 to 27 years of age, who were primarily undergraduates at the University of California, San Diego (UCSD) and participated for monetary compensation and/or course credit. Bilinguals acquired both languages by age 7, and were classified as English dominant using a picture naming test (the Multilingual Naming Test; MINT; Gollan, Weissberger, Runnqvist, Montoya, & Cera, 2012); on average picture-naming test (MINT) scores were 13 (SD = 3; range = 5–17) points higher in English than in Spanish. Bilinguals were recruited from previous experiments that did not involve language switching or reading aloud. The minimum Spanish MINT score was 43/68. These strict selection criteria were applied to ensure inclusion of a relatively homogenous group of bilinguals who were proficient in both languages but also English dominant. See Table 1 for more details about the participants’ language background and demographic characteristics. Participants were excluded for a history of significant head trauma, other neurologic or major psychiatric disorders, alcohol or substance abuse, current pregnancy, and metal objects in the body due to MRI specifications.
Table 1.
Participant demographic and bilingual language background characteristics from the Language History Questionnaire and proficiency testing
| Participant Characteristic | M | SD |
|---|---|---|
| Age | 20.0 | 2.5 |
| Years of education | 13.3 | 1.6 |
| % female | 75 | -- |
| % right-handed | 100 | -- |
| % Hispanic | 96 | -- |
| Age of first exposure to English (years) | 4.1 | 1.7 |
| Age of first exposure to Spanish (years) | 0.2 | 0.4 |
| % English daily use currently | 81.8 | 11.5 |
| % English daily use when growing up | 58.5 | 10.9 |
| Self-rated English speaking proficiencya | 6.8 | 0.5 |
| Self-rated English reading proficiencya | 6.7 | 0.7 |
| Self-rated English writing proficiencya | 6.5 | 0.7 |
| Self-rated English listening proficiencya | 6.9 | 0.4 |
| Self-rated Spanish speaking proficiencya | 6.3 | 0.7 |
| Self-rated Spanish reading proficiencya | 6.3 | 0.7 |
| Self-rated Spanish writing proficiencya | 5.8 | 1.0 |
| Self-rated Spanish listening proficiencya | 6.8 | 0.4 |
| Average caregiver education (years) | 11.5 | 3.9 |
| Average years lived abroad | 2.0 | 2.9 |
| Average English (dominant) MINTb | 61.8 | 2.7 |
| Average Spanish (non-dominant) MINTb | 48.9 | 3.9 |
| Average MINT differenceb | 12.9 | 3.3 |
Self-ratings are based on a 7-point scale: 1 = almost none; 2 = very poor; 3 = fair; 4 = functional; 5 = good; 6 = very good; 7 = like a native speaker
Maximum score is 68 points
Materials and Procedure
fMRI paragraph reading task
A native Spanish-English bilingual research assistant adapted 36 paragraphs from published English-Spanish translations of short stories (modified from previous studies; Gollan & Goldrick, 2016; 2018; Gollan et al., 2014) that ranged from 100–120 words (M=108, SD=4.8) to ensure that an entire paragraph could be projected onto a screen at the foot of the scanner bed. A second native Spanish-English bilingual research assistant checked for errors and confirmed the intended manipulations. The design consisted of a 2 (language) by 3 (condition) structure in which each paragraph was written either primarily in English, or English-default, or primarily in Spanish, or Spanish-default, and either had no language switches, single-language, or had switches on function words or switches on content words. Thus each of the 36 paragraphs was modified so it could be presented in all 6 of the different conditions between subjects. An example paragraph and its condition-specific adaptations are presented in the Appendix. There were 12 language switches in each paragraph (i.e., 6 switch-out of default and 6 switch-back to default points), which were distributed evenly throughout the paragraph. Switch word manipulations were designed to be as natural as possible (though single word switches on function word targets do not conform to habitual constraints on spontaneously occurring switches, but are frequent targets of unintended language switches or intrusion errors; Muysken, 2000; Poulisse & Bongaerts, 1994). None of the switch words were cognates (i.e., translation equivalents that overlap in orthography and phonology such as lemon and limón), proper names, or long words (i.e., words ranged from 1–12 letters) and the same switch word did not appear more than twice in a given paragraph.
Paragraphs were presented in one of six different fixed-order lists; each bilingual was presented with one of these lists. For each list, participants completed 6 consecutive runs of six paragraphs (i.e., blocks; 36 total), with one paragraph per condition in each run (thus each bilingual saw a paragraph in each of the 6 conditions before reading a second paragraph in any of the 6 conditions). In each of the six lists, each paragraph was presented just once. Because the lists were designed for 24 subjects, across all lists, a given paragraph appeared a) four times in each of the six paragraph conditions, b) six times in each of the four run numbers, and c) six times in each of the four within-run positions.
fMRI paragraph reading task procedure
Two practice mixed-language paragraphs were completed prior to the scan to familiarize the participant with the task. During the scan, participants were asked to read the paragraph silently, without moving their head or lips, at a comfortable pace and to press a button when finished reading each paragraph (pilot data indicated that 30 seconds was sufficient to complete reading most if not all paragraphs for most bilinguals). Paragraphs were presented in a fixed-block design with 6 functional runs, where each run consisted of 6 blocks (i.e., 6 paragraphs), which represented all critical conditions. In each run, a fixation cross (15 s) preceded the presentation of each paragraph (30 s), and each run ended with a final fixation cross (15s), for a total time of 4 minutes and 45 seconds (see Figure 1). Stimuli were presented via an LCD projector on a screen at the end of the scanner bed, and were viewed through a mirror mounted on the head cage. A fiber-optic button box designed for use in the magnet recorded button presses. Stimuli were presented with a MacBook Pro laptop using PsychoPy v1.81.
Figure 1.
Order of events and example paragraph stimuli used in the fMRI task.
Post-scan behavioral tasks
Paragraph reading aloud
After the scan, participants read aloud an additional set of paragraphs to provide a measure of individual differences in production of intrusion errors, to examine associations with comprehension of similar switches in the neural data. For the read-aloud task, six paragraphs were selected from news articles (i.e., excerpts from New York Times, CNN, and Huffington Post) that had a mean length of 205 words (SD = 7.3). Half of the paragraphs were English-default and half were Spanish-default. Paragraphs were modified to contain 12 switch words on function words (i.e., 24 switches in total when counting switches out and switches back to the default language). The focus on function words for the read aloud task was because content word switches elicited very few intrusion errors in previous studies (e.g., Gollan et al., 2014; Gollan & Goldrick, 2018). Six different fixed lists were created and counterbalanced and rotated across 24 participants using a Latin Square design. Across all participants, each paragraph was in the first, middle and last position equally often; and every default language was presented as first or second. See example of an English-default and a Spanish-default read-aloud paragraph in Appendix B.
Language switching in single picture naming
To obtain an additional measure of language switching ability in production, participants completed a picture naming task with cued language switches adapted from a previous study (see Kleinman & Gollan, 2016; Experiment 1a for full materials and details; the pseudo-voluntary “bottom-up” block was excluded).1 Participants named 9 black-and-white line drawings of pictures in either English or Spanish based on a visual cue (i.e., a Mexican or a U.S. flag). Bilinguals completed cued switching blocks (consisting of nonswitch or stay trials, and language switch trials), and English-only and Spanish-only blocks (single-language trials), in fully counterbalanced order. There were 108 critical trials in each block. Each trial began with a fixation cross (presented for 350ms) followed by a 150 ms blank screen. A language cue appeared on the screen above the center of the fixation. After 250 ms, the target picture appeared in the center of the screen while the cue remained on the screen. The cue and target remained on the screen until a response, or for a maximum of 3,000ms. An 850 ms inter-trial interval preceded the next trial.
fMRI Specifications and Analysis
Scanning parameters
Participants were scanned on a 3.0 Tesla General Electric (GE) Discovery MR750 whole body imager with an 8-channel head coil. Head movement was constrained with padding and taped to secure head position. A localizer scan was acquired initially to allow selection of the block of slices to be acquired during functional scanning and to assure good head placement in the scanner. Functional BOLD was obtained with a 1-shot gradient echo EPI scan: 22 cm FOV, 64 × 64 matrix, 3.0 mm x 3.0 mm in-plane resolution, TR= 2500 msec, TE= 30 msec, flip angle=90 degrees. Forty-four 3 mm thick sagittal slices covering the whole brain were acquired. Two field maps were collected to correct for distortions in EPI images due to susceptibility artifact. Structural MRI sequence included a high resolution T1-weighted Fast Spoiled Gradient Recall (3D FSPGR) scan to provide anatomic reference: 172 1 mm contiguous sagittal slices, FOV = 25.6 cm, TR = 8 ms, TE = 3.1ms, flip angle = 8, T1 = 600, 256 × 192 matrix, Bandwidth = 31.25 kHZ, frequency direction = S-I, NEX =1, scan time = 8 min 28 sec.
Data processing and analysis
fMRI data were analyzed and overlaid onto structural images with the Analysis of Functional Neuroimaging (AFNI) software package from the NIH (Cox, 1996). To minimize the effects of head motion, each individual’s functional time series was corrected for motion using a three-dimensional iterated, linearized, weighted least-squares method with Fourier interpolation, and time-points with uncorrected motion outliers were excluded from statistical analysis. Slice timing correction was applied and runs were de-trended of low frequency signal drifts. A general linear model (GLM) approach was used on each participant’s time-series to model the hemodynamic response (HDR) associated with every condition using AFNI’s TENT function in 3dDeconvolve. The following predictors were used in the model: a constant, a linear trend, three parameters indicating the degree of motion correction performed in three rotational angles, and 6 stimulus vectors indicating the onset of each individual experimental condition (English default, Spanish default, English default with function switches, English default with content switches, Spanish default with function switches, and Spanish default with content switches). The ‘-gltsym’ command of the TENT function was used to model combined conditions (English default with function switches + Spanish default with function switches; English default with content switches + Spanish default with content switches; English default + Spanish default). An estimated best-fit 7-lag impulse response was used which allowed the hemodynamic response to return to baseline. The modeled hemodynamic responses were subsequently scaled so that beta weights would be equivalent to percent signal change (PSC). Data were smoothed to 6 mm FWHM using AFNI’s 3dBlurToFWHM. Registration to the MNI-152 atlas was performed using FMRIB’s Non-linear Image Registration Tool (FNIRT), part of FSL (http://fsl.fmrib.ox.ac.uk/fsl/), and were resampled at a 3mm3 resolution.
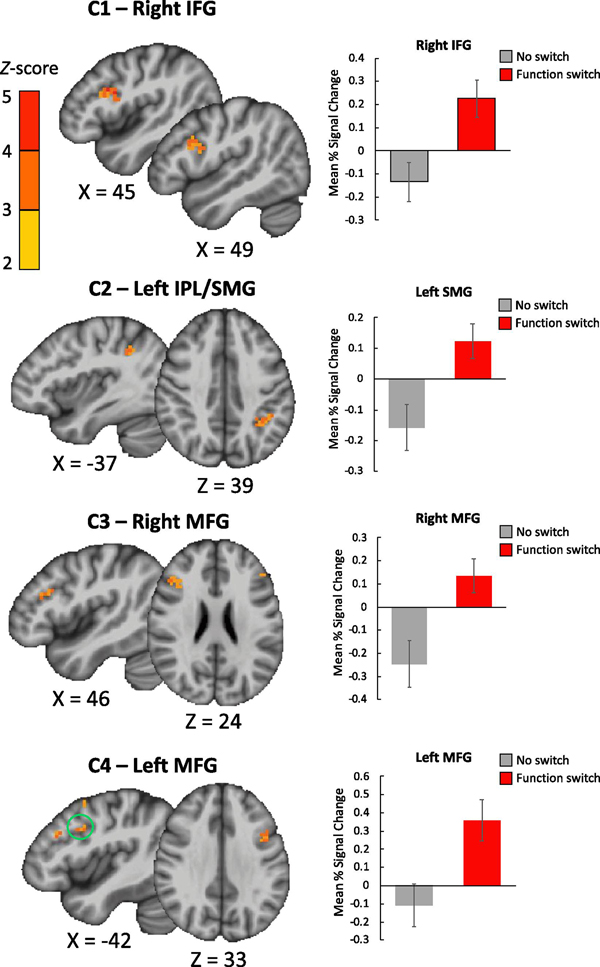
Within-group comparisons for each main contrast of interest—1) Function-word Switches vs Single-Language, 2) Content-word Switches vs Single Language, 3) Function-word Switches vs. Content-word Switches, 4) Spanish-default Switches vs. English-default Switches, 5) Spanish-default Function Switches vs. English-default Function Switches and 6) Spanish-default Content Switches vs. English-default Content Switches, were conducted using voxel-wise paired samples t-tests with percent signal change as the dependent variable (using AFNI’s 3dttest++). Analyses were restricted to a small number of a priori regions of interest (ROIs), as per the recommendation to improve power and reduce an inflated false discovery rate. Five bilateral ROIs associated with cognitive and language control were selected based on prior findings (Abutalebi & Green, 2008; Luk et al., 2011) and included the middle frontal gyrus (MFG; i.e., dorsolateral prefrontal cortex; dlPFC), dorsal anterior cingulate cortex (dACC), inferior frontal gyrus (IFG; pars opercularis and triangularis), supramarginal gyrus (SMG), and the caudate nucleus (CN). ROIs were derived from the Harvard-Oxford atlas. Significance was determined by applying cluster-size correction derived from randomization of voxel-wise t-tests (via AFNI’s Clustsim option in 3dttest++) and then feeding the randomized t-statistic maps onto Monte-Carlo simulations directly for cluster-size threshold determination (using AFNI’s 3dClustSim) as per recommendation in response to reports of inflated false positive rate in fMRI group analysis tools (Eklund, Nichols, & Knutsson, 2016). To determine cluster significance for each ROI we used bi-sided significance testing, a per voxel threshold of p < 0.01, and a Bonferroni-corrected (for 5 ROIs) cluster-wise alpha threshold of p < 0.01 (results meeting a cluster-wise p < 0.05 threshold were reported, and especially for contrasts with halved power—i.e., contrasts 5 and 6 above—are highlighted in Table 2 and interpreted with caution). At the ROI level, the required minimum cluster size was 459 mm^3 (17 contiguous voxels) for the MFG, 486 mm^3 (18 contiguous voxels) for the dorsal ACC, 648 mm^3 (24 contiguous voxels) for the IFG, 540 mm^3 (20 contiguous voxels) for the SMG and 135 mm^3 (5 contiguous voxels) for the caudate.
Table 2.
Brain regions showing significantly higher BOLD response in our regions of interest for 1) reading paragraphs with function switches compared to no switches, 2) reading paragraphs with content switches compared to no switches, and 3) reading paragraphs primarily written in Spanish with English function switches compared to paragraphs primarily in English with Spanish function switches.
| Contrast and Cluster Number | Hemisphere | Anatomical region | BA/Subregion | Cluster volume (mm3) | CM MNI coordinates (x, y, z)* | Z-value at peak intensity | Effect size (r) | Mean PSC | 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| 1) Function-word Switches vs. Single Language | |||||||||
| C1 | R | Inferior Frontal Gyrus | BA 44;45 | 1,188 | 46.1, 12.9, 24.3 | 4.3 | 0.73 | 0.36 | [0.23, 0.50] |
| C2 | L | Inferior Parietal Lobule (Supramarginal Gyrus) | BA 40 | 675 | −36.8, −46.6, 39.5 | 3.4 | 0.70 | 0.28 | [0.17, 0.39] |
| C3 | R | Middle Frontal Gyrus | BA 46;9 | 621 | 41.1, 26.2, 23.8 | 3.5 | 0.66 | 0.38 | [0.22, 0.58] |
| C4 | L | Middle Frontal Gyrus | BA 46;9 | 486 | −44.0, 9.3, 34.3 | 3.3 | 0.59 | 0.47 | [0.27, 0.67] |
| 2) Content-word Switches vs. Single Language** | |||||||||
| L | Inferior Frontal Gyrus | BA 44;45 | 405 | −40.9, 25.2, 18.1 | 3.5 | 0.62 | 0.34 | [0.17, 0.52] | |
| 3) Spanish-default Function Switches vs. English-default Function Switches** | |||||||||
| R | Inferior Parietal Lobule (Supramarginal Gyrus) | BA 40 | 378 | 45.5, −40.5, 51.5 | 3.3 | 0.65 | 0.08 | [0.05, 0.12] | |
PSC = percent signal change; 95% confidence intervals (CI) from 1000 bootstrapping samples using a paired-sample t-test.
Coordinates reflect center of mass (CM) of resulting cluster.
Clusters for these two contrasts did not survive a more stringent correction for multiple comparisons (i.e., these results are presented at a corrected cluster-wise threshold of p < .05 rather than .01; see Methods) and should be interpreted with caution.
Behavioral data analysis
For the post-scan paragraph reading aloud task, a native Spanish-English bilingual research assistant transcribed and classified errors as intrusions (n = 231; e.g., saying “la” instead of “the”), and partial intrusions (n = 61; e.g., starting to produce an intrusion but self-correcting before producing the error). The sum of intrusions and partial intrusions produced by each participant, separated by default language, was used for brain-behavioral correlations reported below.
For the post-fMRI language switching picture naming task, incorrect responses and RTs that were 2.5 standard deviations above or below each participant’s mean RT were excluded from analyses. Mean reaction time (RT) for single, stay, and switch trials were calculated. To examine the associations between the neural cost of switching in reading and the behavioral cost of switching in production, switch costs (RT of switch trials – RT stay trials in mixed blocks) and mixing costs (RT of stay trials in mixed blocks – RT of single trials in single blocks) were calculated to be used as difference scores in the correlations reported below.
Brain-behavior correlations
Percent signal change was extracted from each significant cluster reported in Table 2 (i.e., for function-word switches only) for each individual and condition. Difference scores representing the neural cost of switching were calculated by subtracting the mean percent signal change observed for single-language paragraphs from the mean percent signal change observed for paragraphs with function-word switches for each participant. These values were then correlated with behavioral data using Pearson bivariate correlations. Given the exploratory nature of correlations between neural and behavioral data, we focus on correlations that survived a false discovery rate (FDR) for multiple comparisons using the Benjamini-Hochberg procedure (Thissen, Steinberg, & Kuang, 2002; Williams, Jones & Tukey, 1999). A cut-off of p = .029 was determined by arranging p-values from lowest and highest and choosing the largest p-value that was smaller than the Benjamini-Hochberg critical value as threshold (accepting a FDR of 15%).
Results
fMRI results
Our main questions of interest included: 1) Does silent reading of paragraphs with language switches incur a neural switching cost in the same language control regions as observed in studies of overt language switching (in production)? 2) Does recruitment of language control areas in silent reading differ by part of speech—i.e., content versus function switch words? and 3) Does language control differ for reading Spanish-default versus English-default paragraphs? We combined conditions to maximize power for these 3 main contrasts of interest, such that two conditions were always compared to two conditions in the voxel-wise student t-tests (with 12 paragraphs in each condition). To illustrate, to compute the neural cost associated with function word switching, two conditions were combined to create the overall switching condition (English-default function switches and Spanish-default function switches) and were tested against the combination of two single language conditions (i.e., English-only and Spanish-only paragraphs). Cluster location with coordinates and corresponding Z-values are shown in Table 2.
1). Function-word Switches vs. Single-Language
ROI analyses revealed four significant clusters in the Function-word Switches versus Single-Language contrast; bilinguals showed increased activation when silently reading paragraphs with switches on function words compared to single-language paragraphs without switches in the right IFG, left SMG, and the right and left MFG—or the DLPFC (cluster C1 to C4; see Table 2a and Figure 2).
Figure 2.
Clusters showing significantly greater activation for reading paragraphs requiring switching languages on function words vs. single-language paragraphs in the right IFG (C1), left SMG (C2) and bilateral MFG—or the DLPFC region (C3 and C4). When there are multiple clusters depicted, a green circle represents the significant cluster. Error bars represent the standard error of the mean percent signal change.
2). Content-word Switches vs. Single-Language
In contrast to function-word switches, which revealed a number of significant results, when comparing Content-word Switches to Single-Language paragraphs, no clusters survived the most stringent correction. Only one cluster was significant using a cluster threshold of p < 0.05; this was in the left IFG (Table 2a and Figure 3).
Figure 3.
Cluster in the left IFG showing significantly greater activation for reading paragraphs requiring switching languages on content words vs. single-language paragraphs. The green circle represents the significant cluster. Error bars represent the standard error of the mean percent signal change.
3). Function-word Switches vs. Content-word Switches
Analysis 1 revealed many significant results, whereas Analysis 2 did not, implying a greater neural cost for silent reading of function versus content switches. However, when directly comparing paragraphs with switches on different parts of speech, there were no significant clusters in the same ROIs examined above. As explained above, this analysis collapsed across default-language (to maximize power); a different result emerged in follow-up comparisons presented below.
4). Spanish-default Switches vs. English-default Switches
No significant clusters were identified for switching in Spanish-default paragraphs versus in English-default paragraphs, collapsing across part of speech.
5). Function-word switches in Spanish-default vs. English-default
Increased activation was observed for reading Spanish-default with English function word switches, compared to English-default with Spanish function word switches in the right SMG (Table 2a; Figure 4; this was significant only without the Bonferroni correction, at p < 0.05 cluster threshold level).
Figure 4.
Cluster in the right SMG showing significantly greater activation for reading Spanish-default paragraphs with function switches vs English-default paragraphs with function switches. Error bars represent the standard error of the mean percent signal change.
6). Content word switches in Spanish-default vs. English-default
No significant clusters (even with a p < 0.05 cluster threshold) were identified for switching in Spanish-default with English content word switches, compared to English-default with Spanish content word switches.
Spanish-only vs. English only
Having found some possibly significant differences between switches to Spanish versus English (for function word switches), an important control comparison is needed to determine if the increased activation in the right SMG may simply be an artifact of task difficulty (i.e., if rather than reflecting switching per se, this result reflects reading paragraphs written primarily in Spanish, the non-dominant language for these bilinguals, thus requiring greater recruitment of control regions). To consider this possibility, we compared Spanish-only paragraphs to English-only paragraphs using the more lenient cluster threshold (p < 0.05). No clusters emerged in the right SMG, the region of greatest interest given significant activation for function switches in Spanish-default versus English-default above. Similarly, no clusters emerged in the other four ROIs for this contrast. This suggests that observed activation for switching into English versus switching into Spanish cannot be solely explained by difficulty level.
Behavioral results
Silent paragraph reading RTs during fMRI scan
Mean RTs for each condition, and mean RT costs for reading paragraphs with switches versus single-language paragraphs are reported in Table 3. On some trials participants did not press the button to indicate they had finished reading the paragraph within the allotted time (i.e., 30 seconds; M= 14% of trials, SD = 11%). Of note, one participant’s time-outs were approximately 3 standard deviations above the mean. When this participant was excluded from the analyses reported below, the pattern of activation remained the same. Trials with no responses were coded as the maximum of 30 seconds for the analysis reported below. Of the overall time-outs, 27% were from the Spanish-only condition, 31% from Spanish-default with function switches and 33% from the Spanish-default with content switches. In contrast, only 10% of all timeouts occurred on all English-default paragraphs collapsed (including switch and non-switch paragraphs).
Table 3.
Means and standard deviations (SD) of total paragraph reading times (RT) in seconds, for each condition of interest in the fMRI task, as well as the RT costs associated with reading while switching languages versus reading in a single language
| Paragraph word-order | ||||
|---|---|---|---|---|
| English-default | Spanish-default | |||
| Paragraph Type | M | SD | M | SD |
| Single Language | 20.0 | 3.8 | 25.5 | 2.9 |
| Content Switches | 21.3 | 3.4 | 26.1 | 2.7 |
| Function Switches | 21.8 | 3.4 | 25.7 | 2.7 |
| Content Switch cost | 1.3 | 1.5 | 0.6 | 1.3 |
| Function Switch cost | 1.8 | 1.8 | 0.2 | 1.1 |
A repeated-measures two-way ANOVA with default-language (English, Spanish) and condition (single, function switch, content switch) revealed main effects of default-language, such that bilinguals read English paragraphs faster than Spanish paragraphs (F(1, 23) = 120.4; MSE = 803.8; p < 0.001; η2= 0.8) and condition, such that bilinguals read single-language paragraphs faster than mixed-language paragraphs (F(1, 23) = 14.3; MSE = 15.2; p < 0.001; η2= 0.4). These main effects were qualified by a significant interaction between default-language and condition (F(1, 23) = 8.5; MSE = 7.7; p = .001; η2= 0.3). Follow-up comparisons revealed that in English-default paragraphs, there were significant RT differences between all pairwise conditions; bilinguals read single-language paragraphs slower than paragraphs with content switches, and read paragraphs with function switch slower than those with content switches (ps ≤ 0.04). In contrast, within Spanish-default paragraphs, bilinguals read single-language paragraphs faster than with content switches, but differences between single-language paragraphs and function-word switches, and between function and content word switch paragraphs were not significant (ps = .31 and .24, respectively). When log-transforming reaction times, the main effects and interaction remained unchanged. The difference in sensitivity between English and Spanish-default paragraphs to the switching manipulation could have been magnified by the large number of time-outs in the Spanish-default conditions.
Paragraph reading aloud post-fMRI
Table 4a reports number of intrusion errors bilinguals produced in the read-aloud task separated by each language and switch-out versus switch-back points, and Table 4b reports RTs for each type of paragraph. Bilinguals produced more intrusion errors (t(23) = 4.1; p < 0.001), and also read more slowly (t(23) = 12.2; p < 0.001) Spanish-default than English-default paragraphs, in line with previous findings (Gollan et al., 2014; Gollan & Goldrick, 2018). To maximize power, we collapsed switch-out and switch-back points, and included partial intrusions, when correlating intrusions with neural data2.
Table 4.
(a) Mean, standard deviation (SD), and minimum and maximum number of intrusion and partial intrusion errors produced by participants in the read-aloud task.
| Language | Error | Switch-out | Switch-back | ||||||
| Spanish-default | M | SD | Min | Max | M | SD | Min | Max | |
| Intrusions | 5.4 | 4.0 | 1 | 16 | 1.1 | 1.4 | 0 | 5 | |
| Partial intrusions | 0.4 | 0.6 | 0 | 2 | 1.1 | 1.2 | 0 | 4 | |
| English-default | |||||||||
| Intrusions | 2.7 | 1.7 | 0 | 6 | 0.6 | 0.8 | 0 | 2 | |
| Partial intrusions | 0.2 | 0.4 | 0 | 1 | 0.8 | 0.8 | 0 | 3 | |
| (b) Mean and standard deviation (SD) for reaction times in the read-aloud task | |||||||||
| Paragraph Type | M | SD | |||||||
| Spanish-default with English switches | 100.2 | 20.8 | |||||||
| English-default with Spanish switches | 73.6 | 12.8 | |||||||
Production of language-switches in single picture naming post-fMRI
One bilingual did not complete the picture-naming task. Table 5 reports mean RTs (5a) and error rates (5b) for each trial type, separated by language, as well as difference scores (switch and mixing costs). Table 5c reports mean intrusion errors produced (i.e., naming the picture correctly but not in the language that matched the cue) separated by language of the trial. Bilinguals produced a similar number of intrusions on cued Spanish versus cued English naming trials (t < 1), and a low number of intrusions; thus we collapsed across language for this variable in the correlations.
Table 5.
Means and standard deviations for (a) reaction time (ms) and (b) error rates (%). Table 5c additionally shows minimum and maximum by participant number of picture naming trials produced in the wrong language (i.e., failing to match the cue).
| (a) | ||||
| Language | ||||
| English | Spanish | |||
| Trial Type | M | SD | M | SD |
| Single | 679 | 80 | 674 | 63 |
| Stay | 827 | 117 | 774 | 96 |
| Switch | 892 | 133 | 847 | 137 |
| Mixing cost | 148 | 80 | 100 | 68 |
| Switch cost | 65 | 53 | 73 | 67 |
| (b) | ||||
| Language | ||||
| English | Spanish | |||
| Trial Type | M | SD | M | SD |
| Single | 0.01 | 0.02 | 0.03 | 0.06 |
| Stay | 0.03 | 0.04 | 0.04 | 0.07 |
| Switch | 0.06 | 0.06 | 0.06 | 0.08 |
| Mixing cost | 0.03 | 0.03 | 0.01 | 0.02 |
| Switch cost | 0.03 | 0.05 | 0.02 | 0.05 |
| (c) | ||||
| Language of trial | M | SD | Min | Max |
| Spanish | 1.9 | 3.4 | 0 | 16 |
| English | 2.1 | 1.9 | 0 | 9 |
Switch costs
RTs for condition means were entered into a two-way repeated measures analysis of variance (ANOVA) with language of trial (English, Spanish) and trial type (stay, switch), as repeated measures factors. Participants exhibited significant switch costs, i.e., a main effect of trial type, F(1,22) = 45.4, MSE = 109,415.7; p < 0.001; η2 = 0.7, and responded significantly slower on English trials, a reversed language dominance effect (F (1,22) = 22.0, MSE = 54,683.7; p < 0.001; η2 = 0.5). Switch costs were similarly sized in English and Spanish; the interaction between language and trial type was not significant (F < 1; p = .59). The same ANOVA, but replacing RTs with error rates (in percentage) as the dependent variable, revealed only a main effect of trial type, such that there were significant switch costs in error rates, (F (1,22) = 8.7, MSE = 0.012; p < 0.01; η2 = 0.3).
Mixing costs
A two-way repeated measures ANOVA with language of trial (English, Spanish) and trial type (single, stay), as repeated measures factors to compare non-switch trials across single versus mixed language testing blocks showed that bilinguals paid significant mixing costs, i.e., a main effect of trial type, F(1,22) = 86.2, MSE = 353,776.9; p < 0.001; η2 = 0.8, and named pictures more slowly on English trials, a reversed dominance effect (F (1,22) = 5.7, MSE = 18,687.3; p < 0.05; η2 = 0.2). These main effects were qualified by a significant interaction, such that the mixing costs were bigger in English than in Spanish trials—a mixing cost asymmetry (F (1,22) = 10.0, MSE = 13,690.7; p < 0.01; η2 = 0.3). Another ANOVA with the same trial structure and error rates as the dependent measure revealed a main effect of trial type, such that bilinguals paid significant mixing costs in error rates (F (1,22) = 17.0, MSE = 0.008; p < 0.01; η2 = 0.4), and mixing costs were greater in English than in Spanish (F (1,22) = 7.7, MSE = 0.002; p = 0.01; η2 = 0.3). The main effect of language was not significant in errors, (F (1,22) = 2.1, MSE = 0.008; p = 0.17; η2 = 0.3).
Exploratory correlations between fMRI BOLD response during silent reading and behavioral language switching
To further investigate relationships between cognitive and neural mechanisms underlying switching in silent reading and switching in speech production, we examined correlations, shown in Table 6, between neural costs of switching in silent reading (i.e., fMRI BOLD response) and switching and mixing costs in our picture-naming task, as well as intrusion errors produced in the read-aloud task. If a common language control mechanism serves both production and comprehension of switches, there should be correlations between neural switching costs in silent reading and measures of switching in speech production. Given the similarity between the two paragraph tasks, we anticipated a higher likelihood of finding correlations between neural data and the reading aloud task administered outside the scanner. Additionally, our blocked design did not allow us to distinguish switching from mixing costs in the neural data; however, correlations between neural data and switching versus mixing costs in the picture naming task could shed light on the cognitive mechanism underlying neural costs (e.g., neural costs measured during silent reading could be correlated with switching, mixing, or both costs, or none).
Table 6.
Associations between fMRI data (i.e., percent signal change in significant clusters observed for function switches > single language; i.e., the neural cost of switching with function words) and behavioral measures of language switching.
| Right MFG | Left MFG | Right IFG | Left SMG | English SC a | Spanish SC a | English MC b | Spanish MC b | Picture-naming Intrusions c | Read-aloud intrusions Eng. default d | Read-aloud intrusions Span. default d | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Right MFG | - | ||||||||||
| Left MFG | .446* | - | |||||||||
| Right IFG | .727** | .554** | - | ||||||||
| Left SMG | .485* | .495* | 0.394 | - | |||||||
| English SC a | 0.400 | .466* | .586** | .478* | - | ||||||
| Spanish SC a | 0.328 | 0.173 | 0.164 | 0.089 | 0.313 | - | |||||
| English MC b | −0.085 | −0.260 | −0.310 | −0.229 | 0.011 | .497* | - | ||||
| Spanish MC b | 0.002 | 0.100 | −0.035 | −0.052 | 0.320 | .517* | .507* | - | |||
| Picture-naming intrusions c | −0.190 | −0.209 | −0.386 | 0.240 | 0.054 | 0.028 | 0.060 | 0.030 | - | ||
| Read-aloud intrusions Eng. default d | −0.014 | −0.081 | 0.005 | 0.210 | 0.414* | 0.304 | 0.484* | 0.377 | 0.383 | - | |
| Read-aloud intrusions Span. default d | −0.122 | −0.189 | −0.242 | −0.120 | 0.136 | 0.287 | 0.297 | 0.405 | .292e | 0.611** | - |
SC = switch cost in the picture-naming switching task (i.e., RT switch trials – RT stay trials)
MC = mixing cost in the picture-naming switching task (i.e., RT stay trials – RT single trials)
Picture-naming intrusions = incorrect language used on trials in the picture-naming task (e.g., saying cat instead of gato when cued to name a picture in Spanish)
Sum of intrusions and partial intrusions made at all switch points in the read-aloud paragraphs task administered after the fMRI scan
This correlation was 0.622 before an extreme outlier (5 standard deviations above the average amount of picture naming intrusions produced) was removed.
p < .05
< p = .01
p = .05.
In bold are correlations that survive FDR correction (p ≤ .029). In gray font are correlations that violate independence and/or within-task correlations that are not of interest.
BOLD response for function switches
The neural costs of silent reading with function switches in the right IFG and in the right MFG were positively associated with switching costs in the picture naming task (rs = 0.43 and 0.46; ps = .03 for right IFG and right MFG, respectively). Separating switching costs in picture naming by language, the cost of switching into the dominant language (English) showed a robust correlation with the neural cost in the right IFG (r = 0.59; p = .003) and in the left SMG (r = 0.48; p = 0.02). A similar pattern was found in bilateral MFG, such that the neural costs of switching during reading were positively associated with English switch costs in production (r= 0.47; p = 0.025 and r = 0.40; p = .06 in left and right MFG, respectively), though these did not survive a more stringent FDR correction. Figure 5 illustrates the relationship between English switching costs in the picture naming task, and percent signal change in the significant activation clusters. In contrast, neural costs for function switches in these regions were not significantly correlated with the cost of switching into Spanish (the non-dominant language), or with mixing costs in either language (or overall, all ps ≥ 0.13). Additionally, contrary to our expectation of stronger associations between the two paragraph tasks, neural costs for function switches were not correlated with intrusion errors produced in the read-aloud task with switches on function words (all rs ≤ .24; ps ≥ .26; Table 6).
Figure 5.
Scatterplots of the correlations between switch costs in time in milliseconds (ms) in a single picture naming task with cued language switches and neural switching costs with function word switches in the right IFG, left SMG, and bilateral MFG. The smoothing band represents 95% confidence intervals.
Interestingly, paragraph reading times in the read-aloud task were also not correlated with any neural costs for function switches (all rs < .29) neither for English-default nor for Spanish-default paragraphs (which all had function word switches). Furthermore, switch costs as calculated by behavioral RTs in the scanner were not significantly correlated with corresponding neural switch costs ( all rs < .27).
Cross-task correlations between behavioral measures
Though intrusion errors were not significantly correlated with neural costs, this did not reflect a general failure of intrusions to correlate with any measure of language control. For example, the number of intrusions produced during reading aloud of English-default paragraphs (with switches into Spanish on function words) was significantly correlated with mixing costs for English picture naming trials (r = 0.48; p = .02). This trend was also observed in other mixing costs measures but did not reach significance (see Table 6). Finally, English-default intrusion errors exhibited a trend towards a correlation with English picture-naming switch costs (p = .05), which did not survive FDR correction. In summary, we did not observe many correlations between behavioral measures administered outside the scanner, but to the extent that we did these involved mixing (not switching) costs, thereby exhibiting a different pattern from the neural data, which tended to be correlated with switching (not mixing) costs.
Discussion
This study characterized the neural costs of language switching during silent reading of mixed-language paragraphs using functional MRI. Bilinguals silently read full paragraphs written in just one language, with a handful of switches on either function or content words with explicit instructions to avoid moving the head or mouth, and with no requirement to make decisions about content (only to press a button at completion). Our results revealed the neural costs of silently reading language switches to be similar to previous observations of switch costs in production of language switches (e.g., Ma et al, 2014; Wang et al., 2009; for review of this network see Abutalebi and Green, 2007; 2008; 2016; Green & Abutalebi, 2013; Luk et al., 2011). Specifically, function word switches elicited costs in the bilateral middle frontal gyrus (i.e., dorsolateral prefrontal cortex; dlPFC), right inferior frontal gyrus (RIFG), and left inferior parietal lobule/supramarginal gyrus (IPL/SMG; see Figure 2). By contrast, content word switches no elicited costs, or had a trend towards costs only in the left inferior frontal gyrus (LIFG; Figure 3) (i.e., without Bonferroni correction for multiple ROIs). Similar findings suggested that the neural cost of switching was greater for reading switches to English function words in Spanish-default paragraphs than for reading switches to Spanish function words in English-default paragraphs in the right IPL/SMG (Figure 4), resembling previous findings in production that switching into the dominant language was more costly. Finally, correlations with behavioral data revealed significant relationships between the neural cost of silently reading switches and switch costs found in production. Specifically, the neural cost of function word switches in the RIFG, left MFG, and left SMG was positively correlated with the behavioral cost of switching to the dominant language in cued picture naming Figure 5). These results support the notion of a modality-general switch mechanism that is used to process switches in both language comprehension and production, and is supported by the same (or similar) brain regions as nonlinguistic cognitive control.
Function word switches are more costly
A priori, it was uncertain whether neural costs would be larger for function than content word switches, given that function words are often skipped entirely in reading (e.g., O’Regan, 1979; Saint-Aubin & Klein, 2001), and function word errors are difficult to detect even during explicit attempts at monitoring (Schotter et al., in press; Staub, Dodge & Cohen, 2018). Of greatest interest, function word switches elicited the most robust neural switch costs. This suggests a role of cognitive control for comprehension of language switches even when these occur on a minority of words in connected multi-sentence language processing, and for high frequency words that only provide grammatical information. Interestingly, although unintended switches most often involve function words (and interjections; both in spontaneous speech, Poulisse and Bongaerts, 1994; Poulisse, 1999, and in reading aloud; Gollan et al., 2014; Gollan & Goldrick, 2016; 2018), bilinguals do not typically switch on single function words intentionally (Muysken, 2000), thus making this manipulation less naturalistic (than content switches). Our finding of increased neural costs for function word switches in cognitive control regions is in line with findings by Abutalebi and colleagues (2007) who manipulated regularity of switches in an auditory perception task and found that switches that violate the well-formedness of the sentence structure activated the opercular portion of Broca’s area and the left IPL, two of the same regions observed in our function switch contrast. In contrast, in that study well-formed switches activated inferior temporal regions related to lexical-semantic processing. Abutalebi and colleagues proposed that irregular switches may be first treated as grammatical violations, rather than lexical alternatives, because of their occurrence in unnatural positions. Thus, irregular switches may rely more on regions shown to support syntactic processes (i.e., pars opercularis and IPL; e.g., Caplan et al., 2000; Friederici, 2002 for review). Interestingly, in a MEG study, when the auditory comprehension task was made more naturalistic (i.e., participants listened to recording of spontaneous switching in bilingual conversations), activation in cognitive control areas (i.e., ACC and dlPFC) was not observed, and activation was only significant in the auditory cortex (Blanco-Elorrieta & Pylkkänen, 2017). Taken together, recruitment of cognitive control regions may be dependent on the nature of the task, with unnatural switches eliciting costs and more natural switches being ‘cost-free’ (although null findings must of course be interpreted with caution).
Cognitive control regions recruited in silent reading of language switches
Our most robust cluster was observed in the right IFG, which has been implicated in domain-general inhibition of irrelevant manual or linguistic responses (e.g., in the Simon task in Jahfari et al., 2011; Aron et al., 2004b for review). For example, Aron and colleagues (2004a) compared patients with lesions to the right vs. the left IFG using a stop-signal task (which requires inhibition of a manual response) and found that patients with right (but not left) IFG lesions showed disrupted inhibition of responses, and damage to the right IFG positively correlated with the reaction time needed to inhibit the response. Further, in a language switching study Bruin and colleagues (2014) found significant neural switching costs in the right IFG (and the pre-SMA) for switching into second and third-learned languages, and significant correlations between BOLD response in the right IFG and pre-SMA and a behavioral measure of inhibition (Simon task interference effects in response times). Taken together, the common activation of the right IFG during language switching studies (de Bruin et al., 2014; Fu et al., 2017; the current study) and inhibition of manual responses (as observed in the stop-signal and Simon tasks; Aron et al., 2004a; 2004b; Jahfari et al., 2011) suggests that this region serves a domain-general role in response inhibition, including but not limited to language switching.
Our second largest cluster for switching was observed in the inferior parietal lobule (IPL), and specifically the supramarginal gyrus (SMG). This region was observed in one of the earliest language switching studies by Price, Green, and von Studnitz (1999) who used PET imaging to investigate translation or reading of visually presented words in German, English, or switching languages. They found that switching resulted in activation of the IFG and bilateral SMG. The SMG has been implicated in mapping orthography into phonology (e.g., Graves, Desai, Humphries, Seidenberg & Binder, 2010; for review Paz-Alonso, Oliver, Quiñones, & Carreiras, 2019) and the parietal cortex more generally has been shown to be important for letter identification and early stages of visual processing (Paz-Alonso et al., 2019). Thus it is possible that initial attempts to search for words in the wrong lexicon trigger grapheme to phoneme conversion to a greater extent on switch words, recruiting SMG. The posterior parietal cortex has also been implicated in general executive control and task-switching (e.g., Liston, Matalon, Hare, Davidson & Casey, 2006; see Ye & Zhou, 2009 for the role of the parietal lobe in resolving conflict in sentence comprehension). In line with the idea of a possible bilingual advantage in executive control, a structural neuroimaging study by Mechelli and colleagues (2004) found that bilinguals had increased gray matter volume in the left parietal lobe compared to monolinguals, with the greatest increase in volume present in early high-proficiency bilinguals. Finally, Abutalebi and Green (2008) conceptualize the posterior parietal cortex as biasing selection away from the language not in use or toward the language in use (see also Branzi et al., 2016 suggestion of IPL’s role in engagement and disengagement of inhibitory control). Our finding of significant activation of the IPL suggests that similar processes may be involved during silent reading of switches—that is, the parietal cortex may be important for directing attentional resources to the target language.
Third, we found significant activation of the bilateral dlPFC for function word switches which replicates previous studies relating language-switching in production to the left (Hernandez et a., 2000; Rodriguez-Fornells et al., 2005; Wang et al., 2007) and the right dlPFC (Hernandez et al., 2001; Wang et al., 2007), with the hemispheric distinction and lateralization of the dlPFC’s role in language switching as inconclusive. Bilateral dlPFC plays an important role in response selection and inhibition (Abutalebi & Green, 2007), as well as general attentional control (Aron et al., 2004a) and in particular in sustained top-down control (Braver, Reynolds, & Donaldson, 2003). The dlPFC was also implicated in both language switching and category switching (within a single language) in another study (de Baene et al., 2015) suggesting language control is a subdomain of general cognitive control (e.g., Bialystok, Craik, Klein, & Viswanathan, 2004; Garbin et al., 2010; Abutalebi et al., 2012). Furthermore, our observation of the dlPFC is in line with a study of out-of-context switches in auditory comprehension (in a word picture matching task) in which left dlPFC activation was found when switches were cued (i.e., by pictures of bilingual or monolingual interlocutors or arbitrary colors; Blanco-Elorrieta & Pylkkänen, 2017).
We did not observe significant activation in two other predicted ROIs—the caudate and ACC. The dorsal ACC has been shown to support error detection, task monitoring, and conflict resolution (Aarts, Roelofs & van Turennout, 2008; Kerns et al., 2004). The caudate—a subcortical region traditionally associated with motor control and general cognitive control (Graybiel, 1997; 2000)—has been consistently implicated in language switching and inhibition (e.g., Crinion et al., 2006; Abutalebi & Green, 2008). Previously both the left caudate and the ACC were observed during auditory perception of switches in comprehension in one fMRI study (Abutalebi et al., 2007) and the ACC was implicated in switching in comprehension in two recent MEG studies in which stimuli were isolated words or numbers (Blanco-Elorrieta & Pylkkänen, 2016; 2017). It is possible that the ACC only weakly supports switches in comprehension, and therefore is observed in some studies but not others. However, null effects are difficult to interpret, and methodological differences between studies make direct comparison difficult (e.g., our study involved silent reading whereas others involved auditory comprehension tasks). Setting aside possible debate over which exact regions are implicated, taken together, our findings do at least suggest an important role of the prefrontal and parietal regions in switching in comprehension in the visual domain, possibly supporting a modality-general switch mechanism for these regions.
Inhibition? Switching into the dominant versus the non-dominant language
Language dominance effects are robust in bilingual research—bilinguals generally respond faster when naming pictures in their more dominant (i.e., more proficient) compared to non-dominant language under a single language context (e.g, Gollan, Kleinman & Wierenga, 2014; Ivanova & Costa, 2008). However this pattern reverses in blocks that require language switching—that is, switching into the dominant language is usually more costly than when switching into the non-dominant language, or sometimes language dominance even reverses entirely in mixed-language blocks (e.g., Christoffels et al., 2007; Declerck, Phillipp, & Koch, 2013; Gollan & Ferrerira; 2009; Meuter & Allport, 1999; for review see Kleinman & Gollan, 2018). These findings are often attributed to inhibitory control (e.g., Green, 1998)—that is, to produce words in the non-dominant language, the more dominant language must be inhibited, so that when returning to the dominant language, bilinguals must overcome inhibition, leading to large switch costs (Meuter & Allport, 1999; for review see Declerck & Philipp, 2015). In the current study, language dominance effects were found only in one cluster in the right IPL/SMG (and only without the most stringent correction) when bilinguals read paragraphs written mostly in Spanish with some switches to English on function words (relative to English-default paragraphs with switches to Spanish on function words). This result mirrors published behavioral data—in a read aloud task, bilinguals produced the most intrusion errors on non-dominant default paragraphs with switches into the dominant language (e.g., Fadlon et al., in press; Gollan et al., 2014; Gollan & Goldrick, 2016; 2018; Gollan, Stasenko, Li & Salmon, 2017; Li & Gollan, 2018; Schotter et al., in press). Similarly, in behavioral tasks administered after the fMRI task in the current study, bilinguals exhibited larger switch costs when switching to English than Spanish in picture-naming (see Table 5), and produced the most intrusions in Spanish-default paragraphs in the read-aloud task (see Table 4a). However, note that button press responses for in-scanner paragraph reading times exhibited the opposite pattern, a result we speculate may reflect lack of sensitivity/ceiling effect due to reading times in Spanish being close to our 30-second cutoff.3 Further, the effects we observed in the right IPL/SMG could not be attributed simply to the difficulty associated with completing a task primarily in the non-dominant language rather than something related specifically to switching into a dominant language, given that we found no significant increase in activation in the SMG when comparing Spanish-only to English-only paragraphs.
A similar pattern was found in a neuroimaging study of comprehension. In an auditory semantic categorization task, Hut and colleagues (2017) found that switches from the later-learned English to either of the two native languages in trilinguals resulted in greater activation in the superior temporal gyrus, and this increase was not found for the reverse contrast (i.e. switching from either native language to English). Further, they found that English non-switch trials showed greater activation in the bilateral IFG (compared to non-switch trials in the native languages), and suggested this reflects inhibition of the native languages while using English, even in a receptive task. In a production study, Fu et al., 2017 found that switching into the dominant language elicited greater neural costs in the bilateral IFG, the right dlPFC and the SMA. Thus, while both Hut et al. and Fu et al. suggested their results reveal active suppression of the dominant (or native) language, different brain regions were implicated – and similar underlying mechanisms could be here, though more work is needed to determine which brain regions are involved. The notion of inhibition is highly debated (MacLeod, Dodd, Sheard, Wilson, & Bibi, 2003; Rey-Mermet, Gade, & Oberauer, 2018), and while the observed activation in classic cognitive control regions could signify application of inhibition, it could instead indicate activation of stimulus-relevant information, or possibly both (e.g., Cohen, Dunbar, & McClelland, 1990; Egner & Hirsch, 2005; for review see Aron, 2007).
Clues into the modality-generality of the switch mechanism
The appearance of some control regions in a silent reading task that did not require overt responses, and which arguably could not have elicited response competition (at least not at the level of overtly planning responses)—provides additional constraints on how these effects should be interpreted. To examine the relationships between comprehension and production, we contrasted the neural costs observed in silent reading with post-scan behavioral measures of switching in production. The only production task that was significantly correlated with neural switching costs was the single picture-naming task—specifically switch costs in English—while the task that resembled our silent-reading during fMRI task far more, the paragraph read-aloud task, elicited no significant correlations with neural switching costs. It is unclear why the behavioral task most similar to the fMRI task did not reveal any significant correlations. However, it is not simply the case that intrusions were insufficiently sensitive to switching ability because we found correlations between intrusions in English-default paragraphs and mixing costs for English in the picture naming task. Because we found switching cost correlations consistently for multiple brain regions, this suggests that neural costs found when contrasting single versus mixed language paragraphs reflects operation of a switching mechanism, whereas intrusion errors and mixing costs may measure more sustained forms of control. This pattern appears in line with a production study by Wang and colleagues (2009) who found that sustained language control (defined by mixing costs) versus transient control (defined by switching costs) elicited differential activation patterns. That is, the left inferior parietal lobule was more specific to transient control (and is similar to the robust correlation between behavioral and neural costs in the IPL in the current study), whereas the left MFG was activated for both switching and mixing costs. This interpretation, though speculative, implies the existence of a modality-general switch mechanism that supports processing of language switches in both comprehension and production, and switching in general (both linguistic and nonlinguistic, because of the brain regions involved.
It is reasonable to ask if our scanner task exclusively measured processes associated with reading comprehension processes or if bilinguals may have been covertly producing speech (even though they were instructed to read silently), thus resulting in activation in regions often observed in switching during production. Some aspects of our data seem inconsistent with this interpretation. First, correlations between neural switch cost data and intrusion errors produced in the reading-aloud task administered after the scan (which was very close in design to the task in the scanner) were null. Second, silent reading sometimes involves auditory imagery or a “voice in the head” (Huey, 1908), suggesting that visual word recognition requires processes similar to “sounding out” words —i.e., phonological recoding (e.g., Frost, 1998). Although many reject strong notions of phonological recoding in reading, it is generally agreed that sound-based representations are computed during reading (Rastle & Brysbaert, 2006 for review). These processes are more likely when reading is less proficient and automatic, and reading of orthographically transparent languages such as Spanish. Thus, for our bilinguals Spanish was both less proficient and more transparent, and as such, reading Spanish versus English covertly should also have activated regions known to support phonological recoding (e.g., left posterior fusiform gyrus; see Dietz, Jones, Gareau, Zeffiro, & Eden, 2005). However, this region was not observed in an exploratory whole brain analysis; using an uncorrected p of .001; see Table 7), which showed higher activation for Spanish-only than English-only paragraphs in the right precentral gyrus and in the left thalamus; no significant activation was observed in the reverse direction. Our finding of the precentral gyrus is in line with a meta-analysis by Liu and Cao (2016) who reported that precentral gyri (and bilateral superior/middle temporal gyri) were recruited to a greater degree when bilinguals read in a second language (L2) that was more transparent than the first language (L1). Finally, the few studies that contrasted silent and overt reading suggested that reading aloud elicits more robust activation of the phonological processing system (Barch et al., 1999; Huang, Carr, Cao, 2002), particularly bilateral pre-motor and motor, auditory, and extra-striate regions (Dietz et al., 2005). Similarly, Berken et al. 2015 demonstrated that sequential bilinguals, who in some ways more closely resemble bilinguals in our study, activated speech-motor control areas (e.g., left IFG, left premotor cortex, and left fusiform gyrus) more strongly than simultaneous bilinguals when reading aloud in L2 compared to L1. Because the majority of the above regions were not implicated in the present study, with the exception of the right precentral gyrus (i.e., primary motor cortex) and the left IFG (though a weak effect), it appears less likely that the correlations we observed across modalities reflected covert motoric output or increased phonological decoding involved in switching.
Table 7.
Brain regions showing higher BOLD response for reading single-language paragraphs written in Spanish compared to single-language paragraphs written in English using an exploratory whole-brain analysis (uncorrected voxel-wise p of .001).
| Contrast and Cluster Number | Hemisphere | Anatomical region | BA/Subregion | Cluster volume (mm3) | CM MNI coordinates (x, y, z)* | Z-value at peak intensity |
|---|---|---|---|---|---|---|
| Spanish Only vs. English Only | ||||||
| C1 | R | Precentral gyrus | BA 4 | 108 | 28.5, −13.5, 48 | 3.5 |
| C2 | L | Thalamus | -- | 54 | 1.4, −3, −6 | 3.7 |
Although we cannot definitively rule out the possibility that covert reading or perhaps more likely, automatic phonological recoding during silent reading, played some role in recruiting control regions normally associated with switching in production, what we can say with confidence is that these regions are recruited even when bilinguals do not need to select a single response for production. That is, in our study during the scan bilinguals did not speak, did not move their mouths, and did not need to make any explicit classification of which language they were reading or any other type of decision that might have recruited frontal regions of the brain that support response selection. The significant recruitment of cognitive control regions even in a silent reading task with only 6 switch words per paragraph, and on high frequency function words that are often skipped in reading is surprising and suggests that silent reading should perhaps not be viewed as relatively passive. Instead bilinguals maintain active expectations about language membership of upcoming words, and recruit frontoparietal control regions to resolve conflict between these expectations and violation thereof when reading switch words. Although prediction in language comprehension is not a novel concept, the mechanism of prediction is controversial (e.g., Kuperberg & Jaeger, 2015). For instance, it is possible that language membership may be accomplished by a ‘prediction-by-production’ mechanism as proposed in some comprehension accounts (e.g., Dell & Chang, 2014; Pickering & Gambi, 2018), whereby the reader may retrieve covert production representations (i.e., covert imitation) to predict an upcoming switch, with the aid of context and association.
Limitations and future directions
A notable limitation of the current study is a relatively small sample size, which leaves uncertain how null effects should be interpreted (e.g., lack of significant activation of the ACC). Further, we have assumed throughout that activation of regions previously reported for production of switches (in other studies) in comprehension of switches (in the present study) supports the notion that language switches are processed by at least partially overlapping underlying cognitive mechanisms whether they occur in comprehension or production. By extension, because language switches activated cognitive control regions that were also recruited to support non-linguistic cognitive control processes in bilinguals and monolinguals alike, an even more general purpose switching mechanism might also be supported. However, caution is warranted in interpreting our results to reflect non-linguistic control and requires more explicit confirmation. Fedorenko, Behr, and Kanwisher (2011) cautioned against such interpretations; using fMRI to functionally define classic language regions on an individual subject level, they examined responses in these regions to nonlinguistic (e.g., general working memory, general cognitive control) functions and found little to no overlap between linguistic and non-linguistic functions (with the exception of the left MFG and verbal working memory). Their analysis suggests a high degree of functional specificity within the same brain regions for supporting language versus nonlinguistic functions (for review see Fedorenko, 2014). Thus, additional work with better targeted analyses and that includes both linguistic and non-linguistic tasks will be needed to test if what seems to be shared regions might instead involve functional separation at the level of individual rather than group-based contrasts.
Conclusions
Our finding of neural costs observed for silent reading of function switches, and the correlations between switching costs across fMRI and behavioral tasks, are consistent with proposals that bilinguals rely on shared control mechanisms when producing and comprehending language switches (e.g., van Heuven & Dijkstra, 2010; Grainger et al., 2010). Although open to interpretation and in need of further confirmation, even if control mechanisms are only partly shared across modalities (comprehension and production) and across domains (linguistic and nonlinguistic), bilinguals may be exercising cognitive control regions in the brain more frequently than assumed (if counting only overt production of switches). If so, a greater implication would be that bilingualism constitutes a more intensive mental gym for cognitive control regions. Broadly, this could be relevant for understanding cognitive reserve (Bialystok, Abutalebi, Bak, Burke, & Kroll, 2016; Perani & Abutalebi, 2015), if bilingualism also entails a greater need for cognitive control than monolingualism (an assertion that is disputed; Lehtonen et al., 2018; Paap et al, 2017; Antón, Carreiras & Duñabeitia, 2019; Antón, Fernández-García, Carreiras, Duñabeitia, 2016). Setting this debate aside, while silent reading seems to be a relatively passive task, only a handful of language switches on function words distributed across processing of an entire paragraph was sufficient to elicit significant activation of cognitive control regions of the brain. The possibility of processing mechanisms that supports conflict resolution in both language comprehension and production has broad implications for understanding language processing in bilinguals and monolinguals alike.
Highlights.
Bilinguals silently read paragraphs with and without language switches during fMRI
Frontoparietal brain regions were recruited for switching relative to not switching
Neural costs were largest for switches on function (vs. content) words
Neural switch costs were correlated with behavioral switch costs in production
These results suggest the existence of a modality general switch mechanism
Acknowledgments
We would like to thank Mayra Murillo and Rosa Montoya for help with data collection and coding, and Vic Ferreira, Karen Emmorey, and Mathieu Declerck for helpful discussion. TG was supported by the National Science Foundation (1457159) and the National Institute on Deafness and Other Communication Disorders (011492). CW was supported by VA CSR&D Merit Award [5I01CX000565] and the National Institutes of Health [R01MH113588, R21MH118409]. AS was funded by a Ruth L. Kirschstein National Research Service Award (NRSA) Individual Predoctoral Fellowship (1F31AG058379-01). CH was funded by the National Science Foundation Graduate Research Fellowship Program [2015207525 C.C.H.]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NSF, NIH, or the VA. There were no conflicts of interest in completing this research.
Appendix
A. Example paragraph as it was modified to appear in each of 6 conditions (between participants). Underlines included only in this example for illustration (switches were not underlined in the version participants saw).
English Only
Throughout the Land of the Pig River, the name Mrs. Peace was very well known by almost everyone. It wasn’t so much because of the gossip that traveled from village to village, but due to the many stories that circulated declaring her adventures and mischief. It is said that there was something magnetic and charming about her personality that attracted everyone’s attention. In fact, there was always someone that had something very funny to say about Mrs. Peace. The curious thing is that very few people spoke negatively about her in spite of her eccentric behavior. The truth is that almost everyone admired her—even the youngest ones.
English default-function
Throughout the Land of the Pig River, el name Mrs. Peace was very well known by almost everyone. It wasn’t so much because of the gossip que traveled from village to village, but due to the many stories that circulated declaring her adventures and mischief. It is said that there was something magnetic y charming about her personality that attracted everyone’s attention. In fact, there was always someone that had algo very funny to say about Mrs. Peace. The curious thing is that very poca people spoke negatively about her in spite of her eccentric behavior. La truth is that almost everyone admired her—even the youngest ones.
English default-content
Throughout the Land of the Pig River, the nombre Mrs. Peace was very well known by almost everyone. It wasn’t so much because of the chisme that traveled from village to village, but due to the many stories that circulated declaring her adventures and mischief. It is said that there was something magnetic and encantador about her personality that attracted everyone’s attention. In fact, there was always someone that had something very chistoso to say about Mrs. Peace. The curious thing is that very few gente spoke negatively about her in spite of her eccentric behavior. The verdad is that almost everyone admired her—even the youngest ones.
Spanish Only
Por toda la Tierra del Río Puerco, el nombre doña Paz era muy bien conocido por casi todos. No era tanto por el chisme que corría de pueblo en pueblo, sino por las muchas historias que circulaban declarando sus aventuras y travesuras. Se dice que había algo magnético y encantador de su personalidad que llamaba la atención de todos. De hecho, siempre había alguien que tenía algo muy chistoso que decir de doña Paz. Lo curioso es que muy poca gente hablaba mal de ella a pesar de su comportamiento excéntrico. La verdad es que casi todos la admiraban—hasta los más jóvenes.
Spanish default-function
Por toda la Tierra del Río Puerco, the nombre doña Paz era muy bien conocido por casi todos. No era tanto por el chisme that corría de pueblo en pueblo, sino por las muchas historias que circulaban declarando sus aventuras y travesuras. Se dice que había algo magnético and encantador de su personalidad que llamaba la atención de todos. De hecho, siempre había alguien que tenía something muy chistoso que decir de doña Paz. Lo curioso es que muy few gente hablaba mal de ella a pesar de su comportamiento excéntrico. The verdad es que casi todos la admiraban—hasta los más jóvenes.
Spanish default-content
Por toda la Tierra del Río Puerco, el name doña Paz era muy bien conocido por casi todos. No era tanto por el gossip que corría de pueblo en pueblo, sino por las muchas historias que circulaban declarando sus aventuras y travesuras. Se dice que había algo magnético y charming de su personalidad que llamaba la atención de todos. De hecho, siempre había alguien que tenía algo muy funny que decir de doña Paz. Lo curioso es que muy poca people hablaba mal de ella a pesar de su comportamiento excéntrico. La truth es que casi todos la admiraban—hasta los más jóvenes.
B. Example of an English-default and a Spanish-default paragraph with switches on function words used in the post-scan reading aloud task. Underlines included only in this example for illustration (switches were not underlined in the version participants saw).
“Greenland is Melting Away”
At last the helicopter took off the team’s gear hanging from an attached net sling. Los scientists gazed at the seemingly endless surface of the ice, beneath the chopper, spreading in todo directions, threaded with blue rivers and lakes. After a forty minute flight, el pilot cautiously bounced the helicopter on the ice, making sure it was hard enough to land on.
Stepping out, the scientists were hit por the cold of the Greenland summer, the temperature ranged from 26 to 40 degrees mientras they were there, a constant wind and the glare of the sun.
As the researchers began to set up camp, Bob, un doctoral student from the University of Wyoming, headed toward the river, silent as it sliced through the ice. More than cualquier other member of the team, the success of the mission rested on sus shoulders.
Bob was 31 years old, and grew up kayaking and rafting in Oregon. He designed the complex rope and pulley system, modeled on swift-water boat rescue systems, qu would be crucial to gleaning data de the dangerous waters. Before coming to Greenland, he spent months refining y practicing his rope system on rivers in Wyoming.
“Este es el tiempo que tardas en sacar tu teléfono cuando estás solo”
Si estás solo en una habitación, te tomará un promedio de 44 segundos sacar your teléfono del bolso y empezar a revisarlo, de acuerdo con un experimento hecho en Alemania and el Reina Unido.
The documento reveló que los hombres tardan sólo 21 segundos— menos de la mitad del lapso promedio— en tomar su teléfono, mientras that las mujeres esperan generalmente 57 segundos antes de hacerlo.
Durante el estudio los participantes fueron grabados by una cámara escondida para capturar “objetivamente” el contacto with su celular. El 73% de los participantes usaron sus celulares durante la sesión de espera que duró 10 minutos, y durante that periodo tanto hombres como mujeres lo usaron por 5 minutos.
Sin embargo, almost todos los participantes aseguraron haber pasado entre dos y tres minutos sin recurrir a su teléfono celular.“La gente está más atada a estos dispositivos de lo que creen, sobre todo when están a solas,” estimó Jens Bindert, académico of la Universidad en el Reino Unido. “La inmediatez de la información y las interacciones realizadas a través de our dispositivos móviles hacen que estos sean más than una pieza de tecnología, convirtiéndose en un compañero digital, así como en una conexión con el mundo exterior.
Footnotes
Due to an experimental error, two participants completed the (i.e., voluntary) switching block as well.
The observed patterns did not differ when including only intrusions produced on switch-out points.
This finding of less robust switch costs for switching into L1 as found in behavioral RTs (i.e., Table 5) patterns with previous behavioral studies that did not find a larger cost for switching into L1 than into L2 in comprehension (e.g., Bultena et al, 2015; Reynolds, Schlöffel, & Peressotti, 2016; but see Wang, 2015).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarts E, Roelofs A, & van Turennout TM (2008). Anticipatory activity in anterior cingulate cortex can be independent of conflict and error likelihood. Journal of Neuroscience, 28, 4671–4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abutalebi J, Brambati SM, Annoni J-M, Moro A, Cappa SF, & Perani D (2007). The neural cost of the auditory perception of language switches: an event-related functional magnetic resonance imaging study in bilinguals. The Journal of Neuroscience, 27, 13762–13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abutalebi J, Della Rosa PA, Green DW, Hernandez M, Scifo P, Keim R, Cappa S, & Costa A (2012). Bilingualism tunes the anterior cingulate cortex for conflict monitoring. Cerebral Cortex, 22, 2076–2086. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, & Green D (2007). Bilingual language production: The neurocognition of language representation and control. Journal of Neurolinguistics, 20, 242–275. [Google Scholar]
- Abutalebi DJ, & Green DW (2008). Control mechanisms in bilingual language production: Neural evidence from language switching studies. Language and Cognitive Processes, 23, 557–582. [Google Scholar]
- Abutalebi J, & Green DW (2016). Neuroimaging of language control in bilinguals: neural adaptation and reserve. Bilingualism: Language and Cognition, 19, 689–698. [Google Scholar]
- Antón E, Carreiras M, & Duñabeitia JA (2019). The impact of bilingualism on executive functions and working memory in young adults. PLOS ONE, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antón E, Fernández García Y, Carreiras M, & Duñabeitia JA (2016). Does bilingualism shape inhibitory control in the elderly? Journal of Memory and Language, 90, 147–160. [Google Scholar]
- Aron AR (2007). The neural basis of inhibition in cognitive control. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 13, 214–228. [DOI] [PubMed] [Google Scholar]
- Aron A, Monsell S, Sahakian B, & Robbins T (2004a). A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain, 127, 1561–1573. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, & Poldrack RA (2004b). Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences, 8, 170–177. [DOI] [PubMed] [Google Scholar]
- Barch DM, Sabb FW, Carter CS, Braver TS, Noll DC, & Cohen JD (1999). Overt verbal responding during fMRI scanning: empirical investigations of problems and potential solutions. NeuroImage, 10, 642–657. [DOI] [PubMed] [Google Scholar]
- Berken JA, Gracco VL, Chen J, Watkins KE, Baum S, Callahan M, & Klein D (2015). Neural activation in speech production and reading aloud in native and non-native languages. NeuroImage, 112, 208–217. [DOI] [PubMed] [Google Scholar]
- Bialystok E (2011). Reshaping the mind: The benefits of bilingualism. Canadian Journal of Experimental Psychology, 65, 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialystok E, Abutalebi J, Bak TH, Burke DM, & Kroll JF (2016). Aging in two languages: Implications for public health. Ageing Research Reviews, 27, 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialystok E, Craik FIM, Klein R, & Viswanathan M (2004). Bilingualism, aging, and cognitive control: evidence from the Simon task. Psychology and Aging, 19, 290–303. [DOI] [PubMed] [Google Scholar]
- Bialystok E, Craik FIM, & Luk G (2012). Bilingualism: consequences for mind and brain. Trends in Cognitive Sciences, 16, 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Elorrieta E, & Pylkkänen L (2016). Bilingual language control in perception versus action: MEG reveals comprehension control mechanisms in anterior cingulate cortex and domain-general control of production in dorsolateral prefrontal cortex. Journal of Neuroscience, 36, 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Elorrieta E, & Pylkkänen L (2017). Bilingual language switching in the laboratory versus in the wild: the spatiotemporal dynamics of adaptive language control. The Journal of Neuroscience, 37, 9022–9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branzi FM, Della Rosa PA, Canini M, Costa A, & Abutalebi J (2016). Language control in bilinguals: Monitoring and response selection. Cerebral Cortex, 26, 2367–2380. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, & Donaldson DI (2003). Neural mechanisms of transient and sustained cognitive control during task switching. Neuron, 39, 713–726. [DOI] [PubMed] [Google Scholar]
- Bultena S, Dijkstra T, & Hell JGV (2015). Language switch costs in sentence comprehension depend on language dominance: Evidence from self-paced reading. Bilingualism: Language and Cognition, 18, 453–469. [Google Scholar]
- Caplan D, Alpert N, Waters G, & Olivieri A (2000). Activation of Broca’s area by syntactic processing under conditions of concurrent articulation. Human Brain Mapping, 9, 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Dunbar K, & McClelland JL (1990). On the control of automatic processes: A parallel distributed processing account of the Stroop effect. Psychological Review, 97, 332–361. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Christoffels IK, Firk C, Schiller NO (2007). Bilingual language control: an event-related brain potential study. Brain Res, 1147, 192–208. [DOI] [PubMed] [Google Scholar]
- Crinion J, Turner R, Grogan A, Hanakawa T, Noppeney U, Devlin JT, … Price CJ (2006). Language control in the bilingual brain. Science, 312, 1537–1540. [DOI] [PubMed] [Google Scholar]
- de Baene W, Duyck W, Brass M, & Carreiras M (2015). Brain circuit for cognitive control is shared by task and language switching. Journal of Cognitive Neuroscience, 27, 1752–1765. [DOI] [PubMed] [Google Scholar]
- de Bruin A, Roelofs A, Dijkstra T, & FitzPatrick I (2014). Domain-general inhibition areas of the brain are involved in language switching: FMRI evidence from trilingual speakers. NeuroImage, 90, 348–359. [DOI] [PubMed] [Google Scholar]
- Declerck M, Koch I, Duñabeitia JA, Grainger J, & Stephan DN (2019). What absent \ switch costs and mixing costs during bilingual language comprehension can tell us about language control. Journal of Experimental Psychology: Human Perception and Performance, 45, 771–789. [DOI] [PubMed] [Google Scholar]
- Declerck M, & Philipp AM (2015). A review of control processes and their locus in language switching. Psychonomic Bulletin & Review, 22, 1630–1645. [DOI] [PubMed] [Google Scholar]
- Declerck M, Philipp AM, & Koch I (2013). Bilingual control: Sequential memory in language switching. Journal of Experimental Psychology: Learning, Memory and Cognition, 39, 1793–1806. [DOI] [PubMed] [Google Scholar]
- Dell GS, & Chang F (2013). The P-chain: relating sentence production and its disorders to comprehension and acquisition. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz NAE, Jones KM, Gareau L, Zeffiro TA, & Eden GF (2005). Phonological decoding involves left posterior fusiform gyrus. Human Brain Mapping, 26, 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T & Hirsch J (2005). Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nature Neuroscience. 8, 1784–1790. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols T, Knutsson H. (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences, 113, 7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadlon J, Li C, Prior A, and Gollan TH (in press). Using what’s there: Bilinguals adaptively rely on orthographic and color cues to achieve language control. Cognition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E (2014). The role of domain-general cognitive control in language comprehension. Frontiers in Psychology, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Behr MK, & Kanwisher N (2011). Functional specificity for high-level linguistic processing in the human brain. Proceedings of the National Academy of Sciences of the United States of America, 108, 16428–16433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD (2002). Towards a neural basis of auditory sentence processing. Trends in Cognitive Sciences, 6, 78–84. [DOI] [PubMed] [Google Scholar]
- Frost R (1998). Toward a strong phonological theory of visual word recognition: true issues and false trails. Psychological Bulletin, 123, 71–99. [DOI] [PubMed] [Google Scholar]
- Fu Y, Lu D, Kang C, Wu J, Ma F, Ding G, & Guo T (2017). Neural correlates for naming disadvantage of the dominant language in bilingual word production. Brain and Language, 175, 123–129. [DOI] [PubMed] [Google Scholar]
- Hernandez M, Costa A, & Avila C (2010). Bridging language and attention: brain basis of the impact of bilingualism on cognitive control. NeuroImage, 53, 1272–1278. [DOI] [PubMed] [Google Scholar]
- Gollan TH, & Ferreira VS (2009). Should I stay or should I switch? A cost-benefit analysis of voluntary language switching in young and aging bilinguals. Journal of Experimental Psychology: Learning, Memory, and Cognition, 35, 640–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, & Goldrick M (2016). Grammatical constraints on language switching: Language control is not just executive control. Journal of Memory and Language, 90, 177–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, & Goldrick M (2018). A switch is not a switch: Syntactically driven bilingual l language control. Journal of Experimental Psychology: Learning, Memory, & Cognition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, Kleinman D, & Wierenga CE (2014). What’s easier: Doing what you want, or being told what to do? Cued versus voluntary language and task switching. Journal of Experimental Psychology: General, 143, 2167–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, Schotter ER, Gomez J, Murillo M, & Rayner K (2014). Multiple levels of bilingual language control: Evidence from language intrusions in reading aloud. Psychological Science, 25, 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, Stasenko A, Li C, & Salmon DP (2017). Bilingual language intrusions and other speech errors in Alzheimer’s disease. Brain and Cognition, 118, 27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, Weissberger G, Runnqvist E, Montoya RI, & Cera CM (2012). Self-ratings of spoken language dominance: A multi-lingual naming test (MINT) and preliminary norms for young and aging Spanish-English bilinguals. Bilingualism: Language and Cognition, 15, 594–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger J, Midgley K, & Holcomb PJ (2010). Re-thinking the bilingual interactive-activation model from a developmental perspective (BIA-d) In Kail M & Hickmann M (Eds.), Language acquisition across linguistic and cognitive systems (pp. 267–284). New York: Benjamins. [Google Scholar]
- Graves WW, Desai R, Humphries C, Seidenberg MS, & Binder JR (2010). Neural systems for reading aloud: a multiparametric approach. Cerebral Cortex, 20, 1799–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM (1997). The basal ganglia and cognitive pattern generators. Schizophrenia Bulletin, 23, 459–469. [DOI] [PubMed] [Google Scholar]
- Graybiel AM (2000). The basal ganglia. Current Biology, 10, R509–R511. [DOI] [PubMed] [Google Scholar]
- Green DW (1998). Mental control of the bilingual lexico-semantic system. Bilingualism: Language and Cognition, 1, 67–81. [Google Scholar]
- Green DW, & Abutalebi J (2013). Language control in bilinguals: The adaptive control hypothesis. Journal of Cognitive Psychology, 25, 515–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AE, Dapretto M, Mazziotta J, & Bookheimer S (2001). Language switching and language representation in Spanish–English bilinguals: An fMRI study. NeuroImage, 14, 510–520. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Martinez A, & Kohnert K (2000). In search of the language switch: an FMRI study of picture naming in Spanish–English bilinguals. Brain and Language, 73, 421–431. [DOI] [PubMed] [Google Scholar]
- Hilchey MD, & Klein RM (2011). Are there bilingual advantages on nonlinguistic interference tasks? Implications for the plasticity of executive control processes. Psychonomic Bulletin & Review, 18, 625–658. [DOI] [PubMed] [Google Scholar]
- Huang J, Carr TH, & Cao Y (2002). Comparing cortical activations for silent and overt speech using event-related fMRI. Human Brain Mapping, 15, 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hut SCA, Helenius P, Leminen A, Mäkelä JP, & Lehtonen M (2017). Language control mechanisms differ for native languages: Neuromagnetic evidence from trilingual language switching. Neuropsychologia, 107, 108–120. [DOI] [PubMed] [Google Scholar]
- Ivanova I, & Costa A (2008). Does bilingualism hamper lexical access in speech production? Acta Psychologica, 127, 277–288. [DOI] [PubMed] [Google Scholar]
- Jackson GM, Swainson R, Cunnington R, & Jackson SR (2001). ERP correlates of executive control during repeated language switching. Bilingualism: Language and Cognition, 4, 169–178. [Google Scholar]
- Jahfari S, Waldorp L, Wildenberg, van den WPM, Scholte HS, Ridderinkhof KR, & Forstmann BU (2011). Effective connectivity reveals important roles for both the hyperdirect (fronto-subthalamic) and the indirect (fronto-striatal-pallidal) fronto-basal ganglia pathways during response inhibition. Journal of Neuroscience, 31, 6891–6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, Cho RY, Stenger VA, & Carter CS (2004). Anterior cingulate conflict monitoring and adjustments in control. Science, 303, 1023–1026. [DOI] [PubMed] [Google Scholar]
- Kleinman D, & Gollan TH (2016). Speaking two languages for the price of one: Bypassing language control mechanisms via accessibility-driven switches. Psychological Science, 27, 700–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman D, & Gollan TH (2018). Inhibition accumulates over time at multiple processing levels in bilingual language control. Cognition, 173, 115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg G,R, & Jaeger TF (2015). What do we mean by prediction in language comprehension? Language, Cognition, and Neuroscience, 31, 32–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen M, Soveri A, Laine A, Järvenpää J, de Bruin A, & Antfolk J (2018). Is bilingualism associated with enhanced executive functioning in adults? A meta-analytic review. Psychological Bulletin, 144, 394–425. [DOI] [PubMed] [Google Scholar]
- Li C, & Gollan TH (2018). Cognates facilitate switches and then confusion: Contrasting effects of cascade versus feedback on language selection. Journal of Experimental Psychology. Learning, Memory, and Cognition, 44, 974–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Matalon S, Hare TA, Davidson MC, & Casey BJ (2006). Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron, 50, 643–653. [DOI] [PubMed] [Google Scholar]
- Liu H, & Cao F (2016). L1 and L2 processing in the bilingual brain: A meta-analysis of neuroimaging studies. Brain and Language, 159, 60–73. [DOI] [PubMed] [Google Scholar]
- Luk G, Green DW, Abutalebi J, & Grady C (2011). Cognitive control for language switching in bilinguals: A quantitative meta-analysis of functional neuroimaging studies. Language and Cognitive Processes, 27, 1479–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Hu J, Xi J, Shen W, Ge J, Geng F, Wu Y, Guo J, & Yao D (2014). Bilingual cognitive control in language switching: An fMRI study of English-Chinese late bilinguals. PLoS ONE, 9, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod CM, Dodd MD, Sheard ED, Wilson DE, & Bibi U (2003). In Opposition to Inhibition. In Psychology of Learning and Motivation - Advances in Research and Theory (Vol. 43, pp. 163–214). [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O’Doherty J, Ashburner J, Frackowiak RS, & Price CJ (2004). Neurolinguistics: structural plasticity in the bilingual brain. Nature, 431, 757. [DOI] [PubMed] [Google Scholar]
- Meuter RFI, & Allport A (1999). Bilingual language switching in naming: Asymmetrical costs of language selection. Journal of Memory and Language, 40, 25–40. [Google Scholar]
- Moreno EM, Federmeier KD, & Kutas M (2002). Switching languages, switching palabras (words): an electrophysiological study of code switching. Brain and Language, 80, 188–207. [DOI] [PubMed] [Google Scholar]
- Muysken P (2000). Bilingual speech: A typology of code-switching. Oxford: Cambridge University Press. [Google Scholar]
- O’Regan K (1979). Saccade size control in reading: Evidence for the linguistic control hypothesis. Perception & Psychophysics, 25, 501–509. [DOI] [PubMed] [Google Scholar]
- Paap KR, Johnson HA, & Sawi O (2015). Bilingual advantages in executive functioning either do not exist or are restricted to very specific and undetermined circumstances. Cortex, 69, 265–278. [DOI] [PubMed] [Google Scholar]
- Paap KR, & Sawi O (2014). Bilingual advantages in executive functioning: problems in convergent validity, discriminant validity, and the identification of the theoretical constructs. Frontiers in Psychology, 5, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paap KR, Myuz H Anders RT, Bockelman M, Mikulinsky R & Sawi OM (2017). No compelling evidence for a bilingual advantage in switching or that frequent language switching reduces switch cost, Journal of Cognitive Psychology, 29, 89–112. [Google Scholar]
- Paz-Alonso PM, Oliver M, Quiñones I, & Carreiras M (2019). Neural basis of monolingual and bilingual reading. The Oxford Handbook of Neurolinguistics. [Google Scholar]
- Perani D, & Abutalebi J (2015). Bilingualism, dementia, cognitive and neural reserve. Current Opinion in Neurology, 28, 618–625. [DOI] [PubMed] [Google Scholar]
- Pickering MJ & Gambi C (2018). Predicting while comprehending language: A theory and review. Psychological Bulletin, 144, 1002–1044. [DOI] [PubMed] [Google Scholar]
- Poulisse N (1999). Slips of the tongue: Speech errors in first and second language production. Amsterdam/Philadelphia: John Benjamins. [Google Scholar]
- Poulisse N, & Bongaerts T (1994). First language use in second language production. Applied Linguistics, 15, 36–57. [Google Scholar]
- Price CJ, Green DW, & von Studnitz R (1999). A functional imaging study of translation and language switching. Brain, 122, 2221–2235. [DOI] [PubMed] [Google Scholar]
- Prior A & Gollan TH (2011). Good language-switchers are good task-switchers: Evidence from Spanish-English and Mandarin-English bilinguals. Journal of the International Neuropsychological Society: JINS, 17, 682–691. [DOI] [PubMed] [Google Scholar]
- Prior A, & MacWhinney B (2010). A bilingual advantage in task switching. Bilingualism: Language and Cognition, 13, 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proverbio AM, Leoni G, & Zani A (2004). Language switching mechanisms in simultaneous interpreters: an ERP study. Neuropsychologia, 42, 1636–1656. [DOI] [PubMed] [Google Scholar]
- Rastle K, & Brysbaert M (2006). Masked phonological priming effects in English: Are they real? Do they matter? Cognitive Psychology, 53, 97–145. [DOI] [PubMed] [Google Scholar]
- Rey-Mermet A, Gade M, & Oberauer K (2018). Should we stop thinking about inhibition? Searching for individual and age differences in inhibition ability. Journal of Experimental Psychology. Learning, Memory, and Cognition, 44, 501–526. [DOI] [PubMed] [Google Scholar]
- Reynolds MG, Schlöffel S, & Peressotti F (2016). Asymmetric switch costs in numeral naming and number word reading: Implications for models of bilingual language production. Frontiers in Psychology, 6, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Fornells A, Balaguer RDD, & Münte TF (2006). Executive control in bilingual language processing. Language Learning, 56, 133–190. [Google Scholar]
- Saint-Aubin J, & Klein RM (2001). Influence of parafoveal processing on the missing-letter effect. Journal of Experimental Psychology. Human Perception and Performance, 27, 318–334. [DOI] [PubMed] [Google Scholar]
- Schotter ER, Li C, & Gollan TH (in press). What reading aloud reveals about speaking: Regressive saccades implicate a failure to monitor, not inattention, in the prevalence of intrusion errors on function words. Quarterly Journal of Experimental Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasenko A, Matt G, & Gollan TH (2017). A relative bilingual advantage in switching with preparation: Nuanced explorations of the proposed association between bilingualism and task switching. Journal of Experimental Psychology: General, 146, 1527–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub A, Dodge S, & Cohen AL (2019). Failure to detect function word repetitions and omissions in reading: Are eye movements to blame? Psychonomic Bulletin & Review, 26, 340–346. [DOI] [PubMed] [Google Scholar]
- Thissen D, Steinberg L, & Kuang D (2002). Quick and easy implementation of the Benjamini-Hochberg procedure for controlling the false positive rate in multiple comparisons. Journal of Educational and Behavioral Statistics, 27, 77–83. [Google Scholar]
- van Heuven WJB, & Dijkstra T (2010). Language comprehension in the bilingual brain: fMRI and ERP support for psycholinguistic models. Brain Research Reviews, 64, 104–122. [DOI] [PubMed] [Google Scholar]
- Wang X (2015). Language control in bilingual language comprehension: evidence from the maze task. Frontiers in Psychology, 6, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kuhl PK, Chen C, & Dong Q (2009). Sustained and transient language control in the bilingual brain. NeuroImage, 47, 414–422. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xue G, Chen C, Xue F, & Dong Q (2007). Neural bases of asymmetric language switching in second-language learners: An ER-fMRI study. NeuroImage, 35, 862–870. [DOI] [PubMed] [Google Scholar]
- Weissberger GH, Gollan TH, Bondi MW, Clark LR, & Wierenga CE (2015). Language and task switching in the bilingual brain: Bilinguals are staying, not switching, experts. Neuropsychologia, 66, 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams VSL, Jones LV, & Tukey JW (1999). Controlling error in multiple comparisons, with examples from state-to-state differences in educational achievement. Journal of Educational and Behavioral Statistics, 24, 42–69. [Google Scholar]
- Ye Z, & Zhou X (2009). Conflict control during sentence comprehension: fMRI evidence. NeuroImage, 48, 280–290. [DOI] [PubMed] [Google Scholar]