Abstract
Aims
Myocarditis is a potentially fatal complication of immune checkpoint inhibitors (ICI). Sparse data exist on the use of cardiovascular magnetic resonance (CMR) in ICI-associated myocarditis. In this study, the CMR characteristics and the association between CMR features and cardiovascular events among patients with ICI-associated myocarditis are presented.
Methods and results
From an international registry of patients with ICI-associated myocarditis, clinical, CMR, and histopathological findings were collected. Major adverse cardiovascular events (MACE) were a composite of cardiovascular death, cardiogenic shock, cardiac arrest, and complete heart block. In 103 patients diagnosed with ICI-associated myocarditis who had a CMR, the mean left ventricular ejection fraction (LVEF) was 50%, and 61% of patients had an LVEF ≥50%. Late gadolinium enhancement (LGE) was present in 48% overall, 55% of the reduced EF, and 43% of the preserved EF cohort. Elevated T2-weighted short tau inversion recovery (STIR) was present in 28% overall, 30% of the reduced EF, and 26% of the preserved EF cohort. The presence of LGE increased from 21.6%, when CMR was performed within 4 days of admission to 72.0% when CMR was performed on Day 4 of admission or later. Fifty-six patients had cardiac pathology. Late gadolinium enhancement was present in 35% of patients with pathological fibrosis and elevated T2-weighted STIR signal was present in 26% with a lymphocytic infiltration. Forty-one patients (40%) had MACE over a follow-up time of 5 months. The presence of LGE, LGE pattern, or elevated T2-weighted STIR were not associated with MACE.
Conclusion
These data suggest caution in reliance on LGE or a qualitative T2-STIR-only approach for the exclusion of ICI-associated myocarditis.
Keywords: Cardiovascular magnetic resonance, Immune checkpoint inhibitor, Myocarditis
See page 1744 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa136)
Introduction
Harnessing the power of the immune system has revolutionized cancer treatment.1 , 2 Immune checkpoint inhibitors (ICI) are antibodies that block tumour-driven inhibition of T-cell activation and function and facilitate an immune-mediated attack on cancer cells. These therapies are currently approved for a multitude of cancer indications and the use of ICI is rapidly expanding from late-stage disease to the first line metastatic and adjuvant settings.3 For context, there are currently 2004 immuno-modulatory agents against 303 targets, from 864 companies in 3042 active clinical trials.4 Myocarditis is an uncommon toxicity associated with ICI with wide incidence varying from 0.1% to 1%;5 , 6 however, reporting of ICI-associated myocarditis has increased, likely due to heightened awareness.7 , 8 Myocarditis related to an ICI has a fulminant course, with a case fatality rate of 30–50%.9–12 Cardiovascular magnetic resonance (CMR), with the use of tissue characterization techniques such as late gadolinium enhancement (LGE) and the presence of myocardial oedema, is the gold-standard non-invasive imaging test for diagnosis and risk prediction in myocarditis of other aetiologies.13–18 Endomyocardial biopsy (EMB) is the diagnostic gold standard for myocarditis; however, it is underutilized due to its invasive nature and associated potential complications (rate 0.3–6%).14 , 19 Beyond case reports and small case series, there are sparse data characterizing the use of CMR and correlating with EMB findings in the assessment of ICI-associated myocarditis.20–22 In this study, the largest cohort of ICI-associated myocarditis was leveraged to provide the first data on CMR characteristics, to describe the correlation between CMR findings and histopathology, and to test the association between CMR features and cardiovascular events among patients with ICI-associated myocarditis.
Methods
Patient cohort
Immune checkpoint inhibitor-associated myocarditis is uncommon, and to provide insight an international multicentre registry of ICI-associated myocarditis from 23 sites across the USA, Canada, and Europe (Supplementary material online, Table S1) was established.10 We included consecutive patients who were diagnosed with ICI-associated myocarditis by board-certified cardiologists from the participating sites. The first case in the registry was diagnosed in November 2013, and cases were included in this report until April 2019. The beginning of follow-up was the time of first use of ICI. Patients’ clinical characteristics, CMR features, myocardial biopsy or autopsy data, and outcomes were collected by investigators at each study site. The study complied with the Declaration of Helsinki and was approved by each centre’s institutional review committee; the requirement for written informed consent was waived.
Diagnosis of immune checkpoint inhibitor-associated myocarditis
Immune checkpoint inhibitor-associated myocarditis was diagnosed in one of two ways: (i) standard features present on histopathology23 or (ii) diagnostic criteria for clinically suspected myocarditis based on the European Society of Cardiology (ESC) guidelines.14 This standardized diagnostic strategy has been applied to multiple cohorts.24 , 25
Covariates
Demographics, cardiovascular risk factors, electrocardiograms (ECG), and echocardiograms were extracted from electronic medical records of each study site at the time of the index presentation with myocarditis. Additional covariates included clinical presentation, physical examination, initial and peak cardiac biomarkers, CMR, EMB, and autopsy results. Initial troponin and B-type natriuretic peptide (BNP) were defined as the first measured serum troponin and BNP at the time of admission during the index hospitalization. Peak troponin and BNP were the maximum measured troponin and BNP at the index hospitalization. Cancer-specific covariates included the cancer type, ICI treatment, prior cardiotoxic chemotherapy, and prior radiation.
Cardiovascular magnetic resonance protocol
Patients underwent a CMR at the discretion of the local physicians at the time of presentation with suspected ICI-associated myocarditis. The CMR protocol was not protocol-specified and thus reflected local practice. In summary, all images were acquired with ECG gating, breath-holding, and the patient in a supine position. Subjects were imaged on either a 1.5 or 3 T CMR system. Each CMR protocol included balanced cine steady-state free precession imaging for cardiac function and mass. The typical slice thickness was 8 mm with no gap. The protocol also included black-blood T2-weighted short tau inversion recovery (STIR) imaging sequences in three short-axis slices and a single long-axis view for qualitative assessment of myocardial oedema.26 Qualitative T2-weight STIR signal was evaluated by visual assessment. Where available, the early gadolinium enhancement ratio was acquired in a free-breathing spin echo sequence in four identical short-axis slices (basal, mid-basal, mid-apical, and apical) both before and within the first 3 min after intravenous injection of contrast. Early gadolinium enhancement was defined as the enhancement of myocardium divided by the enhancement of skeletal muscle in a ratio of ≥4.15 , 18 The presence of LGE was determined 10–15 min after contrast administration using both magnitude and phase-sensitive inversion recovery images. Slices were 8 mm thick with 2 mm gaps. In a subset of patients, T1 measurements and T1 mapping were available (n = 15). T1 measurements were performed using a Look-Locker sequence in a single mid-ventricle slice on a 3 T CMR system, pre- and post-contrast, as previously described.27 T1 mapping sequences were only performed pre-contrast on a 3 T system using a 5(3)3 MOLLI in a single mid-ventricle slice. The CMR studies were interpreted at each site by experienced readers as part of clinical care. The LGE pattern was categorized as sub-endocardial/transmural, sub-epicardial, mid-myocardial, and diffuse.28 If more than one pattern was present, the predominant pattern was reported.
Histopathology
Histopathological analysis of cardiac samples obtained by either EMB or post-mortem autopsy was reported. The performance of histopathological sampling was not protocol-specified and thus varied per local practice. It was performed at the time of presentation with myocarditis (EMB) or with death from a cardiovascular complication with ICI-associated myocarditis. Typically, at least five biopsies were preferentially taken from the apical septum of the right ventricle (RV); no left ventricle (LV) biopsies were performed. The findings were reported by pathologists at each study site according to the 2001 consensus statement from the Association for European Cardiovascular Pathology.23
Outcomes
The primary outcome of interest, major adverse cardiovascular events (MACE), was a composite of cardiovascular death, cardiac arrest, cardiogenic shock, and complete heart block (CHB) requiring a pacemaker. In case where cardiac arrest, cardiogenic shock, or CHB led to a death, that case was counted as a cardiac death. When a patient had multiple MACE, the time of MACE was defined as the date of the earliest event. Standard definitions were used for cardiovascular death,29 cardiac arrest,30 cardiogenic shock,31 and CHB. The end of follow-up was on 9 April 2019.
Statistical analysis
Continuous variables were described as mean ± standard deviation or median (interquartile range) and were compared with the use of Student’s t-tests or Wilcoxon Rank Sum tests, as appropriate based on their normality. Normality of continuous variables was tested using the Shapiro–Wilk test. Categorical variables were presented as percentage and were compared using the χ 2 test. The overall agreement and the Cohen’s kappa coefficient between the site read and a blind reviewer for LGE and T2-weighted STIR assessment were assessed. Covariates were compared between patients with and without LGE. Univariable and multivariable [adjusting for age, sex, number of cardiovascular risk factors, and lowest left ventricular ejection fraction (LVEF)] Cox proportional hazards models were performed to examine the association of CMR and histopathology features with MACE. Harrell’s C-statistics was obtained to assess the performance of the survival models.32 Sensitivity analysis was performed by adding study sites in the multivariable-adjusted Cox proportional hazards models. Kaplan–Meier curves for MACE by LGE, myocardial oedema, and pathological fibrosis were presented and compared with the Logrank test. A two-sided P-value <0.05 was considered significant. Analyses were performed with Stata15 (StataCorp, College Station, TX, USA).
Results
Patient characteristics
All 103 ICI-associated myocarditis patients in the registry through April 2019 who had a CMR were included. Of these 103 patients, 56 patients were diagnosed with EMB or autopsy and 47 were diagnosed using the ESC diagnostic criteria for clinically suspected myocarditis (Supplementary material online, Table S2).14 The mean age was 65.6 ± 15.3 years and 29.1% were female (Table 1). More than half of the patients presented with shortness of breath. Other common symptoms included chest pain,33 orthopnoea, paroxysmal nocturnal dyspnoea, and fatigue (Table 2). At the time of presentation, obstructive coronary artery disease (CAD) was excluded in 65 patients using coronary angiography, 16 patients by coronary computed tomography angiography, and 16 patients by stress test with imaging (nuclear stress test or stress echocardiography). The six patients without an ischaemia evaluation all had pathology-proven myocarditis (Supplementary material online, Table S2).
Table 1.
Description of immune checkpoint inhibitor-associated myocarditis with and without late gadolinium enhancement on cardiovascular magnetic resonance
| With CMR (N = 103) | LGE present (N = 49) | LGE absent (N = 54) | P-valuea | |
|---|---|---|---|---|
| Age at start of ICI (years) | 65.6 ± 15.3 | 68.8 ± 10.1 | 62.8 ± 18.3 | 0.057 |
| Female | 30 (29.1) | 13 (26.5) | 17 (31.5) | 0.67 |
| CV risk factors | ||||
| Hypertension | 56 (55.5) | 29 (61.7) | 27 (50.0) | 0.32 |
| Diabetes mellitus | 21 (21.7) | 11 (23.9) | 10 (19.6) | 0.63 |
| No CV risk factors | 29 (28.2) | 11 (22.5) | 18 (33.3) | 0.56 |
| Prior coronary artery disease | 14 (14.7) | 8 (17.4) | 6 (12.2) | 0.57 |
| Prior stroke | 5 (5.3) | 3 (6.7) | 2 (4.0) | 0.67 |
| Prior heart failure | 2 (2.1) | 1 (2.2) | 1 (2.0) | 1.00 |
| Chronic kidney disease | 5 (6.0) | 3 (7.7) | 2 (4.4) | 0.24 |
| Body mass index (kg/m2) | 27.5 ± 6.3 | 26.6 ± 5.2 | 28.4 ± 7.2 | 0.20 |
| Primary cancer type | ||||
| Head and neck | 6 (5.8) | 1 (2.0) | 5 (9.3) | 0.21 |
| Breast | 3 (2.9) | 3 (6.1) | 0 (0) | 0.10 |
| Hodgkin’s lymphoma | 2 (1.9) | 0 (0) | 2 (3.7) | 0.50 |
| Melanoma | 45 (43.7) | 17 (34.7) | 28 (51.9) | 0.11 |
| Non-small-cell lung cancer | 15 (14.6) | 11 (22.5) | 4 (7.4) | 0.048 |
| Pancreatic | 2 (1.9) | 1 (2.0) | 1 (1.9) | 1.00 |
| Renal cell carcinoma | 5 (4.9) | 1 (2.0) | 4 (7.4) | 0.37 |
| Glioblastoma | 1 (1.0) | 1 (2.0) | 0 (0) | 0.48 |
| Prior chemotherapy or radiation | ||||
| Radiation | 31 (30.1) | 16 (32.7) | 15 (27.8) | 0.67 |
| Anthracyclines | 7 (6.8) | 4 (8.2) | 3 (5.6) | 0.71 |
| ICI regimen | ||||
| Monotherapy | 74 (71.8) | 38 (77.6) | 36 (66.7) | 0.28 |
| Anti-PD1 | 67 (90.5) | 35 (92.1) | 32 (88.9) | 0.22 |
| Anti-CTLA4 | 6 (8.1) | 2 (5.3) | 4 (11.1) | 0.68 |
| Anti-PDL1 | 1 (1.4) | 1 (2.6) | 0 (0) | 0.48 |
| Dual therapy | 29 (28.2) | 11 (22.5) | 18 (33.3) | 0.28 |
Values are mean ± SD or n (%).
Anti-CTLA4, anti-cytotoxic T-lymphocyte-associated protein 4; anti-PD1, anti-programmed cell death protein 1; anti-PDL1, anti-programmed death-ligand 1; CMR, cardiovascular magnetic resonance; CV, cardiovascular; ICI, immune checkpoint inhibitors; LGE, late gadolinium enhancement; SD, standard deviation.
Comparison between patients with and without LGE using the Student’s t-tests or Wilcoxon Rank Sum tests for continuous variables, as appropriate based on their normality and the χ 2 test for categorical variables.
Table 2.
Comparison of clinical presentation and outcomes in patients with and without late gadolinium enhancement
| With CMR (N = 103) | LGE present (N = 49) | LGE absent (N = 54) | Relative ratio or difference (95% CI)a | P-valueb | |
|---|---|---|---|---|---|
| Time from starting ICI to admission for myocarditis (days) | 64 (33–133) | 68 (32–97.5) | 74 (29–162) | NA | 0.44 |
| Myocarditis presentation | |||||
| Chest pain | 29 (28.2) | 14 (28.6) | 15 (27.8) | 1.0 (−0.2 to 0.2) | 0.93 |
| Shortness of breath | 57 (55.3) | 26 (53.1) | 31 (57.4) | 0.9 (0.7–1.3) | 0.66 |
| Orthopnoea | 22 (21.8) | 9 (19.2) | 13 (24.1) | 0.7 (0.3–1.4) | 0.31 |
| Paroxysmal nocturnal dyspnoea | 20 (19.6) | 8 (16.7) | 12 (22.2) | 0.6 (0.3–1.4) | 0.26 |
| Fatigue | 35 (38.0) | 13 (32.5) | 22 (42.3) | 0.8 (0.5–1.3) | 0.31 |
| Syncope | 9 (9.6) | 5 (11.9) | 4 (7.7) | 1.7 (0.8–3.8) | 0.20 |
| Sudden cardiac death | 1 (1.1) | 1 (2.4) | 0 (0) | 2.1 (0.7–6.6) | 0.20 |
| Palpitation | 26 (25.5) | 11 (22.9) | 15 (27.8) | 0.8 (0.4–1.5) | 0.47 |
| Physical exam | |||||
| Jugular vein distention | 30 (29.4) | 14 (29.2) | 16 (29.6) | 1.3 (0.8–2.2) | 0.28 |
| Crackles | 39 (38.6) | 18 (37.5) | 21 (39.6) | 1.1 (0.7–1.7) | 0.84 |
| Lower extremity oedema | 35 (34.3) | 16 (33.3) | 19 (35.2) | 1.2 (0.7–1.9) | 0.49 |
| SBP (mmHg) | 126.6 ± 20.2 | 124.7 ± 22.8 | 128.3 ± 17.6 | 3.6 (−4.9 to 12.0) | 0.40 |
| DBP (mmHg) | 72.9 ± 11.3 | 72.3 ± 12.8 | 73.5 ± 9.7 | 1.2 (−3.6 to 5.9) | 0.62 |
| Electrocardiogram at presentation | |||||
| Sinus rhythm | 82 (80.4) | 39 (79.6) | 43 (81.1) | 1.0 (0.8–1.2) | 0.85 |
| ST-segment or T-wave changes | 55 (54.5) | 25 (53.2) | 30 (55.6) | 1.0 (0.7–1.4) | 0.81 |
| Heart rate (beats/min) | 87.0 ± 22.4 | 87.8 ± 25.1 | 86.4 ± 20.5 | −1.4 (−11.5 to 8.6) | 0.78 |
| Biomarkers | |||||
| Initial troponin T (ng/mL) | 0.5 (0.1–1.7) | 1.0 (0.2–6.8) | 0.4 (0.1–1.1) | NA | 0.021 |
| Peak troponin T (ng/mL) | 1.0 (0.1–2.1) | 1.0 (0.2–4.5) | 0.9 (0.1–1.9) | NA | 0.22 |
| Initial BNP (pg/mL) (n = 84) | 589 (194–2413) | 838 (405–4592) | 478.5 (146.5–1350) | NA | 0.09 |
| Peak BNP (pg/mL) (n = 49) | 1088 (242–4873) | 1553.5 (734.5–6542.5) | 922 (194–2567) | NA | 0.16 |
| Echocardiogram | |||||
| Pre-ICI LVEF (%) (n = 66) | 61.1 ± 5.7 | 60.6 ± 5.4 | 61.6 ± 5.9 | 1.0 (−1.8 to 3.8) | 0.48 |
| Lowest LVEF at presentation (%) | 49.8 ± 16.6 | 47.7 ± 16.3 | 51.8 ± 16.8 | 4.2 (−2.3 to 10.7) | 0.20 |
| Change of LVEF (%) (n = 66) | 12.6±14.3 | 12.3 ± 12.9 | 12.9 ± 15.7 | 0.6 (−7.5 to 7.7) | 0.88 |
| LVEF<50% at presentation | 40 (38.8) | 22 (44.9) | 18 (33.3) | 0.8 (0.6–1.1) | 0.23 |
| LVIDD (mm) | 47.8 ± 6.1 | 48.3 ± 6.1 | 47.4 ± 6.1 | −0.8 (−3.6 to 1.9) | 0.53 |
| LVIDS (mm) | 35.0 ± 8.8 | 36.5 ± 8.7 | 33.5 ± 8.8 | −3.0 (−7.3 to 1.4) | 0.18 |
| LA size (mm) | 38.9 ± 7.6 | 39.9 ± 8.1 | 38.1 ± 7.3 | −1.8 (−5.8 to 2.2) | 0.38 |
| Pericardial effusion | 19 (23.5) | 8 (22.2) | 11 (24.4) | 0.9 (0.4–2.0) | 0.82 |
| Global longitudinal strain by echo (%) (n = 79) | −14.3 ± 2.9 | −13.8 ± 3.0 | −14.8 ± 2.8 | −1.1 (−2.4 to 0.2) | 0.099 |
| CMR | |||||
| 1.5 T | 81 (78.6) | 40 (81.6) | 41 (75.9) | 0.8 (0.4–1.6) | 0.48 |
| 3 T | 22 (21.4) | 9 (18.4) | 13 (24.1) | 0.8 (0.4–1.6) | 0.48 |
| LVEDV (mL) | 147.0 ± 39.7 | 149.1 ± 40.8 | 145.0 ± 38.9 | −4.0 (−19.8 to 11.7) | 0.61 |
| LV mass index (g/m2) | 72.4 ± 23.9 | 75.7 ± 26.8 | 69.3 ± 20.5 | −6.4 (−16.0 to 3.1) | 0.18 |
| LVEF by CMR (%) | 49.1 ± 15.1 | 47.5 ± 15.9 | 50.6 ± 14.4 | 3.1 (−2.8 to 9.0) | 0.30 |
| Oedema by T2-weighted STIR | 28 (27.5) | 18 (36.7) | 10 (18.9) | 2.0 (1.0–3.9) | 0.037 |
| Predominant LGE pattern | |||||
| Sub-endocardial/transmural | NA | 3 (6.1) | NA | NA | NA |
| Sub-epicardial | NA | 13 (26.5) | NA | NA | NA |
| Mid-myocardial | NA | 24 (49.0) | NA | NA | NA |
| Diffuse | NA | 9 (18.4) | NA | NA | NA |
| Native T1 value (ms) (n = 15) | 1167.2 ± 32.9 | 1174.3 ± 34.1 | 1162.4 ± 33.2 | −11.9 (−50.1 to 26.3) | 0.51 |
| Extracellular volume (%) (n = 8) | 34.3 ± 2.1 | 34.5 ± 1.9 | 34.0 ± 2.6 | −0.01 (−0.04 to 0.03) | 0.77 |
| Early gadolinium enhancement ratio (n = 15) | 2.8 ± 0.6 | 2.8 ± 0.6 | 2.9 ± 0.6 | 0.1 (−0.5 to 0.8) | 0.72 |
| Histopathology (n = 56) | |||||
| Fibrosis | 31 (55.4) | 11 (50.0) | 20 (58.8) | 0.9 (0.5–1.4) | 0.52 |
| Lymphocytes (T cell) | 55 (98.2) | 21 (95.5) | 34 (100.0) | 1.0 (0.9–1.1) | 0.21 |
| Histiocytes | 4 (7.1) | 1 (4.6) | 3 (8.8) | 0.5 (0.1–4.6) | 0.54 |
| Eosinophils | 4 (7.1) | 2 (9.1) | 2 (5.9) | 1.5 (0.2–10.2) | 0.65 |
| Outcomes | |||||
| Follow-up time for MACEc (days) | 148.5 (62–304) | 136 (63–259) | 162 (62–379) | NA | 0.33 |
| MACE (cumulative incidence)d | 41 (39.8) | 19 (38.8) | 22 (40.7) | 1.0 (0.6–1.5) | 0.84 |
| MACE (incidence rate, per person-year) | 0.63 | 0.68 | 0.59 | 1.2 (0.6–2.2) | 0.32 |
| Complete heart block (cumulative incidence) | 16 (15.8) | 7 (14.6) | 9 (17.0) | 0.9 (0.3–2.1) | 0.74 |
| Complete heart block (incidence rate, per person-year) | 0.25 | 0.26 | 0.25 | 1.0 (0.3–3.1) | 0.47 |
| Cardiogenic shock (cumulative incidence) | 15 (15.2) | 8 (17.0) | 7 (13.5) | 1.3 (0.5–3.2) | 0.62 |
| Cardiogenic shock (incidence rate, per person-year) | 0.24 | 0.29 | 0.20 | 1.5 (0.5–4.9) | 0.22 |
| Cardiac arrest (cumulative incidence) | 15 (15.2) | 7 (14.9) | 8 (15.4) | 0.9 (0.3–2.1) | 0.75 |
| Cardiac arrest (incidence rate, per person-year) | 0.27 | 0.26 | 0.28 | 0.9 (0.3–2.7) | 0.43 |
| CV death (cumulative incidence) | 17 (16.5) | 6 (12.2) | 11 (20.4) | 0.6 (0.2–1.5) | 0.27 |
| CV death (incidence rate, per person-year) | 0.26 | 0.22 | 0.30 | 0.7 (0.2–2.2) | 0.28 |
Values are mean ± SD, n (%), or median (interquartile range).
BNP, B-type natriuretic peptide; CMR, cardiovascular magnetic resonance; CV, cardiovascular; DBP, diastolic blood pressure; ICI, immune checkpoint inhibitors; LA, left atrium; LGE, late gadolinium enhancement; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVIDD, left ventricular internal diameter end diastole; LVIDS, left ventricular internal diameter end systole; LV mass, left ventricular mass; MACE, major adverse cardiovascular events; NA, not applicable; SBP, systolic blood pressure; SD, standard deviation; STIR, short tau inversion recovery.
Relative ratios and 95% CI for categorical variables and difference and 95% CI for normally distributed continuous variables. Cumulative incidence ratio (95% CI) and incidence rate ratio (95% CI) for MACE and individual MACE categories.
Comparison between patients with and without LGE using the Student’s t-tests or Wilcoxon Rank Sum tests for continuous variables, as appropriate based on their normality and the χ 2 test for categorical variables.
Time of the MACE was defined by the date of the earliest event when multiple MACE happened.
Patients may have multiple MACE.
Cancer and treatment characteristics
The most common indications for ICI were melanoma and non-small-cell lung cancer (Table 1). All cases with ICI-associated myocarditis had the ICI permanently discontinued. Most patients (71.8%) received ICI monotherapy and, among them, 90.5% had anti-programmed cell death protein 1 therapy (including nivolumab and pembrolizumab), 8.1% had anti-cytotoxic T-lymphocyte-associated protein 4 therapy (including ipilimumab and tremelimumab), and 1.4% had anti-programmed death-ligand 1 therapy (including avelumab and atezolizumab). Dual ICI therapy was used in 28.2% of patients (Table 1).
Cardiovascular magnetic resonance characteristics
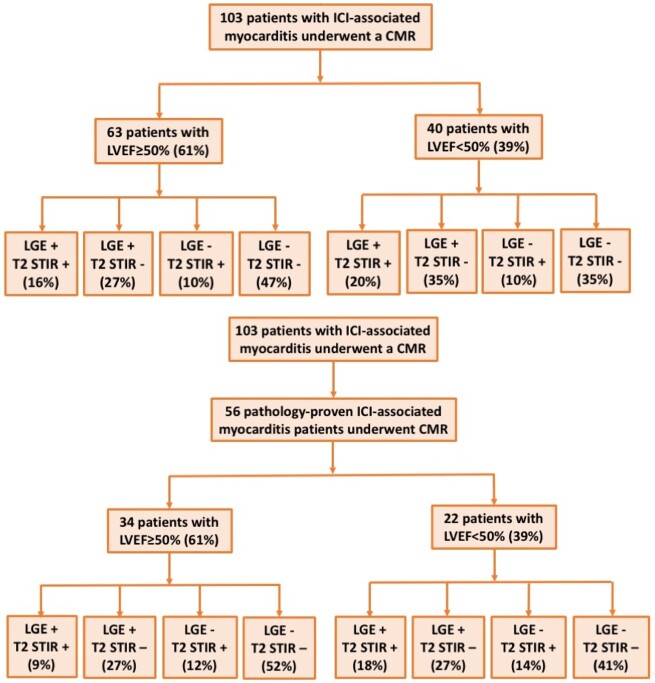
A 1.5 T scanner was used in 81 patients and a 3 T scanner in 22 patients. The mean LVEF, left ventricular end-diastolic volume, and LV mass index were 49.1%, 147.0 mL and 72.4 g/m2, respectively (Table 2). A trivial or small pericardial effusion was noted in 19 patients (23.5%) (Table 2). In total, 40 patients (39%) had an LVEF of <50% and 63 patients (61%) had EF of ≥50% (Figure 1A). Late gadolinium enhancement was present in 49 patients (48%) of the entire cohort, 43% of cases with a preserved LVEF and 55% of cases with a reduced EF (Figure 1A). The predominant LGE pattern included sub-endocardial/transmural (3), sub-epicardial (13), mid-myocardial (24), and diffuse (9) (Figure 2). In the 14 patients with history of CAD before starting ICI, including 5 patients with prior myocardial infarction, 4 patients with prior coronary stenting, 6 patients with prior coronary artery bypass grafting (not mutually exclusive), 8 had LGE; with a sub-epicardial pattern in 2 patients, mid-myocardial in 4 patients, and diffuse pattern in 2 patients. We did not find difference in history of CAD in patients with (17.4%) or without LGE [12.2%, relative ratio (RR) 1.4, 95% confidence interval (CI) 0.5–3.8; P = 0.57]. Late gadolinium enhancement was predominantly distributed at the anteroseptal, inferoseptal, inferior, and inferolateral segments (Supplementary material online, Figure S1A). Two of the three patients with sub-endocardial/transmural pattern had pathology-proven myocarditis. The third patient had LGE in multiple distributions (apical, apical anterior, and apical lateral segments), no obstructive CAD and a clinical presentation consistent with myocarditis. Qualitative myocardial oedema by T2-weighted STIR was present in 28 patients (28%), in 30% of the reduced EF and 26% of the preserved EF cohort. Elevated T2-weighted STIR signal was predominantly distributed at the anteroseptal, inferoseptal, and inferior segments (Supplementary material online, Figure S1B). Eighteen patients had both LGE and elevated T2-weighted STIR signal, 31 patients had LGE and no elevated T2-weighted STIR signal, 10 patients had elevated T2-weighted STIR signal and no LGE, and 43 patients had neither elevated T2-weighted STIR signal or LGE (Figure 1A). Patients with LGE more often had elevated T2-weighted STIR signal (36.7%) than patients without LGE (18.9%, RR 2.0, 95% CI 1.0–3.9; P = 0.037). In 44 randomly selected patients, the overall agreement between the site read and a blind reviewer was 0.97 for LGE assessment and 0.95 for T2-weighted STIR assessment. The Cohen’s kappa coefficient was 0.94 for LGE assessment and 0.85 for T2-weighted STIR assessment.
Figure 1.
Patient cohort of immune checkpoint inhibitor-associated myocarditis. CMR, cardiovascular magnetic resonance; ICI, immune checkpoint inhibitors; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; STIR, short tau inversion recovery.
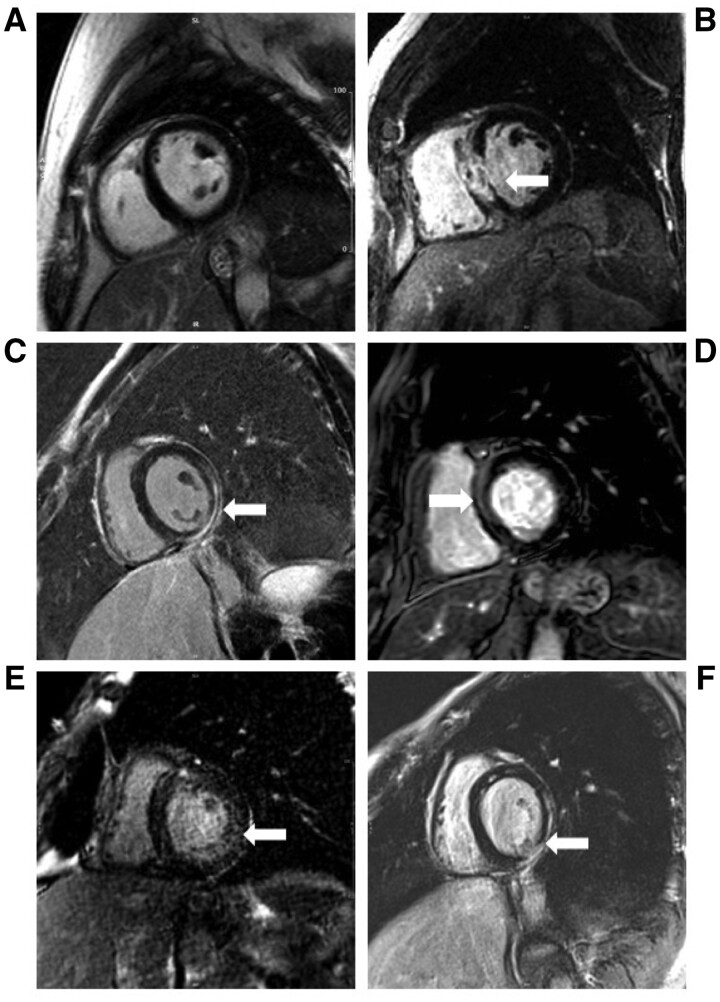
Figure 2.
Representative late gadolinium enhancement pattern. Representative late gadolinium enhancement images from patients with immune checkpoint inhibitor-associated myocarditis, showing a patient with no late gadolinium enhancement (A); a patient with sub-endocardial/transmural late gadolinium enhancement (B); a patient with sub-epicardial late gadolinium enhancement (C); a patient with mid-myocardial late gadolinium enhancement (D); a patient with diffuse late gadolinium enhancement (E); and a patient with mixed late gadolinium enhancement (sub-epicardial, mid-myocardial, and transmural) (F). Regions of late gadolinium enhancement are highlighted using white arrows.
The early gadolinium enhancement ratio was available in a subset of patients and was normal (n = 15, mean 2.8 ± 0.6). Fifteen patients underwent T1 measurements or T1 mapping. The mean native T1 value in these 15 patients was 1167.2 ± 32.9 ms, higher than the normal T1 value at the institution (1100–1150 ms on 3 T; 1000–1100 ms on 1.5 T). The native T1 was similar between patients with and without LGE (1174.3 ± 34.1 vs. 1162.4 ± 33.2 ms, P = 0.51, Table 2). The extracellular volume (ECV) was measured in eight patients with mean value at 34.3 ± 2.1%, higher than normal ECV values of 25.3 ± 3.5% in healthy individuals.34 We did not find difference in ECV between patients with (34.5 ± 1.9%) and without LGE [34.0 ± 2.6%, difference 0.1% (−0.5% to 0.8%), P = 0.77] (Table 2).
The demographics, clinical presentation, cancer characteristics, and outcomes were similar between patients with and without LGE (Tables 1 and 2), except that patients with LGE were more likely to have non-small-cell lung cancer (22.5% vs. 7.4%, P = 0.048) and had higher levels of troponin T on admission (1.0 vs. 0.4 ng/mL, P = 0.021). The characteristics of patients who did (n = 103) and did not (n = 39) have a CMR were mostly similar (Supplementary material online, Tables S3 and S4). However, the percentage of patients with prior heart failure, renal cell carcinoma, and the presence of shortness of breath were higher in patients who did not undergo a CMR. Diastolic blood pressure and follow-up time were greater in patients who underwent a CMR. The characteristics of patients diagnosed by the ESC criteria (n = 68) and by histopathological criteria (n = 74) were also compared (Supplementary material online, Tables S5 and S6).
Histopathology features
Among the 103 patients with a CMR, 56 patients had histopathology data available, either through EMB (46) or autopsy (10), all of which were consistent with myocarditis (Figure 1B). In these pathology-proven patients, analysis reported a lymphocytic infiltration in 55 patients (98%), among whom 21 patients (38%) had LGE and 14 patients (26%) had elevated T2-weighted STIR signal. Thirty-one patients had pathological fibrosis, among whom 11 patients (35%) had LGE. A representative case of pathology-proven ICI myocarditis with normal LGE and normal T2-weighted STIR images is presented in Supplementary material online, Figure S2. Additionally, two representative cases from patients with an autopsy showing diffuse myocarditis in every segment but with normal LGE and normal T2-weighted STIR images are presented in Supplementary material online, Figure S3.
Time from admission to cardiovascular magnetic resonance
To better understand the CMR findings, the association between time from onset of myocarditis to CMR and CMR findings was tested. The time of admission with suspected myocarditis was used as a surrogate of time of symptom onset. Specifically, the time (in days) from admission to CMR in those with and without LGE was compared. The time from admission to CMR was longer in patients with LGE (median time 6 days), compared to patients without LGE (median time 2 days, P < 0.001) (Table 3). The Locally Weighted Scatterplot Smoothing method was performed to graphically demonstrate the relationship between the time from admission to CMR and the presence of LGE (Figure 3).35 The presence of LGE varied with the time from admission to CMR. When a CMR was performed on Day 4 of admission or later, LGE was present in 72.0% of patients. Whereas, when a CMR was performed within 4 days of admission, LGE was only present in 21.6% of patients (P < 0.001, Table 3). Performing a CMR on Day 4 of admission or later was significantly associated with the presence of LGE (odds ratio 9.35, 95% CI 3.77–23.21; P < 0.001). The time from admission to CMR was not different in patients with (median 4 days) or without (median 3 days) elevated T2-weighted STIR signal (P = 0.88). Those patients with positive pathological fibrosis, but negative LGE, had a longer time from admission to biopsy (median time 11 days), but a shorter time from admission to CMR (median time 2 days).
Table 3.
Comparison of time from admission to cardiovascular magnetic resonance and percentage of patients with cardiovascular magnetic resonance at different time between patients with and without late gadolinium enhancement
| LGE | No LGE | P-valuea | |
|---|---|---|---|
| Time from admission to CMR (days) | 6 (4–8) | 2 (1–5) | <0.001 |
| CMR performed ≥4 days | 72.0% (36/50) | 28.0% (14/50) | <0.001 |
| CMR performed <4 days | 21.6% (11/51) | 78.4% (40/51) | <0.001 |
CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement.
The time from admission to CMR was compared using the Wilcoxon Rank Sum tests. The percentage of patients in each time category was compared using the χ 2 test.
Figure 3.
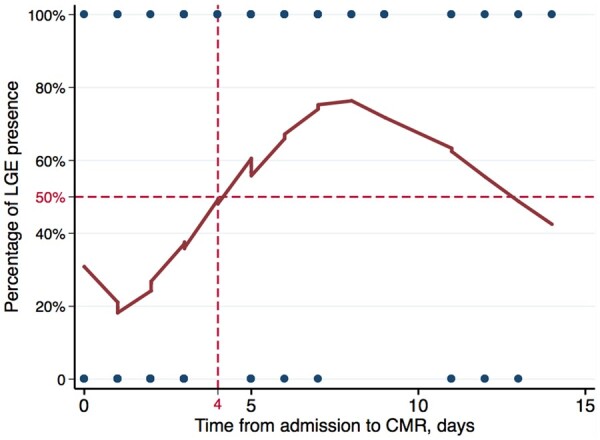
Locally Weighted Scatterplot Smoothing method demonstrating the relationship between the time from admission to cardiovascular magnetic resonance and the presence of late gadolinium enhancement. CMR, cardiovascular magnetic resonance; LGE, late gadolinium enhancement.
Major adverse cardiovascular events
During a median follow-up time of 148.5 days, 41 patients (40%) developed MACE. The presence of LGE, LGE pattern, elevated T2-weighted STIR signal on CMR, or pathological fibrosis were not associated with MACE as their hazard ratios were not statistically significant (Table 4), and Kaplan–Meier curves by subgroups overlapped with each other with a Logrank test P-value >0.05 (Figure 4). In a multivariable model, a reduced EF was significantly associated with higher risk of MACE (hazard ratio 2.07, 95% CI 1.10–3.93; P = 0.025) (Table 4). The results of univariable Cox proportional hazard model were similar (Table 4). Sensitivity analysis by adding study site as a covariate did not change the results meaningfully.
Table 4.
Univariable and multivariable adjusted analysis of association between cardiovascular magnetic resonance and histopathological features and major adverse cardiac events
| Univariable model |
Multivariable model 1a
|
Multivariable model 2b
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | C- statistics | HR (95% CI) | P-value | C- statistics | HR (95% CI) | P-value | C- statistics | |
| CMR | |||||||||
| LGE | 1.03 (0.56–1.91) | 0.92 | 0.504 | 1.16 (0.62–2.18) | 0.65 | 0.550 | 1.19 (0.63–2.25) | 0.58 | 0.681 |
| Sub-endocardial/transmural | 1.95 (0.47–8.04) | 0.36 | 0.506 | 3.33 (0.78–14.12) | 0.10 | 0.531 | 3.39 (0.75–15.37) | 0.11 | 0.677 |
| Sub-epicardial | 0.72 (0.26–1.99) | 0.53 | 0.514 | 0.91 (0.32–2.56) | 0.86 | 0.521 | 1.14 (0.40–3.22) | 0.81 | 0.674 |
| Mid-myocardial | 0.54 (0.25–1.19) | 0.13 | 0.529 | 0.53 (0.24–1.18) | 0.12 | 0.562 | 0.52 (0.24–1.16) | 0.11 | 0.679 |
| Diffuse | 1.80 (0.77–4.20) | 0.17 | 0.514 | 1.80 (0.77–4.23) | 0.18 | 0.536 | 1.46 (0.62–3.44) | 0.39 | 0.677 |
| LVEDV, per SD change | 1.54 (0.98–2.44) | 0.063 | 0.568 | 1.25 (0.98–1.59) | 0.072 | 0.588 | 1.05 (0.79–1.39) | 0.74 | 0.680 |
| LV mass, per SD change | 0.82 (0.56–1.20) | 0.31 | 0.540 | 0.92 (0.63–1.34) | 0.67 | 0.561 | 0.75 (0.48–1.17) | 0.20 | 0.700 |
| LVEF <50% | 2.06 (1.11–3.81) | 0.021 | 0.580 | 1.84 (0.99–3.44) | 0.054 | 0.574 | 2.07 (1.10–3.93) | 0.025 | 0.631 |
| Oedema by T2-weighted STIR | 1.43 (0.74–2.78) | 0.29 | 0.540 | 1.38 (0.69–2.74) | 0.36 | 0.568 | 1.15 (0.57–2.33) | 0.69 | 0.689 |
| Histopathology (n = 56) | |||||||||
| Fibrosis | 1.41 (0.73–2.72) | 0.31 | 0.557 | 1.31 (0.66–2.61) | 0.45 | 0.645 | 1.39 (0.68–2.86) | 0.37 | 0.675 |
CMR, cardiovascular magnetic resonance; HR, hazard ratio; LGE, late gadolinium enhancement; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; SD, standard deviation; STIR, short tau inversion recovery.
Multivariable model 1: Cox proportional hazard model adjusting for age and sex.
Multivariable model 2: Cox proportional hazard model adjusting for age, sex, number of cardiovascular risk factors, and lowest LVEF by echocardiogram during the index hospitalization. When assessing the association of LVEF by CMR with outcomes, LVEF was removed from the model.
Figure 4.
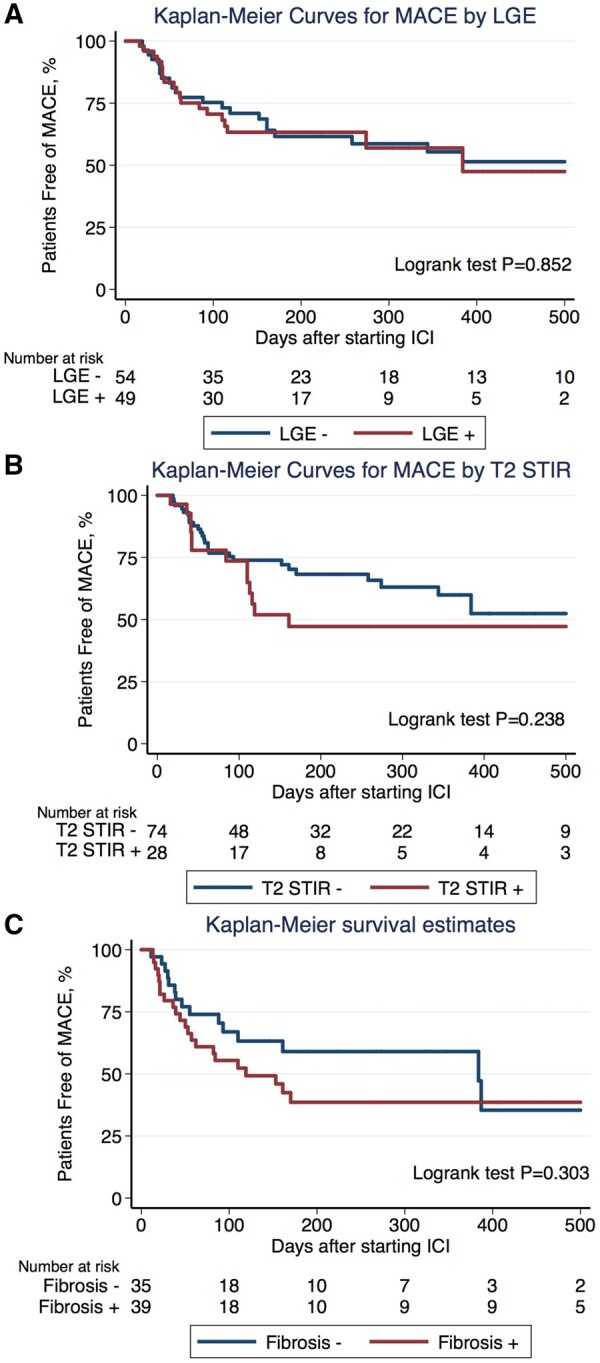
Kaplan–Meier curves for major adverse cardiovascular events by late gadolinium enhancement (A), T2-weighted STIR imaging for oedema (B), and pathological fibrosis (C). LGE, late gadolinium enhancement.
Take home figure.
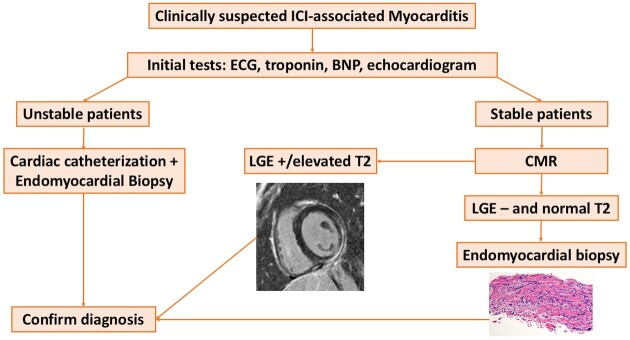
Proposal algorithm for diagnosing immune checkpoint inhibitor-associated myocarditis.BNP, B-type natriuretic peptide; CMR, cardiovascular magnetic resonance; ECG, electrocardiogram; ICI, immune checkpoint inhibitors; LGE, late gadolinium enhancement.
Discussion
There are increased reports of myocarditis related to ICI and this adverse effect is fulminant with a mortality rate of 20–50%.6 , 10 Our understanding of ICI-associated myocarditis needs to improve as these revolutionary therapies are being increasingly applied to a broader range of cancers and to cancers in earlier stages. CMR is the gold-standard imaging test for the diagnosis of myocarditis, and this real-world study is the first to describe the use of CMR in the largest international multicentre cohort of ICI-associated myocarditis. The study reports the following important and novel findings: (i) more than half the cases presented with a preserved LVEF; (ii) LGE was present in less than half of patients with ICI-associated myocarditis and less among those with a preserved LVEF; (iii) qualitative myocardial oedema by T2-weighted STIR was present in less than one-third of patients; (iv) varying patterns of LGE were noted including sub-endocardial/transmural, sub-epicardial, mid-myocardial, and diffuse; (v) the time from admission to CMR affected the likelihood of LGE such as the presence of LGE increased from 21.6% when CMR was performed within 4 days of admission, to 72.0% when a CMR was performed on Day 4 of admission or later; (vi) the presence of LGE or an increase in qualitative T2-weighted STIR signal were not associated with subsequent MACE; and (vii) the correlation between LGE and pathological fibrosis and between myocardial oedema by T2-weighted STIR and lymphocytic infiltration were, at best, modest. Strengths of the current study include the comparatively large sample size of patients with ICI-associated myocarditis and the large subset having both histopathology and CMR data which enabled the unique opportunity to dissect the relationship between LGE and pathological fibrosis in ICI-associated myocarditis.
The strengths of CMR for the diagnosis of myocarditis reside in its excellent spatial resolution and, more importantly, its ability to provide tissue characterization.13 , 14 , 17 , 36 , 37 , 38 Thus, appropriately, CMR is a primary cardiac imaging modality recommended for the evaluation of patients with suspected myocarditis.16 , 17 Beyond research letters and case reports, there are limited data on the use of CMR for the diagnosis of ICI-associated myocarditis and no data comparing CMR to histopathological findings.20 In the current analysis, LGE was present in <50% of cases with ICI-associated myocarditis, and 42% of cases had neither LGE nor an elevated T2-weighted STIR signal. In a research letter with 15 patients with ICI-associated myocarditis who underwent a CMR, Escudier et al. 20 noted LGE among 23% of patients and qualitative oedema among 33% of patients. The rate of LGE and qualitative oedema by T2-weighted STIR is far lower than that reported for acute myocarditis not related to ICI.16 , 17 , 39 For example, in a study with 374 patients with acute myocarditis not related to ICI, LGE was noted in 93% of patients and signs of myocardial oedema by T2-weighted STIR were noted in 94% of patients.17 Similarly, Mahrholdt et al.39 reported that 95% of patients (83/87) diagnosed with active myocarditis had LGE. An important strength of our study is the presence of a pathology-proven myocarditis cohort which noted similar results to the larger cohort. Specifically, 56 of our patients had both a CMR and histopathological analysis of the heart and parallel findings were noted where LGE was present in 39% and elevated T2-weighted STIR signal was present in 25% of patients with pathology-proven myocarditis.
To understand the absence of LGE in patient with myocarditis, factors associated with LGE were tested. Clinical and imaging parameters were similar in patients with or without LGE. Initial troponin T levels were higher in patients with LGE, suggesting more myocardial damage may associate with the presence of LGE on a CMR. The relationship between the timing of the CMR study and the presence of LGE was tested. Time of onset of symptoms was not used because, while many patients had new cardiovascular symptoms, some had vague symptoms. Patients usually had a troponin and/or ECG performed due to these vague symptoms and these abnormal tests triggered the admission. When a CMR was performed on Day 4 of admission or later, the presence of LGE increased from 21.6% to 72.0%. Myocardial fibrosis/scar, reflected by LGE, is considered a subacute or chronic sequel of myocardial inflammation in myocarditis, thus it may take some time for myocardial fibrosis to develop and accumulate before becoming detectable on CMR or biopsy. This finding of a relationship between onset of myocarditis and the presence of fibrosis has also been noted in animal studies of myocarditis. Specifically, in a murine model of viral myocarditis, myocardial fibrin deposition first appeared on Day 3 after infection, and myocardial fibrosis was not detectable until Day 14 after infection.40 In experimental autoimmune myocarditis rat model, LGE was detected in 3 out of 15 rats at 2 weeks after immunization and LGE was detected in 5 of 8 rats at 5 weeks after immunization.41 However, due to the retrospective nature of the registry, the timing of CMR was determined by treating physicians and was likely affected by the severity of presentation and availability of the test and none of the patients underwent serial CMR. Thus, these results generated a hypothesis that the time might affect the presence of LGE in patients with ICI-associated myocarditis and future prospective studies are warranted to test this hypothesis. The finding of a limited association between CMR and histopathology was also consistent among pathology-proven cases. In the current cohort (and illustrated in the case), CMR was typically performed early (median time 2 days) and the histopathology was typically performed later (median time 11 days) in patients with negative LGE and positive histopathological fibrosis. The current results suggest performing CMR later in the clinical course (≥4 days) could potentially improve its diagnostic performance. However, delays in the diagnosis and treatment are not recommended as these delays are likely to have clinical importance. Specifically, in a prior report of 35 cases, earlier treatment of suspected cases was associated with a trend towards a lower rate of MACE.10
These findings indicate that, in clinically suspected ICI-associated myocarditis, the absence of LGE or the absence of increased T2-weighted STIR signal on a CMR does not exclude the potential diagnosis and, until our understanding improves and until future research offers insights into the role of T1 mapping, T2 mapping, and calculation of the ECV, an EMB should still be pursued when clinical suspicion remains after a normal CMR. In addition, it is known that T2-weighted STIR method offers limited sensitivity.42 Late gadolinium enhancement and T2-weighted STIR imaging are dependent on local variations in fibrosis or inflammation to become qualitatively apparent. Therefore, CMR techniques sensitive to myocardial inflammation and oedema, such as T1 mapping, T2 mapping, and calculation of the ECV may be instrumental to identify early changes in myocardium before LGE appears. The native T1 value and ECV of patients with ICI-associated myocarditis appeared to be higher than normal values based on a small subset of our patients. Future studies on T1 mapping and T2 mapping with standard protocol and a large sample size are warranted.
Histopathology has been reported in a small number of cases with ICI-associated myocarditis.6 , 20 In the study by Escudier et al., lymphocytic infiltration was found in eight of nine patients. In the current study, a lymphocytic infiltration was shown in 98% of patients, while fibrosis was found in 55% of the 56 patients who underwent histopathological analysis. Subclinical ICI-associated myocarditis cases have also been reported.22 , 43 For example, in a case of metastatic melanoma treated with ipilimumab and nivolumab, cardiac involvement was clinically unapparent, but patchy fibrosis and diffuse mononuclear infiltrates of myocardium were found in post-mortem autopsy.43 Given the potential of subclinical presentations, the lack of LGE in >50% of patients, the presence of characteristic histopathological findings with a normal troponin, it is reasonable to hypothesize that ICI-associated myocardial injury remains underrecognized and underdiagnosed.44
Outcomes with ICI-associated myocarditis are significantly worse than myocarditis in broad populations. In this cohort, 40% of patients developed MACE and 16.5% of patients had a cardiovascular death during a median follow-up time of ∼5 months. In contrast, among 670 patients admitted to hospital with myocarditis regardless of aetiology, MACE occurred in 15% of patients and death occurred in 4% of patients during a median follow-up time of 4.7 years.16 This several-fold increase in MACE in a very short period highlights the fulminant nature of ICI-associated myocarditis. However, the predictors of such a marked increase in adverse outcomes with ICI-associated myocarditis are not well characterized. In contrast to studies among patients with non-ICI myocarditis, the presence of LGE was not found to have prognostic significance.16 , 17 There are several possible reasons for this discrepancy. First, LGE was only present in <50% of patients and, had the CMR been performed later, there would likely have been more patients with LGE and had an improved statistical power to assess the association between LGE and outcomes. Second, the follow-up time was much shorter (5 months) compared to other studies on prognostic performance of LGE (4–5 years).16 , 17 , 36 Thus, future studies with larger sample size, a longer follow-up time, should outcomes be improved, and better characterization of LGE are warranted.
Limitations
Results of the present study should be interpreted in context. This was a retrospective study and institutional standards were employed. CMR protocol was not pre-specified and CMR was read at local sites. Thus, this study is hypothesis-generating and may have unmeasured confounding caused by different practice pattern and variation between readers. Additional CMR sequences such as T1 mapping, T2 mapping, and measurement of the ECV, which have additive value in non-ICI myocarditis,15 , 18 and in patients at risk of cardiovascular toxicities from cancer therapy,45 were not routinely performed. However, these results reflect CMR practice in real-life clinical settings and reflect the difficulties in describing an evolving disease. These findings will reflect the next stage of this iterative process, where these data have provided the basis for discussions on disease-specific standardization of imaging and non-imaging protocols. In addition, T2-weight STIR imaging was performed in three short-axis slices and a single long-axis view, instead of whole short-axis stack. Endomyocardial biopsy was taken from the apical septum of the RV; no LV biopsies were performed. However, due to the diffuse inflammatory nature of ICI-associated myocarditis as seen in the autopsy samples, the possibility of missing the diagnosis by RV biopsy less. While limited, two CMR/autopsy overlaps were provided and there was pathological myocarditis noted at sites where both the LGE and the black-blood imaging were normal (Supplementary material online, Figure S3). Some of the early cases may be missed due to atypical presentation, reliance on LGE-based approaches, and limited awareness. However, as insight has improved over time, we believe that those included in more recent years are generalizable to the broad population with ICI-associated myocarditis. The data collection protocol was standardized but the definitions for such features as clinical symptoms and physical exam findings (e.g. jugular vein distention) were not standardized. Therefore, it is important to acknowledge that there is likely variability between investigators and sites which limited these findings. Finally, the statistical power is likely limited due to the modest sample size, thus the lack of association between CMR and EMB features and MACE needs to be tested in future studies with a larger sample size.
Conclusions
In this study, the CMR and histopathology features of ICI-associated myocarditis are presented. LGE is present in >80% of patients with non-ICI myocarditis; in contrast, LGE is present in <50% of patients with ICI-associated myocarditis. Increased time between clinical presentation and CMR is associated with greater detection of LGE; however, delays in diagnosis are not recommended as delayed treatment in ICI-associated myocarditis may be associated with an increase in MACE.10 These data suggest caution if using an LGE or qualitative T2-weighted STIR imaging-only approach to diagnose or exclude ICI-associated myocarditis, especially among the majority of patients who have a normal LVEF, and suggest that when there is a clinical suspicion of myocarditis, a biopsy be strongly considered in those with a negative CMR using the sequences applied in this study. Especially while future studies determine if CMR techniques such as T1 and T2 mapping offer improved diagnostic and prognostic value.
Funding
This work was supported by the Sarnoff Cardiovascular Research Foundation to S.S.M. R.J.S was supported, in part, through the National Institutes of Health (NIH)/National Cancer Institute (NCI) (RO1CA229851, UH2CA207355, RO1CA193970). C.L.C and D.G were supported, in part, through the National Institutes of Health (NIH)/National Cancer Institute (NCI) (P30CA008748). P.T. was supported, in part, through the Canadian Institutes of Health Research New Investigator Award (FRN 147814). C.G.T was supported by a Ricerca di Ateneo/Federico II University grant. T.G.N. was supported, in part, through the Kohlberg Foundation, NIH/NHLBI (RO1HL130539 and RO1HL137562) and NIH/Harvard Center for AIDS Research (P30 AI060354).
Conflict of interest: S.S.M. has received consultancy fees from OMR Globus, Alpha Detail, and Opinion Research Team. A.N. has received research support from Amgen and has been a consultant for Takeda Oncology. L.M.H. has received consultancy, advisory board, and speaker fees from MSD, BMS, Roche, Novartis, Amgen, and Curevac. R.J.S. has been a consultant to Merck and Novartis. J.J.M. has served as a consultant/advisor for Novartis, Pfizer, Bristol-Myers Squibb, Takeda/Millennium, Ariad, Acceleron, Vertex, Incyte, Rgenix, Verastem, Pharmacyclics, StemCentRx, Heat Biologics, Daiichi-Sankyo, and Regeneron. J.D.G. has received research support from Amgen. T.G.N. has received advisory fees from Parexel, BMS, H3 Biomedicine, Aprea Therapeutics, and Intrinsic Imaging. All other authors declared no conflict of interest. A.B. has received consulting fees from Bristol-Myers Squibb and Takeda Inc, DSMB for CTI Biopharma.
Supplementary Material
Contributor Information
Lili Zhang, Cardiovascular Imaging Research Center (CIRC), Division of Cardiology, Department of Radiology, Massachusetts General Hospital, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA; Cardio-Oncology Program, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA.
Magid Awadalla, Cardiovascular Imaging Research Center (CIRC), Division of Cardiology, Department of Radiology, Massachusetts General Hospital, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA; Cardio-Oncology Program, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA.
Syed S Mahmood, Cardiology Division, Department of Medicine, New York-Presbyterian Hospital, Weill Cornell Medical Center, 1300 York Avenue, New York, NY 10065, USA.
Anju Nohria, Cardio-Oncology Program, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, 75 Francis St, Boston, MA 02115, USA.
Malek Z O Hassan, Cardiovascular Imaging Research Center (CIRC), Division of Cardiology, Department of Radiology, Massachusetts General Hospital, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA.
Franck Thuny, Department of Cardiology, Aix-Marseille University, Assistance Publique–Hôpitaux de Marseille, Mediterranean university, Cardio-Oncology center (MEDI-CO center), Unit of Heart Failure and Valvular Heart Diseases, Hôpital Nord, Jardin du Pharo, 58 Boulevard Charles Livon 13007, Marseille, France; Groupe Méditerranéen de Cardio-Oncologie (gMEDICO), AP-HM, Chemin des Bourrely, 13015, Marseille, France; Aix-Marseille University, Center for CardioVascular and Nutrition research (C2VN), Inserm 1263, Inra 1260, 13385 Marseille, France.
Daniel A Zlotoff, Cardio-Oncology Program, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA.
Sean P Murphy, Cardiovascular Imaging Research Center (CIRC), Division of Cardiology, Department of Radiology, Massachusetts General Hospital, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA.
James R Stone, Department of Pathology, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St, Boston, MA 02114, USA.
Doll Lauren Alexandra Golden, Cardiovascular Imaging Research Center (CIRC), Division of Cardiology, Department of Radiology, Massachusetts General Hospital, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA.
Raza M Alvi, Cardiovascular Imaging Research Center (CIRC), Division of Cardiology, Department of Radiology, Massachusetts General Hospital, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA.
Adam Rokicki, Cardiovascular Imaging Research Center (CIRC), Division of Cardiology, Department of Radiology, Massachusetts General Hospital, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA; Cardio-Oncology Program, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA.
Maeve Jones-O’Connor, Cardiovascular Imaging Research Center (CIRC), Division of Cardiology, Department of Radiology, Massachusetts General Hospital, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA.
Justine V Cohen, Division of Oncology and Hematology, Department of Medicine, Massachusetts General Hospital, 55 Fruit St, Boston, MA 02114, USA.
Lucie M Heinzerling, Department of Dermatology, University Hospital Erlangen, Friedrich-Alexander-University Erlangen-Nürnberg (FAU), Schloßplatz 4, 91054 Erlangen, Germany.
Connor Mulligan, Cardiovascular Imaging Research Center (CIRC), Division of Cardiology, Department of Radiology, Massachusetts General Hospital, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA.
Merna Armanious, Cardio-Oncology Program, Division of Cardiovascular Medicine, H. Lee Moffitt Cancer Center & Research Institute and University of South Florida, 12902 USF Magnolia Drive, Tampa, FL 33612, USA.
Ana Barac, Cardio-Oncology program, MedStar Heart and Vascular Institute, Georgetown University, 110 Irving St NW, Washington, DC 20010, USA.
Brian J Forrestal, Cardio-Oncology program, MedStar Heart and Vascular Institute, Georgetown University, 110 Irving St NW, Washington, DC 20010, USA.
Ryan J Sullivan, Division of Oncology and Hematology, Department of Medicine, Massachusetts General Hospital, 55 Fruit St, Boston, MA 02114, USA.
Raymond Y Kwong, Noninvasive Cardiovascular Imaging Section, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, 75 Francis St, Boston, MA 02115, USA.
Eric H Yang, UCLA Cardio-Oncology Program, Division of Cardiology, Department of Medicine, University of California at Los Angeles, 757 Westwood Plaza, Los Angeles, CA 90095, USA.
Rongras Damrongwatanasuk, Cardio-Oncology Program, Division of Cardiovascular Medicine, H. Lee Moffitt Cancer Center & Research Institute and University of South Florida, 12902 USF Magnolia Drive, Tampa, FL 33612, USA.
Carol L Chen, Cardiology Division, Memorial Sloan Kettering Cancer Center, Weill Cornell Medical College, 1275 York Avenue, New York, NY 10065, USA.
Dipti Gupta, Cardiology Division, Memorial Sloan Kettering Cancer Center, Weill Cornell Medical College, 1275 York Avenue, New York, NY 10065, USA.
Michael C Kirchberger, Department of Dermatology, University Hospital Erlangen, Friedrich-Alexander-University Erlangen-Nürnberg (FAU), Schloßplatz 4, 91054 Erlangen, Germany.
Javid J Moslehi, Cardio-Oncology Program, Vanderbilt University Medical Center, 1211 Medical Center Dr, Nashville, TN 37232, USA.
Otavio R Coelho-Filho, Cardiology Division, State University of Campinas, Cidade Universitária Zeferino Vaz - Barão Geraldo, Campinas, São Paulo 13083-970, Brazil.
Sarju Ganatra, Cardio-Oncology Program, Division of Cardiovascular Medicine, Lahey Hospital and Medical Center, 41 Burlington Mall Road, Burlington, MA 01805, USA.
Muhammad A Rizvi, Division of Oncology and Hematology, Department of Medicine, Lehigh Valley Hospital, 1200 S Cedar Crest Blvd, Allentown, PA 18103, USA.
Gagan Sahni, Cardiology Division, The Mount Sinai Hospital, 1468 Madison Ave, New York, NY 10029, USA.
Carlo G Tocchetti, Department of Translational Medical Sciences, Federico II University, via S. Pansini 5, 80131 Naples, NA, Italy.
Valentina Mercurio, Department of Translational Medical Sciences, Federico II University, via S. Pansini 5, 80131 Naples, NA, Italy.
Michael Mahmoudi, Faculty of Medicine, University of Southampton, University Road Southampton SO17 1BJ, UK.
Donald P Lawrence, Division of Oncology and Hematology, Department of Medicine, Massachusetts General Hospital, 55 Fruit St, Boston, MA 02114, USA.
Kerry L Reynolds, Division of Oncology and Hematology, Department of Medicine, Massachusetts General Hospital, 55 Fruit St, Boston, MA 02114, USA.
Jonathan W Weinsaft, Cardiology Division, Department of Medicine, New York-Presbyterian Hospital, Weill Cornell Medical Center, 1300 York Avenue, New York, NY 10065, USA; Cardiology Division, Memorial Sloan Kettering Cancer Center, Weill Cornell Medical College, 1275 York Avenue, New York, NY 10065, USA.
A John Baksi, Cardiovascular Research Centre and Cardiovascular Magnetic Resonance Unit, Royal Brompton Hospital, Sydney St, Chelsea, London SW3 6NP, UK; National Heart and Lung Institute, Imperial College London, Kensington, London SW7 2DD, UK.
Stephane Ederhy, UNICO-GRECO cardio-oncology program, sorbonne universite, Hopital Saint Antoine, 27 Rue de Chaligny, 75012 Paris, France.
John D Groarke, Cardio-Oncology Program, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, 75 Francis St, Boston, MA 02115, USA.
Alexander R Lyon, Cardio-Oncology Program, Royal Brompton Hospital, Sydney St, Chelsea, London SW3 6NP, UK; National Heart and Lung Institute, Imperial College London, Cale Street, Chelsea, London, SW3 6LY, United Kingdom.
Michael G Fradley, Cardio-Oncology Program, Division of Cardiovascular Medicine, H. Lee Moffitt Cancer Center & Research Institute and University of South Florida, 12902 USF Magnolia Drive, Tampa, FL 33612, USA.
Paaladinesh Thavendiranathan, Ted Rogers Program in Cardiotoxicity Prevention, Division of Cardiology, Toronto General Hospital, Peter Munk Cardiac Center, University of Toronto, Toronto, ON, Canada.
Tomas G Neilan, Cardiovascular Imaging Research Center (CIRC), Division of Cardiology, Department of Radiology, Massachusetts General Hospital, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA; Cardio-Oncology Program, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA.
References
- 1. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJM, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain J-F, Testori A, Grob J-J, Davidson N, Richards J, Maio M, Hauschild A, Miller WH, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen T-T, Humphrey R, Hoos A, Wolchok JD. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517–2526. [DOI] [PubMed] [Google Scholar]
- 3. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–168. [DOI] [PubMed] [Google Scholar]
- 4. Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol 2018;29:84–91. [DOI] [PubMed] [Google Scholar]
- 5. Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, Berry S, Chartash EK, Daud A, Fling SP, Friedlander PA, Kluger HM, Kohrt HE, Lundgren L, Margolin K, Mitchell A, Olencki T, Pardoll DM, Reddy SA, Shantha EM, Sharfman WH, Sharon E, Shemanski LR, Shinohara MM, Sunshine JC, Taube JM, Thompson JA, Townson SM, Yearley JH, Topalian SL, Cheever MA. PD-1 blockade with pembrolizumab in advanced merkel-cell carcinoma. N Engl J Med 2016;374:2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA Jr, Anders RA, Sosman JA, Moslehi JJ. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018;391:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, Gobert A, Spano JP, Balko JM, Bonaca MP, Roden DM, Johnson DB, Moslehi JJ. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 2018;19:1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol 2018;19:e447–e458. [DOI] [PubMed] [Google Scholar]
- 10. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Awadalla M, Hassan MZO, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Lawrence DP, Groarke JD, Neilan TG. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK, Ancell KK, Balko JM, Bowman C, Davis EJ, Chism DD, Horn L, Long GV, Carlino MS, Lebrun-Vignes B, Eroglu Z, Hassel JC, Menzies AM, Sosman JA, Sullivan RJ, Moslehi JJ, Johnson DB. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018;4:1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Awadalla M, Golden DLA, Mahmood SS, Alvi RM, Mercaldo ND, Hassan MZO, Banerji D, Rokicki A, Mulligan C, Murphy SPT, Jones-O'Connor M, Cohen JV, Heinzerling LM, Armanious M, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Rizvi MA, Sahni G, Lyon AR, Tocchetti CG, Mercurio V, Thuny F, Ederhy S, Mahmoudi M, Lawrence DP, Groarke JD, Nohria A, Fradley MG, Reynolds KL, Neilan TG. Influenza vaccination and myocarditis among patients receiving immune checkpoint inhibitors. J Immunother Cancer 2019;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Biesbroek PS, Hirsch A, Zweerink A, van de Ven PM, Beek AM, Groenink M, Windhausen F, Planken RN, van Rossum AC, Nijveldt R. Additional diagnostic value of CMR to the European Society of Cardiology (ESC) position statement criteria in a large clinical population of patients with suspected myocarditis. Eur Heart J Cardiovasc Imaging 2018;19:1397–1407. [DOI] [PubMed] [Google Scholar]
- 14. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648, 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 15. Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol 2009;53:1475–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grani C, Eichhorn C, Biere L, Murthy VL, Agarwal V, Kaneko K, Cuddy S, Aghayev A, Steigner M, Blankstein R, Jerosch-Herold M, Kwong RY. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol 2017;70:1964–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aquaro GD, Perfetti M, Camastra G, Monti L, Dellegrottaglie S, Moro C, Pepe A, Todiere G, Lanzillo C, Scatteia A, Di Roma M, Pontone G, Perazzolo Marra M, Barison A, Di Bella G. Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY study. J Am Coll Cardiol 2017;70:1977–1987. [DOI] [PubMed] [Google Scholar]
- 18. Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, Friedrich MG. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018;72:3158–3176. [DOI] [PubMed] [Google Scholar]
- 19. Holzmann M, Nicko A, KüHl U, Noutsias M, Poller W, Hoffmann W, Morguet A, Witzenbichler B, TschöPe C, Schultheiss H-P, Pauschinger M, Complication rate of right ventricular endomyocardial biopsy via the femoral approach: a retrospective and prospective study analyzing 3048 diagnostic procedures over an 11-year period. Circulation 2008;118:1722–1728. [DOI] [PubMed] [Google Scholar]
- 20. Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, Monestier S, Grob JJ, Scemama U, Jacquier A, Lalevee N, Barraud J, Peyrol M, Laine M, Bonello L, Paganelli F, Cohen A, Barlesi F, Ederhy S, Thuny F. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation 2017;136:2085–2087. [DOI] [PubMed] [Google Scholar]
- 21. Ganatra S, Neilan TG. Immune checkpoint inhibitor-associated myocarditis. Oncologist 2018;23:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thibault C, Vano Y, Soulat G, Mirabel M. Immune checkpoint inhibitors myocarditis: not all cases are clinically patent. Eur Heart J 2018;39:3553. [DOI] [PubMed] [Google Scholar]
- 23. Leone O, Veinot JP, Angelini A, Baandrup UT, Basso C, Berry G, Bruneval P, Burke M, Butany J, Calabrese F, d'Amati G, Edwards WD, Fallon JT, Fishbein MC, Gallagher PJ, Halushka MK, McManus B, Pucci A, Rodriguez ER, Saffitz JE, Sheppard MN, Steenbergen C, Stone JR, Tan C, Thiene G, van der Wal AC, Winters GL. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol 2012;21:245–274. [DOI] [PubMed] [Google Scholar]
- 24. Hinojar R, Foote L, Arroyo Ucar E, Jackson T, Jabbour A, Yu CY, McCrohon J, Higgins DM, Carr-White G, Mayr M, Nagel E, Puntmann VO. Native T1 in discrimination of acute and convalescent stages in patients with clinical diagnosis of myocarditis: a proposed diagnostic algorithm using CMR. JACC Cardiovasc Imaging 2015;8:37–46. [DOI] [PubMed] [Google Scholar]
- 25. von Knobelsdorff-Brenkenhoff F, Schuler J, Doganguzel S, Dieringer MA, Rudolph A, Greiser A, Kellman P, Schulz-Menger J. Detection and monitoring of acute myocarditis applying quantitative cardiovascular magnetic resonance. Circ Cardiovasc Imaging 2017;1:e005242. [DOI] [PubMed] [Google Scholar]
- 26. h-Ici DO, Ridgway JP, Kuehne T, Berger F, Plein S, Sivananthan M, Messroghli DR. Cardiovascular magnetic resonance of myocardial edema using a short inversion time inversion recovery (STIR) black-blood technique: diagnostic accuracy of visual and semi-quantitative assessment. J Cardiovasc Magn Reson 2012;14:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004;52:141–146. [DOI] [PubMed] [Google Scholar]
- 28. Neilan TG, Coelho-Filho OR, Danik SB, Shah RV, Dodson JA, Verdini DJ, Tokuda M, Daly CA, Tedrow UB, Stevenson WG, Jerosch-Herold M, Ghoshhajra BB, Kwong RY. CMR quantification of myocardial scar provides additive prognostic information in nonischemic cardiomyopathy. JACC Cardiovasc Imaging 2013;6:944–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, Limacher MC, Mahaffey KW, Mehran R, Nissen SE, Smith EE, Targum SL. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 2015;66:403–469. [DOI] [PubMed] [Google Scholar]
- 30. Neilan TG, Farhad H, Mayrhofer T, Shah RV, Dodson JA, Abbasi SA, Danik SB, Verdini DJ, Tokuda M, Tedrow UB, Jerosch-Herold M, Hoffmann U, Ghoshhajra BB, Stevenson WG, Kwong RY. Late gadolinium enhancement among survivors of sudden cardiac arrest. JACC Cardiovasc Imaging 2015;8:414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation 2008;117:686–697. [DOI] [PubMed] [Google Scholar]
- 32. Harrell FE Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA 1982;247:2543–2546. [PubMed] [Google Scholar]
- 33. Yilmaz A, Mahrholdt H, Athanasiadis A, Vogelsberg H, Meinhardt G, Voehringer M, Kispert EM, Deluigi C, Baccouche H, Spodarev E, Klingel K, Kandolf R, Sechtem U. Coronary vasospasm as the underlying cause for chest pain in patients with PVB19 myocarditis. Heart 2008;94:1456–1463. [DOI] [PubMed] [Google Scholar]
- 34. Sado DM, Flett AS, Banypersad SM, White SK, Maestrini V, Quarta G, Lachmann RH, Murphy E, Mehta A, Hughes DA, McKenna WJ, Taylor AM, Hausenloy DJ, Hawkins PN, Elliott PM, Moon JC. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart 2012;98:1436–1441. [DOI] [PubMed] [Google Scholar]
- 35. Austin PC, Steyerberg EW. Graphical assessment of internal and external calibration of logistic regression models by using loess smoothers. Stat Med 2014;33:517–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schumm J, Greulich S, Wagner A, Grun S, Ong P, Bentz K, Klingel K, Kandolf R, Bruder O, Schneider S, Sechtem U, Mahrholdt H. Cardiovascular magnetic resonance risk stratification in patients with clinically suspected myocarditis. J Cardiovasc Magn Reson 2014;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Neilan TG, Shah RV, Abbasi SA, Farhad H, Groarke JD, Dodson JA, Coelho-Filho O, McMullan CJ, Heydari B, Michaud GF, John RM, van der Geest R, Steigner ML, Blankstein R, Jerosch-Herold M, Kwong RY. The incidence, pattern, and prognostic value of left ventricular myocardial scar by late gadolinium enhancement in patients with atrial fibrillation. J Am Coll Cardiol 2013;62:2205–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zamorano J L, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan D J, Lip G Y H, Lyon A R, Lopez Fernandez T, Mohty D, Piepoli M F, Tamargo J, Torbicki A, Suter T M. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. European Heart Journal 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 39. Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, Klingel K, Kandolf R, Sechtem U. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 2006;114:1581–1590. [DOI] [PubMed] [Google Scholar]
- 40. Schnitt SJ, Stillman IE, Owings DV, Kishimoto C, Dvorak HF, Abelmann WH. Myocardial fibrin deposition in experimental viral myocarditis that progresses to dilated cardiomyopathy. Circ Res 1993;72:914–920. [DOI] [PubMed] [Google Scholar]
- 41. Rinkevich-Shop S, Konen E, Kushnir T, Epstein FH, Landa-Rouben N, Goitein O, Ben Mordechai T, Feinberg MS, Afek A, Leor J. Non-invasive assessment of experimental autoimmune myocarditis in rats using a 3 T clinical MRI scanner. Eur Heart J Cardiovasc Imaging 2013;14:1069–1079. [DOI] [PubMed] [Google Scholar]
- 42. Ferreira VM, Piechnik SK, Dall'Armellina E, Karamitsos TD, Francis JM, Ntusi N, Holloway C, Choudhury RP, Kardos A, Robson MD, Friedrich MG, Neubauer S. T(1) mapping for the diagnosis of acute myocarditis using CMR: comparison to T2-weighted and late gadolinium enhanced imaging. JACC Cardiovasc Imaging 2013;6:1048–1058. [DOI] [PubMed] [Google Scholar]
- 43. Koelzer VH, Rothschild SI, Zihler D, Wicki A, Willi B, Willi N, Voegeli M, Cathomas G, Zippelius A, Mertz KD. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors-an autopsy study. J Immunother Cancer 2016;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Neilan TG, Rothenberg ML, Amiri-Kordestani L, Sullivan RJ, Steingart RM, Gregory W, Hariharan S, Hammad TA, Lindenfeld J, Murphy MJ, Moslehi JJ; on behalf of the Checkpoint Inhibitor Safety Working Group. Myocarditis associated with immune checkpoint inhibitors: an expert consensus on data gaps and a call to action. Oncologist 2018;23:874–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Neilan TG, Coelho-Filho OR, Shah RV, Feng JH, Pena-Herrera D, Mandry D, Pierre-Mongeon F, Heydari B, Francis SA, Moslehi J, Kwong RY, Jerosch-Herold M. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. Am J Cardiol 2013;111:717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.