Iron is an essential element for most organisms but causes problems due to poor solubility under oxic conditions and due to toxicity by catalyzing the formation of reactive oxygen species (ROS). Therefore, bacteria have evolved complex regulatory networks for iron homeostasis aiming at a sufficient iron supply while minimizing ROS formation. In our study, the responses of the actinobacterium Corynebacterium glutamicum to iron limitation were analyzed, resulting in a detailed view on the processes involved in iron homeostasis in this model organism. In particular, we provide evidence that iron limitation causes TPP deficiency, presumably due to insufficient activity of the iron-dependent phosphomethylpyrimidine synthase (ThiC). TPP deficiency was deduced from the upregulation of genes controlled by a TPP riboswitch and secretion of metabolites caused by insufficient activity of the TPP-dependent enzymes pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase. To our knowledge, the link between iron starvation and thiamine synthesis has not been elaborated previously.
KEYWORDS: Corynebacterium glutamicum, iron deficiency, thiamine biosynthesis, transcriptome, proteome
ABSTRACT
The response to iron limitation of the Gram-positive soil bacterium Corynebacterium glutamicum was analyzed with respect to secreted metabolites, the transcriptome, and the proteome. During growth in glucose minimal medium, iron limitation caused a shift from lactate to pyruvate as the major secreted organic acid complemented by l-alanine and 2-oxoglutarate. Transcriptome and proteome analyses revealed that a pronounced iron starvation response governed by the transcriptional regulators DtxR and RipA was detectable in the late, but not in the early, exponential-growth phase. A link between iron starvation and thiamine pyrophosphate (TPP) biosynthesis was uncovered by the strong upregulation of thiC. As phosphomethylpyrimidine synthase (ThiC) contains an iron-sulfur cluster, limiting activities of the TPP-dependent pyruvate–2-oxoglutarate dehydrogenase supercomplex probably cause the excretion of pyruvate and 2-oxoglutarate. In line with this explanation, thiamine supplementation could strongly diminish the secretion of these acids. The upregulation of thiC and other genes involved in thiamine biosynthesis and transport is presumably due to TPP riboswitches present at the 5′ end of the corresponding operons. The results obtained in this study provide new insights into iron homeostasis in C. glutamicum and demonstrate that the metabolic consequences of iron limitation can be due to the iron dependency of coenzyme biosynthesis.
IMPORTANCE Iron is an essential element for most organisms but causes problems due to poor solubility under oxic conditions and due to toxicity by catalyzing the formation of reactive oxygen species (ROS). Therefore, bacteria have evolved complex regulatory networks for iron homeostasis aiming at a sufficient iron supply while minimizing ROS formation. In our study, the responses of the actinobacterium Corynebacterium glutamicum to iron limitation were analyzed, resulting in a detailed view on the processes involved in iron homeostasis in this model organism. In particular, we provide evidence that iron limitation causes TPP deficiency, presumably due to insufficient activity of the iron-dependent phosphomethylpyrimidine synthase (ThiC). TPP deficiency was deduced from the upregulation of genes controlled by a TPP riboswitch and secretion of metabolites caused by insufficient activity of the TPP-dependent enzymes pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase. To our knowledge, the link between iron starvation and thiamine synthesis has not been elaborated previously.
INTRODUCTION
Iron is the fourth most abundant element in the Earth’s crust (1). Based on its ability to exist in the ferrous (Fe2+) and ferric (Fe3+) states, iron has become a highly important constituent of many enzymes and is also discussed to be involved in the origin of life (2). In the form of iron centers, heme moieties, and iron-sulfur clusters, iron is crucial for the activity of many enzymes and regulatory proteins. Due to the very poor solubility of ferric iron that predominates under aerobic conditions, a sufficient supply of iron represents a considerable challenge for bacteria (3). However, not only is an insufficient iron supply problematic, but also is excessive “free” iron within the cell, because in the presence of oxygen, Fe2+ catalyzes the formation of reactive oxygen species (ROS), including the hydroxyl radical, which can damage virtually all organic molecules, such as proteins, DNA, and lipids (4). Therefore, bacteria have established sophisticated regulatory networks and mechanisms to ensure iron homeostasis (3 to 6).
We have previously explored various aspects of iron homeostasis in Corynebacterium glutamicum, an intensively studied Gram-positive soil bacterium of the Actinobacteria (7). This species possesses a large arsenal of genes devoted to iron uptake, including several ABC transporters for ferric siderophores or heme and several cytosolic siderophore-interacting proteins. However, C. glutamicum most likely does not produce siderophores on its own, but it makes use of those produced by other bacteria (8). The majority of genes involved in iron uptake are members of the DtxR (diphtheria toxin regulator) regulon. The transcriptional regulator DtxR was characterized as master regulator controlling the expression of more than 50 genes involved in iron metabolism (9, 10). DtxR binds to its target promoters as a complex with ferrous iron and thus functions as a sensor of cytosolic iron concentrations (11). Besides the large set of genes encoding iron uptake systems, several target genes repressed by DtxR themselves encode transcriptional regulators, such as ripA and hrrA. The response regulator HrrA is part of the HrrSA two-component system controlling the utilization of heme as an iron source (12–14). RipA (repressor of iron proteins) is an AraC-type transcriptional regulator repressing the expression of nonessential iron-dependent proteins, such as aconitase or succinate dehydrogenase, under low iron concentrations (15). RipA thereby ensures a prolonged iron supply for essential iron-dependent proteins, such as ribonucleotide reductase, and thus has an analogous function as the small regulatory RNA RyhB in Escherichia coli (16).
Besides transcriptional regulation, we recently identified a posttranslational regulatory mechanism involved in iron homeostasis in C. glutamicum based on pupylation. Pupylation resembles eukaryotic ubiquitination and was initially identified in Mycobacterium tuberculosis, where pupylation usually tags proteins for degradation by the proteasome PrcAB (17, 18). C. glutamicum lacks a proteasome but engages the AAA ATPase forming ring-shaped complexes (ARC; called Mpa in mycobacteria) to unfold pupylated proteins. We found both ferritin and Dps to be subject to pupylation in C. glutamicum, with the small prokaryotic ubiquitin-like protein Pup covalently linked to Lys78 of ferritin and Lys14 of Dps (19). Ferritin pupylation was shown to be important for growth under iron limitation by enhancing the release of stored iron (20).
Despite the characterization of regulatory networks and mechanisms involved in iron homeostasis in C. glutamicum, knowledge about the physiological consequences of low iron availability and the dynamics of the response is still missing. Generally, it has been shown in bacteria that low iron concentrations result in a reduction in tricarboxylic acid (TCA) cycle activity, lower energy production, and, therefore, slowdown or even arrest of growth (21–24). Similar effects were observed also in eukaryotes, such as the yeast Saccharomyces cerevisiae, where iron starvation increased the flux through glycolysis and diminished respiration as well as TCA cycle activity (25). In the present study, we aimed to obtain a detailed view of the iron starvation responses of C. glutamicum wild-type cells. For this purpose, we examined the growth and concomitant formation of metabolic by-products and determined the iron starvation stimulons at the mRNA and protein levels.
RESULTS
Influence of iron limitation on growth and by-product formation.
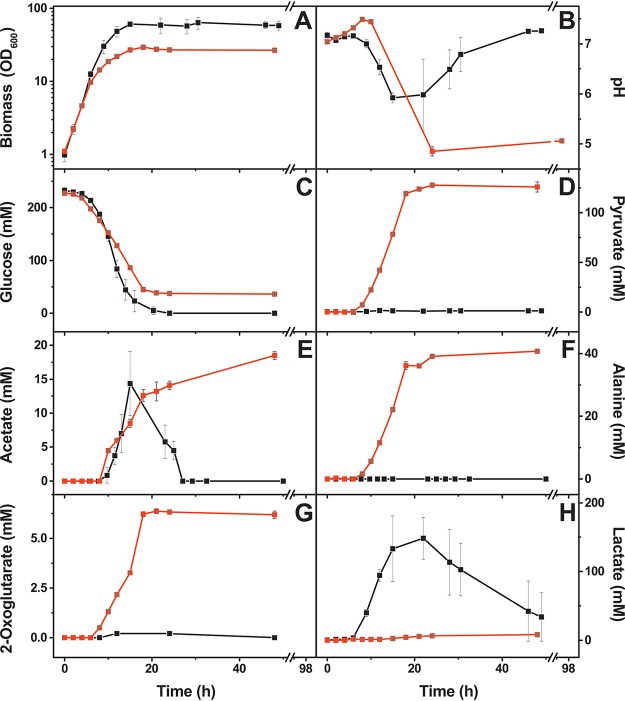
We compared growth, pH, and by-product formation during shake flask cultivation of C. glutamicum in glucose minimal medium with either 1 μM FeSO4 (iron limitation) or 36 μM FeSO4 (iron sufficiency). The data obtained for various parameters are summarized in Table 1. In the cultivations with 222 mM glucose, we observed strong differences at different iron concentrations (Fig. 1). At 1 μM iron, the initial growth rates were only slightly lower than those with 36 μM iron, but the final optical density at 600 nm (OD600) was reduced by 57%, and 36 mM glucose remained unused. Cultures with 36 μM iron temporarily formed high concentrations of l-lactate (149 mM) and some acetate (14 mM), which were reconsumed when glucose became exhausted. The formation of l-lactate is a consequence of oxygen limitation at higher cell densities, allowing the reoxidation of NADH (26). Cultures supplemented with 1 μM iron instead of 36 μM, however, formed only small amounts of l-lactate (8 mM) but high concentrations of pyruvate (128 mM), l-alanine (41 mM), acetate (19 mM), and 2-oxoglutarate (6 mM). Neither the organic acids nor l-alanine was reconsumed. The pH of the cultures with 36 μM iron first dropped from pH 7 to about pH 6 due to the formation of the organic acids and then rose again to pH 7 when the organic acids were reconsumed. In contrast, the pH of the cultures with 1 μM iron dropped to about pH 5 and remained there. The stronger acidification can be explained by the fact that pyruvic acid is a stronger acid (pKa, 2.49) than is lactic acid (pKa, 3.90). The observation that the cells stopped consuming glucose and did not reconsume pyruvate, acetate, 2-oxoglutarate, and l-alanine suggests that the cells became metabolically inactive due to the acidification of the medium (27).
TABLE 1.
Influence of iron limitation on growth-related parameters and by-product formationa
| Parameter | Data by glucose concn, FeSO4 concn |
|||
|---|---|---|---|---|
| 111 mM, 36 μM | 111 mM, 1 μM | 222 mM, 36 μM | 222 mM, 1 μM | |
| Final OD600 | 32 ± 2 | 21 ± 1 | 63 ± 12 | 27 ± 1 |
| μ (h−1) | 0.44 ± 0.01 | 0.39 ± 0.03 | 0.40 ± 0.01 | 0.37 ± 0.00 |
| Maximum pH | 7.7 ± 0.0 | 7.6 ± 0.0 | 7.5 ± 0.1 | 7.5 ± 0.1 |
| Minimum pH | 7.0 ± 0.0 | 7.0 ± 0.0 | 5.9 ± 0.1 | 4.9 ± 0.1 |
| sGUR (nmol min−1 mg [CDW]−1) | 93.7 ± 4.3 | 72.1 ± 6.4 | 89.0 ± 0.6 | 50.9 ± 0.5 |
| Final glucose concn at 48 h (mM) | 0 | 0 | 0 | 36.1 ± 1.8 |
| Maximum acetate concn in: | ||||
| mM | 5.44 ± 2.61 | 7.68 ± 1.09 | 14.4 ± 4.7 | 18.5 ± 0.6 |
| mmol g (CDW)−1 | 0.74 ± 0.38 | 1.64 ± 0.42 | 0.74 ± 0.50 | 2.79 ± 0.14 |
| Maximum lactate concn in: | ||||
| mM | 31.9 ± 6.6 | 1.94 ± 0.11 | 149 ± 31 | 8.32 ± 0.86 |
| mmol g (CDW)−1 | 4.29 ± 1.12 | 0.46 ± 0.04 | 13.0 ± 5.0 | 1.25 ± 0.11 |
| Maximum pyruvate concn in: | ||||
| mM | <1 | 32.4 ± 2.7 | <1 | 128.0 ± 0.6 |
| mmol g (CDW)−1 | <1 | 6.84 ± 0.37 | <1 | 19.0 ± 1.3 |
| Maximum 2-oxoglutarate concn in: | ||||
| mM | <1 | 5.44 ± 0.17 | <1 | 6.37 ± 0.12 |
| mmol g (CDW)−1 | <1 | 1.24 ± 0.11 | <1 | 0.94 ± 0.01 |
| Maximum alanine concn in: | ||||
| mM | <1 | 19.8 ± 2.7 | <1 | 40.8 ± 0.3 |
| mmol g (CDW)−1 | <1 | 4.37 ± 0.73 | <1 | 6.3 ± 0.0 |
For all parameters, mean values and standard deviations derived from three independent biological replicates are given. Maximum concentrations of organic and amino acids in supernatants are shown as absolute values and relative to cell dry weight (CDW). sGUR, specific glucose uptake rate. Values for growth with 222 mM glucose and 36 μM Fe were taken from reference 26.
FIG 1.
Influence of different iron concentrations on growth, pH profile, and by-product formation of C. glutamicum wild type during cultivation in CGXII minimal medium with 222 mM glucose and either 36 μM FeSO4 (black symbols) or 1 μM FeSO4 (red symbols). (A) Growth was measured as the optical density at 600 nm (OD600). (B to H) Aliquots of the culture supernatants were analyzed with respect to pH (B), glucose consumption (C), and formation of pyruvate (D), acetate (E), l-alanine (F), 2-oxoglutarate (G), and lactate (H). Mean values and standard deviations of three biological replicates are shown. The data for the C. glutamicum wild type cultivated with 36 μM FeSO4 were taken from a previous study (26).
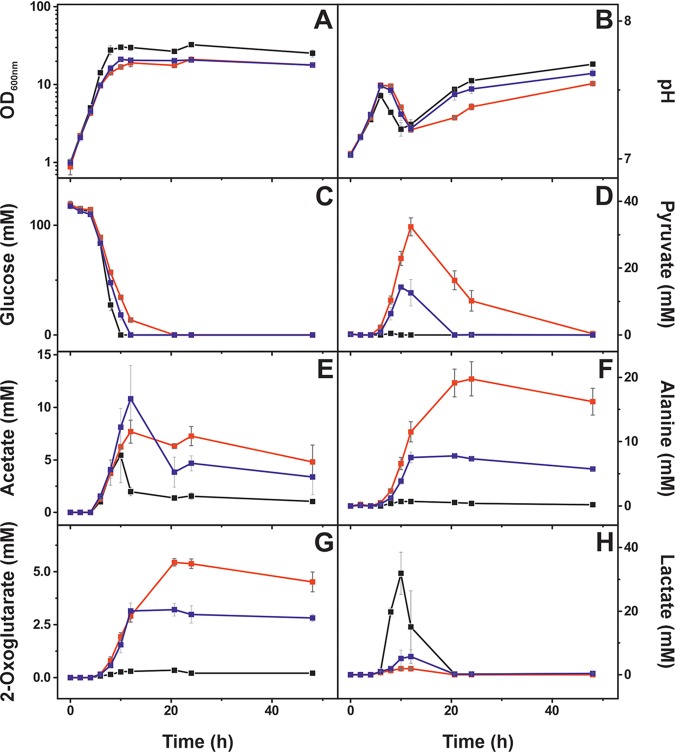
To test this assumption, we repeated the experiment with 111 mM glucose instead of 222 mM. In this case, the cultures grown at 1 μM iron consumed glucose completely and again formed pyruvate (32 mM), l-alanine (20 mM), acetate (8 mM), and 2-oxoglutarate (5 mM) but only minor levels of l-lactate (2 mM) (Fig. 2). Compared to the cultures with 222 mM glucose, the smaller amount of acids formed presumably prevented critically strong acidification, and pyruvate was slowly reconsumed after glucose depletion and partially converted to l-alanine. This result supported the view that a low pH stopped metabolism in the cultivations with 222 mM glucose and 1 μM iron.
FIG 2.
Influence of different iron concentrations and thiamine on growth, pH profile, and by-product formation of C. glutamicum wild type during cultivation in CGXII minimal medium with 111 mM glucose and either 36 μM FeSO4 (black symbols), 1 μM FeSO4 (red symbols), or 1 μM FeSO4 plus 0.6 μM thiamine (blue symbols). (A) Growth was measured as the OD600. (B to H) Aliquots of the culture supernatants were analyzed with respect to pH (B), glucose consumption (C), and formation of pyruvate (D), acetate (E), l-alanine (F), 2-oxoglutarate (G), and lactate (H). Mean values and standard deviations of the results from three biological replicates are shown.
Transcriptional responses to iron-limiting conditions.
It was previously shown by the analysis of C. glutamicum ΔdtxR and ΔripA mutants that DtxR and its subordinate regulator RipA govern the iron starvation response of C. glutamicum (9, 10, 15). However, the temporal onset of the transcriptional iron starvation response in wild-type C. glutamicum is unknown. Therefore, we performed two sets of DNA microarray experiments to compare the mRNA levels of cells cultured under low- and high-iron conditions at different time points. In the first set, we analyzed the transcriptomes in the early exponential-growth phase (OD600, 4 to 5), where cells grew comparably under low- and high-iron conditions (Fig. 1A). Fifty-one genes showed a ≥2-fold altered mRNA level with a P value of ≤0.05, of which 37 were upregulated and 14 were downregulated (see Table S1 in the supplemental material). Although the cells of the low-iron culture had suffered from iron limitation also during precultivation, only four DtxR-regulated genes were slightly upregulated, all of which presumably encode components of siderophore transporters (cg0769, cg0771, cg0922, and cg3404). The majority of the DtxR targets, including the ripA gene, were unaltered. Most of the genes with an increased mRNA level under iron limitation in the early exponential-growth phase were genes of the prophage CGP3 (23 of the total 197 CGP3 prophage genes, with an average mRNA ratio of 3.5). In conclusion, at the chosen time point, the cells of the low-iron culture did not yet severely suffer from iron limitation.
In a second set of DNA microarray experiments, we compared transcript levels in the late-exponential-growth phase (OD600, 12 to 15), when the growth curves of the cultures with 36 μM FeSO4 and 1 μM FeSO4 diverged and the first minute amounts of pyruvate were secreted by the iron-depleted cells (Fig. 1A and D). At this time point, the overall changes were much broader, with a total of 273 genes showing a ≥2-fold altered mRNA level with a P value of ≤0.05. One hundred thirty-nine genes were upregulated, and 134 genes were downregulated (Table S2). A strong iron starvation response was observed, as 37 of the most strongly upregulated genes belong to the DtxR regulon, and 17 of the most strongly downregulated genes represent all known members of the RipA regulon (Table S2). The vast majority of the upregulated DtxR target genes at this time point encode proteins involved in iron and heme uptake. These include three ABC transporters presumably catalyzing siderophore uptake (cg0589-cg0590-cg0591, cg0768-cg0769-cg0770, and cg0926-cg0927-cg0928), eight secreted siderophore-binding lipoproteins (cg0405, cg0748, cg0771, cg0922, cg0924, cg1418, cg2234, and cg3404), and three cytosolic siderophore-interacting proteins (cg0767, cg0921, and cg1405). The upregulated genes involved in heme uptake and utilization comprise one ABC transporter with a secreted heme-binding lipoprotein (cg0467-cg0468-cg0469), four secreted heme transport-associated proteins (cg0466, cg0470, cg0471, and cg3156), and heme oxygenase (cg2445). A number of genes reported to be repressed by DtxR were unaltered in our experiment, such as cg0955, cg1996-cg1998 (cglIM-cglIR-cglIIR), cg3082-cg3083, cg3084-cg3085, cg3112-cg3118 (cysZYMDHXI), and cg3119 (cysJ) (10). Various reasons can be responsible for the missing derepression of these genes that are not directly involved in iron uptake, such as control by additional regulators or generally weak repression by DtxR.
Among the DtxR target genes encoding transcriptional regulators, ripA showed the highest upregulation, with an mRNA ratio of 26, followed by glyR, with a ratio of 8, and hrrA (not listed in Table S2), with a ratio of 1.9. With respect to the targets of these regulators, only those of RipA showed significantly altered expression. All 17 genes of the RipA regulon, most of which encode iron-containing proteins such as aconitase or succinate dehydrogenase, showed strongly reduced expression (Table S2). We assume that RipA economizes iron consumption under iron limitation by reducing the synthesis of nonessential iron-containing proteins, thereby prolonging the availability of iron for essential iron-dependent enzymes, such as ribonucleotide reductase.
Apart from members of the DtxR regulon (37 genes) and the RipA regulon (17 genes), 32 genes of the prophage CGP3 showed increased mRNA levels (average, 2.6-fold) (Table S2), similar to the results of the transcriptome comparison in the early exponential-growth phase. A large functional group with altered expression under iron limitation was formed by 46 genes involved in transport, of which 28 were downregulated and 18 upregulated. The most strongly downregulated operon was formed by cg0508-cg0507-cg0506 encoding an ABC transporter with similarity to the SfuABC Fe3+-ABC transporter of Serratia marcescens (28). This transporter might have a lower affinity for Fe3+ and therefore could be relevant under iron-sufficient conditions. A putative thiamine-regulated ABC transporter for thiamine (cg1727-cg1728-cg1729, 3-fold increased expression), a putative Na+-dependent secondary transporter of the BASS family (cg1419, 5-fold increased expression), and a putative dipeptide ABC transporter (cg2675-cg2676-cg2677-cg2678, 4-fold increased expression) were the most strongly upregulated transporter genes.
Another noticeable functional group with altered expression under iron limitation was formed by genes involved in respiration (29). All genes (ctaD, ctaCF, and ctaE-qcrCAB) encoding the cytochrome bc1-aa3 supercomplex (30, 31) and one of the genes encoding the alternative cytochrome bd oxidase (32) showed 2- to 3-fold decreased expression under iron limitation. The regulators responsible for this effect are not yet known. Previous studies have demonstrated that the cAMP-dependent global regulator GlxR activates the expression of the cytochrome bc1-aa3 supercomplex genes (33) and might be involved in the reduced expression observed in our experiment.
Among the very heterogeneous functional group of genes involved in carbon metabolism that showed altered expression under iron limitation, the most strongly regulated ones were prpD2-prpB2-prpC2, whose mRNA level was more than 7-fold decreased. They encode 2-methylcitrate dehydratase, 2-methylisocitrate lyase, and 2-methylcitrate synthase, key enzymes of the methylcitrate cycle involved in metabolization of propionate and propionyl-coenzyme A (propionyl-CoA) derived from β-oxidation of odd-numbered fatty acids (34). This operon is transcriptionally regulated by several regulators, including PrpR (35), RamA (36), and GlxR (37), allowing responses to various environmental stimuli. Among the group of genes involved in cofactor biosynthesis, the thiC gene encoding phosphomethylpyrimidine synthase was 8-fold upregulated. Three additional genes involved in thiamine biosynthesis (thiO, thiS, thiF) were upregulated about 2-fold. This suggested that iron limitation might lead to thiamine deficiency (see below).
Among the group of further genes with altered expression under iron limitation, cg2962 was highly upregulated (12-fold, Table S2). Sequence analysis of cg2962 revealed similarity to the heme oxygenases HmoA and HmoB of Bacillus subtilis, and the hmoA and hmoB genes are repressed by the transcriptional regulator Fur under iron sufficiency (38–40). Since cg2962 was also >10-fold upregulated in a ΔdtxR mutant compared to the wild type (10), we speculated that it might be another member of the DtxR regulon. Manual inspection of the promoter region indeed revealed a putative DtxR-binding motif (TGTAGTTAGGTTACACTAA), which was located between positions −23 and −5 relative to the transcriptional start site of cg2962 (41), in agreement with a repressor function of DtxR. This binding site was probably not detected before because it contains six mismatches compared to the consensus DNA-binding motif of DtxR (TWAGGTWAGSCTWACCTWA). The results suggest that cg2962 is another member of the DtxR regulon and might function as heme-degrading monooxygenase in C. glutamicum besides HmuO (cg2445).
Relative protein-level changes correlate with the mRNA level changes.
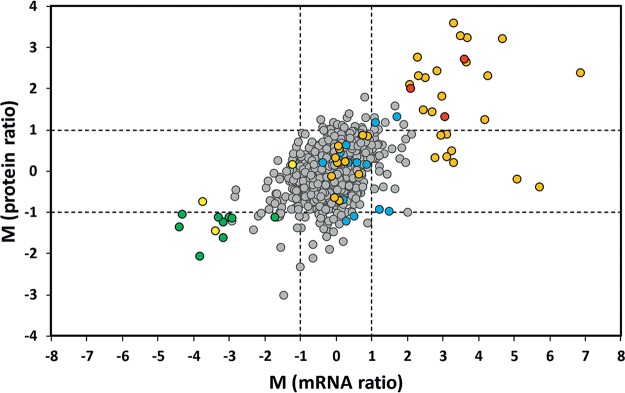
Complementary to the transcriptome analysis, we also analyzed changes at the protein level for C. glutamicum cells grown at high and low iron concentrations and harvested in the late-exponential-growth phase. Between 1,056 and 1,357 proteins were detected (1% false-discovery rate [FDR]) in the 12 measurements (three biological replicates and two technical replicates each for low- and high-iron conditions) with a proteomic shotgun analysis using nano-liquid chromatography–tandem mass spectrometry (nanoLC-MS/MS), covering a total of 1,555 proteins (Table S3). By sequential windowed acquisition of all theoretical fragment ion mass spectrometry (SWATH-MS) measurements, relative levels of 1,123 proteins could be quantified (Table S4). Thereof, 66 proteins exhibited at least 2-fold altered ratios with a P value of ≤0.05 in iron-deprived cells compared to the cells cultivated with 36 μM iron. Thirty-eight proteins showed a ≥2-fold higher level under iron limitation, 16 of which were members of the DtxR regulon (Table S5). The levels of 28 proteins were at least 2-fold lower under iron limitation, 10 of which were members of the RipA regulon. In Table 2, the mRNA and protein ratios of the members of the DtxR and RipA regulon are summarized, showing that the proteome data corroborate the transcriptome data. For the entire data sets, there was a good correlation between mRNA ratios and protein ratios (Fig. 3).
TABLE 2.
mRNA ratios and protein ratios under low- and high-iron conditions of the genes and proteins transcriptionally regulated by DtxR and RipA in C. glutamicuma
| Gene locus | Gene name | Annotation | Ratio of low/high Fe for: |
||
|---|---|---|---|---|---|
| Early exponential, mRNA | Late exponential, mRNA | Late exponential, protein | |||
| DtxR regulon | |||||
| cg0160 | Hypothetical protein | 1.06 | 10.95 | ND | |
| cg0405 | Putative ferric dicitrate ABC transporter, secreted siderophore-binding lipoprotein | 1.24 | 10.05 | 11.95 | |
| cg0465 | Putative membrane protein, conserved | 1.52 | 10.22 | ND | |
| cg0466 | htaA | Secreted heme transport-associated protein | 1.61 | 11.93 | ND |
| cg0467 | hmuT | Heme ABC transporter, secreted heme-binding lipoprotein | 1.26 | 8.83 | 1.82 |
| cg0468 | hmuU | Heme ABC transporter, permease | 1.18 | 7.28 | ND |
| cg0469 | hmuV | Heme ABC transporter, ATPase | 0.84 | 4.27 | ND |
| cg0470 | htaB | Secreted heme transport-associated protein | 1.09 | 118.62 | 5.17 |
| cg0471 | htaC | Secreted heme transport-associated protein | 0.62 | 53.40 | 0.75 |
| cg0527 | glyR | Arsr-type transcriptional regulator of glyA | 1.07 | 7.89 | 1.80 |
| cg0589 | Putative siderophore ABC transporter, ATPase | 1.87 | 9.69 | 1.39 | |
| cg0590 | Putative siderophore ABC transporter, permease | 1.88 | 11.39 | 9.58 | |
| cg0591 | Putative siderophore ABC transporter, permease | 1.34 | 5.94 | ND | |
| cg0748 | Putative siderophore ABC transporter, secreted substrate-binding lipoprotein | 1.07 | 4.93 | 6.70 | |
| cg0767 | Putative cytoplasmic siderophore-interacting protein | 1.12 | 7.25 | 5.36 | |
| cg0768 | Putative siderophore ABC transporter, ATPase | 1.83 | 10.01 | 1.14 | |
| cg0769 | Putative siderophore ABC transporter, permease | 2.77 | 6.87 | ND | |
| cg0770 | Putative siderophore ABC transporter, permease | 1.50 | 10.28 | ND | |
| cg0771 | irp1 | Putative siderophore ABC transporter, secreted siderophore-binding lipoprotein | 2.20 | 12.77 | 6.18 |
| cg0921 | Putative cytoplasmic siderophore-interacting protein | 1.12 | 14.81 | ND | |
| cg0922 | Putative secreted siderophore-binding lipoprotein | 2.20 | 19.62 | 4.87 | |
| cg0924 | Putative siderophore ABC transporter, secreted substrate-binding lipoprotein | 2.12 | 12.97 | 9.29 | |
| cg0926 | Putative siderophore ABC transporter, permease | 1.90 | 6.42 | ND | |
| cg0927 | Putative siderophore ABC transporter, permease | 2.08 | 8.81 | 1.25 | |
| cg0928 | Putative siderophore ABC transporter, ATPase | 1.32 | 4.30 | 4.23 | |
| cg1120 | ripA | AraC family transcriptional regulator of iron proteins | 1.42 | 25.83 | 9.14 |
| cg1405 | Putative cytoplasmic siderophore-interacting protein | 1.02 | 6.63 | 2.69 | |
| cg1418 | Putative secreted siderophore-binding lipoprotein | 1.39 | 7.94 | 3.49 | |
| cg1930 | Putative secreted hydrolase, CGP3 region | 1.36 | 34.35 | 0.86 | |
| cg1931 | Putative secreted protein, CGP3 region | 1.45 | 27.59 | ND | |
| cg2234 | Putative ferric dicitrate ABC transporter, secreted substrate-binding lipoprotein | 1.68 | 6.17 | ND | |
| cg2311 | Putative SAM-dependent methyltransferase | 0.91 | 5.55 | 2.78 | |
| cg2445 | hmuO | Heme oxygenase | 1.85 | 5.05 | 4.88 |
| cg2796 | Homolog of methylcitrate dehydratase PrpD, MmgE/PrpD family protein | 1.06 | 18.29 | 2.33 | |
| cg2797 | Hypothetical protein, conserved | 0.78 | 5.80 | 4.75 | |
| cg2962 | Putative heme-degrading monooxygenase | 1.49 | 12.43 | 6.46 | |
| cg3156 | htaD | Secreted heme transport-associated protein | 1.95 | 37.26 | ND |
| cg3404 | Putative ferric dicitrate ABC transporter, secreted substrate-binding lipoprotein | 2.33 | 6.95 | 1.24 | |
| RipA regulon | |||||
| cg0012 | ssuR | Transcriptional regulator of ROK family | 1.25 | 3.18 | 0.73 |
| cg0310 | katA | Catalase | 1.15 | 0.05 | 0.48 |
| cg0445 | sdhC | Succinate:menaquinone oxidoreductase, cytochrome b | 1.45 | 0.11 | ND |
| cg0446 | sdhA | Succinate:menaquinone oxidoreductase, flavoprotein | 1.30 | 0.08 | 0.59 |
| cg0447 | sdhB | Succinate:menaquinone oxidoreductase, iron-sulfur protein | 1.17 | 0.10 | 0.36 |
| cg0448 | Putative membrane protein | 1.14 | 0.44 | 1.11 | |
| cg1341 | narI | Dissimilatory nitrate reductase, γ-subunit, cytochrome b | 1.49 | 0.07 | ND |
| cg1342 | narJ | Dissimilatory nitrate reductase, δ-subunit, assembly factor | 1.51 | 0.07 | ND |
| cg1343 | narH | Dissimilatory nitrate reductase, β-subunit, iron-sulfur protein | 1.40 | 0.11 | 0.32 |
| cg1344 | narG | Dissimilatory nitrate reductase, α-subunit, Mo cofactor containing | 1.10 | 0.07 | 0.24 |
| cg1345 | narK | Nitrate/nitrite antiporter | 1.33 | 0.10 | ND |
| cg1487 | leuC | Isopropylmalate isomerase, large subunit | 1.24 | 0.13 | 0.45 |
| cg1488 | leuD | Isopropylmalate isomerase, small subunit | 0.93 | 0.31 | 0.45 |
| cg1737 | acn | Aconitase | 1.30 | 0.11 | 0.42 |
| cg1738 | acnR | TetR-type transcriptional repressor of acn gene | 0.95 | 0.38 | ND |
| cg2636 | catA | Catechol 1,2-dioxygenase | 0.88 | 0.05 | 0.39 |
| cg3047 | ackA | Acetate kinase | 1.12 | 0.13 | 0.44 |
| cg3048 | pta | Phosphotransacetylase | 0.82 | 0.10 | 0.45 |
Wild-type cells were cultivated in CGXII medium with 222 mM glucose and either low (1 μM FeSO4) or high (36 μM FeSO4) iron levels. The conditions in the precultures were identical to those of the respective main cultures. Cells were harvested in either the early (Early; OD600, 4 to 5) or late (Late; OD600, 12 to 15) exponential-growth phase to extract RNA or protein. Genes with a ≥2-fold altered mRNA ratio either in the early or the late-exponential phase and a P value of ≤0.05 were used to create the list, and mRNA ratios from the other time point were added irrespective of ratio and P value. The protein data were obtained by SWATH-MS analyses using cells in the late-exponential-growth phase. The mean values from three independent biological replicates are shown. Ratios for which the P value of was ≤0.05 are shown in bold. The complete results of the transcriptome and proteome analyses are shown in Tables S1, S2, and S4. ND, not detected.
FIG 3.
Correlation of mRNA and protein ratios (1 μM FeSO4 versus 36 μM FeSO4) in the late-exponential-growth phase of C. glutamicum. Log2 ratios (M values) are plotted for 1,116 gene-protein pairs for which both the mRNA ratio and protein ratio could be quantified (gray circles). Members belonging to the DtxR (35 members; orange circles) and the RipA (9 members; green circles) regulon or both (3 members; yellow circles) or to prophage CGP3 (14 members; blue circles) are highlighted. The genes thiC (cg1476, phosphomethylpyrimidine synthase), cg2283 (protein of unknown function), and cg2962 (putative heme-degrading monooxygenase), also showing high mRNA and protein ratios, are indicated as red circles. Dashed lines indicate 2-fold ratio change thresholds as log2.
Link between iron deficiency and thiamine biosynthesis.
Among the genes strongly upregulated under iron limitation was thiC (cg1476), encoding phosphomethylpyrimidine synthase, an enzyme involved in thiamine biosynthesis (Tables S2 and S5). Additional genes required for thiamine synthesis (thiO, thiS, and thiF) and genes coding for a putative ABC transporter for thiamine (ykoEDC) were also ≥2-fold upregulated, although not as strongly as thiC. These results suggested that C. glutamicum might face a shortage of thiamine pyrophosphate (TPP) under iron limitation. TPP shortage may result from an insufficient activity of ThiC, as the activity of this enzyme is dependent on an iron-sulfur center (42, 43). Considering that pyruvate and 2-oxoglutarate are oxidized by the TPP-dependent pyruvate dehydrogenase (PDH) and 2-oxoglutarate dehydrogenase (ODH) enzyme complexes, the excretion of large amounts of pyruvate and smaller amounts of 2-oxoglutarate by C. glutamicum under iron limitation might be caused by the limiting activities of these enzymes. To test this idea, we monitored the effect of thiamine addition (0.6 μM) on growth and by-product formation during cultivation in CGXII medium (see Materials and Methods) with 111 mM glucose and 1 μM FeSO4. Supplementation of the medium with 0.6 μM thiamine significantly altered the pattern of secreted products after 6 h (Fig. 2). Growth was slightly improved, and the secretion of pyruvate, 2-oxoglutarate, and l-alanine was reduced by approximately 50%, whereas the formation of acetate and lactate was slightly increased. These changes support the assumed TPP shortage under iron limitation.
DISCUSSION
In the present study, we have characterized the responses of the soil bacterium C. glutamicum to iron limitation with respect to growth, secreted metabolites, and changes in global mRNA and protein profiles. The combination of these results provides a detailed view of the iron starvation response in C. glutamicum and revealed a link to thiamine biosynthesis that may exist also in other microbes.
The transcriptome results obtained for cells cultivated with 1 μM iron and harvested in the early exponential-growth phase (OD600, 4 to 5) revealed that only six DtxR-repressed genes encoding components of siderophore ABC transporters were slightly derepressed (2- to 3-fold), and none of the RipA target genes were repressed (Table S1). A more pronounced iron starvation response might have been hindered by an initial accumulation of the available iron within the cells. Whereas the iron starvation response was moderate, many CGP3 prophage genes were upregulated under iron limitation in the early exponential-growth phase. In a previous study, elevated mRNA levels of CGP3 genes were observed in a ΔdtxR mutant (9). This was explained by an increased excision of CGP3 from the genome and formation of multiple circular CGP3 molecules, possibly triggered by DNA damage caused by elevated intracellular iron concentrations (44). Induction of the prophage was also observed in the wild type but only in a small fraction of the population (44). The 2-fold increase of several CGP3 genes observed in the early exponential-growth phase of iron-starved cells may be due to prophage induction in a minor fraction of the population. The exact mechanism connecting the iron starvation response and CGP3 prophage induction remains to be elucidated.
In contrast to the moderate effects at an OD600 of 4 to 5, we observed a strong iron starvation response at an OD600 of 12 to 15. Transcriptomics revealed that 37 of the known DtxR target genes were strongly upregulated, and 16 of the known RipA targets were 5- to 10-fold downregulated (Table 2 and Fig. 3). We could confirm altered expression of many of these genes also at the protein level using a SWATH-MS approach (45). The transcriptome and proteome data clearly indicate that a large fraction of DtxR was present in the apo-form dissociated from its DNA targets at this time point. Besides known members of the DtxR regulon, we identified a new one, cg2962, which encodes a putative heme monooxygenase based on sequence similarity to the HmoA and HmoB proteins of B. subtilis. The position of the DtxR-binding site upstream of cg2962 agrees with repression by DtxR.
The strong repression of the RipA target genes in the late-exponential phase matches the strong upregulation of RipA (26-fold at the mRNA-level, 9-fold at the protein level). Currently, no mechanisms of activity control other than transcriptional repression by DtxR are known for RipA. In the case of the response regulator HrrA, which exhibited an only 2-fold increased mRNA level under iron limitation, transcriptional control of its target genes requires HrrA phosphorylation by the histidine kinase HrrS, which senses the presence of external heme (13, 14). As heme was not added in our experiments, HrrS presumably remained active as a phosphatase, preventing transcriptional regulation by HrrA. GlyR, another transcriptional regulator repressed by DtxR, was reported to activate the expression of the glyA gene encoding serine hydroxymethyltransferase in the stationary phase (46). Although an 8-fold increased mRNA level and a 2-fold increased protein level of GlyR were detected in the late-exponential phase of iron starvation, the glyA mRNA level remained unchanged, possibly because the cells had not yet entered the stationary phase. In summary, our data reveal the global iron starvation response of wild-type C. glutamicum, which is in excellent agreement with the results obtained previously by transcriptome analysis of the ΔdtxR (9, 10) and ΔripA (15) mutants. In a recent study, the iron starvation response of Corynebacterium pseudotuberculosis was analyzed by comparing global gene expression of cultures grown in the presence and absence of the iron chelator 2,2′-dipyridyl (47). The results obtained for the pathogenic C. pseudotuberculosis show many similarities to those obtained by us for nonpathogenic C. glutamicum, such as upregulation of ripA, hrrA, and genes involved in heme utilization.
In the present study, we observed major differences between iron sufficiency and iron limitation with respect to the by-products secreted into the medium. Under iron sufficiency, cultivation in shake flasks with glucose minimal medium is typically associated with the transient formation of l-lactate, which is a consequence of oxygen limitation at higher cell densities and allows reoxidation of NADH (26). Under iron limitation, however, high concentrations of pyruvate and l-alanine were formed instead of lactate, accompanied by small amounts of 2-oxoglutarate (Fig. 1 and 2 and Table 1). This indicates that the cells were not limited in oxygen but in something else. Pyruvate is usually oxidized to acetyl-coenzyme A (acetyl-CoA) and CO2 by the pyruvate dehydrogenase complex (48). In addition, pyruvate can be oxidized to acetate by pyruvate:quinone oxidoreductase (49). 2-Oxoglutarate is oxidized to succinyl-CoA and CO2 by the 2-oxoglutarate dehydrogenase complex (50), which in C. glutamicum probably forms a supercomplex with the pyruvate dehydrogenase complex (51, 52). None of these enzymes is known to require iron for activity or to be repressed under iron limitation.
However, the analysis of the transcriptome and proteome data hinted to a different link between these enzymes and iron starvation. Cells harvested in the late-exponential-growth phase showed an 8-fold increased mRNA level of thiC and 2-fold increased mRNA levels of thiO, thiS, and thiF, all of which are involved in thiamine biosynthesis. The thiC gene encodes phosphomethylpyrimidine synthase (EC 4.1.99.17), an enzyme involved in TPP biosynthesis that catalyzes the conversion of 5-amino-1-(5-phospho-d-ribosyl)imidazole and S-adenosyl-l-methionine to 4-amino-2-methyl-5-(phosphooxymethyl)pyrimidine, 5′-deoxyadenosine, l-methionine, formate, and carbon monoxide. ThiC contains a [4Fe-4S] cluster on which its activity is strictly dependent (42, 43, 53). Consequently, iron limitation may reduce ThiC activity and thus lead to a limitation in TPP.
TPP is a coenzyme of several enzymes with important roles in cellular metabolism (54). In C. glutamicum, these are, for example, the E1 subunit of the pyruvate dehydrogenase complex (cg2466, AceE), the unusual E1 subunit of the 2-oxoglutarate dehydrogenase complex (cg1280, OdhA), transketolase (cg1774), pyruvate:menaquinone oxidoreductase (cg2891), 1-deoxyxylulose-5-phosphate synthase (cg2083), or acetolactate synthase (cg1435). We could support the assumption that the secretion of pyruvate and 2-oxoglutarate under iron limitation was a consequence of TPP limitation by the fact that thiamine supplementation reduced the formation of these by-products. In addition, the observation that only l-alanine was secreted under iron limitation, but not l-valine, whereas both amino acids were produced by a mutant lacking the E1 subunit of the PDH complex (55), can be explained by a limiting activity of the TPP-dependent acetolactate synthase. In summary, our results suggest that iron limitation can lead to TPP limitation resulting in reduced activities of TPP-dependent enzymes when cells are dependent on endogenous TPP biosynthesis due to a lack of exogenous thiamine or precursors thereof. This link between iron limitation and TPP biosynthesis probably exists not only in C. glutamicum but also in other microbes. In Bacillus licheniformis, for example, the tenA-thiVWX operon encoding a protein (TenA) whose function is ambiguous and an ABC transporter for thiamine was reported to be upregulated 2- to 3-fold under iron starvation (56). Interestingly, the expression of the pdhABCD operon encoding the subunits of the pyruvate dehydrogenase complex was also upregulated in B. licheniformis under these conditions, potentially as a consequence of a limiting TPP availability.
As thiC and the other thiamine biosynthesis genes are not members of the DtxR regulon, their upregulation under iron limitation must be due to expression control by a different transcriptional regulator or by a different mechanism. Comprehensive studies in the past years revealed that expression of TPP biosynthesis and thiamine transport genes in bacteria, but also in archaea and eukaryotes, is regulated by a riboswitch rather than by proteins (57–59). When sufficient TPP is available in the cell, it binds to the riboswitch located at the 5′ end of specific mRNAs, triggering structural changes that either inhibit further transcription by forming a terminator or prevent translation initiation by blocking the ribosome binding site. Also, both transcription and translation may be disturbed by formation of the riboswitch-TPP complex. In E. coli for example, the TPP riboswitch upstream of the thiM coding region inhibits translation but not transcription, whereas the TPP riboswitch upstream of thiC inhibits both transcription and translation (58). In the genome of C. glutamicum, five TPP riboswitches were identified upstream of the coding sequences of thiC (cg1476), thiM (cg1655), thiE (cg2236), ykoE (cg1227), and cg0825 in bioinformatic studies (59, 60). The ykoE gene is the first gene of the ykoE-ykoD-ykoC operon, presumably encoding a transporter for thiamine uptake (61). The thiE gene is the first gene of the thiE-thiO-thiS-thiG-thiF operon encoding enzymes involved in thiamine biosynthesis. The cg0825 gene has been annotated to encode a short-chain dehydrogenase, but its function in thiamine biosynthesis is unknown. Based on the prediction of TPP riboswitches, we assume that the enhanced transcription under iron limitation of thiC and further genes involved in TPP biosynthesis or thiamine transport is caused by the TPP riboswitch as a consequence of TPP shortage due to insufficient activity of the iron-dependent ThiC enzyme. Table 3 summarizes the mRNA and protein ratios under iron limitation of the genes predicted to be controlled by a TPP riboswitch in C. glutamicum. Except for thiM, upregulated mRNA and protein levels were observed for all of them, supporting the view that iron limitation caused TPP deficiency.
TABLE 3.
Expression of genes subject to control by a TPP riboswitch under iron limitation
| Locus taga | Gene | Annotation | mRNA ratio of low/high Fe | P value | Protein ratio of low/high Fe | P value |
|---|---|---|---|---|---|---|
| cg0825 | Short-chain dehydrogenase | 1.37 | 0.03 | 1.61 | <0.01 | |
| cg1227 | ykoE | ABC transporter for hydroxymethylpyrimidine, permease | 3.53 | 0.02 | 1.21 | 0.48 |
| cg1228 | ykoD | ABC transporter for hydroxymethylpyrimidine, ATPase | 3.02 | 0.01 | 1.44 | <0.01 |
| cg1229 | ykoC | ABC transporter for hydroxymethylpyrimidine, permease | 3.18 | 0.01 | 2.97 | <0.01 |
| cg1476 | thiC | Phosphomethylpyrimidine synthase | 8.41 | <0.01 | 2.47 | <0.01 |
| cg1655 | thiM | Hydroxyethylthiazole kinase | 1.26 | 0.27 | 0.95 | 0.63 |
| cg2236 | thiE | Thiamin-phosphate pyrophosphorylase | 1.81 | <0.01 | 1.53 | <0.01 |
| cg2237 | thiO | Putative d-amino acid oxidase flavoprotein oxidoreductase | 2.04 | 0.01 | 1.25 | <0.01 |
| cg2238 | thiS | Sulfur transfer protein involved in thiamine biosynthesis | 2.32 | <0.01 | 1.55 | 0.02 |
| cg2239 | thiG | Thiazole biosynthesis protein | 1.90 | <0.01 | NDb | |
| cg2240 | thiF | Dinucleotide-utilizing enzyme involved in thiamine biosynthesis | 2.09 | <0.01 | 1.21 | 0.06 |
Locus tags with a TPP riboswitch are shown in bold.
ND, not detected.
In Fig. 4, an overview on the currently known iron starvation responses of C. glutamicum is shown, including the findings presented in this study. The scheme depicts four major responses observed or relevant under iron-limiting conditions, i.e., the upregulation of genes involved in iron and heme acquisition, the downregulation of genes for nonessential proteins requiring iron, the pupylation of ferritin and Dps to release stored iron, and the consequences of thiamine limitation on central metabolism. Besides these features that already have been uncovered, there are still many aspects missing in our understanding of the iron starvation response of C. glutamicum, such as the substrates and relevance of the different siderophore transporters, the temporal order of induction of the iron starvation genes, or the hierarchy of iron delivery to iron-dependent enzymes.
FIG 4.
Schematic overview on iron starvation responses of C. glutamicum. put., putative; SAM, S-adenosylmethionine; dep., dependent; Pup, prokaryotic ubiquitin-like protein; PDHc, pyruvate dehydrogenase complex; ODHc, 2-oxoglutarate dehydrogenase complex; TKT, transketolase; DXS, 1-deoxyxylulose-5-phosphate synthase; ALS, acetolactate synthase; PQO, pyruvate:quinone oxidoreductase.
MATERIALS AND METHODS
Strains and cultivation conditions.
The C. glutamicum type strain ATCC 13032 served as the wild type in the present study. Cultivation was performed in CGXII minimal medium containing 111 mM or 222 mM glucose as the main source of carbon and energy and 195 μM protocatechuic acid as an iron chelator (62). To establish iron-limiting conditions, the concentration of iron in the CGXII medium was reduced from 36 μM to 1 μM, as described previously (20). When indicated, thiamine hydrochloride was added to CGXII medium at a concentration of 0.6 μM using a 3 mM stock solution. Growth experiments were started with a first preculture in 5 ml brain heart infusion (BHI) medium (Difco Laboratories) shaken for 8 h at 170 rpm. The second preculture in 20 ml CGXII minimal medium was inoculated using 500 μl of the first preculture and shaken overnight (16 h) at 140 rpm. The second preculture was used to inoculate the main culture in 50 ml CGXII medium to an optical density at 600 nm (OD600) of 1, and growth was monitored using a spectrophotometer at 600 nm. For cultivation under iron limitation, the cells were washed in 0.9% (wt/vol) NaCl solution before inoculating the next cultures.
Quantification of metabolites in culture supernatants by HPLC.
The presence and concentration of organic acids, amino acids, and glucose in culture supernatants were determined using high-performance liquid chromatography (HPLC), as described earlier (26, 63). Culture supernatants were diluted 1:4 (organic acids and glucose) or 1:100 (amino acids) in distilled water (dH2O) and centrifuged for 5 min at 16,000 × g before being used for HPLC analysis.
Transcriptome analyses using DNA microarrays.
For RNA extraction of C. glutamicum samples, cells were cultured in biological triplicates under low- or high-iron conditions and were harvested in the early-exponential (OD600, 4 to 5) and late-exponential (OD600, 12 to 15) phase. A comparison of mRNA levels under these conditions was performed according to established protocols for RNA preparation, cDNA synthesis, hybridization, and data analysis (63). The microarray data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) database and are available in GEO through accession numbers GSE92348 (early-exponential) and GSE92359 (late-exponential), or GSE92397 for both sets.
Sample preparation for proteome analyses.
Cells were harvested in the late-exponential-growth phase (OD600, 12 to 15) from biological triplicate cultures as for the DNA microarray experiments. Cell pellets were resuspended in 1.5 ml buffer (50 mM potassium phosphate [pH 8.0], 20 mM EDTA, and 2 mM dithiothreitol [DTT], including cOmplete Mini protease inhibitor from Roche Diagnostics) and disrupted using a Precellys cell disruptor three times for 30 s at 6,500 rpm. Cell debris was removed by centrifugation (20 min at 16,000 × g and 4°C). Protein concentrations of crude cell extracts were determined using a Bradford assay (64). For tryptic digestion, 50 μg of protein was treated with the Trypsin Singles proteomics-grade kit (Sigma-Aldrich) in a volume of 100 μl, according to the manufacturer’s instructions for solution digestion. Then, 100 μl of 0.1% formic acid in HPLC-grade water was added, and the samples were subjected to ultrafiltration using Nanosep centrifugal devices with Omega membrane 10K (Pall) that were centrifuged at 16,100 × g for 20 min at 4°C to get rid of the remaining particles, undigested proteins, and peptides larger than 10 kDa. The tryptic peptide samples were stored at −20°C until use for MS measurements.
Shotgun proteomic measurement.
After tryptic digestion, the peptides were separated chromatographically on a nanoLC Eksigent ekspert 425 system (Sciex) coupled with a quartz emitter tip (New Objective) to a TripleTof 6600 mass spectrometer (Sciex) and measured as technical duplicates. Sample volumes of 3 μl containing 1.5 μg of digested protein were loaded on a precolumn (ChromXP C18-3 μm, 350 μm by 0.5 mm; Sciex) from the cooled (8°C) autosampler for desalting and enrichment using a flow of 3 μl/min (10 min) of buffer A (0.1% formic acid in HPLC-grade water). The separation of peptides was performed on an analytical column (ChromXP 3C18-CL-120, 150 mm by 0.75 mm; Sciex) with a gradient method (125 min) using buffer A and buffer B (0.1% formic acid in acetonitrile) at 40°C and a flow of 300 nl/min. The gradient conditions were 5% buffer B for 1 min, 5% to 9% for 9 min, 9% to 20% for 50 min, 20% to 40% for 40 min, 40% to 80% for 5 min, and 80% for 4 min. Before injection of the next sample, the column was equilibrated with 5% of buffer B for 16 min. For shotgun measurements by information-dependent acquisition scanning (IDA), the mass spectrometer was operated with a “top 50” method, as follows: initially, a 250-ms survey scan (time of flight-mass spectrometry [TOF-MS] mass range, m/z 400 to 1,500, high-resolution mode) was collected, from which the top 50 precursor ions were automatically selected for fragmentation, whereby each MS/MS event (mass range, m/z 100 to 1,700, high-sensitivity mode) consisted of a 75-ms fragment ion scan. The main selection criterion for parent ion selection was precursor ion intensity. Ions with an intensity exceeding 100 cps and a charge state of +2 to +5 were preferentially selected. Selected precursors were added to a dynamic exclusion list for 22 s. Precursor ions were isolated using a quadrupole resolution of 0.7 atomic mass units (amu) and fragmented in the collision cell with a rolling collision energy (CE) of 10. If fewer than 50 precursor ions fulfilling the selection criteria were detected per survey scan, the detected precursors were subjected to extended MS/MS accumulation time to maintain a constant total cycle time of 4 s. The source and gas settings were 2,200-V spray, 40 lb/in2 curtain gas, 6 lb/in2 ion source gas, and 75°C interface heater.
Proteomic analysis with SWATH-MS.
For SWATH-based MS measurements, optimized Q1 transmission windows were created for the C. glutamicum samples with the SWATH variable windows calculator (v1.0; Sciex) as a set of 30 overlapping windows (1-amu overlap) covering the mass range m/z 200 to 1,600 in high-sensitivity mode. The rolling collision energy was selected with a charge state of +2 and CE of 10. An accumulation time of 49.96 ms was used for each fragment ion scan and for the survey scan acquired at the beginning of each cycle, resulting in a total cycle time of 2.98 s. The source and gas settings were 2,200 V spray, 40 lb/in2 curtain gas, 6 lb/in2 ion source gas, and 75°C interface heater. Each sample was measured as technical duplicate using the same gradient method as for IDA.
Data processing and statistical analysis.
Our analysis aimed at the identification of proteins with significantly altered abundance in C. glutamicum between low- and high-iron conditions. The IDA data from both growth conditions were processed with ProteinPilot (v4.5 beta; Sciex) using the Paragon algorithm for peptide and protein identification to create a spectral library from C. glutamicum for the SWATH measurements. Using this library, the SWATH data were processed with PeakView (v2.1; Sciex) to identify peptides (99% confidence) and proteins (1% FDR). Before quantification, retention time calibration between IDA and SWATH measurements was performed and applied to all peptides in the ion library using manually selected peptides with retention times to cover the complete time span of the gradient. For quantification using PeakView, the peptide process settings were set to 10 peptides per protein, 6 transitions per peptide, 99% peptide confidence threshold, and 75-ppm extract ion current (XIC) width. The list of proteins with quantified intensities was reduced to proteins detected in all samples. Then, the arithmetic mean of six peak areas per protein detected was calculated separately for both conditions. The six values arose from three biological replicates, with two technical replicates each. The ratio of protein levels under iron limitation to those under iron sufficiency was calculated including a two-sided Student's t test to determine the P value. Protein levels were considered significantly altered between the two conditions if they exhibited a fold change of >1.5 and a P value of ≤0.05.
Data availability.
The microarray data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) database under the accession numbers GSE92348 (OD600, 4 to 5) and GSE92359 (OD600, 12 to 15), or under accession no. GSE92397 for both sets. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the data set identifier PXD017349.
Supplementary Material
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Weber KA, Achenbach LA, Coates JD. 2006. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4:752–764. doi: 10.1038/nrmicro1490. [DOI] [PubMed] [Google Scholar]
- 2.Thiel J, Byrne JM, Kappler A, Schink B, Pester M. 2019. Pyrite formation from FeS and H2S is mediated through microbial redox activity. Proc Natl Acad Sci U S A 116:6897–6902. doi: 10.1073/pnas.1814412116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews SC, Robinson AK, Rodríguez-Quiñones F. 2003. Bacterial iron homeostasis. FEMS Microbiol Rev 27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 4.Cornelis P, Wei Q, Andrews SC, Vinckx T. 2011. Iron homeostasis and management of oxidative stress response in bacteria. Metallomics 3:540–549. doi: 10.1039/c1mt00022e. [DOI] [PubMed] [Google Scholar]
- 5.Frawley ER, Fang FC. 2014. The ins and outs of bacterial iron metabolism. Mol Microbiol 93:609–816. doi: 10.1111/mmi.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez GM. 2006. Control of iron metabolism in Mycobacterium tuberculosis. Trends Microbiol 14:320–327. doi: 10.1016/j.tim.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Eggeling L, Bott M (ed). 2005. Handbook of Corynebacterium glutamicum. CRC Press, Boca Raton, FL. [Google Scholar]
- 8.Frunzke J, Bott M. 2008. Regulation of iron homeostasis in Corynebacterium glutamicum, p 241–266 In Burkovski A. (ed), Corynebacteria: genomics and molecular biology. Caister Academic Press, Norfolk, United Kingdom. [Google Scholar]
- 9.Wennerhold J, Bott M. 2006. The DtxR regulon of Corynebacterium glutamicum. J Bacteriol 188:2907–2918. doi: 10.1128/JB.188.8.2907-2918.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brune I, Werner H, Hüser AT, Kalinowski J, Pühler A, Tauch A. 2006. The DtxR protein acting as dual transcriptional regulator directs a global regulatory network involved in iron metabolism of Corynebacterium glutamicum. BMC Genomics 7:21. doi: 10.1186/1471-2164-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White A, Ding X, vanderSpek JC, Murphy JR, Ringe D. 1998. Structure of the metal-ion-activated diphtheria toxin repressor/tox operator complex. Nature 394:502–506. doi: 10.1038/28893. [DOI] [PubMed] [Google Scholar]
- 12.Frunzke J, Gätgens C, Brocker M, Bott M. 2011. Control of heme homeostasis in Corynebacterium glutamicum by the two-component system HrrSA. J Bacteriol 193:1212–1221. doi: 10.1128/JB.01130-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hentschel E, Mack C, Gätgens C, Bott M, Brocker M, Frunzke J. 2014. Phosphatase activity of the histidine kinases ensures pathway specificity of the ChrSA and HrrSA two-component systems in Corynebacterium glutamicum. Mol Microbiol 92:1326–1342. doi: 10.1111/mmi.12633. [DOI] [PubMed] [Google Scholar]
- 14.Keppel M, Davoudi E, Gätgens C, Frunzke J. 2018. Membrane topology and heme binding of the histidine kinases HrrS and ChrS in Corynebacterium glutamicum. Front Microbiol 9:183. doi: 10.3389/fmicb.2018.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wennerhold J, Krug A, Bott M. 2005. The AraC-type regulator RipA represses aconitase and other iron proteins from Corynebacterium under iron limitation and is itself repressed by DtxR. J Biol Chem 280:40500–40508. doi: 10.1074/jbc.M508693200. [DOI] [PubMed] [Google Scholar]
- 16.Massé E, Gottesman S. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc Natl Acad Sci U S A 99:4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearce MJ, Mintseris J, Ferreyra J, Gygi SP, Darwin KH. 2008. Ubiquitin-like protein involved in the proteasome pathway of Mycobacterium tuberculosis. Science 322:1104–1107. doi: 10.1126/science.1163885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Striebel F, Imkamp F, Özcelik D, Weber-Ban E. 2014. Pupylation as a signal for proteasomal degradation in bacteria. Biochim Biophys Acta 1843:103–113. doi: 10.1016/j.bbamcr.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Küberl A, Fränzel B, Eggeling L, Polen T, Wolters DA, Bott M. 2014. Pupylated proteins in Corynebacterium glutamicum revealed by MudPIT analysis. Proteomics 14:1531–1542. doi: 10.1002/pmic.201300531. [DOI] [PubMed] [Google Scholar]
- 20.Küberl A, Polen T, Bott M. 2016. The pupylation machinery is involved in iron homeostasis by targeting the iron storage protein ferritin. Proc Natl Acad Sci U S A 113:4806–4811. doi: 10.1073/pnas.1514529113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smaldone GT, Revelles O, Gaballa A, Sauer U, Antelmann H, Helmann JD. 2012. A global investigation of the Bacillus subtilis iron-sparing response identifies major changes in metabolism. J Bacteriol 194:2594–2605. doi: 10.1128/JB.05990-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pieper R, Huang ST, Parmar PP, Clark DJ, Alami H, Fleischmann RD, Perry RD, Peterson SN. 2010. Proteomic analysis of iron acquisition, metabolic and regulatory responses of Yersinia pestis to iron starvation. BMC Microbiol 10:30. doi: 10.1186/1471-2f180-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fourquez M, Devez A, Schaumann A, Gueneugues A, Jouenne T, Obernosterer I, Blain S. 2014. Effects of iron limitation on growth and carbon metabolism in oceanic and coastal heterotrophic bacteria. Limnol Oceanogr 59:349–360. doi: 10.4319/lo.2014.59.2.0349. [DOI] [Google Scholar]
- 24.Folsom JP, Parker AE, Carlson RP. 2014. Physiological and proteomic analysis of Escherichia coli iron-limited chemostat growth. J Bacteriol 196:2748–2761. doi: 10.1128/JB.01606-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shakoury-Elizeh M, Protchenko O, Berger A, Cox J, Gable K, Dunn TM, Prinz WA, Bard M, Philpott CC. 2010. Metabolic response to iron deficiency in Saccharomyces cerevisiae. J Biol Chem 285:14823–14833. doi: 10.1074/jbc.M109.091710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch-Koerfges A, Kabus A, Ochrombel I, Marin K, Bott M. 2012. Physiology and global gene expression of a Corynebacterium glutamicum ΔF1Fo-ATP synthase mutant devoid of oxidative phosphorylation. Biochim Biophys Acta 1817:370–380. doi: 10.1016/j.bbabio.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Follmann M, Ochrombel I, Krämer R, Trötschel C, Poetsch A, Rückert C, Hüser A, Persicke M, Seiferling D, Kalinowski J, Marin K. 2009. Functional genomics of pH homeostasis in Corynebacterium glutamicum revealed novel links between pH response, oxidative stress, iron homeostasis and methionine synthesis. BMC Genomics 10:621. doi: 10.1186/1471-2164-10-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angerer A, Klupp B, Braun V. 1992. Iron transport systems of Serratia marcescens. J Bacteriol 174:1378–1387. doi: 10.1128/jb.174.4.1378-1387.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bott M, Niebisch A. 2003. The respiratory chain of Corynebacterium glutamicum. J Biotechnol 104:129–153. doi: 10.1016/s0168-1656(03)00144-5. [DOI] [PubMed] [Google Scholar]
- 30.Niebisch A, Bott M. 2001. Molecular analysis of the cytochrome bc1-aa3 branch of the Corynebacterium glutamicum respiratory chain containing an unusual diheme cytochrome c1. Arch Microbiol 175:282–294. doi: 10.1007/s002030100262. [DOI] [PubMed] [Google Scholar]
- 31.Niebisch A, Bott M. 2003. Purification of a cytochrome bc1-aa3 supercomplex with quinol oxidase activity from Corynebacterium glutamicum - Identification of a fourth subunit of cytochrome aa3 oxidase and mutational analysis of diheme cytochrome c1. J Biol Chem 278:4339–4346. doi: 10.1074/jbc.M210499200. [DOI] [PubMed] [Google Scholar]
- 32.Kusumoto K, Sakiyama M, Sakamoto J, Noguchi S, Sone N. 2000. Menaquinol oxidase activity and primary structure of cytochrome bd from the amino-acid fermenting bacterium Corynebacterium glutamicum. Arch Microbiol 173:390–397. doi: 10.1007/s002030000161. [DOI] [PubMed] [Google Scholar]
- 33.Toyoda K, Teramoto H, Inui M, Yukawa H. 2011. Genome-wide identification of in vivo binding sites of GlxR, a cyclic AMP receptor protein-type regulator in Corynebacterium glutamicum. J Bacteriol 193:4123–4133. doi: 10.1128/JB.00384-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bott M. 2007. Offering surprises: TCA cycle regulation in Corynebacterium glutamicum. Trends Microbiol 15:417–425. doi: 10.1016/j.tim.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Plassmeier J, Persicke M, Pühler A, Sterthoff C, Rückert C, Kalinowski J. 2012. Molecular characterization of PrpR, the transcriptional activator of propionate catabolism in Corynebacterium glutamicum. J Biotechnol 159:1–11. doi: 10.1016/j.jbiotec.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Auchter M, Cramer A, Hüser A, Rückert C, Emer D, Schwarz P, Arndt A, Lange C, Kalinowski J, Wendisch VF, Eikmanns BJ. 2011. RamA and RamB are global transcriptional regulators in Corynebacterium glutamicum and control genes for enzymes of the central metabolism. J Biotechnol 154:126–139. doi: 10.1016/j.jbiotec.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Jungwirth B, Sala C, Kohl TA, Uplekar S, Baumbach J, Cole ST, Pühler A, Tauch A. 2013. High-resolution detection of DNA binding sites of the global transcriptional regulator GlxR in Corynebacterium glutamicum. Microbiology 159:12–22. doi: 10.1099/mic.0.062059-0. [DOI] [PubMed] [Google Scholar]
- 38.Gaballa A, Helmann JD. 2011. Bacillus subtilis Fur represses one of two paralogous haem-degrading monooxygenases. Microbiology 157:3221–3231. doi: 10.1099/mic.0.053579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park S, Kim D, Jang I, Oh HB, Choe J. 2014. Structural and biochemical study of Bacillus subtilis HmoB in complex with heme. Biochem Biophys Res Commun 446:286–291. doi: 10.1016/j.bbrc.2014.02.092. [DOI] [PubMed] [Google Scholar]
- 40.Park S, Choi S, Choe J. 2012. Bacillus subtilis HmoB is a heme oxygenase with a novel structure. BMB Rep 45:239–241. doi: 10.5483/bmbrep.2012.45.4.239. [DOI] [PubMed] [Google Scholar]
- 41.Pfeifer-Sancar K, Mentz A, Rückert C, Kalinowski J. 2013. Comprehensive analysis of the Corynebacterium glutamicum transcriptome using an improved RNAseq technique. BMC Genomics 14:888. doi: 10.1186/1471-2164-14-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chatterjee A, Li Y, Zhang Y, Grove TL, Lee M, Krebs C, Booker SJ, Begley TP, Ealick SE. 2008. Reconstitution of ThiC in thiamine pyrimidine biosynthesis expands the radical SAM superfamily. Nat Chem Biol 4:758–765. doi: 10.1038/nchembio.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez-Gomez NC, Downs DM. 2008. ThiC is an [Fe-S] cluster protein that requires AdoMet to generate the 4-amino-5-hydroxymethyl-2-methylpyrimidine moiety in thiamin synthesis. Biochemistry 47:9054–9060. doi: 10.1021/bi8010253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frunzke J, Bramkamp M, Schweitzer JE, Bott M. 2008. Population heterogeneity in Corynebacterium glutamicum ATCC 13032 caused by prophage CGP3. J Bacteriol 190:5111–5119. doi: 10.1128/JB.00310-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gillet LC, Navarro P, Tate S, Rost H, Selevsek N, Reiter L, Bonner R, Aebersold R. 2012. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: a new concept for consistent and accurate proteome analysis. Mol Cell Proteomics 11:O111.016717. doi: 10.1074/mcp.O111.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schweitzer JE, Stolz M, Diesveld R, Etterich H, Eggeling L. 2009. The serine hydroxymethyltransferase gene glyA in Corynebacterium glutamicum is controlled by GlyR. J Biotechnol 139:214–221. doi: 10.1016/j.jbiotec.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Ibraim IC, Parise MTD, Parise D, Sfeir MZT, de Paula Castro TL, Wattam AR, Ghosh P, Barh D, Souza EM, Goes-Neto A, Gomide ACP, Azevedo V. 2019. Transcriptome profile of Corynebacterium pseudotuberculosis in response to iron limitation. BMC Genomics 20:663. doi: 10.1186/s12864-019-6018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schreiner ME, Fiur D, Holatko J, Patek M, Eikmanns B. 2005. E1 enzyme of the pyruvate dehydrogenase complex in Corynebacterium glutamicum: molecular analysis of the gene and phylogenetic aspects. J Bacteriol 187:6005–6018. doi: 10.1128/JB.187.17.6005-6018.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schreiner ME, Eikmanns BJ. 2005. Pyruvate:quinone oxidoreductase from Corynebacterium glutamicum: purification and biochemical characterization. J Bacteriol 187:862–871. doi: 10.1128/JB.187.3.862-871.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Usuda Y, Tujimoto N, Abe C, Asakura Y, Kimura E, Kawahara Y, Kurahashi O, Matsui H. 1996. Molecular cloning of the Corynebacterium glutamicum (“Brevibacterium lactofermentum” AJ12036) odhA gene encoding a novel type of 2-oxoglutarate dehydrogenase. Microbiology 142:3347–3354. doi: 10.1099/13500872-142-12-3347. [DOI] [PubMed] [Google Scholar]
- 51.Hoffelder M, Raasch K, van Ooyen J, Eggeling L. 2010. The E2 domain of OdhA of Corynebacterium glutamicum has succinyltransferase activity dependent on lipoyl residues of the acetyltransferase AceF. J Bacteriol 192:5203–5211. doi: 10.1128/JB.00597-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niebisch A, Kabus A, Schultz C, Weil B, Bott M. 2006. Corynebacterial protein kinase G controls 2-oxoglutarate dehydrogenase activity via the phosphorylation status of the OdhI protein. J Biol Chem 281:12300–12307. doi: 10.1074/jbc.M512515200. [DOI] [PubMed] [Google Scholar]
- 53.Dougherty MJ, Downs DM. 2006. A connection between iron-sulfur cluster metabolism and the biosynthesis of 4-amino-5-hydroxymethyl-2-methylpyrimidine pyrophosphate in Salmonella enterica. Microbiology 152:2345–2353. doi: 10.1099/mic.0.28926-0. [DOI] [PubMed] [Google Scholar]
- 54.Bunik VI, Tylicki A, Lukashev NV. 2013. Thiamin diphosphate-dependent enzymes: from enzymology to metabolic regulation, drug design and disease models. FEBS J 280:6412–6442. doi: 10.1111/febs.12512. [DOI] [PubMed] [Google Scholar]
- 55.Blombach B, Schreiner ME, Holatko J, Bartek T, Oldiges M, Eikmanns BJ. 2007. L-valine production with pyruvate dehydrogenase complex-deficient Corynebacterium glutamicum. Appl Environ Microbiol 73:2079–2084. doi: 10.1128/AEM.02826-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nielsen AK, Breuner A, Krzystanek M, Andersen JT, Poulsen TA, Olsen PB, Mijakovic I, Rasmussen MD. 2010. Global transcriptional analysis of Bacillus licheniformis reveals an overlap between heat shock and iron limitation stimulon. J Mol Microbiol Biotechnol 18:162–173. doi: 10.1159/000315457. [DOI] [PubMed] [Google Scholar]
- 57.Miranda-Ríos J, Navarro M, Soberón M. 2001. A conserved RNA structure (thi box) is involved in regulation of thiamin biosynthetic gene expression in bacteria. Proc Natl Acad Sci U S A 98:9736–9741. doi: 10.1073/pnas.161168098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winkler W, Nahvi A, Breaker RR. 2002. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature 419:952–956. doi: 10.1038/nature01145. [DOI] [PubMed] [Google Scholar]
- 59.Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS. 2002. Comparative genomics of thiamin biosynthesis in procaryotes. New genes and regulatory mechanisms. J Biol Chem 277:48949–48959. doi: 10.1074/jbc.M208965200. [DOI] [PubMed] [Google Scholar]
- 60.Mentz A, Neshat A, Pfeifer-Sancar K, Pühler A, Rückert C, Kalinowski J. 2013. Comprehensive discovery and characterization of small RNAs in Corynebacterium glutamicum ATCC 13032. BMC Genomics 14:714. doi: 10.1186/1471-2164-14-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Josts I, Almeida Hernandez Y, Andreeva A, Tidow H. 2016. Crystal structure of a group I energy coupling factor vitamin transporter S component in complex with its cognate substrate. Cell Chem Biol 23:827–836. doi: 10.1016/j.chembiol.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frunzke J, Engels V, Hasenbein S, Gätgens C, Bott M. 2008. Co-ordinated regulation of gluconate catabolism and glucose uptake in Corynebacterium glutamicum by two functionally equivalent transcriptional regulators, GntR1 and GntR2. Mol Microbiol 67:305–322. doi: 10.1111/j.1365-2958.2007.06020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vogt M, Haas S, Klaffl S, Polen T, Eggeling L, van Ooyen J, Bott M. 2014. Pushing product formation to its limit: metabolic engineering of Corynebacterium glutamicum for l-leucine overproduction. Metab Eng 22:40–52. doi: 10.1016/j.ymben.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 64.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The microarray data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) database under the accession numbers GSE92348 (OD600, 4 to 5) and GSE92359 (OD600, 12 to 15), or under accession no. GSE92397 for both sets. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the data set identifier PXD017349.