Abstract
Optimal therapeutic strategy remains an unmet need in localised high-risk prostate cancer (LHRPC). Androgen biosynthesis inhibition in the preoperative setting may improve outcomes. In this single-centre randomised trial, we looked at therapy outcomes of preoperative treatment with abiraterone acetate + prednisone (AAP) + luteinising hormone-releasing hormone agonist (LHRHa) or LHRHa alone followed by radical prostatectomy in 65 men. We did not see a significant difference of organ-confined carcinoma (p = 0.27). However, tumour volume measures were significantly lower for AAP + LHRHa treatment (p ≤ 0.001). Of note, lower tumour epithelium volume correlated with improved biochemical recurrence-free survival at ≥4-yr follow-up (p = 0.0014). Tumours pretreated with AAP + LHRHa had lower proliferation and androgen signalling expression than LHRHa. On multivariate analysis, glucocorticoid receptor (GR) overexpression correlated with persistent tumours in AAP + LHRHa (p = 0.018). The presence of nuclear androgen receptor splice variant (nARV7) correlated with persistent tumours in both arms. No new safety signals were observed. This is the first study investigating the role of preoperative AAP + LHRHa versus LHRHa alone in LHRPC. We report significant cytoreduction by tumour volume measures inversely correlating with biochemical relapse. Validation of these proposed tumour volume measures is planned. A potential role of GR in resistance to androgen biosynthesis inhibition warrants further study.
Keywords: High-risk localised prostate, cancer, Glucocorticoid receptor, Abiraterone acetate, Androgen deprivation therapy, Neoadjuvant, Preoperative, Radical prostatectomy, Androgen receptor splice variant, Androgen receptor, Tumour volume
Patient summary:
This is the first study of abiraterone acetate plus leuprolide versus leuprolide alone in high-risk localised prostate cancer followed by prostatectomy. Patients in the combination arm had a significantly smaller tumour size.
Localised high-risk prostate cancer (LHRPC) remains a therapeutically unmet need, with 70% of patients relapsing following available therapeutic strategies.
The confirmed increase in efficacy in castration-naïve disease creates the impetus to assess the potential role of preoperative abiraterone acetate in localised high-risk disease [1,2].
Treatment with abiraterone acetate and prednisone (AAP) + luteinising hormone-releasing hormone agonist (LHRHa) results in diminished androgen biosynthesis in LHRPC, but no outcome in comparison with LHRHa is reported [3].
We conducted a randomised, phase 2, open-label, single-institution trial to study persistent cancers after 3 mo of exposure to AAP + LHRHa or LHRHa alone. The goal of this study was to inform prioritisation of biomarkers for the determination of AAP benefit and resistance in LHRPC.
Participants had histologically confirmed prostate adenocarcinoma without evidence of metastasis by conventional imaging and clinical stage ≥T1c with Gleason score 8–10 on biopsy or Gleason score 7 ≥T2b and prostate-specific antigen (PSA) ng/ml.
Sixty-five patients were randomised 2:1 to AAP + LHRHa versus LHRHa alone for 3 mo followed by radical prostatectomy (median age, 61 yr; 25–75th percentiles, 56–68). Fifty-six (85%) patients had biopsy Gleason score ≥8 (Supplementary Table 2).
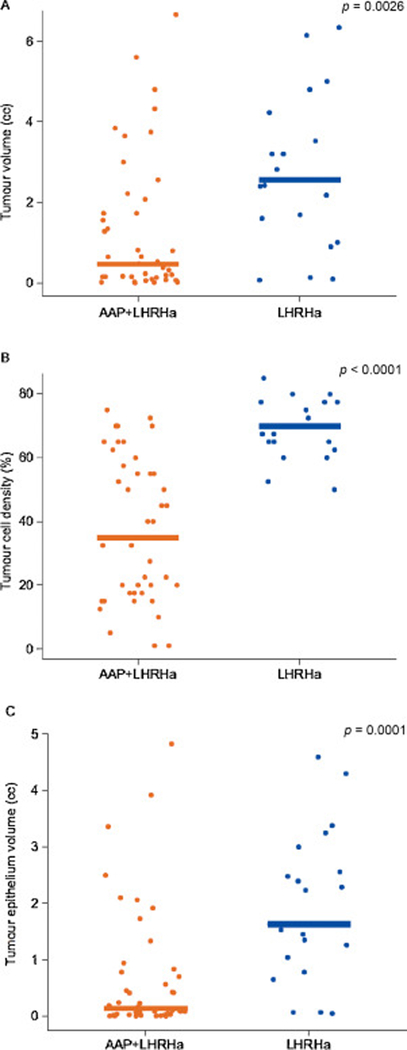
Twenty-one of 44 (48%) AAP + LHRHa-treated tumours and seven of 21 (33%) LHRHa-treated tumours were ypT2N0 (p = 0.27; Table 1). By three quantitative tumour measures, residual tumour was significantly lower in the AAP + LHRHa arm compared with the LHRHa arm (Fig. 1). Thirty-seven (84%) AAP + LHRHa-treated patients and one LHRHa-treated patient had undetectable PSA (≤0.1 ng/ml) before prostatectomy (p < 0.0001 ; Table 1).
Table 1.
Outcomes at prostatectomy for intent-to-treat population
| AAP+LHRHa | LHRHa | p valuea | |
|---|---|---|---|
| N | 44 | 21 | |
| Surgery | |||
| Robotic-assisted prostatectomy | 40 | 18 | |
| Lymph nodes, median (Q1, Q3) | 19.5 (12–25) | 19 (16–22) | |
| Perioperative serious AE | 1 PE | 1 pelvic bleeding | |
| Blood loss (ml), median (Q1, Q3) | 250 (187–300) | 250 (150–300) | |
| Pathology | |||
| ypT2N0, n (%) | 21 (48) | 7 (33) | 0.27 |
| Surgical margin negative, n (%) | 42 (95) | 18 (86) | 0.17 |
| Lymph nodes negative, n (%) | 29(66) | 13 (62) | 0.75 |
| PSA nadirb, n | 44 | 21 | |
| PSA ≤0.1 ng/ml | 37 (84) | 1 (5) | <0.0001 |
AAP = abiraterone acetate plus prednisone; AE = adverse event; LHRHa = luteinising hormone-releasing hormone agoinst; PE = pulmonary embolism; PSA = prostate-specific antigen: Q1 and Q3 =25th and 75th percentiles, respectively.
Based on chi-square test.
Lowest PSA level during study (excluding postprostatectomy visit).
Fig. 1 -.
Residual tumour quantification: (A) tumour volume, (B) tumour cell density, and (C) tumour epithelium volume. The bars represent the median for tumour quantification. AAP = abiraterone acetate plus prednisone; LHRHa = luteinising hormone-releasing hormone agonist.
Adverse events were similar between groups (Supplementary Table 2), and no patient had grade 4 events (Table 2).
Table 2.
Treatment-emergent adverse events (TEAEs) in safety population
| AAP + LHRHa (n = 44) | LHRHa (n = 21) | |||||
|---|---|---|---|---|---|---|
| No. of patients with TEAEs (%)a | 44 (100) | 21 (100) | ||||
| No. of patients with grade 3 TEAEs (%)a | 17 (39) | 5 (24) | ||||
| No. of patients with TESAEs(%)a | 2 (4.5) | 2 (9.5) | ||||
| No. of patients with TEAEs leading to treatment discontinuation (%)a,b | 5 (11.4) | 0 | ||||
| AAP+LHRHa | LHRHa | |||||
| Grade 1/2 | Grade 3d | Total | Grade 1/2 | Grade 3§ | Total | |
| Most frequent TEAEs, n(%)c | ||||||
| Hot flush | 37 (84) | 0 | 37 (84) | 18 (86) | 0 | 18 (86) |
| Anaemia | 23 (52) | 0 | 23 (52) | 8 (38) | 0 | 8 (38) |
| Fatigue | 18 (41) | 1 (2) | 19 (43) | 8 (38) | 2 (10) | 10 (48) |
| ALT increase | 15 (34) | 7 (16) | 22 (50) | 7 (33) | 0 | 7 (33) |
| AST increase | 16 (36) | 1 (2) | 17 (39) | 7 (33) | 0 | 7 (33) |
| Hypertension | 2 (5) | 8 (18) | 10 (23) | 1 (5) | 2 (10) | 3 (14) |
| Hypercholesterolaemia | 9 (21) | 0 | 9 (21) | 5 (24) | 0 | 5 (24) |
| Hyperglycaemia | 9 (21) | 0 | 9 (21) | 3 (14) | 0 | 3 (14) |
| Hyperbilirubinemia | 8 (18) | 0 | 8 (18) | 1 (5) | 0 | 1 (5) |
| Insomnia | 3 (7) | 0 | 3 (7) | 5 (24) | 1(5) | 6 (29) |
(AA = abiraterone acetate; AAP = abiraterone acetate + prednisone; ALT = alanine aminotransferase; AST = aspartate aminotransferase; LHRHa = luteinising hormone-releasing hormone agonist; P = prednisone; TESAE = treatment-emergent serious adverse event.
Adverse events with toxicity grade 5 are not included.
Discontinuation of study medication includes discontinuation of any of the study drugs (AA, P, or LHRHa).
TEAE in ≥15% of patients in either treatment group.
No grade 4 TEAEs were observed.
PSA recurrence was observed in 44% (19/43) and 59% (10/17) of patients in AAP + LHRHa and LHRHa groups, respectively (p = 0.28; Table 3). A lower tumour epithelium volume correlated with improved biochemical recurrence-free survival, with a median follow-up of ≥4 yr (p = 0.0014; Table 3).
Table 3.
PSA recurrencea by treatment and by tumour epithelial volume based on Kaplan-Meier method with log-rank test
| Treatment (p = 0.28) | Tumour epithelial volume (p = 0.001) | Pathological stage (p = 0.0002) | ||||
|---|---|---|---|---|---|---|
| AAP + LHRHa | LHRHa | ≤0.3 cc | >0.3 cc | ypT2N0 | >pT2N0 | |
| 3-yrs RFS(%) | 75 | 71 | 89 | 61 | 89 | 60 |
| Recurrence | 19 | 10 | 8 | 21 | 8 | 21 |
| No recurrence | 24 | 7 | 20 | 11 | 18 | 13 |
| Loss to follow-up | 1 | 2 | 1 | 2 | 1 | 2 |
| Death from other primary reason | 0 | 2 | 0 | 2 | 2 | 0 |
AAP = abiraterone acetate plus prednisone; LHRHa= luteinising hormone-releasing hormone agonist; PSA = prostate-specific antigen; RFS = relapse-free survival.
Defined as a ≥0.1 ng/ml rise in PSA levels.
We explored biomarkers by association with treatment arm and residual volume, and then focused within the abiraterone treatment arm.
We explored biomarkers by association with treatment arm and residual volume, and then focused within the abiraterone treatment arm.
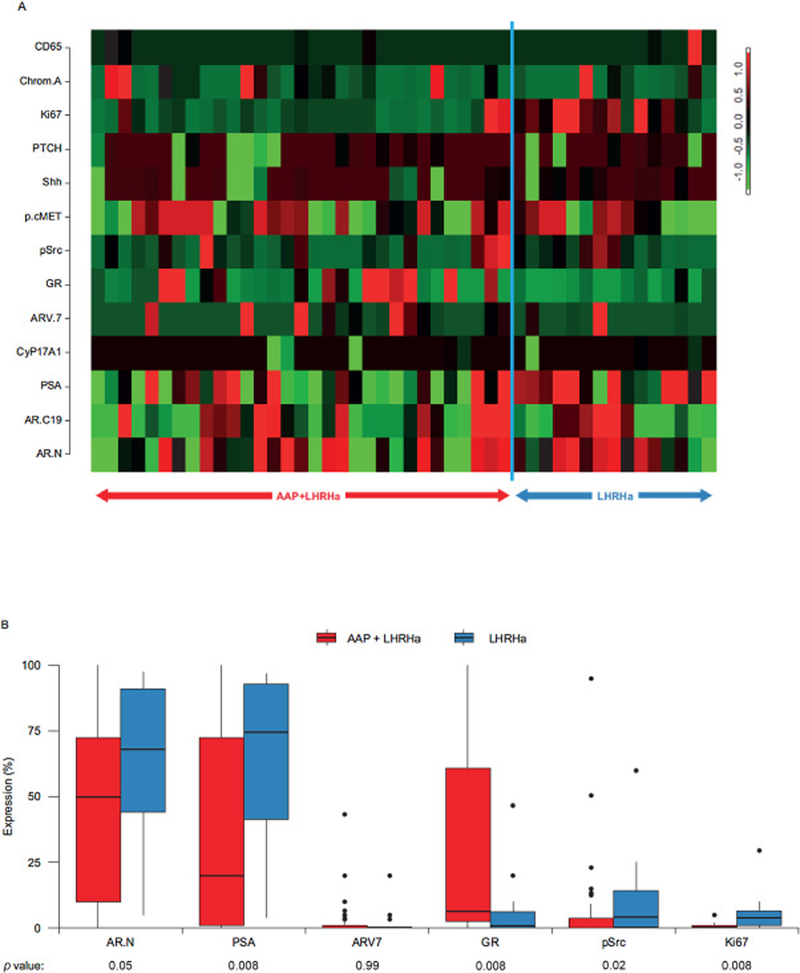
Decreased expression of markers of androgen signalling and proliferation were observed in the AAP + LHRHa-treated arm versus the LHRHa-treated arm (Fig. 2).
Fig. 2 -.
Differential expression of selected markers of interest between treatment arms. (A) Heatmap of markers ordered by domain of interest, including cell cycle regulation and neuroendocrine markers (CD56, chromogranin A, Ki67, PTCH), microenvironment interactions (Shh, p-cMET, p-Src, GR), and AR signalling (ARv7, CYP17, PSA, AR-C terminal, AR-N terminal). The expression of each marker in each sample is represented by the number of standard deviations above (red) or below (green) the mean for that gene across all samples. (B) Protein expression of select markers. AAP = abiraterone acetate plus prednisone; AR = androgen receptor, GR = glucocorticoid receptor, LHRHa = luteinising hormone-releasing hormone agonist; PSA = prostate-specific antigen.
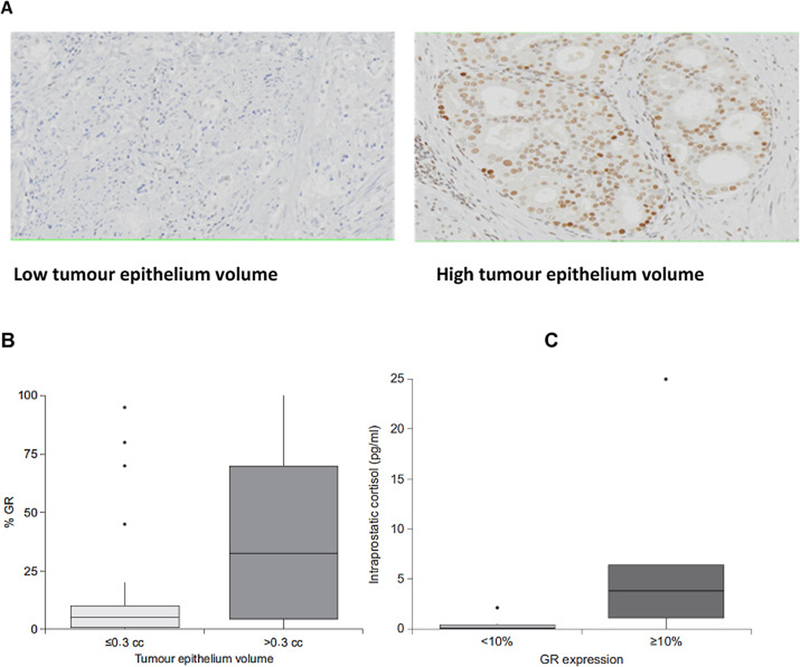
Glucocorticoid receptor (GR) overexpression was more frequent in the AAP + LHRHa arm (p = 0.008). GR overexpression (expressed in ≥10% of tumour cells) was associated with a higher tumour epithelium volume only within the abiraterone-treated arm (r = 0.38, p = 0.018; Fig. 3) and correlated with higher intraprostatic cortisol levels (Fig. 3).
Fig. 3 -.
GR expression by immunohistochemistry in the AAP + LHRHa arm. (A) GR expression in tumour AAP + LHRHa-treated tumour samples. (B) Association of GR expression and tumour volume epithelium. (C) Association of GR expression and cortisol levels. Box plots represent 25th and 75th percentiles; whiskers extend to the minimum and maximum values. Median is indicated by the line within the box. AAP = abiraterone acetate plus prednisone; GR = glucocorticoid receptor; LHRHa = luteinising hormone-releasing hormone agonist.
Higher proliferation (r = 0.39, p = 0.01 p-Src (r = 0.40, p = 0.01), and the presence of nuclear androgen receptor splice variant (ARv7; r = 0.30, p = 0.05) correlated with a higher tumour epithelium volume irrespective of the treatment arm (Supplementary Table 5).
This is the first randomised neoadjuvant study of AAP + LHRHa versus LHRHa alone in LHRPC exploring outcome and markers of AAP + LHRHa treatment resistance. Although there was no significant difference in pathological downstaging between the two treatment arms, tumour volume measures significantly favoured abiraterone acetate addition. Pathology staging may not be an appropriate surrogate for the assessment of neoadjuvant treatment effect, as it appears dissociated from tumour volume changes and extent of tumour infiltration. Moreover, the direct association between persistent higher tumour epithelium volume and shorter time to recurrence supports the relevance of these measures. Our interpretation is supported by the improved outcomes of abiraterone in hormone-naive advanced prostate cancer [1,2].
Serum PSA depletion to ≤0.1 ng/ml was observed in >80% of patients treated with AAP + LHRHa, though in some there was no appreciable cytoreduction. This is consistent with the hypothesis that alternate pathways may be drivers of treatment resistance and progression even at this earlier-stage disease [4]. This underscores the clinical and biological heterogeneity of these primary tumours while showcasing the limitations of PSA.
Our candidate screen for biomarker resistance to abiraterone treatment provided findings with potentially important clinical relevance. Increased GR expression was observed only in the abiraterone-treated tumours and was specific to increased residual tumour measures. Furthermore, high GR expression was associated with higher intraprostatic cortisol levels. This finding is in line with a functional GR signalling axis in some cancers exposed to androgen signalling inhibition.
Neither the mechanism behind the increased GR expression nor its potential role as a survival pathway is understood. While GR activity is reported as a potential resistance mechanism for androgen receptor (AR) inhibition in castration-resistant prostate cancer (CRPC) [5–7], this is the first report associating GR expression with persistence of cancer after abiraterone acetate treatment and could be a response specific to hormone-naive disease. This is supported by the lower AR expression as compared with AAP + LHRHa treatment and is opposite to what we have reported in the metastatic CRPC setting, that is, an increase in AR copy numbers following abiraterone treatment. AR inhibition may serve to reverse AR-mediated repression at the GR locus. GR upregulation may in turn drive the expression of a subset of AR-driven genes, thus allowing the tumour to circumvent AR inhibition [8–11]. Thus, GR activation may contribute to resistance in a subset of these tumours and could be a meritorious target in castration-naïve disease.
The presence of nuclear ARv7 was associated with higher residual tumour (p = 0.05) regardless of the treatment arm.
This is consistent with preclinical and clinical reports in the castration-resistant setting that nuclear ARv7 expression is negatively associated with canonical/physiological androgen signalling and is more likely a marker of genomic instability.
In conclusion, 3-mo neoadjuvant therapy with AAP + LHRHa in high-risk localised disease does not improve conventional pathological stage outcomes as compared with LHRHa alone. However, cytoreduction by tumour volume measures was significantly higher in the abiraterone-treated arm and associated with a lack of biochemical recurrence at a median follow-up of 4 yr. Early emergence of resistance in the abiraterone-treated arm correlated with increased GR expression.
This hypothesis-generating biomarker analysis has several limitations, and data interpretation is hindered by a small sample size. Studies with long-term follow-up are required to confirm the value of proposed tumour volume measures and assess the role of GR-driven resistance in the hormone-naive LHRPC setting.
Supplementary Material
Acknowledgements:
The authors thank Pauline Dieringer, Elsa Li-Ning Tapia, and Ina Prokhorova for their contributions to this study. Writing assistance was provided by Shala Thomas, PhD, and Ira Mills, PhD, and was funded by Janssen Global Services, LLC.
Funding/Support and role of the sponsor: This study was supported by Prostate Cancer Foundation and Janssen Research & Development.
Financial disclosures: Eleni Efstathiou certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Eleni Efstathiou has served as a consultant/advisor for AstraZeneca, Janssen Oncology, Sanofi, Tolmar, and Takeda; has received institutional research funding from Janssen-Cilag, Janssen Oncology, Sanofi, and Takeda. John W. Davis has received institutional research funding from GenomeDx, Hologic, and Janssen, and has received honoraria from Astellas Pharma and Myriad Genetics. Weimin Li, Michael Gormley, Deborah Ricci, NamPhuong Tran, Weimin Peng, Thian Kheoh, and Arturo Molina have been employed by Janssen Research & Development, and have owned stock or held an ownership interest with Johnson & Johnson. Sijin Wen, Mark Titus, Anh Hoang, and Patricia Troncoso have nothing to disclose. Ryan P. McMullin has served as a consultant/advisor for Janssen Research & Development, and has been employed by LabConnect LLC. Amado J. Zurita has served as a consultant/ advisor for Bayer, Incyte, and Pfizer, and has received institutional research funding from Novartis and Pfizer. Christopher J. Logothetis has served as a consultant/advisor for Astellas Pharma, AstraZeneca, Bayer, Bristol-Myers Squibb, Churchill Pharmaceuticals, Clovis Oncology, Helsinn Healthcare, Janssen, Novartis, Pfizer, and Sanofi, and has received institutional research funding from Astellas Pharma, AstraZeneca, Bayer, Bristol-Myers Squibb, Cougar Biotechnology, Exelixis, GlaxoSmithKline, Janssen, Karyopharm Therapeutics, Novartis, Pfizer, and Sanofi.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.eururo.2019.05.010.
References
- [1].James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 2017;377:338–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 2017;377:352–60. [DOI] [PubMed] [Google Scholar]
- [3].Taplin ME, Montgomery B, Logothetis CJ, et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study.J Clin Oncol 2014;32 :3705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Berger MF, Lawrence MS, Demichelis F, et al. The genomic complexity of primary human prostate cancer. Nature 2011;470:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Arora VK, Schenkein E, Murali R, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 2013;155:1309–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Efstathiou E, Titus M, Wen S, et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol 2015;67:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shah N, Wang P, Wongvipat J, et al. Regulation of the glucocorticoid receptor via a BET-dependent enhancer drives antiandrogen resistance in prostate cancer. eLife 2017;6:e27861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Isikbay M, Otto K, Kregel S, et al. Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer. Hormones Cancer 2014;5:72–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li J, Alyamani M, Zhang A, et al. Aberrant corticosteroid metabolism in tumor cells enables GR takeover in enzalutamide resistant prostate cancer. eLife 2017;6:e20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen S, Wang J, Yu G, Liu W, Pearce D. Androgen and glucocorticoid receptor heterodimer formation. A possible mechanism for mutual inhibition of transcriptional activity. J Biol Chem 1997. ;272: 14087–92. [DOI] [PubMed] [Google Scholar]
- [11].Xie N, Cheng H, Lin D, et al. The expression of glucocorticoid receptor is negatively regulated by active androgen receptor signaling in prostate tumors. Int J Cancer 2015; 136:E27–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.