Abstract
Immune checkpoint inhibitors have changed the landscape of classic cancer treatment. However, their use is associated with the emergence of new adverse events. An elderly man with rheumatoid arthritis was started on pembrolizumab for newly diagnosed advanced lung cancer. He subsequently developed hemophagocytic lymphohistiocytosis (HLH), which is potentially fatal but has not been properly established as an immune checkpoint inhibition-induced event. We herein report the case of a patient with pembrolizumab-induced HLH.
Keywords: pembrolizumab, hemophagocytic lymphohistiocytosis, immune checkpoint inhibitors, lung cancer, rheumatoid arthritis, immune-related adverse event
Introduction
Immune checkpoint inhibitors (ICIs) have now become a treatment alternative for a wide range of cancer types. Although they have demonstrated substantial clinical benefits, they have also revealed the new concept of immune-related adverse events (irAEs). Common manifestations include dermatitis, arthritis, endocrinopathy, and hepatitis, and any organ can be affected. Besides non-fatal adverse events, ICIs are reported to occasionally elicit life-threatening conditions, such as myocarditis, pneumonitis, and enterocolitis (1, 2). Hemophagocytic lymphohistiocytosis (HLH) is a fatal condition triggered by various immune-activating events; however, HLH has been rarely reported in the context of lung cancer immunotherapy. We herein report a case of severe HLH developing in an elderly man with rheumatoid arthritis during lung adenocarcinoma treatment with pembrolizumab.
Case Report
A 74-year-old man was diagnosed with rheumatoid arthritis 1 year before presenting to our hospital. He had been successfully treated for rheumatoid arthritis with low-dose oral methotrexate of 6 mg per week. The man visited our hospital for the evaluation of a lung nodule incidentally found on a medical checkup.
Chest computed tomography (CT) revealed an irregularly marginated nodule with pleural indentation in the right lower lobe and several enlarged mediastinal and subclavian lymph nodes (Fig. 1a-c). Endobronchial ultrasound-guided transbronchial needle aspiration was performed; the pathological findings of the specimen indicated the diagnosis of lung adenocarcinoma.
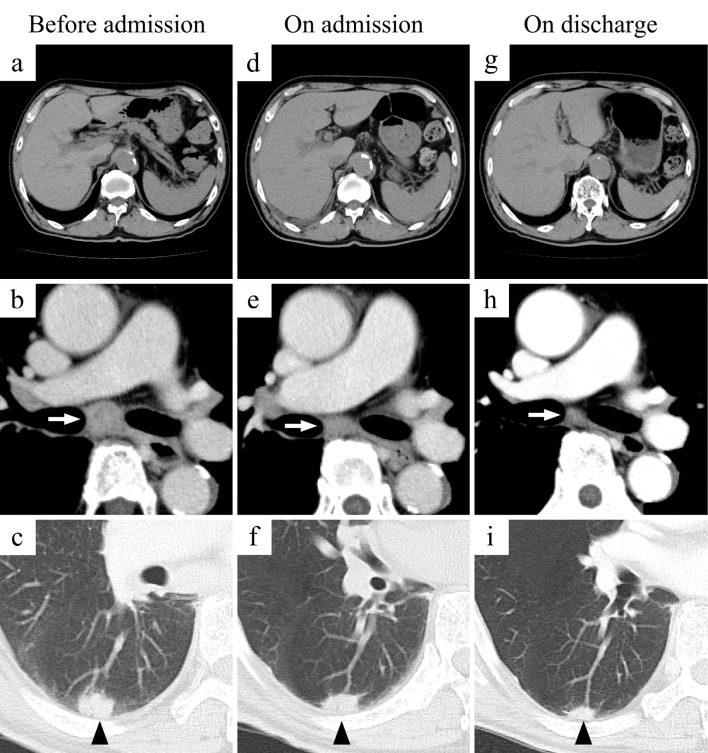
Figure 1.
Changes in CT findings. The upper panels show the liver and spleen: (a) No abnormal liver or spleen findings were seen before admission. (d) Hepatosplenomegaly was detected on admission. (g) The liver and spleen sizes normalized before discharge. The middle panels show the inferior tracheobronchial lymph nodes (white arrows): (b) An enlarged lymph node was confirmed before admission. (e) The lymph node had appeared smaller on admission. (h) The lymph node had nearly normalized in size before discharge. The lower panels show the lung tumor in the right lower lobe (black arrow heads): (c) A lung nodule was seen before admission. (f) The lung nodule appeared smaller on admission. (i) The nodule had significantly decreased in diameter before discharge.
The lung cancer was staged as ШB, T2aN3M0. Immunohistochemistry was negative for both EGFR and ALK mutations, and 60% of the tumor cells were positive for programmed death ligand-1 (PD-L1). Immunotherapy with pembrolizumab was chosen over chemoradiotherapy because definitive radiotherapy was determined to be difficult to perform due to the extensive disease progression involving the bilateral subclavian lymph nodes. Methotrexate had been discontinued by the time the decision to initiate pembrolizumab immunotherapy was made, as no abnormal joint findings or joint pain had been observed for a long period.
Seven days after the initial pembrolizumab course, bilateral finger and limb joint pain and swelling appeared. The second immunotherapy course was suspended, and prednisolone (10 mg/day) and iguratimod were initiated for the flare-up of rheumatoid arthritis. Although the joint events quickly subsided, the patient became febrile 27 days after pembrolizumab administration and visited our hospital's emergency room on the following day.
A laboratory study revealed decreased blood cell counts, prompting hospital admission. The patient had smoked 20 cigarettes daily for 36 years before he quit smoking at the age of 56. He was a school teacher with no exposure to toxic chemical materials, and his medical history was unremarkable. The patient was administered oral anti-hypertensive therapy. Although imaging findings of mild interstitial lung disease and emphysema had been observed on CT before the lung cancer diagnosis, these entities were asymptomatic, so no intervention was performed. A medical interview revealed no family history of hematological conditions, connective tissue disorders, or lung cancer. His medication history included prednisolone, iguratimod, olmesartan, amlodipine, celecoxib, and famotidine.
On admission, the patient's temperature was 38.9℃, and his pulse rate was 82 beats per minute. Oxygen saturation was 96% while breathing ambient air. Bilateral basal fine crackles were audible. The patient's liver and spleen were not palpable, and a macular rash was found to have spread over his face, torso, and extremities (Fig. 2). No other skin changes, including skin nodules or mucosal involvement, were observed, and the joint findings had normalized by this point.
Figure 2.
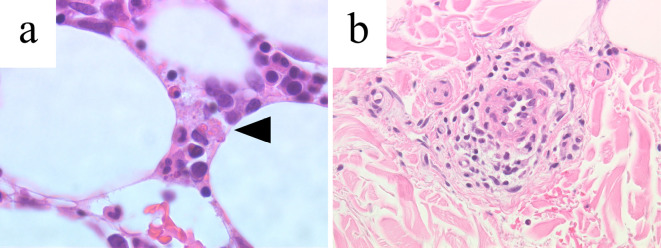
Biopsy specimen. (a) The bone marrow biopsy, Hematoxylin and Eosin (H&E) staining, 400× magnification. The black arrowhead points to an erythrocyte-phagocytosing macrophage. (b) Perivascular lymphocyte infiltration confirmed by a skin biopsy, H&E staining, 400× magnification.
A laboratory test results showed a decreased white cell count of 2,710 /μL, hemoglobin of 12.0 g/dL, and platelet count of 134,000 /μL. An extremely high ferritin level of 28,976 ng/L was detected. The patient's coagulation profile was also abnormal, including a D-dimer level of 156.8 μg/mL. No active viral infection was detected on serology (Table). Blood culture from the sample taken at the emergency room grew no organisms. An electrocardiogram and echocardiography results were unremarkable. CT revealed hepatosplenomegaly. The lung nodule and metastatic lymph nodes were smaller than at the time of the cancer diagnosis (Fig. 1d-f), and a bone marrow biopsy and skin biopsy were planned. The ferritin level and coagulation profile had deteriorated by the next morning, prompting the administration of 1,000 mg of high-dose methylprednisolone and planning of HLH treatment without waiting for the biopsy results. The bone marrow biopsy showed macrophages phagocytosing blood cells and a slightly decreased cellularity. At this point, diagnostic criteria used in the HLH-2004 trial concerning the body temperature, peripheral blood cytopenia, elevated ferritin levels, hemophagocytosis in bone marrow, and hepatosplenomegaly had been met, so an HLH diagnosis was established. The skin biopsy findings were more compatible with drug-induced exanthem than with HLH skin manifestation (Fig. 3).
Table.
Laboratory Data on Admission.
| White cell count | 2,710 | /μL | PT | 14.2 | s |
| Differential count | APTT | 38.9 | s | ||
| Polymorphonuclear cells | 84.8 | % | FDP | 262.8 | μg/mL |
| Lymphocytes | 10.7 | % | Fibrinogen | 494 | mg/dL |
| Monocytes | 3 | % | D-dimer | 156.8 | μg/mL |
| Basophils | 1.1 | % | CEA | 75.6 | ng/mL |
| Eosinophils | 0.4 | % | SLX | 40 | U/mL |
| Hemoglobin | 12.0 | g/dL | Rheumatoid factor | 236 | U/mL |
| MCV | 96.9 | fL | Anti-HCV antibody | Negative | |
| Reticulocyte | 1.1 | % | HBs antigen | Negative | |
| Platelets | 134,000 | /μL | Anti-HBs antibody | 2.0 | U/mL |
| AST | 84 | U/L | Anti-nuclear antibodies | ||
| ALT | 13 | U/L | Homogenous pattern | 40 | × |
| LDH | 614 | U/L | Speckled pattern | 40 | × |
| ALP | 199 | U/L | MMP-3 | 113 | ng/mL |
| Total protein | 6.2 | g/dL | Anti-CCP antibody | 387 | U/mL |
| Albumin | 2.9 | g/dL | IGRA | Negative | |
| Sodium | 127 | mmol/L | Anti-VZV antibodies | ||
| Potassium | 4.4 | mmol/L | IgG | 12.1 | × |
| Chloride | 95 | mmol/L | IgM | 0.04 | × |
| BUN | 17 | mg/dL | Anti-HSV antibodies | ||
| Creatinin | 0.82 | mg/dL | IgG | 0.8 | × |
| HDL-C | 33 | mg/dL | IgM | 0.04 | × |
| LDL-C | 79 | mg/dL | CMV antigen (C10, C11) | Negative | |
| Triglyceride | 88 | mg/dL | Anti-EBV antibodies | ||
| Hemoglobin A1c | 5.8 | % | IgG | 160 | × |
| Haptoglobin | 236.0 | mg/dL | VCA-IgM | Negative | |
| CRP | 8.05 | mg/dL | EA-DR-IgG | Negative | |
| sIL-2R | 4,625 | U/mL | EBNA-IgG | 80 | × |
| Iron | 44 | μg/dL | Anti-HHV-6 antibodies | ||
| TIBC | 217 | μg/dL | IgG | 80 | × |
| Ferritin | 28,976 | ng/mL | IgM | Negative |
ALT: alanine aminotransferase, ALP: alkaline phosphatase, APTT: activated partial thromboplastin time, AST: aspartate aminotransferase, BUN: blood urea nitrogen, CCP: cyclic citrullinated protein, CEA: carcinoembryonic antigen, CMV: cytomegalovirus, CRP: C-reactive protein, EBV: Epstein-Barr virus, FDP: fibrin degradation products, HBs: hepatitis B surface, HCV: hepatitis C virus, HDL-C: high density lipoprotein cholesterol, HHV-6: human herpesvirus-6, HSV: herpes simplex virus, IGRA: interferon-gamma release assay, LDH: lactate dehydrogenase, LDL-C: low density lipoprotein cholesterol, MCV: mean corpuscular volume, MMP-3: matrix metalloprotease-3, PT: prothrombin time, sIL-2R: soluble interleukin-2 receptor, SLX: sialyl SSEA-1, TIBC: total iron binding capacity, VZV: varicella-zoster virus
Figure 3.
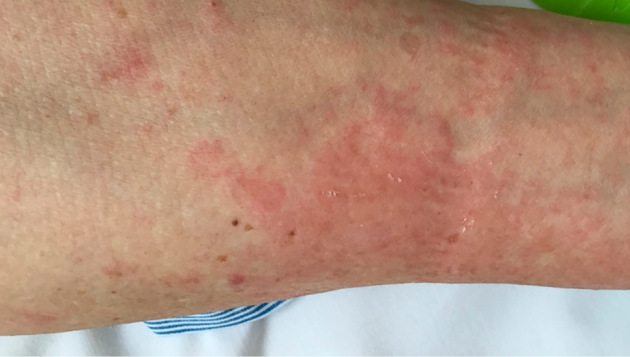
Physical findings. Macular rash observed on the forearm.
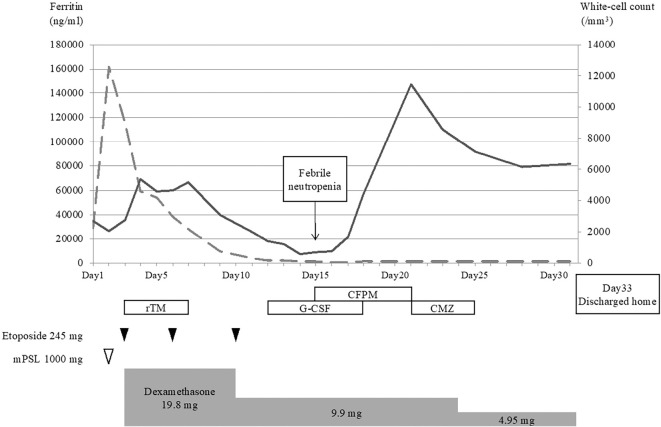
The patient's clinical course is shown in Fig. 4. Treatment with dexamethasone and etoposide was initiated in accordance with the HLH-94 protocol from the third hospital day. Initial dexamethasone and etoposide doses were 10 mg/m2 and 150 mg/m2, respectively. Ferritin levels, which peaked up to 100,000 ng/mL, rapidly fell after HLH-specific treatment initiation, and the blood cell counts also started recovering. Recombinant thrombomodulin was only initially used, as intravascular coagulation was thought to be imminent due to the patient's rapidly deteriorating coagulation profile.
Figure 4.
The patient’s clinical course. Continuous and dashed lines indicate the white cell counts and ferritin levels, respectively. The patient developed febrile neutropenia on the 15th hospital day and was discharged on the 33rd hospital day. CFPM: cefepime, CMZ: cefmetazole, G-CSF: granulocyte colony-stimulating factor, mPSL: methylprednisolone, rTM: recombinant thrombomodulin
However, on the 9th hospital day, blood cell counts began to fall again. As the ferritin levels were controlled and no other signs of HLH worsening were observed at this point, bone marrow suppression due to etoposide use was suspected, and treatment with this agent was discontinued. The blood cell counts did not recover despite the use of granulocyte colony-stimulating factor, and the patient developed febrile neutropenia on the 15th hospital day. Empirical cefepime was started, and a blood culture grew Escherichia coli. Cefepime was subsequently switched to cefmetazole based on sensitivity test results.
The patient responded to antibiotic therapy, and blood cell counts subsequently recovered. As HLH was adequately controlled, dexamethasone was tapered over weeks. CT performed on the 27th hospital day showed a normalized liver and spleen size. The lung tumor and metastatic lymph node diameter had also markedly decreased (Fig. 1g-i). The man was discharged on the 33rd hospital day. At a recent follow-up, he was in complete lung cancer remission without further lung cancer treatment. Disease relapse has not been seen in over seven months.
Discussion
We herein report a case of HLH that developed in a patient with rheumatoid arthritis and advanced lung cancer treated with pembrolizumab. He was successfully rescued by the early initiation of HLH-specific treatment and pembrolizumab discontinuation.
Adult-onset HLH is triggered by immune-activating events, such as infection or treatment initiation, in patients with coexisting morbidity. Accurately identifying the HLH trigger is relevant. Familial HLH was unlikely in the present case, as it almost invariably occurs in children, and adult-onset familial HLH is extremely rare in patients over 60 years old (3). No attributable active infection was observed in this case. Hematological malignancy is one of the common underlying conditions, with solid tumors only accounting for 3.1% of cases in a systematic review of malignancy-associated HLH. Even when a malignancy is underlying HLH development, it is not usually the trigger itself (4). In the present case, the rheumatoid arthritis flare-up episode may have been the HLH trigger. However, the root cause of HLH development in this case is thought to be due to the initiation of lung cancer treatment, as both methotrexate discontinuation and pembrolizumab administration preceded the arthritis flare-up and HLH development.
Whether to continue or discontinue immunosuppressive agents before and during ICI treatment in a patient with a pre-existing autoimmune disease is controversial. It has been reported that irAEs are more frequent in this group of patients and that a considerably large proportion of patients experience flare-ups of their pre-existing conditions during immunotherapy (5). IrAEs or flare-ups in patients with autoimmune diseases are rarely severe enough to require permanent immunotherapy discontinuation (6, 7). In the present case, methotrexate was stopped before pembrolizumab initiation for two reasons: good disease control without noticeable rheumatoid arthritis flare-ups had been observed, and methotrexate persistence would have put the patient at an increased risk of fatal pulmonary injury. He had multiple reported risk factors of methotrexate-induced lung disease, including an advanced age and pre-existing lung disease (8, 9). These risk factors seem to overlap with those of immune checkpoint inhibitor-related pneumonitis (10). We chose to discontinue methotrexate in an attempt to reduce the patient's risk of developing fatal pneumonitis, but methotrexate discontinuation may have led to the patient's ultimate condition. However, methotrexate discontinuation alone cannot entirely explain HLH development, since rheumatoid arthritis is an uncommon autoimmune disorder associated with HLH, and common triggers in patients with connective tissue diseases include infection and immunosuppressive agents, such as infliximab (11).
The present case seems to have involved an ICI-associated event. Although HLH association with advanced malignancies has been described, it is not yet acknowledged as a severe adverse event following ICI administration. To our knowledge, there have only been three previous reports on ICI-related HLH. Two of them involved melanoma and bladder cancer (12, 13). The other one referred to a lung cancer patient on pembrolizumab without rheumatologic conditions (14). In the literature, PD-L1 inhibitors have long been described in the context of their T-cell activating effects. Recent studies have also revealed their driving effects on macrophages (15), the excessive activation of which is an essential mechanism in HLH pathogenesis (16, 17). These clinical reports, in addition to our experience and basic science study results, support the theoretical induction of HLH by overly activated macrophages due to PD-L1 inhibitors. In the present case, based on this scientific body of knowledge and the fact that no other events were equally responsible, the HLH trigger can reasonably be attributed to pembrolizumab.
The HLH prognosis varies and partly depends on the patient background. Idiopathic HLH associated with congenital gene mutations has a poor prognosis (18), while HLH with an obvious modifiable underlying condition, such as rheumatic diseases, has a better prognosis. Regarding ICI efficacy in cases of lung cancer, studies in melanoma and non-small-cell lung cancer have reported that patients experiencing irAEs with ICIs have better survival outcomes than those who do not (19, 20). Despite its fulminant nature, ICI-triggered HLH can be reversible, and the patient may actually benefit from the unprecedented cancer treatment strategy of ICIs. Therefore, awareness of the condition and early treatment institution are important.
In conclusion, we herein report a case of pembrolizumab-induced HLH in a patient with rheumatoid arthritis. The patient survived and remains in complete cancer remission. While ICIs have shown significant efficacy, potentially fatal adverse events may follow. A high degree of suspicion and early intervention are important in order to ensure favorable patient outcomes.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors greatly thank Dr. Takeshi Suzuki of the Department of Rheumatology, Japanese Red Cross Medical Center, Tokyo, Japan, for his general support in the treatment.
References
- 1. Zhang K, Jordan MB, Marsh RA, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 375: 1749-1755, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 4: 1721-1728, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang K, Jordan MB, Marsh RA, et al. Hypomorphic mutations in PRF1, MUNC13-4, and STXBP2 are associated with adult-onset familial HLH. Blood 118: 5794-5798, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang H, Xiong L, Tang W, Zhou Y, Li F. A systematic review of malignancy-associated hemophagocytic lymphohistiocytosis that needs more attentions. Oncotarget 8: 59977-59985, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: A systematic review. Ann Intern Med 168: 121-130, 2018. [DOI] [PubMed] [Google Scholar]
- 6. Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2: 234-240, 2016. [DOI] [PubMed] [Google Scholar]
- 7. Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 28: 368-376, 2017. [DOI] [PubMed] [Google Scholar]
- 8. Alarcón GS, Kremer JM, Macaluso M, et al. Risk factors for methotrexate-induced lung injury in patients with rheumatoid arthritis. A multicenter, case-control study. Methotrexate-Lung Study Group. Ann Intern Med 127: 356-364, 1997. [DOI] [PubMed] [Google Scholar]
- 9. Golden MR, Katz RS, Balk RA, Golden HE. The relationship of preexisting lung disease to the development of methotrexate pneumonitis in patients with rheumatoid arthritis. J Rheumatol 22: 1043-1047, 1995. [PubMed] [Google Scholar]
- 10. Cho JY, Kim J, Lee JS, et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer 125: 150-156, 2018. [DOI] [PubMed] [Google Scholar]
- 11. Atteritano M, David A, Bagnato G, et al. Haemophagocytic syndrome in rheumatic patients. A systematic review. Eur Rev Med Pharmacol Sci 16: 1414-1424, 2012. [PubMed] [Google Scholar]
- 12. Hantel A, Gabster B, Cheng JX, Golomb H, Gajewski TF. Severe hemophagocytic lymphohistiocytosis in a melanoma patient treated with ipilimumab + nivolumab. J Immunother Cancer 6: 73, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shah D, Shrestha R, Ramlal R, Hatton J, Saeed H. Pembrolizumab associated hemophagocytic lymphohistiocytosis. Ann Oncol 28: 1403, 2017. [DOI] [PubMed] [Google Scholar]
- 14. Okawa S, Kayatani H, Fujiwara K, et al. Pembrolizumab-induced autoimmune hemolytic anemia and hemophagocytic lymphohistiocytosis in non-small cell lung cancer. Intern Med 58: 699-702, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gordon SR, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545: 495-499, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood 118: 4041-4052, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crayne CB, Albeituni S, Nichols KE, Cron RQ. The immunology of macrophage activation syndrome. Front Immunol 10: 119, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trottestam H, Horne A, Aricò M, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood 118: 4577-4584, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okada N, Kawazoe H, Takechi K, et al. Association between immune-related adverse events and clinical efficacy in patients with melanoma treated with nivolumab: a multicenter retrospective study. Clin Ther 41: 59-67, 2019. [DOI] [PubMed] [Google Scholar]
- 20. Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 4: 374-378, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]