Abstract
Secondary pulmonary alveolar proteinosis (sPAP) is a complication of myelodysplastic syndrome (MDS). A 60-year-old woman was diagnosed with MDS with excess blasts-1. Fifty-four months after the initial diagnosis, treatment with azacitidine was initiated. Seventy-three months after the diagnosis, a bone marrow examination revealed increased myeloblasts, at which time computed tomography showed diffuse ground-glass opacities and interlobular septal thickening in the bilateral lower lung fields. A lung biopsy revealed the presence of PAP; therefore, the clinical diagnosis of MDS/sPAP was confirmed. Careful attention should be paid to the development of sPAP in MDS patients with pulmonary lesions during azacitidine treatment.
Keywords: myelodysplastic syndromes, secondary pulmonary alveolar proteinosis, azacitidine, umbilical cord blood transplantation
Introduction
Pulmonary alveolar proteinosis (PAP) is a rare lung disease characterized by the abnormal accumulation of surfactant proteins and lipids within alveolar spaces, resulting in respiratory failure (1). PAP is classified into three subtypes: primary, congenital, and secondary. Primary PAP is an autoimmune disorder mediated by an antibody against granulocyte macrophage colony-stimulating factor (GM-CSF) (2). Germline mutations in the GATA2 gene have been reported to include a predisposition to myelodysplastic syndromes (MDS)/acute myeloid leukemia and PAP (3, 4). Secondary PAP (sPAP) is associated with underlying diseases, such as hematological neoplasms, cancers, infections, and the inhalation of silica or titanium, and the anti-GM-CSF antibody is not detected. MDS is the most frequent primary disease; 26 out of 40 sPAP cases (65.0%) were MDS patients (5). Although the cause of sPAP in MDS (MDS/sPAP) remains unclear, the abnormal numbers and functions of alveolar macrophages that originate from MDS cells are considered to result in impaired surfactant clearance (6, 7).
The emergence of sPAP is observed not only at the diagnosis of MDS but also under various clinical conditions (8-12). A previous study showed the development of sPAP at the time of disease progression after treatment with cytotoxic agents (10); however, limited information is currently available on the emergence of sPAP in MDS patients treated with azacitidine.
We herein report a case of MDS/sPAP that was diagnosed during treatment with azacitidine for high-risk MDS.
Case Report
A 60-year-old woman presented with leukocytopenia and thrombocytopenia. A peripheral blood count revealed a leukocyte count of 2.0×109/L (blasts 1%, segment neutrophils 26%, lymphocytes 50%, and monocytes 23%), hemoglobin level of 11.4 g/dL, and platelet count of 100×109/L. A bone marrow examination revealed significantly increased myeloblasts (9.0% of all nucleated cells) and the presence of morphological trilineage dysplasia.
A G-banding chromosomal analysis showed 47, XX, +9 on 10 out of 20 metaphases. The diagnosis of MDS was confirmed as MDS with excess blasts-1 based on the World Health Organization classification revised in 2016 (the international prognostic scoring system: intermediate-2, the revised international prognostic scoring system: intermediate, the World Health Organization classification-based prognostic scoring system: high) (13-16).
Forty-four months into a course observation after the initial diagnosis, the patient was diagnosed with disease progression to MDS with excess blasts-2. We performed target-capture sequencing to detect oncogenic variants in 367 known driver genes implicated in myeloid malignancies (17) and identified Y591N [variant allele frequencies (VAF), 9.9%] and R592Q (VAF, 7.5%) mutations in the MPL gene and a frameshift insertion (P344) (VAF, 2.7%) in the SH2B3 gene. Colonoscopy revealed the presence of a tumor in the transverse colon, and a biopsy confirmed colon cancer. High-resolution computed tomography (HRCT) showed no findings of metastatic lesions, including pulmonary opacities. Transverse colectomy with D3 lymph node dissection was performed, and the cancer was graded as pathological stage II (pT3N0M0) according to the TNM classification.
Due to the lack of a suitable human leukocyte antigen (HLA)-matched sibling or unrelated bone marrow donor from the Japan Marrow Donor Program, the patient started azacitidine treatment at a dose of 75 mg/m2 for 7 days at 54 months after the initial diagnosis and achieved marrow complete remission as the best response after 11 cycles of treatment.
Seventy-three months after the initial diagnosis (18 cycles of azacitidine treatment), a peripheral blood count showed a leukocyte count of 0.93×109/L (blasts 1%, segment neutrophils 9%, lymphocytes 78%, and monocytes 12%), hemoglobin level of 11.7 g/dL, and platelet count of 41×109/L (Table). A bone marrow examination revealed increased myeloblasts (11.6%) and the presence of morphological dysplasia with three lineages. A G-banding chromosomal analysis showed 46, XX on 20 out of 20 metaphases. MPL Y591N (VAF, 4.8%) and R592Q (VAF, 6.8%) as well as a SH2B3 P344 frameshift insertion (VAF, 6.9%) were persistently identified by targeted-capture sequencing. Although a GATA2 deficiency is well known to correlate with PAP (3, 4), mutations in the GATA2 gene were not identified. HRCT for the post-surgical surveillance of colon cancer revealed reticular patterns, ground glass opacities, and interlobular septal thickening in both sides of the lower lung field areas (Fig. 1); however, the patient did not have any symptoms or physical findings of respiratory disorders. Serum anti-GM-CSF antibody was not detected. The respiratory function test showed a slight reduction in the diffusing capacity of the lung for carbon monoxide (68.9%) but normal values for both the forced expiratory volume % in 1 second (117.0%) and % vital capacity (114.4%). The bronchoalveolar lavage fluid had a milky macroscopic appearance (Fig. 2A) and reduced ratios of CD4+/CD8+ T lymphocytes (0.4). The pathological findings of a transbronchial lung biopsy were consistent with PAP (Fig. 2B-D). Based on these results, the diagnosis was confirmed as MDS/sPAP following disease progression of MDS during azacitidine treatment.
Table.
Laboratory Data at the Time of the Diagnosis of Myelodysplastic Syndromes/secondary Pulmonary Alveolar Proteinosis.
| RBC | 351 | ×104/μL | Total bilirubin | 1.0 | mg/dL |
| Hemoglobin | 11.7 | g/dL | AST | 30 | U/L |
| MCV | 99.4 | Fl | ALT | 22 | U/L |
| Hematocrit | 34.9 | % | ALP | 438 | U/L |
| Reticulocytes | 7.69 | ×104/μL | γ-GTP | 37 | U/L |
| WBC | 0.93 | ×109/L | LDH | 355 | U/L |
| Blast | 1 | % | Total protein | 7.0 | g/dL |
| Segment | 9 | % | BUN | 9.3 | mg/dL |
| Lymphocyte | 78 | % | Creatinine | 0.51 | mg/dL |
| Monocyte | 12 | % | KL-6 | 4,213 | U/L |
| Platelets | 41 | ×109/L | SP-D | 21.3 | ng/mL |
RBC: red blood cell, MCV: mean corpuscular volume, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, γ-GTP: γ-glutamyltransferase, LDH: lactate dehydrogenase, BUN: blood urea nitrogen, KL-6: Krebs von der Lungen Nr.6, SP-D: surfactant protein-D
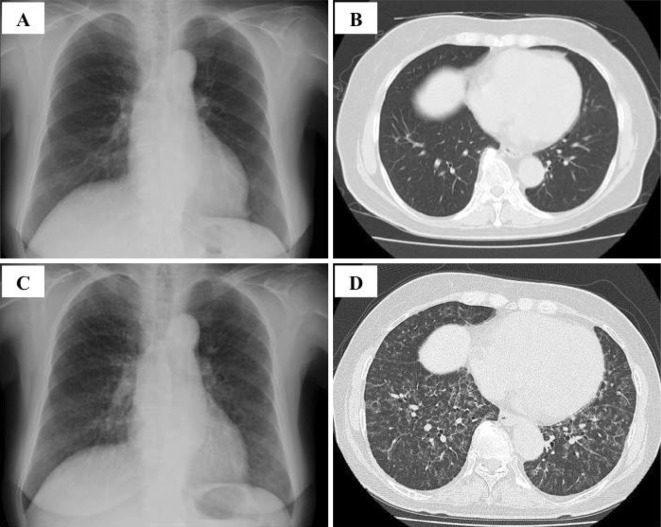
Figure 1.
Chest X-ray and high-resolution computed tomography (HRCT) findings of pulmonary alveolar proteinosis. Scans obtained 57 months after the diagnosis of myelodysplastic syndrome (chest X-ray photograph: A, HRCT image: B) and 73 months after the initial diagnosis (chest X-ray photograph: C, HRCT image: D). Abnormal findings were not initially detected (A, B); however, chest X-ray showed ground glass opacities around the bilateral lower lung fields (C), and HRCT revealed bilateral interlobular septal thickening (D) when the patient was diagnosed with myelodysplastic syndrome progression after azacitidine.
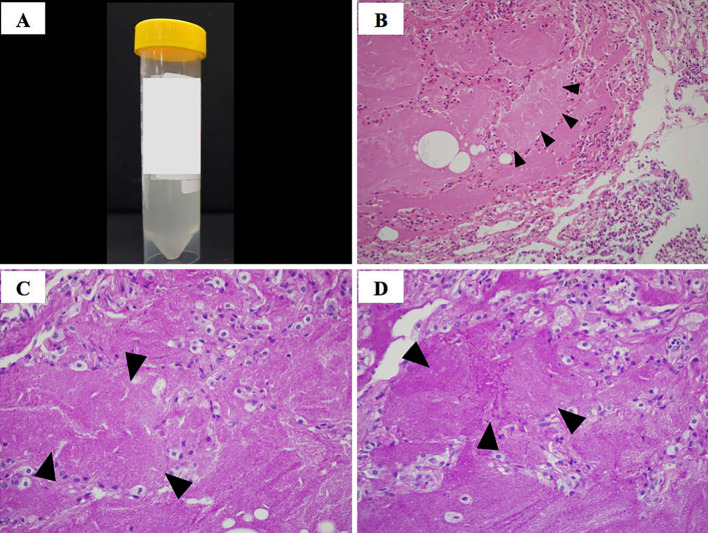
Figure 2.
Appearance of bronchoalveolar lavage fluid and pathological features of the lung obtained by bronchoscopy. The bronchoalveolar lavage fluid had a slightly cloudy, milky appearance (A). Hematoxylin and Eosin staining, 20× (B), periodic acid-Schiff stain, 40× (C), and periodic acid-Schiff stain with predigestion with diastase, 40× (D). Transbronchial lung biopsy specimens showed alveolar spaces filled with eosinophilic materials (B; closed triangles) and granular eosinophilic materials in the alveoli, which were periodic acid-Schiff stain-positive (C; closed triangles). Biopsy specimens also revealed diastase-resistant materials in alveolar spaces (D; closed triangles).
The patient received umbilical cord blood transplantation [total nucleated cell dose, 2.73×107 cells/kg; CD34-positive cell dose, 1.05×105 cells/kg; HLA 2 loci serologically mismatched (HLA-A and HLA-B loci were serologically mismatched), male donor] using reduced-intensity pre-transplant conditioning (fludarabine 30 mg/m2/day for 5 days, melphalan 40 mg/m2/day for 2 days, and total body irradiation 4 Gy, 2 fractions). The patient was considered to be in the high-risk group for post-transplant outcomes according to the hematopoietic cell transplantation-specific comorbidity index due to moderate pulmonary comorbidity and a history of solid tumor (total, 5 score) (18).
The patient achieved neutrophil engraftment with complete donor chimerism on day 19 after transplantation. However, the patient ultimately died of idiopathic pneumonia syndrome on day 30; the bronchoalveolar lavage fluid showed no evidence of infection or sPAP progression. The clinical course is summarized in Fig. 3.
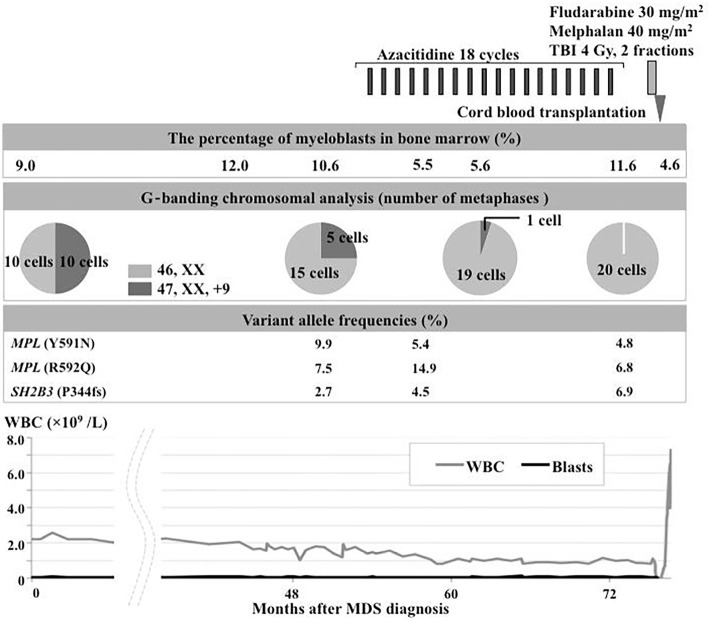
Figure 3.
The clinical course from the diagnosis of MDS to umbilical cord blood transplantation for MDS/sPAP. MDS: myelodysplastic syndrome, MDS/sPAP: myelodysplastic syndrome with secondary pulmonary alveolar proteinosis, TBI: total body irradiation, WBC: white blood cell
Discussion
The diagnosis of MDS/sPAP in this patient was based on the proposed criteria (19): (i) histological findings from a specimen obtained by a transbronchial lung biopsy, (ii) typical findings of HRCT, and (iii) a negative result for serum anti-GM-CSF antibody. To our knowledge, this is the first case report on the development sPAP following disease progression of MDS during azacitidine treatment. The present results provide novel insight into the clinical management and pathogenesis of MDS/sPAP.
The most important result was that the development of sPAP was observed when MDS progressed after marrow complete remission following azacitidine treatment. Azacitidine has been increasingly used as an important therapeutic option for higher-risk MDS because it has a demonstrated survival benefit over conventional care (20). In most MDS cases that achieve any response to the azacitidine treatment, disease relapse, such as an increase in blasts and/or the progression of cytopenia, is observed within two years of the initial response (20, 21). Although the emergence of sPAP may be relatively infrequent, MDS patients who fail to respond to azacitidine need to be carefully managed in order to detect the development of sPAP for better management and the proper selection of therapeutic strategies.
Allogeneic hematopoietic stem cell transplantation has been reported to provide durable remission for patients with MDS/sPAP (8, 9); however, the present case died of idiopathic pneumonia syndrome in the early phase after umbilical cord blood transplantation. The results of the present case suggest that the respiratory dysfunction associated with sPAP negatively affects post-transplant outcomes, although whether or not the persistence of abnormal alveolar macrophages relating to sPAP is a risk factor for idiopathic pneumonia syndrome has not been fully clarified (22, 23). The indication of allogeneic hematopoietic stem cell transplantation for MDS/sPAP should be carefully considered based on prognostic scoring systems, such as the hematopoietic cell transplantation-specific comorbidity index (18, 24, 25). Future studies should investigate the impact of persistent abnormal alveolar macrophages on the post-transplant outcome, including the development of idiopathic pneumonia syndrome.
In terms of the clinical course of genetic and cytogenetic abnormalities during azacitidine treatment, the present case showed 3 significant points: (i) an increased proportion of the normal karyotype from 75% to 100%, (ii) a slightly increased VAF of the SH2B3 P344 frameshift insertion, and (iii) stable VAFs of the MPL Y591N and R592Q mutations before and after azacitidine treatment. Although these gene alterations have been reported in patients with myeloproliferative neoplasm (26-28), the clinical significance of these gene alterations in MDS, particularly in cases with a low VAF at both the diagnosis and the onset of sPAP, remains unclear. However, these mutations might contribute to the abnormal function of alveolar macrophages, which may be related to the development of sPAP. In addition, it is important to note that our analyses did not detect the emergence of acquired genetic and/or cytogenetic abnormalities when sPAP was identified. Based on the findings of a previous study describing changes in the clonal composition under azacitidine (29-31), the disease progression of our case may also have been due to clonal changes. Further studies are warranted in order to clarify the pathogenesis of the development of MDS/sPAP.
In conclusion, clinicians need to perform a screening test for sPAP in MDS patients who fail to respond to azacitidine. Further experimental and clinical investigations are necessary to elucidate the pathogenesis of MDS/sPAP and develop novel therapeutic approaches.
This genetic analysis was approved by the Ethics Committees of Nagasaki University Hospital and Sasebo City General Hospital. Informed consent was obtained from the patient according to the Declaration of Helsinki.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was partially supported by a Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in Aid for Young Scientists (B) (#17K16189). This work was supported by the Japan Society for the Promotion of Science KAKENHI [22134006, 26221308, and 15H05909 (S.O.)].
References
- 1. Rosen SH, Castleman B, Liebow AA, Enzinger FM, Hunt RTN. Pulmonary alveolar proteinosis. N Engl J Med 258: 1123-1142, 1958. [DOI] [PubMed] [Google Scholar]
- 2. Kitamura T, Tanaka N, Watanabe J, et al. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med 190: 875-880, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spinner MA, Sanchez LA, Hsu AP, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood 123: 809-821, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Griese M, Zarbock R, Costabel U, et al. GATA2 deficiency in children and adults with severe pulmonary alveolar proteinosis and hematologic disorders. BMC Pulm Med 15: 87, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ishii H, Tazawa R, Kaneko C, et al. Clinical features of secondary pulmonary alveolar proteinosis: pre-mortem cases in Japan. Eur Respir J 37: 465-468, 2011. [DOI] [PubMed] [Google Scholar]
- 6. Cordonnier C, Fleury-Feith J, Escudier E, Atassi K, Bernaudin JF. Secondary alveolar proteinosis is a reversible cause of respiratory failure in leukemic patients. Am J Respir Crit Care Med 149: 788-794, 1994. [DOI] [PubMed] [Google Scholar]
- 7. Johkoh T, Itoh H, Müller NL, et al. Crazy-paving appearance at thin-section CT: spectrum of disease and pathologic findings. Radiology 211: 155-160, 1999. [DOI] [PubMed] [Google Scholar]
- 8. Tabata S, Shimoji S, Murase K, et al. Successful allogeneic bone marrow transplantation for myelodysplastic syndrome complicated by severe pulmonary alveolar proteinosis. Int J Hematol 90: 407-412, 2009. [DOI] [PubMed] [Google Scholar]
- 9. Fukuno K, Tomonari A, Tsukada N, et al. Successful cord blood transplantation for myelodysplastic syndrome resulting in resolution of pulmonary alveolar proteinosis. Bone Marrow Transplant 38: 581-582, 2006. [DOI] [PubMed] [Google Scholar]
- 10. Shoji N, Ito Y, Kimura Y, et al. Pulmonary alveolar proteinosis as a terminal complication in myelodysplastic syndromes: a report of four cases detected on autopsy. Leuk Res 26: 591-595, 2002. [DOI] [PubMed] [Google Scholar]
- 11. Liu Y, Chen LL, Qiu YY, Xiao YL, Cai HR. Clinical features of secondary pulmonary alveolar proteinosis associated with myelodysplastic syndrome: Two case reports. Medicine (Baltimore) 96: e8481, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohnishi T, Yamada G, Shijubo N, et al. Secondary pulmonary alveolar proteinosis associated with myelodysplastic syndrome. Intern Med 42: 187-190, 2003. [DOI] [PubMed] [Google Scholar]
- 13. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127: 2391-2405, 2016. [DOI] [PubMed] [Google Scholar]
- 14. Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 89: 2079-2088, 1997. [PubMed] [Google Scholar]
- 15. Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol 25: 3503-3510, 2007. [DOI] [PubMed] [Google Scholar]
- 16. Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 120: 2454-2465, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fujioka M, Itonaga H, Kato T, et al. Persistent clonal cytogenetic abnormality with del(20q) from an initial diagnosis of acute promyelocytic leukemia. Int J Hematol. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 18. Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 106: 2912-2919, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ishii H, Seymour JF, Tazawa R, et al. Secondary pulmonary alveolar proteinosis complicating myelodysplastic syndrome results in worsening of prognosis: a retrospective cohort study in Japan. BMC Pulm Med 14: 37, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol 10: 223-232, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol 20: 2429-2440, 2002. [DOI] [PubMed] [Google Scholar]
- 22. Fukuda T, Hackman RC, Guthrie KA, et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventionalconditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood 102: 2777-2785, 2003. [DOI] [PubMed] [Google Scholar]
- 23. Pagliuca S, Michonneau D, Sicre de Fontbrune F, et al. Allogeneic reactivity-mediated endothelial cell complications after HSCT: a plea for consensual definitions. Blood Adv 3: 2424-2435, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Itonaga H, Ishiyama K, Aoki K, et al. Increased opportunity for prolonged survival after allogeneic hematopoietic stem cell transplantation in patients aged 60-69 years with myelodysplastic syndrome. Ann Hematol 98: 1367-1381, 2019. [DOI] [PubMed] [Google Scholar]
- 25. Della Porta MG, Malcovati L, Strupp C, et al. Risk stratification based on both disease status and extra-hematologic comorbidities in patients with myelodysplastic syndrome. Haematologica 96: 441-449, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sangkhae V, Saur SJ, Kaushansky A, Kaushansky K, Hitchcock IS. Phosphorylated c-Mpl tyrosine 591 regulates thrombopoietin-induced signaling. Exp Hematol 42: 477-486, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cabagnols X, Favale F, Pasquier F, et al. Presence of atypical thrombopoietin receptor (MPL) mutations in triple-negative essential thrombocythemia patients. Blood 127: 333-342, 2016. [DOI] [PubMed] [Google Scholar]
- 28. Maslah N, Cassinat B, Verger E, Kiladjian JJ, Velazquez L. The role of LNK/SH2B3 genetic alterations in myeloproliferative neoplasms and other hematological disorders. Leukemia 31: 1661-1670, 2017. [DOI] [PubMed] [Google Scholar]
- 29. da Silva-Coelho P, Kroeze LI, Yoshida K, et al. Clonal evolution in myelodysplastic syndromes. Nat Commun 8: 15099, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong YF, Micklem CN, Taguchi M, et al. Longitudinal analysis of DNA methylation in CD34+ hematopoietic progenitors in myelodysplastic syndrome. Stem Cells Transl Med 3: 1188-1198, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tobiasson M, Abdulkadir H, Lennartsson A, et al. Comprehensive mapping of the effects of azacitidine on DNA methylation, repressive/permissive histone marks and gene expression in primary cells from patients with MDS and MDS-related disease. Oncotarget 8: 28812-28825, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]