Abstract
The present study aimed to determine whether the increased fatigability in old adults during dynamic exercise is associated with age-related differences in skeletal muscle bioenergetics. Phosphorus nuclear magnetic resonance spectroscopy was used to quantify concentrations of high-energy phosphates and pH in the knee extensors of seven young (22.7 ± 1.2 years; six women) and eight old adults (76.4 ± 6.0 years; seven women). Muscle oxidative capacity was measured from the phosphocreatine (PCr) recovery kinetics following a 24 s maximal voluntary isometric contraction. The fatiguing exercise consisted of 120 maximal velocity contractions (one contraction per 2 s) against a load equivalent to 20% of the maximal voluntary isometric contraction. The PCr recovery kinetics did not differ between young and old adults (0.023 ± 0.007 s−1 vs. 0.019 ± 0.004 s−1, respectively). Fatigability (reductions in mechanical power) of the knee extensors was ~ 1.8-fold greater with age and was accompanied by a greater decrease in pH (young = 6.73 ± 0.09, old = 6.61 ± 0.04) and increases in concentrations of inorganic phosphate, [Pi], (young = 22.7 ± 4.8 mM, old = 32.3 ± 3.6 mM) and diprotonated phosphate, [H2PO4−], (young = 11.7 ± 3.6 mM, old = 18.6 ± 2.1 mM) at the end of the exercise in old compared to young adults. The age-related increase in power loss during the fatiguing exercise was strongly associated with intracellular pH (r = –0.837), [Pi] (r = 0.917) and [H2PO4−] (r = 0.930) at the end of the exercise. These data suggest that the age-related increase in fatigability during dynamic exercise has a bioenergetic basis and is explained by an increased accumulation of metabolites within the muscle.
Keywords: ageing, muscle fatigue, 31P-MRS, inorganic phosphate, acidosis, diprotonated phosphate, metabolism
Introduction
Human ageing is accompanied by multiple impairments in the neuromuscular system that lead to a loss of mobility and overall physical function. A portion of the loss in mobility and physical function is a result of the age-related decline in the ability to generate mechanical power(Bassey et al. 1992; Reid & Fielding, 2012) that occurs to a large extent from the age-related loss of muscle mass (Doherty, 2003;Visser et al. 2005),particularly the atrophy of muscle expressing the fast myosin heavy chain isoform (Lexell, 1995; Nilwik et al. 2013; Sundberg et al. 2018a). Another important factor for mobility and physical function is the fatigability of limb muscle (Foulis et al. 2017; Senefeld et al. 2017), which is characterized by an acute reduction in power that occurs in response to contractile activity (Debold et al. 2016; Hunter, 2018). This is particularly true for the old adult population because the age-related decline in the ability to generate power is exacerbated by the increased fatigability that occurs when old adults perform moderate- to high-velocity exercise (McNeil & Rice, 2007; Dalton et al. 2010; Callahan & Kent-Braun, 2011; Dalton et al. 2012; Senefeld et al. 2017; Sundberg et al. 2018b).
The mechanisms responsible for the increased fatigability with ageing are not well understood; however, several studies have localized the primary site for the increased fatigability during dynamic exercise to within the muscle rather than the nervous system (Baudry et al. 2007; Dalton et al. 2010, 2012; Sundberg et al. 2018a; Sundberg et al. 2018b). The leading mechanism purported to be responsible for fatigue within the muscle is the accumulation of metabolites, namely acidosis (H+), inorganic phosphate (Pi) and diprotonated phosphate (H2PO4−), which act to directly inhibit both cross-bridge function and excitation–contraction coupling (Nosek et al. 1987; Wilson et al. 1988; Fitts, 1994; Allen et al. 2008; Kent-Braun et al. 2012; Debold et al. 2016). Thus, it is plausible that age-related changes within the muscle result in the increased production of metabolites and/or an increased sensitivity of the muscle fibres to a given concentration of metabolite accumulation in old compared to young adults. Our recent findings on fibres isolated from muscle biopsies of the vastus lateralis revealed that the decrements in single fibre force, velocity and power elicited by a fatigue-mimicking condition (pH 6.2 + 30 mM Pi) did not differ in fibres from young compared to old men (Sundberg et al. 2018a). These data suggest that the age-related increase in fatigability during dynamic exercise is not likely attributed to an increased sensitivity of the cross-bridge to H+ and Pi, at least not under saturating Ca2+ conditions.
An alternative explanation for the age-related increase in fatigability is that old adults may have impaired skeletal muscle bioenergetics and/or a reduced ability to perfuse the working muscle compared to young adults. Indeed, studies employing phosphorus nuclear magnetic resonance spectroscopy(31P-MRS)revealed anage-related decrease in contractile economy (i.e. old adults require more ATP for a given amount of mechanical power output) when young and old adults perform a dynamic plantarflex or exercise (Layec et al. 2014, 2015b; Layec et al. 2018). Importantly, the age-related increase in the ATP cost of contraction was accompanied by a compensatory hyperemic response that maintained the tissue oxygenation to similar levels in the young and old adults, suggesting that the reduced contractile economy was not a result of impaired muscle perfusion (Layec et al. 2018). Additionally, there is evidence from studies on the knee extensor muscles that old adults have a reduced muscle oxidative capacity compared to young adults (Conley et al. 2000; Johannsen et al. 2012; Larsen et al. 2012), although this is not always observed (Smith et al. 1998; Layec et al. 2015a) particularly for muscle groups other than the knee extensors (Fitzgerald et al. 2016). Interpreted together, it is possible that the large energetic demand during concentric contractions (Ryschon et al. 1997; He et al. 2000; Barclay et al. 2010) coupled with both a reduced contractile economy and lower muscle oxidative capacity is responsible for the increased fatigability with ageing. This should result in an increased reliance on anaerobic metabolic pathways to synthesize ATP and would lead to a greater accumulation of metabolites (H+, Pi and H2PO4−) within the muscle of old compared to young adults. Thus, in the present study, we tested the hypotheses that (i) the age-related increase in power loss during a dynamic fatiguing knee extension exercise will have a bioenergetic basis that results in the increased accumulation of metabolites (H+, Pi and H2PO4−) within the working muscle and (ii) old adults will have a lower muscle oxidative capacity compared to young adults that will be associated with an increased reduction in mechanical power during the dynamic exercise.
Methods
Participants and ethical approval
Seven young(20–23years;sixwomen)and eight old adults (68–87 years; seven women) volunteered and provided their written informed consent to participate in the present study. Participants underwent a general health screening and were excluded from the study if they were taking medications that affect the central nervous system, muscle mass, metabolism or neuromuscular function (e.g. hormone-replacement therapies, anti-depressants, glucocorticoids). All participants were apparently healthy, community dwelling adults and free of any known neurological, musculoskeletal or cardiovascular diseases. All experimental procedures were approved by the Institutional Review Boards at Marquette University and the Medical College of Wisconsin and conformed with the principles of the Declaration of Helsinki.
Experimental protocol
Participants reported to the laboratory on three occasions: twice for familiarization and once for an experimental session to measure the bioenergetics and fatigability of the knee extensors in the magnetic resonance scanner bore. Data collection was restricted to primarily women because the greater strength commonly observed in young men (Sundberg et al. 2018b) resulted in technical difficulties that did not allow us to investigate this cohort in the scanner.
Familiarization sessions
Familiarization sessions were conducted to habituate each participant to performing brief 3–5 s maximal voluntary isometric contractions (MVCs) and maximal voluntary concentric contractions with the knee extensors as described previously (Sundberg et al. 2018a; Sundberg et al. 2018b).Testing was performed on the dominant leg of each participant (preferred kicking leg), except when the participant reported a previous surgical procedure, knee or leg pain, or osteoarthritis of the dominant leg. Familiarization sessions were performed in a Biodex System 4 Dynamometer (Biodex Medical, Shirley, NY, USA) with the participants seated upright in the high-Fowler’s position, where the torso was at 90° flexion with the thigh. The familiarization session also included an assessment of body composition with dual X-ray absorptiometry (Lunar iDXA; GE Heathcare, Madison, WI, USA). Anthropometric measurements for the participants are reported in Table 1.
Table 1.
Anthropometrics of the young and old participants
| Young (n= 7) | Old (n= 8) | P value | ||
|---|---|---|---|---|
| Age | (years) | 22.7 ± 1.2 | 76.4 ± 6.0 | <0.001 |
| Height | (cm) | 169.3 ± 7.9 | 162.7 ± 8.2 | 0.136 |
| Weight | (kg) | 66.0 ± 9.3 | 66.0 ± 11.0 | 0.993 |
| Body mass index | (kg m−2) | 22.9 ± 1.2 | 24.9 ± 3.3 | 0.154 |
| Body fat | (%) | 24.6 ± 6.1 | 34.3 ± 5.2 | 0.005 |
| Lean tissue mass | (kg) | 48.1 ± 7.9 | 41.3 ± 6.0 | 0.080 |
Body fat percentage and lean tissue mass were measured via dual X-ray absorptiometry (Lunar iDXA; GE Healthcare). A significant age difference at P< 0.05 is indicated in bold. Values are the mean ± SD.
Experimental set-up
The experimental session was conducted with participants lying prone in a 3.0 Tesla supraconducting magnet (Discovery MR750; GE Heathcare) operating at 51.7 MHz to measure 31P resonance. A custom 12.7 cm square transmit/receive surface coil tuned for the 31P isotope (Clinical MR Solutions, Brookfield, WI, USA) was placed over the midthigh and secured in position with two Velcro straps and wrapped with an elastic bandage. To maximize the 31P resonance signal-to-noise ratio, participants were positioned with the thigh and surface coil centred in the sagittal plane of the 60 cm bore of the magnet. Once centred, participants were secured to the scanner bed with Velcro straps placed over the hips and distal third of the thigh to constrain hip flexion/extension and minimize extraneous movements (Fig. 1). The participant’s leg was attached to a custom knee extension ergometer via a 5.1 cm wide low-compliance polyester Velcro strap proximal to the malleoli.
Figure 1. Schematic of the custom ergometer to measure the mechanical outputs of the knee extensors within the 3.0 Tesla scanner bore.
Custom ergometer designed and fabricated to accurately measure the mechanical performance of the knee extensors while simultaneously acquiring 31P-MRS to quantify skeletal muscle bioenergetics (A). The ergometer measured force production via an S-type load cell and displacements and velocities via a string potentiometer. Pneumatic dashpots minimized the eccentric portion of the movement cycle and provided a passive return of the limb to the starting position after each contraction. Representative mechanical signals from the beginning and end of the dynamic fatiguing exercise are depicted for a 76-year-old man (B). Also shown are the changes in intracellular pH and [Pi] from the same 76-year-old man performing the 4 min dynamic knee extension exercise (C).
Custom knee extension ergometer
A custom ergometer was designed and fabricated to safely and accurately measure mechanical performances of the knee extensors while simultaneously acquiring 31P-MRS to quantify bioenergetics during both isometric and dynamic knee extension exercises in the 3.0 Tesla scanner bore. The ergometer was fabricated from a variety of non-magnetic and mildly-magnetic materials, including polyvinyl chloride polymer (PVC), brass, aluminum, stainless steel and wood. To secure the ergometer in position on the outside of the scanner bore, two 1.22 m long triangular wood braces were fastened to the ergometer frame which were attached directly to the scanner via two aluminum L brackets.
To measure force output of the knee extensors, the low-compliance polyester Velcro strap attached to the participant’s ankle was secured directly to an S-type load cell (SSM-AJ-2000N; Interface Inc., Scottsdale, AZ, USA) that was adjoined to an 8.9 cm diameter PVC cylinder. The PVC cylinder was sealed on one end and loaded with multiple 0.25–1 kg bags filled with lead pellets to provide the resistance during the dynamic knee extension exercise. To ensure linear displacement of the load during the dynamic contractions, the PVC cylinder was housed within a larger PVC cylinder (diameter 11.4 cm) that was secured to the ergometer frame and equipped with a 32cm long channel system. Linear displacements of the load were measured via a string potentiometer(CelescoSP1–50; Intertechnology Inc., Toronto, ON, Canada) mounted in parallel with the PVC cylinder. A 1.5 cm hole was drilled through the bottom of both PVC cylinders to ‘lock’ the cylinders together for the isometric contractions via a 20 cm long aluminum pin.
To eliminate the eccentric portion of the dynamic knee extension movement cycle, the smaller PVC cylinder was attached to the ergometer frame via two customized, non-magnetic pneumatic dashpots (part # 122162–1; Airpot Corp., Norwalk, CT, USA). The one-way dashpots provided nominal resistance during the concentric portion of the knee extension and after the contraction provided passive return of the limb to the initial position via pull damping. The amount of pull damping was adjusted via two turn-knob vented check valves so that the load was returned to the initial position within ~0.5–1 s.
Force and position signals were calibrated with multiple seven-point calibrations that spanned the range of forces (0–490 N) and displacements (0–30 cm) achieved by the participants. The relationship between the forces applied to the load cell and the resulting resistance changes in the strain gauge circuit were linear across the entire range of applied forces (r2 > 0.99). Similarly, the relationship between the displacements and the resulting voltage divider outputs from the potentiometer were linear across the full range of displacements (r2 = 1.00).
Experimental session and 31P spectral acquisition parameters
The experimental session began with a series of T1-weighted proton localizing images to verify proper placement of the 31P surface coil on the thigh and to identify the region of interest for subsequent high-order shimming (Gruetter, 1993). Magnetic field homogeneity was optimized by high-order shimming of the proton signal from tissue water and 31P resonance was optimized by adjusting the transmit gain to maximize the signal from the PCr resonance. Once optimized, a 4 min scan with long TR (repetition time) intervals (15s) was performed to obtain fully-relaxed spectra of the quiescent knee extensors (Taylor et al. 1983).
Following the 4 min scan, participants performed a minimum of three brief (3–5 s) knee extension MVCs in the prone position within the scanner bore. Participants were provided strong verbal encouragement and visual feedback on their performance with an 85 cm video projector screen mounted ~ 2 m directly in front of their line of vision. Each MVC was interspersed with at least 60 s rest, and MVC attempts were continued until the two highest values were within 5% of each other. The highest MVC in the prone position was recorded and used to calculate the 20% MVC load for the dynamic fatiguing exercise.
Once the MVC was obtained, participants performed a 24 s MVC followed by 10 min of recovery to estimate the muscle oxidative capacity of the knee extensors (Larsen et al. 2009, 2012). Participants were provided strong verbal encouragement to generate their maximal effort throughout the entire 24 s MVC and to fully relax immediately at the end of the contraction. Following the assessment of muscle oxidative capacity, participants were habituated to performing maximal velocity knee extensions against a 20% MVC load for the dynamic fatiguing exercise. This approach was employed because it more closely mimics common daily activities that require moving an object with constant mass but at different velocities.The 31P spectra for the muscle oxidative capacity and fatiguing exercise experiments were both obtained with a TR of 2 s and averaging four repetitions per spectrum to achieve a time resolution of 8 s (Fig. 2). The scan parameters for both the short (2 s) and long (15 s) TR intervals were similar: radiofrequency hard pulse duration = 500 μs, number of excitations = 4, nominal flip angle = 90°, receiver bandwidth = 5 kHz, data points = 2048.
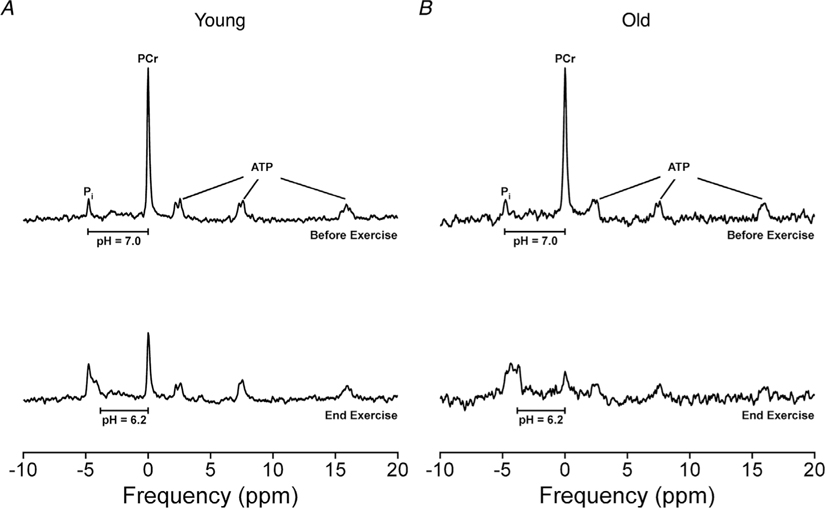
Figure 2. Representative 31P spectra from before and at the end of the fatiguing dynamic exercise.
31P spectra of the knee extensors from before and at the end of the 4 min fatiguing dynamic exercise from a 23-year-old (A) and a 71-year-old woman (B). Spectra were obtained with a TR of 2 s and averaging of four repetitions per spectrum to achieve a time resolution of 8 s. Horizonal bars represent a chemical shift (σ) between the PCr and Pi peaks that correspond to a pH of 7.0 and 6.2. The intracellular milieu from the final 64 s (average of eight spectra) of the fatiguing exercise was pH = 6.79, [Pi] = 22.7 mM and [H2PO4−] = 10.8 mM for the 23-year-old woman and pH=6.57, [Pi]=30.8 mM and [H2PO4−]=18.6 mM for the 71-year-old woman.
Dynamic fatiguing exercise
For the dynamic fatiguing exercise, participants were verbally cued to kick once every 2 s for a total of 4 min (120 contractions). Because of the restricted range of motion that could be attained within the scanner bore (~20 cm linear displacement and 30° angular displacement), the frequency of contractions was increased from the 0.33 Hz used previously (Sundberg et al. 2018a; Sundberg et al. 2018b) to 0.50 Hz in the present study. Participants were exhorted throughout the entire 4 min exercise to generate their maximal effort and to achieve a full range of motion during every contraction. To encourage participants to achieve their maximal volitional shortening velocity, a compliant foam pad was placed on the scanner bed, and participants were instructed to kick as fast as possible and to hit the foam pad as hard as possible with the dorsal surface of their foot. Upon completion of each contraction, the participant was instructed to relax, and the limb was passively returned to the starting position by the ergometer. This approach resulted in an average duty cycle (i.e. the ratio of the duration of muscle force application to the entire duration between contractions) (Sundberg & Bundle, 2015) of 17 ± 3% and 27 ± 5% in the young and old adults, respectively. The higher duty cycle in the old compared to young adults (P <0.001; ) was a result of the longer contractile durations (young = 0.33 ± 0.05 s, old = 0.57 ± 0.12 s; P < 0.001; ) from the slower velocities and not a result of differences in the duration between the start of each contraction (young = 2.01 ± 0.01 s, old = 2.07 ± 0.11 s; P = 0.166; ).The contractile phase of the knee extension was synchronized with the radiofrequency pulse of the scanner to ensure that the leg was in a similar position when the spectral signal was collected during each contraction.
Measurements and data analysis
Mechanical power output
Force and position signals from the S-type load cell and string potentiometer were digitized at 1000 Hz with a Power 1401 A/D converter, stored online with Spike 2 software (Cambridge Electronics Design, Cambridge, UK) and analysed with IGOR Pro, version 8 (WaveMetrics Inc., Oswego, OR, USA). For the dynamic fatiguing exercise, contraction-by-contraction mechanical power outputs (W) were quantified as the product of the applied forces (N) and the first derivative of the linear displacements (m s−1) and averaged over the entire shortening phase of the knee extension cycle. Because power output increased over the first few contractions in some participants as observed previously (Sundberg et al.2018a; Sundberg et al. 2018b), the baseline power output for each participant was the highest average obtained from 10 sequential contractions within the first 20 contractions. To quantify the relative reductions in power for each participant (i.e. fatigability), the average power output from the last 10 contractions was expressed as a percentage of the individual-specific baseline power output.
31P spectral analysis and metabolite calculations
Frequency induction decays (FIDs)from all 31P scans were first apodized with an exponential 5 Hz function. FIDs were then Fourier transformed, and manual zero- and first-order phasing was applied to the resulting spectra. The areas of the peaks corresponding to phosphocreatine (PCr), inorganic phosphate (Pi), phosphomonoesters and the α-, β- and γ-ATP moieties were measured using Lorentzian-shaped curve fitting algorithms (SAGE 7 Dev2007.1; GE Medical Systems). Millimolar (mM) concentrations of the phosphorus metabolites were calculated assuming a resting [ATP] of 8.2 mM (Harris et al. 1974; Taylor et al. 1986) and after correcting for signal saturation using the measured 15 s to 2 s TR ratios of PCr = 1.90 ± 0.13, Pi = 1.83 ± 0.30 and γ-ATP = 1.57 ± 0.11 (Taylor et al. 1983). Intracellular pH was calculated in accordance with Eqn (1):
where σ is the chemical shift in parts per million (ppm) between the PCr and Pi peaks (Hoult et al. 1974). In instances where two Pi peaks were observed (Fig. 2), the total intracellular pH was calculated as a weighted average in accordance with Eqn (2):
The concentration of diprotonated phosphate, [H2PO4−], was calculated in accordance with Eqn (3):
where 6.75 is the pKa of H2PO4− at 38°C and an ionic strength of 0.2 M (Lawson & Veech, 1979; Wilson et al. 1988).
Baseline values for the phosphate moieties and pH were obtained from the average of eight spectra (64 s) when the participant was at rest. To test whether old adults had a greater accumulation of metabolites during the fatiguing dynamic exercise, the pH and metabolite concentrations were recorded from the average of the last eight spectra obtained during the exercise. This conservative approach was taken to help ensure that the end exercise values were an accurate representation of the intracellular milieu and not spuriously high or low values from movement artefact or difficulties fitting the broad Pi peak with a Lorentzian-shaped curve.
Muscle oxidative capacity
Skeletal muscle oxidative capacity was determined by measuring the PCr recovery kinetics following the 24 s isometric MVC. Previous studies on both young (Larsen et al. 2009) and old adults (Larsen et al. 2012) have shown that this protocol depletes [PCr] to ~ 50–60% of resting levels while eliciting minimal changes in muscle pH and [ATP]. Under these conditions, the postcontraction recovery of [PCr] follows a monoexponential time course with the kinetics determined by the ATP resynthesis rates from oxidative phosphorylation (Meyer, 1988; McCully et al. 1993; Paganini et al. 1997; Lanza et al. 2011; Layec et al. 2016). The resynthesis of [PCr] from the end of the isometric contraction through 10 min of recovery was fit for each individual with a residual minimizing, iterative procedure in accordance with Eqn (4):
where PCr (t) is the [PCr] at any time t, PCriso is the [PCr] at the end of the 24 s isometric MVC, PCrrest is the resting [PCr] at the end of the 10 min recovery period, e is the base of the natural logarithm and kPCr is the exponential time constant describing the recovery rate of the [PCr] following the contraction. For ease of comparison with prior literature, the kinetics of PCr recovery are also presented as the inverse time constant, tau (τPCr), which is equal to 1/kPCr (s). The theoretical maximal rate that ATP can be synthesized via oxidative phosphorylation was estimated in accordance with Eqn (5):
where Vmax is the maximal rate of ATP synthesis via oxidative phosphorylation (mM ATP s−1), kPCr is the exponential time constant describing the recovery kinetics of the [PCr] following the 24 s isometric MVC (s−1) and PCrrest is the [PCr] in quiescent muscle (mM) (Meyer, 1988).
Statistical analysis
Mean differences for all variables were compared between the young and old adults with an unpaired independent samples t test. Assumptions for the homogeneity of variance were tested with the Levene’s test before any statistical comparisons. If the assumption of homogeneity of variance was violated, then the non-parametric Kruskal–Wallis test was performed. Simple linear regressions were performed to test for associations between the reductions in mechanical power (fatigability) and the accumulation of metabolites (pH, [Pi], [H2PO4−]) and muscle oxidative capacity (kPCr). All statistical analyses were conducted with and without the two men included. We elected to include the men in the final data set because (i) none of the outcomes were affected by their inclusion and (ii) the mechanisms for the age-related increase in fatigability are similar for both old men and women (Sundberg et al. 2018b). The a priori level of significance for all statistical comparisons was P < 0.05 and all statistics were performed using SPSS, version 25 (IBM Corp., Armonk, NY, USA). Data are presented as the mean ± SD in the text and tables and as the mean ± SE in the figures.
Results
Mechanical performance and fatigability
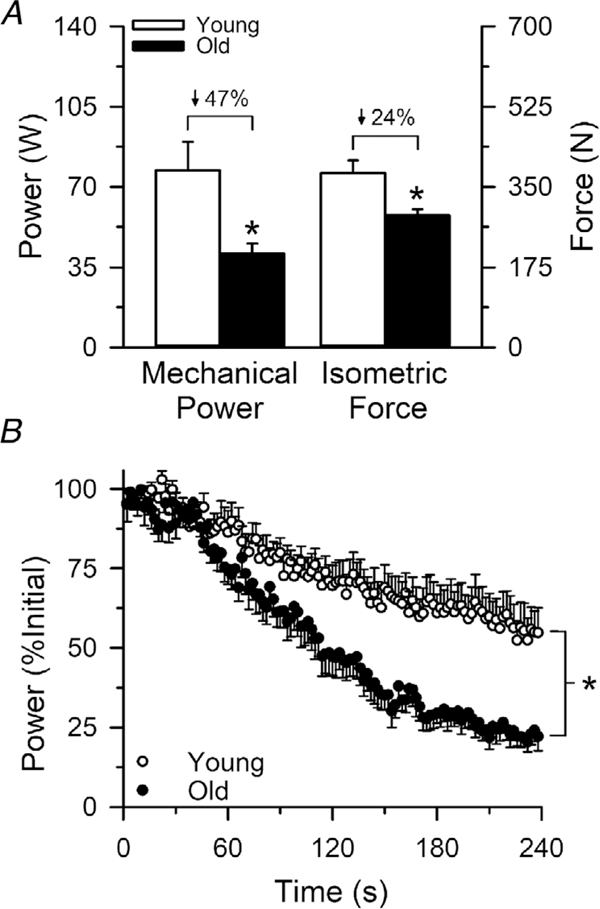
Mechanical performances from the beginning and end of the 4 min dynamic fatiguing exercise are reported in Table 2. The MVC isometric force output of the knee extensors was 24% lower in the old (288.4 ± 35.2 N) compared to the young adults (380.3 ± 73.3 N; P = 0.028; ), whereas the mechanical power outputs from the beginning of the dynamic exercise were 47% lower in the old compared to the young (P = 0.0015; ). Fatigability (reductions in mechanical power) during the 4 min dynamic knee extension exercise was ~1.8 fold greater in the old compared to young adults (P = 0.002; ), with an average relative reduction in power of 8% in the old and 42 ± 18% in the young (Fig. 3).
Table 2.
Mechanical outputs at the beginning and end of the fatiguing dynamic exercise
| Young (n= 7) | Old (n= 8) | P value | ||
|---|---|---|---|---|
| Power | ||||
| Beginning | (W) | 77.2 ± 30.6 | 41.0 ± 12.1 | 0.015 |
| End | (W) | 42.6 ± 20.4 | 9.7 ± 5.2 | 0.001 |
| Force | ||||
| Beginning | (N) | 127.9 ± 31.3 | 89.6 ± 17.0 | 0.028 |
| End | (N) | 99.0 ± 22.3 | 64.6 ± 10.6 | 0.002 |
| Velocity | ||||
| Beginning | (m s−1) | 0.60 ± 0.16 | 0.45 ± 0.08 | 0.040 |
| End | (m s−1) | 0.41 ± 0.12 | 0.15 ± 0.06 | <0.001 |
| Displacement | ||||
| Beginning | (cm) | 21.1 ± 4.7 | 17.9 ± 2.7 | 0.130 |
| End | (cm) | 17.5 ± 3.6 | 11.1 ± 2.1 | 0.001 |
Power, force and velocity are the average values calculated over the entire shortening phase of the knee extension movement cycle. Because power output increased over the first few contractions in some participants, the recorded baseline mechanical outputs for each participant are the highest average obtained from 10 sequential contractions within the first 20 contractions at the beginning of the dynamic exercise. The reported mechanical outputs from the end of the fatiguing dynamic exercise are the average values from the last 10 contractions. A significant age difference at P < 0.05 is indicated in bold. Values are the mean ± SD.
Figure 3. Force, power and fatigability (reductions in power) of the knee extensors in young and old adults.
The mean absolute isometric force and mechanical power output of the knee extensors were 24% and 47% lower, respectively, in the old compared to the young adults (A). Fatigability of the knee extensors was ~1.8-fold greater in the old compared to the young adults, with an average relative reduction in power of 77% in the old and 42% in the young adults (B). ∗Significantly different from young (P< 0.05). Values are the mean ± SE.
Muscle oxidative capacity
The intracellular pH and [PCr], [Pi] and [H2PO4−] of the knee extensors both at rest and at the end of the 24 s isometric MVC did not differ between the young and old adults (Table 3). Similarly, the recovery kinetics of the [PCr] (kPCr) following the 24 s isometric MVC did not differ between the young (0.023 ± 0.007 s−1) and old adults (0.019 ± 0.004 s−1; P =0.250; ), which corresponded with a time constant, τPCr, of 48.5 ± 17.6 s in the young and 54.6 ± 10.8 s in the old adults (P = 0.424; ). Accordingly, the estimated maximal rates of oxidative phosphorylation (Vmax) did not differ between the young (0.61 ± 0.17 mM ATP s−1) and old adults (0.59 ± 0.18 mM ATP s−1; P =0.863; ). Irrespective of age, there was a negative association between kPCr and the relative reductions in mechanical power during the fatiguing exercise (r=–0.619; P=0.014) (Fig. 4).
Table 3.
Metabolite levels at rest and at the end of the 24 s isometric MVC
| Young (n= 7) | Old (n= 8) | P value | ||
|---|---|---|---|---|
| Rest | ||||
| [PCr] | (mM) | 27.4 ± 3.2 | 31.6 ± 7.4 | 0.186 |
| [Pi ] | (mM) | 3.7 ± 1.2 | 4.4 ± 0.8 | 0.196 |
| pH | 6.98 ± 0.04 | 6.99 ± 0.03 | 0.832 | |
| [H2PO4- ] | (mM) | 1.4 ± 0.4 | 1.6 ± 0.3 | 0.184 |
| End contraction | ||||
| [PCr] | (mM) | 14.5 ± 1.7 | 18.1 ± 5.1 | 0.106 |
| [PCr] | (%Rest) | 52.2 ± 6.8 | 56.6 ± 3.7 | 0.136 |
| [Pi ] | (mM) | 17.5 ± 2.8 | 20.7 ± 4.0 | 0.106 |
| pH | 7.00 ± 0.07 | 7.00 ± 0.05 | 0.995 | |
| [H2PO4- ] | (mM) | 6.3 ± 1.4 | 7.6 ± 1.6 | 0.143 |
Millimolar (mM) concentrations of the phosphorus metabolites were calculated assuming a resting [ATP] of 8.2 mM and after correcting for partial signal saturation from using a TR of 2 s. Resting metabolite levels were obtained from the final 64 s (average of eight spectra) of the 10 min recovery period following the 24 s isometric MVC. Values are the mean ± SD.
Figure 4. PCr recovery kinetics to determine muscle oxidative capacity of the knee extensors in vivo.
Mean PCr recovery kinetics (% resting [PCr]) following a 24 s MVC performed with the knee extensors of young and old adults (A). The exponential time constant (kPCr) describing the recovery rate of the [PCr] provides an estimate of the muscle oxidative capacity, which did not differ between the young and old adults. Grey horizontal lines represent the mean kPCr for the young and old adults (B). Irrespective of age, the reductions in power during the dynamic fatiguing exercise were inversely associated with the muscle oxidative capacity (C). Values are the mean ± SE.
Metabolite accumulation during the fatiguing exercise
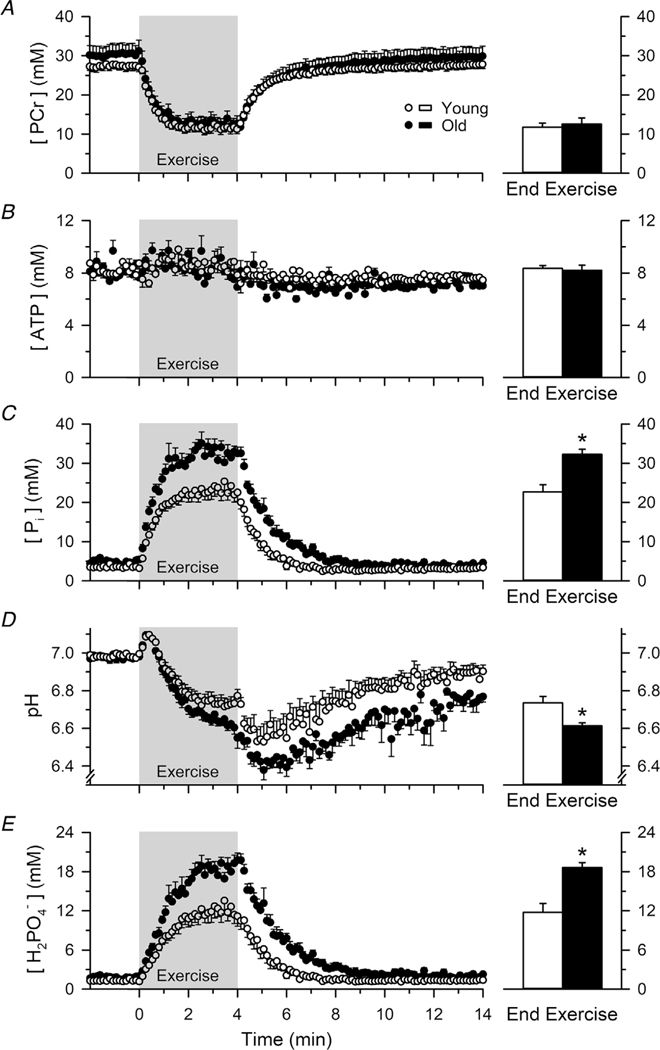
The intracellular [PCr], [ATP], [Pi], pH and [H2PO4−] before, during and in recovery from the fatiguing dynamic exercise are presented for both the young and old adults in Fig. 5. The [PCr] over the final 64 s of the fatiguing dynamic exercise (average of eight spectra) was depleted to similar levels in the young (11.8 ± 2.7 mM) and old adults (12.6 ± 4.4 mM; P = 0.684; ), whereas the [ATP] remained stable in both age cohorts (young = 8.3 ± 0.6 mM, old = 8.2 ± 2.1 mM; P = 0.751; ). By contrast, the [Pi] over the final 64 s of the fatiguing dynamic exercise was ~42% higher in the old (32.3 ± 3.6 mM) compared to the young adults (22.7 ± 4.8 mM; P =0.001; ). Similarly, the pH over the final 64 s of the fatiguing exercise was 0.12 pH units more acidic in the old (6.61 ± 0.04) compared to the young adults (6.73 ± 0.09; P = 0.005; ), which corresponded to a [H+] that was ~30% higher in the old (244.4 ± 24.4 nM) compared to the young adults (187.9 ± 39.8 nM; P = 0.005; ). The greater increase in [Pi] and decrease in pH resulted in an ~59% greater increase in the [H2PO4−] in the old (18.6 ± 2.1 mM) compared to the young adults (11.7 ± 3.6 mM; P < 0.001; ).
Figure 5. Mean profiles of [PCr], [ATP], [Pi], pH and [H2PO4−] before, during and in recovery from the dynamic fatiguing exercise.
The [PCr] over the final 64 s of the fatiguing dynamic exercise (average of eight spectra) was depleted to similar levels in the young and old adults (A), whereas the [ATP] remained stable in both age cohorts (B). By contrast, the [Pi] over the final 64 s of the dynamic fatiguing exercise was ~42% higher in the old compared to the young adults (C). Similarly, the pH at the end of the exercise was 0.12 pH units more acidic in the old compared to the young adults (D). The greater increase in [Pi] and decrease in pH resulted in an ~59% greater increase in the [H2PO4−] in the old compared to the young adults (E). The grey shaded region signifies the data during the 4 min dynamic fatiguing exercise. ∗Significantly different from young (P < 0.05). Values are the mean ± SE.
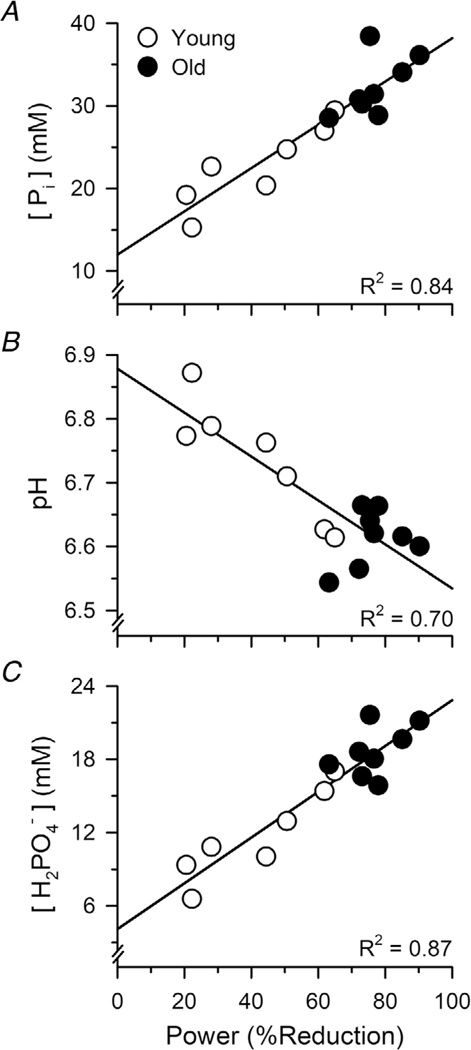
Linear regression analyses revealed that the accumulation of metabolites at the end of the fatiguing exercise was strongly associated with the relative reductions in mechanical power output (i.e. fatigability): [Pi] (r = 0.917; P < 0.001), pH (r = −0.837; P < 0.001) and [H2PO4−] (r = 0.930; P < 0.001) (Fig. 6).
Figure 6. Associations between the accumulation of metabolites and fatigability (reductions in power) during dynamic knee extensions.
Regression analyses revealed that the percent reductions in mechanical power during the fatiguing exercise were closely associated with the [Pi] (A), pH (B) and [H2PO4−] (C) measured over the final 64 s of the exercise.
Discussion
The present study aimed to determine whether the increased fatigability in old adults performing a dynamic knee extensions exercise was the result of age-related differences in skeletal muscle bioenergetics. We provide novel evidence that the age-related increase in power loss during dynamic exercise is accompanied by, and strongly associated with, a greater accumulation of metabolites (Pi, H+ and H2PO4−) within the working muscle (Figs 5 and 6). However, the greater accumulation of metabolites and the concomitant increase in fatigability could not be explained by differences in muscle oxidative capacity between the young and old adults (Fig. 4). These data suggest that the age-related increase in fatigability during dynamic exercise has a bioenergetic basis and is explained by an increased accumulation of metabolites within the muscle.
Is a reduced muscle oxidative capacity an obligatory consequence of ageing?
In contrast to our hypothesis, we found no evidence for a lower muscle oxidative capacity in the knee extensors of the old compared to the young adults (Fig. 4). Specifically, there was no age-related difference in the PCr recovery kinetics following the 24 s MVC (kPCr) or the estimated maximal rates of ATP synthesis via oxidative phosphorylation (Vmax) between the young and old adults. These data are in agreement with several studies on the knee extensors (Smith et al. 1998; Layec et al. 2015a) but in contrast to others that have reported a lower muscle oxidative capacity in old compared to young adults (Conley et al. 2000; Johannsen et al. 2012; Larsen et al. 2012). The explanation for the disparities between studies is unknown but prompts an intriguing biological question as to whether a reduced muscle oxidative capacity is an obligatory consequence of ageing.
Comparing our findings with others gives insight into this important question and provides evidence that a reduced muscle oxidative capacity is not likely an obligatory consequence of ageing. For example, the present study, along with others that used a similar protocol on the knee extensors (Larsen et al. 2009, 2012), observed a large heterogeneity in muscle oxidative capacity between both young and old individuals and considerable overlap in the muscle oxidative capacity values between the age groups. This large intersubject variability of the knee extensors was also observed when an involuntary, electrical stimulation protocol was employed to elicit the contractions (Conley et al. 2000), suggesting that the variability in muscle oxidative capacity is not likely attributed to differences in the ability of individuals to volitionally activate their muscle. The large variability between individuals makes it difficult to detect a difference between young and old adults and may explain the disparate findings between studies regarding the impact of ageing on the muscle oxidative capacity of the knee extensors. In addition, a recent meta-analysis of 22 studies using PCr recovery kinetics to estimate muscle oxidative capacity revealed that the muscle oxidative capacity is actually higher or does not differ in old compared to young adults for muscles of the upper extremity and the ankle plantarflexor and dorsiflexor muscles (Fitzgerald et al. 2016). The disparate findings between muscle groups and across studies on the knee extensors suggests that other factors, such as the patterns of use and disuse of the muscle (Kitahara et al. 2003; Larsen et al. 2009; Homma et al. 2015), may play a more prominent role in altering muscle oxidative capacity rather than ageing per se (Kent & Fitzgerald, 2016).
Age-related increases in fatigability are explained by an increased accumulation of metabolites
Despite the lack of difference in muscle oxidative capacity between the young and old adults, we observed an age-related increase in fatigability during the dynamic knee extension exercise that was accompanied by a greater accumulation of metabolites (H+, Pi and H2PO4−) within the muscle (Fig. 5). The observation that the reductions in power duringthe fatiguing exercisewere closely associated with the extent of intracellular metabolite accumulation suggests that these metabolites play a central role for the increased fatigability in old adults (Fig. 6). The accumulation of metabolites is known to alter muscle force and power production either indirectly via afferent feedback to the central nervous system (Bigland-Ritchie et al. 1986; Gandevia, 2001; Amann et al. 2011) or directly by disrupting excitation–contraction coupling and cross-bridge function (Fitts, 1994; Allen et al. 2008; Debold et al. 2016). However, investigations employing non-invasive stimulations to the nervous system indicated that the age-related increase in fatigability of the knee extensors during dynamic exercise is not a result of suboptimal neural drive or an inability of the action potential to propagate from the neuromuscular junction across the sarcolemma (Dalton et al. 2012; Sundberg et al. 2018a; Sundberg et al. 2018b). By contrast, the age-related increase in power loss during a dynamic exercise that was similar to the one used in the present study was closely associated with the reductions in the amplitude and rate of torque development of the electrically-evoked twitch in both men and women (Sundberg et al. 2018a; Sundberg et al. 2018b).These findings in conjunction with the results in the present study (Figs 5 and 6) suggest that the age-related increase in power loss during dynamic fatiguing exercise is the result of an increased accumulation of metabolites (H+, Pi and H2PO4−) that act to directly inhibit contractile function within the muscle.
Although the role of H+ in skeletal muscle fatigue remains a topic of intense debate (Fitts, 2016; Westerblad, 2016), there is evidence from both human (Sundberg et al. 2018a) and animal studies (Metzger & Moss, 1987, 1990; Debold et al. 2004; Debold et al. 2006; Knuth et al. 2006; Karatzaferi et al. 2008; Nelson & Fitts, 2014; Nelson et al. 2014) that elevated levels of H+ and Pi inhibit force and power production through their effects on the cross-bridge and Ca2+ release from the sarcoplasmic reticulum (Allen et al. 2011), but do so through different mechanisms (Debold et al. 2016). One central argument against H+ as an important mediator of muscle fatigue (Westerblad, 2016) is that the intracellular acidosis of skeletal muscle rarely reaches levels below 6.5 to 6.4 (Taylor et al. 1986; Wilson et al. 1988; Cady et al. 1989). A cursory look at the data in the present study supports this assertion (Fig. 5); however, the pH values are based on a weighted spatial average (Eq. 2) from a muscle made up of a heterogeneous mixture of fibre types. It was recently proposed that this approach likely underestimates the pH of individual fibres, particularly fibres expressing the fast (IIa and IIx) myosin heavy chain isoforms (Debold et al. 2016). Based on closer examination of the chemical shift between the PCr and Pi peaks in the present study, we observed regions of the muscle in the old participants that reached a pH of 6.2 or below during the fatiguing exercise (Fig. 2), and the pH continued to decrease for ~1–2 min after the exercise in both young and old adults (Fig. 5). These data provide evidence that the findings from prior cellular (Metzger & Moss, 1987, 1990; Debold et al. 2004; Debold et al. 2006; Knuth et al. 2006; Karatzaferi et al. 2008; Nelson & Fitts, 2014; Nelson et al. 2014; Sundberg et al. 2018a) and molecular studies (Debold et al. 2008; Debold et al. 2012; Debold et al. 2013; Longyear et al. 2014; Jarvis et al. 2018; Woodward & Debold, 2018) that used a fatigue-mimicking environment of pH 6.2–6.5 and ~20–30 mM Pi are similar to the metabolic environment that can be achieved volitionally in vivo. Interpreted together, the greater accumulation of H+ and Pi in old compared to young adults (Fig. 5) likely caused, at least in part, the age-related increase in power loss during the fatiguing exercise through both the combined effects of H+ and Pi on the cross-bridge (Karatzaferi et al. 2008; Nelson & Fitts, 2014; Nelson et al. 2014; Sundberg et al. 2018a) and the effects of Pi on Ca2+ release from the sarcoplasmic reticulum (Allen et al. 2011; Allen & Trajanovska, 2012).
One of the primary questions that remains is what causes the greater accumulation of metabolites within the muscle of old compared to young adults during dynamic fatiguing exercise? Clearly, the old adults relied more on anaerobic metabolic pathways to synthesize the ATP necessary to perform the exercise compared to the young (Fig. 5). However, the lack of difference in the PCr recovery kinetics between the young and old adults (Fig. 4), which provides an index of in vivo mitochondrial capacity (Lanza et al. 2011; Layec et al. 2016), suggests that the increased accumulation of intracellular metabolites and the concomitant increase in fatigability was not are sult of age-related impairments in mitochondrial function. In support of this finding, the age-related increase in power loss during dynamic exercise has also been observed in the ankle dorsiflexors (Baudry et al. 2007; McNeil & Rice, 2007) and plantarflexors (Dalton et al. 2010), as well as the elbow flexors (Senefeld et al. 2017), which are all muscle groups with similar or greater muscle oxidative capacity in old compared to young adults (Fitzgerald et al. 2016; Kent & Fitzgerald, 2016). By contrast, there is evidence from the ankle plantarflexors that old adults have an increased ATP cost of contraction during dynamic exercise compared to young adults, which is independent of the ability of the cardiovascular system to perfuse the working muscle (Layec et al. 2014, 2015b; Layec et al. 2018). Our observation that the old adults showed a disproportionate increase in [Pi] relative to the decrease in [PCr] (Fig. 5) may provide further support for the hypothesis of a greater ATP turnover during dynamic exercise with age. However, surprisingly, we did not observe a greater age-related depletion in the[ATP]during the exercise suggesting that the old adults may have relied to a greater extent on the adenylate kinase reaction to maintain a stable [ATP]. Although the explanation remains unclear, the disproportionate increase in [Pi] relative to the decrease in [PCr] was also observed when the ATP cost of contraction was increased in young men by attenuating group III//IV afferent feedback via lumbar intrathecal fentanyl administration (Broxterman et al. 2017; Broxterman et al. 2018). Whether the reduced contractile economy with ageing is a result of inefficiencies in neural activation of the muscle, chemomechanical coupling of the myosin-actin interaction (myofibrillar ATPase) and/or ion transport (Ca2+ and/or Na+-K+ ATPases) is unknown and worth further investigation.
Conclusions
The present study provides novel evidence that the age-related increase in fatigability during a dynamic knee extension exercise is associated with a greater accumulation of H+ and Pi within the working muscle of old compared to young adults. Importantly, the lack of a difference in the PCr recovery kinetics between the young and old adults suggests that the increased accumulation of intracellular metabolites (Pi, H+ and H2PO4−) was not likely the result of age-related impairments in mitochondrial function. We conclude that the increased fatigability of old adults during dynamic exercise is the result of age-related impairments in skeletal muscle bioenergetics and is explained by an increased accumulation of H+ and Pi within the muscle.
Key points.
The mechanisms for the age-related increase in fatigability during dynamic exercise remain elusive.
We tested whether age-related impairments in muscle oxidative capacity would result in a greater accumulation of fatigue causing metabolites, inorganic phosphate (Pi), hydrogen (H+) and diprotonated phosphate (H2PO4−), in the muscle of old compared to young adults during a dynamic knee extension exercise.
The age-related increase in fatigability (reduction in mechanical power) of the knee extensors was closely associated with a greater accumulation of metabolites within the working muscle but could not be explained by age-related differences in muscle oxidative capacity.
These data suggest that the increased fatigability in old adults during dynamic exercise is primarily determined by age-related impairments in skeletal muscle bioenergetics that result in a greater accumulation of metabolites.
Acknowledgements
We thank Mary Cleary and Jose Delgadillo for their assistance with data acquisition during the experimental sessions; Dr Andrew Nencka for assistance with developing the data acquisition parameters in the preliminary phase of the study; and Jeff Rainwater for the illustration of the experimental set-up in Figure 1. We also thank the research participants for their willingness to provide the rigorous efforts necessary to make this study possible.
Funding
This work was supported by a National Institute of Aging Ruth L. Kirschstein predoctoral fellowship (AG052313) and an American Heart Association postdoctoral fellowship (19POST34380411) to Christopher Sundberg, as well as a National Institute of Aging R01 (AG048262) to Robert Fitts and Sandra Hunter.
Biography

Christopher Sundberg is a postdoctoral research associate in the Department of Biological Sciences at Marquette University, Milwaukee, Wisconsin, USA, working under the tutelage of Professors Robert Fitts and Sandra Hunter. His research interests are to identify the aetiologies of muscle fatigue and the physiological processes that limit human performance in health, disease and ageing. His current studies aim to develop a comprehensive understanding of the mechanisms responsible for the age-related loss in muscle power and increased fatigability by integrating techniques to study these phenomena in both the intact neuromuscular system and the isolated muscle cell.
Footnotes
Competing interests
The authors declare that they have no competing interests.
References
- Allen DG, Clugston E, Petersen Y, Roder IV, Chapman B & Rudolf R (2011). Interactions between intracellular calcium and phosphate in intact mouse muscle during fatigue. J Appl Physiol (1985) 111, 358–366. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD & Westerblad H (2008). Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88, 287–332. [DOI] [PubMed] [Google Scholar]
- Allen DG & Trajanovska S (2012). The multiple roles of phosphate in muscle fatigue. Front Physiol 3, 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA (2011). Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol 589, 5299–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay CJ, Woledge RC & Curtin NA (2010). Is the efficiency of mammalian (mouse) skeletal muscle temperature dependent? J Physiol 588, 3819–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassey EJ, Fiatarone MA, O’Neill EF, Kelly M, Evans WJ & Lipsitz LA (1992). Leg extensor power and functional performance in very old men and women. Clin Sci (Lond) 82, 321–327. [DOI] [PubMed] [Google Scholar]
- Baudry S, Klass M, Pasquet B & Duchateau J (2007). Age-related fatigability of the ankle dorsiflexor muscles during concentric and eccentric contractions. Eur J Appl Physiol 100, 515–525. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie BR, Dawson NJ, Johansson RS & Lippold OCJ (1986). Reflex origin for the slowing of motoneuron firing rates in fatigue of human voluntary contractions. J Physiol 379, 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxterman RM, Hureau TJ, Layec G, Morgan DE, Bledsoe AD, Jessop JE, Amann M & Richardson RS (2018). Influence of group III/IV muscle afferents on small muscle mass exercise performance: a bioenergetics perspective. J Physiol 596, 2301–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxterman RM, Layec G, Hureau TJ, Morgan DE, Bledsoe AD, Jessop JE, Amann M & Richardson RS (2017). Bioenergetics and ATP synthesis during exercise: role of group III/IV muscle afferents. Med Sci Sports Exerc 49, 2404–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady EB, Jones DA, Lynn J & Newham DJ (1989). Changes in force and intracellular metabolites during fatigue of human skeletal muscle. J Physiol 418, 311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan DM & Kent-Braun JA (2011). Effect of old age on human skeletal muscle force-velocity and fatigue properties. J Appl Physiol (1985) 111, 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley KE, Jubrias SA & Esselman PC (2000). Oxidative capacity and ageing in human muscle. J Physiol 526 (Pt 1), 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton BH, Power GA, Vandervoort AA & Rice CL (2010). Power loss is greater in old men than young men during fast plantar flexion contractions. J Appl Physiol (1985) 109, 1441–1447. [DOI] [PubMed] [Google Scholar]
- Dalton BH, Power GA, Vandervoort AA & Rice CL (2012). The age-related slowing of voluntary shortening velocity exacerbates power loss during repeated fast knee extensions. Exp Gerontol 47, 85–92. [DOI] [PubMed] [Google Scholar]
- Debold EP, Dave H & Fitts RH (2004). Fiber type and temperature dependence of inorganic phosphate: implications for fatigue. Am J Physiol Cell Physiol 287, C673–C681. [DOI] [PubMed] [Google Scholar]
- Debold EP, Romatowski J & Fitts RH (2006). The depressive effect of Pi on the force-pCa relationship in skinned single muscle fibers is temperature dependent. Am J Physiol Cell Physiol 290, C1041–C1050. [DOI] [PubMed] [Google Scholar]
- Debold EP, Beck SE & Warshaw DM (2008). Effect of low pH on single skeletal muscle myosin mechanics and kinetics. Am J Physiol Cell Physiol 295, C173–C179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debold EP, Fitts RH, Sundberg CW & Nosek TM (2016). Muscle fatigue from the perspective of a single crossbridge. Med Sci Sports Exerc 48, 2270–2280. [DOI] [PubMed] [Google Scholar]
- Debold EP, Longyear TJ & Turner MA (2012). The effects of phosphate and acidosis on regulated thin-filament velocity in an in vitro motility assay. J Appl Physiol (1985) 113, 1413–1422. [DOI] [PubMed] [Google Scholar]
- Debold EP, Walcott S, Woodward M & Turner MA (2013). Direct observation of phosphate inhibiting the force-generating capacity of a miniensemble of myosin molecules. Biophys J 105, 2374–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty TJ (2003). Invited review: aging and sarcopenia. J Appl Physiol (1985) 95, 1717–1727. [DOI] [PubMed] [Google Scholar]
- Fitts RH (1994). Cellular mechanisms of muscle fatigue. Physiol Rev 74, 49–94. [DOI] [PubMed] [Google Scholar]
- Fitts RH (2016). The role of acidosis in fatigue: pro perspective. Med Sci Sports Exerc 48, 2335–2338. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LF, Christie AD & Kent JA (2016). Heterogeneous effects of old age on human muscle oxidative capacity in vivo: a systematic review and meta-analysis. Appl Physiol Nutr Metab 41, 1137–1145. [DOI] [PubMed] [Google Scholar]
- Foulis SA, Jones SL, van Emmerik RE & Kent JA (2017). Post-fatigue recovery of power, postural control and physical function in older women. PLoS ONE 12, e0183483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC (2001). Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81, 1725–1789. [DOI] [PubMed] [Google Scholar]
- Gruetter R (1993). Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med 29, 804–811. [DOI] [PubMed] [Google Scholar]
- Harris RC, Hultman E & Nordesjo LO (1974). Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest 33, 109–120. [PubMed] [Google Scholar]
- He ZH, Bottinelli R, Pellegrino MA, Ferenczi MA & Reggiani C (2000). ATP consumption and efficiency of human single muscle fibers with different myosin isoform composition. Biophys J 79, 945–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma T, Hamaoka T, Osada T, Murase N, Kime R, Kurosawa Y, Ichimura S, Esaki K, Nakamura F & Katsumura T (2015). Once-weekly muscle endurance and strength training prevents deterioration of muscle oxidative function and attenuates the degree of strength decline during 3-week forearm immobilization. Eur J Appl Physiol 115, 555–563. [DOI] [PubMed] [Google Scholar]
- Hoult DI, Busby SJ, Gadian DG, Radda GK, Richards RE & Seeley PJ (1974). Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature 252, 285–287. [DOI] [PubMed] [Google Scholar]
- Hunter SK (2018). Performance fatigability: mechanisms and task specificity. Cold Spring Harb Perspect Med 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis K, Woodward M, Debold EP & Walcott S (2018). Acidosis affects muscle contraction by slowing the rates myosin attaches to and detaches from actin. J Muscle Res Cell Motil 39, 135–147. [DOI] [PubMed] [Google Scholar]
- Johannsen DL, Conley KE, Bajpeyi S, Punyanitya M, Gallagher D, Zhang Z, Covington J, Smith SR & Ravussin E (2012). Ectopic lipid accumulation and reduced glucose tolerance in elderly adults are accompanied by altered skeletal muscle mitochondrial activity. J Clin Endocrinol Metab 97, 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatzaferi C, Franks-Skiba K & Cooke R (2008). Inhibition of shortening velocity of skinned skeletal muscle fibers in conditions that mimic fatigue. Am J Physiol Regul Integr Comp Physiol 294, R948–R955. [DOI] [PubMed] [Google Scholar]
- Kent JA & Fitzgerald LF (2016). In vivo mitochondrial function in aging skeletal muscle: capacity, flux, and patterns of use. J Appl Physiol (1985) 121, 996–1003. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Fitts RH & Christie A. (2012). Skeletal muscle fatigue. Compr Physiol 2, 997–1044. [DOI] [PubMed] [Google Scholar]
- Kitahara A, Hamaoka T, Murase N, Homma T, Kurosawa Y, Ueda C, Nagasawa T, Ichimura S, Motobe M, Yashiro K, Nakano S & Katsumura T (2003). Deterioration of muscle function after 21-day forearm immobilization. Med Sci Sports Exerc 35, 1697–1702. [DOI] [PubMed] [Google Scholar]
- Knuth ST, Dave H, Peters JR & Fitts RH (2006). Low cell pH depresses peak power in rat skeletal muscle fibres at both 30°C and 15°C: implications for muscle fatigue. J Physiol 575, 887–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Bhagra S, Nair KS & Port JD (2011). Measurement of human skeletal muscle oxidative capacity by 31P-MR spectroscopy: a cross-validation with in vitro measurements. J Magn Reson Imaging 34, 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RG, Callahan DM, Foulis SA & Kent-Braun JA (2009). In vivo oxidative capacity varies with muscle and training status in young adults. J Appl Physiol (1985) 107, 873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RG, Callahan DM, Foulis SA & Kent-Braun JA. (2012). Age-related changes in oxidative capacity differ between locomotory muscles and are associated with physical activity behavior. Appl Physiol Nutr Metab 37, 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson JW & Veech RL (1979). Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem 254, 6528–6537. [PubMed] [Google Scholar]
- Layec G, Gifford JR, Trinity JD, Hart CR, Garten RS, Park SY, Le Fur Y, Jeong EK & Richardson RS (2016). Accuracy and precision of quantitative 31P-MRS measurements of human skeletal muscle mitochondrial function. Am J Physiol Endocrinol Metab 311, E358–E366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layec G, Hart CR, Trinity JD, Le Fur Y, Jeong EK & Richardson RS (2015a). Skeletal muscle work efficiency with age: the role of non-contractile processes. Clin Sci (Lond) 128, 213–223. [DOI] [PubMed] [Google Scholar]
- Layec G, Trinity JD, Hart CR, Kim SE, Groot HJ, Le Fur Y, Sorensen JR, Jeong EK & Richardson RS (2014). In vivo evidence of an age-related increase in ATP cost of contraction in the plantar flexor muscles. Clin Sci (Lond) 126, 581–592. [DOI] [PubMed] [Google Scholar]
- Layec G, Trinity JD, Hart CR, Kim SE, Groot HJ, Le Fur Y, Sorensen JR, Jeong EK & Richardson RS (2015b). Impact of age on exercise-induced ATP supply during supramaximal plantar flexion in humans. Am J Physiol Regul Integr Comp Physiol 309, R378–R388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layec G, Trinity JD, Hart CR, Le Fur Y, Zhao J, Reese V, Jeong EK & Richardson RS (2018). Impaired muscle efficiency but preserved peripheral hemodynamics and mitochondrial function with advancing age: evidence from exercise in the young, old, and oldest-old. J Gerontol A Biol Sci Med Sci 73, 1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexell J (1995). Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50, 11–16. [DOI] [PubMed] [Google Scholar]
- Longyear TJ, Turner MA, Davis JP, Lopez J, Biesiadecki B & Debold EP (2014). Ca++-sensitizing mutations in troponin, Pi, and 2-deoxyATP alter the depressive effect of acidosis on regulated thin-filament velocity. J Appl Physiol (1985) 116, 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully KK, Fielding RA, Evans WJ, Leigh JS Jr. & Posner JD (1993). Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol (1985) 75, 813–819. [DOI] [PubMed] [Google Scholar]
- McNeil CJ & Rice CL (2007). Fatigability is increased with age during velocity-dependent contractions of the dorsiflexors. J Gerontol A Biol Sci Med Sci 62, 624–629. [DOI] [PubMed] [Google Scholar]
- Metzger JM & Moss RL (1987). Greater hydrogen ion-induced depression of tension and velocity in skinned single fibres of rat fast than slow muscles. J Physiol 393, 727–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RA (1988). A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol Cell Physiol 254, C548–C553. [DOI] [PubMed] [Google Scholar]
- Nelson CR, Debold EP & Fitts RH (2014). Phosphate and acidosis act synergistically to depress peak power in rat muscle fibers. Am J Physiol Cell Physiol 307, C939–C950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CR & Fitts RH (2014). Effects of low cell pH and elevated inorganic phosphate on the pCa-force relationship in single muscle fibers at near-physiological temperatures. Am J Physiol Cell Physiol 306, C670–C678. [DOI] [PubMed] [Google Scholar]
- Nilwik R, Snijders T, Leenders M, Groen BB, van Kranenburg J, Verdijk LB & van Loon LJ (2013). The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol 48, 492–498. [DOI] [PubMed] [Google Scholar]
- Nosek TM, Fender KY & Godt RE (1987). It is diprotonated inorganic phosphate that depresses force in skinned skeletal muscle fibers. Science 236, 191–193. [DOI] [PubMed] [Google Scholar]
- Paganini AT, Foley JM & Meyer RA (1997). Linear dependence of muscle phosphocreatine kinetics on oxidative capacity. Am J Physiol Cell Physiol 272, C501–C510. [DOI] [PubMed] [Google Scholar]
- Reid KF & Fielding RA (2012). Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev 40, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryschon TW, Fowler MD, Wysong RE, Anthony A & Balaban RS (1997). Efficiency of human skeletal muscle in vivo: comparison of isometric, concentric, and eccentric muscle action. J Appl Physiol (1985) 83, 867–874. [DOI] [PubMed] [Google Scholar]
- Senefeld J, Yoon T & Hunter SK (2017). Age differences in dynamic fatigability and variability of arm and leg muscles: associations with physical function. Exp Gerontol 87, 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Montain SJ, Matott RP, Zientara GP, Jolesz FA & Fielding RA (1998). Creatine supplementation and age influence muscle metabolism during exercise. J Appl Physiol (1985) 85, 1349–1356. [DOI] [PubMed] [Google Scholar]
- Sundberg CW & Bundle MW (2015). Influence of duty cycle on the time course of muscle fatigue and the onset of neuromuscular compensation during exhaustive dynamic isolated limb exercise. Am J Physiol Regul Integr Comp Physiol 309, R51–R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg CW, Hunter SK, Trappe SW, Smith CS & Fitts RH (2018a). Effects of elevated H+ and Pi on the contractile mechanics of skeletal muscle fibres from young and old men: implications for muscle fatigue in humans. J Physiol 596, 3993–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg CW, Kuplic A, Hassanlouei H & Hunter SK (2018b). Mechanisms for the age-related increase in fatigability of the knee extensors in old and very old adults. J Appl Physiol (1985) 125, 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DJ, Bore PJ, Styles P, Gadian DG & Radda GK (1983). Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Med 1, 77–94. [PubMed] [Google Scholar]
- Taylor DJ, Styles P, Matthews PM, Arnold DA, Gadian DG, Bore P & Radda GK (1986). Energetics of human muscle: exercise-induced ATP depletion. Magn Reson Med 3, 44–54. [DOI] [PubMed] [Google Scholar]
- Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM & Harris TB (2005). Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 60, 324–333. [DOI] [PubMed] [Google Scholar]
- Westerblad H (2016). Acidosis is not a significant cause of skeletal muscle fatigue. Med Sci Sports Exerc 48, 2339–2342. [DOI] [PubMed] [Google Scholar]
- Wilson JR, McCully KK, Mancini DM, Boden B & Chance B (1988). Relationship of muscular fatigue to pH and diprotonated Pi in humans: a 31P-NMR study. J Appl Physiol (1985) 64, 2333–2339. [DOI] [PubMed] [Google Scholar]
- Woodward M & Debold EP (2018). Acidosis and phosphate directly reduce myosin’s force-generating capacity through distinct molecular mechanisms. Front Physiol 9, 862. [DOI] [PMC free article] [PubMed] [Google Scholar]