Abstract
Objective:
To examine the impact of secondary ADHD (SADHD) on long-term global and executive functioning in adolescents after traumatic brain injury (TBI).
Setting:
Three tertiary cared children’s hospitals and one general hospital.
Participants:
120 children (54 TBI; 66 orthopedic injury [OI]) without pre-injury ADHD assessed ~6.8 years post injury.
Design:
Cross-sectional data analysis from a prospective, longitudinal study.
Main Measures:
Outcomes included functional impairment (Child and Adolescent Functional Assessment Scale [CAFAS]) and executive functioning (Behavior Rating Inventory of Executive Functioning [BRIEF]).
Results:
SADHD moderated the association of injury type with the BRIEF-Behavioral Regulation Index (F(1,113) = 4.42, p = .04) and CAFAS (F(1,112) = 8.95, p = .003).TBI was only associated with poorer outcomes in the context of SADHD. SADHD was also associated with poorer outcomes on the BRIEF-Global Executive Composite (BRIEF-GEC; F(1,113) = 52.92, p <.0001) and BRIEF-Metacognitive Index scores (F(1,113) = 48.64, p<.0001) across groups. Adolescents with TBI had greater BRIEF-GEC scores than those with OI (F(1,113) = 5.00, p = .03).
Conclusions:
Although SADHD was associated with poorer functioning across groups, its adverse effects on behavioral regulation and overall functioning were amplified following TBI. TBI + SADHD may confer elevated risk for significant impairments in early adolescence.
Traumatic brain injury (TBI) remains a major cause of mortality and morbidity in children and adolescents.1 In the United States, more than one million children, adolescents, and young adults evaluated in emergency departments for TBI annually.2 While a TBI occurs suddenly, ongoing residual deficits may persist for months, years, or longer after injury. An estimated 165,000 children and adolescents are currently living with residual problems following childhood TBI.3
Cognitive and behavioral problems are common after pediatric TBI; there is anincreased risk for the development of psychiatric disorders.4,5 The rate of new onset psychiatric disorders following childhood TBI is 49% compared to 13% in children with orthopedic injury (OI).6 Psychiatric disorders associated with TBI in children and adolescents include attention disorders, anxiety, depression, and other mood disorders.6–11 The most common psychiatric disorder in children following TBI is Attention-Deficit/Hyperactivity Disorder (ADHD).5,12
ADHD is characterized by developmentally inappropriate hyperactivity, impulsivity, and inattention across multiple settings.9,13,14 The development of ADHD symptoms after an injury, such as TBI, is referred to as secondary ADHD (SADHD).15 The prevalence of ADHD in children with history of TBI is approximately 16% six months post-injury,16 indicating significantly higher risk compared to the estimated prevalence of ADHD in the United States of 7.8%.17 Similarly, children with moderate TBI display almost twice the risk of developing SADHD compared to those with OI, and children with severe TBI demonstrating risks four times higher than those with OI.18 Risk factors associated with the development of SADHD include male sex, lower levels of maternal education, and family dysfunction.18
The association of SADHD with behavioral and cognitive recovery is poorly understood. The presence of SADHD after TBI is associated with poorer perfomance on measures of attention, executive function, and memory one year post injury.12,19–21 However, previous research is hampered by a lack of consideration of the effects of SADHD, independent of pre-morbid ADHD, on long-term outcomes. While pre-morbid ADHD is known to be associated with worse outcomes following early-childhood TBI, the implications of SADHD for long-term recovery of cognitive and behavioral functions are unclear.17
The present study aimed to characterize long-term global and executive functioning in emerging adolescence after early childhood TBI among youth with and without new-onset attention problems post-injury. We hypothesized that SADHD after TBI would be associated with greater impairment relative to TBI without SADHD and to OI with or without post-injury ADHD.
Method
Participants
Institutional review boards of participating hospitals approved all procedures and written informed consent/assent was obtained prior to participation. The parent study used a concurrent cohort/prospective design. Consecutive admissions (from 2003-2006) of children between 3 and 7 years old, hospitalized overnight for TBI or OI, were screened at three tertiary care children’s hospitals and one general hospital. Participants returned for a final follow-up assessment from December 2009-April 2015.
Children with OI were recruited as a comparison group to account for pre-injury child and family factors, including child attention problems that increase the likelihood of sustaining an injury requiring hospitalization.22 Inclusion in the OI group required a documented bone fracture in an area of the body other than the head requiring an overnight hospital stay, as well as the absence of any evidence of findings suggestive of brain injury.
Additional eligibility criteria included accidental cause of injury, no pre-injury neurological problems or developmental delays, and English as the primary language in the home. Children were not excluded if they had a pre-injury history of learning or attention problems. Assessments were completed at baseline (~1 month after injury), at 6, 12, and 18 months post injury, and at longer-term follow-ups an average of 3.4 years and 6.8 years post injury. Children were invited for the final assessment when they transitioned to middle school, with the expectation that deficits in executive function and attention might emerge at this time given increasing expectations for independent planning and problem solving.23,24 Previous manuscripts have reported on behavioral and executive functioning of this cohort throughout their trajectory of recovery 25(e.g. 25–29, and more recently authors explored factors associated with the development of SADHD over time after injury in this cohort.30 The present study extends these findings by exploring how the development of SADHD throughout recovery may impact areas of functional impairment, particularly during the chronic phase of recovery. In order to focus on long term effects of early childhood TBI, and explore how the presence of ADHD may influence impairment or emerging deficits during this critical period of emerging adolescence, individuals were included in the current analyses if they returned for the extended follow-up an average of 6.8 years (SD=1.05) after injury. Individuals who completed the long-term follow-up were not significantly different from those who did not complete it in terms of injury type, age at injury, time since injury at baseline, sex, race, or SES.
Measures
Family and Background Questionnaire
A background questionnaire was used to collect demographic information, as well as pre-injury history of ADHD diagnosis and medication history. To evaluate socioeconomic status (SES), we averaged z-scores for maternal education and median income for the census tract in which the family resided.
Executive Functioning
The parent-report Behavior Rating Inventory of Executive Functioning (BRIEF)31 was administered to assess behavioral outcomes related to attention and everyday executive function . The BRIEF is widely used to assess behavioral manifestations of executive functioning in children following TBI.32 The Global Executive Composite (GEC), Metacognitive Index (MI), and Behavioral Regulation Index (BRI) scores (T-scores; M = 50, SD = 10) were used as dependent variables. The Metacognitive Index reflects planning, organization, and working memory abilities and the Behavioral Regulation Index reflects emotion and behavioral control abilities. The Global Executive Composite provides a summary of the two scales. Higher scores indicate greater executive dysfunction, with a score of 65 or higher indicating clinical impairment.31
Functional Impairment
The Child and Adolescent Functional Assessment Scale (CAFAS) was used to assess functioning in everyday settings.33,34 The CAFAS uses information from structured interviews with key informants (e.g., parents) to generate standardized ratings of functioning across the following domains: school, home, community, behavior toward others, moods/emotions, self-harmful behaviors, substance abuse, and thinking. Functioning in each domain is rated on an ordinal scale, ranging from 0 (unimpaired) to 30 (severe impairment) in 10-point increments, with higher scores reflecting greater impairment. A total score, created by summing domain scores, was used in the present analyses. Totals range from 0 to 240 and scores ˃50 are considered to be impaired. The CAFAS has excellent validity and interrater reliability, ranging from 0.74 to 0.99.33,35 Two research personnel were certified as CAFAS trainers. Additional raters were trained to achieve interrater reliability >80% as recommended by the creator of the CAFAS. Ten percent of interviews were taped and jointly rated, yielding an overall interrater reliability of 98.7%.
ADHD symptoms
ADHD symptoms were assessed using the Child Behavior Checklist (CBCL).36 The CBCL is a parent-report measure of child emotional and behavioral problems with high test-retest reliability and criterion validity, and has been shown to be sensitive to behavioral problems following TBI.37,38 Parents completed the CBCL at all assessments, with report at baseline reflective of their child’s behavior prior to their injury and all other reports reflective of current behavior. The Attention-Deficit/Hyperactivity DSM-oriented scale T-score was used to identify patients with elevated levels of attention problems. Clinically significant elevations on CBCL ratings of attention problems are robustly associated with diagnoses made via structured diagnostic interview, highlighting the clinical utility of this approach.39
Defining ADHD
In the current study, ADHD was defined based on T-scores ≥ 65 on the Attention-Deficit/Hyperactivity DSM-oriented scale on the CBCL, parent reported history of an ADHD diagnosis, or parent report of treatment with stimulant medication. Participants who met one or more of these ADHD criteria at the baseline assessment (when parents were asked to report on behavior, ADHD diagnosis, and treatment prior to injury) were considered to have primary ADHD (PADHD). Children who did not meet the definition for PADHD but met the definition for ADHD at any subsequent assessment visit were considered to have SADHD. While SADHD is typically associated with TBI-related changes in attentional functioning, in the present paper it is used to describe new attention problems following either TBI or OI. In order to focus on the long-term functioning of children with SADHD, those with PADHD were excluded from analyses.
Analyses
General linear modeling was used to examine the effect of injury (TBI vs OI), SADHD status (SADHD vs. No ADHD), and their interaction on global and executive functioning outcomes after controlling for age at injury, sex, and SES. Significance threshold was defined as p<0.05. Logistic regression models, with the same factors described above, were used to examine the role of SADHD status, injury, and their interaction on the likelihood of BRIEF and CAFAS scores in the clinical range.
Results
A total of 221 participants were enrolled in the study. To focus on the consequences of more severe injuries, 15 children with uncomplicated mild TBI (GCS > 13 with no clinically significant neuroimaging findings) were excluded from analyses. An additional 16 children (5 TBI, 11 OI) with PADHD were excluded, and 3 additional children were excluded because they were missing parent rating scales at the baseline assessment. Of the 187 children who were identified as eligible for the analyses, 120 children (TBI: n=54, OI: n=66) and their caregivers completed the extended follow-up, an average 6.8 years (SD=1.05) after injury. Individuals who completed the long-term follow-up were not significantly different from those who did not complete it in terms of injury type, age at injury, time since injury at baseline, sex, race, or SES. See Table 1 for demographic information for individuals included in the analyses. At the extended follow-up, 25 (46.3%) children in the TBI group and 9 (13.6%) children in the OI group were identified as having SADHD (p < .001). Descriptive statistics, as well as frequency and proportion of clinically elevated scores, for all outcome variables by injury group and SADHD status are presented in Table 2.
Table 1.
Demographics information for all injury groups.
| OI (n=66) | TBI (n=54) | Total Sample (n=120) | |
|---|---|---|---|
| Age at injury | 5.16 (1.05) | 5.19 (1.14) | 5.17 (1.09) |
| Time since injury (years) | 6.71 (.95) | 6.89 (1.16) | 6.79 (1.05) |
| Males, n (%) | 34 (51.5%) | 31 (57.4%) | 65 (54.2%) |
| Race, n (%) nonwhite | 15 (22.7%) | 15 (27.8%) | 30 (25%) |
| zSES | .14 (.90) | −.05 (.95) | .05 (.93) |
| SADHD | 9 (13.6%) | 25 (46.3) | 34 (28.3%) |
Note: OI = Orthopedic Injury, TBI = Traumatic Brain Injury, zSES = z-score of socioeconomic status, SADHD = Secondary ADHD.
The TBI group had a greater proportion of participants with SADHD than the OI group (p<.0001). No other group differences for any of the demographic variables were noted.
Table 2.
Mean (standard deviations) of all outcome variables by injury group and SADHD status.
| OI (n = 66) | TBI (n = 54) | Full sample (n=120) | ||||||
|---|---|---|---|---|---|---|---|---|
| No ADHD (n=57) | SADHD (n=9) | All OI | No ADHD (n=29) | SADHD (n=25) | All TBI | No ADHD (n=86) | SADHD (n=34) | |
| BRIEF-BRI | 47.02 (8.79) | 55.78 (9.35) | 48.21 (9.30) | 49.93 (8.25) | 67.32 (12.25) | 57.65 (13.64) | 47.79 (8.63) | 64.26 (12.53) |
| BRIEF – MI | 46.88 (8.66) | 62.56 (12.23) | 49.02 (10.60) | 50.52 (8.52) | 64.84 (10.32) | 57.15 (11.77) | 48.10 (8.74) | 64.24 (10.71) |
| BRIEF – GEC | 46.56 (8.95) | 61.00 (11.47) | 48.53 (10.50) | 49.93 (8.28) | 67.12 (9.93) | 57.89 (12.48) | 47.70 (8.83) | 65.50 (10.54) |
| CAFAS | 11.96 (17.21) | 22.22 (22.79) | 13.38 (18.22) | 15.86 (19.18) | 56.40 (31.61) | 34.63 (32.60) | 13.29 (17.89) | 47.35 (32.97) |
Note: OI = Orthopedic Injury, TBI = Traumatic Brain Injury, ADHD = Attention Deficit Hyperactivity Disorder, SADHD = Secondary Attention Deficit Hyperactivity Disorder, BRIEF-BRI = Behavior Rating Inventory of Executive Function – Behavior Regulation Index, BRIEF – MI = Behavior Rating Inventory of Executive Function – Metacognitive Index, BRIEF - GEC Behavior Rating Inventory of Executive Function – Global Executive Composite; CAFAS = Child and Adolescent Functional Assessment Scale.
Executive Functioning - Behavior Rating Inventory of Executive Functioning
Global Executive Composite (GEC).
The analyses revealed main effects of injury type (F(1,113) = 5.00, p = .03) and SADHD status (F(1,113) = 52.92, p <.0001). Individuals with TBI had higher GEC scores than those with OI , and those with SADHD had greater GEC scores than those with no ADHD, see Table 2. The interaction of injury type by ADHD status was not significant. Further, SADHD status was significantly associated with the likelihood of having a GEC score within the clinically elevated range (X2(1) = 16.49, p<.01). Specifically, a greater proportion of those with SADHD had clinically elevated GEC scores compared to the no ADHD group , see Table 3. Neither injury type nor the interaction of injury type and SADHD status was associated with risk for clinically elevated GEC scores.
Table 3.
Frequency, and proportion of clinically elevated scores on each of the dependent variables by injury group and SADHD status
| OI (n = 66) | TBI (n = 54) | Full Sample (n = 120) | ||||||
|---|---|---|---|---|---|---|---|---|
| No ADHD (n = 57) | SADHD (n=9) | All OI | No ADHD (n=29) | SADHD (n = 25) | All TBI | All No ADHD (n = 86) | All SADHD (n=34) | |
| BRIEF-BRI | 3 (5.3%) | 2 (22.2%) | 5 (7.6%) | 2 (6.9%) | 16 (64%) | 18 (33.3%) | 5 (5.8%) | 18 (52.9%) |
| BRIEF – MI | 4 (7.0%) | 5 (55.6%) | 9 (13.6%) | 1 (3.5%) | 13 (52.0%) | 14 (25.9%) | 5 (5.8%) | 18 (52.9%) |
| BRIEF – GEC | 5 (8.8%) | 3 (33.3%) | 8 (12.1%) | 1 (3.5%) | 18 (72%) | 19 (35.2%) | 6 (7.0%) | 21 (61.8%) |
| CAFAS | 2 (3.7%) | 1 (11.1%) | 3 (4.6%) | 1 (3.5%) | 11 (55.0%) | 12 (22.2%) | 3 (3.5%) | 12 (35.3%) |
Note: OI = Orthopedic Injury, TBI = Traumatic Brain Injury, ADHD = Attention Deficit Hyperactivity Disorder, SADHD = Secondary Attention Deficit Hyperactivity Disorder, BRIEF-BRI = Behavior Rating Inventory of Executive Function – Behavior Regulation Index, BRIEF – MI = Behavior Rating Inventory of Executive Function – Metacognitive Index, BRIEF - GEC Behavior Rating Inventory of Executive Function – Global Executive Composite; CAFAS = Child and Adolescent Functional Assessment Scale.
Behavioral Regulation Index (BRI).
The main effects of injury type (F(1,113) = 10.20, p = .002) and SADHD status (F(1,113) = 33.12, p <.0001) were significant. The interaction of injury type by SADHD status was also significant, F(1,113) = 4.42, p = .04 (see Table 2, Figure 1). The effect of injury type was only significant among those with SADHD, such that those with TBI+SADHD had higher BRI scores than those with OI+SADHD (t(113) = 3.07, p = .003), with no significant difference between OI and TBI patients without SADHD. The effect of SADHD was significant in both injury groups, such that those with SADHD had higher BRI scores in both the TBI (t(113)=6.44, p<.0001) and OI (t(113) = 2.46, p = .02) groups. SADHD status was significantly associated with obtaining BRI scores in the clinically elevated range (X2(1) = 12.96, p<.01). A larger proportion of those with SADHD had clinically elevated BRI scores compared to the no ADHD group, see Table 3. Neither injury type nor the interaction of injury and SADHD status was associated with risk for clinically elevated BRI scores.
Figure 1.
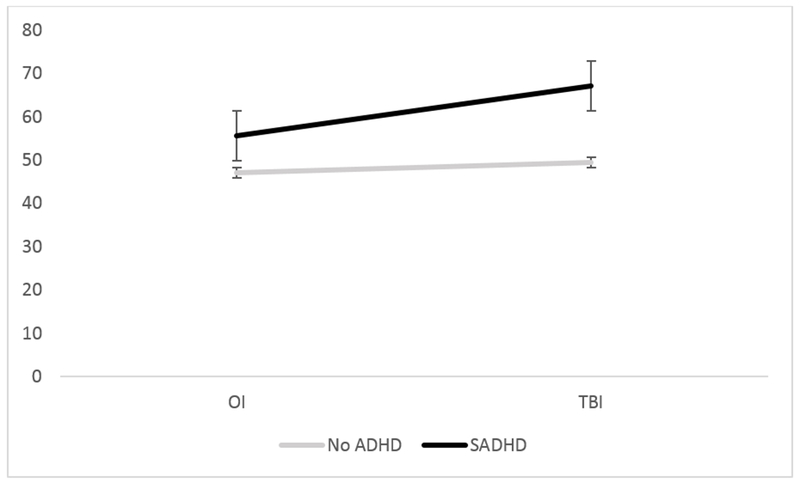
ADHD by Injury type interaction on Behavioral Regulation Index (BRI) on parent reported Behavioral Rating Index of Executive Function (BRIEF).
Metacognitive Index (MI)
The main effect of SADHD status was significant (F(1,113) = 48.64, p<.0001), such that those with SADHD had higher MI scores than those without SADHD regardless of injury type, see Table 2. Neither injury type nor the injury type by SADHD status interaction was significant. Finally, SADHD status was predictive of clinically elevated MI scores (X2(1) = 18.88, p<.01). Again, a larger proportion of those in the SADHD group had clinically elevated MI scores than those in the no ADHD group, see Table 3. Neither injury type nor the interaction of injury type and SADHD status was associated with risk for clinically elevated MI scores.
Global Functioning – Child and Adolescent Functional Assessment Scale (CAFAS)
The main effects of injury type (F(1,112) = 15.35, p = 0002) and SADHD status (F(1,112) = 16.47, p <.0001) were significant on the CAFAS. The interaction of injury type by SADHD status was also significant, F(1,112) = 8.95, p = .003 (see Table 2, Figure 2). The effect of injury type was only significant among those with SADHD, such that those with TBI+SADHD had higher CAFAS scores than those with OI+SADHD (t(112) = 4.01, p=.0001), with no significant difference between those with OI and TBI without ADHD. Further, the effect of SADHD was only significant among those with TBI (t(112) = 5.72, p<.0001); CAFAS scores did not differ among children with OI with and without SADHD. Finally, both SADHD status (X2(1) = 4.31, p = .04) and the interaction of injury type and SADHD status (X2(1) = 5.03, p = .02) were associated with the likelihood of clinically elevated functional impairment. Specifically, children with TBI + SADHD had a greater likelihood of clinically elevated CAFAS score compared to those with TBI and no ADHD (X2(1) = 22.82, p<.001) and those with OI + SADHD (X2(1) = 7.40, p=.007), see Table 3.
Figure 2.
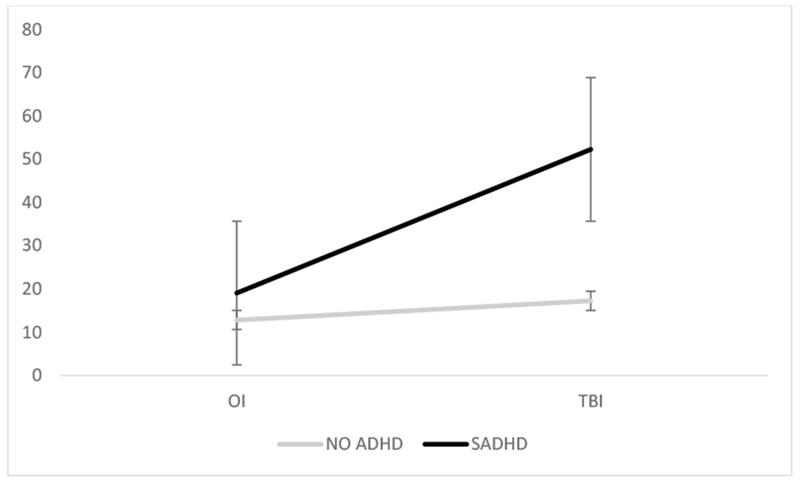
ADHD by Injury type interaction on global functioning as measured by the CAFAS
Discussion
The current findings provide further evidence of the significant association of SADHD with functional outcomes following early childhood TBI. SADHD was associated with poorer functioning on all outcomes examined. However, for parent-reported behavioral regulation and independent ratings of overall functioning, the adverse effects of SADHD were amplified for children with TBI. Among those with both SADHD and TBI, average Behavioral Regulation Index and Global Executive Composite scores were within the clinically impaired range. These findings suggest that children with TBI+SADHD may be at elevated risk for significant impairments in early adolescence as they transition from more structured environments to greater autonomy. Contrary to expectations, TBI was not independently associated with parent-rated metacognitive deficits after controlling for SADHD. Thus, identifying and effectively treating SADHD may result in improved executive and global functioning during this critical developmental period.
Not surprisingly, SADHD, after either TBI or OI, was associated with poorer executive function behaviors and functioning in everyday settings. The observed effect of SADHD on both MI and GEC suggests that SADHD impacts metacognitive skills and global executive functioning skills similarly across all adolescents, regardless of injury type. These findings are consistent with prior research demonstrating functional impairments 40–44 and deficits in executive functioning 40,45–48 among individuals with ADHD. The impact of TBI on overall executive functioning also was consistent with existing research;28,49,50 however, the lack of association of injury type with meta-cognitive skills was unexpected. Most research has shown a robust effect of severe TBI. 45,51–53 Collapsing across TBI severity groups may have hindered our ability to detect injury related differences.
The interaction of SADHD and injury type, observed on the Behavioral Regulation Index of the BRIEF and Child and Adolescent Functional Assessment Scale total score, suggest that SADHD is associated with worse outcomes when it occurs in association with TBI as opposed to OI. SADHD had a significant impact on behavioral regulation for all adolescents regardless of injury type, but with a more pronounced effect in the TBI group. This may suggest that SADHD after TBI is unique in the greater demonstration of difficulties with behavioral control, emotional instability, and disinhibition, while SADHD after OI may be more reflective of developmental ADHD emerging after injury. Similarly, the impact of SADHD on global functioning was only significant among children with TBI, with no effect of SADHD seen among children with OI. Although most previous research has focused on understanding the risk factors associated with the development of SADHD following TBI, the additive adverse effects of TBI and SADHD on long-term functioning as measured by the BRIEF and CAFAS are consistent with previous research examining other outcomes, including neuropsychological sequelae,20 attention, executive functioning, memory,19 inhibitory control,54 and adaptive and intellectual functioning.55
The elevated risk of clinical impairment highlights the critical need to track children with TBI over time to identify and manage emerging SADHD symptoms. However, the evidence for management approaches (medical or behavioral) for attention problems following TBI in children is quite limited, with significant heterogeneity in sample characteristics and outcomes preventing definitive conclusions. 56 Some evidence suggests that medication used to treat developmental ADHD may also be effective for SADHD57 and may also result in improvement in EF.57,58 Assessing and addressing SADHD symptoms in early adolescence in this population may be especially important for promoting a successful transition to adulthood.
This study was limited by a reliance on parent report for assessing SADHD. Although ratings on the CBCL show a close correspondence with clinical diagnosis,59 a structured/semi-structured clinical interview and clinician derived diagnosis with the integration of information from multiple sources would provide more robust evidence of SADHD. Similarly, there may exist some overlap in items on the CBCL (used in part to define the ADHD sample) and the BRIEF (used to identify weaknesses in executive functioning). While executive functioning is theorized to underly the symptoms of ADHD, they are not one in the same. Future work would benefit not only from a clinician derived diagnosis of ADHD, but also objective performance based assessments to better understand the patterns of executive functioning weaknesses in this population. Along these lines, additional psychiatric comorbidities were not assessed. Previous work has documented the high rate of comorbidity, particularly personality changes, among children/adolescents with SADHD.39,60 Because the Child and Adolescent Functional Assessment Scale taps into multiple domains of functioning, future studies should control for the presence of behavioral comorbidities when examining the impact of SADHD on impairment. Although the current sample was representative of enrolled children, attrition may have biased the sample in unknown ways. Further, children with PADHD were excluded from analysis. We were able to explore the impact of new onset symptoms, but the small number of children with PADHD precluded study of the relative effects of PADHD in the two injury groups. Other authors have noted a different pattern of executive functioning 20 and inhibitory control 61,62 in children with SADHD compared to those with PADHD. In addition, due to the young age at injury of the sample, it is possible that that a portion of those in the SADHD group may have had PADHD that had not yet been diagnosed due to their young age. To help minimize the complication of this, parent report of pre-injury behavior, rather than relying solely on previous diagnosis of ADHD, was used to identify children with potentially elevated levels of ADHD symptoms prior to their injury. Further, although the current study used an OI comparison group, children in this group may have been more prone to pre-injury ADHD-symptom traits compared to a non-injured, healthy control group.18 Future research would benefit from including a non-injured healthy comparison group to further elucidate the effects of significant early childhood injury and ADHD on functional outcomes. Finally, the current study aims to identify groups of individuals who may be at greater risk for poor outcome after pediatric TBI, and therefore looked at the moderating effect of clinically significant levels of ADHD symptoms. Future studies may benefit from focusing on understanding symptoms of ADHD as a mediator of poor outcome, or mechanism of impairment.
In sum, the current findings provide important information about the effects of SADHD on long-term functioning following early TBI. Consistent with the model of managing TBI from a chronic disease model 37with the goal of optimizing functioning 37, findings underscore the importance of tracking children over time and managing SADHD to mitigate associated functional impairments. Future research is needed to identify behavioral or family-centered treatments, in addition to pharmacotherapy, to prevent or ameliorate impairments.
Acknowledgments
Funding Source:
This publication was supported by grant R01 HD42729 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and Trauma Research grant from the State of Ohio Emergency Medical Services. Additional support was provided through Grant 8 UL1 TR000077 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health. Dr. Narad was supported by grant 1F32HD088011-01 from the NICHD. Ms. Riemersma and Ms. Morrison were supported by a grant from the Rehabilitation Research Experience for Medical Students. Dr. Kurowski was supported by Mentored Patient-Oriented Research Career Development Award K23 HD07468303 from the NICHD. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Disclosures: The authors have no conflicts to disclose
References
- 1.Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007;22(5):341–353. [PubMed] [Google Scholar]
- 2.Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dewan MC, Mummareddy N, Wellons JC, Bonfield CM. Epidemiology of global pediatric traumatic brain injury: Qualitative Review. World Neurosurg. 2016;91(e491):497–509. [DOI] [PubMed] [Google Scholar]
- 4.Babikian T, Asarnow R. Neurocognitive outcomes and recovery after pediatric TBI: meta-analytic review of the literature. Neuropsychology. 2009;23(3):283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloom DR, Levin HS, Ewing-Cobbs L, et al. Lifetime and novel psychiatric disorders after pediatric traumatic brain injury. J Am Acad Child Psych. 2001;40(5):572–579. [DOI] [PubMed] [Google Scholar]
- 6.M JE, Schachar RJ, Ornstein TJ. Preinjury and secondary attention-deficit/hyperactivity disorder in pediatric traumatic brian injury forensic cases In: S EMS, B BL, eds. Pediatric Forensic Neuropsychology. New York, NY: Oxford University Press; 2012:258–274. [Google Scholar]
- 7.Konigs M, Heij HA, van der Sluijs JA, et al. Pediatric Traumatic Brain Injury and Attention Deficit. Pediatrics. 2015;136(3):534–541. [DOI] [PubMed] [Google Scholar]
- 8.Max JE, Levin HS, Schachar RJ, et al. Predictors of personality change due to traumatic brain injury in children and adolescents six to twenty-four months after injury. J Neuropsych Clin N. 2006;18(1):21–32. [DOI] [PubMed] [Google Scholar]
- 9.Max JE, Keatley E, Wilde EA, et al. Depression in children and adolescents in the first 6 months after traumatic brain injury. Int J Dev Neurosci. 2012;30(3):239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luis CA, Mittenberg W. Mood and anxiety disorders following pediatric traumatic brain injury: a prospective study. J Clin Exp Neuropsych. 2002;24(3):270–279. [DOI] [PubMed] [Google Scholar]
- 11.Max JE, Wilde EA, Bigler ED, et al. Psychiatric disorders after pediatric traumatic brain injury: a prospective, longitudinal, controlled study. J Neuropsych Clin N. 2012;24(4):427–436. [DOI] [PubMed] [Google Scholar]
- 12.Yang LY, Huang CC, Chiu WT, Huang LT, Lo WC, Wang JY. Association of traumatic brain injury in childhood and attention-deficit/hyperactivity disorder: a population-based study. Pediatr Res. 2016;80(3):356–362. [DOI] [PubMed] [Google Scholar]
- 13.Berger I Diagnosis of Attention Deficit Hyperactivity Disorder: Much Ado about Something. Isr Med Assoc J. 2011;13(9):571–574. [PubMed] [Google Scholar]
- 14.Sharma A, Couture J. A Review of the Pathophysiology, Etiology, and Treatment of Attention-Deficit Hyperactivity Disorder (ADHD). Ann Pharmacother. 2014;48(2):209–225. [DOI] [PubMed] [Google Scholar]
- 15.Mychasiuk R, Hehar H, Esser MJ. A mild traumatic brain injury (mTBI) induces secondary attention-deficit hyperactivity disorder-like symptomology in young rats. Behavioural Brain Research. 2015;286:285–292. [DOI] [PubMed] [Google Scholar]
- 16.Yeates KO, Armstrong K, Janusz J, et al. Long-term attention problems in children with traumatic brain injury. J Am Acad Child Adolesc Psy. 2005;44(6):574–584. [DOI] [PubMed] [Google Scholar]
- 17.Bonfield CM, Lam S, Lin YM, Greene S. The impact of attention deficit hyperactivity disorder on recovery from mild traumatic brain injury Clinical article. J Neuros-Pediatr. 2013;12(2):97–102. [DOI] [PubMed] [Google Scholar]
- 18.Narad ME, Kennelly M, Zhang N, et al. Secondary Attention-Deficit/Hyperactivity Disorder in Children and Adolescents 5 to 10 Years After Traumatic Brain Injury. JAMA Pediatr. 2018;172(5):437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slomine BS, Salorio CF, Grados MA, Vasa RA, Christensen JR, Gerring JP. Differences in attention, executive functioning, and memory in children with and without ADHD after severe traumatic brain injury. J Int Neuropsychol Soc. 2005;11(5):645–653. [DOI] [PubMed] [Google Scholar]
- 20.Ornstein TJ, Sagar S, Schachar RJ, et al. Neuropsychological performance of youth with secondary attention-deficit/hyperactivity disorder 6- and 12-months after traumatic brain injury. J Int Neuropsychol Soc. 2014;20(10):971–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levin H, Hanten G, Max J, et al. Symptoms of attention-deficit/hyperactivity disorder following traumatic brain injury in children. J Dev Behav Pediatr. 2007;28(2):108–118. [DOI] [PubMed] [Google Scholar]
- 22.DiScala C, Lescohier I, Barthel M, Li G. Injuries to children with attention deficit hyperactivity disorder. Pediatrics. 1998;102(6):1415–1421. [DOI] [PubMed] [Google Scholar]
- 23.Dupaul GI, Stoner G. ADHD in the Schools: Assessment and Intervention Strategies. 2nd ed New York: Guildford; 2003. [Google Scholar]
- 24.Feldlaufer H, Midgley C, Eccles JS. Student, teacher, and observer perceptions of the classroom environment before and after the transition to junior high school. J Early Adolescence. 1998;8:133–156. [Google Scholar]
- 25.Narad ME, Treble-Barna A, Peugh J, et al. Recovery Trajectories of Executive Functioning After Pediatric TBI: A Latent Class Growth Modeling Analysis. J Head Trauma Rehabil. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurowski BG, Taylor HG, Yeates KO, Walz NC, Stancin T, Wade SL. Caregiver ratings of long-term executive dysfunction and attention problems after early childhood traumatic brain injury: family functioning is important. PM R. 2011;3(9):836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karver CL, Wade SL, Cassedy A, et al. Age at injury and long-term behavior problems after traumatic brain injury in young children. Rehabil Psychol.. 2012;57(3):256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganesalingam K, Yeates KO, Taylor HG, Walz NC, Stancin T, Wade S. Executive functions and social competence in young children 6 months following traumatic brain injury. Neuropsychology. 2011;25(4):466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapman L, Wade SL, Walz NC, Taylor HG, Stancin T, Yeates KO. Clinically significant behavior problems during the intial 18 months following early childhood traumatic brain injury Rehabil Psychol. 2010;55(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narad ME, Kennelly M, Zhang N, et al. Secondary Attention-Deficit/Hyperactivity Disorder in Children and Adolescents 5 to 10 Years After Traumatic Brain Injury. JAMA Pediatr. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gioia G, Isquith PK, Guy SC, Kenworthy L. BRIEF: Behavior Rating Inventory of Executive Function. Lutz, FL: Psychological Assessment Resources, Inc; 2000. [Google Scholar]
- 32.McCauley SR, Wilde EA, Anderson VA, et al. . Recommendations for the use of common outcome measures in pediatric traumatic brain injury research J Neurotram. 2012;29(4):678–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodges K, Wong MM. Psychometric Characteristics of a Multidimensional Measure to Assess Impairment: The Child and Adolescent Functional Assessment Scale. J Child Fam Stud. 1996;5(4):445–467. [Google Scholar]
- 34.Hodges K, Wong MM, Latessa M. Use of the Child and Adolescent Functional Assessment Scale (CAFAS) as an outcome measure in clinical settings. J Behav Health Serv Res. 1998;25(3):325–336. [DOI] [PubMed] [Google Scholar]
- 35.Xue Y, Hodges K, Wotring J. Predictors of outcome for children with behavior problems served in public mental health. J Clin Child Adolesc Psychol. 2004;33(3):516–523. [DOI] [PubMed] [Google Scholar]
- 36.Achenbach TM, Rescorla L. Manual for the ASEBA school-age forms & profiles : an integrated system of multi-informant assessment. Burlington, VT: ASEBA; 2001. [Google Scholar]
- 37.Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma. 2010;27(8):1529–1540. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz L, Taylor HG, Drotar D, Yeates KO, Wade SL, Stancin T. Long-term behavior problems following pediatric traumatic brain injury: prevalence, predictors, and correlates. J Pediatri Psychol. 2003;28(4):251–263. [DOI] [PubMed] [Google Scholar]
- 39.Gerring J, Brady KD, Chen A, Vasa R, Grados M, Bandeen-Roche KJ, Bryan N, Denckla MB Premorbid prevalence of ADHD and development of secondary ADHD after closed head injury J Am Acad Child Psy. 1998;37(6):647–654. [DOI] [PubMed] [Google Scholar]
- 40.Biederman J Impact of comorbidity in adults with attention-deficit/hyperactivity disorder. J Clin Psychiat. 2004;65 Suppl 3:3–7. [PubMed] [Google Scholar]
- 41.Garner AA, O’Connor B C, Narad ME, Tamm L, Simon J, Epstein JN. The relationship between ADHD symptom dimensions, clinical correlates, and functional impairments. J Dev Behav Pediatr. 2013;34(7):469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strine TW, Lesesne CA, Okoro CA, et al. Emotional and behavioral difficulties and impairments in everyday functioning among children with a history of attention-deficit/hyperactivity disorder. Prev Chronic Dis. 2006;3(2):A52. [PMC free article] [PubMed] [Google Scholar]
- 43.Nixon E The social competence of children with attention deficit hyperactivity disorder: A review of the literature. Child Psychology and Psychiatry Review. 2001;6:172–180. [Google Scholar]
- 44.Bagwell CL, Molina BS, Pelham WE Jr., Hoza B. Attention-deficit hyperactivity disorder and problems in peer relations: predictions from childhood to adolescence. J Am Acad Child Adolesc Psychiatry. 2001;40(11):1285–1292. [DOI] [PubMed] [Google Scholar]
- 45.Gioia GA, Isquith PK, Kenworthy L, Barton RM. Profiles of everyday executive function in acquired and developmental disorders. Child Neuropsychol. 2002;8(2):121–137. [DOI] [PubMed] [Google Scholar]
- 46.McCandless S, L OL. The Clinical Utility of the Behavior Rating Inventory of Executive Function (BRIEF) in the diagnosis of ADHD. J Atten Disord. 2007;10(4):381–389. [DOI] [PubMed] [Google Scholar]
- 47.Reddy LA, Hale JB, Brodzinsky LK. Discriminant Validity of the Behavior Rating Inventory of Executive Function Parent Form for Children With Attention-Deficit/Hyperactivity Disorder. School Psychol Quart. 2011;26(1):45–55. [Google Scholar]
- 48.Toplak ME, Bucciarelli SM, Jain U, Tannock R. Executive functions: performance-based measures and the behavior rating inventory of executive function (BRIEF) in adolescents with attention deficit/hyperactivity disorder (ADHD). Child Neuropsychol. 2009;15(1):53–72. [DOI] [PubMed] [Google Scholar]
- 49.Hanten G, Bartha M, Levin HS. Metacognition following pediatric traumatic brain injury: a preliminary study. Dev Neuropsychol. 2000;18(3):383–398. [DOI] [PubMed] [Google Scholar]
- 50.Sesma H, Slomine B, Ding R, McCarthy M, Grp CS. Executive functioning in the first year after pediatric traumatic brain injury. Pediatrics. 2008;121(6):E1686–E1695. [DOI] [PubMed] [Google Scholar]
- 51.Beauchamp M, Catroppa C, Godfrey C, Morse S, Rosenfeld JV, Anderson V. Selective changes in executive functioning ten years after severe childhood traumatic brain injury. Dev Neuropsychol. 2011;36(5):578–595. [DOI] [PubMed] [Google Scholar]
- 52.Muscara F, Catroppa C, Anderson V. The impact of injury severity on executive function 7–10 years following pediatric traumatic brain injury. Dev Neuropsychol. 2008;33(5):623–636. [DOI] [PubMed] [Google Scholar]
- 53.Nadebaum C, Anderson V, Catroppa C. Executive function outcomes following traumatic brain injury in young children: a five year follow-up. Dev Neuropsychol. 2007;32(2):703–728. [DOI] [PubMed] [Google Scholar]
- 54.Schachar R, Levin HS, Max JE, Purvis K, Chen S. Attention deficit hyperactivity disorder symptoms and response inhibition after closed head injury in children: do preinjury behavior and injury severity predict outcome? Dev Neuropsychol. 2004;25(1-2):179–198. [DOI] [PubMed] [Google Scholar]
- 55.Max JE, Lansing AE, Koele SL, et al. Attention deficit hyperactivity disorder in children and adolescents following traumatic brain injury. Dev Neuropsychol. 2004;25(1-2):159–177. [DOI] [PubMed] [Google Scholar]
- 56.Backeljauw B, Kurowski BG. Interventions for Attention Problems After Pediatric Traumatic Brain Injury: What Is the Evidence? PM&R. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurowski BG, Epstein JN, Pruitt DW, Horn PS, Altaye M, Wade SL. Benefits of Methylphenidate for Long-Term Attention Problems After Traumatic Brain Injury in Childhood: A Randomized, Double-Masked, Placebo-Controlled, Dose-Titration, Crossover Trial. J Head Trauma Rehabil. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.LeBlond E, Smith-Paine J, Riemersma JJ, Horn PS, Wade SL, Kurowski BG. Influence of methylphenidate on long-term neuropsychological and everyday functioning adter traumatic brain injury in children with secondary attention problems. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wassenberg R, Max JE, Koele SL, Firme K. Classifying psychiatric disorders after traumatic brain injury and orthopaedic injury in children: adequacy of K-SADS versus CBCL. Brain Inj. 2004;18(4):377–390. [DOI] [PubMed] [Google Scholar]
- 60.Max JE, Schachar RJ, Levin HS, et al. Predictors of secondary attention-deficit/hyperactivity disorder in children and adolescents 6 to 24 months after traumatic brain injury. J Am Acad Child Psy. 2005;44(10):1041–1049. [DOI] [PubMed] [Google Scholar]
- 61.Konrad K, Gauggel S, Manz A, Scholl M. Inhibitory control in children with traumatic brain injury (TBI) and children with attention deficit/hyperactivity disorder (ADHD). Brain Inj. 2000;14(10):859–875. [DOI] [PubMed] [Google Scholar]
- 62.Sinopoli KJ, Schachar R, Dennis M. Traumatic brain injury and secondary attention-deficit/hyperactivity disorder in children and adolescents: the effect of reward on inhibitory control. J Clin Exp Neuropsych. 2011;33(7):805–819. [DOI] [PMC free article] [PubMed] [Google Scholar]


