Abstract
Objective
Relative to dementia, little is known about informant bias in Mild Cognitive Impairment (MCI). We investigated the influence of informant demographic and relational characteristics on reports of everyday functioning using the Functional Activities Questionnaire (FAQ).
Method
4,284 MCI participants and their informants from the National Alzheimer’s Coordinating Center Uniform Data Set were included. Informants were stratified according to cohabitation, relationship, visit frequency, race/ethnicity, education, and sex. Informant-rated mean FAQ score was compared across these groups using univariate general linear model analyses and post-hoc tests. Interactions were tested between informant variables. The predictive contribution of informant variables to FAQ score was explored using hierarchical linear regression. Analyses covaried for participant cognition using a cognitive composite score, and for participant age, sex and depression.
Results
After controlling for participant cognition, depression, age, and sex, informant-rated FAQ scores varied significantly across all informant variables (p’s<.005, ηp2’s ≤ .033) except sex and visit frequency. FAQ scores were higher (more impaired) among informants who cohabitate with the participant, among paid caregivers, spouses, and adult children, and among informants with higher levels of education. Scores were lowest (less impaired) among Black/African American informants as compared to all other racial/ethnic groups.
Conclusions
Demographic and relational characteristics of informants influence the perception and reporting of instrumental activities of daily living (IADL) in adults with MCI. As everyday functioning is crucial for differential diagnosis and treatment outcome measurement, it is important to be aware of sources of informant report discrepancies.
MeSH Keywords: Observer Bias, Mild Cognitive Impairment, Informant, Activities of Daily Living, Cognitive Aging, Alzheimer’s disease, Observer Variation
Introduction
As the global population ages and the proportion of medically complex elders continues to grow (Bluethmann, Mariotto, & Rowland, 2016), an effective and comprehensive approach toward combating dementia has become a public health priority. Disappointing results from clinical trials have shifted treatment targets to prodromal and preclinical stages before neuronal degeneration begins (Cummings, Morstorf, & Zhong, 2014; Schelke et al., 2016; Siemers et al., 2016; Sperling et al., 2014). This shift in treatment focus makes the accurate diagnosis of the prodromal stage of dementia, Mild Cognitive Impairment (MCI), critical for early detection and intervention. The present study examined a potential source of inaccurate MCI diagnosis, namely, informant bias.
Several iterations of diagnostic criteria for MCI exist (Albert et al., 2011; Jack et al., 2018; Petersen, 2004; Petersen et al., 2009). The Petersen (2004) criteria are the most widely used and require subjective cognitive concern from the patient or informant, objective evidence of cognitive decline or cognitive performance lower than expected based on age, along with essentially normal functional activities. Limitations of these criteria have been identified within the context of the Alzheimer’s Disease Neuroimaging Initiative (ADNI; Mueller et al., 2005), a large multisite longitudinal study in which MCI diagnosis was based on impairment on a single cognitive test score, subjective cognitive complaints, and clinical judgement of mild impairment (global CDR score of 0.5). Edmonds et al. (2014) found that subjective cognitive complaints were overestimated among cognitively intact individuals and underestimated among those with amnestic MCI, which may be attributable to lack of awareness secondary to memory impairments (Edmonds, Delano-Wood, Galasko, Salmon, & Bondi, 2014). Bondi et al. (2014) identified nearly a third of MCI participants performing within normal limits on other neuropsychological tests, and reversion rates of up to 8.5% in this subgroup of MCI participants. More recently, Thomas et al. (2019) found that inconsistent application of episodic memory cutoff scores by diagnosing clinicians led to greater reliance on subjective rating scales and an artificially high rate of MCI diagnoses. These pathways to inaccurate MCI diagnosis have been explored and corresponding recommendations have been made (Bondi et al., 2014; Edmonds et al., 2014; Thomas et al., 2019). Another potential yet less explored route to inaccurate diagnosis depends on informants’ reporting for the functional criterion.
Consideration of instrumental activities of daily living (IADL), defined as complex activities including home maintenance, shopping, and money management (Lawton & Brody, 1969), is critical to the differential diagnosis of MCI. The Petersen (2004) criteria state that IADL should be “essentially intact” and the National Institute on Aging and Alzheimer’s Association (NIA-AA) criteria state that persons with MCI “generally maintain their independence of function in daily life, with minimal aids or assistance” (Albert et al., 2011). However, mild difficulties during complex activities that require higher cognitive demand (e.g., managing finances and keeping appointments) occur frequently in most older adults (Jekel et al., 2015; Nygard, 2003), making it challenging to determine when subtle IADL difficulties reflect pathological changes in cognition. Nonetheless, mounting evidence demonstrates that IADL difficulties occur as early as the prodromal stage of dementia (i.e., MCI) (Teng et al., 2010) and represent a reliable predictor of conversion from MCI to dementia (Pérès et al., 2006; Triebel et al., 2009). Therefore, the precise characterization of everyday functioning is important for accurate diagnosis and prognosis.
Informant reports represent the most common method of assessing IADL in MCI; thus, it is critical to consider potential inaccuracies in informants’ perceptions, especially as this information is typically acquired in the absence of objective evaluation. Several factors have been shown to influence the degree to which informants and caregivers rate impairment in IADL in studies of dementia cohorts as well as mixed MCI and dementia cohorts. Caregiver burden is often reported as a factor that strongly influences informant report in studies of dementia, in that higher burden promotes underestimation of functional abilities (Conde-Sala et al., 2013; Mangone et al., 1993; Zanetti, Geroldi, Frisoni, Bianchetti, & Trabucchi, 1999). Another factor known to contribute to informant bias in dementia is cohabitation, such that caregivers who live with the person with dementia assign lower quality of life ratings (Bosboom, Alfonso, Eaton, & Almeida, 2012). Relationship represents an additional influencing factor. It has been shown that spouse informants of both MCI and AD participants report higher quality of life (Lin, Brook, Grill & Teng 2017) and higher cognitive ability ratings (Persson, Braekhus, Selbaek, Kirkevold & Engedal, 2015) as compared to non-spouses, and are more accurate in assessing everyday functioning than adult children informants (Loewenstein et al., 2001). However, these variables have been largely overlooked in cohorts that are restricted to MCI participants, and it is unclear whether they may be less relevant when cognitive and functional impairments are more subtle.
The influence of ethnicity and race is an additional area of consideration. The perception, subjective experience, and meanings assigned to dementia vary across different ethnic and cultural groups, which in turn can influence the reporting of functional symptoms (Dilworth-Anderson & Gibson, 2002). A study from Burns, Nichols, Graney, Martindale-Adams, and Lummus (2006) showed that African-American caregivers of individuals with dementia generally overrated their care recipient’s cognitive abilities, whereas White caregivers underestimated cognitive functioning of their care recipients. This difference remained significant even after controlling for caregiver age, sex, education, income, and burden (Burns et al., 2006). Results align with previous research indicating that Black caregivers may view caregiving situations more favorably and optimistically than White caregivers (Farran, Miller, Kaufman, & Davis, 1997; Raczynski et al., 1994), leading to an overestimation of abilities. A separate study showed this same trend for older adults with MCI (Potter et al., 2009). These differences may be due to multiple factors, including beliefs that dementia symptoms reflect normal aging (Connell, Roberts, McLaughlin, & Akinleye, 2009).
There are no studies to our knowledge that simultaneously examine the role of multiple informant variables that may influence reporting of subtle IADL difficulties in people with MCI. The aim of this study was to evaluate potential sources of informant bias on the frequently used Functional Activities Questionnaire (FAQ) in a large MCI cohort to extend findings from the dementia literature. We explored this question using participant data from the Uniform Data Set, a large multisite research database compiled by the National Alzheimer’s Coordinating Center (NACC) (Beekly et al., 2004; 2007). We examined the influence of informant-participant relationship, cohabitation, frequency of in-person visits, and informant race, ethnicity, education, and sex on informant-reported FAQ ratings. We controlled for MCI participant features known to influence everyday function, including demographic variables, neuropsychological test scores, and depressive symptoms (Mcalister, Schmitter-Edgecombe & Lamb, 2016; Rog et al., 2014). After controlling for these variables, we interpreted discrepancies in FAQ ratings amongst different informants as evidence for potential bias. For example, after accounting for differences in cognition and other MCI participant covariates, if we observed significantly discrepant FAQ ratings between spouse and child informants, we would infer that relationship of the informant may influence their perception of functional abilities. Specifically, we predicted that 1) informants with closer relationships to participants (i.e., spouses vs. children, cohabiting vs. not cohabiting, higher frequency of visits) would be more likely to report greater functional difficulties; and 2) informants of particular racial/ethnic groups would report functional difficulties at varying rates. To our knowledge there is no empirical research on the influence of informant sex and education on functional reporting; however, because these factors may influence the interpretation of behavioral symptoms, we included them in our analyses.
METHOD
Participants
Data from 4,284 MCI participants (see Table 1) and their informants (M age 65.2 ± 13.2; M education 15.5 ± 2.7; 68.6% female) were acquired from the publicly available National Alzheimer’s Coordinating Center (NACC) database. The NACC consists of approximately 39 National Institute on Aging (NIA)-funded Alzheimer’s Disease Centers (ADCs) from throughout the United States collecting a longitudinal, standardized protocol of clinical and neuropathological data (Beekly et al., 2004; 2007; Morris et al., 2006). ADCs were overseen by their local Institutional Review Board, and informed consent was obtained accordingly. Participants completed approximately yearly examinations by a trained clinician involving collection of a Uniform Data Set (UDS; Beekly et al., 2007), including information on demographics, medical history, clinical evaluations, functional assessment, and neuropsychological data. Diagnosis of MCI was made by a clinician or consensus conference (Morris, 2008; Morris et al., 2006; Weintraub et al., 2009) using modified Petersen criteria (Petersen, 2004; Petersen et al., 2001; 2009). The majority of ADCs do not use the FAQ for consensus MCI diagnosis (Teng et al., 2010). Access to the NACC database is available to researchers at ADC and non-ADC institutions by request (Beekly et al., 2004; 2007). Data collected from September 2005 to December 2016 were analyzed. The present study included participants with a diagnosis of MCI (single-domain and multi-domain, amnestic and non-amnestic) at the initial assessment, who are native English speakers and at least 50 years old. Individuals with a history or presence of stroke, Parkinson’s disease, traumatic brain injury, seizures, schizophrenia, bipolar disorder, primary progressive aphasia, frontotemporal dementia, Down’s syndrome, or CNS neoplasm were excluded.
Table 1.
Descriptive characteristics of MCI Participants (N = 4,284)
| Mean (SD) | Range (Minimum-Maximum) | |
|---|---|---|
| Demographic Variables | ||
| Age | 73.9 (8.7) | 50–109 |
| Education | 15.4 (3.1) | 1–30 |
| Sex (% female) | 52% | --- |
| Race (% White) | 78% | --- |
| Informant-rated FAQ Score | ||
| Mean FAQ | 0.34 (.46) | 0–3 |
| Cognition | ||
| Mini Mental State Exam | 27.2 (2.3) | 16–30 |
| Logical Memory IIA Delayed | 6.7 (4.7) | 0–22 |
| Recall | ||
| Digit Span Backward | 5.9 (2.1) | 0–12 |
| Semantic Fluency (animals) | 16.0 (5.0) | 2–48 |
| Trail Making Test Part A (sec) | 43.6 (21.6) | 1–150 |
| Trail Making Test Part B (sec) | 136.7 (75.6) | 13–300 |
| Digit Symbol | 37.9 (11.9) | 0–93 |
| Cognitive composite | 0 (1) | −3.9–3.1 |
| Mood | ||
| Geriatric Depression Scale (GDS) | 2.3 (2.5) | 0–15 |
FAQ = Functional Activities Questionnaire.
Measures
MCI Participant Demographic, Mood, and Cognitive Variables
MCI participant variables expected to be associated with functional abilities, including age, sex, years of education, Geriatric Depression Scale scores (15-item version) (GDS; Yesavage & Sheikh, 1986), and neuropsychological test scores, were collected to use as potential covariates in primary analyses (see Table 1). Neuropsychological tests that are sensitive to MCI and associated with functional abilities were included (i.e., attention, processing speed, executive functions, memory, and language (Weintraub et al., 2009; Schmitter-Edgecombe & Parsey, 2014)) and are described in Supplemental Table 1. A cognitive composite was created including test scores that were significantly associated with the FAQ, as outlined in the Statistical Analyses section.
Informant Variables
To examine potential bias in informant ratings, we obtained basic demographic variables of the informant including race, ethnicity, years of education, and sex. In addition, the informant’s relationship to the MCI participant, whether the informant lives with the participant (cohabitation), and approximate frequency of in-person visits (if no cohabitation) were collected. Informants were stratified according to these variables as outlined in Table 2.
Table 2.
Variables used to characterize informants (N = 4,284)
| Cohabitation | Yes (65%) | No (35%) |
| Relationship | Spouse, partner, or companion (60%) | Child (22%) | Sibling (4%) | Other relative (3%) | Friend, neighbor, or someone known through family, friends, work, or community (10%) | Paid caregiver, healthcare provider, or clinician (<1%) |
| Frequency of in-person visits (if no cohabitation) | Daily (12%) | At least 3 times per week (25%) | Weekly (29%) | At least 3 times per month (13%) | Monthly (13%) | Less than once a month (8%) |
| Race/ethnicity | Black/African American (15%) | Multiracial (3%) | White non-Hispanic (78%) | White Hispanic (2%) | Asian (2%) |
| Education | < 12 years (3%) | 12 years (18%) | 13–15 years (20%) | ≥16 years (59%) |
| Sex | Male (31%) | Female (69%) |
Functional Activities Questionnaire (FAQ)
Informant ratings on the FAQ were examined as the dependent variable. The FAQ is a 10-item scale designed to assesses functioning across activities that are common to older adults in the community and minimally influenced by socioeconomic status (Pfeffer, Kurosaki, Harrah, Chance, & Filos, 1982). Activities assessed include shopping, financial management, playing games, remembering appointments, cooking, paying attention and traveling. Each item is assigned a score of 0 (normal); 1 (has difficulty, but does by self); 2 (requires assistance); or 3 (dependent) such that higher scores indicate more restricted functioning. FAQ scores have been shown to discriminate between cognitively normal individuals vs. those with dementia (Pfeffer et al., 1982); cognitively normal vs. MCI; and MCI vs. dementia (Brown, Devanand, Liu, Caccappolo, & Alzheimer’s Disease Neuroimaging, 2011; Teng et al., 2010). A cut score of FAQ sum > 5 (corresponding to a mean FAQ > .50; or two items with ratings > 3) was originally proposed to distinguish individuals with mild dementia from those with healthy cognition (Pfeffer et al., 1982), with subsequent studies suggesting cut scores ranging from an FAQ sum of 4 to 7; see Castilla‐Rilo et al. (2007). A cut score between FAQ sum 5–6 and between mean FAQ .436-.437 has been proposed to distinguish people with MCI vs. mild dementia (Teng et al., 2010). Despite lack of clarity regarding precise cut scores, the FAQ is the primary measure of functional status in the NACC database and was administered to an informant as part of the UDS at each annual visit (Morris, 2008; Morris et al., 2006; Weintraub et al., 2009). The score at the visit associated with initial MCI diagnosis was used for this study. The mean FAQ score was calculated for each participant using all valid responses (Mean FAQ). Table 1 includes descriptive information of Mean FAQ across all MCI participants.
STATISTICAL ANALYSES
Analyses were performed using SPSS, version 24. Because our dependent variable (Mean FAQ) was positively skewed, analyses were performed using nonparametric methods and square root transformation. All results were considered significant at p<.005, as recommended by Ioannidis (2018).
First, we used bivariate Spearman’s rank-order correlations to examine associations between MCI participant variables (i.e., demographics, mood, cognition) and Mean FAQ for determination of covariates in subsequent analyses. Neuropsychological test scores that were significantly (p<.005) associated with Mean FAQ were entered into a Principal Component Analysis (PCA) to form a composite score(s). Factors/components with eigenvalues greater than 1, with a maximum iteration of 25, were extracted (Field, 2013).
Next, we used Pearson chi-square tests of independence to measure associations among informant features. Significant (p<.005) and substantial (Cramer’s V ≥ .40) associations among informant variables informed our analytic plan for multivariate analyses (Rea & Parker, 1992).
Finally, to determine the effect of informant features on informant-rated Mean FAQ, above and beyond the effect of participant variables (i.e., demographics, mood, cognition), Mean FAQ (square root transformed) was compared across stratified informant groups using univariate general linear model (GLM) analyses with post-hoc tests while covarying for participant features. Interactions between informant variables also were tested. Due to the non-parametric distribution of Mean FAQ, predicted values of Mean FAQ (covarying for participant features) were calculated for each participant and were used to visualize main effects results within violin plots. Hierarchical linear regressions (with participant variables in Block 1 and non-overlapping informant variables in Block 2) were conducted to determine the relative contribution of informant and participant variables on Mean FAQ in a multivariate model.
RESULTS
Relations between Participant Variables and Everyday Function (FAQ)
As shown in Table 3, participant age, sex, and GDS score were significantly (although weakly) correlated with Mean FAQ. Also shown in Table 3, seven of the 10 neuropsychological tests listed in Supplemental Table 1 were significantly associated with Mean FAQ and were entered into PCA to create a cognitive composite(s). Results of the PCA yielded a single component explaining 45.5% of the variance, with a Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy = .80 (Supplemental Table 2). Regression-based factor scores were used as a single cognitive composite covariate, with lower scores reflecting poorer overall cognitive performance.
Table 3.
Nonparametric Spearman’s correlation coefficients for Mean FAQ x MCI participant variables
| Mean FAQ Spearman’s rho | |
|---|---|
| MCI Participant Demographic Variables | |
| Age | .061* |
| Education | −0.009 |
| Sex | −.051*a |
| MCI Participant Mood | |
| Geriatric Depression Scale (GDS) | .172* |
| MCI Participant Cognitive Measures | |
| Logical Memory IIA Delayed | −.225* |
| Recall | |
| Benson Figure Delayed Recall | −0.081 |
| Digit Span Forward | −0.021 |
| Digit Span Backward | −.043* |
| Semantic Fluency (animals) | −.115* |
| Trail Making Test Part A | .103* |
| Trail Making Test Part B | .119* |
| Digit Symbol | −.155* |
| Boston Naming Test | −0.012 |
| Letter fluency | 0.056 |
| Cognitive composite | −.169* |
Male=1; Female =2
p<.005.
Relations among Informant Variables
Pearson chi-square tests identified a significant association between the informant variables of cohabitation and relationship (χ2 (6, N=4302) = 3298.79, p<.001, V = .88), such that informants who live with the MCI participant are more likely to be spouses (91%) than non-spouses. Therefore, to avoid multicollinearity in our regression analysis, two separate regressions were conducted including either cohabitation and not relationship or relationship and not cohabitation. Relations between other informant variables were identified as significant (p<.005) according to chi-square tests; however, they did not reach a level of moderate association (Cramer’s V’s ≤.23) and as such were included together in regression analyses (Supplemental Table 3).
Main Effects of Informant Features on FAQ after Controlling for Participant Variables
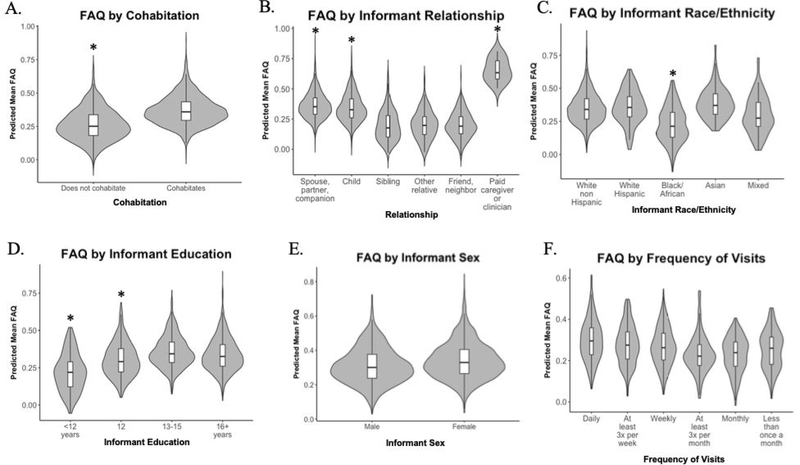
Univariate GLM analyses of square root transformed Mean FAQ with MCI participant age, sex, depression, and cognition as covariates demonstrated significant main effects of informant cohabitation (F(1,3473) = 76.44; p<.001; np2 = .022), relationship with participant (F(6,3468) = 17.31; p<.001; np2 = .029), race/ethnicity (F(4,3415) = 29.47; p<.001; np2 = .033), and education (F(3,3301) = 8.71; p<.001; np2 = .008). (Figure 1A–D). There was no significant main effect of informant sex (F(1,3473) = .73; p = .39; np2 <.001) or frequency of in-person visits (F(5,1221) = .29; p = .92; np2 = .001) (Figure 1E, 1F). As shown in Figure 1A, informants who live with the MCI participant assigned significantly higher (more impaired) FAQ ratings than those who do not. Figure 1B shows that among informant relationships, paid caregivers/clinicians, spouses/partners/companions, and adult children assigned significantly higher ratings than siblings, friends/neighbors, or other relatives. The main effect of informant race/ethnicity was driven by significantly lower (less impaired) ratings among informants who identify as Black or African American as compared to all other groups (Figure 1C). Finally, Figure 1D demonstrates that lower levels of informant education were associated with lower (less impaired) FAQ ratings. These results remained significant with Kruskal Wallis & Mann-Whitney tests for non-normally distributed data.
Fig. 1.
Violin plots with integrated box plots depicting distributions of predicted Mean FAQ scores covarying for participant age, sex, depression and cognition across informant variables. Significant main effects found in: A. Cohabitation, B. Relation to participant, C. Race/ethnicity, D. Education. Non-significant main effects found in: E. Sex, F. Frequency of in-person visits.*p<.005 for pairwise comparisons indicating significant difference from other groups (except in B. Relationship: Paid caregiver is significantly higher than all groups except Spouse and Child). FAQ = Functional Activities Questionnaire.
Interactions also were tested. There was an interaction trending towards significance between informant sex and cohabitation (F(1,3471) = 7.52; p = .006; np2 = .002) such that among informants who live with the participant, female informants reported more functional impairment, whereas the opposite pattern was observed (male informant > female informant) among informants who do not live with the participant. No other interactions were significant (p’s >.2).
Multivariate Analysis of Informant Variables as Predictors of FAQ
To examine the effect of all informant variables in a single analysis, hierarchical multivariate linear regression analyses were performed (Tables 4–5). Participant variables (age, sex, depression, and cognition) were entered in Block 1 and informant variables were analyzed in Block 2. Results of Block 1 showed that three participant covariates (sex, depression, cognition) contributed significantly to the model and accounted for 5.6% of the variance in informant-rated Mean FAQ. In the first regression (including cohabitation and not relationship), adding informant variables that demonstrated significant main effects (cohabitation, race/ethnicity, and education) to Block 2 significantly improved the prediction model and explained an additional 3.5% of the variance in Mean FAQ (Table 4). Together, all seven variables accounted for 8.9% of the variance in FAQ. In the second regression (including relationship and not cohabitation), adding informant variables to Block 2 (relationship, race/ethnicity, and education) significantly improved the prediction model and explained an additional 3.3% of the variance in Mean FAQ (Table 5). Together, all seven variables accounted for 8.7% of the variance in FAQ.
Table 4.
Hierarchical linear regression of MCI participant (Block 1) and informant (Block 2) variables predicting Mean FAQ score (square root transformed)
| Block | Predictors | Adjusted R2 | R2 Change | F (df) | β | t | P |
|---|---|---|---|---|---|---|---|
| 1 | Participant sex | −0.06 | −3.53 | <.001* | |||
| Participant age | 0.055 | 0.056 | 48.45 | 0.009 | 0.516 | 0.606 | |
| Participant GDS | (4,3265)* | 0.14 | 7.87 | <.001* | |||
| Participant cognition | −0.18 | −9.81 | <.001* | ||||
| 2 | Participant sex | 0.004 | 0.196 | 0.844 | |||
| Participant age | 0.008 | 0.461 | 0.645 | ||||
| Participant GDS | 0.131 | 7.67 | <.001* | ||||
| Participant cognition | 0.089 | 0.035 | 46.67 | −0.226 | −12.29 | <.001* | |
| Informant cohabitation | (7,3262)* | 0.136 | 7.42 | <.001* | |||
| Informant | −0.105 | −5.87 | <.001* | ||||
| race/ethnicity | |||||||
| Informant education | 0.073 | 4.27 | <.001* | ||||
Note. Dependent variable (Mean FAQ) was square root transformed. Excluding informant relationship variable from Block 2.
p<.001.
FAQ = Functional Activities Questionnaire; GDS = Geriatric Depression Score.
Table 5.
Hierarchical linear regression of MCI participant (Block 1) and informant (Block 2) variables predicting Mean FAQ score (square root transformed)
| Block | Predictors | Adjusted R2 | R2 Change | F (df) | β | t | P |
|---|---|---|---|---|---|---|---|
| 1 | Participant sex | −0.06 | −3.53 | <.001* | |||
| Participant age | 0.055 | 0.056 | 48.45 | 0.009 | 0.516 | 0.606 | |
| Participant GDS | (4,3265)* | 0.14 | 7.87 | <.001* | |||
| Participant cognition | −0.18 | −9.81 | <.001 | ||||
| 2 | Participant sex | −0.011 | −0.653 | 0.513 | |||
| Participant age | −0.001 | −0.077 | 0.939 | ||||
| Participant GDS | 0.131 | 7.68 | <.001* | ||||
| Participant cognition | 0.087 | 0.033 | 45.72 | −0.222 | −12.05 | <.001* | |
| Informant | (7,3262)* | −0.104 | −5.80 | <.001* | |||
| race/ethnicity | |||||||
| Informant education | 0.062 | 3.62 | <.001* | ||||
| Informant relationship | −0.124 | −7.00 | <.001* | ||||
Note. Dependent variable (Mean FAQ) was square root transformed. Excluding informant cohabitation variable from Block 2.
p<.001.
FAQ = Functional Activities Questionnaire; GDS = Geriatric Depression Score.
DISCUSSION
Our findings demonstrate that demographic and relational features of informants influence the perception and reporting of IADL in adults with MCI. Specifically, informant cohabitation with and relationship to the person with MCI, as well as informant education and race/ethnicity were each significantly associated with informant-rated FAQ scores. Importantly, the influence of these informant variables was observed even after controlling for demographics, cognition, and depressive symptoms of the person with MCI, which may have otherwise explained differences in everyday functioning.
Understanding discrepancies in informant reporting of everyday functioning is important for several reasons. Clinically, IADL functioning is a core criterion distinguishing MCI from dementia, making accurate assessment of function critical for differential diagnosis. That is, according to current clinical diagnostic criteria, individuals with the exact same cognitive test scores may be diagnosed with either mild dementia or MCI depending on their level of functional ability. Further, it has been shown that even small differences in the FAQ can have a robust threshold effect, such that any increase over 1 point on the FAQ sum is associated with a threefold increased risk of conversion from MCI to dementia (Mis, Devlin, Drabick, & Giovannetti, 2019) and a significantly greater risk of conversion to either MCI or dementia in healthy controls (Nowrangi, Rosenberg, & Leoutsakos, 2016). Therefore, even small effects of informant features, such as the modest effect sizes observed in this study (.008 < ηp2’s ≤ .033), may have a meaningful impact on predictive validity of the FAQ. With regard to clinical trials, researchers have emphasized the need for more clinically relevant outcomes (Posner et al., 2017) and the FDA now requires demonstrated improvements in valid and meaningful functional endpoints for medications targeting dementia prevention and treatment (Kozauer & Katz, 2013). Given the predictive validity of subtle declines in functioning (Farias, Lau, Harvey, Denny, Barba, & Mefford, 2017; Nowrangi, Rosenberg, & Leoutsakos, 2016), it is likely that clinical trials will begin to implement formal IADL assessment as part of inclusion and exclusion criteria for studies targeting MCI and pre-MCI populations, in addition to the use of IADLs as endpoints. Our results suggest that caution is warranted when relying upon informant-reported functioning for differential diagnosis, prognosis, and outcome studies.
Nonetheless, informant report is a widely used tool. In a review of studies that examined IADL restrictions in MCI patients as compared to healthy controls and those with AD, Jekel et al. (2015) identified that 15 of the 35 studies relied solely on informant report questionnaires to assess IADL. This finding, along with the results from our study, raise the important question of how we might account for discrepancies in informant reporting. It may be possible to adjust for informant features in a similar manner to the current use of demographically adjusted neuropsychological scores. This method would be especially useful in situations with conflicting information; for instance, when informant reporting indicates more apparent functional difficulties but patient reporting and cognitive test scores indicate sub-threshold impairment. Consideration of informant features (race, relationship, level of education, etc.) may be useful in interpreting the informant report in these situations. The influence of informant features in clinical trials has been largely ignored. In a review of six Alzheimer’s Disease Cooperative Study randomized clinical trials, the distribution of study partners was similar to that of the present study, with 63% spouses and 23% adult children (Grill, Raman, Ernstrom, Aisen, Karlawish, 2013). Our findings showing significantly different FAQ ratings between spouses and adult children suggest that the distribution of study-partner type should be carefully considered in trials that rely on informant report of IADL.
The results of our study contribute important insights to the extant literature on discrepancies in informant reporting. Here, we saw that closer relationships (e.g., spouses vs. children vs. siblings or friends) were associated with higher ratings of functional impairment. On the contrary, in a mixed population of persons with MCI and dementia, Persson et al. (2015) reported that spouses underestimated functional deficits relative to non-spouses, which they posited may have been due to gradual functional decline or disease denial. In individuals with MCI we believe that closer relationships and cohabitation may afford certain informants more opportunities to observe everyday errors, which, paired with high levels of concern among a non-community-based study cohort, may lead to over-reporting.
Our finding of lower FAQ ratings among informants who identify as Black or African American aligns with studies suggesting that differences in the perception, subjective experience, and meanings assigned to dementia may lead Black caregivers to view caregiving situations from a more optimistic lens (Burns et al., 2006; Dilworth-Anderson & Gibson, 2002; Farran, Miller, Kaufman, & Davis, 1997; Raczynski et al., 1994). Although we did not find significant overall interactions between race/ethnicity and other informant variables, some studies examining caregiver ethnicity and relationship have suggested that discrepancies between informants of different racial or ethnic identities should be interpreted in the context of relationship and sex, as individuals from different racial and ethnic groups assign different caregiving responsibilities to unique members of the family (Azar et al., 2017; Mintzer et al., 1992). While these studies examined caregiving attitudes for individuals with dementia as opposed to MCI, possibly rendering these findings less relevant in MCI settings, these interactions between race/ethnicity and relationship should still be examined in other datasets with sufficient racial and ethnic diversity.
Finally, the observed association between high levels of informant education and higher FAQ ratings (reflecting more IADL difficulties) suggest that high levels of education may be associated with greater awareness of the symptoms of MCI and dementia and an increased sensitivity to observed functional limitations. This is an interesting finding and relevant given the uneven distribution of highly educated participants in studies like the NACC. Replication of these findings within a less educated and more diverse cohort will be important.
There are several limitations worth noting. We analyzed only a single timepoint (visit at initial MCI diagnosis), though it would be beneficial to explore whether/how informant features are associated with longer term accuracy of future progression. In addition, it is noteworthy that in regression analyses the MCI participant variables accounted for 5.5% of the variance and the informant variables accounted for an additional 3.5%, leaving 91% of variance in FAQ ratings unexplained. Although the aim of the present study was to explore informant effects and not to account for all of the sources of variance in the FAQ, unexplored variables may account for some of this remaining variance. Regarding the informant variables that were investigated, the NACC database does not include measures of caregiver burden, informant depression, informant cognition, and other confounds previously explored in the literature and found to influence informant ratings in dementia (Conde-Sala et al., 2013; DeBettignies, Mahurin, & Pirozzolo, 1993). Although caregiver burden is likely less relevant in MCI where impairments are more subtle, informant depression and cognition may be important influencing features that should be evaluated in future research. With respect to MCI participant variables, analyses covaried for MCI participant demographics, cognition, and mood to determine whether informant features influenced functional ratings above and beyond these variables; however, this methodology assumes that measures of cognition can serve as a proxy for true real-world function, which is not always true (Royall, Lauterbach, Kaufer, Malloy, Coburn, & Black, 2007; Mcalister et al., 2016). While these variables (demographic, cognition, and depression) were the most valid available controls for function, our findings would be strengthened by covarying for either a) a performance-based task that measures objective functional ability, or b) additional MCI participant variables that may also contribute to differences in functioning and subsequent discrepancies in FAQ ratings. These additional covariates might include features such as literacy and physical functioning which were not available for analysis, but should be included in future research. Of note, we did not expect MCI participant physical functioning to play a large role in informant FAQ discrepancies because 1) any sensory difficulties would be captured in cognitive test performance which already served as a covariate, 2) per the standard administration instructions, informants are instructed to only report functional limitations on the FAQ that are due to cognitive problems, and not to penalize based on physical limitations, and 3) we excluded participants with a history of major comorbid neurologic conditions that would be expected to influence physical functioning. Nonetheless, controlling for physical functioning and/or general health is an important future direction.
Taken together, these results suggest that certain informant features influence functional reporting above and beyond participant demographic, cognitive, and mood features. There are several future directions that should be explored. First, as Jekel et al. (2015) note, studies comparing multiple functional assessment modalities (self-report, informant-report, and performance-based) in the same sample are needed to clarify underlying causes of discrepancy. An additional question that may be pursued is whether informant discrepancies are specific to MCI subtype (e.g., amnestic versus non-amnestic) or to the participant’s cognitive profile. Further, it is likely that neither current cognitive composites nor existing measures of function are sensitive enough to detect changes in pre-MCI populations (Posner et al., 2017). There is a clear need to develop sensitive tasks that are capable of measuring the range of performance and subtle changes that occur in preclinical AD and MCI. A promising initial result in this direction includes findings from Farias et al. (2017) whose study of a group of cognitively normal individuals demonstrated that self- and informant-rated functional limitations on the Everyday Cognition (ECog) scale were associated with significantly greater risk of diagnostic conversion to MCI, even after controlling for baseline cognitive abilities. Performance-based tasks have also shown great promise in characterizing in real time the subtle difficulties that emerge in pre-dementia populations, but they can be burdensome to administer and score. Fortunately, new technologies may provide a solution by translating performance-based tasks to increasingly scalable platforms. Using a non-immersive tablet-based virtual reality task modeled after the Naturalistic Action Task (Schwartz, Segal, Veramonti, Ferraro & Buxbaum, 2002), Giovannetti et al. (2018) have shown that the Virtual Kitchen task can quantify subtle and discrete types of micro-errors that occur during the completion of an everyday task, and that performance on this task is associated with underlying cognition. Other novel measures that leverage computer- and phone-based platforms include the Harvard Automated Phone Task Marshall et al.. (2015) and the Czaja Functional Assessment Battery (Czaja, Loewenstein, Lee, Fu, & Harvey, 2017; Czaja et al., 2017). Measures like these will provide more objective and ecologically valid representations of specific and subtle functional difficulties that emerge over the course of aging, and will allow for more accurate and efficient characterization of everyday functioning in both clinical and research settings.
Supplementary Material
Acknowledgements
The authors have no conflicts of interest and are grateful to Molly Ungrady for her contributions in the development of Figure 1 plots. Funding for Katherine Hackett and Tania Giovannetti’s effort was partially provided by NIA/NIH grant R21AG060422 to TG. Preliminary results from this project were presented at the 2019 North American meeting of the International Neuropsychological Society.
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, … Petersen RC (2011). The diagnosis of mild cognitive impairment due to alzheimer’s disease: Recommendations from the national institute on aging-alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association. (2019). 2019 Alzheimer’s Disease Facts and Figures. Alzheimers Dement, 15(3), 321–87. [Google Scholar]
- Azar M, Zhu C, DeFeis B, Gu Y, Ornstein K, Lawless S, & Cosentino S (2017). Increased reporting accuracy of alzheimer disease symptoms in caribbean hispanic informants. Alzheimer Disease & Associated Disorders, doi: 10.1097/WAD.0000000000000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, … Kukull WA (2007). The national alzheimer’s coordinating center (NACC) database: The uniform data set. Alzheimer Disease & Associated Disorders, 21(3), 249–258. doi: 10.1097/wad.0b013e318142774e [DOI] [PubMed] [Google Scholar]
- Beekly DL, Ramos EM, Van Belle G, Deitrich W, Clark AD, Jacka ME, & Kukull WA (2004). The national alzheimer’s coordinating center (NACC) database: An alzheimer disease database. Alzheimer Disease & Associated Disorders, 18(4), 270–277. [PubMed] [Google Scholar]
- Bluethmann SM, Mariotto AB, & Rowland JH (2016). Anticipating the “silver tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the united states. Cancer Epidemiology, Biomarkers & Prevention, 25(7), 1029–1036. doi: 10.1158/1055-9965.EPI-16-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, … & Salmon DP (2014). Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. Journal of Alzheimer’s Disease, 42(1), 275–289. doi: 10.3233/JAD-140276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosboom PR, Alfonso H, Eaton J, & Almeida OP (2012). Quality of life in alzheimer’s disease: Different factors associated with complementary ratings by patients and family carers. International Psychogeriatrics, 24(5), 708–721. doi: 10.1017/S1041610211002493 [DOI] [PubMed] [Google Scholar]
- Brown PJ, Devanand DP, Liu X, Caccappolo E, & Alzheimer’s Disease Neuroimaging I (2011). Functional impairment in elderly patients with mild cognitive impairment and mild alzheimer disease. Archives of General Psychiatry.Pages = {617–626}, 68(6) doi: 10.1001/archgenpsychiatry.2011.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R, Nichols LO, Graney MJ, Martindale-Adams J, & Lummus A (2006). Cognitive abilities of alzheimer’s patients: Perceptions of black and white caregivers. International Journal of Aging & Human Development, 62(3), 209–219. doi: 10.2190/3GG6-8YV1-ECJG-8XWN [DOI] [PubMed] [Google Scholar]
- Castilla‐Rilo J, López‐Arrieta J, Bermejo‐Pareja F, Ruiz M, Sánchez‐Sánchez F, & Trincado R (2007). Instrumental activities of daily living in the screening of dementia in population studies: a systematic review and meta‐analysis. International Journal of Geriatric Psychiatry: A journal of the psychiatry of late life and allied sciences, 22(9), 829–836. doi: 10.1002/gps.1747 [DOI] [PubMed] [Google Scholar]
- Conde-Sala J, Re-Ramrez R, Turr-Garriga O, Gascn-Bayarri J, Juncadella-Puig M, Moreno-Cordn L, … Garre-Olmo J (2013). Factors associated with the variability in caregiver assessments of the capacities of patients with alzheimer disease. Journal of Geriatric Psychiatry and Neurology, 26(2), 86–94. doi: 10.1177/0891988713481266 [DOI] [PubMed] [Google Scholar]
- Connell CM, Roberts JS, McLaughlin SJ, & Akinleye D (2009). Racial differences in knowledge and beliefs about alzheimer disease. Alzheimer Disease & Associated Disorders, 23(2), 110–116. doi: 10.1097/WAD.0b013e318192e94d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Morstorf T, & Zhong K (2014). Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimer’s Research & Therapy, 6(4), 37. doi: 10.1186/alzrt269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaja SJ, Loewenstein DA, Lee CC, Fu SH, & Harvey PD (2017). Assessing functional performance using computer-based simulations of everyday activities. Schizophrenia research, 183, 130–136. doi: 10.1016/j.schres.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaja SJ, Loewenstein DA, Sabbag SA, Curiel RE, Crocco E, & Harvey PD (2017). A novel method for direct assessment of everyday competence among older adults. Journal of Alzheimer’s Disease, 57(4), 1229–1238. doi: 10.3233/JAD-161183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBettignies BH, Mahurin RK, & Pirozzolo FJ (1993). Functional status in alzheimer’s disease and multi-infarct dementia. Clinical Gerontologist, 12(4), 31–49. doi: 10.1300/J018v12n04_03 [DOI] [PubMed] [Google Scholar]
- Dilworth-Anderson P, & Gibson BE (2002). The cultural influence of values, norms, meanings, and perceptions in understanding dementia in ethnic minorities. Alzheimer Disease & Associated Disorders, 16, S63. [DOI] [PubMed] [Google Scholar]
- Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, & Bondi MW (2014). Subjective cognitive complaints contribute to misdiagnosis of mild cognitive impairment. Journal of the International Neuropsychological Society, 20(8), 836–847. doi: 10.1017/S135561771400068X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Lau K, Harvey D, Denny KG, Barba C, & Mefford AN (2017). Early functional limitations in cognitively normal older adults predict diagnostic conversion to mild cognitive impairment. Journal of the American Geriatrics Society, 745 65(6), 1152–1158. doi: 10.1111/jgs.14835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farran CJ, Miller BH, Kaufman JE, & Davis L (1997). Race, finding meaning, and caregiver distress. Journal of Aging and Health, 9(3), 316–333. doi: 10.1177/089826439700900303 [DOI] [PubMed] [Google Scholar]
- Field A (2013). Discovering statistics using IBM SPSS statistics. sage. [Google Scholar]
- Giovannetti T, Seligman SC, Britnell P, Brennan L, & Libon DJ (2015). Differential effects of goal cues on everyday action errors in alzheimer’s disease versus parkinson’s disease dementia. Neuropsychology, 29(4), 592. doi: 10.1037/neu0000167 [DOI] [PubMed] [Google Scholar]
- Giovannetti T, Yamaguchi T, Roll E, Harada T, Rycroft SS, Divers R, … & Hackett K (2018). The Virtual Kitchen Challenge: preliminary data from a novel virtual reality test of mild difficulties in everyday functioning. Aging, Neuropsychology, and Cognition, 1–19. doi: 10.1080/13825585.2018.1536774 [DOI] [PubMed] [Google Scholar]
- Grill JD, Raman R, Ernstrom K, Aisen P, & Karlawish J (2013). Effect of study partner on the conduct of alzheimer disease clinical trials. Neurology, 80(3), 282–288. doi: 10.1212/WNL.0b013e31827debfe [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn JL, & Cattell RB (1967). Age differences in fluid and crystallized intelligence. Acta psychologica, 26, 107–129. doi: 10.1016/0001-6918(67)90011-X [DOI] [PubMed] [Google Scholar]
- Ioannidis JP (2018). The proposal to lower P value thresholds to. 005. Jama, 319(14), 1429–1430. doi: 10.1001/jama.2018.1536 [DOI] [PubMed] [Google Scholar]
- Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, … & Liu E. (2018). NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s & Dementia, 14(4), 535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekel K, Damian M, Wattmo C, Hausner L, Bullock R, Connelly PJ, … Graessel E. (2015). Mild cognitive impairment and deficits in instrumental activities of daily living: A systematic review. Alzheimer’s Research & Therapy, 7(1), 17. doi: 10.1186/s13195-015-0099-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, & Weintraub S (1983). The boston naming test. 2nd Philadelphia: Lea & Febiger, [Google Scholar]
- Kozauer N, & Katz R (2013). Regulatory innovation and drug development for early-stage alzheimer’s disease. New England Journal of Medicine, 368(13), 1169–1171. doi: 10.1056/NEJMp1302513 [DOI] [PubMed] [Google Scholar]
- Lawton MP, & Brody EM (1969). Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist, 9(3), 179–186. Retrieved from http://www-ncbi-nlm-nih-gov.libproxy.temple.edu/pubmed/5349366 [PubMed] [Google Scholar]
- Lin A, Brook J, Grill JD, & Teng E (2017). Participant-informant relationships affect quality of life ratings in incipient and clinical alzheimer disease. The American Journal of Geriatric Psychiatry : Official Journal of the American Association for Geriatric Psychiatry, 25(3), 297–307. doi: 10.1016/j.jagp.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein DA, Arguelles S, Bravo M, Freeman RQ, Arguelles T, Acevedo A, & Eisdorfer C (2001). Caregivers’ judgments of the functional abilities of the alzheimer’s disease patient: A comparison of proxy reports and objective measures. The Journals of Gerontology, Series B, 56(2), P78. doi: 10.1093/geronb/56.2.P78 [DOI] [PubMed] [Google Scholar]
- Mangone CA, Sanguinetti RM, Baumann PD, Gonzalez RC, Pereyra S, Bozzola FG, … Sica REP (1993). Influence of feelings of burden on the caregiver’s perception of the patient’s functional status. Dementia and Geriatric Cognitive Disorders, 4(5), 287–293. doi: 10.1159/000107335 [DOI] [PubMed] [Google Scholar]
- Marshall GA, Dekhtyar M, Bruno JM, Jethwani K, Amariglio RE, Johnson KA, Sperling RA, & Rentz DM (2015). The Harvard Automated Phone Task: New performance-based activities of daily living tests for early Alzheimer’s disease. The Journal of Prevention of Alzheimer’s Disease, 2(4), 242. doi: 10.14283/jpad.2015.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcalister C, Schmitter-Edgecombe M, & Lamb R (2016). Examination of variables that may affect the relationship between cognition and functional status in individuals with mild cognitive impairment: A meta-analysis. Archives of Clinical Neuropsychology, 31(2), 123–147. doi: 10.1093/arclin/acv089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintzer J, Rubert M, Loewenstein D, Gamez E, Millor A, Quinteros R, … Eisdorfer C. (1992). Daughters caregiving for hispanic and non-hispanic alzheimer patients: Does ethnicity make a difference? Community Mental Health Journal, 28(4), 293–303. doi: 10.1007/BF00755796 [DOI] [PubMed] [Google Scholar]
- Mis R, Devlin K, Drabick D, & Giovannetti T (2019). Heterogeneity of Informant-Reported Functional Performance in Mild Cognitive Impairment: A Latent Profile Analysis of the Functional Activities Questionnaire. Journal of Alzheimer’s Disease, (Preprint), 1–14. doi: 10.3233/JAD-180975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC (2008). National alzheimer’s coordinating center: NACC uniform data set (UDS) coding guidebook for initial visit packet. Washington University: ADC Clinical Task Force, [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, … Clark C. (1989). The consortium to establish a registry for alzheimers disease (CERAD). part I. clinical and neuropsychological assessment of alzheimers disease. Neurology, 39(9), 1159–1165. Retrieved from https://www.scopus.com/inward/record.uri?eid=2-s2.0-0024453856&partnerID=40&md5=fd2c5775c7225672d7b5dc5338ed3c27 [DOI] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, DeCarli C, Ferris S, … Peskind ER. (2006). The uniform data set (UDS): Clinical and cognitive variables and descriptive data from alzheimer disease centers. Alzheimer Disease & Associated Disorders, 20(4), 210–216. doi: 10.1097/01.wad.0000213865.09806.92 [DOI] [PubMed] [Google Scholar]
- Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack C, Jagust W, … & Beckett L. (2005). The Alzheimer’s disease neuroimaging initiative. Neuroimaging Clinics, 15(4), 869–877. doi: 10.1016/j.nic.2005.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowrangi MA, Rosenberg PB, & Leoutsakos JS (2016). Subtle changes in daily functioning predict conversion from normal to mild cognitive impairment or dementia: An analysis of the NACC database.28(12), 2009–2018. doi: 10.1017/S1041610216000995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygard L (2003). Instrumental activities of daily living: A stepping-stone towards alzheimer’s disease diagnosis in subjects with mild cognitive impairment? Acta Neurologica Scandinavica, 107, 42–46. doi: 10.1034/j.1600-0404.107.s179.8.x [DOI] [PubMed] [Google Scholar]
- Pérès K, Chrysostome V, Fabrigoule C, Orgogozo JM, Dartigues JF, & Barberger-Gateau P (2006). Restriction in complex activities of daily living in MCI: Impact on outcome. Neurology, 67(3), 461–466. doi: 10.1212/01.wnl.0000228228.70065.f1 [DOI] [PubMed] [Google Scholar]
- Persson K, Braekhus A, Selbaek G, Kirkevold O, & Engedal K (2015). Burden of care and patient’s neuropsychiatric symptoms influence carer’s evaluation of cognitive impairment. Dementia and Geriatric Cognitive Disorders, 40(5–6), 256–267. doi: 10.1159/000437298 [DOI] [PubMed] [Google Scholar]
- Petersen RC (2004). Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine, 256(3), 183–194. doi: 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, … Winblad B (2001). Current concepts in mild cognitive impairment. Archives of Neurology, 58(12), 1985–1992. doi: 10.1001/archneur.58.12.1985 [DOI] [PubMed] [Google Scholar]
- Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, … Jack CR (2009). Mild cognitive impairment: Ten years later. Archives of Neurology, 66(12), 1447–1455. doi: 10.1001/archneurol.2009.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer R,Kurosaki I, T. T, Harrah C,H, Chance J,M, & Filos S (1982). Measurement of functional activities in older adults in the community. Journal of Gerontology, 37(3), 323–329. doi: 10.1093/geronj/37.3.323 [doi] [DOI] [PubMed] [Google Scholar]
- Posner H, Curiel R, Edgar C, Hendrix S, Liu E, Loewenstein DA, … Harvey PD (2017). Outcomes assessment in clinical trials of alzheimer’s disease and its precursors: Readying for short-term and long-term clinical trial needs. Innovations in Clinical Neuroscience, 14(1–2), 22. [PMC free article] [PubMed] [Google Scholar]
- Potter GG, Plassman BL, Burke JR, Kabeto MU, Langa KM, Llewellyn DJ, … Steffens DC (2009). Cognitive performance and informant reports in the diagnosis of cognitive impairment and dementia in african americans and whites. Alzheimers & Dementia, 5(6), 445–453. doi: 10.1016/j.jalz.2009.04.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raczynski JM, Taylor H, Cutter G, Hardin M, Rappaport N, & Oberman A (1994). Diagnoses, symptoms, and attribution of symptoms among black and white inpatients admitted for coronary heart disease. American Journal of Public Health, 84(6), 951–956. doi: 10.2105/AJPH.84.6.951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea LM, & Parker RA (1992). Designing and conducting survey research. San Francisco: Jossey-Boss. [Google Scholar]
- Reitan RM (1993). Wolfson D (Ed.), The halstead-reitan neuropsychological test battery : Theory and clinical interpretation (2nd ed. ed.). Tucson, Ariz.: Tucson, Ariz. : Neuropsychology Press. [Google Scholar]
- Rog LA, Park LQ, Harvey DJ, Huang CJ, Mackin S, & Farias ST (2014). The independent contributions of cognitive impairment and neuropsychiatric symptoms to everyday function in older adults. The Clinical Neuropsychologist, 28(2), 215–236. doi: 10.1080/13854046.2013.876101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royall DR, Lauterbach EC, Kaufer D, Malloy P, Coburn KL, & Black KJ (2007). The cognitive correlates of functional status: a review from the Committee on Research of the American Neuropsychiatric Association. The Journal of neuropsychiatry and clinical neurosciences, 19(3), 249–265. [DOI] [PubMed] [Google Scholar]
- Schelke MW, Hackett K, Chen JL, Shih C, Shum J, Montgomery ME, … Isaacson RS (2016). Nutritional interventions for alzheimer’s prevention: A clinical precision medicine approach. Annals of the New York Academy of Sciences, 1367(1), 50–56. doi: 10.1111/nyas.13070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2009). When does age-related cognitive decline begin?. Neurobiology of aging, 30(4), 507–514. doi: 10.1016/j.neurobiolaging.2008.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW (1994). The course of adult intellectual development. American Psychologist, 49, 304–313. doi: 10.1037/0003-066X.49.4.304 [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, & Parsey CM (2014). Cognitive correlates of functional abilities in individuals with mild cognitive impairment: Comparison of questionnaire, direct observation, and performance-based measures. The Clinical Neuropsychologist, 28(5), 726–746. doi: 10.1080/13854046.2014.911964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Segal M, Veramonti T, Ferraro M, & Buxbaum LJ (2002). The Naturalistic Action Test: A standardised assessment for everyday action impairment. Neuropsychological rehabilitation, 12(4), 311–339. doi: 10.1080/09602010244000084 [DOI] [Google Scholar]
- Siemers ER, Sundell KL, Carlson C, Case M, Sethuraman G, Liu-Seifert H, … Demattos R (2016). Phase 3 solanezumab trials: Secondary outcomes in mild alzheimer’s disease patients. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 12(2), 110–120. doi: 10.1016/j.jalz.2015.06.1893 [DOI] [PubMed] [Google Scholar]
- Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, & Aisen P (2014). The A4 study: Stopping AD before symptoms begin? Science Translational Medicine, 6(228), 228fs13. doi: 10.1126/scitranslmed.3007941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng E, Becker BW, Woo E, Knopman DS, Cummings JL, & Lu PH (2010). Utility of the functional activities questionnaire for distinguishing mild cognitive impairment from very mild alzheimer’s disease. Alzheimer Disease and Associated Disorders, 24(4), 348. doi: 10.1097/WAD.0b013e3181e2fc84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KR, Eppig JS, Weigand AJ, Edmonds EC, Wong CG, Jak AJ, … & Bondi MW (2019). Artificially low mild cognitive impairment to normal reversion rate in the Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s & Dementia. doi: 10.1016/j.jalz.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triebel KL, Martin R, Griffith HR, Marceaux J, Okonkwo OC, Harrell L, … Marson DC (2009). Declining financial capacity in mild cognitive impairment A 1-year longitudinal study. Neurology, 73(12), 928–934. doi: 10.1212/WNL.0b013e3181b87971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler A (1987). Wechsler adult intelligence scale-revised. San Antonio, Texas: Psychological Corporation. [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, … Galasko D (2009). The alzheimer’s disease centers’ uniform data set (UDS): The neuropsychological test battery. Alzheimer Disease and Associated Disorders, 23(2), 91. doi: 10.1097/WAD.0b013e318191c7dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, & Sheikh JI (1986). Geriatric depression scale (GDS) recent evidence and development of a shorter version. Clinical Gerontologist, 5(1–2), 165–173. [Google Scholar]
- Zanetti O, Geroldi C, Frisoni GB, Bianchetti A, & Trabucchi M (1999). Contrasting results between caregiver's report and direct assessment of activities of daily living in patients affected by mild and very mild dementia: the contribution of the caregiver's personal characteristics. Journal of the American Geriatrics Society, 47(2), 196–202. doi: 10.1111/j.1532-5415.1999.tb04578.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.