Abstract
Background:
Barth syndrome (BTHS) is a rare X-linked condition resulting in cardiomyopathy however; the effects of BTHS on myocardial substrate metabolism and its relationships with cardiac high-energy phosphate metabolism and left ventricular function are unknown. We sought to characterize myocardial glucose, fatty acid and leucine metabolism in BTHS and unaffected controls and examine their relationships with cardiac high-energy phosphate metabolism and left ventricular function.
Methods/Results:
Young adults with BTHS (n=14) and unaffected controls (n=11, Control, total n=25) underwent bolus injections of 15O-water and 1-11C-glucose, palmitate and leucine and concurrent positron emission tomography (PET) imaging. Left ventricular function and cardiac high-energy phosphate metabolism were examined via echocardiograpy and 31P magnetic resonance spectroscopy, respectively. Myocardial glucose extraction fraction (21 ± 14 vs. 10 ± 8 %, P =0.03) and glucose utilization (828.0 ± 470.0 vs. 393.2 ± 361.0 μmol•g−1•min−1, P =0.02) were significantly higher in BTHS vs. Control. Myocardial fatty acid extraction fraction (31 ± 7 vs. 41 ± 6 %, P <0.002) and uptake (0.25 ± 0.04 vs. 0.29 ± 0.03 ml•g−1•min−1, P <0.002) were significantly lower in BTHS vs. Control. Altered myocardial metabolism was associated with lower cardiac function in BTHS.
Conclusions:
Myocardial substrate metabolism is altered and may contribute to left ventricular dysfunction in BTHS.
Introduction
Barth syndrome (BTHS) is a rare (~1 in 300,000 live births)1 X-linked recessive condition that results in heart failure, skeletal muscle myopathy, and exercise intolerance.2,3 Mutations in the tafazzin gene (TAZ) cause BTHS by altering the phospholipid-acyltransferase tafazzin and result in abnormal mitochondrial cardiolipin.4 Alterations in cardiolipin manifest as mitochondrial abnormalities including smaller and fragmented mitochondria5, disruptions in supercomplex formation6, inner mitochondrial membrane instability7 and reduced respiratory capacity5.
Although cardiomyopathy is a defining feature of BTHS, clinical presentation is often variable, as many individuals will have normal or low-normal resting cardiac function by conventional echocardiography and modestly lower left ventricular (LV) function by speckle-tracking echocardiography.8–10 In contrast, a subset of individuals will require heart transplantation as infants, or less frequently, as adults.9 Our group previously found in adolescents and young adults with BTHS that although resting LV function was only mildly affected, cardiac contractility was significantly blunted during exercise, and contributed to severe exercise intolerance.11
Mechanisms that underlie cardiomyopathy in BTHS are not fully clear. We recently found impaired cardiac high-energy phosphate metabolism (i.e. phosphocreatine/ATP) in children, adolescents, and young adults with BTHS but phosphate metabolism was not associated with resting LV function.10 In addition, we recently demonstrated impaired whole-body fat oxidation and enhanced glucose metabolism during moderate exercise in individuals with BTHS.12 In other non-BTHS related heart failure conditions, alterations in myocardial glucose and fatty acid metabolism have been associated with reduced cardiac function.13,14 In addition, abnormal myocardial amino acid metabolism has been previously shown in non-BTHS models of heart failure. Abnormalities in amino acid metabolism appear to also be characteristic in BTHS as we found altered whole-body leucine metabolism in a previous pilot study of adolescents and young adults with BTHS.15–18 However, the role of myocardial substrate metabolism in cardiac dysfunction in BTHS is unknown. Therefore, the primary objective of the current study was to characterize myocardial glucose, fatty acid, and leucine metabolism in young adults with BTHS compared to unaffected controls. Our secondary objective was to examine potential relationships between myocardial substrate metabolism, cardiac high-energy phosphate metabolism and function in BTHS. Given that myocardial fatty acid metabolism is the primary source of energy for the adult mammalian heart, and that typically in heart failure patients without BTHS there is increased myocardial glucose metabolism, we hypothesized that myocardial fatty acid metabolism would be lower and glucose and leucine metabolism would be elevated in individuals with BTHS compared to healthy, unaffected controls. We secondarily hypothesized that alterations in myocardial glucose and fatty acid metabolism would be associated with lower cardiac function and cardiac high-energy phosphate metabolism. Information regarding perturbations of cardiac metabolism in mitochondrial disease may be add to the knowledge of the mechanisms of and possibly inform interventions to manipulate metabolism for other more common diseases such as heart failure.
Participants
Twenty-five (n=25) participants were studied, including n=14 young adults with BTHS and n=11 young adult unaffected controls (Control, Table 1). Participants with BTHS were recruited through the Barth Syndrome Registry located at the University of Florida and controls were recruited from the Greater St. Louis community. Inclusion criteria included 1) confirmed diagnosis of BTHS via genetic testing or healthy control, 2) age 18–36 years, 3) sedentary (physically active less than 2×/wk), and 4) medically stable and stable on medications for ≥ 3 months (BTHS only). Participants with BTHS were currently taking: beta blockers (71%), ACE inhibitors (50%), digoxin (57%), granulocyte colony-stimulating factor (29%), and supplemental amino acids (43%). Supplemental amino acids were not taken 3 days prior to and during the study. This study was approved by the Human Research Protection Office at Washington University in St. Louis (WUSTL). All participants provided written informed consent.
Table 1:
Participant demographic and anthropometric data
| Control (n=11) | BTHS (n=14) | p-value | |
|---|---|---|---|
| Age (years) | 24 ± 5 | 26 ± 4 | 0.26 |
| Height (cm) | 176.6 ± 5.3 | 177.2 ± 7.3 | 0.80 |
| Weight (kg) | 81.1 ± 11.7 | 68.1 ± 12.8 | 0.01 |
| BMI | 26.1 ± 3.9 | 21.6 ± 3.4 | <0.001 |
| FFM (kg) | 63.8 ± 6.8 | 41.1 ± 6.1 | <0.001 |
| FFM (%) | 80 ± 7 | 63 ± 12 | <0.001 |
| FM (kg) | 16.8 ± 7.3 | 26.1 ± 10.7 | 0.03 |
| FM (%) | 20 ± 7 | 37 ± 12 | <0.001 |
| Hemoglobin (g/dL) | 14.4 ± 0.8 | 14.1 ± 10 | 0.50 |
| Hematocrit (%) | 43.1 ± 2.1 | 41.7 ± 3.2 | 0.26 |
| ANC (K/cumm) | 3.3 ± 1.1 | 1.3 ± 1.0 | <0.001 |
| TG (mg/dL) | 96.7 ± 53.3 | 91.5 ± 33.6 | 0.76 |
| Total Chol (mg/dL) | 167.3 ± 24.1 | 126.1 ± 25.2 | <0.001 |
| HDL (mg/dL) | 47.3 ± 11.1 | 40.0 ± 12.7 | 0.12 |
| LDL (mg/dL) | 101.4 ± 23.2 | 67.7 ± 23.2 | 0.001 |
Values are means ± SD. FFM: fat free mass, FM: fat mass, ANC: absolute neutrophil count, ALC: absolute lymphocyte count AMC: absolute monocyte count, AEC: absolute eosinophil count, TG: triglyceride, Chol: cholesterol, HDL: high-density lipoprotein, LDL: low-density lipoprotein.
Methods
Procedures were performed over a two-day period at the Clinical Research Unit, the Center for Clinical Imaging Research, and Cardiovascular Imaging and Clinical Research Core Laboratory at WUSTL.
Day #1 of Study Visit
Body Composition
Following a medical history and physical examination from the study physician, fat mass and fat-free mass (kg and %) in all participants were determined through air-displacement plethymosgraphy (Bod Pod, Life Measurements Inc., Concord, CA).
Echocardiography
All participants underwent conventional two-dimensional (2-D), pulsed-wave Doppler, and tissue Doppler echocardiography (General Electric Vivid E9; Waukesha, WI, USA). LV mass was determined by 2D echocardiography according to recommendations of the American Society of Echocardiography.19 LV ejection fraction, fractional shortening, tissue-Doppler imaging and 2D speckle tracking echocardiographic-derived global peak systolic strain was determined as previously described.10
Cardiac High Energy Phosphate Metabolism
Cardiac high-energy phosphate metabolism (PCr/ATP) was determined by 31Phosphorus Magnetic Resonance Spectroscopy (31P-MRS, Prisma 3T MRI, Siemens Medical Systems, Erlangen, Germany) using a home built 31P transmit-receive RF coil as previously described.10 Phosphocreatine serves as an important energy reserve as it transfers the high-energy phosphate bond from the site of ATP production (mitochondria) to the site of ATP utilization (myofibrils).20 Briefly, resting participants were positioned with their heart at the center of the magnet, and the 31P surface coil was positioned on the chest with the center of the coil just below the mitral valve of the heart using proton scout images. A small fiducial marker placed at the center of the RF coil to adjust the positioning of the coil relative to the heart. A non-localized 31P spectrum was then acquired and RF transmit frequency was centered on the PCr resonance. Data were acquired following 2.56 ms adiabatic half passage pulse radiofrequency pulses applied at 6 s intervals and with a spectral width of 2 kHz and 64 averages per spectrum. Spectra were processed offline using the jMRUI (Java-Based Magnetic Resonance User Interface) software.21 Spectra were fitted in time domain by using a nonlinear least-squares algorithm (AMARES)22. ATP, PCr, and 2,3-diphosphoglycerate (DPG) signals were fitted to Lorentzian line shapes. The three ATP peaks were fitted as two doublets and one triplet, with equal amplitudes and line widths and prior knowledge for the J-coupling constant. After fitting, the γ-ATP peak area was corrected for blood contamination according to the amplitude of the 2,3-diphosphoglycerate (2,3-DPG) peak as described previously. PCr/ATP ratio was calculated and corrected for partial saturation.23 The coefficient of variation for PCr/ATP measurement by 31P-MRS is 7.73%.24
Graded Exercise Test
Participants performed a graded exercise test on a recumbent cycle ergometer to characterize exercise tolerance (VO2peak) as previously described.12
Evening Prior to Day #2 of Study Visit:
The evening prior to Day 2 of the study visit, participants consumed a standardized meal containing a total of 12 kcal/kg body weight (55% of total energy from carbohydrates, 30% from fat, and 15% from protein) prepared by the Bionutrition Research Kitchen at the WUSTL Clinical Research Unit. At 1900 h, participants ingested a carbohydrate beverage (80 gm carbohydrates, 12.2 gm fat, 17.6 gm protein, Boost; Nestle, Vevey, Switzerland) to ensure adequate muscle and hepatic glycogen stores. Participants were instructed to fast from 2000 h (except water) until after the study visit the following morning.
Day #2 of Study Visit
Myocardial Substrate Metabolism Study
Participants reported to the WUSTL Clinical Research Unit at 0700h for catheter placement. An 18-gauge catheter was inserted into an antecubital vein for radiopharmaceutical infusion, and a second catheter was inserted into a contralateral hand vein (heated to 55°C) for arterialized venous blood sampling. Participants were then transported to the WUSTL Center for Clinical Imaging Research via wheelchair. Positron emission tomography (PET) imaging was performed using a PET/CT tomograph (Biograph TruePoint/TrueView PET/CT, Siemens Medical Systems, Knoxville, TN) and was started at 0800h to standardize for potential circadian variations.25 Blood pressure and heart rate were monitored throughout the imaging study. PET imaging was used to quantify myocardial blood flow (MBF) after 15O-water injection, myocardial glucose extraction fraction, uptake and utilization after 1-11C-glucose injection, leucine turnover rate after 1-11C-leucine injection, and fatty acid (FA) extraction fraction, uptake, utilization, oxidation and esterification after 1-11C–palmitate injection. All PET acquisition followed previously described procedures.26–28 We examined myocardial leucine metabolism because 1) leucine is a branched-chain amino acid that can undergo oxidation in the TCA cycle and 2) we previously found altered whole-body leucine metabolism in adolescents and young adults with BTHS18. Data acquisition procedures and compartmental modeling equations are described in detail in Supplemental Materials.
Statistics
This study was a secondary analysis under a larger parent project (NCT#01625663) and was powered under the primary outcome.12 Demographics, energetic, cardiac function, peak exercise testing, and PET variables between groups were compared by independent-sample t-tests and Mann Whitney U tests, depending on normality. All variables in correlation analyses were determined to have a normal distribution using Shapiro-Wilk tests and were analyzed by Pearson Product Correlation Coefficient Analyses. Statistical significance was defined as a P value of 0.05 or less.
Results
Demographics
Participant demographics and anthropomorphic data are provided in Table 1. All participants were male, and all were Caucasian except for one African American (AA) adult participant with BTHS, one AA adult control, one adult participant with BTHS who was of Middle Eastern descent, and two Asian adult controls. Age was not different between BTHS vs. Control. Body weight and body mass index were significantly lower in BTHS vs. Control. Absolute fat-free mass (kg and %) were significantly lower and absolute fat mass (kg and %) was higher in BTHS vs. Control.
Fasting Hormones and Metabolites
Plasma total and low-density cholesterol concentrations were significantly lower in both in BTHS vs. Control. In contrast, plasma free fatty acid concentration was significantly higher and plasma insulin concentration tended to be higher in BTHS vs. Control (Table 2).
Table 2:
Cardiac Metabolism, Function and Energetics
| Variables | Control | BTHS | p-value |
|---|---|---|---|
| Myocardial Metabolism | |||
| MBF (ml•g−1•min−1) | 0.72 ± 0.10 (0.66, 0.78) | 0.83 ± 0.18 (0.74, 0.92) | 0.09 |
| GLU-EF (%) | 9 ± 7 (4, 14) | 21 ± 14 (13, 27) | 0.03* |
| MGUp (ml•g−1•min−1) | 0.08 ± 0.07 (0.03, 0.13) | 0.16 ± 0.09 (0.11, 0.21) | 0.03 |
| MGU (μmol•g−1•min−1) | 373.2 ± 346.2 (147.0, 599.4) | 828.0 ± 470.0 (572.5, 1083.5) | 0.02 |
| FA-EF (%) | 40 ± 6 (36, 44) | 31 ± 7 (27, 35) | 0.002 |
| FAOX-EF (%) | 82 ± 11 (75, 89) | 78 ± 11 (72, 84) | 0.43 |
| FAES-EF (%) | 18 ± 11 (11, 25) | 22 ± 11 (16, 28) | 0.43 |
| MFAOX (μmol•g−1•min−1) | 104.5 ± 61.4 (66.5, 142.6) | 114.8 ± 28.3(100.0, 129.6) | 0.58 |
| MFAES (μmol•g−1•min−1) | 21.8 ± 18.3 (10.5, 33.1) | 33.5 ± 19.5 (23.3, 43.7) | 0.58 |
| MFAUp-R (ml•g−1•min−1) | 0.29 ± 0.03 (0.27, 0.31) | 0.25 ± 0.03 (0.23, 0.27) | 0.02 |
| MFAUT (μmol•g−1•min−1) | 126.3 ± 72.6 (81.3, 171.3) | 148.4 ± 39.1 (127.9, 168.9) | 0.34 |
| Leucine K2 (min−1) | 0.24 ± 0.19 (0.12, 0.36) | 0.19 ± 0.4 (0.17, 2.1) | 0.33* |
| Plasma Substrates | |||
| FFA (mmol•L−1) | 0.43 ± 0.26 | 0.60 ± 0.14 | 0.05 |
| Glucose (mmol•L−1) | 5.2 ± 0.49 | 5.1 ± 0.48 | 0.44 |
| Insulin (uU•mL−1) | 4.0 ± 2.7 | 7.6 ± 5.7 | 0.09 |
| Myocardial Energetic Reserve | |||
| Cardiac ATP/PCr | 2.2 ± 0.2 (2.1, 2.3) | 1.8 ± 0.2 (1.7, 1.9) | 0.003 |
| Cardiac Function | |||
| Resting HR (bpm) | 65.8 ± 9.5 (60.2, 71.4) | 78.8 ± 9.0 (74.1, 83.5) | 0.002 |
| Resting SBP (mmHg) | 130.7 ± 14.4 (122.2, 139.2) | 105.2 ± 9.5 (100.2, 110.2) | <0.0001 |
| Resting DBP (mmHg) | 74.8 ± 6.3 (71.1, 78.5) | 65.4 ± 8.5 (61.0, 69.9) | 0.005 |
| RPP (bpm × mmHg) | 8558 ± 1321 (7778, 9338) | 8319 ± 1464 (7553, 9085) | 0.68 |
| Left Ventricular Mass (g) | 153.5 ± 27.9 (137.0, 170.0) | 142.2 ± 44.1 (116.1, 168.3) | 0.72 |
| FS (%) | 37± 7 (33, 41) | 32 ± 6 (29, 36) | 0.04 |
| EF (%) | 62 ± 6 (59, 66) | 57 ± 13 (49, 65) | 0.16 |
| S’ septal | 7.7 ± 1.7 (6.7, 8.7) | 7.2 ± 1.3 (6.4, 8.0) | 0.31 |
| E’ septal | 11.6 ± 2.6 (10.0, 13.2) | 9.6 ± 2.1 (8.4, 10.8) | 0.07 |
| Global Strain (%) | −17.4 ± 2.1 (−18.6, −16.2) | −15.5 ± 3.0 (−17.3, −13.7) | 0.07 |
| Whole-Body Peak Exercise | |||
| VO2peak (ml•kg−1•min−1) | 41.3 ± 10.7 (35.0, 47.6) | 11.9 ± 3.5 (10.1, 13.7) | <0.0001 |
| Peak Work Rate (watts) | 240.9 ± 44.4 (214.7, 267.1) | 60.7 ± 11.4 (54.7, 66.7) | <0.0001 |
| Peak HR (bpm) | 182.6 ± 11.2 (176.0, 189.2) | 156.6 ± 13.1 (149.7, 163.5) | <0.0001 |
| Peak RER | 1.2 ± 0.1 (1.1, 1.3) | 1.5 ± 0.2 (1.4, 1.6) | <0.0001 |
Values are means ± SD and 95% confidence intervals for the mean (CIs). μmol: micromolar, MBF: myocardial blood flow, GLU-EF: myocardial glucose extraction fraction, MGUp: myocardial glucose uptake, MGU: myocardial glucose utilization, FA-EF: myocardial fatty acid extraction fraction. FA-EF: myocardial fatty acid extraction fraction, FAOX: myocardial fatty acid oxidation extraction fraction, MFES: myocardial esterification extraction fraction, MFAOX: myocardial fatty acid oxidation, MFAES: myocardial fatty acid esterification, MFAUp: myocardial fatty acid uptake (ml/g/min), MFAU: myocardial fatty acid utilization (umol/g/min), leucine K2: leucine turnover, ATP: adenosine triphosphate, PCr: phosphocreatine, HR: heart rate, SBP: systolic blood pressure, DBP: diastolic blood pressure, RPP: rate pressure product, LVM: left ventricular mass, FS: fractional shortening, EF: ejection fraction, S’: S’ velocity, E’: E’ velocity, VO2peak: peak volume of oxygen consumption, RER: respiratory exchange ratio.
All P-values from independent t-tests except those denoted * where Mann-Whitney U-tests were used.
Peak Exercise Testing and Cardiac Function
Peak exercise tolerance (i.e., VO2peak), work rate, and heart rate were all significantly lower in BTHS vs. Control (Table 2).
Cardiac High Energy Phosphate Metabolism and LV Function
Cardiac PCr/ATP was significantly lower in participants with BTHS vs. Control (Table 2). Resting heart rate was higher and resting systolic blood pressure, diastolic blood pressure, and fractional shortening were lower in BTHS vs. Control. Although LV ejection fraction was not different between the groups, peak global strain tended (p=0.07) to be reduced in BTHS vs. Control (Table 2).
Myocardial Substrate Metabolism
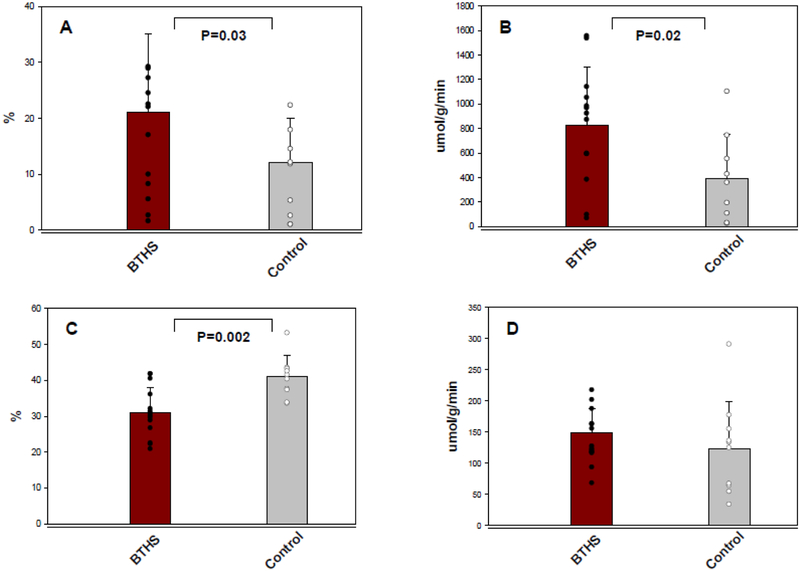
Myocardial glucose extraction fraction, uptake and utilization rates were significantly higher in individuals with BTHS vs. Control (Figure 1A, 1B, Table 2). Conversely, myocardial fatty acid extraction fraction (Figure 1C) and uptake (Table 2) were lower in BTHS vs. Control. Myocardial fatty acid oxidation (Table 2) and utilization rates (Figure 1D) and leucine turnover rate were not significantly different between the two groups (Table 2).
Figure 1: Myocardial Glucose and Fatty Acid Metabolism.
A. Myocardial glucose extraction fraction, B. Myocardial glucose utilization rate, C. Myocardial fatty acid extraction fraction, D. Myocardial fatty acid utilization rate. BTHS: Barth syndrome, nmol: nanomole, g: gram, min: minute.
Relationships between Myocardial Substrate Metabolism, Cardiac Phosphate Metabolism and Function
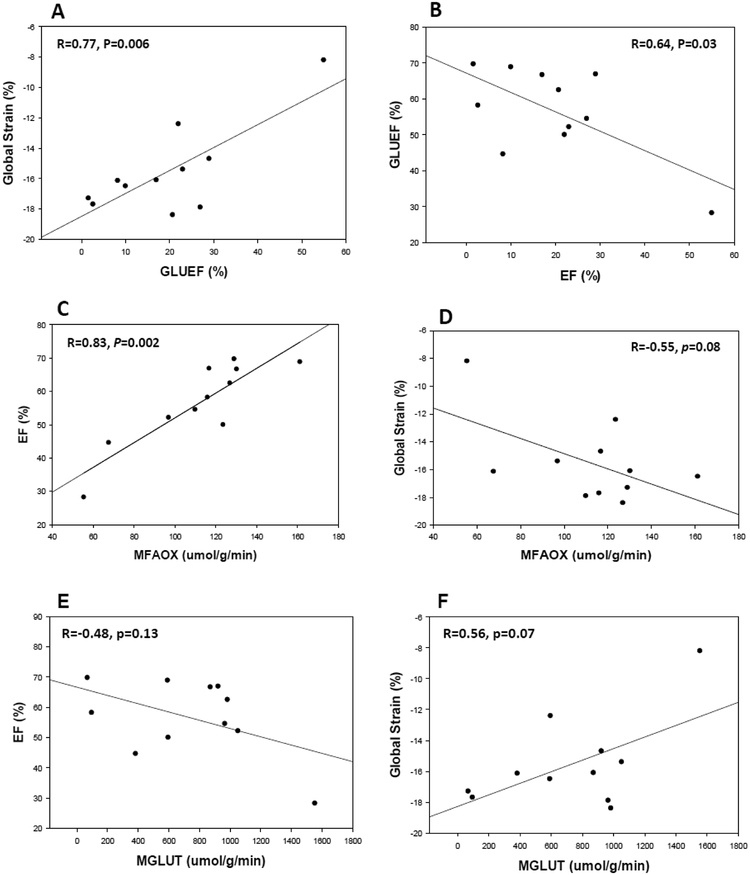
With BTHS analyzed independently, higher myocardial glucose extraction fraction was significantly associated with reduced global strain (Figure 2A), ejection fraction (Figure 2B) and S’ velocity (Table 3) and tended to be associated with lower E’ velocity (Table 3). Higher myocardial glucose utilization tended to be associated with lower ejection fraction and global strain (Figure 2 E & F, Table 3). Lower myocardial fatty acid utilization was significantly associated with lower ejection fraction and lower S’ velocity (Table 3). Lower myocardial fatty acid oxidation was significantly associated with lower ejection fraction (Figure 2C), lower S’ velocity (Table 3) and tended to be associated with reduced global strain (Figure 2D). There were no significant relationships between myocardial glucose, fatty acid or leucine metabolism kinetics and cardiac high energy phosphate metabolism (PCr/ATP).
Figure 2: Relationships between Myocardial Substrate Metabolism and Cardiac Function in BTHS.
A. Myocardial glucose extraction fraction vs. global strain, B Myocardial glucose extraction fraction vs. ejection fraction, C. Myocardial fatty acid oxidation vs. ejection fraction, D. Myocardial fatty acid oxidation vs. global strain, E. Myocardial glucose utilization vs ejection fraction, F. Myocardial glucose utilization vs. global strain. GLU-EF: myocardial glucose extraction fraction, EF: ejection fraction, MFAOX: myocardial fatty acid oxidation rate, MGLUT: myocardial glucose utilization, umol: micromole, g: gram, min: minute.
Table 3:
Relationships between Myocardial Metabolism and Function in BTHS
| EF (%) | S’ (cm•s−1) | E’ (cm•s−1) | Global Strain (%) | |
|---|---|---|---|---|
| MBF (ml•g −1 •min −1 ) | 0.52 P=0.10 | 0.84, P=0.001 | P >0.10 | −0.67 P<0.03 |
| GLU-EF (%) | −0.64 P=0.03 | −0.53 P=0.10 | −0.60 P=0.07 | 0.77, P=0.006 |
| MGU (μmol•g −1 •min −1 ) | P>0.10 | P>0.10 | P>0.10 | 0.56, P=0.07 |
| MFAOX (μmol•g −1 •min −1 ) | 0.88 P=0.001 | 0.71 P=0.01 | P >0.10 | −0.55 P=0.08 |
| MFAUp (ml•g −1 •min −1 ) | 0.61 P=0.05 | 0.59 P=0.05 | P>0.10 | P>0.10 |
| MFAU (μmol•g −1 •min −1 ) | 0.63 P=0.04 | 0.69 P=0.02 | P>0.10 | P>0.10 |
R values determined by Pearson Correlation Coefficient analyses, μmol: micromole, MBF: myocardial blood flow, GLU-EF: myocardial glucose extraction fraction, MGU: myocardial glucose utilization rate, MFAOX: myocardial fatty acid oxidation rate, MFAUp: myocardial fatty acid uptake rate, MFAU: myocardial fatty acid utilization rate, FS: fractional shortening, EF: ejection fraction, S’: S’ velocity, E’: E’ velocity.
When controls were analyzed independently, myocardial glucose extraction fraction (r= −0.78, p=0.003) and utilization (r= −0.60, p=0.04) were associated with LV ejection fraction. Also, myocardial glucose extraction fraction (r= 0.82, p=0.001) and utilization (r= 0.73, p=0.007) were associated with LV global strain. Lastly, myocardial blood flow was associated with global strain (r= 0.70, p=0.008).
Discussion
The primary finding of this study was that young adults with BTHS even without frank heart failure had markedly higher myocardial glucose metabolism compared to non-affected controls. In addition, we found that myocardial fatty acid extraction fraction and uptake were lower in BTHS compared to controls but this deficit appeared to be compensated by elevated plasma free fatty acid concentration in order to normalize myocardial fatty acid utilization. Further, we found that alterations in myocardial glucose and fatty acid metabolism were associated with lower cardiac function. Taken together, these novel data suggest that abnormalities in myocardial fatty acid and glucose metabolism may contribute to the cardiac pathophysiology in BTHS.
In our study, myocardial glucose extraction fraction, uptake, and utilization were markedly higher in young adult patients with BTHS without frank heart failure compared to healthy, age-matched controls. On average, glucose extraction fraction, uptake and utilization were approximately double that of controls. It has been historically thought that a shift away from myocardial fatty acid metabolism to higher myocardial glucose metabolism in the failing heart was advantageous because glucose use is more oxygen efficient. However, recent studies in both humans29 and animals with non-BTHS heart failure30,31 suggest that decreasing myocardial fatty acid metabolism overall worsens LV function and that a shift away from higher myocardial fatty acid metabolism might be maladaptive. Glucose oxidation produces significantly less ATP than fatty acid oxidation (~20% vs. 80%). Thus, a shift toward more myocardial glucose metabolism could limit ATP production in the failing heart. In addition to producing an energy deficit, elevated myocardial glycolysis out of proportion to glucose oxidation could lead to intracellular acidosis. This in turn, could reduce cardiac contractility through the desensitization of contractile proteins to Ca2+ and reduce slow Ca2+ current.32,33 However, we did not directly measure myocardial glycolysis and glucose oxidation, thus it is unclear if higher myocardial glucose metabolism in BTHS was caused by higher glycolysis, glucose oxidation, or a combination of both. Our previous findings of elevated whole-body glucose turnover (i.e. disposal) in individuals with BTHS12 also did not differentiate whether whole-body glycolysis or glucose oxidation contributed to elevated glucose turnover. Findings in the BTHS murine model suggest that glycolytic metabolism is upregulated in BTHS. For example, a previous proteomics evaluation of BTHS mouse hearts revealed a significant increase in the expression of UDP-galactose 4-epimerase, the enzyme that performs the final step in the Leloir pathway of galactose metabolism through catalyzing the reversible conversion of UDP-galactose the UDP-glucose.34 In addition, hepatic glycogenolysis was higher during exercise in BTHS vs. wild type mice.35 Hence, it appears that in BTHS both skeletal and cardiac muscle have an increased dependence on glucose metabolism, and likely, glycolytic metabolism, although more definitive studies that measure both cardiac and skeletal muscle glycolysis and glucose oxidation are warranted.
Myocardial fatty acid metabolism was also altered in BTHS with participants demonstrating lower myocardial extraction fraction and uptake compared to unaffected controls. However, it is important to note that myocardial fatty acid utilization and oxidation rates were not different. This appears to be at least in part due to elevated plasma free fatty acid levels (i.e. higher fatty acid availability) in BTHS as myocardial fatty acid utilization is the product of plasma free fatty acid concentration, extraction fraction, and myocardial blood flow. The shift toward less fatty acid extraction and uptake in BTHS is reminiscent of the general shift away from fatty acid metabolism found in most studies of non-BTHS heart failure. This is in contrast, however, to individuals with specific types of inherited cardiomyopathy other than BTHS, including acyl-CoA dehydrogenase and 3-hydroxyacl-CoA deficiency, who have lower fatty acid uptake and lower myocardial fatty acid oxidation rates compared to healthy controls.36,37 Importantly, palmitate extraction fraction is lower in those with non-BTHS-inherited cardiomyopathy. However, unlike participants with BTHS in the current study, plasma free fatty acid levels were similar to controls, likely contributing to the lower fatty acid oxidation seen in these studies. It is unclear why myocardial fatty acid oxidation and utilization rates in those with BTHS were similar to controls while glucose utilization rates were increased. Although speculative, it is possible that mitochondrial uncoupling is increased in BTHS as seen in non-BTHS heart failure where higher mitochondrial uncoupling has been associated with lower efficiency of the failing heart.38 Uncoupling proteins are stimulated by circulating free fatty acids and reactive oxygen species39; both elevated in BTHS.5 However, the role of uncoupling proteins and myocardial metabolism in the pathophysiology of BTHS require further study.
Mechanisms contributing to alterations in myocardial glucose and fatty acid metabolism in BTHS are unclear. Lower myocardial fatty acid extraction fraction and uptake suggest that fatty acid transport, either across the sarcolemma or into the mitochondria (or both), might be impaired. While in non-BTHS inherited fatty acid transport disorders the mechanism of disease is known40, the precise and likely diverse effects of cardiolipin deficiency on fatty acid uptake in BTHS are less clear. In our previous study, impairments in whole-body fatty acid oxidation was only apparent during exercise and not rest, whereas enhancements in glucose metabolism were seen at rest, during exercise and during post-exercise recovery.12 This phenomenon might also be true in cardiac muscle as defects in myocardial fatty acid oxidation in BTHS might only be fully elucidated during exercise. This notion is supported by the finding that cardiac function is normal to low-normal during rest in BTHS, but functional improvement with exercise is blunted.11 Ultimately, measurement of myocardial metabolism in individuals with BTHS should be performed under stress (e.g. exercise, dobutamine) in order to reveal the full spectrum of potential alterations not seen during the resting condition.
It should be noted that greater than half of participants with BTHS were taking beta-blocker therapy for the prevention of heart failure. Beta-blocker medications reduce adipose tissue lipolysis through receptor-mediated blockade thus tending to lower plasma free fatty acid concentration in individuals41 However, in our cohort, plasma free fatty acid concentration was actually greater in BTHS compared to unaffected controls, likely because the participants with BTHS demonstrated higher adipose tissue lipolytic rate compared to unaffected controls (data not shown).12 In addition, in non-BTHS populations, most data suggest that beta-blockers reduce rather than enhance glucose metabolism.42 Therefore, it is unlikely that BTHS-related alterations in myocardial glucose and fatty acid metabolism seen in the current study were primarily due to effects of beta-blocker therapy.
Myocardial leucine metabolism was not different in BTHS compared to controls. We originally hypothesized that since myocardial fatty acid metabolism would be impaired, alternative substrate utilization, including glucose and oxidizable amino acids (e.g. leucine), would be higher to compensate for the energy deficit from lower fatty acid metabolism. Although leucine metabolism contributes only a small proportion to myocardial oxygen consumption43, abnormal myocardial amino acid metabolism has been previously shown in non-BTHS models of heart failure.15–17 In addition, abnormalities in plasma amino acid profiles have been demonstrated in BTHS.18,44 However, we did not find any differences in myocardial leucine metabolism between BTHS and unaffected controls but it is unclear if other amino acids might be affected in BTHS. Of note, our previous proteomics evaluation of BTHS mouse hearts did reveal a significant increase in mitochondrial isovaleryl-CoA dehydrogenase, an enzyme involved in L-leucine catabolism which may indicate this form of compensatory metabolic flexibility is possible at specific points in BTHS disease progression34. Additional research is required to fully characterize myocardial amino acid metabolism in BTHS.
Alterations in myocardial fatty acid and glucose metabolism were associated or tended to be associated with lower cardiac function in young adults with BTHS. Higher myocardial fatty acid oxidation and utilization was associated with both better LV function while higher glucose metabolism were associated or tended to be associated with worse LV function suggesting that alterations in glucose and fatty acid metabolism might contribute to LV dysfunction in BTHS.
Limitations
There were limitations to our study. First, this was a small sample size and this study was a secondary aim of a parent study that was powered on the primary outcome.12 We were also not able to study children and adolescents due to the radiation burden associated with radioisotope administration. Limiting this study to only young adults with BTHS could have led to selection bias as only individuals surviving to young adulthood could be studied and those who do not survive into adulthood might have more profound abnormalities. Another limitation included the inability to obtain full-echocardiograms on all participants with BTHS due to limited imaging windows from pectus excavatum in three individuals. Lastly, it is important to note that we found significant relationships between myocardial glucose and fatty acid metabolism and LV function but these relationships do not imply causation.
Conclusion
In summary, we found altered myocardial glucose and fatty acid metabolism in young adults with BTHS that was associated with lower LV function. Abnormalities in myocardial lipid and glucose metabolism might contribute to the cardiac pathophysiology of BTHS and represent key outcome measures to be considered in future evaluations of potential BTHS therapeutics.
New Knowledge Gained
Myocardial glucose and fatty acid metabolism is altered in young adults with BTHS. These metabolic abnormalities are associated with lower left ventricular function and might contribute to the pathophysiology of cardiomyopathy in BTHS and represent a novel area for therapeutic development.
Supplementary Material
Acknowledgments
We thank the nursing staff at the WUSTL Institute for Clinical and Translational Sciences Clinical Research Unit for their hard work and altruism. We especially thank Kitty Krupp, RN, for her work with study radioisotope tracer administration and blood draws and the staff at the WUSTL CCIR for acquisition of the PET scans. Lastly, we thank the participants and their families for their dedication and effort to travel to St. Louis and participate in this study.
Sources of Funding: This work was supported by the National Institutes of Health R01HL107406–01, K01EB010171, P30DK056341, P30DK020579, HD007434, S10RR024532 and UL1TR000448 from the National Center for Research Resources and NIH Roadmap for Medical Research. Additional support provided by the Barnes-Jewish Hospital Foundation to the Cardiovascular Imaging and Clinical Research Core Laboratory.
Abbreviations
- BTHS
Barth syndrome
- WUSTL
Washington University in St. Louis
- LV
left ventricular
- 2D
two-dimensional
- PET
Positron emission tomography
- PCr/ATP
phosphocreatine to adenosine triphosphate ratio
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Clinical Trials #: NCT01625663
Conflict of Interest: None
References
- 1.Barth Syndrome Foundation. About Barth Syndrome-Frequently Asked Questions. 2018; https://www.barthsyndrome.org/about-barth-syndrome.
- 2.Clarke SL, Bowron A, Gonzalez IL, et al. Barth syndrome. Orphanet J Rare Dis. February122013;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barth PG, Scholte HR, Berden JA, et al. An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J Neurol Sci. December1983;62(1–3):327–355. [DOI] [PubMed] [Google Scholar]
- 4.Bione S, D’Adamo P, Maestrini E, Gedeon AK, Bolhuis PA, Toniolo D. A novel X-linked gene, G4.5. is responsible for Barth syndrome. Nat Genet. April1996;12(4):385–389. [DOI] [PubMed] [Google Scholar]
- 5.Wang G, McCain ML, Yang L, et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med. June2014;20(6):616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Y, Phoon CK, Berno B, et al. Loss of protein association causes cardiolipin degradation in Barth syndrome. Nat Chem Biol. August2016;12(8):641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang M, Mileykovskaya E, Dowhan W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J Biol Chem. November152002;277(46):43553–43556. [DOI] [PubMed] [Google Scholar]
- 8.Spencer CT, Bryant RM, Day J, et al. Cardiac and clinical phenotype in Barth syndrome. Pediatrics. August2006;118(2):e337–346. [DOI] [PubMed] [Google Scholar]
- 9.Kang SL, Forsey J, Dudley D, Steward CG, Tsai-Goodman B. Clinical characteristics and outcomes of cardiomyopathy in Barth syndrome: The UK Experience. Pediatr Cardiol. January2016;37(1):167–176. [DOI] [PubMed] [Google Scholar]
- 10.Bashir A, Bohnert KL, Reeds DN, et al. Impaired cardiac and skeletal muscle bioenergetics in children, adolescents, and young adults with Barth syndrome. Physio Rep. February2017;5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spencer CT, Byrne BJ, Bryant RM, et al. Impaired cardiac reserve and severely diminished skeletal muscle O(2) utilization mediate exercise intolerance in Barth syndrome. Am J Physiol Heart Circ Physiol. November2011;301(5):H2122–2129. [DOI] [PubMed] [Google Scholar]
- 12.Cade WT, Bohnert KL, Peterson LR, et al. Blunted fat oxidation upon submaximal exercise is partially compensated by enhanced glucose metabolism in children, adolescents and young adults with Barth syndrome. J Inherit Metab Dis. 2019May;42(3):480–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davila-Roman VG, Vedala G, Herrero P, et al. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. July172002;40(2):271–277. [DOI] [PubMed] [Google Scholar]
- 14.Tuunanen H, Engblom E, Naum A, et al. Decreased myocardial free fatty acid uptake in patients with idiopathic dilated cardiomyopathy: evidence of relationship with insulin resistance and left ventricular dysfunction. J Card Fail. October2006;12(8):644–652. [DOI] [PubMed] [Google Scholar]
- 15.Peterson MB, Mead RJ, Welty JD. Free amino acids in congestive heart failure. J Mol Cell Cardiol. April1973;5(2):139–147. [DOI] [PubMed] [Google Scholar]
- 16.Lai L, Leone TC, Keller MP, et al. Energy metabolic reprogramming in the hypertrophied and early stage failing heart: a multisystems approach. Circ Heart Fail. November2014;7(6):1022–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sansbury BE, DeMartino AM, Xie Z, et al. Metabolomic analysis of pressure-overloaded and infarcted mouse hearts. Circ Heart Fail. July2014;7(4):634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cade WT, Spencer CT, Reeds DN, et al. Substrate metabolism during basal and hyperinsulinemic conditions in adolescents and young-adults with Barth syndrome. J Inherit Metab Dis. January2013;36(1):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. December2005;18(12):1440–1463. [DOI] [PubMed] [Google Scholar]
- 20.Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J. January11992;281 (Pt 1):21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naressi A, Couturier C, Devos JM, et al. Java-based graphical user interface for the MRUI quantitation package. Magma. May2001;12(2–3):141–152. [DOI] [PubMed] [Google Scholar]
- 22.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. November1997;129(1):35–43. [DOI] [PubMed] [Google Scholar]
- 23.El-Sharkawy AM, Schar M, Ouwerkerk R, Weiss RG, Bottomley PA. Quantitative cardiac 31P spectroscopy at 3 Tesla using adiabatic pulses. Magn Reson Med. April2009;61(4):785–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bashir A, Gropler R. Reproducibility of creatine kinase reaction kinetics in human heart: a (31) P time-dependent saturation transfer spectroscopy study. NMR Biomed. June2014;27(6):663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young ME, Razeghi P, Cedars AM, Guthrie PH, Taegtmeyer H. Intrinsic diurnal variations in cardiac metabolism and contractile function. Circ Res. December72001;89(12):1199–1208. [DOI] [PubMed] [Google Scholar]
- 26.Bergmann SR, Herrero P, Markham J, Weinheimer CJ, Walsh MN. Noninvasive quantitation of myocardial blood flow in human subjects with oxygen-15-labeled water and positron emission tomography. J Am Coll Cardiol. September1989;14(3):639–652. [DOI] [PubMed] [Google Scholar]
- 27.Herrero P, Weinheimer CJ, Dence C, Oellerich WF, Gropler RJ. Quantification of myocardial glucose utilization by PET and 1-carbon-11-glucose. J Nucl Cardiol. Jan-Feb 2002;9(1):5–14. [DOI] [PubMed] [Google Scholar]
- 28.Bergmann SR, Weinheimer CJ, Markham J, Herrero P. Quantitation of myocardial fatty acid metabolism using PET. J Nucl Med. October1996;37(10):1723–1730. [PubMed] [Google Scholar]
- 29.Tuunanen H, Engblom E, Naum A, et al. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation. November142006;114(20):2130–2137. [DOI] [PubMed] [Google Scholar]
- 30.Kolwicz SC Jr., Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ Res. August312012;111(6):728–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He L, Kim T, Long Q, et al. Carnitine palmitoyltransferase-1b deficiency aggravates pressure overload-induced cardiac hypertrophy caused by lipotoxicity. Circulation. October22012;126(14):1705–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD. Targeting fatty acid and carbohydrate oxidation--a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta. July2011;1813(7):1333–1350. [DOI] [PubMed] [Google Scholar]
- 33.Fiolet JW, Baartscheer A. Cellular calcium homeostasis during ischemia; a thermodynamic approach. Cardiovasc Res. January12000;45(1):100–106. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki-Hatano S, Saha M, Soustek MS, et al. AAV9-TAZ Gene Replacement Ameliorates Cardiac TMT Proteomic Profiles in a Mouse Model of Barth Syndrome. Mol Ther Methods Clin Dev June142019;13:167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schweitzer GG, Finck BN, Cade WT. Increased anaerobic metabolism during exercise in Barth syndrome may result from augmented liver glycogenolysis. Barth Syndrome Foundation: International Scientific, Medical, & Family Conference.; 2018; Clearwater Beach, FL (Abstract). [Google Scholar]
- 36.Kelly DP, Mendelsohn NJ, Sobel BE, Bergmann SR. Detection and assessment by positron emission tomography of a genetically determined defect in myocardial fatty acid utilization (long-chain acyl-CoA dehydrogenase deficiency). Am J Cardiol. March151993;71(8):738–744. [DOI] [PubMed] [Google Scholar]
- 37.Bergmann SR, Herrero P, Sciacca R, et al. Characterization of altered myocardial fatty acid metabolism in patients with inherited cardiomyopathy. J Inherit Metab Dis. November2001;24(6):657–674. [DOI] [PubMed] [Google Scholar]
- 38.Murray AJ, Cole MA, Lygate CA, et al. Increased mitochondrial uncoupling proteins, respiratory uncoupling and decreased efficiency in the chronically infarcted rat heart. J Mol Cell Cardiol. April2008;44(4):694–700. [DOI] [PubMed] [Google Scholar]
- 39.Sluse FE. Uncoupling proteins: molecular, functional, regulatory, physiological and pathological aspects. Adv Exp Med Biol. 2012;942:137–156. [DOI] [PubMed] [Google Scholar]
- 40.Knottnerus SJG, Bleeker JC, Wust RCI, et al. Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle. Rev Endocr Metab Disord. 2018March;19(1):93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cleroux J, Van Nguyen P, Taylor AW, Leenen FH. Effects of beta 1- vs. beta 1 + beta 2-blockade on exercise endurance and muscle metabolism in humans. J Appl Physiol (1985). February1989;66(2):548–554. [DOI] [PubMed] [Google Scholar]
- 42.Fonseca VA. Effects of beta-blockers on glucose and lipid metabolism. Curr Med Res Opin. March2010;26(3):615–629. [DOI] [PubMed] [Google Scholar]
- 43.Ichihara K, Neely JR, Siehl DL, Morgan HE. Utilization of leucine by working rat heart. Am J Physiol. December1980;239(6):E430–436. [DOI] [PubMed] [Google Scholar]
- 44.Vernon HJ, Sandlers Y, McClellan R, Kelley RI. Clinical laboratory studies in Barth Syndrome. Mol Genet Metab. June2014;112(2):143–147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.