Figure 3. Binding mechanisms of hRpn13 for K48-diubiquitin.
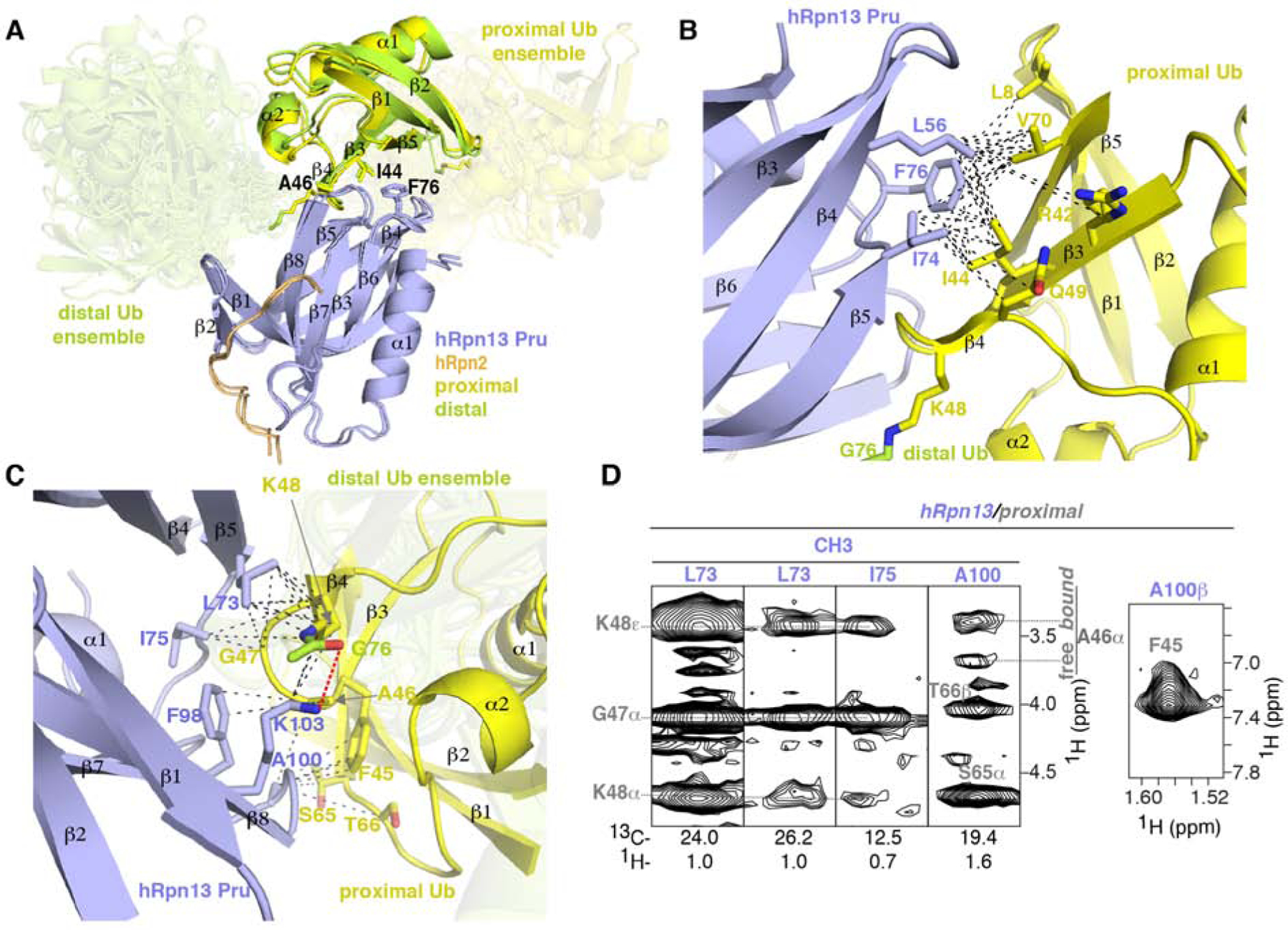
(A) Superposition of Ternary-P (displayed as in Figure 1E) and Ternary-D (displayed as in Figure 1F) overlaying the hRpn13-bound ubiquitin moieties. Key interacting amino acid sidechains are shown, including hRpn13 F76 and ubiquitin I44 and A46.
(B and C) Enlarged view of the interactions between hRpn13 and proximal ubiquitin for Ternary-P, shown as in (A). Black dashed lines represent intermolecular NOE interactions involving hRpn13 L56, I74 or F76 and proximal ubiquitin L8, R42, I44, K48, Q49 or V70 (B) as well as hRpn13 L73, I75, F98, A100 or K103 and proximal ubiquitin F45-K48 or S65-T66 (C). In (C), a hydrogen bond between the hRpn13 K103 ε-ammonium group and the carbonyl of isopeptide bonded ubiquitin G76 is represented by a red dashed line. Nitrogen and oxygen atoms at the interaction surface are colored in blue and red, respectively.
(D) Selected regions from a 13C-half-filtered NOESY experiment (100 ms mixing time) acquired on sample Ternary-13C-D (Figure 1A) highlighting interactions displayed in (C) between hRpn13 (labeled purple) and proximal ubiquitin (labeled gray) of K48-diubiquitin. Two sets of signals are observed for ubiquitin A46 and labeled according to chemical shift position overlapping with free ubiquitin (free) or shifted by hRpn13 binding (bound). See also Figures S1 and S3.
