Figure 4. Unbound ubiquitin is only partially constrained in complex with hRpn2:hRpn13.
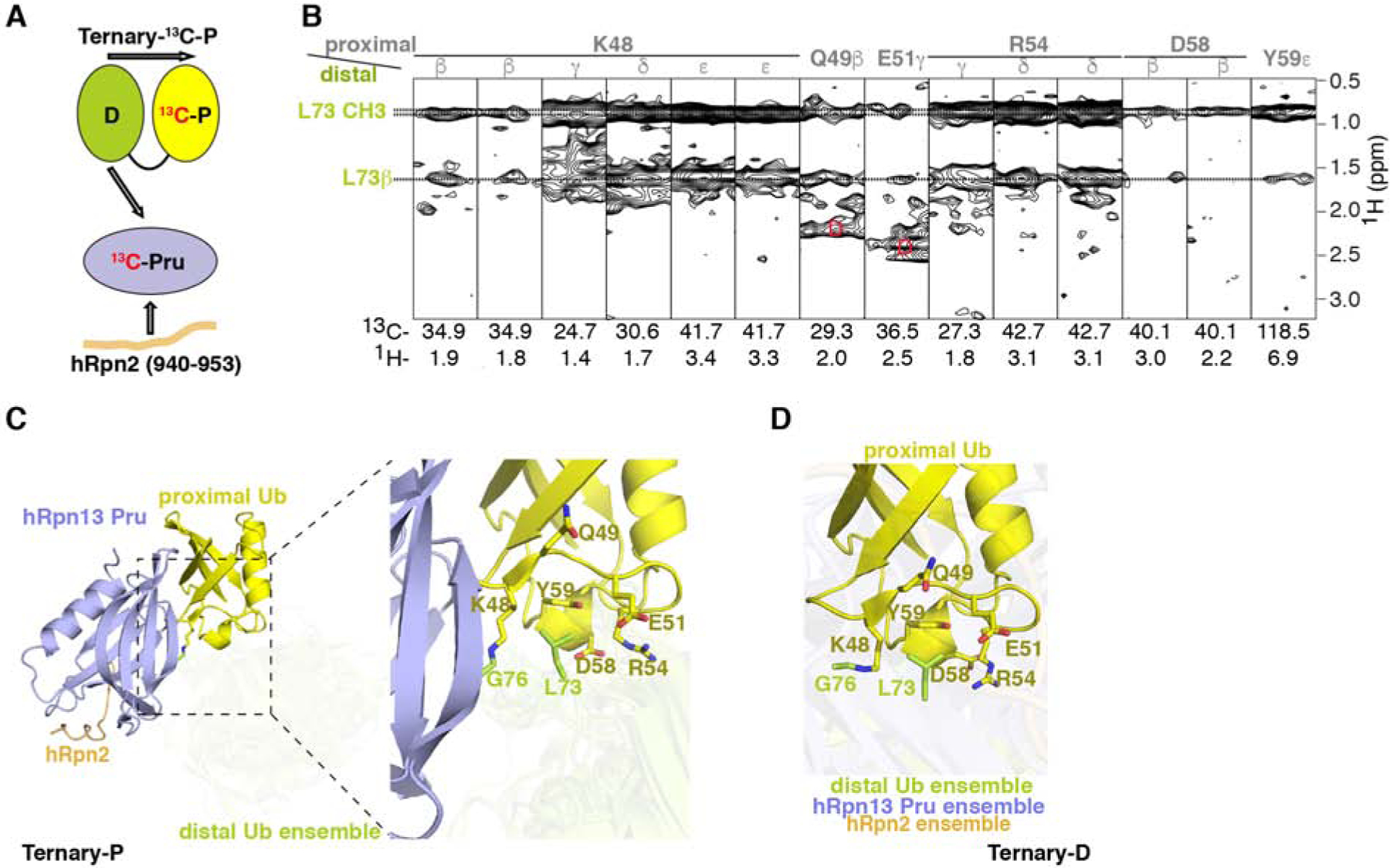
(A) Schematic representation of interactions in Ternary-13C-P detectable by the 13C-half-filtered NOESY experiment (indicated by arrows) with the coloring of Figure 1A.
(B) Selected regions from a 13C-half-filtered NOESY experiment (100 ms mixing time) acquired on sample Ternary-13C-P (A) highlighting interactions between the two ubiquitin moieties, with proximal ubiquitin assignments in gray and distal ubiquitin assignments in green. Breakthrough signals at the diagonal are labeled by the letter D (red).
(C and D) Representative Ternary-P (C, displayed as in Figure 3C) and Ternary-D (D) structures illustrating interactions between distal ubiquitin L73 and proximal ubiquitin K48, Q49, E51, R54, D58 and Y59. The region expanded to the right in (C) is indicated by a dashed rectangle. In (D), a representative structure is displayed for the superposition of proximal ubiquitin when hRpn2 (940–953):hRpn13 Pru is bound to distal ubiquitin with the conformational ensemble for the components not superimposed displayed in a transparent view. See also Figure S4.
