Abstract
Objectives:
Alcohol and cannabis remain the substances most widely used by adolescents. Better understanding of the dynamic relationship between trajectories of substance use in relation to neuropsychological functioning is needed. The aim of this study was to examine the different impact of within-and between-person changes in alcohol and cannabis use on neuropsychological functioning over multiple time points.
Methods:
Hierarchical linear modeling examined the effects of alcohol and cannabis use on neuropsychological functioning over the course of 14 years in a sample of 175 adolescents (ages 12–15 at baseline).
Results:
Time-specific fluctuations in alcohol use (within-person effect) predicted worse performance across time on the WASI Block Design subtest (B = −0.05, SE = 0.02, p =.01). Greater mean levels of percent days of cannabis use across time (between-person effect) was associated with an increased contrast score between DKEFS Color Word Inhibition and Color Naming conditions (B = 0.52, SE = 0.14, p <.0001) and poorer performance over time on Block Design (B = −.08, SE = 0.04, p =.03). Neither alcohol and/or cannabis use over time was associated with performance in the verbal memory and processing speed domains.
Conclusions:
Greater cumulative cannabis use over adolescence may be linked to poorer inhibitory control and visuospatial functioning performance, whereas more proximal increases in alcohol consumption during adolescence may drive alcohol-related performance decrements in visuospatial functioning. Results from this prospective study add to the growing body of literature on the impact of alcohol and cannabis use on cognition from adolescent to young adulthood.
Keywords: Adolescence, Alcohol, Cannabis, Neuropsychological Trajectories, Inhibitory Control, Visuospatial Functioning
Introduction
Substantial changes occur in the in the functional integration and organization of brain functional networks from adolescence and through adulthood (Kundu. et al., 2018). While these neural changes lead to significant improvements in complex cognitive functions, the elevations in novelty seeking, risk-taking behaviors, and increases in peer-directed social interactions make adolescence a period of heightened vulnerability for the onset of alcohol and drug use (Spear, 2000). The triadic model of adolescent motivated behavior (Ernst, 2014) proposes triangular relationship between three functional neural systems (the PFC, the striatum, and the amygdala) and how the predetermined order in which these neural systems mature impacts adolescent behavior.
Alcohol and cannabis remain the substances most widely used by adolescents, with 59% of students having consumed alcohol by the end of high school and one in seventeen 12th graders smoking cannabis daily (Johnston et al., 2019). Importantly, the neurotoxic effects of substance use may have serious long-lasting implications on the developing brain (Meruelo, Castro, Cota, & Tapert, 2017; Squeglia, Jacobus, & Tapert, 2009a). While negative effects of alcohol and cannabis on adolescent cognition have been widely reported in the literature, there are significant limitations in the research thus far (Gonzalez, Pacheco-Colón, Duperrouzel, & Hawes, 2017; Luciana et al., 2018). Important limitations to consider relate to the cross-sectional structure of many study designs and assignment of participants into categorical groups (e.g., heavy using adolescents, adolescents with substance use disorder) and comparing them to nonusers or those with minimal substance use, despite the dimensional nature of the data. Additionally, alcohol and cannabis use have commonly been modeled as static variables (i.e., the extent of use modeled as cumulative use and thus one predictor) in previous longitudinal studies, which ignores the frequently changing nature of substance use and cognition across adolescence. Better understanding of the dynamic relationship between trajectories of substance use in relation to brain and cognitive development is needed. Longitudinal research that examines trajectories of use will allow for such evaluation.
Previous studies indicate that in comparison to light and nondrinkers, adolescents who engage in heavy drinking, including binge drinking, show worse neuropsychological performance across several domains, such as learning and memory (Brown, Tapert, Granholm, & Delis, 2000; Green et al., 2010; Nguyen-Louie et al., 2015; Sneider, Cohen-Gilbert, Crowley, Paul, & Silveri, 2013), visuospatial functioning (Nguyen-Louie et al., 2015; Squeglia, Spadoni, Infante, Myers, & Tapert, 2009b; Tapert & Brown, 1999; Tapert, Granholm, Leedy, & Brown, 2002), executive function (Giancola, Mezzich, & Tarter, 1998; Gil-Hernandez et al., 2017; Parada et al., 2012; Thoma et al., 2011; Winward, Hanson, Bekman, Tapert, & Brown, 2014a), as well as attention and processing speed (Ferrett, Carey, Thomas, Tapert, & Fein, 2010; Nguyen-Louie et al., 2015; Tapert et al., 2002; Tarter, Mezzich, Hsieh, & Parks, 1995; Thoma et al., 2011). However, the strict use of categorical classification in these studies is a limitation, as the alcohol use groups often include a wide range of alcohol consumption and therefore alterations in cognition related to changing patterns of alcohol use may not be detected (Nguyen-Louie et al., 2016).
The impact of adolescent cannabis use on cognition has been less consistent. Compared to non-users, moderate to heavy adolescent cannabis users tend to show poorer performance on measures of attention, memory, processing speed, and executive functioning (Dahlgren, Sagar, Racine, Dreman, & Gruber, 2016; Fontes et al., 2011; Gonzalez et al., 2017; Jacobus et al., 2015; Mathias et al., 2011; Scott et al., 2018; Winward, Hanson, Tapert, & Brown, 2014b). While protracted cannabis use has been linked to subtle cognitive weaknesses, the magnitude of such effects has been inconsistent across studies (Jacobus & Tapert, 2014; Scott et al., 2018). Using a co-twin control study design, Jackson and colleagues (2016) prospectively showed that, compared to non-users, youth who use cannabis exhibit decreases on the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) Vocabulary subtest. However, there were no significant differences in performance between users and non-users on the remaining five WASI subtests. Further, there were no significant differences in cognitive performances between adolescent cannabis users and their twins after adjusting for demographic and covarying factors (i.e., age, sex, race, zygosity, socioeconomic status, and other substance use). Similarly, Meier and colleagues (2018) found some evidence for a cannabis-related working memory impairment, but not IQ or executive functioning, using a co-twin design.
There is evidence to suggest that cognitive functioning improves with cannabis abstinence (Crean, Crane, & Mason, 2011; Scott et al., 2018), though this may be domainspecific. Decrements in attention and working memory have been found to resolve following abstinence, ranging from days to weeks after cessation of use. In contrast, some investigations also report poorer performance on tests of learning and memory (Medina et al., 2007) and aspects of executive functioning (i.e., decision-making and risk-taking) after prolonged abstinence from cannabis (Verdejo-Garcia, Rivas-Perez, Lopez-Torrecillas, & Perez-Garcia, 2006). Studies that are able to assess neuropsychological functioning prior to cannabis exposure and well into young adulthood with multiple assessments are needed to provide more clarity on cannabis-related alterations on cognitive development in the short and long term (Volkow et al., 2018).
The aim of this study is to examine the different longitudinal associations between alcohol and cannabis use and cognitive function measured over 14 years in a sample of adolescents ages 12–15 at baseline. This study expands on several earlier investigations from our team that examined this sample while the study was ongoing and thus includes shorter follow-up periods (3–4 years on average). For instance, Squeglia et al., (2009) found that initiation of alcohol use was associated with poorer neuropsychological performance over a 3-year follow-up period. Nguyen-Louie et al., (2015) found that more days of alcohol and cannabis use was associated with poorer neuropsychological performance over 4 years of follow-up. Data collection is no longer ongoing and therefore the present study is the first to examine substance-related behaviors on a continuous spectrum in the entire sample over a 14-year period for all subjects that have three or more follow-up time points available. This study is also the first to address our previous study limitations by closer examination of within-person variability of alcohol and cannabis use on neuropsychological performance over time. Thus, allowing for the examination of: (1) the independent effects of high levels of substance use across time and (2) time-specific fluctuations in substances use (i.e., deviations from the person’s mean percent use days, which varied across time) on neuropsychological test performance measured over multiple time points. Specifically, we focused on four cognitive domains that have previously been shown to be affected by alcohol and cannabis use, namely processing speed, executive functioning, learning and memory, and visuospatial functioning. (Jacobus et al., 2015; Nguyen-Louie et al., 2015; Squeglia et al., 2009b; Winward et al., 2014b). It was anticipated that neuropsychological performance at any given time point would be influenced by both between-person variability (i.e., a person being a more-frequent drinker, on average, across years) and within-person variability (i.e., a person drinking or using cannabis more frequently than usual during the year) in substance use. Based on previous studies, we hypothesized that increases in alcohol and cannabis use would be associated with worse performance over time on tests in these domains (between-person variability). Within-person variability was also examined; however, no hypothesis was made regarding this effect given the novelty of the literature in this area.
Methods
Participants & Procedures
The sample included all data available from a longitudinal study of 295 youth with and without identified environmental risk factors and genetic liability for substance use disorder at study enrollment (Brumback et al., 2016; Nguyen-Louie et al., 2018; Nguyen-Louie et al., 2016; Squeglia et al., 2015). At baseline, participants in the parent project were healthy adolescents aged 12 to 15 years with very little to no experience with alcohol or other substances and recruited from San Diego area middle schools via flyers sent to the students’ households. Baseline exclusionary criteria included: any report of prenatal alcohol (> 2 drinks during a given week) or illicit substance exposure; premature birth (prior to 35th gestational week); history of any neurological or DSM-IV(American Psychiatric Association, 2000) Axis I disorder; history of head trauma or loss of consciousness (> 2 min) or chronic medical illness; learning disability or mental retardation; psychoactive medication use; history of alcohol use that exceeds 10 total lifetime drinking days or > 2 drinks per week; history of other substance use above minimal levels (defined as ≥ 3 lifetime experiences with cannabis or use in the past 3 months, ≥ 5 lifetime cigarette uses, or any other intoxicant use); English non-fluency; and noncorrectable sensory problems. Written informed assent for adolescent participants and consent of the parent/legal guardian were obtained prior to participation in accordance with the University of California San Diego Human Research Protections Program.
At baseline, eligible youths were administered detailed, structured clinical interviews assessing demographic and psychosocial functioning, Axis I psychiatric disorders, and substance use history. An informant (a biological parent in the majority of cases) was also interviewed on demographic and family history to corroborate the report of the youth. Follow-up assessments were administered in a similar manner. Youth were followed up, on average, 5.1 times (sd=1.4; range: 3–11) after baseline. All participants were asked to abstain from alcohol and recreational drug use for at least 24 hours prior to all baseline and follow-up appointments and abstinence was confirmed via breath alcohol concentration and urine drug screen in the laboratory. Additional study details are available in previous publications (Nguyen-Louie et al., 2015; Squeglia, Schweinsburg, Pulido, & Tapert, 2011).
Measures
Demographics.
Participant age and sex at the time of assessment were acquired as part of the standard interview procedure. The Hollingshead Index of Social Position score, an index of socioeconomic status (SES) (Hollingshead, 1965), was calculated for each participant at baseline using parental socioeconomic background information (i.e., educational attainment, occupation, and salary of each parent) to characterize the youth’s rearing environment. Higher values on this measure indicate lower SES.
Substance use measures.
The Customary Drinking and Drug Use Record (CDDR; Brown et al., 1998) is a structured interview that examines the use patterns and severity of substance involvement including alcohol and cannabis. The percentage of alcohol and cannabis use days in the past year were individually calculated at baseline and each follow-up time point. Alcohol and cannabis recency were defined as the number of days prior to neuropsychological assessment participants last used alcohol or cannabis; larger values represent less recent use.
Neuropsychological test measures.
A comprehensive neuropsychological battery was administered at baseline and follow-up to assess cognitive functioning in the parent study. In the current study, baseline and follow-up neuropsychological data included: Wechsler Intelligence Scale for Children – Third Edition (WISC-III; Wechsler, 1991) Digit Span and Digit Symbol subtests; WASI Block Design subtest; Delis-Kaplan Executive Function System (D-KEFS; Delis, Kaplan, & Kramer, 2001) Color Word Interference (CWI) and Trail Making Test (TMT) subtests; and the California Verbal Learning Test - Children’s Version (CVLT-C; Delis, Kramer, Kaplan, & Ober, 1994). At follow-up, participants 18 years and older were administered the adult versions of the CVLT - Second Edition (CVLT-II; Wechsler, 1997) and Wechsler Adult Intelligence Scale – Third Edition (WAIS-III; Wechsler, 1997). Alternate version of the CVLT-C and CVLT-Second Edition were used to avoid practice effects in the learning and memory domain.
Statistical analysis
Hierarchical linear modeling (HLM) examined the effects of alcohol and cannabis use on neuropsychological functioning over the course of 14 years of assessments in 175 participants (age range 12–29 across follow-up). The use of HLM allowed for examination of both constant (i.e, sex) and time-varying covariates (i.e., age and alcohol/cannabis use recency) and for assessing within-and between-person changes in substance use over multiple time points (Curran & Bauer, 2011; Worley et al., 2014). All models included random person-level intercepts; random slope for time was also included when inclusion improved model fit. Neuropsychological and substance use data were derived from 14 time points, assessed yearly, from 2004 to 2018. Subjects provided data from as few as three to as many as 14 time points, and all available time points were included in models using maximum likelihood estimation, with missing data assumed to be missing at random. For each follow -up year, analyses revealed no differences in missing data on the basis of age (ps = 0.12 – 0.78), sex (ps = 0.07 – 0.90), or substance use (past year alcohol use days: ps = 0.08 – 0.98; past year marijuana use days: ps = 0.07 – 0.92), supporting this assumption. Data from one participant whose missing data were due to substance use treatment were excluded. To assess linear trends in substance use and cognitive functioning, participants with only two years of data (i.e., baseline and one follow-up time point) were excluded from the current analyses.
The neuropsychological outcome measures of interest included raw scores from the WISC-III (at baseline) or WAIS-III (at follow-up) Digit Span forward, Digit Span backward, and Digit Symbol subtests, WASI Block Design subtest, and the CVLT Short Delay Free Recall, Long Delay Free Recall, List A Trials 1–5 total, and List A Trial 5 indices. Contrast scores (i.e., the difference in scaled scores between the two conditions) for D-KEFS CWI Inhibition versus Color Naming condition and TMT Letter-Number Sequencing versus Motor Speed condition tasks were also investigated to better assess the effects of substance use on inhibitory control and cognitive flexibility. A positive contrast score indicates greater time required to complete the CWI Inhibition and TMT Letter-Number Sequencing conditions, independent of reading and psychomotor speed, respectively (Delis et al. 2001). Raw scores from WISC-III and WAIS-III Digit Span subtests were converted to percent correct to account for differences in the maximum total score between versions. These particular tests were chosen based on evidence from prior studies in our laboratory and others demonstrating their significant associations with alcohol and cannabis use in adolescents (Jacobus et al., 2015; Nguyen-Louie et al., 2015; Nguyen-Louie et al., 2017; Squeglia et al., 2009b). The percent of drinking days and percent of cannabis use days in the past year were log-transformed and included in all models as independent predictors.
We expected that neuropsychological performance in any given year would be influenced by both between-person variability (i.e., a person being a more-frequent drinker, on average, across years) and within-person variability (i.e., a person drinking more frequently than usual during the year) in substance use. To model these effects independently, substance use indices (i.e. percent days of alcohol or cannabis use in the past year) were grand mean-centered and decomposed into two variables for both cannabis and alcohol: 1) a variable representing each person’s mean percent use days, which was constant across time, and 2) a variable representing time-specific deviations from the person’s mean percent use days, which varied across time. This modeling strategy allows for examination of both the independent effects of chronically high levels of substance use and, importantly, time-specific fluctuations in substance use on neuropsychological performance. It also reduces the degree of correlation between the substance use variables and age. To examine the association between alcohol and cannabis use and neuropsychological functioning separately, eight models were estimated, one model for each unique pairing of neuropsychological outcome and substance (i.e., cannabis or alcohol). Age, sex, and alcohol/cannabis use recency were included as covariates and retained in final full models if statistically significant. Estimates of f2 effect sizes were calculated using recommended procedures for multilevel models (Selya et al., 2012). All statistical analyses were performed in Stata 14.2 (StataCorp, 2007).
Results
Description of Sample
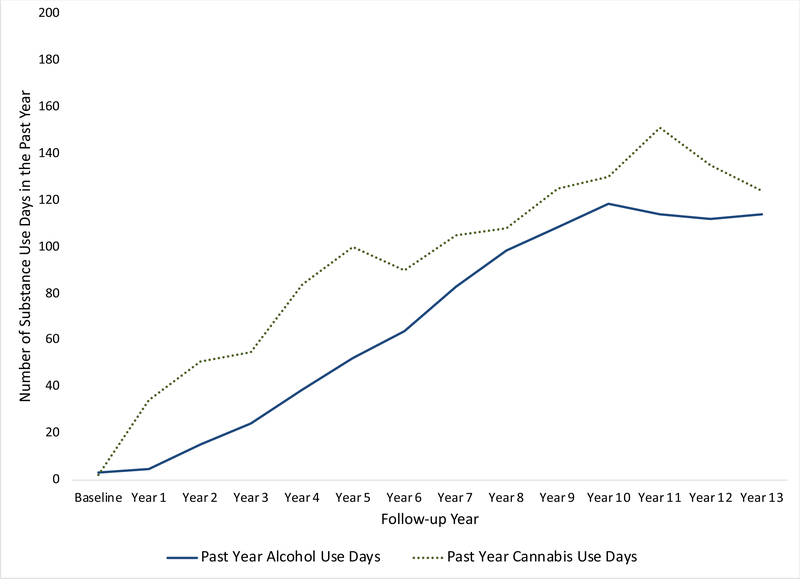
At baseline, participants (N = 175; 43% female) were between 12–15 years old (M = 13. SD = 0.80). Sixty-eight percent of participants were White, 17% were African-American/Black, and 6% were Asian-American. Youth were primarily from middle class families, with a median Hollingshead Index of Social Position score of 23.1 (SD = 13.7); however, the sample represented youth from a range of SES backgrounds, with scores between 11 and 73. At baseline, 88% of youth had never tried alcohol, 95% had never tried cannabis, 87% had never tried any substances. On average, youth initiated drinking at age 16.4 (SD = 2.26) and cannabis at 16.8 (SD = 2.34). Trajectories of alcohol and cannabis use by year are presented in Figure 1.
Figure 1.
Trajectories of Substance Use Over the Course of 14 years.
Covariates
Age was positively associated with nine of ten neuropsychological measures over time, such that individuals performed significantly better on these measures as their age increased (ps <.05). Age was not associated with CVLT List A Trials 1–5 total (p =.780). More recent alcohol use was significantly associated with poorer performance on Digit Span Forward (B = 0.04, SE = 0.02, p = .04). Alcohol use recency was not a significant predictor of any other neuropsychological performance (ps > .05). Independent of age, women performed significantly better than men over time on WAIS Digit Symbol, CVLT Short and Long Delay Free Recall, List A Trials 1–5 total, and List A Trial 5, and D-KEFS Number-Letter Switching-Motor Speed contrast (ps < .05). Sex was not a significant predictor of performance on Digit Span Forward and Backward, WASI Block Design or D-KEFS CWI Inhibition-Color Naming contrast. The appropriate covariates were included in all models in which associations were found with the corresponding outcome (e.g., alcohol recency and Digit Span Forward).
Effects of alcohol on cognition
Accounting for the effects of age, greater mean percent days of drinking across time (‘between-person effect’) was associated with better performance on WAIS Digit Span Forward (B = 0.10, SE = 0.02, p =.03, f2 < 0.01). When the model included alcohol use recency, this relationship was no longer significant (p = .15).
Controlling for age, time-specific fluctuations in alcohol use (i.e., drinking more frequently than usual within the year; ‘within-person effect’) predicted worse performance across time on Block Design (B = −0.05, SE = 0.02, p = .01, f2 < 0.01). There were no significant effects of between-person alcohol use on WASI Block Design (p = .09). There were no significant effects of within-or between-person alcohol use on D-KEFS CWI or TMT contrast scores, WAIS Symbol Digits, or CVLT Short and Long Delay Free Recall, List A Trial 5, or List A Trials 1–5 total (ps > .05). See Table 1 for results. Neuropsychological test scores for baseline and each follow-up year are presented in Table 2.
Table 1.
Effects of covariates and within-and between-person changes in alcohol and cannabis use over 14 years on neuropsychological functioning among adolescents.
| Covariates |
Main Effects: Percent of use days in the past year |
||||
|---|---|---|---|---|---|
| Age | Gendera | Recency of use |
Between-personb | Within-personc | |
|
b (SE) p-value |
b (SE) p-value |
||||
| Alcohol Use | |||||
| Digit Span forward 1 |
0.05 (0.01) <0.0001 |
-- | −0.04 (1.7) 0.326 |
0.07 (0.05) 0.147 |
0.02 (0.02) 0.482 |
| Digit Span backward 1 |
0.17 (0.01) <0.0001 |
-- | -- | 0.04 (0.03) 0.301 |
−0.00 (0.02) 0.891 |
| Digit Symbol 1 |
0.08 (0.01) <0.0001 |
−0.56 (0.11) <0.0001 |
-- | −0.05 (0.04) 0.231 |
−0.02 (0.02) 0.447 |
| Block Design 2 |
0.03 (0.01) 0.002 |
-- | -- | −0.07 (0.04) 0.090 |
−0.05 (0.02) 0.009 |
| List A Trial 5 3 |
0.03 (0.02) 0.022 |
−0.27 (0.17) 0.023 |
-- | 0.02 (0.04) 0.691 |
−0.00 (0.03) 0.903 |
| List A Trials 1–5 total 3 | -- |
−0.28 (0.12) 0.018 |
-- | 0.04 (0.04) 0.356 |
0.01 (0.02) 0.669 |
| Short Delay Free Recall 3 |
0.04 (0.01) 0.010 |
-- | -- | −0.04 (0.05) 0.412 |
−0.02 (0.03) 0.476 |
| Long Delay Free Recall 3 |
0.03 (0.02) 0.047 |
−0.32 (0.13) 0.011 |
-- | 0.01 (0.05) 0.902 |
−0.02 (0.03) 0.527 |
| Inhibition-Color Naming contrast 4 |
−0.13 (0.05) 0.017 |
-- | -- | 0.19 (0.14) 0.192 |
0.03 (0.11) 0.766 |
| Letter-Number Sequencing – Motor Speed contrast 4 |
0.05 (0.01) <0.0001 |
−0.15 (0.06) 0.004 |
-- | −0.01 (0.02) 0.778 |
−0.02 (0.02) 0.293 |
| Cannabis Use | |||||
| Digit Span forward 1 |
0.09 (0.01) <0.0001 |
-- | -- | 0.01 (.04) 0.822 |
0.1 (0.2) 0.427 |
| Digit Span backward 1 |
0.14 (0.01) <0.0001 |
-- | -- | 0.02 (0.03) 0.639 |
0.01 (0.02) 0.565 |
| Digit Symbol 1 |
0.14 (0.01) <0.0001 |
−0.52 (0.10) <0.0001 |
-- | −0.06 (0.03) 0.096 |
0.02 (0.02) 0.187 |
| Block Design 2 |
0.10 (0.01) <0.0001 |
-- | -- |
−0.08 (0.04) 0.031 |
0.00 (0.1) 0.816 |
| List A Trial 5 3 |
0.04 (0.01) <0.0001 |
−0.27 (0.11) 0.011 |
-- | −0.02 (0.04) 0.497 |
−0.02 (0.02) 0.333 |
| List A Trials 1–5 total 3 | -- |
−0.31 (0.11) 0.006 |
−0.04 (0.04) 0.316 |
−0.00 (0.02) 0.993 |
|
| Short Delay Free Recall 3 |
0.04 (0.01) <0.0001 |
-- | -- | −0.04 (0.04) 0.242 |
0.02 (0.02) 0.385 |
| Long Delay Free Recall 3 |
0.03 (0.01) 0.001 |
−0.22 (0.11) 0.041 |
-- | −0.012 (0.04) 0.693 |
−0.00 (0.02) 0.635 |
| Inhibition-Color Naming contrast 4 |
−0.49 (0.04) <0.0001 |
-- | -- |
0.52 (0.14) <0.0001 |
−0.09 (0.09) <0.321 |
| Letter-Number Sequencing – Motor Speed contrast 4 |
−0.05 (0.01) <0.0001 |
−0.13 (0.06) 0.017 |
-- | −0.01 (0.02) 0.656 |
0.01 (.01) 0.622 |
Note: Bolded values are statistically significant, p < .05. Age, sex, and alcohol/cannabis use recency were included as covariates and retained in final full models if only statistically significant. Covariates removed in the final model are indicated with “--” in the appropriate cell.
Wechsler Intelligence Scale for Children – Third Edition at baseline, Wechsler Adult Intelligence Scale – Third Edition at follow-up
Wechsler Abbreviated Scale of Intelligence
California Verbal Learning Test - Children’s Version at baseline, California Verbal Learning Test – Second Edition at follow-up
Delis-Kaplan Executive Function System
Positive values indicate better performance by men
Between-person differences in overall alcohol or marijuana use frequency in the past year
Within-person differences in yearly alcohol or marijuana frequency compared to the year prior
Table 2.
Neuropsychological performance for baseline and each follow-up year.
| Time point |
N | Digit Span Forward1 |
Digit Span Backward1 |
Digit Symbol1 |
Block Design2 |
List A Trial 53 |
List A Trials 1–5 Total3 |
Short Delay Free Recall3 |
Long Delay Free Recall3 |
Inhibition- Color Naming contrast4 |
Letter- Number Sequencing – Motor Speed contrast4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
M (SD) |
|||||||||||
| Baseline | 175 | 9.7 (1.9) | 6.1 (1.9) | 60.9 (11.2) | 44.8 (12.6) | 12.7 (1.7) | 12.7 (1.7) | 11.7 (2.0) | 11.9 (1.9) | −0.9 (2.6) | 0.6 (2.4) |
| Year 1 | 84 | 9.8 (2.0) | 6.1 (2.2) | 64.4 (14.3) | 50.8 (11.5) | 12.7 (1.7) | 12.7 (1.7) | 12.0 (2.1) | 12.3 (1.8) | −1.0 (2.4) | 1.1 (2.0) |
| Year 2 | 97 | 10.0 (1.9) | 6.4 (2.0) | 72.7 (13.5) | 55.9 (9.5) | 13.4 (1.3) | 13.4 (1.3) | 12.6 (1.7) | 13.2 (1.7) | −1.0 (3.0) | 1.2 (2.1) |
| Year 3 | 81 | 10.4 (2.3) | 6.5 (2.3) | 74.5 (13.4) | 56.3 (10.8) | 13.6 (1.5) | 13.6 (1.5) | 12.8 (1.7) | 13.0 (2.1) | −1.2 (2.0) | 1.6 (1.8) |
| Year 4 | 83 | 10.8 (2.2) | 7.0 (2.3) | 81.5 (11.1) | 56.6 (10.3) | 13.5 (1.8) | 55.6 (7.7) | 12.7 (2.5) | 13.2 (2.2) | −1.1 (1.5) | 1.2 (2.1) |
| Year 5 | 92 | 10.7 (2.2) | 7.4 (2.4) | 86.6 (14.5) | 59.8 (8.7) | 13.6 (1.9) | 56.0 (8.2) | 12.6 (2.4) | 12.9 (2.3) | −1.3 (1.7) | 1.5 (1.9) |
| Year 6 | 88 | 11.2 (2.0) | 7.6 (2.2) | 86.5 (14.5) | 61.5 (7.5) | 14.3 (1.6) | 59.4 (7.3) | 13.5 (2.3) | 13.4 (2.7) | −1.8 (1.9) | 1.8 (1.7) |
| Year 7 | 57 | 11.3 (2.1) | 9.5 (2.8) | 80.5 (13.7) | 53.3 (9.4) | 13.9 (2.2) | 57.4 (9.4) | 13.1 (2.8) | 13.2 (2.8) | −1.0 (1.6) | 1.9 (1.4) |
| Year 8 | 48 | 11 (1.80) | 9.4 (2.2) | 76.9 (14.8) | 55.6 (7.3) | 13.8 (1.9) | 58.6 (8.5) | 13.0 (2.2) | 13.2 (2.5) | −1.0 (1.4) | 1.9 (2.1) |
| Year 9 | 21 | 11.5 (1.8) | 9.3 (2.1) | 79.7 (14.1) | 55.9 (7.1) | 14.9 (1.4) | 62.1 (7.7) | 14.5 (1.7) | 14.7 (1.6) | −1.1 (1.5) | 1.2 (1.4) |
| Year 10 | 17 | 11.1 (1.9) | 9.2 (2.4) | 75.6 (13.0) | 53.9 (6.2) | 13.9 (1.8) | 58.4 (8.7) | 13.4 (2.4) | 14.2 (1.8) | −1.4 (1.5) | 1.1 (1.6) |
| Year 11 | 25 | 11.4 (1.6) | 9.8 (2.6) | 78.8 (15.3) | 55.0 (7.2) | 14.4 (1.7) | 60.0 (7.2) | 14.0 (2.1) | 14.0 (2.3) | −1.5 (1.7) | 1.8 (2.0) |
| Year 12 | 18 | 10.9 (1.8) | 9.0 (2.6) | 77.1 (12.7) | 54.4 (7.9) | 14.3 (2.1) | 59.4 (10.2) | 13.0 (2.7) | 13.5 (2.8) | −1.3 (1.9) | 1.1 (2.1) |
| Year 13 | 6 | 13.0 (1.3) | 10.7 (1.4) | 79.3 (9.2) | 54.0 (9) | 12.8 (1.9) | 53.3 (7.9) | 11.7 (2.3) | 12.3 (2.0) | −0.7 (1.5) | 3.2 (1.3) |
All scores are unstandardized, raw scores. Inhibition-Color Naming and Letter Number Sequencing-Motor Speed contrast scores indicate the difference in scaled scores between the two respective conditions. The number of missing data for each follow up year can be calculated as 175-N, where N indicates the number of adolescents assessed in the table above.
Wechsler Intelligence Scale for Children – Third Edition at baseline, Wechsler Adult Intelligence Scale – Third Edition at follow-up
Wechsler Abbreviated Scale of Intelligence
California Verbal Learning Test - Children’s Version at baseline, California Verbal Learning Test – Second Edition at follow-up
.Delis-Kaplan Executive Function System
Effects of cannabis on cognition
Accounting for the effects of age, greater mean levels of percent days of cannabis use across time (‘between-person effect’) were associated with an increased contrast score between DKEFS CWI Inhibition and Color Naming conditions (B = 0.52, SE = 0.14, p < .001, f2 < 0.01). Follow-up analyses revealed that this effect was largely driven by the association between greater cannabis use over time and worse performance over time on the Inhibition condition (p < .001) versus the Color Naming condition, suggesting poorer inhibitory control with more cannabis use. Greater mean percent days of cannabis use across time (‘between-person effect’) also predicted poorer performance over time on WASI Block Design (B = −.08, SE = 0.04, p = .031, f2 < 0.01). There were no significant effects of time-specific fluctuations in cannabis use (‘within-person effect’) on Block Design (p = .816), nor within-or between-person effects of cannabis use on D-KEFS TMT contrast scores, WAIS Symbol Digits, or CVLT Short and Long Delay Free Recall, List A Trial 5, or List A Trials 1–5 total (ps > .05). See Table 1 for results. Notably, cannabis use recency was not significantly associated with performance on any neuropsychological measures (ps > .05). Neuropsychological test scores for baseline and each follow-up year are presented in Table 2.
Discussion
This study examined the longitudinal association between alcohol and cannabis use and cognition among a group of typically developing healthy adolescents with minimal substance use at baseline (ages 12–14). Our results showed three key findings: (1) after accounting for the effects of age, greater mean percent days of cannabis use over time was associated with worse performance on a measure of inhibitory control (DKEFS CWI Inhibition-Color Naming contrast); (2) after accounting for the effects of age, greater mean percent days of cannabis use over time was associated with worse performance on a visuospatial functioning task (WASI Block Design); and (3) an individual drinking more frequently than usual predicted worse performance on the WASI Block Design test. Greater mean percent days of alcohol use across time was not associated with worse performance in the cognitive domains assessed. Contrary to our hypothesis we found no association between alcohol and/or cannabis use over time on test performance in the verbal memory and processing speed domains.
In our sample, greater percent days of cannabis use was associated with deficits in inhibitory control over time. There is growing evidence that executive functions continue to develop throughout late adolescence and into young adulthood (Barber, Caffo, Pekar, & Mostofsky, 2013; Rubia, 2013; Rubia, Smith, Taylor, & Brammer, 2007). The inhibitory control circuit, in particular, may be particularly vulnerable to cannabis use in adolescence (Fontes et al., 2011; Yanes et al., 2018). In accordance with our findings, previous studies have found that adolescent cannabis use is associated with inhibitory control deficits (Dahlgren et al., 2016; Fontes et al., 2011; Jacobus et al., 2015; Lisdahl & Price, 2012; Mathias et al., 2011). Such deficits might result in a vulnerability and/or failure to inhibit maladaptive behavior; more specifically, adolescent cannabis users may experience greater difficulty abstaining from cannabis in the presence of cannabis cues. Inhibitory control deficits might further increase the likelihood of engaging in other risky behaviors (Spear, 2000).
At the neural level, alterations in brain response patterns (Gruber, Dahlgren, Sagar, Gönenc, & Killgore, 2012; Gruber & Yurgelun-Todd, 2005; Solowij et al., 2012) and connectivity (Behan et al., 2014) have been reported among adolescents and young adult cannabis users during tasks of inhibitory control. Even after prolonged abstinence, regular cannabis use has been associated with altered neural activation in the executive and default mode network (Blest-Hopley, Giampietro, & Bhattacharyya, 2019). Pre-existing vulnerabilities in the inhibitory control circuitry have also been associated with substance use initiation and other risk behaviors (Giancola & Parker, 2001). Thus, it is possible that pre-existing neurodevelopmental vulnerabilities compounded with the impact of cannabis on the developing brain overtime results in neuropsychological deficits in cognitive control.
Deficits in visuospatial functioning (Block Design) were associated with both a person drinking more frequently than usual and reporting more cumulative cannabis use across time. Despite differences in study design and neuropsychological tests used to asses visuospatial functioning, the impact of adolescent alcohol use on visuospatial functioning has been frequently documented (Nguyen-Louie et al., 2015; Squeglia, Spadoni, Infante, Myers, & Tapert, 2009; Tapert & Brown, 1999; Tapert, Granholm, Leedy, & Brown, 2002). For example, in a previous report from our group (a subgroup of the current sample), Nguyen-Louie et al., 2015 found worsening visuospatial functioning over a 4-year period after initiation of heavy drinking. Similarly, Squeglia et al. (2009b) found that greater number of drinking days predicted worsening visuospatial functioning performance among adolescent girls who initiated moderate to heavy drinking. Our findings expand on our previous research and suggest that deficits in visuospatial functioning might be more sensitive to a “spike” in drinking pattern versus cumulative reports of drinking behaviors. The impact of cannabis use on visuospatial functioning has been less consistent (Gonzalez et al., 2017; Scott et al., 2018). In support of our findings, a study by Lyons et al. (2004) compared monozygotic twin pairs who were discordant for regular cannabis use and found that out of 16 neuropsychologist tests, cannabis users performed worse than non-users on the WAIS-R Block Design subtest. In a different sample, our group found that heavy cannabis users (ages 16–22) performed worse on visuospatial functioning tasks compared to demographically matched non-users (Jacobus et al., 2015). In contrast, others have found no impact of cannabis use on visuospatial functioning (Jackson et al., 2016; Meier et al., 2012). Our findings suggest both alcohol and cannabis use throughout adolescence may impact visusopatial functioning. The Block Design subtest has been found to be a predictor of everyday spatial ability (Groth-Marnat & Teal, 2000). This has important implications as adolescents become more independent and begin to drive. Block design is also frequently associated with central executive/frontal lobe function (Lezak, Howieson, & Loring, 2004) and has been used as a measure of central executive functioning in previous studies (Brown et al., 2012). Thus, we cannot discard the possibility that the observed deficits in Block Design might be the result of visuospatial planning and organization deficits. Future studies using a wide range of neuropsychological measures are needed to definitely disentangle the impact of alcohol and cannabis use of visuospatial skills.
Notably, we did not find alcohol or cannabis use to be associated with deficits in verbal memory. This was surprising given results from previous studies, including those from our group, that indicate associations between poorer performance on verbal memory tests and frequent alcohol and cannabis use (Green et al., 2010; Jacobus et al., 2015; Nguyen-Louie et al., 2015; Nguyen-Louie et al., 2016; Sneider et al., 2013; Solowij et al., 2011; Winward et al., 2014b. Similarly, we did not find alcohol and/or cannabis use to impact neuropsychological performance on working memory and/or processing speed tasks. This is in contrast to some previous studies that show decrements in processing speed among cannabis users even after 3 weeks of abstinence (Winward et al., 2014). Although the notable length of follow up period (314 years) might have played a role as other well-designed prospective studies have also identified modest or no differences in these domains (Gonzalez et al., 2017; Lyons et al., 2004; Scott et al., 2018).
Examining patterns of substance use on cognitive functioning from early-mid adolescence and into young adulthood is critical given changes in substance throughout this period as well as dramatic changes in brain development and cognition. Despite the strengths of this prospective study, including the statistical design that incorporates time-specific fluctuations in substance use, the large sample size, and the number of assessment points, our study has some limitations that are worth noting. Only a limited number of covariates and confounders were evaluated in order to preserve statistical power. The potential for self-report bias to decrease the precision of adolescent alcohol and cannabis use estimates is another important limitation. Lack of information on cannabis product types, potency, and cannabis constituents is another limitation given the increasing heterogeneity of cannabis products available (Wilson, Freeman, & Mackie, 2019). A separate model for each neuropsychology measure was tested to best address issues of multicollinearity. However, there remains the possibility that Type I error is inflated given multiple models were examined. Nevertheless, our models were established a priori and given the larger sample size and established validity of the measures used our effect sizes should be fairly representative of the population. Practice effects of repeated neuropsychological testing should also be considered. Lastly, our study was conducted with a high SES and predominantly Caucasian sample and the findings may not generalize to other populations.
Results from this study suggest that throughout adolescence and young adulthood, greater lifetime cannabis use may be associated with poorer inhibitory control and visuospatial functioning, whereas alcohol-related neurocognitive alterations may be more sensitive to proximal fluctuations in use severity, particularly in the domain of visuospatial processing. These findings add to the growing body of literature on the impact of alcohol and cannabis use on cognition from adolescence to young adulthood. The reliance on retrospective self-report is a limitation, and while errors in recall may impact the validity of some self-reported substance use estimates, we used well-validated substance use assessment measures (e.g., Timeline Follow back) to minimize bias in substance use estimation. Nevertheless, replication is important and we will continue to examine to what extent differences in neurocognitive outcomes are driven by pre-existing environmental and biological factors versus substance-related exposure in large sample prospective studies (Luciana et al., 2018). Examining the unique trajectories of alcohol and cannabis use (the most widely used substances by adolescents) and impact on cognition will help inform policy makers, prevention strategies, and targets for novel interventions to reduce adolescent substance use.
Acknowledgements
This study was supported by National Institute on Alcohol Abuse and Alcoholism (J.J., A.I., R01 AA013419); National Institute on Drug Abuse (J.J., A.I., C.C., U01 DA041089; J.J., K.E.C. R21 DA047953; M.W. K23DA039348); National Institute of Mental Health (K.E.C., T32 MH018399National Center for Advancing Translational Science (J.J., KL2 TR001444); and the California Tobacco-Related Disease Research Grants Program Office of the University of California (J.J., K.E.C., Grant 580264). The authors would like to thank participating schools in the San Diego Unified School District, participating families, and the Adolescent Brain Imaging Project laboratory.
Footnotes
The authors have no financial relationships or conflicts of interest to disclose.
References
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed., text revision). Washington, DC: American Psychiatric Association. [Google Scholar]
- Barber AD, Caffo BS, Pekar JJ, & Mostofsky SH (2013). Developmental changes in within-and between-network connectivity between late childhood and adulthood. Neuropsychologia, 51, 156–167. doi: 10.1016/j.neuropsychologia.2012.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan B, Connolly CG, Datwani S, Doucet M, Ivanovic J, Morioka R, … Garavan H (2014). Response inhibition and elevated parietal-cerebellar correlations in chronic adolescent cannabis users. Neuropharmacology, 84, 131–137. doi: 10.1016/j.neuropharm.2013.05.027 [DOI] [PubMed] [Google Scholar]
- Blest-Hopley G, Giampietro V, & Bhattacharyya S (2019). Regular cannabis use is associated with altered activation of central executive and default mode networks even after prolonged abstinence in adolescent users: Results from a complementary metaanalysis. Neurosci Biobehav Rev, 96, 45–55. doi: 10.1016/j.neubiorev.2018.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LA, Brockmole JR, Gow AJ, & Deary IJ (2012). Processing speed and visuospatial executive function predict visual working memory ability in older adults. Experimental Aging Research, 38, 1–19. doi: 10.1080/0361073X.2012.636722 [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, & Vik PW (1998). Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol, 59, 427–438. doi: 10.15288/jsa.1998.59.427 [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, & Delis DC (2000). Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol Clin Exp Res, 24, 164–171. [PubMed] [Google Scholar]
- Brumback TY, Worley M, Nguyen-Louie TT, Squeglia LM, Jacobus J, & Tapert SF (2016). Neural predictors of alcohol use and psychopathology symptoms in adolescents. Dev Psychopathol, 28, 1209–1216. doi: 10.1017/s0954579416000766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Crane NA, & Mason BJ (2011). An evidence based review of acute and longterm effects of cannabis use on executive cognitive functions. J Addict Med, 5, 1–8. doi: 10.1097/ADM.0b013e31820c23fa [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran PJ, & Bauer DJ (2011). The disaggregation of within-person and between-person effects in longitudinal models of change. Annu Rev Psychol, 62, 583–619. doi: 10.1146/annurev.psych.093008.100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren MK, Sagar KA, Racine MT, Dreman MW, & Gruber SA (2016). Marijuana Use Predicts Cognitive Performance on Tasks of Executive Function. J Stud Alcohol Drugs, 77, 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, & Kramer JH (2001). The Delis-Kaplan Executive Function System: Examiner’s Manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, & Ober BA (1994). Manual for the California Verbal Learning Test–Children’s Version. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Ferrett HL, Carey PD, Thomas KG, Tapert SF, & Fein G (2010). Neuropsychological performance of South African treatment-naïve adolescents with alcohol dependence. Drug Alcohol Depend, 110, 8–14. doi: 10.1016/j.drugalcdep.2010.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR, … Lacerda AL (2011). Cannabis use before age 15 and subsequent executive functioning. Br J Psychiatry, 198, 442–447. doi: 10.1192/bjp.bp.110.077479 [DOI] [PubMed] [Google Scholar]
- Giancola PR, Mezzich AC, & Tarter RE (1998). Disruptive, delinquent and aggressive behavior in female adolescents with a psychoactive substance use disorder: relation to executive cognitive functioning. J Stud Alcohol, 59, 560–567. [DOI] [PubMed] [Google Scholar]
- Giancola PR, & Parker AM (2001). A six-year prospective study of pathways toward drug use in adolescent boys with and without a family history of a substance use disorder. J Stud Alcohol, 62, 166–178. [DOI] [PubMed] [Google Scholar]
- Gil-Hernandez S, Mateos P, Porras C, Garcia-Gomez R, Navarro E, & Garcia-Moreno LM (2017). Alcohol Binge Drinking and Executive Functioning during Adolescent Brain Development. Front Psychol, 8, 1638. doi: 10.3389/fpsyg.2017.01638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Pacheco-Colón I, Duperrouzel JC, & Hawes SW (2017). Does Cannabis Use Cause Declines in Neuropsychological Functioning? A Review of Longitudinal Studies. J Int Neuropsychol Soc, 23, 893–902. doi: 10.1017/S1355617717000789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A, Garrick T, Sheedy D, Blake H, Shores EA, & Harper C (2010). The effect of moderate to heavy alcohol consumption on neuropsychological performance as measured by the repeatable battery for the assessment of neuropsychological status. Alcohol Clin Exp Res, 34, 443–450. doi: 10.1111/j.1530-0277.2009.01108.x [DOI] [PubMed] [Google Scholar]
- Groth-Marnat G, & Teal M (2000). Block design as a measure of everyday spatial ability: A study of ecological ability. Perceptual & Motor Skills, 90, 522–526. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gönenc A, & Killgore WD (2012). Age of onset of marijuana use impacts inhibitory processing. Neurosci Lett, 511, 89–94. doi: 10.1016/j.neulet.2012.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, & Yurgelun-Todd DA (2005). Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res Cogn Brain Res, 23, 107–118. doi: 10.1016/j.cogbrainres.2005.02.016 [DOI] [PubMed] [Google Scholar]
- Hollingshead AB (1965). Two-Factor Index of Social Position. New Haven, CT: Yale University Press. [Google Scholar]
- Jackson NJ, Isen JD, Khoddam R, Irons D, Tuvblad C, Iacono WG, … Baker LA (2016). Impact of adolescent marijuana use on intelligence: Results from two longitudinal twin studies. Proc Natl Acad Sci U S A, 113, E500–508. doi: 10.1073/pnas.1516648113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Infante MA, Castro N, Brumback T, Meruelo AD, & Tapert SF (2015). Neuropsychological performance in adolescent marijuana users with cooccurring alcohol use: A three-year longitudinal study. Neuropsychology, 29, 829–843. doi: 10.1037/neu0000203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, & Tapert SF (2014). Effects of cannabis on the adolescent brain. Curr Pharm Des, 20, 2186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, & Patrick ME (2019). Monitoring the Future national survey results on drug use 1975–2018 Ann Arbor: Institute for Social Research, University of Michigan. [Google Scholar]
- Kundu P, Benson BE, Rosen D, Frangou S, Leibenluft E, Luh WM, … Ernst M (2018). The integration of functional brain activity from adolescence to adulthood. The Journal of Neuroscience, 38, 3559–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, & Loring DW (2004). Neuropsychological assess-ment (4th ed.). Oxford, UK: Oxford University Press. [Google Scholar]
- Lisdahl KM, & Price JS (2012). Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. J Int Neuropsychol Soc, 18, 678–688. doi: 10.1017/S1355617712000276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Bjork JM, Nagel BJ, Barch DM, Gonzalez R, Nixon SJ, & Banich MT (2018). Adolescent neurocognitive development and impacts of substance use: Overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev Cogn Neurosci, 32, 67–79. doi: 10.1016/j.dcn.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MJ, Bar JL, Panizzon MS, Toomey R, Eisen S, Xian H, & Tsuang MT (2004). Neuropsychological consequences of regular marijuana use: a twin study. Psychol Med, 34, 1239–1250. [DOI] [PubMed] [Google Scholar]
- Mathias CW, Blumenthal TD, Dawes MA, Liguori A, Richard DM, Bray B, … Dougherty DM (2011). Failure to sustain prepulse inhibition in adolescent marijuana users. Drug Alcohol Depend, 116, 110–116. doi: 10.1016/j.drugalcdep.2010.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, & Tapert SF (2007). Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc, 13, 807–820. doi: 10.1017/s1355617707071032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, … Moffitt TE (2012). Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A, 109, E2657–2664. doi: 10.1073/pnas.1206820109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo AD, Castro N, Cota CI, & Tapert SF (2017). Cannabis and alcohol use, and the developing brain. Behav Brain Res, 325, 44–50. doi: 10.1016/j.bbr.2017.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Louie TT, Castro N, Matt GE, Squeglia LM, Brumback T, & Tapert SF (2015). Effects of Emerging Alcohol and Marijuana Use Behaviors on Adolescents’ Neuropsychological Functioning Over Four Years. J Stud Alcohol Drugs, 76, 738–748. doi: 10.15288/jsad.2015.76.738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Louie TT, Matt GE, Jacobus J, Li I, Cota C, Castro N, & Tapert SF (2017). Earlier Alcohol Use Onset Predicts Poorer Neuropsychological Functioning in Young Adults. Alcohol Clin Exp Res, 41, 2082–2092. doi: 10.1111/acer.13503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Louie TT, Simmons AN, Squeglia LM, Alejandra Infante M, Schacht JP, & Tapert SF (2018). Earlier alcohol use onset prospectively predicts changes in functional connectivity. Psychopharmacology (Berl), 235, 1041–1054. doi: 10.1007/s00213-0174821-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Louie TT, Tracas A, Squeglia LM, Matt GE, Eberson-Shumate S, & Tapert SF (2016). Learning and Memory in Adolescent Moderate, Binge, and Extreme-Binge Drinkers. Alcohol Clin Exp Res, 40, 1895–1904. doi: 10.1111/acer.13160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada M, Corral M, Mota N, Crego A, Rodríguez Holguín S, & Cadaveira F (2012). Executive functioning and alcohol binge drinking in university students. Addict Behav, 37, 167–172. doi: 10.1016/j.addbeh.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Rubia K (2013). Functional brain imaging across development. Eur Child Adolesc Psychiatry, 22, 719–731. doi: 10.1007/s00787-012-0291-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, & Brammer M (2007). Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp, 28, 1163–1177. doi: 10.1002/hbm.20347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Slomiak ST, Jones JD, Rosen AFG, Moore TM, & Gur RC (2018). Association of Cannabis With Cognitive Functioning in Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Psychiatry, 75, 585–595. doi: 10.1001/jamapsychiatry.2018.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selya AS, Rose JS, Dierker LC, Hedeker D, & Mermelstein RJ (2012). A Practical Guide to Calculating Cohen’s f2, a Measure of Local Effect Size, from PROC MIXED. Frontiers in Psychology . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneider JT, Cohen-Gilbert JE, Crowley DJ, Paul MD, & Silveri MM (2013). Differential effects of binge drinking on learning and memory in emerging adults. J Addict Res Ther, Suppl 7. doi: 10.4172/2155-6105.s7-006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, … Yücel M (2011). Verbal learning and memory in adolescent cannabis users, alcohol users and nonusers. Psychopharmacology (Berl), 216, 131–144. doi: 10.1007/s00213-011-2203-x [DOI] [PubMed] [Google Scholar]
- Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, … Yucel M (2012). Reflection impulsivity in adolescent cannabis users: a comparison with alcoholusing and non-substance-using adolescents. Psychopharmacology (Berl), 219, 575–586. doi: 10.1007/s00213-011-2486-y [DOI] [PubMed] [Google Scholar]
- Spear LP (2000). The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev, 24, 417–463. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, & Tapert SF (2009a). The influence of substance use on adolescent brain development. Clin EEG Neurosci, 40, 31–38. doi: 10.1177/155005940904000110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Schweinsburg AD, Pulido C, & Tapert SF (2011). Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol Clin Exp Res, 35, 1831–1841. doi: 10.1111/j.1530-0277.2011.01527.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, & Tapert SF (2009b). Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav, 23, 715–722. doi: 10.1037/a0016516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, & Pfefferbaum A (2015). Brain development in heavy-drinking adolescents. Am J Psychiatry, 172, 531–542. doi: 10.1176/appi.ajp.2015.14101249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, & Brown SA (1999). Neuropsychological correlates of adolescent substance abuse: four-year outcomes. J Int Neuropsychol Soc, 5, 481–493. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, & Brown SA (2002). Substance use and withdrawal: neuropsychological functioning over 8 years in youth. J Int Neuropsychol Soc, 8, 873883. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Mezzich AC, Hsieh YC, & Parks SM (1995). Cognitive capacity in female adolescent substance abusers. Drug Alcohol Depend, 39, 15–21. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Monnig MA, Lysne PA, Ruhl DA, Pommy JA, Bogenschutz M, … Yeo RA (2011). Adolescent substance abuse: the effects of alcohol and marijuana on neuropsychological performance. Alcohol Clin Exp Res, 35, 39–46. doi: 10.1111/j.1530-0277.2010.01320.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Rivas-Perez C, Lopez-Torrecillas F, & Perez-Garcia M (2006). Differential impact of severity of drug use on frontal behavioral symptoms. Addict Behav, 31, 1373–1382. doi: 10.1016/j.addbeh.2005.11.003 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, … Weiss SRB (2018). The conception of the ABCD study: From substance use to a broad NIH collaboration. Dev Cogn Neurosci, 32, 4–7. doi: 10.1016/j.dcn.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1997). Wechsler Adult Intelligence Scale (3rd ed.). New York: The Psychological Corporation. [Google Scholar]
- Wechsler D (1999). Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wilson J, Freeman TP, & Mackie CJ (2019). Effects of increasing cannabis potency on adolescent health. Lancet Child Adolesc Health, 3, 121–128. doi: 10.1016/S2352-4642(18)30342-0 [DOI] [PubMed] [Google Scholar]
- Winward JL, Hanson KL, Bekman NM, Tapert SF, & Brown SA (2014a). Adolescent heavy episodic drinking: neurocognitive functioning during early abstinence. J Int Neuropsychol Soc, 20, 218–229. doi: 10.1017/s1355617713001410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winward JL, Hanson KL, Tapert SF, & Brown SA (2014b). Heavy alcohol use, marijuana use, and concomitant use by adolescents are associated with unique and shared cognitive decrements. J Int Neuropsychol Soc, 20, 784–795. doi: 10.1017/s1355617714000666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley MJ, Trim RS, Tate SR, Roesch SC, Myers MG, & Brown SA (2014). Selfefficacy and social networks after treatment for alcohol or drug dependence and major depression: disentangling person and time-level effects. Psychol Addict Behav, 28, 1220–1229. doi: 10.1037/a0037901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanes JA, Riedel MC, Ray KL, Kirkland AE, Bird RT, Boeving ER, … Sutherland MT (2018). Neuroimaging meta-analysis of cannabis use studies reveals convergent functional alterations in brain regions supporting cognitive control and reward processing. J Psychopharmacol, 32, 283–295. doi: 10.1177/0269881117744995 [DOI] [PMC free article] [PubMed] [Google Scholar]