Abstract
Prior studies in the Child Health and Development Studies (CHDS) found in utero exposure to the pesticide, dichlorodiphenyltrichloroethane (DDT), increased breast cancer risk by age 52. Mammographic density is considered a primary risk factor for breast cancer. We conducted a study of 309 daughters from the CHDS to examine in utero DDT exposure and mammographic density in midlife. Among daughters with high (>75th percentile) exposure to p,p’-Dichlorodiphenyldichloroethylene (DDE), p,p’-DDT was significantly correlated with increased dense area and percent density regardless of her body mass in midlife. In the subset of women with lower (<75th percentile) p,p-DDE, p,p’-DDT was associated with increased non-dense breast area. This was explained by adjustment for midlife BMI suggesting that p,p’-DDT may be obesogenic. In aggregate our findings indicate that early life p,p’-DDT exposure impacts breast density in a complex way that depends on the hosts biological ability to sequester and process DDT and levels of exposure.
Keywords: Mammographic breast density, dichlorodiphenyltrichloroethane (DDT), Child Health and Development Studies (CHDS), in utero exposure, breast cancer, dense area, percent density, non-dense area
1. Intro
Previously, Child Health and Development Studies (CHDS) daughters were found to have increased risk of breast cancer by age 52 associated with in utero exposure to the pesticide, 2,4-dichlorodiphenyltrichloroethane (o,p’-DDT) [1]. Technical DDT consisted of p,p’-DDT (the primary component), o,p’-DDT (a minor component/contaminant) and its major degradation product is p,p’-dichlorodiphenyldichloroethylene (DDE) While previous in utero exposure to environmental contaminants cannot be changed there might be steps along the life course that can be modified to reduce the health risk caused by exposure. Additionally, future in utero exposures can be prevented using policy that weighs both the benefits and potential long term risks of using DDT in chemical vector control. As stated by Bouwman (2011) there are situations where DDT will provide the best achievable health benefit as a malaria control measure, but maintaining that DDT is safe ignores the results of many studies [2].
Density is considered an important intermediate marker of breast cancer risk [3] and a potentially modifiable risk factor [4, 5]. For example, pharmaceuticals such as tamoxifen have been shown to change breast density [4, 5]. Epidemiologic studies have consistently supported a 4 to 6-fold increase in breast cancer incidence for women with dense breast tissue for up to 10 years following measurement [6–11]. Mammographic density (75% or greater) has also been associated with a nine-fold increase in carcinoma in situ or atypical hyperplasia and a twelve-fold increase in risk of developing hyperplasia [3]. Increased risk associated with mammographic density has been shown for both premenopausal and postmenopausal breast cancer [9]. Density is shaped by cumulative hormonal exposures throughout life, but in particular during the intrauterine, menarche, pregnancy and lactation time periods [12].
Body Mass Index (BMI) has a complex relationship with breast cancer risk and breast density. BMI is associated with increased breast fat [13] and lower percent breast density. BMI before menopause has an inverse relationship with both breast density and breast cancer risk [14, 15]. BMI after menopause is positively associated with postmenopausal breast cancer risk, whereas percent breast density is lower after menopause [14, 16]. Evidence suggests that examining the window of susceptibility when mammary tissue is developing and differentiating is critical in establishing breast cancer risk [16] and in untangling the nexus between breast density and breast cancer. These windows include: in utero, childhood, puberty, before first term pregnancy, during pregnancy and during the perimenopause [16–18]. These periods are characterized by different structure, function and hormonal control mechanisms and thus risk factors for breast cancer during each period might differ. Predictors of breast density and the role of breast density in the etiology of breast cancer may depend on age, biological stage, and underlying vulnerability, and may interact with other risk factors such as genetic susceptibility and exposures during critical windows.
DDTs have been shown to change cancer risk and also act as obesogens [1, 19–21]. In utero exposure to DDT and its break-down product DDE are associated with increased BMI in humans [19, 20]. Animal studies suggest DDE may affect offspring obesity due to its effects on lipid metabolism [20, 22, 23] Thus DDTs may impact breast density via increases in BMI. It is also possible that DDTs may exert an impact on breast density via alternative, BMI-independent pathways. Just as BMI associations with breast cancer can depend on age and menopausal status at the time of diagnosis, DDT associations with breast density may depend on age and menopausal status or other underlying susceptibility features including genetic predisposition. In a companion analysis, McDonald et al. (2019) investigates whether family history of breast cancer modifies associations between in utero of DDT exposure and daughter’s mammographic density women in early menopause [24]. Here, we test whether mammographic density is affected by in utero exposure to environmental DDT compounds in women in their 40s. We also investigate the role, if any, of daughter’s BMI in mediating or modifying this association given that DDTs are obesogens.
2. Methods
2.1. Study Population
The Child Health and Development Studies (CHDS) is a pregnancy cohort designed to examine the association between prenatal exposures and health and development over the life course for parents and children. The CHDS recruited women residing in the Oakland, California area who were members of the Kaiser Permanente Foundation Health Plan and who sought obstetric care for pregnancies between 1959 and 1966 (with deliveries extending into 1967) [25]. More than 98% of all eligible women enrolled. The CHDS founding mothers voluntarily participated in an in-person interview and gave permission to access their own medical records and those of their children to researchers. The mothers’ blood specimens were collected at several times through pregnancy and 1–3 days after delivery. The CHDS regularly links to the California Cancer Registry (CCR). Maternal breast cancer cases were identified using linkage to CCR records to identify cancer diagnoses, including year and age at diagnosis. Daughter breast cancer cases were identified by both linkage to the California Cancer Registry and by self-report during a computer-assisted telephone interview in an adult follow-up study of CHDS daughters from 2010 to 2013[1].
Between 2005 −2007, 567 daughters born into the CHDS participated in an adult follow-up study described by Terry et al. (2011) [26]. A 45 minute telephone interview was conducted to collect personal health history and medication use, family history of cancer, sociodemographic factors, alcohol and tobacco use, detailed reproductive history and anthropometric measures. Yearly mammographic density screening was recommended as standard practice beginning at age 40 from 1997 to 2015 [27] which encompasses the time frame of the present study. We collected and were able to read 348 digital screening mammograms (61%) of the 567. Of the 348, 12 were missing on menopausal status, 9 women were previously diagnosed with breast cancer, 8 were missing weight at interview, 7 were missing maternal weight, two were missing lipid measures, and age at menopause was unknown for one woman. Our analysis includes 309 CHDS daughters who had never been diagnosed with breast cancer, had adult mammographic density data and maternal perinatal serum measurements of DDT and lipids. Of the 309 women, 192 had no siblings in the study and 117 had at least one sibling. There were 33 women, 11% of our sample, with mothers who had a breast cancer diagnosis. The present study was reviewed and approved by the Institutional Review Boards of the Public Health Institute and Columbia University.
2.2. Samples and Assays
Blood samples were collected from pregnant women in each trimester and in the early post-partum period between 1959 and 1967, processed to isolate serum, and stored since then at −20 Celsius. DDT-related compounds (p,p’-DDT and o,p’-DDT, and p,p’-DDE) were examined for a number of different studies and assay methods are described in previous publications [1, 28]. Briefly, samples were measured using gas chromatography with electron capture detection (GC/ECD) with a capillary column (10%) or GC/ECD with dual columns (86%) or using gas chromatography triple quadrupole mass spectrometry (4%). Sholtz et al. 2011 [28] showed DDT congeners measured in different labs and using different techniques could be combined without risking meaningful misclassification due to variation. This allows us to conserve irreplaceable archived serum samples.
Total cholesterol and triglycerides were measured enzymatically in a lab certified by the Centers for Disease Control and Prevention/National Heart, Lung, and Blood Institute Lipid Standardization Program.
2.3. Mammographic Density Data
Collection and measurement of screening mammograms are described by Terry et al. (2011) [26]. Briefly, researchers requested films from radiological facilities of the participants, digitized the films using a Kodak Lumisys Film Digitizer (Kodak LS85), and promptly returned the films to the facilities. We assessed mammographic density using Cumulus, a computer-assisted thresholding program [29] in which the reader outlines the total breast area and dense area, and the software measures the size by identifying the number of pixels within the outlined areas. We calculated absolute breast area and dense area by converting the measure from pixels to cm2. Non-dense breast area is calculated by taking absolute breast area and subtracting dense area. Percent mammographic density was calculated as dense area divided by total breast area multiplied by 100. If both left and right images were available, we only read left breast images. We used the film taken closest to the date of interview in our analyses.
2.4. Statistical Analysis
Dense area, non-dense area, percent density, o,p’-DDT and p,p’-DDT were natural log transformed. p,p’-DDE was categorized according to quartiles based on the distribution among the 567 women who participated in the adult follow-up study. Quartile cutpoints for the 567 were nearly identical to those for the 309 in the analysis sample. For analysis, DDE was dichotomized as ≤75th percentile (≤58 ng/mL) versus >75th percentile (>58 ng/mL). Associations were estimated in Proc Mixed, SAS using log-log models to adjust for sibling clustering using SAS 9.3 (SAS Institute Inc., Cary, NC). Our starting models were models that included variables found to be related to mammographic density and breast cancer risk from previous CHDS studies and in the literature. Using backward selection, we generated the most parsimonious models. Models 1 and 2 were adjusted for mother’s age during pregnancy (years), and mother’s weight (dichotomized as body mass index,BMI ≥25kg/m2, vs. BMI <25kg/m2), o,p’-DDT (continuous natural log transformed), mother’s total cholesterol (continuous, mg/dL), mother’s total triglycerides (continuous, mg/dL), daughter’s age at mammogram (continuous, years). Model 2 was further adjusted for daughter’s weight at interview (BMI ≥ 25kg/m2, vs. <25kg/m2). Further models were run adjusting for maternal breast cancer history and menopausal status, but did not change results (results not shown). Model 1 and model 2 were also run using continuous BMI and results did not change (Supplemental Table 1).
3. Results
The maternal and daughter characteristics of the analysis subset are presented in Table 1. Mothers were a median age of 26 years when they were pregnant with their daughters. Daughters were a median age of 44 years at study interview and similarly had a median age of 43 years at the time of their mammogram. At time of study interview, most daughters had not entered menopause (72%).Table 1 also shows distributions for maternal perinatal serum levels of DDTs. It has been established that organochlorine levels are consistent across all trimesters of pregnancy and soon after delivery [30], thus these levels represent proxy in utero exposures for offspring daughters.
Table 1.
Maternal and daughter characteristics of the analysis subset (n=309)
| F0 | Min | 25% | Median | 75% | Max |
|---|---|---|---|---|---|
| Height (m) | 1.42 | 1.6 | 1.63 | 1.68 | 1.78 |
| Weight (kg) * | 44 | 55.03 | 60.69 | 69.33 | 102.58 |
| BMI†* | 16.86 | 21.26 | 22.72 | 25.42 | 44.17 |
| p,p’-DDT (ng/ml) | 0.60 | 6.00 | 9.12 | 14.10 | 84.90 |
| p,p’-DDE (ng/ml) | 11 | 32 | 46 | 61 | 163 |
| o,p’-DDT (ng/ml) | 0.0043 | 0.21 | 0.36 | 0.64 | 3.59 |
| Total Cholesterol (mg/dL) | 75 | 200 | 240 | 290 | 560 |
| Triglycerides (mg/dL) | 45 | 152 | 190 | 245 | 56 ‘ |
| Age at birth of daughter | 16 | 23 | 26 | 31 | ‘3 |
| N (%) | |||||
| BMI ≥ 25 kg/m2 | 89 (29) | ||||
| Breast Cancer | 33 (11) | ||||
| F1 | Min | 25% | Median | 75% | Max |
| Height (m)Δ | 1.49 | 1.6 | 1.65 | 1.73 | 1.88 |
| Weight (kg)Δ | 41.40 | 63.00 | 72.00 | 89.10 | 154.80 |
| BMI† | 14.01 | 23.11 | 26.24 | 31.37 | 62.42 |
| Age at interview | 39 | 42 | 44 | 44 | 47 |
| Age at mammogram | 34 | 42 | 43 | 45 | 47 |
| Dense area | 1.41 | 17.99 | 30.43 | 45.46 | 127.71 |
| Non-dense area | 10.12 | 44.75 | 84.33 | 137.73 | 431.35 |
| Percent Density | 1.26 | 13.29 | 29.71 | 46.38 | 79.09 |
| N (%) | |||||
| BMI ≥ 25 kg/m2 | 181 (59) | ||||
| Menopausal statusΔ | |||||
| Premenopausal | 224 (72) | ||||
| Transition | 49 (16) | ||||
| Post-menopausal | 36 (12) | ||||
| Race | |||||
| White | 229 (74) | ||||
| Black | 43 (14) | ||||
| Hispanic | 19 (6) | ||||
| Asian | 10 (3) | ||||
| Mixed | 6 (2) | ||||
| Other | 2 (1) |
Adjusted weight for 101 days of pregnancy
BMI = weight (kg)/[height(m)]2
at interview
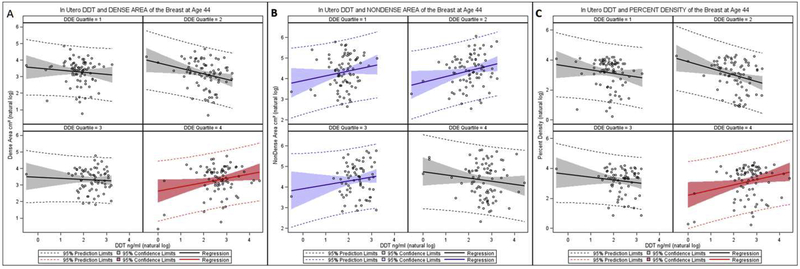
High (>75th percentile) co-exposure to p,p’-DDT and p,p’-DDE in utero predicted higher dense area (Table 2 and Figure 1a) and higher percent density (Table 2 and Figure 1c). Among daughters who had both high p,p’-DDE and high p,p’-DDT exposures in utero, p,p’-DDT was significantly positively correlated with dense area and percent density regardless of her BMI in midlife. Model 2 shows that each 10 percent increase in in utero p,p’-DDT in women with high p,p’-DDE, there was a predicted 2.5 percent increase in dense area of the breast and a 2.9 percent increase in percent density. Further, we estimated the change in percent density associated with the mid-points of the interquartile range for the natural log of p,p’-DDT within the 4th quartile of natural log p,p’-DDE. Among women who were highly exposed to p,p’-DDT (median of 4th quartile vs. median of 1st quartile) within the highest exposure of p,p’-DDE (4th quartile), we observed 6% increase in percent density (95% CI=1.5% to 10.8%, p-value=0.01).
Table 2.
In utero p,p’-DDT and adult breast density before age 50 by in utero p,p’-DDE levels
| ln p,p’-DDTf Correlations with Breast Density Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| In Dense Areag | In Non-Dense Areag | In Percent Densityg | |||||||
| 95% CI | 95% CI | 95% CI | |||||||
| coefficient | lower | upper | coefficient | lower | upper | coefficient | lower | upper | |
| Model 1a | |||||||||
| p,p’-DDE | |||||||||
| Lowd | −0.13 | −0.31 | 0.05 | 0.25 | 0.07 | 0.42 | −0.26 | −0.48 | −0.04 |
| Highe | 0.29 | 0.05 | 0.53 | −0.17 | −0.41 | 0.06 | 0.39 | 0.10 | 0.68 |
| p-value for interactionc | <0.005 | <0.005 | <0.001 | ||||||
| Model 2b | |||||||||
| p,p’-DDE | |||||||||
| Lowd | −0.09 | −0.27 | 0.10 | 0.09 | −0.06 | 0.24 | −0.12 | −0.32 | 0.08 |
| Highe | 0.26 | 0.03 | 0.50 | −0.06 | −0.26 | 0.13 | 0.30 | 0.04 | 0.57 |
| p-value for interaction | <0.02 | NS | <0.02 | ||||||
Bold coefficients indicates significant finding (p<.05); ln, natural log
adjusted for age at mammogram, o,p’-DDT ng/ml (natural log transformed), maternal age at pregnancy, maternal weight adjusted weight for 101 days of pregnancy (Body Mass Index (BMI) ≥ 25 kg/m2 vs. BMI <25 kg/m2), maternal total serum cholesterol and total serum triglycerides.
additional adjustment of daughters weight at interview defined as BMI ≥ 25 kg/m2 or BMI <25 kg/m2.
The interaction is tested by a product term of the p,p’-DDT and p,p’-DDE variables.
High p.p’-DDE is defined by >75th percentile, >58 ng/mL
Low p.p’-DDE is defined by <75th percentile, ≤58 ng/mL
Natural log transformed; ng/ml
Natural log transformed; Dense Area and Non-Dense area, cm2
Figure 1.
In Utero p,p’-DDT and Adult Breast Density Parameters Before Age 50 by in Utero p,p’-DDE quartile
Panel A. Dense Area
Panel B, Non-dense Area
Panel C. Percent Density
High p,p’-DDT in daughters with lower perinatal p,p’-DDE (<75th percentile) predicted higher non-dense breast area (Table 2, model 1 and Figure 2b). However, adjusting for BMI ≥ 25kg/m2 accounted for this association (Table 2, Model 2). In the subset of women with lower p,p-DDE, the associations between in utero exposures to p,p’-DDT and non-dense breast area in adult women support evidence that DDT acts as an obesogen and impact non-dense breast tissue by increasing fat in the breast. The coefficient for daughters’ BMI in Model 2, for the non-dense area outcome provides a quantitative estimate of the impact of daughter’s BMI on non-dense area: β = 0.89, 95% CI= 0.7446 to 1.04, p-value < .0001)
Interactions between p,p’-DDT and p,p’-DDE were statistically significant for both dense area (p<0.02) and percent density (p<0.02). We did not see an association between o,p-DDT exposure and breast density. It is worth noting however, when we used the threshold for low o,p-DDT exposure based on a previous breast cancer study in daughters [1], we did see a significant association only with non-dense area of the breast in this sample (β=0.27, 95% CI= 0.06 to 0.48, p-value=0.01; Supplemental Table 1).
Findings were not explained by menopausal status or maternal age, maternal serum lipids or maternal body mass. Adjusting for maternal breast cancer history, menopausal status at interview or race did not change results. Adjusting for continuous maternal or daughters BMI did not change the significance or interpretation of our results (Supplemental Table 2).
4. Discussion
While many epidemiologic studies have not reported associations between DDT exposure and breast cancer risk [31, 32], many of those studies fail to measure DDT congeners [32, 33] and fail to look at windows of susceptibility [17]. Both are essential to understanding the relationship of environmental exposures to breast cancer risk and intermediate markers of breast cancer risk. The CHDS provides a unique opportunity to study both. Previous CHDS research has already shown a relation between in utero DDT exposure and risk of breast cancer in daughters [1]. Examining intermediate risk factors at different biological time points during periods of breast development and proliferation when the cells are more susceptible to programming errors [34], offers the possibility of understanding the path to breast cancer and also an opportunity to find an intermediate point for intervening to modify risk before disease onset.
Of the women who were highly exposed to p,p’-DDT (median of 4th quartile vs. median of 1st quartile) within the highest exposure of p,p’-DDE (4th quartile), we observed 6% increase in percent density. This difference in breast density associated with high exposure is important given that breast density is a strong intermediate marker for breast cancer [35, 36]. In a recent examination of the association between percent breast density and the long-term excess risk of breast cancer, Rebolj et al. (2019) found a 3% increase in breast cancer risk per percentage point increase in percent density [37]. Applying this risk estimate to our findings would suggest that women highly exposed to DDTs in utero could have in excess of an 18% increased risk of breast cancer.
Our o,p’-DDT findings for non-dense area suggest that there are different effects for the different DDTs: high co-exposure to p,p’-DDT and DDE were associated with percent density and dense area, but higher o,p’-DDT exposure was associated with non-dense area. Additional evidence of differential effects for the different DDT compounds is provided in our companion paper in this issue showing an o,p’-DDT association with dense area but only in daughters with a maternal history of breast cancer [24]. Taken together these findings suggest a complex role for DDT, depending on timing of exposure, target tissue and outcome measure.
This study highlights the importance of measuring all DDT related compounds as they have varying effects at different concentrations on different aspects of breast density in women. The underlying explanation for a differential relationship between levels of p,p-DDT and p,p’-DDE are unknown but could result from one or more underlying mechanism including: 1) an indicator of chronic and current exposure to the technical pesticde [38, 39]; 2) biological ability to metabolize p,p’-DDT to p,p’-DDE; and/or 3) the ability to sequester p,p’-DDT in fat before metabolizing it to p,p’-DDE.
In this study percent density of women was affected by in utero exposures to DDTs in two ways: increased dense area from high co exposure to p,p’-DDT and p,p’-DDE; and increased non-dense area with increased p,p’-DDT levels in women with low DDE. Adjusting for BMI accounted for the p,p’-DDT association with non-dense area (fat and stroma). Our findings provide evidence that high levels of p,p’-DDT may act as an obesogen, increasing BMI and non-dense area. Previous studies have found different results for obesity looking at DDT congeners. A meta-analysis of prospective human studies across the world demonstrates a consistent positive association between prenatal exposure to the DDT pesticide metabolite DDE and childhood obesity [20, 40]. A study in young children recently exposed to DDTs, found a positive association with p,p’ DDT and weight and body composition in girls [41]. The CHDS cohort found that o,p’-DDT was associated with higher BMI and waist circumference in midlife [42]. Possible pathways for DDT associations with obesity include impaired adipocyte differentiation and dysfunction [41, 43] and changes in the epigenome [44]. We find DDT increases non-dense area of the breast via increased BMI, lending supportive evidence for the obesogenic effects of DDT compounds. Both higher postmenopausal BMI and dense breasts are considered breast cancer risk factors. DDTs may be playing dual and corresponding roles in breast cancer risk by increasing both breast density (dense area and percent density) and late life BMI. DDTs potentially can be increasing breast cancer risk both in the premenopause as indicated by the DDT-associated risk with dense area and percent density as seen in this study; and potentially in the postmenopause through increased BMI. To further complicate the narrative, it has been shown that higher pre-menopausal and adolescent BMI is associated with reduced risk of all breast cancer [13, 15, 45]. Additionally there is evidence of a strong association for increased non-dense area with lower breast cancer risk [13], suggesting that fat at younger ages may play a protective role against breast cancer.
High levels of both p,p’-DDT and p,p’-DDE could be reflective of chronic and/or current exposure [38, 39]. Even so, animals, including humans, have developed defenses against natural and synthetic carcinogenic toxins [46, 47]. Since DDTs are lipophilic, obesity and higher body fat might be the body’s own protection against possible toxic DDT effects. Women who do not store DDTs in their fat as effectively might be the women we see with the highest serum levels of p,p’-DDT and p,p’-DDE. Women who are unable to utilize fat to store DDTs in pregnancy would theoretically have higher circulating DDT levels and would expose their daughters to higher levels in utero. The differences in p,p’-DDT effects on breast density parameters by p,p’-DDE level might also be an indicator of susceptible maternal metabolism. Relative levels of DDT and DDE resulting from differential metabolism could be used to create a metric for identifying women at higher risk of breast cancer. Future studies, particularly metabolomics, would need to be done to disentangle the genetic, epigenetic and exposure interactions that lead to differences in breast density parameters related to in-utero exposure to DDTs.
Supplementary Material
Highlights.
DDTs may alter breast cancer risk through DDT-related changes in breast density.
In utero DDT may affect breast cancer depending on co-exposure to DDT compounds.
Findings support the hypothesis that DDTs can act as obesogens.
5. Acknowledgements and Funding
The authors acknowledge the late Jacob Yerushalmy and Barbara van den Berg; Roberta Christianson and all CHDS staff for their contributions to this study. We thank the CHDS families that made this study possible. Funding was provided by NIH (R01ES013736, R01CA104842, HHSN275201100020C)
Abbreviations:
- CHDS
Child Health and Development Studies
- DDT
dichlorodiphenyltrichloroethane
- DDE
Dichlorodiphenyldichloroethylene
- BMI
Body Mass Index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Cohn BA, La Merrill M, Krigbaum NY, Yeh G, Park JS, Zimmermann L, Cirillo PM, DDT Exposure in Utero and Breast Cancer, Journal of Clinical Endocrinology and Metabolism 100(8) (2015) 2865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bouwman H, van den Berg H, Kylin H, DDT and malaria prevention: addressing the paradox, Environ Health Perspect 119(6) (2011) 744–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Boyd NF, Jensen HM, Cooke G, Han HL, Relationship between mammographic and histological risk factors for breast cancer, J Natl Cancer Inst 84(No. 15) (1992) 1170–9. [DOI] [PubMed] [Google Scholar]
- [4].Cuzick J, Warwick J, Pinney E, Warren RM, Duffy SW, Tamoxifen and breast density in women at increased risk of breast cancer, J Natl Cancer Inst 96(8) (2004) 621–8. [DOI] [PubMed] [Google Scholar]
- [5].Kim J, Han W, Moon HG, Ahn S, Shin HC, You JM, Han SW, Im SA, Kim TY, Koo H, Chang J, Cho N, Moon W, Noh DY, Breast density change as a predictive surrogate for response to adjuvant endocrine therapy in hormone receptor positive breast cancer, Breast Cancer Res 14(4) (2012) R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Boyd NF, Lockwood GA, Byng JW, Little LE, Yaffe MJ, Tritchler DL, The relationship of anthropometric measures to radiological features of the breast in premenopausal women, British Journal of Cancer 78(No. 9) (1998) 1233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, Lockwood GA, Tritchler DL, Yaffe MJ, Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study, 87(No. 9) (1995) 670–5. [DOI] [PubMed] [Google Scholar]
- [8].Ciatto S, Zappa M, A prospective study of the value of mammographic patterns as indicators of breast cancer risk in a screening experience, Eur. J. Radiol 17(No. 2) (1993) 122–5. [DOI] [PubMed] [Google Scholar]
- [9].Byrne C, Schairer C, Wolfe J, Parekh N, Salane M, Brinton L, Hoover R, Haile R, Mammographic features and breast cancer risk: effects with time, age, and menopause status, J Natl Cancer Inst 87(No. 21) (1995) 1622–9. [DOI] [PubMed] [Google Scholar]
- [10].Kato I, Beinart C, Bleich A, Su S, Kim M, Toniolo PG, A nested case-control study of mammographic patterns, breast volume, and breast cancer (New York City, NY, United States), 6(No. 5) (1995) 431–8. [DOI] [PubMed] [Google Scholar]
- [11].Oza AM, Boyd NF, Mammographic parencymal patterns: a marker of breast cancer risk, Epidemiologic Reviews 15(No. 1) (1993) 196–208. [DOI] [PubMed] [Google Scholar]
- [12].Boyd NF, Lockwood GA, Martin LJ, Byng JW, Yaffe MJ, Tritchler DL, Mammographic density as a marker of susceptibility to breast cancer: a hypothesis, IARC Sci Publ 154 (2001) 163–9. [PubMed] [Google Scholar]
- [13].Pettersson A, Tamimi RM, Breast fat and breast cancer, Breast Cancer Research and Treatment 135(1) (2012) 321–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Warner ET, Hu R, Collins LC, Beck AH, Schnitt S, Rosner B, Eliassen AH, Michels KB, Willett WC, Tamimi RM, Height and Body Size in Childhood, Adolescence, and Young Adulthood and Breast Cancer Risk According to Molecular Subtype in the Nurses’ Health Studies, Cancer Prev Res (Phila) 9(9) (2016) 732–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].The Premenopausal Breast Cancer Collaborative Group, Association of body mass index and age with subsequent breast cancer risk in premenopausal women, JAMA Oncology (2018) e181771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Harris HR, Tamimi RM, Willett WC, Hankinson SE, Michels KB, Body size across the life course, mammographic density, and risk of breast cancer, Am J Epidemiol 174(8) (2011) 909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fenton SE, Birnbaum LS, Timing of Environmental Exposures as a Critical Element in Breast Cancer Risk, The Journal of Clinical Endocrinology & Metabolism 100(9) (2015) 3245–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Russo J, Mailo D, Hu Y-F, Balogh G, Sheriff F, Russo IH, Breast Differentiation and Its Implication in Cancer Prevention, Clinical Cancer Research 11(2) (2005) 931s–936s. [PubMed] [Google Scholar]
- [19].Valvi D, Mendez MA, Martinez D, Grimalt JO, Torrent M, Sunyer J, Vrijheid M, Prenatal concentrations of polychlorinated biphenyls, DDE, and DDT and overweight in children: a prospective birth cohort study, Environ Health Perspect 120(3) (2012) 451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].La Merrill M, Birnbaum LS, Childhood obesity and environmental chemicals, Mt. Sinai J. Med 78(1) (2011) 22–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cohn BA, Wolff MS, Cirillo PM, Sholtz RI, DDT and breast cancer in young women: new data on the significance of age at exposure, Environ Health Perspect 115(10) (2007) 1406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].La Merrill M, Karey E, Moshier E, Lindtner C, La Frano MR, Newman JW, Buettner C, Perinatal Exposure of Mice to the Pesticide DDT Impairs Energy Expenditure and Metabolism in Adult Female Offspring, PLoS ONE 9(7) (2014) e103337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sanyal S, Agarwal N, Dudeja PK, Mahmood A, Subrahmanyam D, Effect of a single oral dose of DDT on lipid metabolism in protein-calorie malnourished monkeys, Indian J. Biochem. Biophys 19(2) (1982) 111–4. [PubMed] [Google Scholar]
- [24].McDonald JA, Cirillo PM, Tehranifar P, Krigbaum NY, Engman N, Cohn BA, Terry MB, In Utero DDT Exposure and Breast Density in Early Menopause by Maternal History of Breast Cancer, Reprod. Toxicol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].van den Berg BJ, Christianson RE, Oechsli FW, The California Child Health and Development Studies of the School of Public Health, University of California at Berkeley, Paediatr. Perinat. Epidemiol 2(3) (1988) 265–82. [DOI] [PubMed] [Google Scholar]
- [26].Terry MB, Schaefer CA, Flom JD, Wei Y, Tehranifar P, Liao Y, Buka S, Michels KB, Prenatal smoke exposure and mammographic density in mid-life, Journal of Developmental Origins of Health and Disease 2(Special issue 6) (2011) 340–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].The American Cancer Society medical and editorial content team, History of ACS Recommendations for the Early Detection of Cancer in People Without Symptoms, 2018. https://www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/overview/chronological-history-of-acs-recommendations.html. (Accessed 10/4/2019.
- [28].Sholtz RI, McLaughlin KR, Cirillo PM, Petreas M, Park JS, Wolff MS, Factor-Litvak P, Eskenazi B, Krigbaum N, Cohn BA, Assaying organochlorines in archived serum for a large, long-term cohort: implications of combining assay results from multiple laboratories over time, Environ Int 37(4) (2011) 709–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ, The quantitative analysis of mammographic densities, Phys. Med. Biol 39(10) (1994) 1629–38. [DOI] [PubMed] [Google Scholar]
- [30].Longnecker MP, Klebanoff MA, Gladen BC, Berendes HW, Serial levels of serum organochlorines during pregnancy and postpartum., Arch Environ Health 54(2) (1999) 110–4. [DOI] [PubMed] [Google Scholar]
- [31].Park JH, Cha ES, Ko Y, Hwang MS, Hong JH, Lee WJ, Exposure to Dichlorodiphenyltrichloroethane and the Risk of Breast Cancer: A Systematic Review and Meta-analysis, Osong Public Health Res Perspect 5(2) (2014) 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lopez-Cervantes M, Torres-Sanchez L, Tobias A, Lopez-Carrillo L, Dichlorodiphenyldichloroethane burden and breast cancer risk: a meta-analysis of the epidemiologic evidence, Environ Health Perspect 112(2) (2004) 207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Laden F, Hankinson S, Wolff M, Colditz G, Willett W, Speizer F, Hunter D, Plasma organochlorine levels and the risk of breast cancer: an extended follow-up in the Nurses’ Health Study., Int. J. Cancer 91(4) (2001) 568–74. [DOI] [PubMed] [Google Scholar]
- [34].Walker CL, Ho SM, Developmental reprogramming of cancer susceptibility, Nat Rev Cancer 12(7) (2012) 479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Boyd NF, Martin LJ, Yaffe MJ, Minkin S, Mammographic density and breast cancer risk: current understanding and future prospects, Breast Cancer Res 13(6) (2011) 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Evans DG, Warwick J, Astley SM, Stavrinos P, Sahin S, Ingham S, McBurney H, Eckersley B, Harvie M, Wilson M, Beetles U, Warren R, Hufton A, Sergeant JC, Newman WG, Buchan I, Cuzick J, Howell A, Assessing individual breast cancer risk within the U.K. National Health Service Breast Screening Program: a new paradigm for cancer prevention, Cancer Prev Res (Phila) 5(7) (2012) 943–51. [DOI] [PubMed] [Google Scholar]
- [37].Rebolj M, Blyuss O, Chia KS, Duffy SW, Long-term excess risk of breast cancer after a single breast density measurement, Eur. J. Cancer 117 (2019) 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jaga K, Dharmani C, Global surveillance of DDT and DDE levels in human tissues, Int. J. Occup. Med. Environ. Health 16(1) (2003) 7–20. [PubMed] [Google Scholar]
- [39].Ahlborg UG, Lipworth L, Titus-Ernstoff L, Hsieh C-C, Hanberg A, Baron J, Trichopoulos D, Adami H-O, Organochlorine Compounds in Relation to Breast Cancer, Endometrial Cancer, and Endometriosis: An Assessment of the Biological and Epidemiological Evidence Critical Reviews in Toxicology 25(6) (1995) 463–531. [DOI] [PubMed] [Google Scholar]
- [40].Cano-Sancho G, Salmon AG, La Merrill MA, Association between exposure to p,p’-DDT and its metabolite p,p’-DDE with obestity: integrated systemic review and meta analysis, environmental Health Perspetives in press (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Coker E, Chevrier J, Rauch S, Bradman A, Obida M, Crause M, Bornman R, Eskenazi B, Association between prenatal exposure to multiple insecticides and child body weight and body composition in the VHEMBE South African birth cohort, Environ Int 113 (2018) 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Merrill ML, Krigbaum N, Cirillo P, Cohn B, Association between maternal exposure to the pesticide dichlorodiphenyltrichloroethane (DDT) and risk of obesity in middle age, Int J Obesity Under Revision (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].La Merrill M, Emond C, Kim MJ, Antignac JP, Le Bizec B, Clement K, Birnbaum LS, Barouki R, Toxicological function of adipose tissue: focus on persistent organic pollutants, Environ Health Perspect 121(2) (2013) 162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Heindel JJ, Vom Saal FS, Blumberg B, Bovolin P, Calamandrei G, Ceresini G, Cohn BA, Fabbri E, Gioiosa L, Kassotis C, Legler J, La Merrill M, Rizzir L, Machtinger R, Mantovani A, Mendez MA, Montanini L, Molteni L, Nagel SC, Parmigiani S, Panzica G, Paterlini S, Pomatto V, Ruzzin J, Sartor G, Schug TT, Street ME, Suvorov A, Volpi R, Zoeller RT, Palanza P, Parma consensus statement on metabolic disruptors, Environ Health 14(1) (2015) 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Baer HJ, Tworoger SS, Hankinson SE, Willett WC, Body fatness at young ages and risk of breast cancer throughout life, Am J Epidemiol 171(11) (2010) 1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ames BN, Mutagenesis and carcinogenesis: Endogenous and exogenous factors, Environmental and Molecular Mutagenesis 14(S16) (1989) 66–77. [DOI] [PubMed] [Google Scholar]
- [47].Ames BN, Profet M, Gold LS, Nature’s chemicals and synthetic chemicals: comparative toxicology, Proceedings of the National Academy of Sciences 87(19) (1990) 7782–7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.