Abstract
Background and aims
Clinical evidence exists that patients with diabetes are at higher risk for Coronavirus disease 2019 (COVID-19). We investigated the physiological origins of this clinical observation linking diabetes with severity and adverse outcome of COVID-19.
Methods
Publication mining was applied to reveal common physiological contexts in which diabetes and COVID-19 have been investigated simultaneously. Overall, we have acquired 1,121,078 publications from PubMed in the time span between 01-01-2000 and 17-04-2020, and extracted knowledge graphs interconnecting the topics related to diabetes and COVID-19.
Results
The Data Mining revealed three pathophysiological pathways linking diabetes and COVID-19. The first pathway indicates a higher risk for COVID-19 because of a dysregulation of Angiotensin-converting enzyme 2. The other two important physiological links between diabetes and COVID-19 are liver dysfunction and chronic systemic inflammation. A deep network analysis has suggested clinical biomarkers predicting the higher risk: Hypertension, elevated serum Alanine aminotransferase, high Interleukin-6, and low Lymphocytes count.
Conclusions
The revealed biomarkers can be applied directly in clinical practice. For newly infected patients, the medical history needs to be checked for evidence of a long-term, chronic dysregulation of these biomarkers. In particular, patients with diabetes, but also those with prediabetic state, deserve special attention.
Keywords: SARS-Coronavirus-2, Hypertension, ACE2, Liver dysfunction, Inflammation
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by the Severe Acute Respiratory Syndrome - Coronavirus-2 (SARS-CoV-2), has emerged as a rapidly spreading communicable disease affecting practically all countries across the globe. The overall death rate of COVID-19 is rather low at ca. 1.4–2.3% [1,2], depending on the normalisation and estimations of non-symptomatic patients; however, several risk groups have been identified as being at higher risk of developing a more severe form of the disease and, subsequently, have higher mortality. In particular, cardiovascular diseases, hypertension, chronic respiratory diseases, Metabolic Syndrome (MS), and Diabetes Mellitus (DM) appear to play an important role in developing a more severe form of the disease with several complications [[3], [4], [5], [6], [7], [8]]. The prevalence of particular diseases and their impact on the death rate remains to be elucidated, because the pre-existing diseases are not isolated adequately and may appear simultaneously. DM, for example, is very often related to cardiovascular disorders and hypertension [3], accompanied by obesity [9], and, in several cases, also with smoking [6].
Clear evidence has been provided that DM is one of the leading risk factors for COVID-19 [[4], [5], [6], [7], [8]]. A more comprehensive review of the growing body of references reporting more severe disease and higher mortality rate of patients with DM is provided in the Supplement (see Table S1). The International Diabetes Federation (IDF), according to the WHO declaration, has also published a special note on the risk of individuals with DM [10]. It should be noted that, despite the COVID-19 epidemic, at the same time, we are facing the epidemic of Type 2 Diabetes Mellitus (T2DM). According to the WHO Global Report on Diabetes [11] an estimated 422 million adults worldwide were living with DM in 2014, compared to 108 million in 1980. The IDF estimates that, in 2019, ca. 463 million adults (20–79 years) were living with DM, and by 2045 this will rise to approx. 700 million [12]. Thus, the global increase in DM should be considered as a public health crisis that needs to be considered simultaneously in providing prevention services and treatment tools for COVID-19.
Besides the clinical evidence that DM is an important risk factor for COVID-19, we are still lacking research analyses that would give us better understanding of the physiological processes involved in the interrelation between DM and COVID-19. We analysed recent publications focused on COVID-19 and DM, and make parallels with previous studies analysing the interrelations between DM and the Severe Acute Respiratory Syndrome - Coronavirus (SARS-CoV) infection. This is promising because of similarities between SARS-CoV-2 and SARS-CoV. The genetic sequence of SARS-CoV-2 shares more than 80% of the identity of SARS-CoV [3], and both viruses use the same receptor, the Angiotensin-converting enzyme 2 (ACE2), as the cellular entry point [3,[13], [14], [15]]. We have applied Data Mining analysis of publications in PubMed to investigate the common physiological links between DM, SARS-CoV-2, and SARS-CoV. The results revealed three main pathophysiological pathways linking DM and COVID-19 via ACE2, liver dysfunction, and chronic inflammation. Accordingly, the clinical biomarkers are proposed for predicting better an adverse outcome of COVID-19, which might help clinicians for taking appropriate measures. We discuss the importance of these findings for patients with DM, and those with dysregulated biomarkers due to their metabolic disorders and prediabetic state, but not yet being diagnosed for DM.
2. Methods
We have developed a Python-based framework for publication mining and knowledge graph extraction. Publication mining on PubMed was performed using the Python library Entrez [16]. We searched PubMed for selected queries which are related to COVID-19 and DM. Overall, we acquired 1,121,078 publications in the time span between 01-01-2000 and 17-4-2020. From those publications we extracted query-specific knowledge graphs. The latter are graph-based abstractions of a specific knowledge embedded in the medium from which we extracted them [17]. We have identified 14 topics, which are important either from the perspective of DM or COVID-19. These topics are the nodes of the graphs, and by applying text mining techniques on the acquired publications, we can show which topics are related and how strong their interrelations are. Lastly, we merged the separated knowledge graphs into one single knowledge graph, highlighting maximal topic-pair occurrence frequencies, when viewed from a different research perspective. A detailed description of the method, topics and queries is provided in the Supplement.
3. Results
3.1. Diabetes and COVID-19 are linked via ACE2
In the studies investigating cellular and molecular mechanisms that could be responsible for an increased risk of individuals with DM for COVID-19, ACE2 has appeared as one of the crucial ones [1,7,18]. Initially, ACE2 has been identified from human heart failure and lymphoma cDNA libraries [19,20], and was later shown to serve as the receptor for the SARS-CoV [[21], [22], [23], [24]]. Recently, it has been shown that ACE2 is also the cellular entry point for the virus SARS-CoV-2 [3,14], and SARS-CoV-2 has an even higher affinity than SARS-CoV for ACE2 [13]. The expression and distribution of the ACE2 in the human body may indicate the potential routes of the infection and the organs most targeted by SARS-CoV-2. ACE2 is expressed differently in tissue and organs. High ACE2 expression was identified in the alveolar cells of the lung, oesophagus epithelial cells, absorptive enterocytes from the ileum and colon, cholangiocytes, myocardial cells, kidney proximal tubule cells, bladder urothelial cells, and epithelial cells of the oral mucosa [14]. The high ACE2 expression in lungs and kidneys corresponds with the most common acute conditions: Acute Respiratory Distress (ARD) and Acute Renal Failure (ARF). In one of the largest studies analysing characteristics of 14,860 COVID-19 patients dying in Italy, ARD was observed in most of the patients (96.5%), followed by ARF (24.3%) [25].
The expression and distribution of ACE2 changes with age, and is influenced by several diseases. In particular, in providing a healthy metabolism, ACE2 should be fine regulated, like a label: Not too little, not too much, just right [1]. In patients with DM, the expression of ACE2 depends on the progression of the disease. In an early phase of DM, the ACE2 is upregulated [26,27]. This increased expression of ACE2 is likely to be an adaptive response to counter the Angiotensin-converting enzyme (ACE) overactivity, and the ACE2 could truly be seen as “good” ACE [26]. Several studies have shown that the ACE2/Ang-(1–7)/Mas axis plays an important anti-inflammatory and anti-fibrogenic role (for review see Ref. [28]). In later phases of DM, the ACE2 is downregulated [27]. One of the reasons that ACE2 expression is reduced in patients with DM could be glycosylation [1]. The deficiency of ACE2 in the progression of DM causes complications, e.g., diabetic nephropathy [1,27,29,30], and may also contribute to enhanced oxidative stress in the pancreas, and to decreased glucose tolerance and impaired insulin secretion [26,31]. Therefore, the upregulation of ACE2 is promising to restore the positive effects of ACE2. Experimental evidence exists that an increase in ACE2 expression can indeed improve pancreatic beta-cell function in db/db mice [32]. To this end, several drugs, in particular ACE inhibitors (ACEi) and Angiotensin Receptor Blockers (ARBs), have been developed with the end effect of increasing the ACE2.
In the context of metabolic syndrome and DM, the ACE2 regulation appears as a “double-edged sword” [1]. On the one hand, the ACE2/Ang-(1–7) system plays an important anti-inflammatory and antioxidant role, protecting the lungs against Acute Respiratory Distress Syndrome (ARDS), and ACE2 has been shown to be protective against lethal avian influenza A H5N1 infection and others [33,34]. On the other hand, however, the expression of ACE2 is markedly increased in patients with DM (and hypertension), being treated with ACEi or ARBs as an adaptive response to counteract the elevated levels of Ang-II and Ang-I [1]. Thus, the implementation of ACE2-stimulating drugs could facilitate the entry of SARS-CoV- 2 into pneumocytes and other ACE2-rich tissues, and, consequently, it might result in more severe and fatal disease, as hypothesised by some researchers [1,7,35]. At present this is rather speculative, and the question remains open for further studies to clarify this issue.
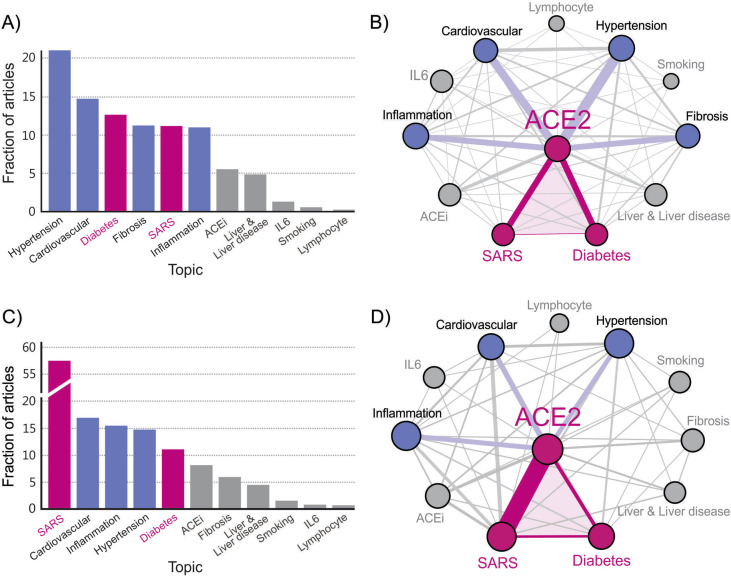
To investigate a broader context and get a deeper insight into the interrelation between DM and COVID-19 via ACE2, we applied Data Mining analysis of publications in PubMed. The analysis is focused on topics which have been found to play a key role in the development and outcome of COVID-19 disease. The task of our Data Mining algorithm was to extract the main topics in the acquired publications by counting the PubMed entries with a particular topic and calculating the frequency of co-appearances of topic pairs in the database. A detailed description of the Data Mining procedure and the corresponding analysis are given in the Supplement. Regarding the topic ACE2, we have analysed publications separately for the period before 2020, and separately for the year 2020. The results for the period before 2020 are shown in Fig. 1 A and B. The fraction of articles containing a particular topic in the ACE2 database is presented in Fig. 1A. Fig. 1B shows the corresponding interrelation between the extracted topics in the form of a weighted knowledge graph. The results of the Data Mining analysis for 2020 are presented in Fig. 1C and D. In analogy with Fig. 1A and B, Fig. 1C and D shows the fraction of articles containing a particular topic in the ACE2 publication database, and the weighted knowledge graph visualises the interrelation between the extracted topics, respectively.
Fig. 1.
Results of Data Mining for the ACE2 publication database: Frequency of topic usage for the period before 2020 (A) and for the year 2020 (C), and the corresponding extracted weighted knowledge graph for the period before 2020 (B) and for the year 2020 (D).
In both Fig. 1B and D, the triangle DM-ACE2-COVID-19 can be well recognised, highlighting the interrelation between DM and COVID-19 via ACE2 (“ACE2 axis”). Importantly, our analysis shows that only a weak direct link exists between DM and COVID-19, which obscures the actual interrelation between the two diseases. Therefore, Data Mining can contribute importantly to a deeper insight into the interrelation between the topics; they might not always be strongly connected with a direct link, but via other neighbouring topics that might not be otherwise recognised as trivial “inter-hops”. Our results show that, in the context of the interrelated topics in PubMed, the ACE2 appears as an important link between the topic Diabetes and the topic SARS in both cases.
As expected, because of the recent epidemic, the link between ACE2 and SARS is much stronger in 2020 (compare Fig. 1B and D), and SARS has replaced the leading topic Hypertension before 2020. The topic Hypertension remains among the most related topics with ACE2 also in 2020, and plays a crucial role in influencing the triangle DM-ACE2-COVID-19. This result agrees with the fact that hypertension is the leading comorbidity in COVID-19 [25]. It is a blurred line between hypertension and DM because of the fact that ca. 70% of patients with DM have hypertension [3]. So, hypertension, together with DM, can be seen as the leading risk factor for complications and fatal outcomes of COVID-19 [[3], [4], [5],7].
3.2. Liver dysfunction, elevated ALT
Artificial Intelligence (AI) analysis [36] revealed that a mildly elevated liver enzyme Alanine aminotransferase (ALT) is the most predictive clinical biomarker in newly infected patients with SARS-CoV-2 for later development of ARDS. The AI model that learned from historical data of patients from two hospitals in Wenzhou, Zhejiang, China, achieved 70%–80% accuracy in predicting severe cases of COVID-19 [36]. Several other research groups have also found a relationship between COVID-19 and liver injury, in particular, abnormal levels of ALT and Aspartate aminotransferase (AST) (for review see Ref. [5]). The common observation is that abnormal liver aminotransferase levels are more prevalent in severe cases than in mild cases of COVID-19. It appears that a major disease burden might be chronic liver diseases, including chronic viral hepatitis, Non-Alcoholic Fatty Liver Disease (NAFLD), and other metabolic disorders, including MS and DM [5,37].
From the physiological point of view, it is of interest to understand why these chronic liver disorders, and, in particular, the elevated ALT levels, are so tightly related to COVID-19. It should be noted that liver disorders are in very close relation with MS, prediabetes, and DM, and there is a huge body of evidence supporting this close interrelationship. Similar to the recent finding of AI [36] that ALT is a promising predictor for the severity of COVID-19, Sattar et al. [38] have reported that ALT was recognised as a reliable predictor of DM. In this clinical study by Sattar et al. [38] the participants that have developed DM over 5 years of follow-up, the ALT, but not AST, levels increased progressively with the increasing number of MS abnormalities, and those with ALT in the top quartile had an elevated risk for DM versus those in the bottom quartile. Several other studies have also found this high correlation between elevated ALT concentrations and the DM, MS, and NAFLD [[38], [39], [40], [41], [42], [43], [44]]. Interestingly, elevations of ALT must not be very high; already mild, but chronic elevations of transaminases often reflect underlying insulin resistance and DM [45].
Taken together, if a chronic liver disease, mostly characterised by elevated ALT, is one of the major concerns for DM, and the same is true for COVID-19, then COVID-19 and DM have a “common enemy”. To understand the physiological role of this “common enemy”, we have applied Data Mining analysis of publications in PubMed on a broader query list: DM, COVID-19, ACE2, Inflammation, ALT, and Liver & Liver disease (for details see the Supplement). The results are shown in Fig. 2 . The network presentation shows the main nodes and their connections. The link between SARS and ALT, via the topic Liver & Liver disease, is the most evident one, and, hence, fully in agreement with the predictions of the AI investigations by Jiang et al. [36]. It should be noted, however, that our analysis carries different information than that of Jiang et al. [36], but the results are complementary, pointing out the important role of ALT in COVID-19. According to our analysis, this link between ALT and COVID-19 is not a direct link, and, therefore, intuitively not recognised so easily. The nodes ALT and SARS are connected through the hub Liver & Liver disease that is also connected with DM, and provides the connection between DM-ALT-SARS (see Fig. 2). We call this connection “Liver axis”, linking DM and COVID-19 via ALT, representing a reliable biomarker for liver dysfunctions involved in DM and COVID-19.
Fig. 2.
Merged knowledge graph computed by Data Mining for the SARS, Diabetes, ACE2, ALT, Inflammation, and Liver & Liver disease databases acquired from PubMed for all years.
The results presented in Fig. 2 demonstrate a comprehensive view of the relationships between the diseases, their pathophysiological pathways, and the corresponding biomarkers. The basic two diseases considered here are COVID-19 and DM. We start with the corresponding nodes in the network, i.e. SARS and Diabetes, and highlight them by colouring the corresponding nodes red. The link between SARS and Diabetes is rather weak, which means that the two diseases are not very often related in the literature. However, there exist three very important indirect links between SARS and Diabetes, provided via the three most connected first neighbours of both SARS and Diabetes, i.e. ACE2, Liver & Liver disease, and Inflammation (marked blue in Fig. 2). Among these first most connected common neighbours, we can find the just introduced “Liver axis” and the previously discussed “ACE2 axis”. Both axes have their clinical biomarkers, and to reveal them, we look at the next most connected neighbours, marked green in the network (Fig. 2). In the “Liver axis”, the most connected node among the next neighbours of Liver & Liver disease is ALT, indicating that ALT is an important clinical biomarker for COVID-19, which also corresponds with the previously discussed predictions of the AI investigations by Jiang et al. [36]. In the “ACE2 axes”, the next most connected neighbour of ACE2 is the node Hypertension, and hypertension is indeed the most important clinical biomarker indicating the highest risk for COVID-19, as discussed in the previous section [25].
In addition to the “Liver axis”, discussed in this section, and the “ACE2 axis”, discussed in the previous section, our analysis also points to the third very important link between DM and COVID-19, i.e. inflammation. Notably, this is not an acute inflammatory state being coevolved with COVID-19, but it corresponds to a pre-existing, chronic low-grade inflammation. The revealed 3rd axis interconnecting DM and COVID-19, i.e. “Inflammatory axis”, deserves a separate presentation and is discussed in the next section.
3.3. Chronic (low-grade) inflammation
Inflammation represents an important link between DM and COVID-19. Fig. 2 reveals that inflammation is one of the most connected first common neighbours of both SARS and Diabetes (marked in blue in Fig. 2). Looking at the next most connected neighbours of the Inflammation node (marked in green in Fig. 2), we can recognise three important biomarkers, i.e., fibrosis, Interleukin-6 (IL-6), and lymphocytes. The serum biomarkers IL-6 and the count of lymphocyte are determined routinely, and in patients with COVID-19 these biomarkers were shown to be considerably dysregulated [4,18]. For example, in a group of non-survivors the serum values of IL-6 were much higher, 2–5-times, in comparison with the values measured in survivors, and the lymphocyte count was considerably lower, 2–5-times, in the group of non-survivors [4].
To understand the physiological and clinical background of this “Inflammatory axis”, linking DM and COVID-19 better, we provide a short review of publications. A large body of references exists linking DM with chronic inflammation (for a recent review see Ref. [46]); and, on the other hand, several reports for COVID-19 show a clear correlation between the severity of the disease and the level of dysregulated biomarkers for systemic inflammation [4,18,47]. Since the outbreak of COVID-19, there has simply not been enough time for extensive research that could explain comprehensively all the physiological mechanisms linking COVID-19 with chronic inflammation. However, indirect evidence exists that might help in understanding the link between DM and COVID-19 via chronic inflammation. The fact is that both T2DM and the severity of COVID-19 are more prevalent in the elderly population. The interrelation between ageing and inflammatory processes has been well established, and we know that inflammation is an important concomitant cause of many major age-associated pathologies, such as cancer, neurodegeneration, and DM [48,49]. During the process of ageing the immune system becomes compromised, and is associated with an increasing inflammation [50]. In particular, chronic inflammation, the so-called inflammaging, and metabolic function are affected strongly by the ageing processes [49,51,52].
Although, chronic inflammation is a coevolving process with ageing, and in elderly groups associated with several pathologies, it is not trivial to understand why and to what extent the inflammatory processes are involved in other diseases, and how they influence comorbidity. In particular, the comorbidity is difficult to assess when attributed to the disease connections on the molecular level, such as dysregulated genes, protein–protein interactions, and metabolic pathways as potential causes of comorbidity [49]. From the perspective of Genetics, for example, a pair of diseases can be connected if they are both associated with the same genes being dysregulated [49,53,54]. We are still lacking data for SARS-CoV-2; however, for the previous SARS-CoV infection, it has been shown that the NFKBIA gene plays the most important role for comorbidities of this disease [49]. Remarkably, in age-related inflammatory processes leading to Chronic Inflammatory Diseases (CIDs), the first genes being dysregulated are NFKBIA and Akt1 [49]. Thus, the link between CIDs and SARS-CoV infection can be established at the very early stages of the development of CIDs. The number of shared dysregulated genes between SARS-CoV infection and CID should be analysed to assess the comorbidity risk of a given CID in a more progressive stage. Importantly, it has been found that T2DM shares the highest number of dysregulated genes with SARS-CoV infection [49]. This gives a solid base for the comorbidity of T2DM for SARS-CoV infection via inflammation that can, at least from the genetical and epigenetical point of view, be explained by the same gene dysregulations, starting with dysregulations of NFKBIA and Akt1.
These results from genetic studies agree with findings indicating that PI3K-Akt activation is induced by insulin [55], and any defect in the PI3K-Akt signalling pathway is associated with insulin resistance and DM [56]. Moreover, it has been found that, not only DM, but also obesity, is related to imbalances in the PI3K-Akt signalling pathway [57]. This causes additional concern, as, in several countries, obesity is reaching the characteristics of an epidemic that spread out quickly and even more aggressively than DM. Therefore, we should be concerned about a broad range of metabolic disorders that lead to chronic inflammatory states, and, in most cases, do not evoke enough attention because of their “silent”, i.e. low-grade, appearance. In the present COVID-19 epidemic all these chronic inflammatory states, closely related to metabolic disorders, even though not (yet) treated as DM or any other CIDs, deserve special attention, and should be considered seriously as an important risk factor for the severity and mortality of patients infected with SARS-CoV-2.
4. Limitations of the study
This study has some limitations: The number of published articles that have been analysed for this year is relatively small, and some interrelations could strengthen in the future. In addition, it would be beneficial to add other data repositories in the analysis, including Protein–Protein Interaction networks, in combination with drug information resources for approved drugs. By knowing the therapeutic targets, drug metabolism, and other molecular interaction network information, the knowledge graph could be enriched and expanded to highlight even more complex relationships.
5. Conclusions
The motivation for this study comes from the clinical evidence indicating DM as an important risk factor for COVID-19. A large body of clinical reports confirms an adverse development and higher mortality of patients with DM (see Supplement, Table S1). Our analyses, based on Data Mining of publications in PubMed, reveal that DM and COVID-19 are interrelated via three main axes: The “ACE2 axis”, “Liver axis”, and “Inflammatory axis”. All these axes are further interrelated, in particular, the “Liver axis” and the “Inflammatory axis”, with common biomarkers, and form a complex network (Fig. 2). We have extracted the most important clinical biomarkers linking DM and COVID-19: The hypertension, elevated values of ALT and IL-6, and a decreased count of lymphocytes, which is in good agreement with the results of other studies reporting the indicators for higher risk of COVID-19 [4,18,25].
We found it rather surprising that DM and COVID-19, and even more generally DM and infections with SARS-CoV and SARS-CoV-2, do not coappear more frequently in publications. The Data Mining analysis showed that the link between these two topics, Diabetes and SARS, is rather weak (Fig. 2). The whole research todate was obviously concentrated on the “nearest neighbours” of DM and SARS separately. Our analysis revealed the “common nearest neighbours” of both DM and SARS, and shows how Data Mining and network analysis can contribute to better and deeper insight into the interrelation between the diseases. Moreover, we were able to identify the “outer neighbours”, in our case the important biomarkers, and when these biomarkers correspond with the clinical data, indicating that they are indeed closely related with the diseases, as in our case, the blood pressure, ALT, IL-6, lymphocytes count, for example, these sophisticated numerical approaches are justified.
The proposed biomarkers can be applied directly in clinical practice. It is recommended that the medical history of newly infected patients with SARS-CoV-2 should be checked for evidence of pre-existing, long-term, chronic dysregulations of these biomarkers. In general, patients with DM and elderly people are at higher risk. However, not all of them have these dysregulations, whereas, on the other hand, in a world full of stress, less time for preparing good food, and less physical activity, younger populations and people not even being diagnosed for DM, can have considerable dysregulations of these biomarkers, putting them at higher risk for COVID-19. Therefore, these findings may help in preventing masses of people being unaware of their metabolic disorders and prediabetic state. It is true that, at present, the main concern is COVID-19, as the most rapidly spreading epidemic with a readily recognised severe outcome, and with a concern of crashing complete national health systems, also in developed countries. However, we should not forget that some of the most persistent, silently spreading epidemics with a huge and permanently increasing annual rate of morbidity remain obesity, metabolic syndrome, and DM. It is hard to imagine, but for sure we would be less concerned about the COVID-19-epidemic if we did not live in the middle of a DM-epidemic.
Credit author statement
Marko Marhl conceived the idea of the study and wrote the main part of the paper. Vladimir Grubelnik designed the Figures and participated in the writing of the section Results. Marša Magdič has reviewed references concerning the relationship between DM and COVID-19, and edited Table S1 with all the references. Rene Markovič provided the Data Mining analysis and wrote the Supplement. The manuscript was proofread and edited by all authors.
Funding
This work was supported by the Slovenian Research Agency (Research Core Funding, No. P1-0055).
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dsx.2020.05.013.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Pal R., Bhansali A. COVID-19, diabetes mellitus and ACE2: the conundrum. Diabetes Res Clin Pract. 2020;162:108132. doi: 10.1016/j.diabres.2020.108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Muniyappa R., Gubbi S. COVID-19 pandemic, corona viruses, and diabetes mellitus. Am J Physiol Metab. 2020;2020 doi: 10.1152/ajpendo.00124.2020. ajpendo.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brake S.J., Barnsley K., Lu W., McAlinden K.D., Eapen M.S., Sohal S.S. Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19) J Clin Med. 2020;9:841. doi: 10.3390/jcm9030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta R., Ghosh A., Singh A.K., Misra A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab Syndr Clin Res Rev. 2020;14:211–212. doi: 10.1016/j.dsx.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietz W., Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity. 2020 doi: 10.1002/oby.22818. oby.22818. [DOI] [PubMed] [Google Scholar]
- 10.International Diabetes Federation COVID-19 and Diabetes n.d. www.idf.org/aboutdiabetes/what-is-diabetes/covid-19-and-diabetes.html April 22, 2020.
- 11.Roglic G., Organization W.H. World Health Organization; Geneva, Switzerland: 2016. Global report on diabetes. [Google Scholar]
- 12.IDF Atlas 9th edition and other resources n.d. https://www.diabetesatlas.org/en/resources/.
- 13.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94 doi: 10.1128/JVI.00127-20. e00127-20,/jvi/94/7/JVI.00127-20.atom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchmann J.P., Holmes E.C. Entrezpy: a Python library to dynamically interact with the NCBI Entrez databases. Bioinformatics. 2019;35:4511–4514. doi: 10.1093/bioinformatics/btz385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nickel M., Murphy K., Tresp V., Gabrilovich E. A review of relational machine learning for knowledge graphs. Proc IEEE. 2016;104:11–33. doi: 10.1109/JPROC.2015.2483592. [DOI] [Google Scholar]
- 18.Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 20.Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 21.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner A.J., Hiscox J.A., Hooper N.M. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25:291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofmann H., Pyrc K., van der Hoek L., Geier M., Berkhout B., Pöhlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc Natl Acad Sci Unit States Am. 2005;102:7988–7993. doi: 10.1073/pnas.0409465102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bindom S.M., Lazartigues E. The sweeter side of ACE2: physiological evidence for a role in diabetes. Mol Cell Endocrinol. 2009;302:193–202. doi: 10.1016/j.mce.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmieri L., Andrianou X., Bella A., Bellino S., Boros S., Canevelli M. Report based on available data on March 26th. 2020. Characteristics of COVID-19 patients dying in Italy. [Google Scholar]
- 26.Batlle D., Jose Soler M., Ye M. ACE2 and diabetes: ACE of ACEs? Diabetes. 2010;59:2994–2996. doi: 10.2337/db10-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel V.B., Parajuli N., Oudit G.Y. Role of angiotensin-converting enzyme 2 (ACE2) in diabetic cardiovascular complications. Clin Sci. 2014;126:471–482. doi: 10.1042/CS20130344. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues Prestes T.R., Rocha N.P., Miranda A.S., Teixeira A.L., Simoes-e-Silva A.C. The anti-inflammatory potential of ACE2/angiotensin-(1-7)/mas receptor Axis: evidence from basic and clinical research. Curr Drug Targets. 2017;18:1301–1313. doi: 10.2174/1389450117666160727142401. [DOI] [PubMed] [Google Scholar]
- 29.Tikellis C., Johnston C.I., Forbes J.M., Burns W.C., Burrell L.M., Risvanis J. Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension. 2003;41:392–397. doi: 10.1161/01.HYP.0000060689.38912.CB. [DOI] [PubMed] [Google Scholar]
- 30.Wysocki J., Ye M., Soler M.J., Gurley S.B., Xiao H.D., Bernstein K.E. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55:2132–2139. doi: 10.2337/db06-0033. [DOI] [PubMed] [Google Scholar]
- 31.Graus-Nunes F., Souza-Mello V. The renin-angiotensin system as a target to solve the riddle of endocrine pancreas homeostasis. Biomed Pharmacother. 2019;109:639–645. doi: 10.1016/j.biopha.2018.10.191. [DOI] [PubMed] [Google Scholar]
- 32.Bindom S.M., Hans C.P., Xia H., Boulares A.H., Lazartigues E. Angiotensin I-converting enzyme type 2 (ACE2) gene therapy improves glycemic control in diabetic mice. Diabetes. 2010;59:2540–2548. doi: 10.2337/db09-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cole-Jeffrey C.T., Liu M., Katovich M.J., Raizada M.K., Shenoy V. ACE2 and microbiota: emerging targets for cardiopulmonary disease therapy. J Cardiovasc Pharmacol. 2015;66:540–550. doi: 10.1097/FJC.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou Z., Yan Y., Shu Y., Gao R., Sun Y., Li X. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun. 2014;5:3594. doi: 10.1038/ncomms4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diaz J.H. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Trav Med. 2020 doi: 10.1093/jtm/taaa041. taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang X., Coffee M., Bari A., Wang J., Jiang X., Huang J. Towards an artificial intelligence framework for data-driven prediction of coronavirus clinical severity. Comput Mater Continua (CMC) 2020;62:537–551. doi: 10.32604/cmc.2020.010691. [DOI] [Google Scholar]
- 37.Zhang Y., Zheng L., Liu L., Zhao M., Xiao J., Zhao Q. Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single center in Wuhan city, China. Liver Int. 2020 doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 38.Sattar N., Scherbakova O., Ford I., O'Reilly D.S.J., Stanley A., Forrest E. Elevated alanine aminotransferase predicts new-onset type 2 diabetes independently of classical risk factors, metabolic syndrome, and C-reactive protein in the west of scotland coronary prevention study. Diabetes. 2004;53:2855–2860. doi: 10.2337/diabetes.53.11.2855. [DOI] [PubMed] [Google Scholar]
- 39.Esteghamati A., Jamali A., Khalilzadeh O., Noshad S., Khalili M., Zandieh A. Metabolic syndrome is linked to a mild elevation in liver aminotransferases in diabetic patients with undetectable non-alcoholic fatty liver disease by ultrasound. Diabetol Metab Syndrome. 2010;2:65. doi: 10.1186/1758-5996-2-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahran H.N., Saber L.M., Alghaithy A.A., Elareefy A.A. The role of elevated alanine aminotransferase (ALT), FasL and atherogenic dyslipidemia in type II diabetes mellitus. J Taibah Univ Med Sci. 2017;12:8–13. doi: 10.1016/j.jtumed.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandal A., Bhattarai B., Kafle P., Khalid M., Jonnadula S.K., Lamicchane J. Elevated liver enzymes in patients with type 2 diabetes mellitus and non-alcoholic fatty liver disease. Cureus. 2018;10 doi: 10.7759/cureus.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alzahrani S.H., Baig M., Bashawri J.I., Aashi M.M., Shaibi F.K., Alqarni D.A. Prevalence and association of elevated liver transaminases in type 2 diabetes mellitus patients in jeddah, Saudi arabia. Cureus. 2019;11:e5166. doi: 10.7759/cureus.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noordam R., Vermond D., Drenth H., Wijman C.A., Akintola A.A., van der Kroef S. High liver enzyme concentrations are associated with higher glycemia, but not with glycemic variability, in individuals without diabetes mellitus. Front Endocrinol. 2017;8:236. doi: 10.3389/fendo.2017.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanyal D., Mukherjee P., Raychaudhuri M., Ghosh S., Mukherjee S., Chowdhury S. Profile of liver enzymes in non-alcoholic fatty liver disease in patients with impaired glucose tolerance and newly detected untreated type 2 diabetes. Indian J Endocrinol Metab. 2015;19:597. doi: 10.4103/2230-8210.163172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris E.H. Elevated liver function tests in type 2 diabetes. Clin Diabetes. 2005;23:115–119. doi: 10.2337/diaclin.23.3.115. [DOI] [Google Scholar]
- 46.Tsalamandris S., Antonopoulos A.S., Oikonomou E., Papamikroulis G.-A., Vogiatzi G., Papaioannou S. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. 2019;14:50. doi: 10.15420/ecr.2018.33.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng F., Tang W., Li H., Huang Y.-X., Xie Y.-L., Zhou Z.-G. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in changsha. Eur Rev Med Pharmacol Sci. 2020;24:3404–3410. doi: 10.26355/eurrev_202003_20711. [DOI] [PubMed] [Google Scholar]
- 48.Tieri P., Termanini A., Bellavista E., Salvioli S., Capri M., Franceschi C. Charting the NF-κB pathway interactome map. PloS One. 2012;7 doi: 10.1371/journal.pone.0032678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moni M.A., Liò P. Network-based analysis of comorbidities risk during an infection: SARS and HIV case studies. BMC Bioinf. 2014;15:333. doi: 10.1186/1471-2105-15-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Magalhães J.P. How ageing processes influence cancer. Nat Rev Canc. 2013;13:357–365. doi: 10.1038/nrc3497. [DOI] [PubMed] [Google Scholar]
- 51.Calçada D., Vianello D., Giampieri E., Sala C., Castellani G., de Graaf A. The role of low-grade inflammation and metabolic flexibility in aging and nutritional modulation thereof: a systems biology approach. Mech Ageing Dev. 2014;136–137:138–147. doi: 10.1016/j.mad.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Ferrucci L., Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goh K.-I., Cusick M.E., Valle D., Childs B., Vidal M., Barabási A.-L. The human disease network. Proc Natl Acad Sci Unit States Am. 2007;104:8685–8690. doi: 10.1073/pnas.0701361104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feldman I., Rzhetsky A., Vitkup D. Network properties of genes harboring inherited disease mutations. Proc Natl Acad Sci Unit States Am. 2008;105:4323–4328. doi: 10.1073/pnas.0701722105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferreira G.D., Germeyer A., de Barros Machado A., do Nascimento T.L., Strowitzki T., Brum I.S. Metformin modulates PI3K and GLUT4 expression and Akt/PKB phosphorylation in human endometrial stromal cells after stimulation with androgen and insulin. Eur J Obstet Gynecol Reprod Biol. 2014;175:157–162. doi: 10.1016/j.ejogrb.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Z., Liu H., Liu J. Akt activation: a potential strategy to ameliorate insulin resistance. Diabetes Res Clin Pract. 2019;156:107092. doi: 10.1016/j.diabres.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Huang X., Liu G., Guo J., Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. 2018;14:1483–1496. doi: 10.7150/ijbs.27173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.