Highlights
-
•
Patients having COVID-19 pneumonia are at risk of venous thromboembolism.
-
•
Prophylaxis versus anticoagulation for severely ill patients is currently debated.
-
•
No specific guidelines for the management of severe pulmonary embolism exist.
-
•
Endovascular pulmonary embolism therapy may play a critical role in severe COVID-19.
As in any acute infection, severe coronavirus disease (COVID-19) is associated with a systemic inflammatory response, which can lead to coagulation disorders, sometimes referred to as sepsis-induced coagulopathy [1]. Disturbed coagulation in COVID-19 has been identified as a significant indicator of poor prognosis [2], and recent reports attempted to understand COVID-19-related coagulopathy and raised awareness of acute pulmonary embolism (PE) events [3,4].
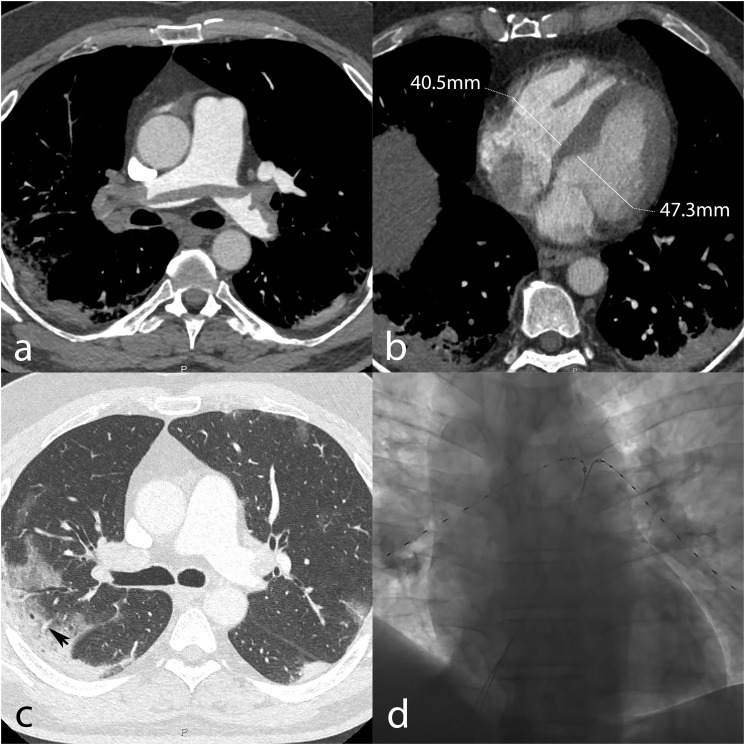
In a man in his 50s admitted due to low-grade fever, dyspnea, vomiting, and progressively worsening coughing for five days, CT showed typical features of COVID-19 pneumonia (Fig. 1 , c). However, saddle PE was found as well (Fig. 1, a) with a CT obstruction index of 75% and a normal right-to-left ventricular ratio (Fig. 1, b). His heart rate was 112/min, blood pressure 105/75 mmHg, respiratory rate 23/min while on oxygen (flow rate: 10 l/min), with a sO2 of 89%. Blood sampling showed normal leukocyte count (7.0 × 109/l), elevated C-reactive protein (111 mg/l), elevated D-dimers (35′000 ng/ml), elevated high-sensitivity cardiac troponin (60 ng/l), prolonged prothrombin time (45 s [normal range: 26–37 s]), and mild thrombopenia 126 × 109/l. Arterial PaO2 was markedly decreased (28.7 mmHg). Although the patient was at intermediate-low risk for PE death at the time of diagnosis, the simultaneous presence of COVID-19 pneumonia, PE with a high clot burden and distal perfusion impairment, disturbed coagulation factors, and elevated inflammatory markers raised concern for exacerbation of COVID-19 and development of severe acute respiratory syndrome. For these reasons, the patient was treated with catheter-directed ultrasound-assisted thrombolysis (Fig. 1, d) using two 6-French catheters inserted through the right common femoral vein and placed in the right and left lung, respectively. A total of 30 mg of rt-PA were infused over 15 h, and the patient's clinical condition improved rapidly. The next day, his heart rate was 85/min, respiratory rate 25/min, and PaO2 raised to 86.9 mmHg under a reduced oxygen flow rate (5 l/min). A follow-up pulmonary CT angiogram demonstrated marked clot burden reduction, with a CT obstruction index of 27.5%.
Fig. 1.
Pre-therapy pulmonary CT angiography demonstrating saddle pulmonary embolism with a high clot burden (a), and normal right-to-left ventricular ratio (0.86) (b). Lung window shows typical COVID-19 pneumonia appearance, including peripheral mixed consolidative and ground-glass opacities with vascular enlargement (black arrow, c). Placement of catheters in the right and left pulmonary arteries for thrombolysis (d).
The incidence of acute PE in COVID-19 remains unknown; nevertheless, growing evidence suggests that critically ill patients are at increased risk of thromboembolism, and elevated D-dimer levels are associated with high mortality in these patients [5]. The concept of inflammation-mediated downregulation of physiological anticoagulant mechanisms supports these observations. To prevent thromboembolic complications, the International Society on Thrombosis and Heamostatis (ISTH) promptly released a guidance paper advocating antithrombotic prophylaxis for all patients admitted due to COVID-19 in the absence of contraindication, regardless of their clinical status. Some authors even suggest that critically ill patients with COVID-19 should receive systemic anticoagulation [6].
Most patients with symptomatic PE have arterial hypoxemia, mostly due to a mismatch between ventilation and perfusion. In more severe PE, acute right ventricular (RV) failure, resulting in low systemic output, is considered the leading cause of death. Accordingly, the most recent guidelines for PE management recommend pharmacomechanical reperfusion only in high-risk patients who are in shock [7]. In this regard, the case we present here had not fulfilled all these criteria, and should have been managed with anticoagulation as per the guidelines of the European Society of Cardiology. Nonetheless, since PE with high obstruction index developing on top of pneumonia may adversely contribute to the patient's condition, the care team decided to pursue more aggressive therapy, which led to rapid improvement of the patient's clinical status. In this case, the concept of PE worsening the respiratory function was relevant because the pulmonary arterial blockage caused by clots did not necessarily follow the COVID-19 lesion distribution. Considering the currently limited knowledge concerning the interaction between thromboembolic disorders and COVID-19 pneumonia, this non-evidence-based management strategy was applied after careful review of the patient's clinical course by a multidisciplinary team. While not representative of the standard of care, systemic rescue thrombolysis was recently suggested in patients having intermediate-risk PE in case of deterioration under anticoagulation therapy [8], and other authors proposed thrombolysis for severely ill COVID-19 patients with acute PE [9].
Furthermore, induced bleeding is a major concern when using aggressive therapies such as thrombolysis, and must be considered when assessing benefits and risks – particularly in intermediate-risk patients. For this reason, our team used catheter-directed ultrasound-assisted thrombolysis; this system presents promising results regarding the risk of major bleeding complications [10], at least through a reduced thrombolytic agent dose.
In conclusion, because COVID-19 pneumonia is associated with an increased thromboembolic risk, thrombosis prophylaxis is recommended for all inpatients. On the other hand, guidelines to manage patients having COVID-19 pneumonia complicated by PE with a high CT obstruction index are currently lacking. In our case, endovascular treatment was used to improve lung function efficiently because of concern related to the combined hypoxemic effects of impaired arterial perfusion and infectious lung inflammation, adding up and possibly exacerbating the clinical course of COVID-19 pneumonia. However, this strategy is not recommended by the current guidelines. Awaiting further evidence, it should necessarily receive consensus from a multidisciplinary team, and informed consent from the patient.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgments
Acknowledgments
The authors gratefully acknowledge all healthcare actors who cared for the patient.
Funding
No funding was received for the writing of this opinion.
References
- 1.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020 doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L., Yan X., Fan Q. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klok F.A., Kruip M., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rotzinger D.C., Beigelman-Aubry C., von Garnier C., Qanadli S.D. Pulmonary embolism in patients with COVID-19: Time to change the paradigm of computed tomography. Thromb Res. 2020;190:58–59. doi: 10.1016/j.thromres.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escher R., Breakey N., Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett C.D., Moore H.B., Yaffe M.B., Moore E.E. ISTH interim guidance on recognition and management of coagulopathy in COVID-19: A Comment. J Thromb Haemost. 2020 doi: 10.1111/jth.14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konstantinides S.V., Meyer G. The 2019 ESC Guidelines on the Diagnosis and Management of Acute Pulmonary Embolism. Eur Heart J. 2019;40:3453–3455. doi: 10.1093/eurheartj/ehz726. [DOI] [PubMed] [Google Scholar]
- 8.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. Journal of the American College of Cardiology. 2020 doi: 10.1016/j.jacc.2020.04.031:27284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poor H.D., Ventetuolo C.E., Tolbert T. COVID-19 critical illness pathophysiology driven by diffuse pulmonary thrombi and pulmonary endothelial dysfunction responsive to thrombolysis. medRxiv. 2020 doi: 10.1101/2020.04.17.20057125:2020.2004.2017.20057125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arora S., Lahewala S., Patel P. Abstract 15240: catheter-directed thrombolysis versus systemic thrombolysis in pulmonary embolism: predictors of in-hospital mortality and major bleeding. Circulation. 2016;134:A15240. [Google Scholar]