LIKE its predecessors, severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle Eastern respiratory syndrome coronavirus, SARS-CoV-2 is a coronavirus that can be transmitted to humans and cause significant respiratory disease.1 As of June 4, 2020, there were more than 6.56 million reported SARS-CoV-2–positive patients globally, resulting in at least 388,000 deaths.2 The disease associated with SARS-CoV-2 infection is now known as coronavirus-2019 (COVID-19). Although most SARS-CoV-2 infections cause very mild symptoms, approximately 5% of patients develop acute respiratory distress syndrome (ARDS), with some cases progressing to multiorgan dysfunction. This disease has been reported to have a case fatality ratio of 1% to 4%.3 In just more than 1 month, COVID-19 became the leading cause of death in the United States in 2020, overtaking both heart disease and cancer.4 Currently, many hospitals around the world are struggling to meet the needs for mechanical ventilators and expand intensive care unit (ICU) capacity.5 The reserve capacity for ventilators is necessary because of the expected surge in hypoxemic patients presenting with progressive COVID-19 and an uncertain future when seasons change.5 The aim of this stand-alone editorial is to examine the role of helmet-delivered continuous positive airway pressure (CPAP) noninvasive ventilation (NIV) as an adjunct to mechanical ventilation in patients requiring respiratory support for COVID-19.
The recent “Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019” consensus statement agreed that after admission for COVID-19, each patient’s condition may progress at a variable rate to either recovery, with minimal oxygen requirements and no ventilatory support, or a worsening of the disease process and the need for an escalation in NIV and mechanical ventilation.6 When COVID-19 progression is identified, the current critical care management recommendation is to initiate early endotracheal intubation and mechanical ventilation.6 This recommendation aims to avoid emergency intubation in a rapidly decompensating patient should worsening hypoxemia develop if intubation is delayed. This recommendation also is aimed at source control, decreasing the risk of cross-contamination from droplet and aerosolized viral particles to other patients and healthcare workers (HCWs).6 All persons under investigation for COVID-19 and all COVID-19–positive patients should wear a mask.6 , 7 It also is recommended that all HCWs should wear droplet and contact personal protective equipment to provide a mechanical barrier to droplet spread and ideally be more than 2 meters from the patient.6 , 7 Additional airborne personal protective equipment is required during any aerosol-generating medical procedures in these patients.6 , 7 These recommendations are based on reports that 11% of critically ill patients in Wuhan, China, required high-flow nasal cannulae (HFNC) and this increased the risk of viral aerosolization and droplet transmission.3 , 6 , 8 , 9 While HFNC poses significant risks for providers, patient mortality associated with mechanical ventilations also significant. Mortality among COVID-19 patients older than 65 years in the Seattle, WA, area was at least 62%.10 A recent report from the experience in New York found the mortality associated with intubation and mechanical ventilation in 5,700 patients hospitalized with COVID-19 was 76% in the those ages 18 to 65 years and 87% in patients older than 65 years.11
The usual features of typical ARDS, recently termed the “H-type”, in COVID-19 patients are a progressive deteriorating lung compliance requiring increased inspired oxygen concentration (FiO2), high positive end-expiratory pressure, prone ventilation, sedation with paralysis, and inotropic support.12 There is growing evidence that a subset of COVID-19 patients present with atypical ARDS, which recently has been termed “L-type” ARDS, for severe hypoxemia but well-preserved pulmonary mechanics, good lung compliance, and low lung congestion12 , 13 (Table 1 ).11, 12, 13, 14, 15 The respiratory support requirements for COVID-19 patients with L-type atypical respiratory ARDS physiology may require different respiratory support principles from those usually provided to patients presenting with the typical H-type classic ARDS.14 As a result, several have advocated a ventilation strategy focused on the principle less is more.12, 13, 14 , 16 Mechanical ventilation should be delivered with low tidal volumes, low plateau pressure, and a low positive end-expiratory pressure level,16 albeit with a higher FiO2.14 It is postulated that the hypoxemia in this subset of COVID-19 patients with more compliant lungs may be due to a large shunt secondary to the loss of the protective mechanism of lung perfusion regulation and the loss of pulmonary hypoxic vasoconstriction and microthrombi in the pulmonary vasculature.12 , 17 As a result of the coagulopathy seen in this disease, anticoagulation in the treatment algorithms is an important therapeutic modality in COVID-19.18 The fact that many of these COVID-19 patients with L-type ARDS with good lung compliance show improved oxygen saturation during prone positioning may be related to improved lung perfusion and the force of gravity affecting pulmonary blood flow.12
Table 1.
L-Type and H-Type Presentation of COVID-19, Goals of NIV Helmet CPAP Therapy, and Indications for Initiation of Endotracheal Intubation and Mechanical Ventilation11, 12, 13, 14, 15
| Phenotypes of COVID-19 | Atypical ARDS or L-Type Disease | Typical ARDS or H-Type Disease |
|---|---|---|
| Clinical features | Hypoxemia accompanied by high pulmonary compliance and little shortness of breath | Hypoxemia accompanied by loss of alveolar air space, congested lungs, and shortness of breath |
| Pulmonary mechanics | Low elastance, low ventilation-to-perfusion ratio, low lung weight on CT, low lung recruitment, and reasonably aerated lung tissue | High elastance, high right-to-left shunt, high lung weight, and high lung parenchyma recruitment |
| Respiratory support | NIV helmet CPAP therapy recommended in Italy | Intubation and mechanical ventilation |
| Respiratory support goals | Mild-to-moderate respiratory effort, normal respiratory rate | Ventilation strategies: Less is more Low tidal volumes, low PEEP, and low plateau pressure in order to prevent VILI |
| Signs of improvement | Normal-to-increased PaCO2, low respiratory rate, maintain a PaO2/FiO2 ratio of 150 | Decrease in the need for mechanical ventilatory support, weaning |
| Need for endotracheal intubation and mechanical ventilation | Increasing respiratory rate, excessive patient inspiratory and expiratory effort, low PaCO2, FiO2 >80% after 1 h of initiating helmet CPAP therapy |
Abbreviations: ARDS, acute respiratory distress; COVID-19, coronavirus-2019; CPAP, continuous positive airway pressure; CT, computed tomography; FiO2, fraction of inspired oxygen; NIV, noninvasive ventilation; PaO2, partial pressure of oxygen; PaCO2, partial pressure of carbon dioxide; PEEP, positive end-expiratory pressure; SILI, self-induced lung injury; VILI, ventilator-induced lung injury
The Role of NIV in Hypoxemic Acute Respiratory Failure Associated With COVID-19
The high mortality associated with intubation and mechanical ventilation in COVID-19 patients, coupled with the concerns over provider risk from aerosolization via traditional forms of NIV, have led to the following questions: “Do many mild-to-moderate COVID-19 patients undergo endotracheal intubation too early in order to limit aerosolized and droplet viral particles, and does this potentially delay or worsen some patients' recovery?”8 and “What is the role of helmet-based CPAP via NIV for respiratory support in COVID-19 patients to limit the spread of aerosolized viral particles and potentially avoid endotracheal intubation?”19 , 20
The main reason for early endotracheal intubation over initiating NIV support in patients with COVID-19 is to limit possible aerosolization of COVID-19 particles from HFNC and NIV, as was reported in the early Chinese experience.6 Airway procedures in these patients are all classified as medical aerosol-generating procedures (MAGPs). These MAGPs include NIV and HFNC bag-mask ventilation, endotracheal intubation or tube suctioning, bronchoscopy, transport, tracheostomy tube change, and high-frequency oscillatory ventilation.6 , 21 , 22
It is known that NIV can play a significant role in respiratory support. A recent systematic review and meta-analysis concluded that NIV can improve survival in the acute care setting when it is applied early for respiratory failure; however, the benefit is lost when it is used too late in respiratory deterioration.19 CPAP is a mode in NIV used to treat hypoxemic acute respiratory failure (hARF). This mode of respiratory support delivers a constant positive airway pressure during both inspiration and expiration.23 , 24
Helmet CPAP is an important and evidence-based airway adjunct.19 , 20 , 25 , 26 Even though it is not intended to replace endotracheal intubation and mechanical ventilatory support in the critically ill patient, helmet CPAP can be used for more patients than can intubation and confines aerosolized viral particle spread within the helmet. Determining which patients will undergo rapid progression from mild respiratory disease, or the L-type better compliance form of respiratory failure, to overt H-type ARDS in COVID-19, often is not clear in the early course of the disease.13 , 14 Recent literature suggested that although only 10% to 14% of COVID-19 patients need the ICU, 60% to 70% of those will develop progressive ARDS and 20% to 25% will require endotracheal intubation and ventilation.6 , 27 , 28
Helmet CPAP, as used in Italy, can play a significant role in helping to determine patient severity. It provides good respiratory support in the moderately ill patient in the earlier stages of the disease.20 These patients still can breathe well on their own but remain significantly hypoxic despite conventional treatment. The helmet can be fitted at this stage. It provides a significant increase in inspired oxygen with up to 10 cmH2O CPAP through adaption with a traditional CPAP machine or wall oxygen regulated by a simple device. It further enables patient self-proning to improve oxygenation, which limits the need for multiple personnel to perform this in the intubated patient. The helmet allows for a safe means of containing droplet and aerosolization of virus particles by the use of a heat- moisture exchange filter on the inspiratory and expiratory limbs of the helmet. The comfort of the helmet also limits the need for sedation and subsequent inotropic support compared with endotracheal intubation. With the use of the helmet, the need for rapid early intubation often can be delayed safely while a patient is observed carefully for any improvement in disease or deterioration in condition. This may enable endotracheal intubation to be avoided in a subset of patients.
Previous data supported the use of helmet CPAP as a safe and effective evidence-based approach to respiratory failure. An independent meta-analysis, including randomized clinical trials in Italy, found helmet CPAP to be a beneficial mode of respiratory support when used for the correct indication. Helmet CPAP significantly increased the partial pressure of oxygen/FiO2 (weighted mean difference 73.40, 95% confidence interval (95% CI) 43.92-102.87; p < 0.00001) and decreased arterial carbon dioxide levels (weighted mean difference –1.92, 95% CI –3.21 to –0.63; p = 0.003), intubation rate (relative risk 0.21, 95% CI 0.11-0.40; p < 0.00001), and in-hospital mortality rate (relative risk 0.22, 95% CI 0.09-0.50; p = 0.0004) in 4 studies that included 377 patients.29 Due to the diverse clinical diagnoses and variations in the timing of blood gas analysis in the studies, the authors recommended additional large randomized controlled trials to test the outcome of the CPAP helmet for hARF more rigorously.26 , 30 , 31 A single-center randomized clinical trial of 83 patients with ARDS requiring NIV was conducted in the medical ICU at the University of Chicago from October 3, 2012–September 21, 2015. Patients were randomly assigned to receive CPAP via helmet or a face mask. The trial was terminated early because the helmet arm was found to be superior. The intubation rate was 61.5% (n = 24) for the face mask group and 18.2% (n = 8) for the helmet group (absolute difference –43.3%; 95% CI –62.4% to –24.3%; p < 0.001). The number of ventilator-free days also was significantly greater in the helmet group (28 v 12.5; p < 0.001). At 90 days, 15 patients (34.1%) in the helmet group died compared with 22 patients (56.4%) in the face mask group (absolute difference –22.3%; 95% CI –43.3 to –1.4; p = 0.02). Adverse events included 3 interface-related skin ulcers for each group (ie, 7.6% in the face mask group had nose ulcers and 6.8% in the helmet group had neck ulcers).26 An additional systematic review in 2016 that included 11 studies involving 621 patients found that the overall hospital mortality was 17.53% in the helmet NIV group versus 30.67% in the control group. Use of the helmet CPAP was associated with lower hospital mortality (odds ratio [OR] 0.43, 95% CI 0.26-0.69; p = 0.0005), intubation rate (OR 0.32, 95% CI 0.21-0.47; p < 0.00001), and complications (OR 0.6, 95% CI 0.4-0.92; p = 0.02). In contrast, there were no significant differences in gas exchange and ICU stay (p > 0.05). Subgroup analysis found that the helmet reduced mortality mainly in hARF patients (p < 0.05), and a lower intubation rate was shown. In addition, the effect of the helmet on partial pressure of carbon dioxide was influenced by type of acute respiratory failure and ventilation mode (p < 0.00001).29 The authors in this study concluded that NIV with a helmet was associated with reduced hospital mortality and endotracheal intubation requirement. The helmet was as effective as the mask in gas exchange with no additional advantage. Large randomized controlled trials are needed to provide more robust evidence.29
Patients presenting with mild-to-moderate COVID-19 or the L-type ARDS initially supported with HFNC low CPAP or NIV must be observed very closely for any clinical deterioration due to disease progression.6 , 12 , 14 , 32 One of the early signs of disease progression is excessive inspiratory work generated by the patient. If this is observed, the patient should undergo endotracheal intubation because any increased work of breathing and the generation of excessive negative intrathoracic pressure to move air has been shown to cause a self-inflicted lung injury.14 , 15 Determining patient breathing effort during progression of respiratory disease is not always easy. To more precisely quantify patient breathing work effort, esophageal manometry, although not commonly used, may be needed in these patients to measure the generation of changes in intrathoracic pressure.32 Esophageal pressure changes of 5 to 10 cmH2O may be well-tolerated. However, if an esophageal pressure change >15 cmH2O is generated, the risk of self-inflicted lung injury is increased, and endotracheal intubation should be performed promptly.14 If endotracheal intubation is delayed in this situation, and a further sudden clinical deterioration occurs, it can be associated with hypoxemia and cardiovascular collapse, and an emergency endotracheal intubation may be required, which puts the HCW team at risk during the MAGP.6 , 7 , 33 Naturally, it is not practical to monitor respiratory effort with manometry in a pandemic situation.
It remains unclear that delaying intubation in the COVID-19 patient who ultimately requires intubation has any benefit. However, the data previously discussed were promising that some patients may benefit and others may avoid intubation at all. The current Surviving Sepsis Campaign recommendations for COVID-19 discuss the use of helmet NIV and CPAP compared with mask NIV.6 Helmet CPAP is certainly a therapeutic option that has been used in Italy for more than a decade and has been used extensively during the COVID-19 pandemic.8 , 19 , 34 However, in the current Surviving Sepsis Campaign recommendations, consensus could not be reached on its safety or efficacy in COVID-19, especially in patients who ultimately require endotracheal intubation and mechanical ventilatory support.6
Challenges and Clinical Use of Helmet-Delivered NIV
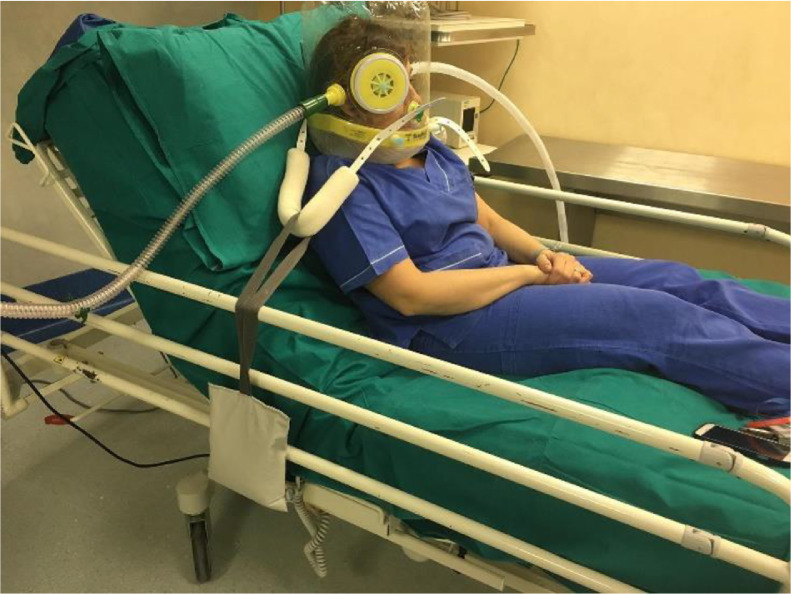
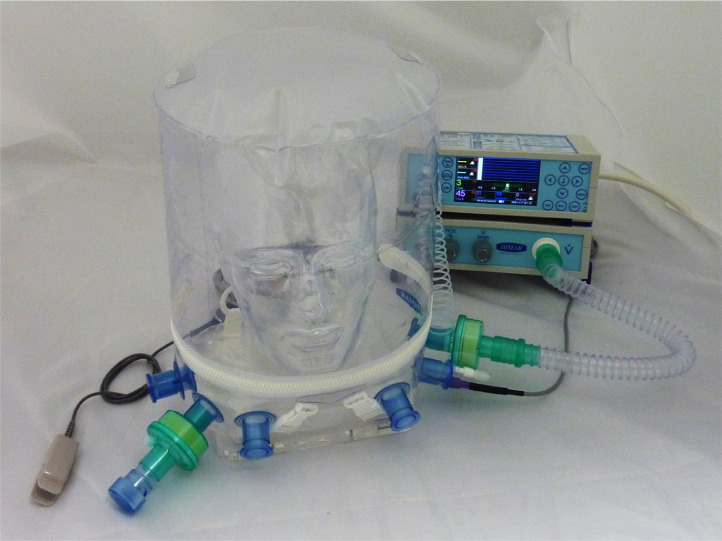
The first helmet prototypes were developed in 1991 by Maurizio Borsari. One of the problems with CPAP helmets available in other parts of the world is that they are not all approved by the Food and Drug Administration and most US physicians are unfamiliar with the helmet (Fig 1 ) (Fig 2 ). However, the concept and fitting of the mask are relatively simple. Patients typically can sit up or lie down on some pillows. It is likely that the CPAP helmet NIV is best used in the early phase of the disease or during recovery after extubation. The CPAP helmet consists of a transparent plastic hood that surrounds the patient’s head. The helmet does not have any pressure points on the face, thereby reducing patient discomfort and improving device tolerance without the risk of skin necrosis.26 The helmet allows the patient to see, read, talk, and interact more easily than do other NIV respiratory devices. It is available in various sizes that can fit small children and adults.24 It has a soft, latex-free collar constructed of silicon polyvinyl chloride that creates a pneumatic seal around the patient’s neck. The presence of 2 or more inlet and outlet ports enable connections of standard respiratory inspiratory and expiratory limb tubing. A high-efficiency particulate filter is placed on the expiratory limb of the circuit to minimize exhaled aerosolized viral particles.8 In addition, there is a distal variable CPAP valve. The extra ports allow for a sealed site for the insertion of a nasogastric tube or the administration of a nebulizer. This port also enables the patient to drink from a straw. A monitor controls the gas flow in the helmet (n = 30-60 L to prevent rebreathing and carbon dioxide retention), temperature, and FiO2. The presence of a zipper in the helmet allows for easy access if needed. The noise level in the CPAP helmet is equal to 100 dB, which can be reduced with a heat- moisture exchange filter on the helmet gas inspiratory limb.35 Helmet CPAP requires a fairly cooperative patient with an intact neuromuscular system, but tolerance appears to be excellent, especially in patients who feel claustrophobic with a tight-fitting CPAP face mask. Armpit straps can be replaced with a counterweight system that results in better patient comfort, and humidification can be added to the system.8 Occasionally, it may be necessary to reduce anxiety with the administration of very light sedation.
Fig. 1.
An example of an older version of the Italian helmet continuous positive airway pressure. Note the counterweight for added patient comfort.
Used with permission from Lucchini et al.8
Fig. 2.
The new version of the Italian helmet continuous positive airway pressure.
Used with permission from author Francesco Bellia.
Patients in a CPAP helmet must be monitored closely. An inability to maintain a partial pressure of oxygen/FiO2 ratio of 150 during use, with no reduction in respiratory rate and an increasing FiO2 requirement, defined as an FiO2 >80% after 1 hour of initiating helmet CPAP therapy, are considered indications for endotracheal intubation and mechanical ventilation (see Table 1). The challenge in COVID-19 patients is to identify the patients most likely to benefit from CPAP helmet NIV and to monitor them closely for any signs of worsening respiratory symptoms that would require an escalation to endotracheal intubation and mechanical ventilation.34 Patients with COVID-19 all are recommended to receive regular respiratory therapy to help mobilize inspissated respiratory secretions associated with this disease.34
Conclusion
In this critical time of unparalleled medical challenges of caring for vast numbers of COVID-19 hypoxemic patients requiring respiratory support, any alternative respiratory support device with evidence of extensive use in other parts of the world deserves consideration. The authors suggest that the helmet-delivered NIV pressure- support device could be a low-cost addition to the ventilatory options for COVID-19 patients.
Conflict of Interest
None.
References
- 1.Ren LL, Wang YM, Wu ZQ. Identification of a novel coronavirus causing severe pneumonia in human: A descriptive study. Chin Med J (Engl) 2020;133:1015–1024. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins University and Medicine. Available at: https://coronavirus.jhu.edu Accessed June 4, 2020.
- 3.Guan WJ, Ni ZY, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danilychev M. Covid-19 daily deaths vs. top 15 causes of death (ave/day) in the US. Available at: https://public.flourish.studio/visualisation/1727839/. Accessed April 25, 2020.
- 5.Moghadas SM, Shoukat A, Fitzpatrick MC. Projecting hospital utilization during the COVID-19 outbreaks in the United States. Proc Natl Acad Sci U S A. 2020;117:9122–9126. doi: 10.1073/pnas.2004064117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alhazzani W, Moller MH, Arabi YM. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Crit Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lockhart SL, Duggan LV, Wax RS. Personal protective equipment (PPE) for both anesthesiologists and other airway managers: Principles and practice during the COVID-19 pandemic [E-pub ahead of print] Can J Anaesth. 2020 doi: 10.1007/s12630-020-01673-w. doi: 10.1007/s12630-020-01673-w. Accessed April 23, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucchini A, Giani M, Isgro S. The “helmet bundle: in COVID-19 patients undergoing non invasive ventilation. [E-pub ahead of print] Intensive Crit Care Nurs. 2020 doi: 10.1016/j.iccn.2020.102859. doi: 10.1016/j.iccn.2020.102859 Accessed June 4, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui DS, Chow BK, Ng SS. Exhaled air dispersion distances during noninvasive ventilation via different Respironics face masks. Chest. 2009;136:998–1005. doi: 10.1378/chest.09-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatraju PK, Ghassemieh BJ, Nichols M. Covid-19 in critically ill patients in the Seattle region - case series [E-pub ahead of print] N Engl J Med. 2020 doi: 10.1056/NEJMoa2004500. doi: 10.1056/NEJMoa20045002020. Accessed June 4, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson S, Hirsch JS, Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area [E-pub ahead of print] JAMA. 2020 doi: 10.1001/jama.2020.6775. doi: 10.1001/jama.2020.6775 Accessed June 4, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattinoni L, Coppola S, Cressoni M. Covid-19 does not lead to a “typical” acute respiratory distress syndrome [E-pub ahead of print] Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202003-0817LE. Accessed June 4, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24(1):154. doi: 10.1186/s13054-020-02880-z. Accessed June 4, 2020. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gattinoni L, Chiumello D, Caironi P. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? [E-pub ahead of print] Intensive Care Med. 2020 doi: 10.1007/s00134-020-06033-2. Accessed June 4, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grieco DL, Menga LS, Eleuteri D. Patient self-inflicted lung injury: Implications for acute hypoxemic respiratory failure and ARDS patients on non-invasive support. Minerva Anestesiol. 2019;85:1014–1023. doi: 10.23736/S0375-9393.19.13418-9. [DOI] [PubMed] [Google Scholar]
- 16.Gattinoni L, Quintel M, Marini JJ. “Less is more” in mechanical ventilation. Intensive Care Med. 2020;46:780–782. doi: 10.1007/s00134-020-05981-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang T, Sun LX, Feng RE. [Comparison of clinical and pathological features between severe acute respiratory syndrome and coronavirus disease 2019] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E040. doi: 10.3760/cma.j.cn112147-20200311-00312. Accessed June 4, 2020. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Bikdeli B, Madhavan MV, Jimenez D. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up [E-pub ahead of print] J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.031. Accessed June 4, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabrini L, Landoni G, Oriani A. Noninvasive ventilation and survival in acute care settings: A comprehensive systematic review and metaanalysis of randomized controlled trials. Crit Care Med. 2015;43:880–888. doi: 10.1097/CCM.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 20.Cabrini L, Landoni G, Zangrillo A. Minimise nosocomial spread of 2019-nCoV when treating acute respiratory failure. Lancet. 2020;395:685. doi: 10.1016/S0140-6736(20)30359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cinesi Gomez C, Penuelas Rodriguez O, Lujan Torne ML. Clinical consensus recommendations regarding non-invasive respiratory support in the adult patient with acute respiratory failure secondary to SARS-CoV-2 infection [E-pub ahead of print] Rev Esp Anestesiol Reanim. 2020 doi: 10.1016/j.redar.2020.03.006. doi: 10.1016/j.redar.2020.03.006Accessed June 4, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 2019;11(10) doi: 10.3390/v11100940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta S, Hill NS. Noninvasive ventilation. Am J Respir Crit Care Med. 2001;163:540–577. doi: 10.1164/ajrccm.163.2.9906116. [DOI] [PubMed] [Google Scholar]
- 24.Piastra M, Antonelli M, Chiaretti A. Treatment of acute respiratory failure by helmet-delivered non-invasive pressure support ventilation in children with acute leukemia: A pilot study. Intensive Care Med. 2004;30:472–476. doi: 10.1007/s00134-003-2103-6. [DOI] [PubMed] [Google Scholar]
- 25.Brambilla AM, Aliberti S, Prina E. Helmet CPAP vs. oxygen therapy in severe hypoxemic respiratory failure due to pneumonia. Intensive Care Med. 2014;40:942–949. doi: 10.1007/s00134-014-3325-5. [DOI] [PubMed] [Google Scholar]
- 26.Patel BK, Wolfe KS, Pohlman AS. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: A randomized clinical trial. JAMA. 2016;315:2435–2441. doi: 10.1001/jama.2016.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian S, Hu N, Lou J. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80:401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X, Yu Y, Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Q, Gao Y, Chen R. Noninvasive ventilation with helmet versus control strategy in patients with acute respiratory failure: A systematic review and meta-analysis of controlled studies. Crit Care. 2016;20:265. doi: 10.1186/s13054-016-1449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo Y, Luo Y, Li Y. Helmet CPAP versus oxygen therapy in hypoxemic acute respiratory failure: A meta-analysis of randomized controlled trials. Yonsei Med J. 2016;57:936–941. doi: 10.3349/ymj.2016.57.4.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Squadrone V, Massaia M, Bruno B. Early CPAP prevents evolution of acute lung injury in patients with hematologic malignancy. Intensive Care Med. 2010;36:1666–1674. doi: 10.1007/s00134-010-1934-1. [DOI] [PubMed] [Google Scholar]
- 32.Gattinoni L, Giosa L, Bonifazi M. Targeting transpulmonary pressure to prevent ventilator-induced lung injury. Expert Rev Respir Med. 2019;13:737–746. doi: 10.1080/17476348.2019.1638767. [DOI] [PubMed] [Google Scholar]
- 33.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 34.Jancin B. European COVID-19 insights: Try helmet CPAP. Available at: https://www.mdedge.com/chestphysician/article/221215/coronavirus-updates/european-covid-19-insights-try-helmet-cpap. Accessed April 24, 2020.
- 35.Lucchini A, Valsecchi D, Elli S. [The comfort of patients ventilated with the Helmet Bundle] Assist Inferm Ric. 2010;29:174–183. [PubMed] [Google Scholar]