Highlights
-
•
The role of pre-existing immunosuppression on COVID-19 risk and outcomes is unclear
-
•
Immunotherapy is being evaluated for COVID-19-related cytokine release syndrome
-
•
We report a fingolimod-treated MS patient who developed severe COVID-19
-
•
COVID-19 recovery occurred after stopping fingolimod and treating with tocilizumab
Keywords: COVID-19, Multiple sclerosis, Fingolimod, Tocilizumab
Abstract
Background
Treatment decisions in patients with multiple sclerosis (MS) during the coronavirus disease 2019 (COVID-19) pandemic are challenging. It is not known whether and how various disease modifying therapies, especially immunosuppressive drugs, affect COVID-19 risk and disease course.
Methods
Case report
Results
We report a fingolimod-treated MS patient who developed severe COVID-19 but recovered after treatment with tocilizumab.
Conclusion
This report suggests that a brief course of tocilizumab for the treatment of severe COVID-19 may be effective while not aggravating pre-existing MS.
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by SARS-CoV-2 and is currently pandemic. COVID-19 appears to have a mild course in most people but individuals over 50 years of age and those with pre-existing conditions may fare worse with relatively high rates of need for ventilatory assistance and subsequent death. As a novel disease entity without herd immunity, an available vaccine, or proven therapy, COVID-19 presents additional challenges for patients taking immunosuppressant drugs, including some multiple sclerosis (MS) disease modifying therapies (DMTs). Immunosuppressed patients are plausibly at higher risk for a more severe COVID-19 course although this is not established. Guidance for management of MS DMTs during the pandemic have been issued by national professional societies and patient organizations but is largely speculative as little has yet been reported on COVID-19 course and outcomes in MS patients with or without use of DMTs (Brownlee et al., 2020).
A contrasting hypothesis concerns the potential benefit of some immunotherapies for COVID-19 infection, proposing a protective role via limitation of the hyperactive inflammatory response (cytokine release syndrome (CRS) or “cytokine storm”) associated with clinical deterioration in COVID-19. A recent report of a MS patient who did well despite COVID-19 infection while on the B cell-depleting drug ocrelizumab illustrates this general hypothesis (Novi et al., 2020).
More specifically, tocilizumab, a humanized monoclonal antibody that targets the interleukin-6 (IL-6) receptor, has been reported to improve outcomes for patients with severe COVID-19 infection and is the subject of controlled trials (Luo et al., 2020).
We present a patient with MS who developed COVID-19 while treated with fingolimod. After suspension of fingolimod, she developed CRS and acute respiratory distress syndrome (ARDS) and was successfully treated with tocilizumab.
2. Case report
A 58-year-old female presented to the emergency department with three days of fever and dry cough. Her son had previously developed similar symptoms. She was diagnosed with relapsing MS in 2007 and was previously treated with interferon beta 1a, glatiramer acetate, and natalizumab; she had been taking fingolimod since 2011. Her most recent examination showed a stable Expanded Disability Status Scale score of 6, and there was no evidence of disease activity for several years. Her past history included migraine, diabetes mellitus, hypertension, hyperlipidemia, obesity, and transient ischemic attack.
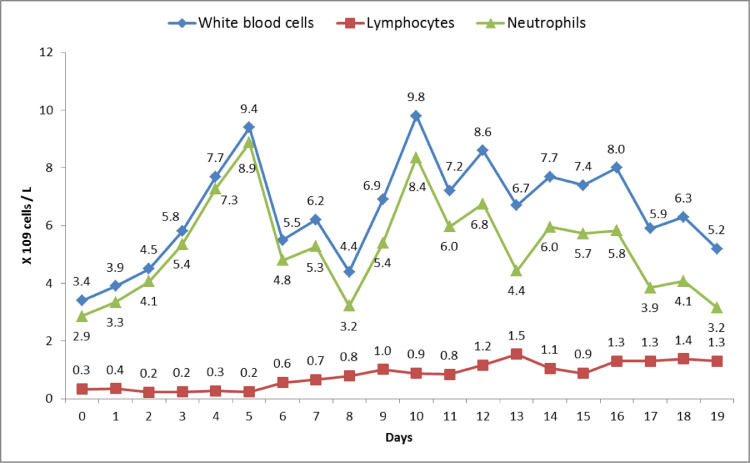
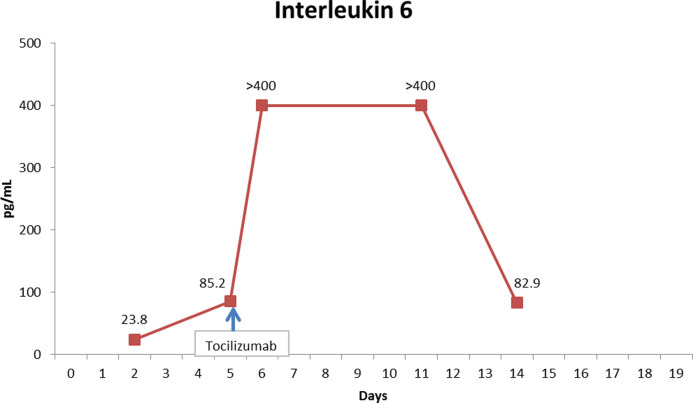
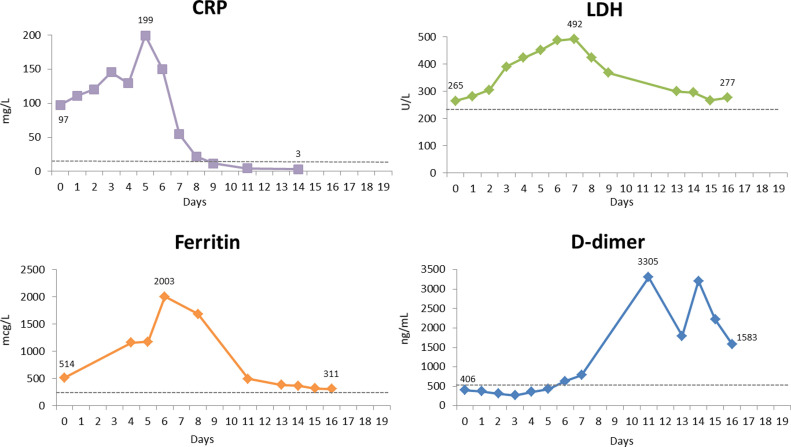
Upon presentation, oxygen saturation was 95% and she was afebrile. PCR for SARS-CoV-2 on nasal swab was positive. Chest x-ray was unremarkable. She was advised to self-isolate at home and take acetaminophen. She returned 5 days later with worsening dyspnea, and chest x-ray showed multifocal pneumonia. Chest computed tomography showed multiple bilateral peripheral bilateral ground glass opacities. Absolute lymphocyte count (ALC) was 0.33 × 109/L (fig. 1 ), similar to five months prior to hospitalization (0.3 × 109/L) and the range since treatment onset (0.19-0.39 × 109/L). She had elevated C-reactive protein (CRP), ferritin and lactate dehydrogenase, and D-dimer was normal (fig. 2 ). The patient was admitted and hydroxychloroquine and azithromycin were initiated. Fingolimod was discontinued. Two days after admission, IL-6 was elevated at 23.6 pg/mL (normal <1.8), and inflammatory markers were higher (figs. 2 and 3 ). Three days later, the patient developed increasing oxygen needs and chest X-ray revealed worsening airspace opacities in both lungs. She required intubation due to respiratory failure and was transferred to the intensive care unit (ICU). She received one dose of intravenous tocilizumab 600 mg. She did not receive corticosteroids. One day after tocilizumab, IL-6 peaked to >400 pg/mL. Her lymphocyte count normalized 9-12 days after fingolimod discontinuation. While in the ICU, she also required hemodynamic support with vasopressors. After 10 days, she was extubated. Over the following four days, she continued to improve clinically, CRP level normalized and IL-6 improved. She was discharged 4 days post-extubation. Fingolimod was reinitiated prior to discharge, 18 days after discontinuation. At the follow up video visit one week after discharge, the patient continued self-isolation, and she reported hyposmia and dysgeusia.
Fig. 1.
White blood cell, lymphocyte and neutrophil counts trend during hospitalization
Reference ranges: White blood cell count 3.4-9.6 × 109 cells/L, lymphocyte count 0.95-3.07 × 109 cells/L and neutrophil count 1.56-6.45 × 109 cells/L.
Fig. 2.
Interleukin-6 trend during hospitalization
Fig. 3.
C-reactive protein (CRP), ferritin, lactate dehydrogenase (LDH) and D-dimer trends during hospitalization
Reference ranges: CRP <8mg/L, ferritin 11-307 mcg/L, LDH 122-222 U/L and D-dimer <500 ng/mL.
3. Discussion
We describe a fingolimod-treated MS patient who developed severe COVID-19, ARDS and CRS that responded to tocilizumab therapy. Fingolimod was discontinued upon hospital admission owing to concern that immunosuppression may worsen COVID-19. (Brownlee et al., 2020; Giovannoni et al., 2020). Fingolimod is a sphingosine-1-phosphate receptor modulator that reduces the egress of lymphocytes from secondary lymphoid organs into the circulation and is associated with increased risk of infections, especially herpes viruses, in patients with MS. Our patient's ALC was low since initiation of fingolimod (0.25-0.39 × 109/L); although this may have increased her risk of COVID-19 infection or its complications, this degree of lymphopenia is characteristic of fingolimod effect and neither efficacy nor adverse events, including viral infections, are significantly correlated with peripheral lymphocyte count. It is also possible that suspension of fingolimod actually increased her risk of later developing CRS and ARDS, though this would require loss of immunosuppression over just a few days, shorter than typically seen with fingolimod discontinuation. The patient's ALC normalized earlier than expected with lymphocyte reconstitution seen after fingolimod discontinuation (usually 4–8 weeks), which might have been related to the active infection (Ghadiri et al., 2017). Interestingly, fingolimod is currently under investigation as a potential treatment for COVID-19-associated ARDS (clinicaltrials.gov Identifier: NCT04280588).
COVID-19 severity and outcomes appear to be related to the degree and characteristics of the immune response to SARS-Cov-2. Patients who develop severe respiratory failure might have a dysregulated “cytokine storm” with high circulating levels of proinflammatory cytokines that result in direct tissue injury, especially in the lungs. IL-6 is the primary cytokine involved in this inflammatory cascade and higher serum levels have been associated with worse outcomes. Early identification and treatment of CRS improves outcomes (Luo et al., 2020).
Tocilizumab has been used in the treatment of CRS observed in patients with chimeric antigen receptor T-cell (CAR-T) therapies. Given the resemblance of the inflammatory response observed in patients with COVID-19, tocilizumab has been recommended for treatment of severe COVID-19 (Luo et al., 2020). Following administration of tocilizumab in the setting of CAR-T therapy, serum IL‑6 levels may increase (Chen et al., 2016), presumably by prevention of the IL‑6 receptor-mediated uptake of IL‑6 into peripheral tissue (Nishimoto et al., 2008). Nishimoto et al found that as long as free tocilizumab was detectable, IL-6 receptors were saturated by tocilizumab and IL-6 signal was completely inhibited. A similar post-tocilizumab increase in serum IL-6, also observed in our patient, has been reported in other cases of COVID-19 (Luo et al., 2020) and may be associated with worse outcome but confirmatory data are needed.
Interleukin-6 may be involved in MS pathogenesis by induction of IL-17 producing T-cells. The effects of acute lL-6 inhibition on MS disease activity are not clear. Evidence from the experimental autoimmune encephalomyelitis model shows conflicting data regarding effects of anti-IL-6 monoclonal antibodies (Serada et al., 2008). Human data is limited to case reports with repeated treatment; one patient with rheumatoid arthritis (RA) developed MS during treatment with tocilizumab (Beauchemin et al., 2016), whereas another patient with RA and MS was treated with anti-IL-6 therapy for more than 5 years without experiencing a MS exacerbation (Sato et al., 2014). Although these reports suggest that IL-6 inhibition could cause or trigger CNS inflammatory demyelination, a report describes an adolescent with a tumefactive cervical demyelinating lesion who was successfully treated with tocilizumab (Hoshino et al., 2020). Therefore, the MS-related implications of both acute and chronic IL-6 therapy remain uncertain.
Our report illustrates the complexity of interpreting the effects of adding or suspending immunotherapies in MS patients who develop COVID-19. Some monoclonal antibodies, such as the anti-CD19 ocrelizumab and the anti-CD52 alemtuzumab, have cell depleting effects that last many months and are not reversible. In contrast, suspension of fingolimod results in a rapid return of sequestered lymphocytes to the peripheral circulation, likely reversing most of its immunosuppressive properties and allowing opportunity for intervention. The risk of “rebound” MS disease after fingolimod discontinuation is delayed 2–4 months and is probably mitigated by restarting the drug after COVID-19 recovery, as in our case.
Our case also suggests that a brief course of tocilizumab for the treatment of severe COVID-19 may be effective while not aggravating pre-existing MS. This is also likely the case for COVID-19 that develops in patients with neuromyelitis optica spectrum disorder, for which IL-6 inhibition has demonstrable efficacy for attack prevention (Yamamura et al., 2019). Ongoing registries will provide more insight about the outcomes of patients with MS who develop COVID-19 while taking various DMTs and who receive other immunotherapies.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
Dr. Valencia-Sanchez reports no conflicts of interest. Dr. Wingerchuk reports receiving consulting fees from MedImmune, Novartis, Biogen, Celgene, Genentech, TG Therapeutics, Arcus Medica, Third Rock Ventures, Reistone and research support paid to Mayo Clinic from Alexion and TerumoBCT.
References
- Beauchemin P, Carruthers R. MS arising during Tocilizumab therapy for rheumatoid arthritis. Mult. Scler. 2016;22(2):254–256. doi: 10.1177/1352458515623862. [DOI] [PubMed] [Google Scholar]
- Brownlee W, Bourdette D, Broadley S, Killestein J, Ciccarelli O. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurology. 2020 doi: 10.1212/WNL.0000000000009507. pii: 10.1212/WNL.0000000000009507[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Chen F, Teachey DT, Pequignot E. Measuring IL-6 and sIL-6R in serum from patients treated with tocilizumab and/or siltuximab following CAR T cell therapy. J. Immunol. Methods. 2016;434:1–8. doi: 10.1016/j.jim.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadiri M, Fitz-Gerald L, Rezk A. Reconstitution of the peripheral immune repertoire following withdrawal of fingolimod. Mult. Scler. 2017;23(9):1225–1232. doi: 10.1177/1352458517713147. [DOI] [PubMed] [Google Scholar]
- Giovannoni G, Hawkes C, Lechner-Scott J, Levy M, Waubant E, Gold J. The COVID-19 pandemic and the use of MS disease-modifying therapies. Mult. Scler. Relat. Disord. 2020 doi: 10.1016/j.msard.2020.102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino H, Shirai Y, Konishi H, Yamamura T, Shimizu N. Efficacy of tocilizumab for fulminant multiple sclerosis with a tumefactive cervical lesion: a 12-year-old boy. Mult. Scler. Relat. Disord. 2020;37 doi: 10.1016/j.msard.2019.101460. [DOI] [PubMed] [Google Scholar]
- Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: A single center experience. J. Med. Virol. 2020 doi: 10.1002/jmv.25801. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N, Terao K, Mima T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112:3959–3964. doi: 10.1182/blood-2008-05-155846. [DOI] [PubMed] [Google Scholar]
- Novi G, Mikulska M, Briano F. COVID-19 in a MS patient treated with ocrelizumab: does immunosuppression have a protective role? Mult. Scler. Relat. Disord. 2020;42 doi: 10.1016/j.msard.2020.102120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Kobayashi D, Abe A. Tocilizumab treatment safety in rheumatoid arthritis in a patient with multiple sclerosis: A case report. BMC Res. Notes. 2014;7:641–643. doi: 10.1186/1756-0500-7-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serada S, Fujimoto M, Mihara M. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U S A. 2008;105(26):9041–9046. doi: 10.1073/pnas.0802218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura T, Kleiter I, Fujihara K. Trial of Satralizumab in Neuromyelitis Optica Spectrum Disorder. N Engl. J. Med. 2019;381(22):2114–2124. doi: 10.1056/NEJMoa1901747. [DOI] [PubMed] [Google Scholar]