Abstract
Background
The coronavirus disease 2019 pandemic has had an impact on healthcare systems around the world with 3 million people contracting the disease and 208,000 cases resulting in death as of this writing. Information regarding coronavirus infection in pregnancy is still limited.
Objective
This study aimed to describe the clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnant women with positive laboratory testing for severe acute respiratory syndrome coronavirus 2.
Study Design
This is a cohort study of pregnant women with severe or critical coronavirus disease 2019 hospitalized at 12 US institutions between March 5, 2020, and April 20, 2020. Severe disease was defined according to published criteria as patient-reported dyspnea, respiratory rate >30 per minute, blood oxygen saturation ≤93% on room air, ratio of partial pressure of arterial oxygen to the fraction of inspired oxygen <300 mm Hg, or lung infiltrates >50% within 24–48 hours on chest imaging. Critical disease was defined as respiratory failure, septic shock, or multiple organ dysfunction or failure. Women were excluded from the study if they had presumed coronavirus disease 2019, but laboratory testing was negative. The primary outcome was median duration from hospital admission to discharge. Secondary outcomes included need for supplemental oxygen, intubation, cardiomyopathy, cardiac arrest, death, and timing of delivery. The clinical courses are described by the median disease day on which these outcomes occurred after the onset of symptoms. Treatment and neonatal outcomes are also reported.
Results
Of 64 hospitalized pregnant women with coronavirus disease 2019, 44 (69%) had severe disease, and 20 (31%) had critical disease. The following preexisting comorbidities were observed: 25% had a pulmonary condition, 17% had cardiac disease, and the mean body mass index was 34 kg/m2. Gestational age was at a mean of 29±6 weeks at symptom onset and a mean of 30±6 weeks at hospital admission, with a median disease day 7 since first symptoms. Most women (81%) were treated with hydroxychloroquine; 7% of women with severe disease and 65% of women with critical disease received remdesivir. All women with critical disease received either prophylactic or therapeutic anticoagulation during their admission. The median duration of hospital stay was 6 days (6 days [severe group] and 10.5 days [critical group]; P=.01). Intubation was usually performed around day 9 on patients who required it, and peak respiratory support for women with severe disease was performed on day 8. In women with critical disease, prone positioning was required in 20% of cases, the rate of acute respiratory distress syndrome was 70%, and reintubation was necessary in 20%. There was 1 case of maternal cardiac arrest, but there were no cases of cardiomyopathy or maternal death. Thirty-two of 64 (50%) women with coronavirus disease 2019 in this cohort delivered during their hospitalization (34% [severe group] and 85% [critical group]). Furthermore, 15 of 17 (88%) pregnant women with critical coronavirus disease 2019 delivered preterm during their disease course, with 16 of 17 (94%) pregnant women giving birth through cesarean delivery; overall, 15 of 20 (75%) women with critical disease delivered preterm. There were no stillbirths or neonatal deaths or cases of vertical transmission.
Conclusion
In pregnant women with severe or critical coronavirus disease 2019, admission into the hospital typically occurred about 7 days after symptom onset, and the duration of hospitalization was 6 days (6 [severe group] vs 12 [critical group]). Women with critical disease had a high rate of acute respiratory distress syndrome, and there was 1 case of cardiac arrest, but there were no cases of cardiomyopathy or maternal mortality. Hospitalization of pregnant women with severe or critical coronavirus disease 2019 resulted in delivery during the clinical course of the disease in 50% of this cohort, usually in the third trimester. There were no perinatal deaths in this cohort.
Key words: coronavirus, COVID-19, pregnancy, SARS-CoV-2
Introduction
The 2019 novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused a coronavirus disease 2019 (COVID-19) pandemic, with 3 million people contracting the disease and 208,131 cases resulting in death globally at the time of this writing (April 27, 2020).1 COVID-19 is usually mild in 81%, severe in 14%, and critical in 5% of nonpregnant patients,2 percentages which are similar (86%, 9%, and 5%, respectively) to pregnant women in early reports.3
AJOG MFM at a Glance.
Why was this study conducted?
This study aimed to describe the clinical course of severe or critical coronavirus disease 2019 (COVID-19) in hospitalized pregnant patients so that healthcare providers have guidance regarding antepartum and delivery management.
Key findings
Pregnant women with severe or critical COVID-19 were admitted to the hospital about 7 days after the onset of symptoms and stayed in the hospital for 6 days (median, 6 days [severe group] and 10.5 days [critical group]; P=.01). Intubation was usually performed around day 9 on patients who required it; peak respiratory support for women with severe conditions was performed on day 8. Hospitalization of pregnant women with severe or critical COVID-19 resulted in delivery during the course of the disease in 50% of this cohort. Furthermore, 75% of all women with critical COVID-19 had mostly iatrogenic preterm delivery.
What does this add to what is known?
These data add significantly to the body of literature regarding the clinical course of severe or critical COVID-19 in pregnancy and suggest that the clinical course of severe or critical COVID-19 in hospitalized pregnant women may be shorter than in hospitalized nonpregnant patients.
The clinical course of severe or critical COVID-19 in nonpregnant patients includes hospital admission occurring at a median of 7 days after the onset of symptoms, dyspnea on day 8, sepsis on day 9, acute respiratory distress syndrome (ARDS) on days 9–12, intensive care unit (ICU) admission and mechanical ventilation on days 10.5–12, and death or discharge for survivors on day 21; the median length of hospital stay in nonpregnant patients is about 12 days.4, 5, 6; however, there is very limited information regarding the clinical course of severe and critical COVID-19 in hospitalized pregnant women. In a cohort of 15 pregnant women in China, the interval from symptom onset to admission varied from 2 to 10 days, and no other clinical course details were provided.7
Knowledge of the clinical course of the disease is vital to answering questions about the management of pregnant women with COVID-19. The physiological changes in pregnancy affect lung volumes and immune response and therefore have the potential to affect the clinical course of COVID-19 in pregnancy. To be able to answer questions about optimal management, timing of delivery, and various medications used for obstetrical indications, the clinical course of the maternal disease must be understood. The physiological changes in pregnancy, particularly those related to disease severity, progression, and outcomes, also need to be more clearly elucidated.
This study aimed to describe the clinical course of severe and critical COVID-19 in pregnant women during hospitalization.
Materials and Methods
Study design
A multicenter cohort study was performed on pregnant women admitted to the hospital for treatment of severe or critical COVID-19 from March 5, 2020 to April 20, 2020, to determine the clinical course of the disease. To maximize the number of cases, this study was of both retrospective and prospective nature. It was designed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cohort studies before data collection.8 Institutional review board (IRB) approval was obtained from the primary site, Thomas Jefferson University (IRB control number 20E.397). Collaborating sites obtained IRB approval or data use agreements with Thomas Jefferson University, when required by their institution.
Inclusion criteria were all pregnant and current postpartum women with positive laboratory testing for SARS-CoV-2, criteria that meet the diagnosis of severe or critical COVID-19 as defined by the Chinese Center for Disease Control and Prevention.9 Severe COVID-19 was defined as dyspnea, respiratory rate ≥30 breaths per minute, blood oxygen saturation ≤93% on room air, ratio of partial pressure of arterial oxygen to fraction of inspired oxygen <300 mm Hg, or lung infiltrates >50% within 24–48 hours of symptom onset.9 For the purpose of the definition of severe disease in this study, “dyspnea” was defined as patient-reported dyspnea at rest. Critical COVID-19 was defined as respiratory failure requiring mechanical ventilation, septic shock, or multiple organ dysfunction or failure.9 Respiratory failure was defined as a need for invasive mechanical ventilation. Septic shock was defined as ≥2 Sequential (sepsis-related) Organ Failure Assessment (SOFA) criteria (decline in ratio of partial pressure of oxygen to fraction of inspired oxygen, decline in platelets, rising bilirubin, decline in mean arterial pressure [MAP], decline in Glasgow Coma Scale, and rise in serum creatinine) and need for vasopressors to maintain a MAP of ≥65 mm Hg and serum lactate of >2 mmol/L even with sufficient volume resuscitation.10 Women were considered to have multiple organ dysfunction or failure if they had evidence of at least 2 of the following: renal impairment or failure (defined as a 3-fold increase in baseline creatinine or need for dialysis11), liver failure (international normalized ratio [INR]>1.5), refractory hypoglycemia as diagnosed by the treating institution, or hepatic encephalopathy.12 Women with inconclusive or negative testing for SARS-CoV-2 (even if clinical suspicion was high) and women who received a diagnosis of COVID-19 >7 days after delivery were excluded.
The primary outcome was median duration (from hospital admission to discharge) overall and for women with severe vs critical COVID-19. This outcome was chosen as a surrogate for the duration of illness requiring support. Secondary maternal outcomes focused on the clinical course of COVID-19, with the day of symptom onset being defined as disease day 1. The variables of the clinical course examined included disease day of oxygen supplementation, other respiratory support, intubation and extubation, day of peak respiratory support, and day of return to room air. Data were also recorded on new-onset cardiomyopathy, cardiac arrest, and death. Disease day of delivery, if applicable, was included. Peak levels of various serum laboratory markers as well as the peak day of laboratory value if it was drawn ≥3 times were recorded. Additional data were collected on the duration of hospitalization, treatment modalities, obstetrical outcomes, and neonatal outcomes.
Data collection
Investigators at each institution reviewed the electronic medical record of women and any delivered neonate, and deidentified data were recorded and stored in a standardized computer spreadsheet. Data were reviewed by coinvestigators and compiled for analysis.
Statistical analysis
Statistical analysis included means and standard deviations for normally distributed data, medians with interquartile ranges (IQRs), and percentages. The groups were compared using the t-test, analysis of variance, Mann-Whitney U test, χ2 test, and Fisher exact test. Analysis was performed for severe and critical disease separately, as well as for the entire cohort.
Results
Twelve institutions provided data: Pennsylvania (n=6), New York (n=3), New Jersey (n=2), and Ohio (n=1). Of 64 women included, 44 (69%) had severe disease, and 20 (31%) met the criteria for critical COVID-19 (Figure 1 ).
Figure 1.
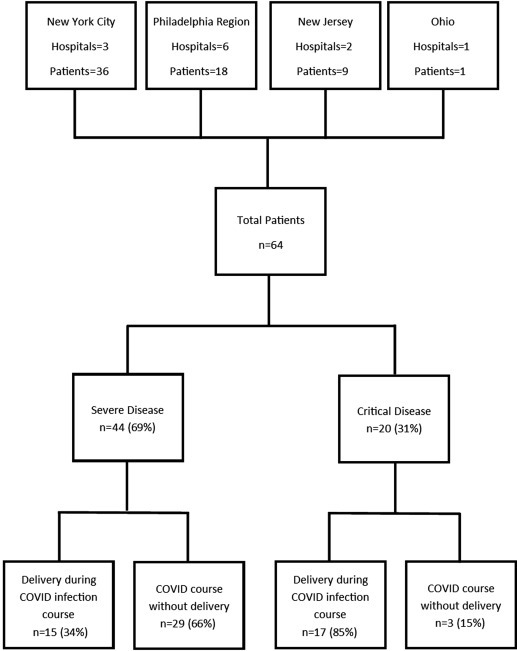
Flow diagram of included women
Flowchart of women with severe or critical COVID-19 during pregnancy.
COVID-19, coronavirus disease 2019.
Pierce-Williams et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. AJOG MFM 2020.
Maternal demographics are shown in Table 1 . Overall, the mean age was 33 years; women with critical disease were significantly older than women with severe disease. Average body mass index was 34 kg/m2. Among 64 women, 21 (31%) were Hispanic, 18 (28%) were non-Hispanic black, and 16 (25%) were non-Hispanic white. Preexisting pulmonary conditions (ie, obstructive sleep apnea, asthma, chronic obstructive pulmonary disease) were present in 25% of women, and preexisting cardiac disease (ie, chronic hypertension, cardiomyopathy) were present in 17% of women.
Table 1.
Maternal demographics
| Characteristic | All (N=64) | Severe group (n=44) | Critical group (n=20) | P value |
|---|---|---|---|---|
| Maternal age (y) | 33.2±5.8 | 32.0±6.0 | 35.9±4.3 | .011 |
| BMI kg/m2 | 33.5±7.1 | 35.3±7.3 | 29.7±5.1 | .003 |
| Race and ethnicity | .21 | |||
| Non-Hispanic black | 18 (28) | 13 (30) | 5 (25) | |
| Non-Hispanic white | 16 (25) | 9 (20) | 7 (35) | |
| Hispanic | 20 (31) | 17 (39) | 3 (15) | |
| Asian/Pacific Islander | 3 (5) | 1 (2) | 2 (10) | |
| Other | 7 (11) | 4 (9) | 3 (15) | |
| Singleton pregnancy | 63 (98) | 44 (100) | 19 (95) | .135 |
| Multiparous | 46 (72) | 30 (68) | 16 (80) | .33 |
| Insurance type | .014 | |||
| State | 26 (41) | 21 (48) | 5 (25) | |
| Private | 35 (55) | 23 (52) | 12 (60) | |
| Uninsured | 3 (5) | 0 (0) | 3 (15) | |
| Pulmonary pathology (OSA, asthma, COPD, etc) | 16 (25) | 12 (27) | 4 (20) | .76 |
| Cardiac disease (including chronic hypertension, cardiomyopathy) | 11 (17) | 8 (18) | 3 (15) | 1.00 |
Data are presented as mean±standard deviation or n (%).
BMI, body mass index; COPD, chronic obstructive pulmonary disease; OSA, obstructive sleep apnea.
Pierce-Williams et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies. AJOG MFM 2020.
The average gestational age at symptom onset of COVID-19 was 29.9±5.8 weeks (range 16.6–39.1 weeks), with hospital admission at 30.7±5.7 weeks. No patients included in this study were postpartum at the onset of symptoms or hospitalization. Only 23% of women with severe disease experienced symptom onset at <24 weeks, whereas all women with critical disease experienced symptom onset at >24 weeks (P=.024). Sixty-nine percent of women developed symptoms at <34 weeks, and 86% of women developed symptoms at <37 weeks. Most women (98%) had nasopharyngeal testing to confirm the presence of the disease. Two women with critical disease initially tested negative: 1 subsequently tested positive by bronchoalveolar lavage (after 3 negative nasopharyngeal swabs over 6 days), and the other tested positive by repeat nasopharyngeal testing (Table 2 ). The vast majority of women in this study were treated with hydroxychloroquine (81%) (Table 3 ). Remdesivir was used in 16 of 64 (25%) women with COVID-19 (7% with severe disease and 65% with critical disease). Convalescent serum was used in 1 patient with critical disease. Of 64 women, 15 (23%) (4% with severe disease and 55% with critical disease) received steroids for maternal indications (rather than for fetal maturity). Of 20 women with critical disease, 12 (60%) received prophylactic anticoagulants, and 8 (40%) received therapeutic anticoagulants during admission. Furthermore, of 44 women with severe disease, 25 (57%) received prophylactic anticoagulants, and 2 (5%) received therapeutic anticoagulants (Table 3).
Table 2.
Initial findings of COVID-19
| Characteristic | All (N=64) | Severe group (n=44) | Critical group (n=20) | P value |
|---|---|---|---|---|
| Gestational age at symptom onset (wk) | 29.9±5.8 | 29.9±6.3 | 29.7±4.6 | .899 |
| Symptom onset at <24 wk | 10 (15.6) | 10 (22.7) | 0 (0) | .024 |
| Symptom onset at <34 wk | 44 (68.8) | 29 (65.9) | 15 (75) | .47 |
| Symptom onset at <37 wk | 55 (85.9) | 36 (81.8) | 19 (95) | .16 |
| Gestational age at hospitalization (wk) | 30.7±5.7 | 30.8±6.2 | 30.6±4.5 | .898 |
| Initial negative testing | 2 (3) | 0 (0) | 2 (10) | .094 |
| Source of sample | ||||
| Nasopharyngeal | 63 (98) | 44 (100) | 19 (95) | .14 |
| BAL | 1 (2) | 0 (0) | 1 (5) | |
Data are presented as mean±standard deviation or n (%).
BAL, bronchoalveolar lavage; COVID-19, coronavirus disease 2019.
Pierce-Williams et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies. AJOG MFM 2020.
Table 3.
Management of patients with COVID-19
| Characteristic | All (N=64) | Severe group (n=44) | Critical group (n=20) | P value |
|---|---|---|---|---|
| Hydroxychloroquine | 52 (81) | 33 (75) | 19 (95) | .06 |
| Antibiotic treatment for CAP | 36 (56) | 22 (50) | 14 (70) | .14 |
| Remdesivir | 16 (25) | 3 (7) | 13 (65) | <.001 |
| Convalescent serum | 1 (2) | 0 (0) | 1 (5) | .31 |
| Steroids for maternal treatment | 15 (23) | 4 (9) | 11 (55) | <.001 |
| Anticoagulants during admission | ||||
| Prophylactic heparin/LMWH | 37 (58) | 25 (57) | 12 (60) | .81 |
| Therapeutic heparin/LMWH | 10 (16) | 2 (5) | 8 (40) | <.001 |
| Supplemental O2 | 52 (81) | 32 (73) | 20 (100) | .01 |
| High-flow nasal cannula | 16 (25) | 5 (11) | 11 (55) | <.0001 |
| BiPAP/CPAP | 5 (8) | 1 (2) | 4 (20) | .03 |
| Intubated | 19 (30) | 0 (0) | 19 (95) | <.001 |
| Reintubated | 4 (6) | 0 (0) | 4 (20) | .008 |
| Prone positioning | 4 (6) | 0 (0) | 4 (20) | .008 |
| ECMO | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| ARDS | 14 (22) | 0 (0) | 14 (70) | <.001 |
| New-onset maternal cardiomyopathy | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Maternal cardiac arrest | 1 (2) | 0 (0) | 1 (5) | <.001 |
| Maternal mortality | 0 (0) | 0 (0) | 0 (0) | 1.00 |
Data are presented as n (%).
ARDS, acute respiratory distress syndrome; BiPAP, bilevel positive airway pressure; CAP, community-acquired pneumonia; COVID-19, coronavirus disease 2019; CPAP, continuous positive airway pressure; ECMO, extracorporeal membrane oxygenation; LMWH, low-molecular-weight heparin.
Pierce-Williams et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies. AJOG MFM 2020.
In this cohort, 32 of 44 (73%) women with severe disease required O2 supplementation, whereas 19 of 20 (95%) women with critical disease required intubation (Table 3). In women with critical disease, 14 of 20 (70%) developed ARDS, 4 of 20 (20%) were placed in prone positioning (gestational ages 26–31 weeks), and 4 of 20 (20%) required reintubation. At the time of writing, there were no cases of cardiomyopathy or maternal mortality. One woman experienced cardiac arrest after a prolonged clinical course of the disease. She was hospitalized 14 days after symptom onset and intubated 6 days later at 20 days from symptom onset. She remained intubated for approximately 1 week, with initial signs of recovery and extubation; however, she again developed respiratory failure and experienced cardiac arrest on day 31. Cardiopulmonary resuscitation and reintubation were performed, and return of spontaneous circulation (ROSC) was achieved. She gave birth through cesarean delivery after ROSC. She was intubated for more than 2 weeks after delivery before she underwent tracheostomy placement.
In this cohort and at the time of writing, we observed that the median day (with IQR) of events from the first day of reported symptoms were as follows (Table 4 ; Figure 2 ): hospital admission on day 7 (IQR, 5–9), initiation of supplemental O2 on day 8 (IQR, 5–10), intubation (among women with critical disease) on day 9 (IQR, 6–11.5), peak respiratory support on day 8.5 (IQR, 6–11), hydroxychloroquine treatment on day 8 (IQR, 6–11), remdesivir treatment on day 10.5 (IQR, 8.8–12.5), discontinuation of supplemental O2 on day 13 (IQR, 9–17) (2 patients with severe disease and 11 patients with critical disease remained on supplemental O2 at the time of writing), and symptom resolution on day 15 (IQR, 11.5–20) (for patients who have achieved resolution). Of women who gave birth during the clinical course of the disease, delivery occurred on median disease day 10 (IQR, 1–6). When comparing women with severe disease vs women with critical disease, the clinical course was longer for the critical group, requiring the use of invasive mechanical ventilation, later cessation of supplemental oxygen (median day 12 [IQR, 8–16] vs day 17 [IQR, 14–22]; P=.014), last fever (median day 9 [IQR, 6–10] vs day 17.5 [IQR, 14–20.5]; P<.00001), symptoms (median day 13.5 [IQR, 10–18.5] vs day 20 [IQR, 15.5–22]; P=.029), and later hospital discharge (median day 12 [IQR, 9.3–15.8] vs day 17 [IQR, 16–25]; P=.0056). The Supplemental Table shows the disease day of events in the clinical course of COVID-19 by gestational age cutoffs.
Table 4.
Average disease day of events in clinical course of COVID-19 (day 1, first day of onset of symptoms)
| Characteristic | All (N=64) | Severe group (n=44) | Critical group (n=20) | P value |
|---|---|---|---|---|
| Inpatient admission | 7 (5–9) | 7 (5–9) | 7 (5–10) | .98 |
| Hydroxychloroquine | 8 (6–11) | 8 (6–11) | 8 (5.5–10.5) | .77 |
| Remdesivir | 10.5 (8.8–12.5) | 10 (9.5–10.5) | 11 (8–14) | .80 |
| Antibiotics for CAP | 8 (5–10) | 8 (6–10) | 8 (5–9) | .43 |
| Supplemental O2 started | 8 (5–10) | 8 (6–9) | 7 (5–10) | .54 |
| Peak respiratory support | 8.5 (6–11) | 8 (7–11) | 9 (6–11.5) | .89 |
| High-flow nasal cannula | 9 (7–11) | 9 (7.8–10) | 9 (6–11) | .80 |
| BiPAP/CPAP | 12 (10–19) | 10 (–)a | 15.5 (10–20) | — |
| Intubation | 9 (6–11.5) | —b | 9 (6–11.5) | — |
| Reintubation | 23 (20.3–25.8) | — | 23 (20.3–25.8) | — |
| Final extubationc | 15.5 (12–18.8) | — | 15.5 (12–18.8) | — |
| Supplemental O2 discontinued (n=50)d | 13 (9–17) | 12 (8–16) | 17 (14–22) | .014 |
| Last fever | 9 (7–15) | 9 (6–10) | 17.5 (14–20.5) | <.00001 |
| Hospital discharge (n=51)e | 12 (10–16.5) | 12 (9.3–15.8) | 17 (16–25) | .0056 |
| Symptom resolutionf | 15 (11.5–20) | 13.5 (10–18.5) | 20 (15.5–22) | .029 |
| Delivery (n=32) | 10 (6–13) | 8 (6–12) | 12 (6–13) | .35 |
| Maternal cardiac arrest (n=1)g | 31 | — | 31 | — |
Data are presented as median (interquartile range).
BiPAP, bilevel positive airway pressure; CAP, community-acquired pneumonia; COVID-19, coronavirus disease 2019; CPAP, continuous positive airway pressure.
Pierce-Williams et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies. AJOG MFM 2020.
No interquartile range for BiPAP/CPAP in severe group, as n=1
No patients were intubated in severe group
Five women remained intubated at the time of writing
Two patients with severe disease remained on O2 and admitted at time of writing
Eleven patients with critical disease remained on O2 and admitted at time of writing
Twenty-nine patients have achieved resolution of symptoms
Only 1 woman had maternal cardiac arrest.
Figure 2.
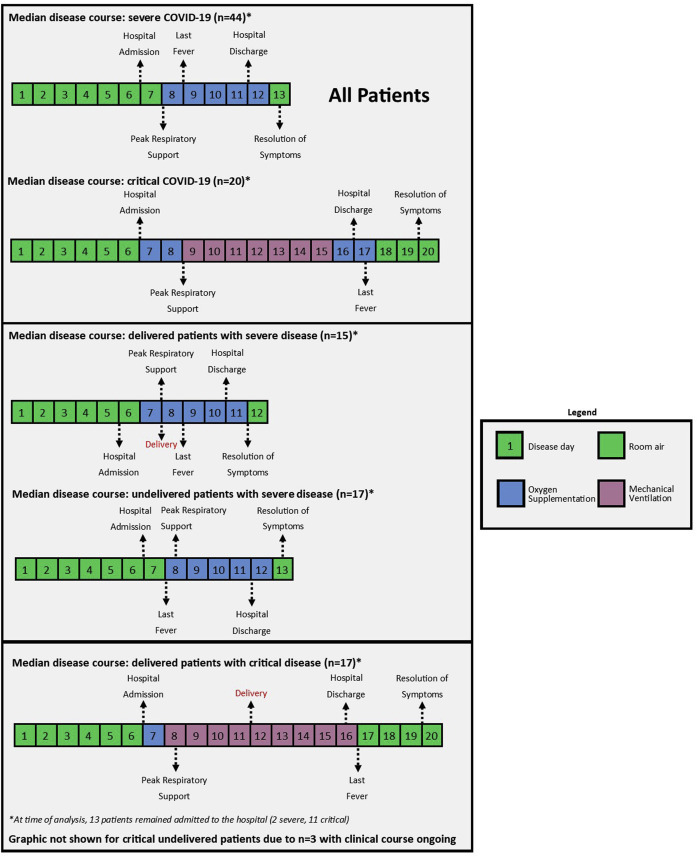
Median clinical course
Median clinical course of the disease, separated by patient groups (severe and critical) and delivery (delivered and not delivered). Of note, dates of oxygen discontinuation, hospital discharge, and symptom resolution may appear falsely low because of publication before completion of disease course in some patients.
COVID-19, coronavirus disease 2019.
Pierce-Williams et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. AJOG MFM 2020.
If laboratory studies typically utilized to predict disease severity were used as a proxy for the most severe point in the clinical course of the disease in this study, the average peak disease day was 11 (Table 5 ). Patients with critical disease had higher interleukin-6 (IL-6) levels, ferritin, fibrin split products, platelets, procalcitonin, troponins, C-reactive protein, creatine phosphokinase, and lactate dehydrogenase compared with patients with severe disease.
Table 5.
Peak laboratory prognostic markers
| Laboratory valuesa | All (N=64) | Severe group (n=44) | Critical group (n=20) | P value |
|---|---|---|---|---|
| IL-6 (≤1.8 pg/mL)b | 70.4±68.1 | 40.0±27.6 | 93.2±81.8 | .008 |
| Ferritin (5–130 ng/mL) | 311.0±647.5 | 195.7±192.8 | 469.5±967.3 | .0003 |
| Disease day of peak | 13.0±6.1 | 11.1±4.3 | 15.2±7.4 | .007 |
| Fibrin split products (μg/mL) | 690.8±405.7 | 843.0±181.7 | 462.5±647.0 | .0006 |
| Disease day of peak | 11.1±4.3 | 8.7±1.2 | 18 | — |
| Liver enzymes (5–35 U/L) | ||||
| AST | 74.0±70.2 | 71.4±82.2 | 79.3±35.6 | .68 |
| ALT | 60.2±59.8 | 53.0±58.4 | 74.8±61.5 | .18 |
| Disease day of peak | 11.4±6.1 | 10.0±5.0 | 13.9±7.3 | .015 |
| Platelets (150–420 109/L) | 350.1±153.1 | 307.8±123.2 | 441.2±173.7 | .001 |
| Disease day of peak | 13.0±6.5 | 11.1±5.7 | 16.8±6.5 | .0007 |
| D-dimer (100–1500 ng/mL) | 1325.6±3388.5 | 745.5±979.5 | 2014.4±4893.1 | .101 |
| Disease day of peak | 13.3±6.8 | 12.3±7.6 | 14.5±6.0 | .26 |
| PT (10–13 s) | 12.9±1.4 | 12.3±0.9 | 13.8±1.6 | <.0001 |
| PTT (24–38 s)c | 37.1±20.0 | 31.6±3.4 | 44.8±29.5 | .004 |
| Disease day of peak | 11.2±6.1 | 9.0±5.0 | 13.7±6.4 | .002 |
| Procalcitonin (0.01–0.1 ng/mL) | 0.6±1.2 | 0.3±0.4 | 1.1±1.7 | .004 |
| Disease day of peak | 9.3±5.8 | 8.4±5.3 | 10.5±6.5 | .18 |
| Troponin (<0.04 mg/dL) | 0.7±3.0 | 0.01±0.01 | 1.5±4.5 | .03 |
| Disease day of peak | 12.0±7.5 | 10.4±4.6 | 13.1±9.1 | .12 |
| CRP (<20 mg/dL) | 83.7±82.0 | 43.3±45.9 | 141.9±88.6 | .0001 |
| Disease day of peak | 10.9±4.7 | 9.9±4.4 | 12.2±5.0 | .068 |
| CPK (13–101 U/L) | 280.4±286.5 | 92.8±83.7 | 485.1±290.9 | .0001 |
| Disease day of peak | 13.2±7.0 | 14.4±5.5 | 12.4±8.1 | .25 |
| LDH (80–450 U/L) | 364.9±164.3 | 317.0±157.6 | 467.6±131.2 | .0004 |
| Disease day of peak | 11.1±6.0 | 9.4±5.1 | 14.6±6.4 | .0009 |
Data are presented as (reference range value in pregnancy, unit) and mean day±standard deviation.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPK, creatine phosphokinase; CRP, C-reactive protein; IL-6, interleukin-6; LDH, lactate dehydrogenase; PT, prothrombin time; PTT, partial thromboplastin time.
Pierce-Williams et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies. AJOG MFM 2020.
Many of the reference ranges for these laboratory tests change by trimester
Reference range is for nonpregnant patients
Seventy-four percent of women received prophylactic or therapeutic anticoagulation, which will alter coagulation study results.
Table 6 shows the median duration of COVID-19 hospital characteristics for 51 discharged women. Symptoms lasted 15 days for those whose symptoms had resolved (13 days for women with severe disease vs 19 days for women with critical disease; P=.099). For the primary outcome, the length of hospital stay was on average 8±5 days (median duration 6 days). Women with severe disease were hospitalized for 6 days (IQR, 4.3–7), and women with critical disease were hospitalized for 12 (IQR, 7–16) days (P=.0031). At the time of writing, several women remained hospitalized (2 with severe disease and 11 with critical disease). Women required supplemental O2 for a duration of 6 days (5 [IQR, 3–7] days for those with severe disease and 10 [IQR, 7–16] for those with critical disease; P=.0038). Of the women who were intubated and had been discharged, the median duration of intubation was 3 days (IQR, 2–8).
Table 6.
Duration of COVID-19 hospital characteristics for discharged women
| Characteristic | All | Severe group | Critical group | P value |
|---|---|---|---|---|
| Symptoms (n=29)a | 15 (11–19) | 13 (10–19) | 19 (14–22) | .099 |
| Hospital stay | 6 (5–8) | 6 (4.3–7) | 12 (7–18) | .0031 |
| Supplemental O2 | 6 (3–8) | 5 (3–7) | 10 (7–16) | .0038 |
| Intubation | 3 (2–8) | N/A | 3 (2–8) | — |
Data are presented as median (interquartile range) of days.
COVID-19, coronavirus disease 2019; N/A, not applicable.
Pierce-Williams et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies. AJOG MFM 2020.
Only for women who became asymptomatic.
Among women who delivered during hospitalization, preterm delivery at <34 weeks’ gestation and <37 weeks’ gestation occurred in 10 (31.2%) and 19 (59.4%) women, respectively; however, when evaluating only women with critical disease who gave birth (n=17), 15 of 17 (88%) delivered preterm (Table 7 ). Spontaneous preterm labor occurred in only 2 (6%) women who delivered, both in the critical group. During the clinical course of COVID-19, 50% of women delivered (34% of women with severe disease and 85% of women with critical disease) (Table 7). Maternal status was the primary indication in most cases (69% overall, 60% severe, 76% critical). The remainder was for fetal indications. Women with critical COVID-19 delivered at an earlier mean gestational age than those with severe disease (32±4 weeks’ gestation vs 37±2 weeks’ gestation; P<.0001). There were no cases of intrauterine fetal demise. Delivery route was cesarean for 53% and 94% of women with severe and critical disease, respectively. Preeclampsia or gestational hypertension occurred in only 2 (3%) women; however, 50% of our cohort did not give birth at the time of writing. The rate of postpartum hemorrhage, defined as blood loss of >1000 mL at time of vaginal or cesarean delivery or symptomatic hypovolemia within 24 hours associated with blood loss, was 9% overall (13% in the severe disease group and 6% in the critical disease group). The rate of presumed intrauterine infections (chorioamnionitis or endometritis) was 9% (20% in the severe disease group and 0% in the critical disease group).
Table 7.
Pregnancy outcomes
| Characteristic | All (N=64) | Severe group (n=44) | Critical group (n=20) | P value |
|---|---|---|---|---|
| Steroids for fetal maturity | 16 (25) | 7 (16) | 9 (45) | .013 |
| Magnesium sulfate for neuroprotection | 6 (9) | 0 (0) | 6 (30) | .001 |
| Hypertensive disorders of pregnancy | 2 (3) | 2 (5) | 0 (0) | 1.00 |
| Magnesium sulfate for preeclampsia | 1 (2) | 1 (2) | 0 (0) | 1.00 |
| IUGR | 2 (3) | 0 (0) | 2 (10) | .094 |
| Stillbirth | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Oligohydramnios | 1 (2) | 1 (2) | 0 (0) | 1.00 |
| Delivered with COVID-19 during hospitalization (any cause) | 32 (50) | 15 (34) | 17 (85) | <.001 |
| Delivered for maternal status | 22 (69) | 9 (60) | 13 (76) | .32 |
| Delivered for fetal status | 3 (9) | 0 (0) | 3 (18) | .23 |
| Delivered for obstetrical indications | 7 (22) | 6 (40) | 1 (6) | .033 |
| Presumed chorioamnionitis or endometritis | 3 (9) | 3 (20) | 0 (0) | .092 |
| Postpartum hemorrhagea | 3 (9) | 2 (13) | 1 (6) | 0.590 |
| Preterm delivery at <37 wk | 19 (59) | 4 (27) | 15 (88) | .001 |
| Preterm delivery at <34 wk | 10 (31) | 0 (0) | 10 (59) | <.0001 |
| Spontaneous preterm labor | 2 (6) | 0 (0) | 2 (12) | .49 |
| PPROM | 1 (3) | 1 (7) | 0 (0) | .47 |
| Delivery route | ||||
| Vaginal (includes operative vaginal delivery) | 8 (25) | 7 (47) | 1 (6) | .009 |
| Cesarean delivery | 24 (75) | 8 (53) | 16 (94) | |
Data are presented as n (%).
COVID-19, coronavirus disease 2019; IUGR, intrauterine growth restriction; PPROM, preterm premature rupture of membranes.
Pierce-Williams et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies. AJOG MFM 2020.
Postpartum hemorrhage is defined as an estimated blood loss of ≥1000 mL with noticeable symptoms of blood loss/vital sign changes.
The average neonatal birthweight was 2403.3±858.0 g (2945.8±509.2 g in the severe disease group and 1924.6±846.6 g in the critical disease group); however, the difference is likely attributed to earlier gestational age of delivery in the critical disease group. Sixty-four percent of neonates required ICU admission (40% in the severe disease group and 83% in the critical disease group). Additional neonatal outcomes are reported in Table 8 . Detailed data on neonatal testing and method of testing were not available. One of 33 neonates received a diagnosis of COVID-19; in this newborn, the first test for SARS-CoV-2 at 24 hours of life was negative, but a repeat test at 48 hours returned with a positive result. The neonate showed no signs or symptoms of COVID-19.
Table 8.
Neonatal outcomes
| Characteristic | All (N=33)a | Severe group (n=15) | Critical group (n=18) | P value |
|---|---|---|---|---|
| Gestational age at delivery (wk) | 34.5±4.2 | 37.7±1.6 | 31.9±3.8 | <.0001 |
| Birthweight (g)a | 2403.3±858.0 | 2945.8±509.2 | 1924.6±846.6 | <.001 |
| NICU admission | 21 (63.6) | 6 (40) | 15 (83.3) | 0.014 |
| 5-minute Apgar scores | 7.9±1.7 | 8.8±0.8 | 7.2±2.0 | 0.0067 |
| Neonatal death | 0 (0) | 0 (0) | 0 (0) | 1.00 |
Data are presented as mean±standard deviation or n (%).
NICU, neonatal intensive care unit.
Pierce-Williams et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies. AJOG MFM 2020.
One twin pregnancy in the critical group.
Discussion
Principal findings
In this cohort, hospitalized pregnant women with severe or critical COVID-19 were typically admitted on disease day 7 (as measured from the first day of onset of symptoms), and the median duration of hospitalization was 6 days (6 days vs 10.5 days for women with severe and critical disease, respectively). Intubation was usually performed around disease day 9 on those who required it (approximately 2 days after hospital admission), and peak severity of nonintubated (severe) disease, determined by highest oxygen use, occurred on disease day 9. Fifty percent of women with COVID-19 delivered during the clinical course of the disease, with most deliveries due to maternal disease. About half of the women with severe disease delivered vaginally, whereas more than 90% of women with critical disease gave birth through cesarean delivery. The average gestational age at delivery was 37 weeks’ gestation for women with severe disease and 32 weeks’ gestation for women with critical disease. Most preterm deliveries were due to maternal status; however, data on exact etiologies were not collected. There were no maternal deaths as of this manuscript’s submission.
Results in the context of what is known
The clinical course of the disease in hospitalized pregnant women with severe or critical COVID-19 seems to be similar to nonpregnant patients in the limited available studies. Of note, these comparisons are limited because the comparison criteria include a broad age range, an international community, nonpregnant patients, men, and varied indications for admission. In a series of 41 nonpregnant patients in China, hospital admission occurred on disease day 7 with onset of dyspnea on day 8. ARDS occurred on day 9, and ICU admission and mechanical ventilation on day 10.5.4 In another study of nonpregnant patients from China, the length of hospital stay was 12 days.6 In a cohort of 191 hospitalized nonpregnant patients, ARDS and ICU admission occurred on day 12, death occurred on day 18.5, and discharge occurred on day 22.5 In a cohort of 15 pregnant women in China, the interval from symptom onset to admission was 2–10 days. It must be noted that this cohort does not include any patients who required invasive mechanical ventilation.7 It is important to note that these nonpregnant populations were mostly older men, and an appropriate nonpregnant reproductive age control group is not yet available in the literature.
A current challenge lies in the prediction of patients presenting with COVID-19 who will progress to develop critical disease. Consistently across studies in nonpregnant populations, the risk of adverse outcomes increases with age and comorbidities, as well as certain laboratory values and radiologic findings.5 , 13, 14, 15, 16 In 1 study, patients requiring ICU care were more likely to have comorbid hypertension, cardiovascular disease, diabetes, and cerebrovascular disease.16 In terms of prognostic laboratory parameters, more severe lymphopenia has been noted in patients with worse outcomes.4 , 5 , 13 , 16, 17, 18 Other laboratory markers linked with severe illness include elevated D-dimer, IL-6, and serum ferritin levels.5 , 16 , 18
Because there are currently few reports of severe or critical COVID-19 in pregnant women, this study aimed to describe these patients and their clinical progression. In previous outbreaks of respiratory pathogens, pregnant populations experienced increased severity of illness and mortality. However, current data in the COVID-19 pandemic suggest that pregnant women experience similar, or even lower, rates of severe disease than nonpregnant patients.3 , 19 Despite these early findings, there is concern that pregnant women may still be at a higher risk for complications. For example, case reports have indicated cardiomyopathy in the setting of COVID-19.20 Further data are needed on when this occurs and what predicts its occurrence. It is therefore critical to determine the unique factors in pregnant women that predict a more severe disease course to guide clinical management as well as the optimal time of delivery.
Clinical implication and research implications
These data can help guide obstetricians in deciding when a patient should be symptomatically improving or at risk for deterioration. This is particularly important for the obstetrician who needs to consider timing and mode of delivery for patients with severe or critical COVID-19.21 Typically, attempts should be made to support the mother with oxygen as needed to maintain an oxygen saturation of >95% and reassuring fetal monitoring. Markers of developing critical disease seem to be valid in pregnancy as well (Table 5), but further research is needed given that the physiology of pregnancy changes the normal ranges of several of these markers, with the ultimate aim of developing a tool to predict those who would benefit from delivery. One useful variable is time: in our data, pregnant women with COVID-19 who developed critical disease and needed intubation progressed quickly from admission on day 7 from symptom onset to intubation on day 9. As the standard deviation for time to intubation is 4.5 days, this means that 95% of women requiring intubation will do so within 20 days of symptom onset; this is still a long period when management is most difficult. As most women with severe disease do not develop critical illness, expectant supportive management is probably reasonable for most. On the other end of the spectrum, for women at >34 weeks’ gestation with several risk factors for critical disease such as advanced age; comorbidities including obesity, pulmonary or cardiac disease, diabetes, and others; predictive laboratory abnormalities; and predictive respiratory requirements, delivery may be indicated before onset of critical disease. These are challenging cases to manage, often requiring individualized decisions, given that we do not know whether cesarean delivery increases the severity of the clinical course and given the significant fluid shifts. A predictive tool to determine the patients with COVID-19 who would benefit from delivery during hospitalization could be developed as we acquire more data.
Strengths and limitations
To date, we are not aware of a larger cohort study examining specifically the disease course of hospitalized pregnant women with molecular test confirming severe or critical COVID-19. A current report of COVID-19 in pregnant women from Wuhan, China, examined 118 pregnant women with COVID-19. In this cohort, only 10 women had severe or critical disease, and no data described the clinical course of the disease.22
Our study is limited by the nature of the cohort study (nonrandomized, lack of control group for comparison). In addition, management of patients with COVID-19 varied across institutions. Because of the urgency of obtaining information on COVID-19 in pregnancy, to guide physicians in managing patients during this pandemic, we were unable to report on long-term pregnancy or neonatal outcomes in this study.
These data are also limited by the fact that 13 women (2 with severe disease and 11 with critical disease) remained hospitalized, with several still on invasive mechanical ventilation, at the time of data collection; therefore, data on complete hospital courses and complete COVID-19 courses and pregnancy outcomes are limited. In this study, 50% of women did not give birth, which can be seen as a strength in that delivery can be delayed; however, complete outcomes on pregnancy are not yet available. In particular, effects of COVID-19 on pregnancy, including fetal growth restriction and amniotic fluid abnormalities, could not be elucidated because of the timing of data collection. Longer-term analysis will be required in the future.
Recommendations on pharmacotherapy cannot be made on the basis of these data. Larger case-control studies of the course of COVID-19 in pregnancy and randomized-controlled trials (RCTs) of interventions are necessary to best determine prognosis and management of these women. Hydroxychloroquine was used in 81% of women in our study; however, current recommendations are for its use only in the setting of clinical trials owing to unclear risks and benefits.23 Based on limited data from an RCT on the treatment of Ebola virus with remdesivir, the medication appears safe in pregnancy.24 One-fourth (25%) of our cohort received remdesivir. RCTs are ongoing on the efficacy of remdesivir for COVID-19 treatment. At some of our institutions, RCTs on the use of convalescent plasma are ongoing and include pregnant women. Use of anticoagulants remains controversial, with more research needed. By study design, no data are provided for mild COVID-19.
Conclusions
Pregnant women with severe or critical COVID-19 were typically admitted to the hospital on disease day 7 and stayed hospitalized for 6 days. Intubation was usually performed around day 9 on patients who required it, and peak severity of nonintubated disease occurred on day 9 as well. Laboratory and treatment management can reasonably follow nonpregnant guidelines; however, differing reference ranges for some laboratory values in pregnancy must be considered. This clinical course is not markedly different from that of hospitalized nonpregnant patients with COVID-19, except that days in hospital are less, with lower mortality; however, this comparison is made with nonpregnant patients who are much older on average. This suggests that pregnancy should not be considered an independent risk factor for severe or critical COVID-19. Maternal mortalities have been rarely reported so far in the literature25 , 26; however, the sample sizes of pregnancy studies remain small, and further research is necessary. Unique to pregnancy, complications such as preterm delivery and need for delivery are common for women with severe or critical COVID-19. This information is important in counseling women who received diagnosis at the early stage of disease on the potential for progression to a severe or critical stage. As management of these cases is complex, we recommend a multidisciplinary approach with maternal-fetal medicine, infectious disease, and critical care physicians. We encourage healthcare providers to use these data when deciding on pregnancy management decisions and in counseling family about the progression of COVID-19.
Acknowledgments
The authors thank the members of the IRB at their institutions, Doreen Kornrumpf, Donald Polizzi, and Brandy Firman, for their help in coordinating this multicenter study and patients for their participation in this study.
Footnotes
This paper is part of a supplement that represents a collection of COVID-related articles selected for publication by the editors of AJOG MFM without additional financial support.
The authors report no conflict of interest.
No financial support was received for this study.
Cite this article as: Pierce-Williams RAM, Burd J, Felder L, et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am J Obstet Gynecol MFM 2020;2:100134.
Supplementary Material
References
- 1.Johns Hopkins University and Medicine Coronavirus Resource Center COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) https://coronavirus.jhu.edu/map.html Available at:
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Breslin N., Baptiste C., Gyamfi-Bannerman C., et al. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100118. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu D., Li L., Wu X., et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020 doi: 10.2214/AJR.20.23072. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 9.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team Vital surveillances: the epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Wkly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 10.Society for Maternal-Fetal Medicine (SMFM) Electronic address: pubs@smfm.org, Plante LA, Pacheco LD, Louis JM. SMFM Consult Series #47: sepsis during pregnancy and the puerperium. Am J Obstet Gynecol. 2019;220:B2–B10. doi: 10.1016/j.ajog.2019.01.216. [DOI] [PubMed] [Google Scholar]
- 11.Hilton R. Defining acute renal failure. CMAJ. 2011;183:1167–1169. doi: 10.1503/cmaj.081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernal W., Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525–2534. doi: 10.1056/NEJMra1208937. [DOI] [PubMed] [Google Scholar]
- 13.Chen J., Qi T., Liu L., et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80:e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim E.S., Chin B.S., Kang C.K., et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean cohort study on COVID-19. J Korean Med Sci. 2020;35:e142. doi: 10.3346/jkms.2020.35.e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020 doi: 10.1515/cclm-2020-0369. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Cheng B., Jiang T., Zhang L., et al. Clinical characteristics of pregnant women with coronavirus disease 2019 in Wuhan, China. SSRN Electron J. 2020 doi: 10.2139/ssrn.3555240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juusela A., Nazir M., Gimovsky M. Two cases of coronavirus 2019–related cardiomyopathy in pregnancy. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100113. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnettler W.T., Al Ahwel Y., Suhag A. Severe ARDS in COVID-19-infected pregnancy: obstetric and intensive care considerations. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100120. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L., Li Q., Zheng D., et al. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N Engl J Med. 2020 doi: 10.1056/NEJMc2009226. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhimraj A., Morgan R.L., Shumaker A.H., et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa478. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulangu S., Dodd L.E., Davey R.T., Jr., et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaigham M., Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020 doi: 10.1111/aogs.13867. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karami P., Naghavi M., Feyzi A., et al. Mortality of a pregnant patient diagnosed with COVID-19: a case report with clinical, radiological, and histopathological findings. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101665. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


