Abstract
The versatility of colloidal particles endows the particle stabilized or Pickering emulsions with unique features and can potentially enable the fabrication of a wide variety of derived materials. We review the evolution and breakthroughs in the research on the use of colloidal particles for the stabilization of Pickering emulsions in recent years for the particle categories of inorganic particles, polymer-based particles, and food-grade particles. Moreover, based on the latest works, several emulsions stabilized by the featured particles and their derived functional materials, including enzyme immobilized emulsifiers for interfacial catalysis, 2D colloidal materials stabilized emulsions as templates for porous materials, and Pickering emulsions as adjuvant formulations, are also summarized. Finally, we point out the gaps in the current research on the applications of Pickering emulsions and suggest future directions for the design of particulate stabilizers and preparation methods for Pickering emulsions and their derived materials.
Keywords: Pickering emulsions, Colloidal particles at interfaces, Interfacial catalysis, Biomedicine, Functional materials
Graphical abstract
This review describes the various applications derived from Pickering emulsions focusing on several different categories based on the particle stabilizers comprised by inorganic particles, polymer particles, food-grade particles, and several composite particles and newly-discovered 2D material particles.
Highlights
-
•
We review the evolution and breakthroughs in the research on the use of colloidal particles for the stabilization of Pickering emulsions in recent years for the particle categories of inorganic particles, polymer-based particles, and food-grade particles.
-
•
We discuss recent emulsions stabilized by the featured particles and their derived functional materials, including enzyme immobilized emulsifiers for interfacial catalysis, 2D colloidal materials stabilized emulsions as templates for porous materials, and Pickering emulsions as adjuvant formulations.
-
•
We point out the gaps in the current research on the applications of Pickering emulsions and suggest future directions for the design of particulate stabilizers and preparation methods for Pickering emulsions and their derived materials.
Introduction
An emulsion is a system consisting of two immiscible liquids where one of the liquids is dispersed in the other. Owing to the high surface energy of the interface between the two immiscible phases, emulsions are thermodynamically unstable. Conventional emulsions are stabilized by surfactants or amphiphilic polymers that reduce the oil–water interfacial tension and form a molecular film around the liquid droplets. In addition to molecular emulsifiers, solid particles can also serve as emulsion stabilizers. Particle-stabilized emulsions known as ‘Pickering emulsions’ were first observed and described by Ramsden and Pickering in the early 20th century [1,2]. In recent decades, benefiting from the rapid development of particle synthesis techniques and discovery of new colloids with tunable surface properties, the applications of Pickering emulsions and derivative materials have attracted increasing attention. Although conventional surfactant-stabilized emulsions have been successfully and extensively used in various fields such as crude oil recovery, food, paints and coatings, and water treatment, the large-scale use of surfactants in industrial applications or personal care products is not cost effective and in some cases may cause adverse effects such as irritation and hemolytic behavior. Therefore, the use of colloidal particles may be a promising alternative to the use of surfactants, motivating the recent trend in the studies of Pickering emulsions of seeking to enable the commercial manufacturing and practical use of these materials.
Pickering emulsions are often considered to be highly stable due to the nearly irreversible interfacial adsorption of particulate stabilizers [3], and the stabilization mechanism of Pickering emulsions has been explored by many researchers [3,4]. Owing to the high energy of particle desorption from the interface, the resultant superior stability of Pickering emulsions can effectively protect the encapsulated actives within the emulsion droplets for a long time, and even proteins and enzymes can be well preserved in Pickering emulsions. Based on their fundamental properties, previous academic research on Pickering emulsions has mainly focused on microencapsulation, controlled release, drug delivery, and biphasic catalysis. In addition to their use in emulsions, advanced materials templated or fabricated from Pickering emulsions have also been reported extensively in recent studies, with most of the materials obtained in the form of foams and capsules. Pickering emulsions are excellent templates for the facile preparation of foam materials because the emulsion droplets naturally provide void structures with adjustable pore sizes after evaporation or washing. Meanwhile, the good stability of the Pickering emulsions is beneficial for foam formation during the freezing–drying or polymerization process. Some examples of new foam materials obtained from Pickering emulsions are also included in this review. Unlike the method of using the sacrificial seed template particles, the preparation of capsules or colloidosomes from Pickering emulsions does not require the subsequent removal of the template particles, and furthermore, cargos can be encapsulated inside the particles in advance [5]. Recently, Bago Rodriguez and Binks [6] have reviewed the methods and applications of Pickering emulsion templated capsules and discussed the typical examples of the research studies conducted in recent years.
Among the various applications of Pickering emulsions and their derivative materials, particle stabilizers are particularly significant because they not only serve as the emulsifiers for the emulsion stabilization but can also be equipped with desired functionalities such as oxidation resistance, UV protection, environmental responsiveness, and even electromagnetic properties. The widely varying characteristics of these particles enable their use in a wide range of applications, and the interfacial assembly of these particles by Pickering emulsions offers a new opportunity for more effective exploitation of their performance.
Considering the fact that colloidal particles are the key component in the formation of Pickering emulsions and that a wide range of particles such as inorganic particles, polymer particles, and food-grade particles have been found to stabilize Pickering emulsions, in this review, we will first concisely review the various types of particle-stabilized Pickering emulsions, with several important recent studies chosen as the typical illustrative examples. In addition, with the explosive increase in the study of functional materials, such as 2D materials and biomedical materials, many of these materials have been proven to greatly decrease the oil–water interfacial energy, and the combination of Pickering emulsions and these new materials is becoming an increasingly popular research topic compared with the traditional applications of Pickering emulsions. In this review, we will also survey the recently reported novel applications of Pickering emulsions and the functional materials stabilized by these novel particles, including interfacial biocatalysis, biomedical adjuvants, and foam materials.
Pickering emulsions stabilized by different types of particles
Pickering emulsions stabilized by inorganic particles
Inorganic particles were the first colloidal particles studied in the preparation and stabilization of Pickering emulsions and are still the most studied colloidal particles for this purpose. Owing to the high stability of inorganic particles, many Pickering emulsions stabilized by inorganic particles have been explored in the past decades. Among these, silica colloidal particles have been the most popular materials because of their good resistance to acidic and basic environments, easy surface modification, and controllable size and structure.
Monodispersed silica particles can be synthesized by the Stöber method [7], and the diameter of the silica particles can be controlled in the range from tens of nanometers to several micrometers. In 1988, Levine et al [8] successfully fabricated an oil-in-water (o/w) emulsion stabilized by hydrophilic silica nanoparticles with the diameter of ∼572 nm and found that a monolayer of silica nanoparticles was formed at the oil–water interface. Because the Pickering emulsion is actually formed by the assembly of colloidal particles at the liquid–liquid interface, the size of the emulsion droplet is inevitably limited by the particulate stabilizers used, and hence the majority of the reported silica-stabilized Pickering emulsions are in the micrometer size range, hindering their use in a wide variety of medical-related applications, and particularly in the applications that require cell penetration.
Recently, Jiang et al. [9] have successfully fabricated a submicron Pickering emulsion stabilized solely by modified silica nanoparticles (Figure 1 a). They first synthesized monodispersed silica nanoparticles with different diameters (50, 130, and 230 nm) and then chemically modified the silica nanoparticles to make them hydrophobic to stabilize the water-in-oil (w/o) Pickering emulsions. It was found that the size of the obtained emulsion droplets could be reduced through the decrease in the diameter of the silica stabilizers, and the diameter of the emulsion droplets can even be smaller than 500 nm when 50 nm silica nanoparticles were used as the emulsifiers. By using the submicron Pickering emulsion as the template, tetraethyl orthosilicate was introduced into the oil phase, and a silica shell was formed at the oil–water interface, simultaneously fixing the adsorbed silica nanoparticles. The obtained all-silica submicron capsule can be applied for the encapsulation of hydrophilic cargos, and the release of the actives can be responsively triggered by ethanol or surfactant.
Figure 1.
Examples of Pickering emulsions stabilized by inorganic particles. (a) Left to right, Scanning Electron Microscopy (SEM) image of 50 nm solid silica nanoparticles, Transmission Electron Microscopy (TEM) image of submicron colloidosome templated from w/o emulsion stabilized by 50 nm silica, detailed TEM image of submicron silica colloidosome with inset SEM image of the submicron silica capsule, respectively. (Reproduced from the study by Jiang et al [9] with permission from Wiley) (b) From left to right and down, TEM image of nanopores of mesoporous silica, water-solid-air three phase contact angles of the mesoporous silica nanoparticles, appearance and corresponding optical microscopy images of Pickering emulsions stabilized by different mesoporous silicas with ethyl acetate as the oil, respectively. (Reproduced from the study by Xue et al [10] with permission from American Chemical Society) (c) Scheme of the preparation of hybrid Pickering capsules via CaCO3 crystallization, and the optical microscopy image of the Pickering emulsion, SEM image of the CaCO3 capsule. (Reproduced from the study by Komatsu et al [14] with permission from American Chemical Society) (d) TEM images of the Ag/carbon quantum dots microspheres templated from inverse Pickering emulsion. (Reproduced from the study by Zhai et al [15] with permission from Elsevier) (e) SEM and TEM (insert) images of synthesized α-ZrP particles. (Reproduced from the study by Yu et al [19] with permission from Elsevier) (f) Pickering emulsion stabilized by a mixture of s-TiO2 and t-Fe3O4 nanoparticles. Inset: image of a water drop in hexane lying on a substrate formed with particles. (Reproduced from the study by Xie et al [21] with permission from American Chemical Society).
Xue et al. [10] synthesized mesoporous silica nanospheres with tunable interfacial activity, and both o/w and w/o Pickering emulsions were successfully obtained (Figure 1b). In the synthesis of such mesoporous silica nanoparticles (MSNs), hydrophilic zwitterionic groups and hydrophobic groups were simultaneously incorporated on the shell so that the obtained Pickering emulsions can switch from o/w to w/o and were proven to be highly stable against coalescence even at high-salinity conditions. Meanwhile, polyoxometalate was carried in the pores of the mesoporous silica, promoting the efficiency of the epoxidation reactions and eliminating the need to stir the emulsions during catalysis. Gao et al. [11] synthesized dendritic MSNs for stabilizing the o/w Pickering emulsions. While the synthesized MSNs were relatively small with a diameter of only 130 nm, the maximal pore size of these nanoparticles reached ∼7 nm, which is larger than that for most MSNs. This enabled the absorption of Candida antarctica lipase B into the pores of the MSNs with the enzyme loading of 166.3 mg/gsupport, and a MSN-stabilized Pickering emulsion platform for interfacial enzymatic catalysis was obtained. In addition to the Pickering emulsions stabilized by solid and mesoporous silica, Bao et al. [12] recently used hollow silica nanoparticles for the stabilization of Pickering emulsions and found that hollow silica nanoparticles exhibited strong adsorption. The cavity structure endowed the hollow silica nanoparticles with a low density, which may be the reason for the stability of the prepared Pickering emulsions.
Furthermore, many other inorganic colloidal particle including nonmetallic oxide particles, magnetic particles, and elemental particles exhibit good performance for the stabilization of Pickering emulsions. For example, laponite clay consists of disc-like particles, and a toluene-in-water Pickering emulsion was successfully prepared using these particles by Ashby and Binks [13] approximately 20 years ago. In another work, Komatsu et al [14] fabricated a calcium carbonate (CaCO3) capsule templated from the Pickering emulsion stabilized by calcium carbonate and showed that the CaCO3 capsule is soluble under acidic conditions (Figure 1c). In addition, carbon-based particles such as carbon quantum dots [15] (Figure 1d), carbon nanotubes [16,17], and graphene oxide (GO) particles [18] have been shown to be excellent materials for use as Pickering stabilizers for applications in water purification, biosensors, and electrochemistry-related fields. On the other hand, metal salts have been rarely used in Pickering emulsions. In the two example of such studies, Yu et al [19] synthesized highly uniform zirconium phosphate (α-ZrP) nanoparticles to produce α-ZrP nanodisk-stabilized o/w emulsions (Figure 1e), and Chen et al. used α-ZrP nanosheets for forming a Pickering emulsion during the flooding process to enhance oil recovery [20]. Metal oxide particles such as iron (III) oxide (Fe3O4) nanoparticles [21] (Figure 1f) and titanium oxide (TiO2) particles [22] have been a popular choice for application to Pickering emulsions. The Fe3O4 nanoparticles endow the emulsion with magnetic-responsive functionality, while the TiO2 nanoparticles can help to screen UV radiation and enhance the photocatalytic performance. In addition to these two examples, many other inorganic particles such as gold nanospheres have been used as the stabilizers for Pickering emulsions [23].
With the ongoing progress in the development of particle synthesis, the focus of particle design and synthesis studies has advanced beyond obtaining particles with a uniform and controlled size, and currently, an increasing research effort is devoted to the control of the particle structure, morphology, and functionality, particularly for the well-known classical colloidal particles such as silica, TiO2, and gold nanoparticles. These particles have been synthesized to obtain various shapes and engineered structures or modified with different functional groups via surface chemistry to meet the needs of various applications. The breakthroughs in particle synthesis have also strongly influenced the fabrication of Pickering emulsions, leading to the realization of novel and interesting Pickering emulsion systems. It is expected that this trend will continue in the future.
Pickering emulsions stabilized by polymer-based particles
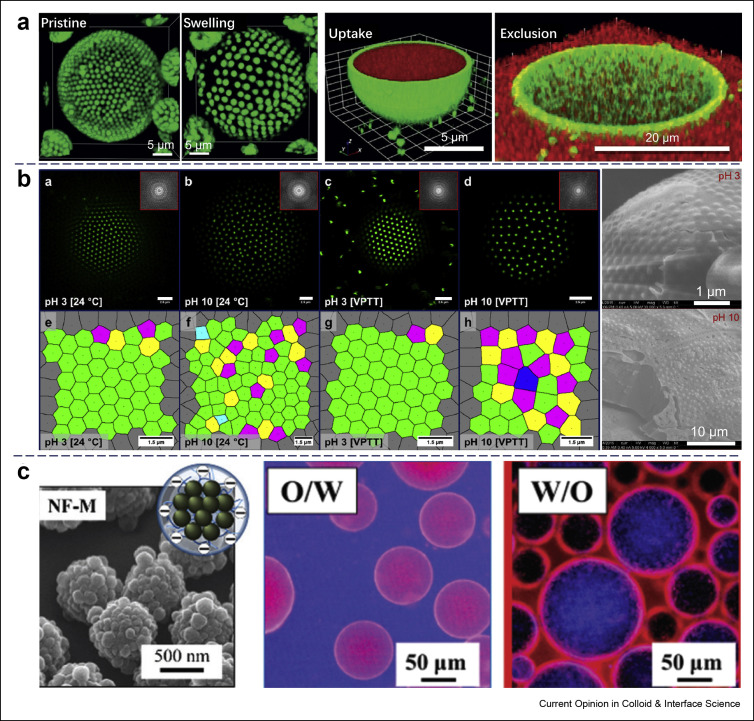
The inorganic particles used for Pickering emulsions have been mainly elemental particles, as well as the salts and oxides of inorganic elements, and therefore they are usually rigid and difficult to deform. By contrast, polymer colloidal particles are composed of three-dimensional polymer networks that are entangled and chemically cross-linked. Polystyrene (PS) particles are a classical example of such polymer particles and were used by Dinsmore et al. [24] to fabricate a ‘colloidosome’ capsule that was templated from an o/w Pickering emulsion stabilized by monodispersed PS spheres. The colloidosome structure was fabricated from the emulsion with the full coverage of the PS spheres at the oil droplet. Although the PS spheres were polymer based, they still behaved as rigid particles due to the high glass transition temperatures (Tg) of the PS polymer. Recently, Douliez et al. [25] reported a hydrogelled colloidosome that was derived from a water-in-water (w/w) Pickering emulsion stabilized by amine-modified PS latex beads. The internal phase of the w/w emulsion was an aqueous liquid filled with dextran, while the continuous phase consisted of a polyethylene oxide–enriched solution. After the gelation of the core of the prepared w/w Pickering emulsions, the obtained colloidosome with molecularly crowded interiors displayed reversible swelling, as shown in Figure 2 a. Unlike the w/o emulsions, this w/w colloidosome allowed the uptake and exclusion of macromolecular payloads, making it a novel Pickering system for microencapsulation.
Figure 2.
Examples of Pickering emulsions stabilized by polymer-based particles. (a) Left to right, confocal images of pristine and swollen hydrogel colloidosomes stabilized with 1 μm PS latex beads, uptake and exclusion of payloads by the hydrogelled colloidosomes, respectively. (Reproduced from the study by Douliez et al [25] with permission from Wiley) (b) Left (a–h): confocal microscope images of colloidal lattices at droplet surfaces and corresponding constructed Voronoi diagrams of the colloidal lattices at varying pH and temperature, right: Cryo-scanning electron microscopy (cryo-SEM) images of the interface of IL-in-water droplets covered by composite microgel particles at different pH, respectively, the scale bars in a-d are 2.5 μm and in e-h are 1.5 μm (reproduced from the study by Chen et al [39] with permission from Elsevier) (c) Scanning Electron Microscopy (SEM) images of the nanocomposite microgel and fluorescence microscopy images of as-stabilized o/w and w/o emulsions. (Reproduced from the study by Watanabe et al [41] with permission from The Royal Society of Chemistry). IL, ionic liquid.
Responsive microgels [26, 27, 28] are a new type of particulate emulsifiers that have been used to stabilize Pickering emulsions [29]. Similar to PS spheres, microgels are polymer-based colloidal particles. However, the microgels are more flexible than the rigid PS particles and are very hydrophilic and thus can easily absorb water within the polymer networks. Moreover, microgels can switch between the states of swelling and collapse, with the switching triggered by the changes in the environmental conditions such as temperature [30,31] and pH [32,33]. In the swollen state, the microgel is soft and highly deformable, and the polymer chains are stretched. On the other hand, the collapsed microgel resembles a rigid particle due to the shrinkage of polymer chains [34]. In the synthesis of microgels, functional and responsive groups are introduced to endow the microgels with environmentally dependent behaviors.
For the conventional rigid particle stabilized Pickering emulsions, the stabilization mechanism is explained well by the high desorption energy from the interface that can be calculated from the contact angle and the oil–water interfacial tension. However, the stabilization mechanism underlying the preparation of the microgel-stabilized emulsions is far from being completely understood [35,36]. Compared with the rigid colloids, the microgels are soft, fuzzy, and deformable [26] so that the concept of the contact angle may be not applicable to the microgels at the oil–water interface. In the emulsification process, the microgel is more similar to a polymer emulsifier because it itself is composed of polymers. Thus, the stabilization mechanism may also be explained by the ability of the microgel to reduce the oil–water interfacial tension. A previous study has indicated that soft particles reduced the oil–water interfacial tension more effectively than rigid particles [37]. Moreover, the soft particles also exhibited significantly higher interfacial activity at the oil–water interface than the rigid particles, and a clear correlation between the deformability of the microgels and the emulsions stability arising from the core–shell structure of the microgels was demonstrated [38].
One of the signature characteristics of microgel particles is their flexible and easily adjustable structure due to the preparation of the microgel particles by the polymerization of monomers. Specifically, based on the knowledge of polymer chemistry, microgel particles with various functional groups can be synthesized by copolymerizing with different monomers. For instance, temperature- and pH-sensitive microgels can be synthesized by the copolymerization of N-isopropylacrylamide and methacrylic acid, and then the obtained poly(N-isopropylacrylamide-co-methacrylic acid) (PNIPAM-MAA) microgel particles can endow the microgel-stabilized emulsions with controllable stability that is governed by both the temperature and pH.
Chen et al. [39] synthesized core–shell microgel particles for stabilizing ionic liquid-in-water Pickering emulsions, and the monolayer of the microgels adsorbed at the interface even displayed a colloidal lattice structure (Figure 2b). The microgel particle was composed of a PS core and a poly (N-isopropylacrylamide-co-acrylic acid) gel shell, and the whole microgel particle was thermosensitive and pH responsive. To visualize how the interfacial behavior of the microgel particles at the ionic liquid–water interface is influenced by the changes in the environment, the microgel was labeled with a fluorescence agent, and confocal microscopy and Cryo-scanning electron microscopy (cryo-SEM) characterizations clearly demonstrated the packing difference of the microgels at the different conditions.
Generally, microgel particles are easily swollen by water and are always considered to be highly hydrophilic. Although they are superior emulsifiers for stabilizing the o/w Pickering emulsion and even a small amount of microgels is enough to prepare an o/w internal phase Pickering emulsions with a high oil volume fraction close to 0.9 [40], the preparation of w/o Pickering emulsions by these water-swollen microgel particles is difficult. To strongly stabilize w/o Pickering emulsions by microgels, the microgels that are dispersed in water should also somehow show hydrophobicity to improve their affinity to the oil component so as to strongly adsorb at the water–oil interface in the preparation of the w/o emulsions. To overcome this issue, nanocomposite microgels with adjustable hydrophobicity and surface roughness were synthesized by Watanabe et al. [41] First, poly(N-isopropylacrylamide-co-fumaric acid) microgels were synthesized and prepared as the seed particles. Then, nanocomposite microgels were synthesized by seeded emulsion polymerization using the seed microgels, and different monomers including methyl methacrylate and styrene were used for linking the seed microgels. Interestingly, it was found that both the hydrophobicity and the surface roughness of the microgels can influence the interfacial behavior of the nanocomposite microgels, and suitable tuning of these properties can control the emulsion type (o/w or w/o) and stabilize the water-in-polar oil or water-in-nonpolar oil Pickering emulsions, as illustrated in Figure 2c. While further studies are still necessary to provide an in-depth understanding of the stabilization mechanism, the existing work on the microgel stabilization of Pickering emulsions has opened a new avenue for the research on Pickering emulsions stabilized by soft particles.
Pickering emulsions stabilized by food-grade particles
The formulations of many common foods such as chocolate and butter are based on emulsions [42]. However, the applications of particle-stabilized emulsions in food industry are challenging due to the strict limitations on the particulate emulsifiers that can be used in foods. Safety is perhaps the most important issue for the food industry, and safety concerns restrict the range of the possible Pickering stabilizers to edible materials only [43, 44, 45]. In addition, food production is always carried out on a large scale, imposing the requirement for the use of inexpensive stabilizers only.
Several food-grade particles of natural origin have been successfully explored for forming Pickering emulsions. Tan et al. [46] successfully fabricated a high internal phase emulsion (HIPE) (dispersed phase volume fraction exceeds 0.74) that was stabilized by gelatin particles, and subsequently, numerous edible particle stabilized HIPEs were reported. In Table 1 , for reference, we summarized the studies of edible particles used for the stabilization of Pickering HIPEs reported in the literature in the last two years.
Table 1.
High internal phase emulsions stabilized by edible colloidal particles.
| Particle material | Form of stabilizer | Oil phase | Emulsion type | Highlights | Reference |
|---|---|---|---|---|---|
| Starch | Starch nanocrystals (SNCs) | Soy oil | o/w | Sole effective stabilizer, stable and gel-like HIPEs were formed | [47] |
| Mixture of chitosan/octenyl succinic anhydride (OSA) starch | Docosahexaenoic acid (DHA) -rich algae oil | o/w | OSA starch–chitosan complex facilitated the formation of stable HIPEs | [48] | |
| OSA-modified starch | Camellia oils | o/w | Encapsulation and improvement of β-carotene stability and bioaccessibility | [49] | |
| Chitin | Chitin nanofibrils | Sunflower oil, cyclohexane | o/w | Emulgel inks suitable for 3D printing | [50] |
| Zein | Zein–pectin hybrid particle | Corn oil | o/w | Robust and ordered interfacial structure, HIPEs with ideal oxidant stability | [51] |
| Zein–tannic acid complex particles | Sunflower oil | o/w | Controllable rheological behavior of high internal phase emulsion gels | [52] | |
| Zein–propylene glycol alginate–rhamnolipid complex particles | Medium-chain triglyceride (MCT) oil | o/w | Relatively small oil droplets (<20 μm), the HIPEs had good stability in a range of environmental conditions | [53] | |
| Gliadin | Gliadin particles | Algal oil | o/w | Antioxidation, in vitro digestion showed retarded lipid oxidation | [54] |
| Gliadin–chitosan complex particles | Hexane | o/w | Water-insoluble protein porous materials for oil absorption | [55] | |
| Gliadin nanoparticles/gum Arabic | Corn oil | o/w | Improved stability of β-carotene encapsulated in the oil droplets | [56] | |
| Whey | Whey protein microgels | Corn oil | o/w | Higher stability than surfactant-stabilized HIPEs | [57] |
| Whey protein isolate microgels | Grape seed oil | o/w | Encapsulation of L. plantarum within HIPEs successfully increased the cell viability after pasteurization processing | [58] | |
| Peanut | Natural peanut-protein-isolate (PPI) microgel | Peanut oil, hexane | o/w | A substitute for partially hydrogenated vegetable oils, protein-based foam scaffolds | [59] |
| Gelatin | Gelatin nanoparticles | Hexane | o/w | Hierarchical porous protein scaffold, enhanced cell adhesion | [60] |
| Casein | Casein nanogels | Olive oil | o/w | Potential application in drug delivery | [61] |
| Chitosan-caseinophosphopeptides nanocomplexes | Corn oil, linseed oil | o/w | Enhanced lipid oxidative stability and curcumin bioaccessibility | [62] |
HIPEs, high internal phase emulsions.
Despite the intense research effort devoted to the preparation and stabilization of Pickering HIPEs with food-grade particles, all of the HIPEs obtained to date have been of the o/w type, and commercial applications and products that are suitable for mass manufacturing have been rarely developed. Since Pickering emulsion is a powerful tool, more attention should be devoted to food-relevant applications in future research.
Featured emulsions stabilized by particles for functional materials
Colloidal particles are the essential components in the formation of a Pickering emulsion, and generally, the materials, texture, and the functionality of the particles determine the specific applications of the prepared emulsions. While the previous section discussed the recent development of different types of particles and the corresponding Pickering emulsions, in this section, we will review several emulsions stabilized by functional particles that have been applied in recent years in the popular fields such as catalysis, biomedicine, and advanced materials.
Pickering interfacial catalysis
Pickering emulsions are an ideal platform for biphasic catalysis because of their enhanced stability, large interfacial area, facile product separation, and easy catalyst recycling. Consequently, the exploration of Pickering emulsions for biphasic catalysis is a hot topic in the study of applications of Pickering emulsions. Different catalysts and even enzymes have been successfully encapsulated in Pickering emulsions to increase the catalytic reaction rate, and the results demonstrated that the use of Pickering emulsions is indeed an efficient approach for both preserving the activity of biocatalysts and improving their catalytic performance [63, 64, 65]. This field has seen ongoing advances, and in recent years, cascade catalysis and interfacial catalysis have attracted increasing attention in the studies of Pickering emulsion–based catalysis.
Sun et al. [66] developed a Pickering emulsion stabilized by a special colloidal stabilizer and demonstrated that the interfacial cascade reaction was catalyzed by the stabilizers (Figure 3 a). In other words, the colloidal stabilizers acted as both the emulsifiers for the stabilization of the emulsion and as the catalytic sites. In this work, an enzyme–polymer conjugate was innovatively synthesized by the grafting of the enzyme onto the polymer, and the enzyme–polymer conjugates were proven to stabilize the o/w Pickering emulsions. The synthesis of this special kind of particle requires certain skills in both polymer chemistry and biochemistry because the enzyme protein must be linked to the polymer while maintaining its activity, and atom transfer radical polymerization was used in the synthesis. Nevertheless, this novel particulate stabilizer paved the way for the use of the protein-stabilized Pickering emulsions for interfacial biphasic biocatalysis, and it was shown that two different enzymes, namely benzaldehyde lyase and glucose oxidase, can be effectively conjugated with the polymer and retain their activity at the liquid–liquid interface. By cooperating with additional enzymes, a Pickering emulsion system for interfacial cascade biocatalysis was built up, significantly broadening the range of application of Pickering emulsion catalysis.
Figure 3.
Examples of Pickering emulsions for interfacial catalysis. (a) Left: scheme of preparation of active enzyme–PNIPAAm conjugates to stabilize Pickering emulsions and interfacial biocatalysis, right: (a) Photograph of the emulsion. (b & c) Optical microscopy image and confocal image of the emulsion. (d) Cryo-SEM image of emulsion droplets after UV cross-linking. (e) TEM picture of emulsion droplets after solvent evaporation. (Reproduced from the study by Sun et al [66] with permission from Wiley) (b) TEM micrograph of particles mixture after dispersion in water (left) and Cryo-scanning electron microscopy (cryo-SEM) image of water-in-toluene emulsions stabilized with particles mixture (right), the inset shows a representation of the particles in water and at the surface droplets. (Reproduced from the study by Yang et al [69] with permission from The Royal Society of Chemistry) (c) Optical microscopy images of w/o emulsions stabilized with microgels and various amounts of silica nanoparticles. (Reproduced from the study by Jiang et al [68] with permission from American Chemical Society) (d) Left to right, scheme of wettability adjustment of E@Alg@s-TiO2 microparticles at the water–hexane interface by the chain length of grafted silane, optical microscopy image of interfacial catalysis system of the water-in-hexane Pickering emulsion stabilized by the microparticles, conversion comparison of the interfacial catalysis, respectively. (Reproduced from the study by Yang et al [67] with permission from The Royal Society of Chemistry) (e) Left to right, illustration of the concept of a liquid−solid hybrid catalyst and its utilization in continuous flow reactions, comparison of specific activity of the kinetic resolution of the alcohols over the liquid–solid hybrid catalysts with different enzyme loadings, respectively. (Reproduced from the study by Zhang et al [72] with permission from American Chemical Society).
Yang et al. [67] immobilized the lipase enzyme in the alginate gel microparticles and then used the microparticles as the stabilizers for a w/o Pickering emulsion, also achieving the goal of interfacial biocatalysis (Figure 3d). Unlike in the work of Ref. [66], an inverse w/o Pickering emulsion was prepared, and a very different method was used for enzyme immobilization. To fix the enzyme in the alginate particle, the lipase was first trapped in the gel network of the alginate, and then the alginate gel was emulsified by silane-grafted titania nanoparticles and surfactant Span 80 to obtain a w/o emulsion with the paraffin oil as the oil phase. After gelation by calcium chloride, a TiO2-coated alginate microparticle was formed with the lipase confined inside the microparticle. Then, the wettability of microparticles was changed to stabilize the w/o Pickering emulsion, enabling the catalysis of an esterification reaction at the interface. More recently, the strategy of the use of binary particles for the stabilization of w/o Pickering emulsions for interfacial biocatalysis was developed in our group [68]. In the binary particles, the first component was a pH-responsive microgel that simultaneously encapsulated the enzymes and served as a catalyst carrier, while hydrophobic silica nanoparticles served as the second component that improved the stability of the resultant w/o emulsion, and the optical microscopy images of prepared w/o emulsions are illustrated in Figure 3c. It was found that the obtained inverse Pickering emulsion showed ideal catalytic behavior for the reactions at the water–oil interface. The enzyme immobilization method was facile and did not involve chemical bonding, and the Pickering emulsion was also prepared in the normal manner by homogenization. This approach may inspire new insights and methods for the fabrication and applications of Pickering stabilized systems.
In another approach, Yang et al [69] fabricated Pickering emulsions for interfacial catalysis using two kinds of nanoparticles while controlling the self-assembled behavior of the two colloidal particles, and the tandem reaction was catalyzed continuously (Figure 3b). Researchers [70, 71, ∗72, 73] filled the catalyst-containing Pickering emulsions stabilized with a variety of particles in continuous flow column reactors and designed a series of macroscale flow reaction systems for improving the catalytic and recycling efficiencies (Figure 3e). This work provides an approach for amplifying the interfacial catalysis by Pickering emulsions that has great potential for industrial use.
Biomedical applications
A number of biomedical applications related to Pickering emulsions have been reported based on the good stability, high payload capacity, and most importantly the biocompatibility of the particle stabilizers. Therefore, many naturally derived particles and biodegradable particles, including chitosan, poly(lactic-co-glycolic acid) (PLGA), gelatin, and protein-based particles, have been applied for emulsion stabilization [74].
While surfactant-stabilized emulsions have been explored as adjuvants for vaccine formulations [75], the application of Pickering emulsions in immunologic agents is still challenging. With the progress of modern medicine, enhanced vaccination has become extremely important, and Pickering emulsions are likely to be used in this field in the future. As an initial demonstration, Xia et al. [76] developed a PLGA nanoparticle stabilized squalene-in-water Pickering emulsion adjuvant system for exploiting the force-dependent deformability and lateral mobility. Compared with the conventional emulsions stabilized by surfactants and solid particles, Pickering emulsions displayed an enhanced antigen uptake and promoted humoral and cellular immune responses. More practically, the PLGA nanoparticle-stabilized Pickering emulsion adjuvant system (PPAS) was further used in clinical-relevant vaccinations, and immune protection by H1N1 vaccine with PPAS as the adjuvant was investigated experimentally, with the results obtained on mice indicating that PPAS may be a good candidate adjuvant. Unfortunately, the appearance of a number of epidemic diseases and novel viruses such as the influenza, severe acute respiratory syndrome, Middle East respiratory syndrome, and the novel Coronavirus Disease (COVID-19) that appeared in 2019 has made development of vaccines increasingly urgent. Although the vaccine itself is the most direct and powerful tool for preventing the virus-borne epidemics, the adjuvant plays an important role in the vaccine formulations. It is clear that good adjuvants can promote the efficiency of the vaccine, and Pickering emulsions should be investigated as adjuvants.
Pioneering works on the use of Pickering emulsions in biomedical applications have also extensively focused on drug encapsulation and delivery (such as topical drug delivery and oral drug delivery) [77,78], bioimaging [79], and stimuli-responsive materials [80]. For further information regarding biomedical-relevant applications, the readers are referred to the latest review on the biomedical applications of Pickering emulsions [74].
Functional materials
The 21st century is the century of materials, and research on advanced materials including nanomaterials, biomedical materials, and energy materials has been extremely popular in recent years. In particular, porous foam materials have been shown to be promising materials for use in a variety of fields. As a pristine three-dimensional structure, Pickering emulsion is a natural template for the fabrication of hierarchically structured foam materials. Although many methods can be used for producing porous foams, such as freezing drying, bubbling, and particle templating [81], the Pickering emulsion templating technique has several unique advantages: (1) the polydispersity and size of the emulsion droplets can be facilely controlled by the emulsification parameters, enabling the controlled adjustment of the porosity of the templated foam; (2) owing to the high stability of Pickering emulsions at high temperatures or in other harsh conditions, polymerization reactions can be easily introduced to polymerize the continuous phase, and a number of monomers and multiple reaction routes can be chosen; and (3) the removal of interior liquid is feasible and can be carried out merely by freeze drying or heat-induced evaporation.
Among the Pickering emulsion templated foams, the HIPE templating has been the most extensively studied because this unique Pickering emulsion endows the obtained scaffold materials with low density and high porosity and void volume. Macroporous foams produced from Pickering emulsions have attracted the interest of many researchers in the fields of biochemistry, interfacial science, engineering, environmental science, and energy materials.
Studies of foams derived from Pickering emulsion have been popular in the field of cell culture and tissue engineering. Similar to the biomedical applications of Pickering emulsions, the use of Pickering emulsions in cell culture and tissue engineering imposes the requirement for the biocompatibility of the particle stabilizers. Many polymer-based particles have been used for the stabilization of emulsions and have demonstrated good biocompatibility with normal growth of the cells in the templated scaffolds [82,83]. In recent years, the applications of Pickering stabilized foams for cell culture have been more focused on the concept of green chemistry because many protein-based colloidal particles are suitable for the preparation of Pickering HIPEs, and the polymerization or cross-linking can be achieved in a milder manner [84].
In more practical and engineering applications, HIPE templated foams have been widely used for oil–water separation, oil capture, and water treatment. Because the internal volume fraction of the HIPE is very high, the templated foam material displays a low density after liquid removal. When the primary emulsion is of the w/o type, polymerization of the oil liquid can always obtain a hydrophobic scaffold foam. One of the advantages of such foam is that it can freely float on water, and the large voids inside the foam can absorb a large amount of oil, endowing these foams with great potential for the treatment of marine oil spills. In recent years, many works have reported on the innovative use of HIPE-templated foams in water treatment [85], oil capture [86,87], oil–water separation [88], and CO2 or pH-switchable oil/water absorption [88,89]. However, the majority of the aforementioned emulsion-templated foams are derived from surfactant-stabilized emulsions. While Pickering-type polyHIPEs have been reported for more than a decade [90,91], the use of Pickering HIPE-templated foams for applications relevant to oil–water separation has been rare. The bridging of the gaps between the properties of the current state-of-the-art Pickering-type polyHIPEs and the properties required for the use of these polyHIPEs for practical oil–water separation may be due to the closed cell structure of the Pickering templated foams, and the simultaneous incorporation of surfactants into Pickering polyHIPEs is a possible direction for future research in this field.
Currently, the study of the preparation and applications of 2D materials is one of the hottest areas in science and technology research. Owing to their outstanding conductivity, unique electronic structure, and large surface area, 2D materials are considered to have a very high potential for applications in the electrochemistry and energy fields [92]. However, the performance of 2D materials is severely hindered by their tendency to aggregate or stack, with the solutions of this issue including the generation of interlayer space, creation of hierarchical structures, and assembly of the 2D material flakes into a 3D macroscopic structure [93]. Thus, the use of Pickering emulsions is an approach for the interfacial assembly of 2D materials.
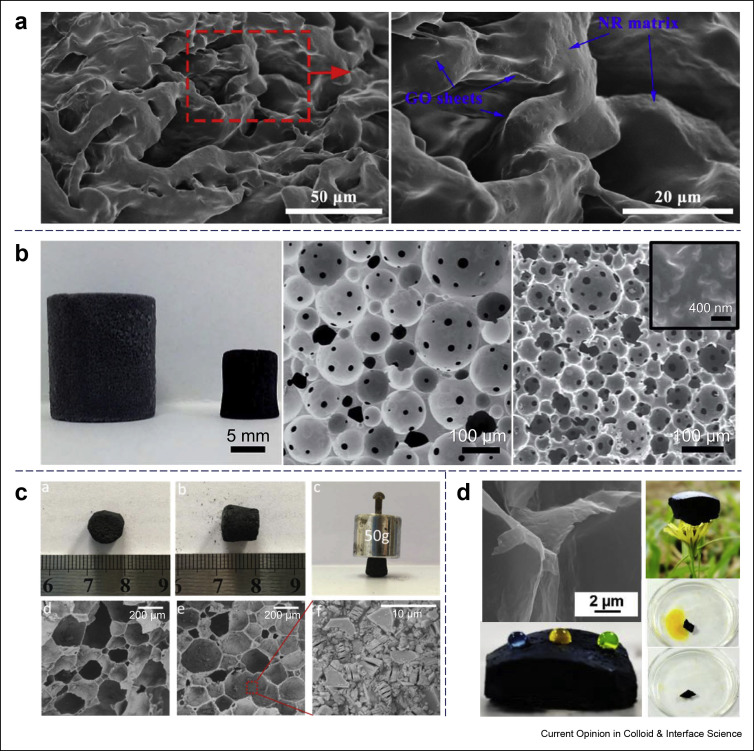
As the oldest and most intensely investigated 2D material, GO has attracted considerable attention since the first discovery of graphene in 2004 [94]. Owing to its amphiphilic nature and extraordinary interface chemistry properties, GO acts as a sheet structure colloidal particle for the fabrication of Pickering emulsions, while the GO sheets can assemble in a 3D structure at the liquid–liquid interface [95]. Kim et al. [96] have studied the stabilization of Pickering emulsions by GO sheets since 2010, and many researchers have found that the GO particles can act as the surfactants for stabilizing the interface [97], and GO-stabilized capsules [98], HIPEs [99,100], and foams [101, 102, 103, 104, 105] have been continuously reported in the literature, and some of examples are illustrated in Figure 4 a and b. For further information regarding the current understanding of the interfacial behavior of GO at the liquid–liquid or air–liquid interfaces, the readers can refer to a recent review [95]. Beyond graphene and GO, boron nitride, transition-metal dichalcogenides (MoS2, WS2, and so on), phosphorene, and 2D transition metal carbides or nitrides (MXenes) have also been added to the family of 2D materials in recent years. Recently, an o/w Pickering HIPE was successfully prepared using modified Ti3C2Tx-MXene flakes, and a templated macroporous foam material was fabricated [106]. (Figure 4c) Similarly, Shi et al. [107] demonstrated that cooperative assembly of amine-functionalized polyhedral oligomeric silsesquioxane (POSS–NH2) and Ti3C2Tx-Mxene sheets improved the interfacial activity at the water–oil interface. The obtained w/o emulsion was concentrated by centrifugation to fabricate the MXene aerogel, and it was shown that the aerogel has a macroporous structure and low density and can be potentially used for oil absorption and electromagnetic interference shielding (Figure 4d). In addition, the interfacial behavior and jamming of the Ti3C2Tx nanosheets at the oil–water interface was examined and described. [108] It is believed that the development of 2D-material–stabilized Pickering emulsions and derived functional materials will be a new trend in colloidal and material science.
Figure 4.
Example of Pickering emulsions stabilized by 2D materials and derived foams. (a) Cross-section SEM images of freeze-dried Pickering emulsions stabilized with GO platelets. (Reproduced from the study by yang et al [104] with permission from Elsevier) (b) Photograph of interconnected poly(DVB)HIPEs (left) and the carbonized foam (right) and the corresponding SEM images. (Reproduced from the study by Woodword et al [103] with permission from The Royal Society of Chemistry) (c) (a-c) Photographs of porous material derived from Ti3C2Tx stabilized Pickering emulsion and the robustness of the porous material under a weight of 50 g, (d-e) SEM images of the Ti3C2Tx stabilized HIPE templated porous material, (f) SEM image of the wall of the porous material embedded with Ti3C2Tx particles. (Reproduced from the study by Bian et al [106] with permission from The Royal Society of Chemistry) (d) SEM image of close-up view of MXene aerogels prepared from emulsion templates, illustration of the low density of the obtained aerogel, and application in oil absorption of the aerogel, respectively. (Reproduced from the study by Shi et al [107] with permission from Wiley). HIPE, high internal phase emulsion.
Conclusions and future directions
Pickering emulsions have received considerable attention in recent years, and the applications of Pickering emulsions have been extended to a variety of fields, including material science, engineering, biochemistry, food, and cosmetics. This review briefly describes the various applications derived from Pickering emulsions focusing on several different categories based on the particle stabilizers comprised by inorganic particles, polymer particles, food-grade particles, and several composite particles and newly discovered 2D material particles. Owing to the recent breakthroughs in particle synthesis, the functionalities of the particle stabilizers endow the as-prepared Pickering emulsions with unique features that are unavailable in conventional surfactant-stabilized emulsions, and many studies have demonstrated the importance of these Pickering emulsions in interfacial catalysis, biomedicine, drug delivery, functional materials, and other fields. We believe that the design of engineered colloidal particles will be a popular topic of future research.
While to date, most Pickering emulsions have been stabilized by particles of a single type, there have been some reports in the literature on the emulsions stabilized with mixed rigid particles, and in several rare cases, binary particles with contrasting features (e.g. softness, hydrophobicity, surface charge) have been used for the costabilization of Pickering emulsions. The different emulsifiers can endow the as-prepared Pickering emulsions with multiple functionalities and responses, and the fundamental study of multiparticle behavior at the emulsion interface and its specific applications should also be considered a promising direction for future research. In addition, while to date, the vast majority of the reported Pickering emulsions have been the single oil-in-water (o/w) or water-in-oil (w/o) emulsions, and future work may concentrate on the design of particles with suitable amphiphilicity for stabilizing emulsions with multiple compartments, such as water-in-oil-in-water (w/o/w) or oil-in-water-in-oil (o/w/o) double emulsions. In one example of such work, Lei et al. [109] synthesized poly(2-(diethylamino)ethyl methacrylate) microgel particles for fabricating an HIPE with the double emulsion morphology via a simple one-step emulsification method.
A survey of the literature shows that food-grade or naturally derived colloidal particles such as cellulose, gelatin, and proteins have been widely extracted or modified for the preparation of Pickering emulsions, particularly for HIPEs. By contrast, investigations of the use of formulations based on Pickering emulsion in cosmetics have been rarely reported. Although emulsification technology is widely used in cream, lotion, foundation, and other cosmetic dispersions, the majority of the emulsion-based products are stabilized by surfactants. The use of Pickering emulsion formulations for cosmetic applications to avoid the skin irritation and sensitization issues caused by surfactants should be a promising concept for future research, particularly due to the large size of the cosmetics market. For these studies, it may be useful to draw on the experience of the studies of the Pickering emulsions stabilized by food-grade particles to address the safety and biocompatibility issues. Finally, we believe that Pickering emulsification is an efficient approach for the assembly of 2D materials at the interface for the construction of 3D functional foams that is envisioned for enabling the applications of the Pickering emulsion–derived materials in the fields of electronics, energy, and even wearable devices.
Conflict of interest statement
Nothing declared.
Acknowledgements
The authors gratefully acknowledge the financial support from the Hong Kong Special Administration Region (HKSAR) General Research Fund (CUHK14306617 and 2130535) and the Impact Postdoctoral Fellowship Scheme of The Chinese University of Hong Kong.
This review comes from a themed issue on Emulsions and Microemulsions
Edited by Carlos Rodriguez-Abreu and Kenji Aramaki
References
- 1.Pickering S.U. Cxcvi.—emulsions. J Chem Soc Trans. 1907;91:2001–2021. [Google Scholar]
- 2.Ramsden W., Gotch F. Separation of solids in the surface-layers of solutions and ‘suspensions’ (observations on surface-membranes, bubbles, emulsions, and mechanical coagulation).—preliminary account. Proc Roy Soc Lond. 1904;72:156–164. [Google Scholar]
- 3.Binks B.P. Particles as surfactants—similarities and differences. Curr Opin Colloid Interface Sci. 2002;7:21–41. [Google Scholar]
- 4.Leunissen M.E., van Blaaderen A., Hollingsworth A.D., Sullivan M.T., Chaikin P.M. Electrostatics at the oil-water interface, stability, and order in emulsions and colloids. Proc Natl Acad Sci U S A. 2007;104:2585–2590. doi: 10.1073/pnas.0610589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossier-Miranda F.J., Schroën C.G.P.H., Boom R.M. Colloidosomes: versatile microcapsules in perspective. Colloids Surf, A. 2009;343:43–49. [Google Scholar]
- 6.Bago Rodriguez A.M., Binks B.P. Capsules from Pickering emulsion templates. Curr Opin Colloid Interface Sci. 2019;44:107–129. [Google Scholar]
- 7.Stöber W., Fink A., Bohn E. Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci. 1968;26:62–69. [Google Scholar]
- 8.Levine S., Bowen B.D., Partridge S.J. Stabilization of emulsions by fine particles i. Partitioning of particles between continuous phase and oil/water interface. Colloid Surface. 1989;38:325–343. [Google Scholar]
- Jiang H., Hong L., Li Y., Ngai T. All-silica submicrometer colloidosomes for cargo protection and tunable release. Angew Chem Int Ed. 2018;57:11662–11666. doi: 10.1002/anie.201805968. [DOI] [PubMed] [Google Scholar]; Submicron w/o Pickering emulsions and templated colloidosomes solely stabilized by silica nanoparticles are fabricated, with the adjustable shell thickness, and tunable release of encapsulated cargos.
- Xue F., Zhang Y., Zhang F., Ren X., Yang H. Tuning the interfacial activity of mesoporous silicas for biphasic interface catalysis reactions. ACS Appl Mater Interfaces. 2017;9:8403–8412. doi: 10.1021/acsami.6b16605. [DOI] [PubMed] [Google Scholar]; Mesoporous silica stabilized Pickering emulsions can switch from o/w to w/o, and were proven to be highly stable against coalescence even at high-salinity conditions, also catalyst can be encapsulated in the pores of silica for promoting catalytic reaction without stirring.
- Gao J., Wang Y., Du Y., Zhou L., He Y., Ma L. Construction of biocatalytic colloidosome using lipase-containing dendritic mesoporous silica nanospheres for enhanced enzyme catalysis. Chem Eng J. 2017;317:175–186. [Google Scholar]; A MSNs-stabilized Pickering emulsion platform for interfacial enzymatic catalysis was obtained by using mesoporous silica with large pore size, and lipase B was successfully carried in the MSNs for interfacial catalysis.
- 12.Bao Y., Zhang Y., Liu P., Ma J., Zhang W., Liu C. Novel fabrication of stable Pickering emulsion and latex by hollow silica nanoparticles. J Colloid Interface Sci. 2019;553:83–90. doi: 10.1016/j.jcis.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Ashby N.P., Binks B.P. Pickering emulsions stabilised by laponite clay particles. Phys Chem Chem Phys. 2000;2:5640–5646. [Google Scholar]
- 14.Komatsu S., Ikedo Y., Asoh T.-A., Ishihara R., Kikuchi A. Fabrication of hybrid capsules via CaCO3 crystallization on degradable coacervate droplets. Langmuir. 2018;34:3981–3986. doi: 10.1021/acs.langmuir.8b00148. [DOI] [PubMed] [Google Scholar]
- 15.Zhai X., Gao J., Wang X., Mei S., Zhao R., Wu Y. Inverse Pickering emulsions stabilized by carbon quantum dots: influencing factors and their application as templates. Chem Eng J. 2018;345:209–220. [Google Scholar]
- 16.Briggs N.M., Weston J.S., Li B., Venkataramani D., Aichele C.P., Harwell J.H. Multiwalled carbon nanotubes at the interface of Pickering emulsions. Langmuir. 2015;31:13077–13084. doi: 10.1021/acs.langmuir.5b03189. [DOI] [PubMed] [Google Scholar]
- 17.Briggs N., Raman A.K.Y., Barrett L., Brown C., Li B., Leavitt D. Stable Pickering emulsions using multi-walled carbon nanotubes of varying wettability. Colloids Surf, A. 2018;537:227–235. [Google Scholar]
- 18.Luo Q., Wang Y., Yoo E., Wei P., Pentzer E. Ionic liquid-containing Pickering emulsions stabilized by graphene oxide-based surfactants. Langmuir. 2018;34:10114–10122. doi: 10.1021/acs.langmuir.8b02011. [DOI] [PubMed] [Google Scholar]
- 19.Yu Y.-H., Chen Y.-P., Zeng M., Cheng Z. Microwave-assisted rapid synthesis of hexagonal α-zirconium phosphate nanodisks as a Pickering emulsion stabilizer. Mater Lett. 2016;163:158–161. [Google Scholar]
- 20.Chen H., Qing S., Ye Z.B., Han L.J., Wang X., Xu L. Experimental investigation of hydrophobically modified alpha-zrp nanosheets for enhancing oil recovery in low-permeability sandstone cores. ACS Omega. 2019;4:22178–22186. doi: 10.1021/acsomega.9b03481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie C.-Y., Meng S.-X., Xue L.-H., Bai R.-X., Yang X., Wang Y. Light and magnetic dual-responsive Pickering emulsion micro-reactors. Langmuir. 2017;33:14139–14148. doi: 10.1021/acs.langmuir.7b03642. [DOI] [PubMed] [Google Scholar]
- 22.Fessi N., Nsib M.F., Chevalier Y., Guillard C., Dappozze F., Houas A. Photocatalytic degradation enhancement in Pickering emulsions stabilized by solid particles of bare TiO2. Langmuir. 2019;35:2129–2136. doi: 10.1021/acs.langmuir.8b03806. [DOI] [PubMed] [Google Scholar]
- 23.Liu D., Zhou F., Li C., Zhang T., Zhang H., Cai W. Black gold: plasmonic colloidosomes with broadband absorption self-assembled from monodispersed gold nanospheres by using a reverse emulsion system. Angew Chem Int Ed. 2015;54:9596–9600. doi: 10.1002/anie.201503384. [DOI] [PubMed] [Google Scholar]
- 24.Dinsmore A.D., Hsu M.F., Nikolaides M.G., Marquez M., Bausch A.R., Weitz D.A. Colloidosomes: selectively permeable capsules composed of colloidal particles. Science. 2002;298:1006. doi: 10.1126/science.1074868. [DOI] [PubMed] [Google Scholar]
- Douliez J.-P., Martin N., Beneyton T., Eloi J.-C., Chapel J.-P., Navailles L. Preparation of swellable hydrogel-containing colloidosomes from aqueous two-phase Pickering emulsion droplets. Angew Chem Int Ed. 2018;57:7780–7784. doi: 10.1002/anie.201802929. [DOI] [PubMed] [Google Scholar]; A hydrogelled colloidosome was derived from a water-in-water (w/w) Pickering emulsion stabilized by amine-modified polystyrene latex beads, and the obtained colloidosome with molecularly crowded interiors displayed reversible swelling, allowing the uptake and exclusion of macromolecular payloads.
- 26.Plamper F.A., Richtering W. Functional microgels and microgel systems. Acc Chem Res. 2017;50:131–140. doi: 10.1021/acs.accounts.6b00544. [DOI] [PubMed] [Google Scholar]
- 27.Lyon L.A., Fernandez-Nieves A. The polymer/colloid duality of microgel suspensions. Annu Rev Phys Chem. 2012;63:25–43. doi: 10.1146/annurev-physchem-032511-143735. [DOI] [PubMed] [Google Scholar]
- 28.Karg M., Pich A., Hellweg T., Hoare T., Lyon L.A., Crassous J.J. Nanogels and microgels: from model colloids to applications, recent developments, and future trends. Langmuir. 2019;35:6231–6255. doi: 10.1021/acs.langmuir.8b04304. [DOI] [PubMed] [Google Scholar]
- 29.Kwok M.-h., Sun G., Ngai T. Microgel particles at interfaces: phenomena, principles, and opportunities in food sciences. Langmuir. 2019;35:4205–4217. doi: 10.1021/acs.langmuir.8b04009. [DOI] [PubMed] [Google Scholar]
- 30.Nolan C.M., Gelbaum L.T., Lyon L.A.H. NMR investigation of thermally triggered insulin release from poly(n-isopropylacrylamide) microgels. Biomacromolecules. 2006;7:2918–2922. doi: 10.1021/bm060718s. [DOI] [PubMed] [Google Scholar]
- 31.Ngai T., Behrens S.H., Auweter H. Novel emulsions stabilized by pH and temperature sensitive microgels. Chem Commun. 2005;3:331–333. doi: 10.1039/b412330a. [DOI] [PubMed] [Google Scholar]
- 32.Fujii S., Read E.S., Binks B.P., Armes S.P. Stimulus-responsive emulsifiers based on nanocomposite microgel particles. Adv Mater. 2005;17:1014–1018. [Google Scholar]
- 33.Das M., Mardyani S., Chan W.C.W., Kumacheva E. Biofunctionalized pH-responsive microgels for cancer cell targeting: rational design. Adv Mater. 2006;18:80–83. [Google Scholar]
- 34.Mourran A., Wu Y., Gumerov R.A., Rudov A.A., Potemkin, Pich A. When colloidal particles become polymer coils. Langmuir. 2016;32:723–730. doi: 10.1021/acs.langmuir.5b03931. [DOI] [PubMed] [Google Scholar]
- 35.Li Z., Ngai T. Microgel particles at the fluid–fluid interfaces. Nanoscale. 2013;5:1399–1410. doi: 10.1039/c2nr33503d. [DOI] [PubMed] [Google Scholar]
- 36.Richtering W. Responsive emulsions stabilized by stimuli-sensitive microgels: emulsions with special non-Pickering properties. Langmuir. 2012;28:17218–17229. doi: 10.1021/la302331s. [DOI] [PubMed] [Google Scholar]
- 37.Li Z., Harbottle D., Pensini E., Ngai T., Richtering W., Xu Z. Fundamental study of emulsions stabilized by soft and rigid particles. Langmuir. 2015;31:6282–6288. doi: 10.1021/acs.langmuir.5b00039. [DOI] [PubMed] [Google Scholar]
- 38.Destribats M., Lapeyre V., Wolfs M., Sellier E., Leal-Calderon F., Ravaine V. Soft microgels as Pickering emulsion stabilisers: role of particle deformability. Soft Matter. 2011;7:7689–7698. [Google Scholar]
- Chen H., Nofen E.M., Rykaczewski K., Dai L.L. Colloidal lattices of environmentally responsive microgel particles at ionic liquid–water interfaces. J Colloid Interface Sci. 2017;504:440–447. doi: 10.1016/j.jcis.2017.04.083. [DOI] [PubMed] [Google Scholar]; Core-shell microgel particles with both temperature and pH responsiveness were used for stabilizing ionic liquid (IL)-in-water Pickering emulsions, and the monolayer of the microgels adsorbed at the interface even displayed a colloidal lattice structure.
- 40.Li Z., Ming T., Wang J., Ngai T. High internal phase emulsions stabilized solely by microgel particles. Angew Chem Int Ed. 2009;48:8490–8493. doi: 10.1002/anie.200902103. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Takizawa M., Jiang H., Ngai T., Suzuki D. Hydrophobized nanocomposite hydrogel microspheres as particulate stabilizers for water-in-oil emulsions. Chem Commun. 2019;55:5990–5993. doi: 10.1039/c9cc01497g. [DOI] [PubMed] [Google Scholar]; Nanocomposite microgels with adjustable hydrophobicity and surface roughness were synthesized to stabilize o/w and w/o Pickering emulsions, while both water-in-polar oil and water-in-nonpolar oil Pickering emulsions can be successfully prepared.
- 42.Norton J.E., Fryer P.J., Parkinson J., Cox P.W. Development and characterisation of tempered cocoa butter emulsions containing up to 60% water. J Food Eng. 2009;95:172–178. [Google Scholar]
- 43.Berton-Carabin C.C., Schroën K. Pickering emulsions for food applications: background, trends, and challenges. Ann Rev Food Sci Technol. 2015;6:263–297. doi: 10.1146/annurev-food-081114-110822. [DOI] [PubMed] [Google Scholar]
- 44.Dickinson E. Colloids in food: ingredients, structure, and stability. Ann Rev Food Sci Technol. 2015;6:211–233. doi: 10.1146/annurev-food-022814-015651. [DOI] [PubMed] [Google Scholar]
- 45.Linke C., Drusch S. Pickering emulsions in foods - opportunities and limitations. Crit Rev Food Sci Nutr. 2018;58:1971–1985. doi: 10.1080/10408398.2017.1290578. [DOI] [PubMed] [Google Scholar]
- 46.Tan H., Sun G., Lin W., Mu C., Ngai T. Gelatin particle-stabilized high internal phase emulsions as nutraceutical containers. ACS Appl Mater Interfaces. 2014;6:13977–13984. doi: 10.1021/am503341j. [DOI] [PubMed] [Google Scholar]
- 47.Yang T., Zheng J., Zheng B.-S., Liu F., Wang S., Tang C.-H. High internal phase emulsions stabilized by starch nanocrystals. Food Hydrocolloids. 2018;82:230–238. [Google Scholar]
- 48.Yan C., McClements D.J., Zhu Y., Zou L., Zhou W., Liu W. Fabrication of osa starch/chitosan polysaccharide-based high internal phase emulsion via altering interfacial behaviors. J Agric Food Chem. 2019;67:10937–10946. doi: 10.1021/acs.jafc.9b04009. [DOI] [PubMed] [Google Scholar]
- 49.Yan C., McClements D.J., Zou L., Liu W. A stable high internal phase emulsion fabricated with osa-modified starch: an improvement in β-carotene stability and bioaccessibility. Food Funct. 2019;10:5446–5460. doi: 10.1039/c9fo00508k. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Y., Huan S., Bai L., Ketola A., Shi X., Zhang X. High internal phase oil-in-water Pickering emulsions stabilized by chitin nanofibrils: 3d structuring and solid foams. ACS Appl Mater Interfaces. 2020 doi: 10.1021/acsami.9b23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou F.-Z., Huang X.-N., Wu Z.-l., Yin S.-W., Zhu J.-h., Tang C.-H. Fabrication of zein/pectin hybrid particle-stabilized Pickering high internal phase emulsions with robust and ordered interface architecture. J Agric Food Chem. 2018;66:11113–11123. doi: 10.1021/acs.jafc.8b03714. [DOI] [PubMed] [Google Scholar]
- 52.Zou Y., Yang X., Scholten E. Tuning particle properties to control rheological behavior of high internal phase emulsion gels stabilized by zein/tannic acid complex particles. Food Hydrocolloids. 2019;89:163–170. [Google Scholar]
- 53.Dai L., Yang S., Wei Y., Sun C., McClements D.J., Mao L. Development of stable high internal phase emulsions by Pickering stabilization: utilization of zein-propylene glycol alginate-rhamnolipid complex particles as colloidal emulsifiers. Food Chem. 2019;275:246–254. doi: 10.1016/j.foodchem.2018.09.122. [DOI] [PubMed] [Google Scholar]
- 54.Zhou F.Z., Zeng T., Yin S.W., Tang C.H., Yuan D.B., Yang X.Q. Development of antioxidant gliadin particle stabilized Pickering high internal phase emulsions (HIPEs) as oral delivery systems and the in vitro digestion fate. Food Funct. 2018;9:959–970. doi: 10.1039/c7fo01400g. [DOI] [PubMed] [Google Scholar]
- 55.Zhou F.-Z., Yu X.-H., Zeng T., Yin S.-W., Tang C.-H., Yang X.-Q. Fabrication and characterization of novel water-insoluble protein porous materials derived from Pickering high internal-phase emulsions stabilized by gliadin–chitosan-complex particles. J Agric Food Chem. 2019;67:3423–3431. doi: 10.1021/acs.jafc.9b00221. [DOI] [PubMed] [Google Scholar]
- 56.Ma L., Zou L., McClements D.J., Liu W. One-step preparation of high internal phase emulsions using natural edible Pickering stabilizers: gliadin nanoparticles/gum Arabic. Food Hydrocolloids. 2020;100:105381. [Google Scholar]
- 57.Zamani S., Malchione N., Selig M.J., Abbaspourrad A. Formation of shelf stable Pickering high internal phase emulsions (HIPE) through the inclusion of whey protein microgels. Food Funct. 2018;9:982–990. doi: 10.1039/c7fo01800b. [DOI] [PubMed] [Google Scholar]
- 58.Su J., Wang X., Li W., Chen L., Zeng X., Huang Q. Enhancing the viability of lactobacillus plantarum as probiotics through encapsulation with high internal phase emulsions stabilized with whey protein isolate microgels. J Agric Food Chem. 2018;66:12335–12343. doi: 10.1021/acs.jafc.8b03807. [DOI] [PubMed] [Google Scholar]
- 59.Jiao B., Shi A., Wang Q., Binks B.P. High-internal-phase Pickering emulsions stabilized solely by peanut-protein-isolate microgel particles with multiple potential applications. Angew Chem Int Ed Engl. 2018;57:9274–9278. doi: 10.1002/anie.201801350. [DOI] [PubMed] [Google Scholar]
- 60.Tan H., Tu Z., Jia H., Gou X., Ngai T. Hierarchical porous protein scaffold templated from high internal phase emulsion costabilized by gelatin and gelatin nanoparticles. Langmuir. 2018;34:4820–4829. doi: 10.1021/acs.langmuir.7b04047. [DOI] [PubMed] [Google Scholar]
- 61.Chen S., Zhang L.-M. Casein nanogels as effective stabilizers for Pickering high internal phase emulsions. Colloids Surf, A. 2019;579:123662. [Google Scholar]
- 62.Huang X.-N., Zhou F.-Z., Yang T., Yin S.-W., Tang C.-H., Yang X.-Q. Fabrication and characterization of Pickering high internal phase emulsions (HIPEs) stabilized by chitosan-caseinophosphopeptides nanocomplexes as oral delivery vehicles. Food Hydrocolloids. 2019;93:34–45. [Google Scholar]
- 63.Wu C., Bai S., Ansorge-Schumacher M.B., Wang D. Nanoparticle cages for enzyme catalysis in organic media. Adv Mater. 2011;23:5694–5699. doi: 10.1002/adma.201102693. [DOI] [PubMed] [Google Scholar]
- 64.Cao W., Huang R., Qi W., Su R., He Z. Self-assembly of amphiphilic janus particles into monolayer capsules for enhanced enzyme catalysis in organic media. ACS Appl Mater Interfaces. 2015;7:465–473. doi: 10.1021/am5065156. [DOI] [PubMed] [Google Scholar]
- 65.Qu Y., Huang R., Qi W., Su R., He Z. Interfacial polymerization of dopamine in a Pickering emulsion: synthesis of cross-linkable colloidosomes and enzyme immobilization at oil/water interfaces. ACS Appl Mater Interfaces. 2015;7:14954–14964. doi: 10.1021/acsami.5b03787. [DOI] [PubMed] [Google Scholar]
- Sun Z., Glebe U., Charan H., Böker A., Wu C. Enzyme–polymer conjugates as robust Pickering interfacial biocatalysts for efficient biotransformations and one-pot cascade reactions. Angew Chem Int Ed. 2018;57:13810–13814. doi: 10.1002/anie.201806049. [DOI] [PubMed] [Google Scholar]; The enzyme–polymer conjugate was innovatively synthesized by the grafting of the enzyme onto the polymer, and the enzyme–polymer conjugates were proven to stabilize the o/w Pickering emulsions with the functionality of interfacial catalysis.
- Yang X., Wang Y., Bai R., Ma H., Wang W., Sun H. Pickering emulsion-enhanced interfacial biocatalysis: tailored alginate microparticles act as particulate emulsifier and enzyme carrier. Green Chem. 2019;21:2229–2233. [Google Scholar]; Lipase enzyme was successfully immobilized in the tailored alginate microparticles, and the microparticles served as both emulsion stabilizers and catalytic sites.
- Jiang H., Liu L., Li Y., Yin S., Ngai T. Inverse Pickering emulsion stabilized by binary particles with contrasting characteristics and functionality for interfacial biocatalysis. ACS Appl Mater Interfaces. 2020;12:4989–4997. doi: 10.1021/acsami.9b16117. [DOI] [PubMed] [Google Scholar]; Inverse Pickering emulsions were stabilized by binary particles with contrasting characteristics, the binary particles were composed of hydrophobic silica nanoparticles and pH-responsive microgels, and enzyme was encapsulated in the microgel in a mild way for interfacial catalysis.
- Yang B., Leclercq L., Schmitt V., Pera-Titus M., Nardello-Rataj V. Colloidal tectonics for tandem synergistic Pickering interfacial catalysis: oxidative cleavage of cyclohexene oxide into adipic acid. Chem Sci. 2019;10:501–507. doi: 10.1039/c8sc03345e. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pickering emulsions for interfacial catalysis were fabricated by using two kinds of nanoparticles, while controlling the self-assembled behavior of the two colloidal particles, and tandem reaction was catalyzed continuously.
- 70.Chen H., Zou H., Hao Y., Yang H. Flow Pickering emulsion interfaces enhance catalysis efficiency and selectivity for cyclization of citronellal. ChemSusChem. 2017;10:1989–1995. doi: 10.1002/cssc.201700318. [DOI] [PubMed] [Google Scholar]
- 71.Zhang M., Ettelaie R., Yan T., Zhang S., Cheng F., Binks B.P. Ionic liquid droplet microreactor for catalysis reactions not at equilibrium. J Am Chem Soc. 2017;139:17387–17396. doi: 10.1021/jacs.7b07731. [DOI] [PubMed] [Google Scholar]
- Zhang X., Hou Y., Ettelaie R., Guan R., Zhang M., Zhang Y. Pickering emulsion-derived liquid–solid hybrid catalyst for bridging homogeneous and heterogeneous catalysis. J Am Chem Soc. 2019;141:5220–5230. doi: 10.1021/jacs.8b11860. [DOI] [PubMed] [Google Scholar]; The concept of a liquid–solid hybrid catalyst and utilization in continuous flow reactions was proposed.
- 73.Meng Z., Zhang M., Yang H. Pickering emulsion droplets hosting ionic liquid catalysts for continuous-flow cyanosilylation reaction. Green Chem. 2019;21:627–633. [Google Scholar]
- 74.Harman C.L.G., Patel M.A., Guldin S., Davies G.-L. Recent developments in Pickering emulsions for biomedical applications. Curr Opin Colloid Interface Sci. 2019;39:173–189. [Google Scholar]
- 75.Jansen T., Hofmans M.P.M., Theelen M.J.G., Manders F., Schijns V.E.J.C. Structure- and oil type-based efficacy of emulsion adjuvants. Vaccine. 2006;24:5400–5405. doi: 10.1016/j.vaccine.2006.03.074. [DOI] [PubMed] [Google Scholar]
- Xia Y., Wu J., Wei W., Du Y., Wan T., Ma X. Exploiting the pliability and lateral mobility of Pickering emulsion for enhanced vaccination. Nat Mater. 2018;17:187. doi: 10.1038/nmat5057. [DOI] [PubMed] [Google Scholar]; PLGA nanoparticle stabilized squalene-in-water Pickering emulsion was explored as the adjuvant system for exploiting the force-dependent deformability and lateral mobility, while the formulation displayed an enhanced antigen uptake, and promoted humoral and cellular immune responses.
- 77.Ruiz-Rodriguez P.E., Meshulam D., Lesmes U. Characterization of Pickering o/w emulsions stabilized by silica nanoparticles and their responsiveness to in vitro digestion conditions. Food Biophys. 2014;9:406–415. [Google Scholar]
- 78.Simovic S., Ghouchi-Eskandar N., Prestidge C.A. Pickering emulsions for dermal delivery. J Drug Deliv Sci Technol. 2011;21:123–133. [Google Scholar]
- 79.Phan-Quang G.C., Lee H.K., Phang I.Y., Ling X.Y. Plasmonic colloidosomes as three-dimensional sers platforms with enhanced surface area for multiphase sub-microliter toxin sensing. Angew Chem Int Ed. 2015;54:9691–9695. doi: 10.1002/anie.201504027. [DOI] [PubMed] [Google Scholar]
- 80.Wu J., Ma G.-H. Recent studies of Pickering emulsions: particles make the difference. Small. 2016;12:4633–4648. doi: 10.1002/smll.201600877. [DOI] [PubMed] [Google Scholar]
- 81.Thompson B.R., Horozov T.S., Stoyanov S.D., Paunov V.N. Hierarchically structured composites and porous materials from soft templates: fabrication and applications. J Mater Chem A. 2019;7:8030–8049. [Google Scholar]
- 82.Hu Y., Gu X., Yang Y., Huang J., Hu M., Chen W. Facile fabrication of poly(l-lactic acid)-grafted hydroxyapatite/poly(lactic-co-glycolic acid) scaffolds by Pickering high internal phase emulsion templates. ACS Appl Mater Interfaces. 2014;6:17166–17175. doi: 10.1021/am504877h. [DOI] [PubMed] [Google Scholar]
- 83.Yang T., Hu Y., Wang C., Binks B.P. Fabrication of hierarchical macroporous biocompatible scaffolds by combining Pickering high internal phase emulsion templates with three-dimensional printing. ACS Appl Mater Interfaces. 2017;9:22950–22958. doi: 10.1021/acsami.7b05012. [DOI] [PubMed] [Google Scholar]
- 84.Tan H., Wei J., Sun G., Mu C., Lin W., Ngai T. Interconnected macroporous 3d scaffolds templated from gelatin nanoparticle-stabilized high internal phase emulsions for biomedical applications. Soft Matter. 2017;13:3871–3878. doi: 10.1039/c7sm00706j. [DOI] [PubMed] [Google Scholar]
- 85.Pan J., Zeng J., Cao Q., Gao H., Gen Y., Peng Y. Hierarchical macro and mesoporous foams synthesized by HIPEs template and interface grafted route for simultaneous removal of λ-cyhalothrin and copper ions. Chem Eng J. 2016;284:1361–1372. [Google Scholar]
- 86.Zhang T., Guo Q. Continuous preparation of polyHIPE monoliths from ionomer-stabilized high internal phase emulsions (HIPEs) for efficient recovery of spilled oils. Chem Eng J. 2017;307:812–819. [Google Scholar]
- 87.Zhang N., Zhou Y., Zhang Y., Jiang W., Wang T., Fu J. Dual-templating synthesis of compressible and superhydrophobic spongy polystyrene for oil capture. Chem Eng J. 2018;354:245–253. [Google Scholar]
- 88.Lei L., Zhang Q., Shi S., Zhu S. Highly porous poly(high internal phase emulsion) membranes with “open-cell” structure and CO2-switchable wettability used for controlled oil/water separation. Langmuir. 2017;33:11936–11944. doi: 10.1021/acs.langmuir.7b02539. [DOI] [PubMed] [Google Scholar]
- 89.Guo Z., Gu H., Chen Q., He Z., Xu W., Zhang J. Macroporous monoliths with pH-induced switchable wettability for recyclable oil separation and recovery. J Colloid Interface Sci. 2019;534:183–194. doi: 10.1016/j.jcis.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 90.Menner A., Ikem V., Salgueiro M., Shaffer M.S.P., Bismarck A. High internal phase emulsion templates solely stabilised by functionalised titania nanoparticles. Chem Commun. 2007:4274–4276. doi: 10.1039/b708935j. [DOI] [PubMed] [Google Scholar]
- 91.Ikem V.O., Menner A., Bismarck A. High internal phase emulsions stabilized solely by functionalized silica particles. Angew Chem Int Ed. 2008;47:8277–8279. doi: 10.1002/anie.200802244. [DOI] [PubMed] [Google Scholar]
- 92.Khazaei M., Mishra A., Venkataramanan N.S., Singh A.K., Yunoki S. Recent advances in MXenes: from fundamentals to applications. Curr Opin Solid State Mater Sci. 2019;23:164–178. [Google Scholar]
- 93.Shang T., Lin Z., Qi C., Liu X., Li P., Tao Y. 3d macroscopic architectures from self-assembled MXene hydrogels. Adv Funct Mater. 2019;29 [Google Scholar]
- 94.Dreyer D.R., Park S., Bielawski C.W., Ruoff R.S. The chemistry of graphene oxide. Chem Soc Rev. 2010;39:228–240. doi: 10.1039/b917103g. [DOI] [PubMed] [Google Scholar]
- 95.McCoy T.M., Turpin G., Teo B.M., Tabor R.F. Graphene oxide: a surfactant or particle? Curr Opin Colloid Interface Sci. 2019;39:98–109. [Google Scholar]
- 96.Kim J., Cote L.J., Kim F., Yuan W., Shull K.R., Huang J. Graphene oxide sheets at interfaces. J Am Chem Soc. 2010;132:8180–8186. doi: 10.1021/ja102777p. [DOI] [PubMed] [Google Scholar]
- 97.Gudarzi M.M., Sharif F. Self assembly of graphene oxide at the liquid–liquid interface: a new route to the fabrication of graphene based composites. Soft Matter. 2011;7:3432–3440. [Google Scholar]
- 98.Zhang Y., Zheng X., Wang H., Du Q. Encapsulated phase change materials stabilized by modified graphene oxide. J Mater Chem A. 2014;2:5304–5314. [Google Scholar]
- 99.Zheng Z., Zheng X., Wang H., Du Q. Macroporous graphene oxide–polymer composite prepared through Pickering high internal phase emulsions. ACS Appl Mater Interfaces. 2013;5:7974–7982. doi: 10.1021/am4020549. [DOI] [PubMed] [Google Scholar]
- 100.Yi W., Wu H., Wang H., Du Q. Interconnectivity of macroporous hydrogels prepared via graphene oxide-stabilized Pickering high internal phase emulsions. Langmuir. 2016;32:982–990. doi: 10.1021/acs.langmuir.5b04477. [DOI] [PubMed] [Google Scholar]
- 101.Chen Y., Wang Y., Shi X., Jin M., Cheng W., Ren L. Hierarchical and reversible assembly of graphene oxide/polyvinyl alcohol hybrid stabilized Pickering emulsions and their templating for macroporous composite hydrogels. Carbon. 2017;111:38–47. [Google Scholar]
- 102.Liu C., Zhang J., Sang X., Kang X., Zhang B., Luo T. CO2/water emulsions stabilized by partially reduced graphene oxide. ACS Appl Mater Interfaces. 2017;9:17613–17619. doi: 10.1021/acsami.7b02546. [DOI] [PubMed] [Google Scholar]
- 103.Woodward R.T., Markoulidis F., De Luca F., Anthony D.B., Malko D., McDonald T.O. Carbon foams from emulsion-templated reduced graphene oxide polymer composites: electrodes for supercapacitor devices. J Mater Chem A. 2018;6:1840–1849. [Google Scholar]
- 104.Yang Z., Liu H., Wu S., Tang Z., Guo B., Zhang L. A green method for preparing conductive elastomer composites with interconnected graphene network via Pickering emulsion templating. Chem Eng J. 2018;342:112–119. [Google Scholar]
- 105.Yang L., Liu Y., Filipe C.D.M., Ljubic D., Luo Y., Zhu H. Development of a highly sensitive, broad-range hierarchically structured reduced graphene oxide/polyhipe foam for pressure sensing. ACS Appl Mater Interfaces. 2019;11:4318–4327. doi: 10.1021/acsami.8b17020. [DOI] [PubMed] [Google Scholar]
- Bian R., Lin R., Wang G., Lu G., Zhi W., Xiang S. 3D assembly of Ti3C2-Mxene directed by water/oil interfaces. Nanoscale. 2018;10:3621–3625. doi: 10.1039/c7nr07346a. [DOI] [PubMed] [Google Scholar]; An o/w Pickering HIPE was successfully prepared using CTAB-modified Ti3C2Tx-MXene flakes, and the prepared emulsion acted as template for the fabrication of macroporous foam materials.
- Shi S., Qian B., Wu X., Sun H., Wang H., Zhang H.-B. Self-assembly of MXene-surfactants at liquid–liquid interfaces: from structured liquids to 3d aerogels. Angew Chem Int Ed. 2019;58:18171–18176. doi: 10.1002/anie.201908402. [DOI] [PubMed] [Google Scholar]; MXene aerogel was successfully prepared that was templated from Mxene stabilized w/o Pickering emulsion, and it was shown that the aerogel has a macroporous structure and low density, and can be potentially used for oil absorption and electromagnetic interference (EMI) shielding.
- Cain J.D., Azizi A., Maleski K., Anasori B., Glazer E.C., Kim P.Y. Sculpting liquids with two-dimensional materials: the assembly of Ti3C2Tx MXene sheets at liquid–liquid interfaces. ACS Nano. 2019;13:12385–12392. doi: 10.1021/acsnano.9b05088. [DOI] [PubMed] [Google Scholar]; The interfacial behavior and jamming of the Ti3C2Tx nanosheets at the oil-water interface was examined and described.
- 109.Lei L., Zhang Q., Shi S., Zhu S. High internal phase emulsion with double emulsion morphology and their templated porous polymer systems. J Colloid Interface Sci. 2016;483:232–240. doi: 10.1016/j.jcis.2016.08.034. [DOI] [PubMed] [Google Scholar]