Abstract
Background and purpose
The novel severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2), now named coronavirus disease 2019 (COVID-19), may change the risk of stroke through an enhanced systemic inflammatory response, hypercoagulable state, and endothelial damage in the cerebrovascular system. Moreover, due to the current pandemic, some countries have prioritized health resources towards COVID-19 management, making it more challenging to appropriately care for other potentially disabling and fatal diseases such as stroke. The aim of this study is to identify and describe changes in stroke epidemiological trends before, during, and after the COVID-19 pandemic.
Methods
This is an international, multicenter, hospital-based study on stroke incidence and outcomes during the COVID-19 pandemic. We will describe patterns in stroke management, stroke hospitalization rate, and stroke severity, subtype (ischemic/hemorrhagic), and outcomes (including in-hospital mortality) in 2020 during COVID-19 pandemic, comparing them with the corresponding data from 2018 and 2019, and subsequently 2021. We will also use an interrupted time series (ITS) analysis to assess the change in stroke hospitalization rates before, during, and after COVID-19, in each participating center.
Conclusion
The proposed study will potentially enable us to better understand the changes in stroke care protocols, differential hospitalization rate, and severity of stroke, as it pertains to the COVID-19 pandemic. Ultimately, this will help guide clinical-based policies surrounding COVID-19 and other similar global pandemics to ensure that management of cerebrovascular comorbidity is appropriately prioritized during the global crisis. It will also guide public health guidelines for at-risk populations to reduce risks of complications from such comorbidities.
Key Words: SARS-CoV-2, COVID-19, Pandemic, Stroke, Incidence, Mortality, National crisis, Health policy
Introduction
An emergence of pneumonia cases in Wuhan, Hubei Province, China, was first reported to the World Health Organization (WHO) on December 31, 2019.1 A novel coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2), which had not been previously identified in humans, was subsequently isolated from these patients, and was identified as the source of the acute respiratory illnesses, now named coronavirus disease 2019 (COVID-19). On March 11, 2020, COVID-19 was subsequently declared a global pandemic by the World Health Organization (WHO) and has spread to over 212 countries/territories worldwide so far.2 , 3 This has surpassed the spread of other viral respiratory tract diseases such as severe acute respiratory syndrome (SARS).4 , 5 Specifically, as of April 30th, 2020, there are 3,090,445 reported laboratory-confirmed cases worldwide3, and this number is expected to continue increasing. This has resulted in an unprecedented international public health crisis that has prompted numerous countries to implement nation-wide lockdowns to mitigate the spread of the virus and reduce the burden on healthcare systems.
The public health burden of COVID-19 has reached beyond that of any other infectious respiratory illness, as it may have associations with non-communicable diseases (NCDs), including cerebrovascular or cardiovascular disorders (CVDs). Patients with COVID-19 often have vascular risk factors and comorbidities,6 , 7 which may increase the need for hospitalization, and need for intensive care unit admissions and/or intubation. It may also induce vascular injury that would promote the risk of stroke.8, 9, 10, 11
While prevention and management of COVID-19 is a top global health priority, this should not come at the cost of compromising the treatment and management of other potentially life-threatening diseases.12 The present multicenter, international study has been designed to assess changes in stroke rates, severity, subtypes (ischemic/hemorrhagic), and outcomes before, during, and after the COVID-19 pandemic. The results of this initiative can be used as a monitoring model for public health authorities and policymakers regarding the system's capacity during a global crisis of this magnitude. Ultimately, enhanced simultaneous surveillance of NCD and COVID-19 comorbidity in populations would generate the empirical data needed to better understand the dual-disease burden and to target coordinated care.
Study design
The CASCADE Study (Call to Action: SARS-Cov-2 and Cerebrovascular DisordEr) is a retrospective and prospective international multicenter cohort study comparing the rate, severity and outcomes of stroke before, during, and following the COVID-19 outbreak.
The study aims to create a multicenter stroke registry to: (1) describe changes in stroke clinical care approach (including imaging, stroke-unit care admission, thrombolysis, thrombectomy, neurosurgery availability, and outpatient assistance in different countries during the COVID-19 outbreak); (2) evaluate the incidence of CVD in COVID-19 positive patients, as well as identifying clinical markers predictive of CVD occurrence in COVID-19 positive patients; (3) identify the clinical, neuroimaging and prognostic factors of CVD during the pandemic in comparison to the same period in 2018 and 2019, and subsequently, 2021.
Study population and data collection
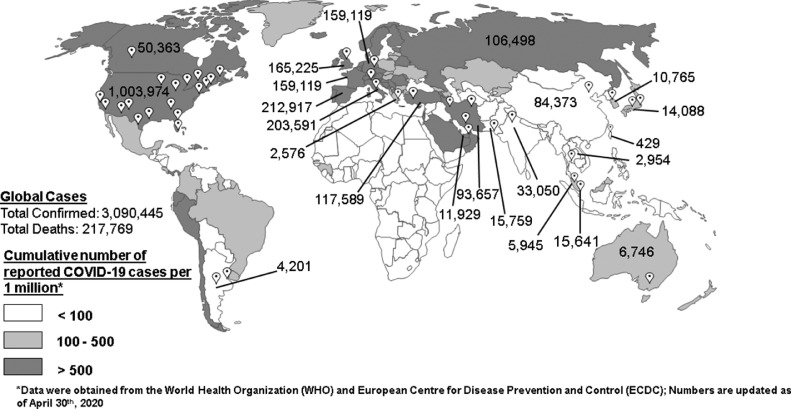
CASCADE is a new initiative, and we foresee continuous collaboration across multiple participating centers worldwide (Fig. 1 ). Neurological and stroke centers, evaluating cerebrovascular disease patients (aged > 18) during the COVID-19 pandemic will be included in the project. For each participating center, the overall types of acute phase stroke therapy utilized, the crude and age-standardized stroke rate, vascular risk factors, stroke severity, and stroke outcomes such as in-hospital mortality will be tracked and compared with the previous corresponding months in 2018 and 2019, and in 2021 or 2022 if the disease progresses through 2021.
Fig. 1.
World map depicting participating centers (white push pins) and their total COVID-19 cases, and total cases in the top 10 afflicted countries. Geographic distribution of the cumulative number of reported COVID-19 cases per 1 million population is illustrated in a color-gradient. Data on COVID-19 cases are updated as of April 30, 2020 (countries with more than 100 cases per one million of their population are highlighted).
Other important data include the date of the first report of COVID-19, the date of the latest quarantine, the date of the end of quarantine, the date and nature of phased social distancing and lock-down restrictions, and the date and nature of relaxation of social distancing and lock-down measures (if available) in each corresponding country/region, according to official local and governmental reports. We will also obtain data regarding COVID-19 from international databases; e.g. the European Centre for Disease Prevention and Control (ECDC),13 and the United Nations Educational, Scientific and Cultural Organization (UNESCO)-COVID-19 Educational Disruption and Response.14 Environmental data, specifically, national air quality, will be obtained through NASA's Giovanni OMI visualization tool.15 We have developed a set of common variables with their definitions to facilitate standardization of data-collection among participating centers (Supplement Table 1). The final database will include data regarding important variables, such as stroke incidence, stroke severity, vascular risk factors, medications, and outcome (e.g. in-hospital mortality and disability).
Stroke will be defined according to the Stroke Council of the American Heart Association/American Stroke Association census definition.16 The severity of stroke will be measured according to the National Institutes of Health Stroke Scale (NIHSS). Stroke disability will be assessed using the modified Rankin Scale (mRS) on admission, at discharge, and longitudinally (30 days and 3 months follow-ups), if available. Depending on the participating centers' registry, we will include all stroke (first-ever-stroke and recurrence) cases, stroke subtypes such as acute ischemic stroke (AIS), intracerebral hemorrhage (ICH), cerebral venous thrombosis, and subarachnoid hemorrhage (SAH). In addition, if available, we will monitor prospectively cases admitted with COVID-19 who develop strokes in hospital or within two weeks after their discharge. We will also identify stroke patients with a laboratory-confirmed COVID-19 diagnosis.17 Further, given the limited availability of COVID-19 laboratory testing in some centers, we will gather data regarding those clinically suspected of having COVID-19 following a stroke. With this approach, we can provide a broader understanding of the larger incidence of both the communicable and non-communicable related disease.
Ethical considerations
Local Ethics Committee approval will be obtained for each participating center. Data sharing will be according to the institutional Ethics Committee's policies and official agreements. We will use anonymized data, secured in protected servers. The confidential data will be saved in an encrypted and secured dataset (e.g., RedCap, http://projectredcap.org/, Vanderbilt University). Anonymized data obtained by each center will be pooled together to be analyzed by the designated data processing center. A subject ID coding will be designed to have a specific designation code for each participating center that is only shared with the data processing center and the corresponding site.
Statistical analysis plans
Crude and standardized hospitalization rates of stroke per month will be calculated using a direct method. We will use a Poisson regression model with a log link to compare the number of stroke hospitalizations during the COVID-19 pandemic, as compared to corresponding months in the previous and, possibly, upcoming years. We will calculate risk ratios [with 95% confidence intervals (CIs)] of the number of stroke hospitalizations within the pandemic period, as compared to the periods before and after COVID-19. We will use Kaplan-Meier survival method within the study period and Cox-regression model to estimate the hazard ratio (HR) with 95% CI of in-hospital mortality. An interrupted time series (ITS) analysis will be used to assess the change in stroke hospitalization rates after COVID-19 in each center. We will compare the proportion of different stroke care managements (e.g. outpatient assistance, craniectomy utilization, discharge setting, supported discharge) between the COVID-19 pandemic and previous periods. These variables will be added as a time-varying confounder in the ITS regression model. Prevalence of stroke subtypes and important neuroimaging features (e.g. cortical vs. subcortical, anterior vs. posterior circulation, stroke size and location, etc.) will be estimated in proportion to total admitted strokes. These variables will also be analyzed in the ITS model as a time-varying confounder. Using an autoregressive integrated moving average (ARIMA) model (according to the Box-Jenkins-Tiao strategy), serial autocorrelations of the hospitalization rates will be assessed. The Q-statistic will test analytically whether the autocorrelation function is different from a white noise process. A lag plot will be created using the sample autocorrelation coefficients, plotted against the corresponding lags. We will calculate the Durbin-Watson (DW) statistic to test the autocorrelation of the errors in the segmented regression models.
Discussion
CASCADE is a new multicenter initiative to assess the impact of the COVID-19 pandemic on stroke occurrence, severity, care, and outcomes. The results of this initiative will have multiple clinical and public health implications. Similar to other major global crises (e.g. WWII), critical periods of time surrounding COVID-19 can be categorized as periods before, during, and after the pandemic. Since the 1918 flu pandemic,18 the world has not experienced a pandemic of this magnitude. Therefore, the pooling of epidemiological, clinical, and laboratory data on the COVID-19 pandemic is imperative to the success of national and global public health initiatives directed towards this novel virus, and future national and international health crises. In addition to the COVID-19 viral epidemiology, a successful model should also include other major diseases, including NCDs, identifying the risk for vulnerable populations (i.e. those with prior comorbidities), and prioritizing preventive measures through strengthening public protections and improving access to health services. Combining these preventive measures for COVID-19 with measures surrounding life-threatening NCD, as well as focusing on screening and treatment programs during and after the pandemic is of major importance, and one of the priorities of the CASCADE study.
COVID-19 can affect stroke rate and outcomes through various direct and indirect pathways. Among 20,812 patients confirmed to have COVID-19 in China as of February 11, 2020, 2683 patients (12.8%) had hypertension, and 873 (4.2%) had cardiovascular disease.6 Similarly, in Italy, in a subset of 355 patients who died of COVID-19, 352 were found to have comorbidities, including diabetes in 35.5%, ischemic heart disease in 30%, atrial fibrillation in 24.5%, and stroke in 9.6% of patients,7 The systemic inflammatory response from SARS-Cov-2 may trigger the rupture or erosion of atherosclerotic plaque,8 , 11 destabilize previous stroke or coronary artery disease,9 and may be associated with acute cardiac injury such as myocarditis.8 , 10
SARS-Cov-2 may also have a peripheral vascular detrimental effect due to its ability to act on Angiotensin-Converting Enzyme 2 (ACE2) receptors, which play a major role in regulating cardiovascular function.11 , 19 Furthermore, it also has a potential negative neurotropic effect due to the expression of ACE2 receptors on neurons and cerebral endothelial cells. This facilitates the rupture of the blood-brain barrier and cerebral arteries,20 which suggests the development of more severe and devastating strokes21 in the presence of COVID-19. Finally, stroke-induced suppression of the innate and adaptive immune system is well described22 and may impact susceptibility to, and severity of, SARS-Cov-2 infection in the acute phase of stroke, ultimately impacting clinical outcomes.
Given that COVID-19 is closely related to SARS,23 we anticipate similarities in cardiovascular presentations, such as arrhythmias. Specifically, in a cohort study (n=121), tachycardia (71.9%) and bradycardia (14.9%) were reported in patients with SARS.24 In addition, hypoxemia resulting from severe respiratory illness in COVID-19, particularly in those with underlying CVDs such as stroke and heart failure, may also lead to atrial fibrillation7 and increased mortality rate.25 Finally, COVID-19 has been associated with abnormalities in the coagulation cascade.26
Similar to many other global health crises, COVID-19 may also indirectly affect the rate and severity of CVDs. Prioritization of medical resources towards COVID-19 precautions and assistance, as seen in many countries, may result in a reduction of resources for other potentially fatal diseases.27 This is important as a healthcare system collapse under significant burden from COVID-19 may, directly and indirectly, affect all other major health conditions (e.g., stroke), resulting in higher mortality and morbidity among both infected and non-infected patients. It is necessary to note that most countries heavily impacted by COVID-19 are those that are typically more impacted by the presence of concurrent NCD's, rather than communicable diseases in general (paper in preparation). As such, it is possible that the lack of prior exposure to a pandemic of this magnitude could have resulted in a lack of preparedness in terms of preventive and emergency management plans.
COVID-19 may also indirectly affect CVDs through heightened emotional responses, such as stress.28 Specifically, within the 3 weeks following the 2011 Great East Japan Earthquake and Tsunami disaster, there was a significant increase in the number of cardiovascular events, including acute coronary syndrome or congestive heart failure, without any change in the rate of stroke.29 A study observed a French population over 4 Football World Cup events, a time period that is considered stressful for the audience viewing the matches.30 Interestingly, the study found a decrease in the rate of stroke, potentially due to a state of the euphoria associated with viewing the football game.30 A similar study in Germany during the 2006 World Cup found that the incidence of cardiac emergencies increased significantly on days that the German team was playing.31 The difference in the incidence of CVDs during these national disasters and events may be explained by the heterogeneous nature of CVD occurrences.29 Further, due to the implementation of mitigation strategies that have resulted in physical isolation of large subsets of the population, COVID-19 has indirectly, and beneficially, impacted air quality and pollution. Recent observational satellite images obtained from NASA depicted improvements in air quality over China during periods of quarantine in February 2020, a time which had seen nation-wide business closures and prohibited vehicular transportation. Specifically, up to 30% reductions in NO2 emissions, and up to 40% reductions in CO2 emissions have been observed.32 , 33 While similar reductions are normally seen during Chinese Lunar New Year due to business closures, the emissions typically increase once again in the following weeks.34 However, due to the quarantine, the number of festivities and outdoor events have been reduced. Similar trends have been observed in states that have implemented “shelter-in-place” orders within the United States.35 As of March 9, 2020, Italy, which had also implemented a nation-wide lockdown, has also seen improvements in air quality. Specifically, from March 14 until March 25, 2020, Rome had seen up to a 35% decrease in NO2 as compared to the same period in 2019.36 This is particularly important as air quality has been shown to have effects on stroke and other NCD's.37 Conversely, it is possible that prolonged homestay due to the quarantine may lead to increased sedentary behaviors, physical inactivity, and unhealthy dietary intake habits, leading to increasing or worsening chronic health conditions.38
The CASCADE study is feasible and can provide scientific answers for many of the aforementioned hypotheses. The data will be gathered in a challenging, resource-limited situation during the COVID-19 pandemic, which is having an enormous impact on healthcare and the economy in many countries. The proposed initiative can provide a wide range of information, from individual-based health related issues to changes in health policy in high, middle, and low-income countries. As well, the CASCADE registry can also be combined with other international stroke registries to expand the knowledge in this field. In addition, our data will reflect the real-world and systemic healthcare challenges currently facing many centers around the world. Data will be gathered based on available stroke information at each center. It is possible that those with transient ischemic attacks (TIAs) and minor strokes may not present to Emergency Rooms due to fear of contracting COVID-19 from the hospital, and/or social distancing measures. However, we would ultimately not expect to see a significant decrease in the rate of severe and fatal stroke presentations. Therefore, any changes in the rate, severity, and outcome of stroke within the study period will likely reflect the direct and indirect consequences of COVID-19.
Conclusion
The worldwide convergence of NCD and communicable infectious diseases has the potential to overstretch global health systems. The CASCADE initiative can potentially shed light on challenges related to stroke care as a major NCD within this context. Identifying and analyzing the current health-related data during the COVID-19 period will help to optimize resource allocation, conduct proper assessments to objectively identify the nature of the problem, implement strategies to address the issue in the most effective and efficient way, and better prepare for future viral outbreaks.
Acknowledgments
Acknowledgment
We wanted to acknowledge Dr. Matias Alet, MD, representing Stroke Center, Vascular Neurology, FLENI, Buenos Aires, Argentina, who joined the CASCADE initiative following submission.
Declaration of Competing Interest
Authors and investigators have no conflict of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jstrokecerebrovasdis.2020.104938.
Appendix. Supplementary materials
References
- 1.World Health Organization (WHO). Pneumonia of unknown cause – China2020. https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/. Accessed 2020 Jan 05.
- 2.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coronavirus disease 2019 (COVID-19) World Health Organization; 2020. Situation Report – 101 [Internet]https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200430-sitrep-101-covid-19.pdf?sfvrsn=2ba4e093_2 [cited April 30, 2020]. Available from. [Google Scholar]
- 4.Li B, Yang J, Zhao F. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020 doi: 10.1007/s00392-020-01626-9. Epub 2020 March 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coronavirus Disease 2019 (COVID-19), Cases and Latest Updates [Internet]. Centers for Disease Control and Prevention. 2020[cited March 11, 2020]. Available from:https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/world-map.html.
- 6.Novel Coronavirus Pneumonia Emergency Response Epidemiology T . Vol. 41. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi; 2020. pp. 145–151. ([The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]). [DOI] [PubMed] [Google Scholar]
- 7.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. Epub 2020 March 23. [DOI] [PubMed] [Google Scholar]
- 8.Madjid M, Safavi-Naeini P, Solomon SD. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. Epub 2020 March 27. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Hu B, Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Z, Shi L, Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. Epub 2020 March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y-Y, Ma Y-T, Zhang J-Y. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020 doi: 10.1038/s41569-020-0360-5. Epub 2020 March 05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuurbier S, Vanacker P.Tomorrow's care starts today: European Stroke Organization (ESO); 2020. https://eso-stroke.org/eso/tomorrows-care-starts-today/Accessed April 14, 2020.
- 13.Coronavirus Pandemic (COVID-19)[Internet]. Our World in Data. 2020. Available from: https://ourworldindata.org/coronavirus.
- 14.United Nations Educational Scientific and Cultural Organization (UNESCO). COVID-19 educational disruption and response: UNESCO; 2020. https://en.unesco.org/covid19/educationresponse. Accessed 2020 April 17.
- 15.Atmospheric Composition, Water & Energy Cycles and Climate Variability [Internet]. NASA's Goddard earth sciences data and information services center (GES DISC). 2020 [cited March 31, 2020]. Available from:https://giovanni.gsfc.nasa.gov/giovanni/#service=TmAvMp& starttime=&endtime=&variableFacets=dataFieldDiscipline%3AAtmospheric%20Chemistry %3BdataFieldMeasurement%3ANO2%3B.
- 16.Sacco RL, Kasner SE, Broderick JP. An Updated Definition of Stroke for the 21st Century. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Global surveillance for COVID-19 caused by human infection with COVID-19 virus: interim guidance2020. Geneva: World Health Organization, 2020Contract No.: WHO/2019-nCoV/SurveillanceGuidance/2020.6.
- 18.Morens DM, Taubenberger JK, Harvey HA. The 1918 influenza pandemic: lessons for 2009 and the future. Crit Care Med. 2010;38:e10–e20. doi: 10.1097/CCM.0b013e3181ceb25b. 00003246-201004001-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann M, Kleine-Weber H, Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. Epub 2020 March 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palasca O, Santos A, Stolte C. TISSUES 2.0: an integrative web resource on mammalian tissue expression. Database. 2018:2018. doi: 10.1093/database/bay003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Xu X, Chen Z. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.03.031. Epub 2020 March 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malone K, Amu S, Moore AC. The immune system and stroke: from current targets to future therapy. Immunol Cell Biol. 2019;97:5–16. doi: 10.1111/imcb.12191. [DOI] [PubMed] [Google Scholar]
- 23.Wrapp D, Wang N, Corbett KS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. 32075877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu CM, Wong RSM, Wu EB. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J. 2006;82:140–144. doi: 10.1136/pgmj.2005.037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen N, Zhou M, Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med202020200188. Epub 2020 March 16. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed]
- 27.Bersano A, Pantoni L. On being a neurologist in Italy at the time of the COVID-19 outbreak. Neurology. 2020 doi: 10.1212/WNL.0000000000009508. Epub 2020 Apr 10. [DOI] [PubMed] [Google Scholar]
- 28.Wang C, Pan R, Wan X. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in china. Int J Environ Res Public Health. 2020;17:1729. doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nozaki E, Nakamura A, Abe A. Occurrence of cardiovascular events after the 2011 Great East Japan Earthquake and tsunami disaster. International heart journal. 2013;54:247–253. doi: 10.1536/ihj.54.247. 24097211. [DOI] [PubMed] [Google Scholar]
- 30.Aboa-Eboulé C, Béjot Y, Cottenet J. The impact of world and european football cups on stroke in the population of Dijon, France: a longitudinal study from 1986 to 2006. J Stroke Cerebrovasc Dis. 2014;23:e229–e235. doi: 10.1016/j.jstrokecerebrovasdis.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Wilbert-Lampen U, Leistner D, Greven S. Cardiovascular events during World Cup Soccer. N Eng J Med. 2008;358:475–483. doi: 10.1056/NEJMoa0707427. 18234752. [DOI] [PubMed] [Google Scholar]
- 32.Myllyvirta L.Analysis: coronavirus has temporarily reduced China's CO2 emissions by a quarter: CarbonBrief; 2020. https://www.carbonbrief.org/analysis-coronavirus-has-temporarily-reduced-chinas-co2-emissions-by-a-quarter. Accessed Ferbuary 19, 2020.
- 33.Patel K.Airborne nitrogen dioxide plummets over China: earth observatory; 2020. https://earthobservatory.nasa.gov/images/146362/airborne-nitrogen-dioxide-plummets-over-china. Accessed Febrauary 25, 2020.
- 34.Feng J, Sun P, Hu X. The chemical composition and sources of PM2.5 during the 2009 Chinese New Year's holiday in Shanghai. Atmos Res. 2012;118:435–444. doi: 10.1016/j.atmosres.2012.08.012. [DOI] [Google Scholar]
- 35.Holcombe M, O'Key S.Satellite images show less pollution over the US as coronavirus shuts down public places: Cable News Network (CNN); 2020. https://edition.cnn.com/2020/03/23/health/us-pollution-satellite-coronavirus-scn-trnd/index.html. Accessed 26 March 2020.
- 36.Morgan S.Air pollution plummets by up to 50% as virus curbs traffic, new data reveals: EurActiv; 2020. https://www.euractiv.com/section/transport/news/air-pollution-plummets-as-virus-curbs-traffic-new-data-reveals/. Accessed 25 March 2020.
- 37.Béjot Y, Reis J, Giroud M. A review of epidemiological research on stroke and dementia and exposure to air pollution. Int J Stroke. 2018;13:687–695. doi: 10.1177/1747493018772800. 29699457. [DOI] [PubMed] [Google Scholar]
- 38.Owen N, Sparling PB, Healy GN. Sedentary behavior: emerging evidence for a new health risk. Mayo Clin Proc. 2010;85:1138–1141. doi: 10.4065/mcp.2010.0444. 21123641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.