Highlights
-
•
IVIG, combined with moderate-dose of corticosteroids, might improve patient outcomes.
-
•
The use of corticosteroids might accelerate recovery from COVID-19.
-
•
No controlled clinical trials exist on the use of corticosteroids for COVID-19.
-
•
IL6 correlates with severity, criticality, viral load, and prognosis of COVID-19.
-
•
Tocilizumab, an anti-IL6, can confer benefit in patients with COVID-19 and high IL6.
Keywords: COVID-19, Treatment, Immunoglobulin, Targeted therapy, Corticosteroids, Interleukin 6
Abstract
The novel coronavirus, SARS-CoV2, can cause a potentially fatal disease, COVID-19, in humans. Here, we will provide an overview of therapeutic options for COVID-19. Plasma from patients recovered from COVID-19 that contains antibodies against SARS-CoV2 has shown promising results in patients with severe COVID-19. Also, IVIG, combined with moderate-dose of corticosteroids, might improve patient outcomes. Evidence links COVID-19 to variable degrees of inflammation. Studies show that the use of corticosteroids might accelerate recovery from COVID-19. There are, however, no controlled clinical trials that show whether the use of corticosteroids can reduce COVID-19-related death. Also, the pro-inflammatory cytokine IL6 is the best-documented cytokine in COVID-19 correlated with severity, criticality, viral load, and prognosis of patients with COVID-19. Tocilizumab, a monoclonal antibody against IL6, could confer clinical benefit in patients with high IL6 levels. Essential elements that process SARS-CoV2 cell entry and specific characteristics that allow SARS-CoV2 to escape the immune system have the potential as targets for COVID-19 therapy.
1. Introduction
Several generations have been exposed to COVID-19 under different conditions of life at varying locations around the world. As long as the COVID-19 continues to spread, its power of genome modification would probably be increased. What concerns us more is that the 2019-nCoV, by the process of modification of genome structure, might become more and more fitted to humans to profoundly affect those who have already escaped – children and young people without a pre-existing condition. No one can tell how much time it takes to reach a new level of perfection, and that the development of vaccines for active immunization is a long process.
Moreover, most of the best available anti-viral agents are not helpful in the treatment of COVID-19. A randomized controlled trial of 199 hospitalized adults with confirmed COVID-19 demonstrated no actual difference between patients who received lopinavir-ritonavir and patients who received standard care alone in clinical improvement, death rate, and positive virus test rate at day 28 [1]. Here we review intermediate indicators of the pathogenesis of COVID-19 that have the potential of being considered for the treatment of COVID-19.
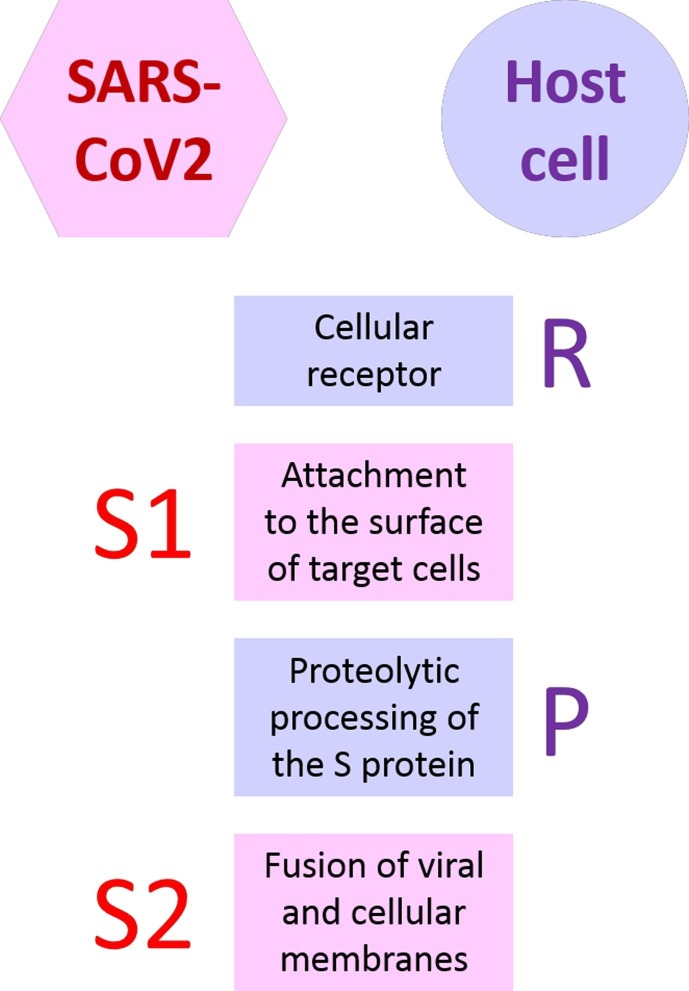
2. SARS-CoV2 cell entry is not complex
Fig. 1 is a schematic illustration of the minimum proteins required to mediate the SARS-CoV2 cell entry. The trimeric, transmembrane spike (S) glycoprotein of the virus, SARS-2-S, includes two main functional subunits: S1 and S2. The former, in turn, consists of the four core domains, S1A, S1B, S1C, and S1D, that contribute to the attachment to the surface receptor of target cells. Then, the latter coordinates the fusion of viral and cellular membranes. Receptor binding activates proteases that can carry out proteolytic cleavage of the S protein. In coronaviruses, cleavage occurs at two sites: the S1/S2 junction and at the S2′, a region close to the viral fusion peptide [2]. Proteolytic cleavage of the S protein causes conformational changes so that they cannot revert to the original structure and profound enough to prime the S2 subunit for the fusion of viral and cellular membranes.
Fig. 1.
Proteins required for the SARS-CoV2-cell entry.
3. Certain characteristics of SARS-CoV2
3.1. SARS-CoV2 has acquired an S glycoprotein that highly underwent genetic variation and glycosylation
3.1.1. Polybasic cleavage site
As evidenced by sequence analysis, there is a residue insertion formed of four amino acids (12 nucleotides) at the boundary between S1 and S2 subunits of the SARS-CoV 2 S. It defines a polybasic furin cleavage site of RRAR for the human SARS-CoV 2 that was absent in human SARS-CoV, bat SARS-like CoVs, and pangolin SARS-like CoV while might be present in other species [3]. After the introduction of mutation to the residue insertion and furin cleavage site, the S1/S2 cleavage of the SARS-CoV 2 S did not longer take place. However, the SARS-CoV 2 S entry raised for VeroE6 cells and remained high in BHK cells that express human ACE. Therefore, it seems that SARS-CoV2 transmissibility does not depend on the S1/S2 cleavage.
A polybasic cleavage site explains a virus that is highly-pathogenic for humans while it is low-pathogenic for other species. For example, using reverse genetic tools, an avian paramyxovirus type 7 (APMV-7) was developed by mutating the fusion (F) protein cleavage site [4]. The constructed APMV-7 showed furin cleavage and increased replication and syncytium formation in cell cultures. However, chicken exposed to the virus did not exhibit infection.
3.1.2. Glycosylation
Glycosylation and its related products, i.e., glycans, introduce changes to the viral envelope that make the virus fitted for interaction with the host cell membrane [5]. Generally, glycans are oligosaccharides linked to the dense decoration of the spike glycoprotein. In particular, these oligosaccharides have shown to influence the folding of the S protein and proteolytic process so that they facilitate the virus cell entry. Moreover, a virus with the glycosylated glycoprotein gains an extra feature for an escape from the immune responses. Therefore, glycans are a good target for vaccine design.
Two main types of glycans are N-linked and O-linked glycans. Both are released from glycoproteins. Whereas enzymes fulfill the construction of N-glycans, chemical methods perform the release of O-glycans. N-glycans are linked to the amino acid asparagine (Asn) residues (Asn-any amino acids except for proline- Ser or Thr) utilizing an N-glycosidic bond, mostly N-acetylglucosamine. O-glycans are attached to the amino acid serine (Ser) and threonine (Thr) residues by the addition of an N-acetyl galactosamine (GalNAc). For example, N-glycans exist in Hendra virus, SARS-CoV, influenza virus, hepatitis virus, HIV-1, and West Nile virus [6], and O-glycans have occurred in the Ebola virus.
The 2019-nCoV S protein includes 13 and 9N-linked glycosylation sequons in the S1 and S2 subunit, respectively [3]. All of these have previously occurred in the SARS-CoV S glycoprotein, except for four-linked glycosylation sequons in the S1. Also, due to the existence of an amino acid proline in the polybasic cleavage site, which makes the inserted sequence PRRA, there are three O-linked glycans introduced to the 2019-nCoV RBD residues S673, T678, and S686 [7].
3.1.3. SARS-CoV2 receptor binding domain contains six amino acids providing favorable positions for binding to human ACE2
When compared to SARS-CoV SB, the motif binding the human ACE2 to the SARS-CoV 2 SB showed a relatively higher binding affinity for human ACE2 as indicated in smaller equilibrium dissociation constant (2.9 nM vs. 7.7 nM) [3]. Structural analysis has demonstrated that fourteen positions critically take part in the receptor-binding domain of the SARS-CoV SB that contain eight conserved (T402, Y436, Y440, N473, Y475, T486, G488, and Y491) positions and six semi-conserved (R426 to N448, Y442 to L464, L472 to F495, N479 to Q502, Y484 to Q507, and T487 to N510) substitutions with respect to the SARS-CoV 2 SB.
3.1.4. SARS-CoV2 receptor binding domain contains cyclic regions that can make interaction with cell-surface GRP78
Pep42 is a cyclic oligopeptide that, with its hydrophobic character, can selectively interact with cell surface glucose‐regulated protein 78 (GRP78), a member of 70 kDa heat shock proteins. GRP78, also known as BiP or HSPA5, under endoplasmic reticulum stress, can be translocated from the endoplasmic reticulum to the membrane and helps to maintain cellular integrity under physiologic and pathological stress. It critically contributes to various functions ranging from protein folding, transportation, and degradation to cell-signaling, proliferation, survival, apoptosis, inflammation, and immunity. The expression of GRP78 decreases with age.
On the 2019-nCoV spike protein, 13 disulfide bonds are corresponding to 13 different cyclic regions thought to be similar to the cyclic form of Pep42 [8]. Among these, four regions I-IV take place in the outer surface of a putative receptor-binding domain (RBD) on the viral spike., including C361, C379, C391, and C480. These regions share sequence similarity with Pep42, ranging from 15.38% to 46.15%. However, only one of them, i.e., region IV (GRAVY = 0.08), is a hydrophobic region, like Pep42 (GRAVY = 1.1). Structural models evaluate the energy contribution for region IV as a part of region III to the GRP78 to be about (−9.8 out of −14.0 kcal/mol), and the docking platform proposes region IV as the best region binding to GRP78. The region IV can be linked to the substrate-binding domain β (SBDB) of GRP78 using five H-bonds (through P479, N481, E484, and N487) and four hydrophobic interactions (through T478, E484, and F486).
4. Passive immunization
There are two main ways to induce protection against infections: active and passive immunization. Active immunization comes to exist when the body's own immune system can produce antibodies actively following exposure to a viral antigen. On the contrary, there is a passive working mode of the immune system that would appear following the transfer of antibodies that act directly to neutralize viral infectivity.
4.1. Serum therapy
4.1.1. Convalescent plasma from patients recovered from COVID-19
4.1.1.1. Hypothesis: Plasma from patients recovered from COVID-19 contain antibodies against 2019-nCoV
A look at the history of viral outbreaks offers convalescent plasma as the only remedy to avoid further fatalities. The most recent examples include the pandemic of influenza A H1N1 (H1N1pdm09) in virus infection 2009, the Western African Ebola virus epidemic in 2014, and the outbreak of MERS-CoV in 2015 [9]. Meta-analysis studies have shown a reduced risk of mortality in patients with SARS-CoV and influenza receiving convalescent plasma [10].
4.1.1.2. Rationale: Convalescent plasma from patients recovered from COVID-19 can treat patients with severe COVID-19
A pilot study [11] recently has investigated the safety and effect of convalescent plasma that contain antibody levels higher than 1:640 combined with regular anti-viral agents and standard supportive care on clinical outcomes of ten patients with severe COVID-19. The study showed clinical improvement of all the ten patients accompanied by an increase in lymphocyte count and a decrease in CRP. Following transfusion of convalescent plasma, all the seven patients who were SARS-CoV2 RNA positive before transfusion of convalescent plasma turned SARS-CoV2 negative. There were no control groups receiving convalescent plasma alone or standard therapy without convalescent therapy to evaluate the main effect of convalescent plasma.
4.1.2. Serum from bats with SARS-like CoV
4.1.2.1. Hypothesis: whole-genome sequencing and phylogenetic analysis shed light on the origin of the 2019-nCoV virus – It has been probably introduced from bats to men
Genome sequencing of a fecal bat sample, Rp3, could detect an isolate of coronaviruses, which was almost identical to the causative agent of the SARS-CoV outbreak of 2002–2003. Hence, it attained the name SARS-like coronavirus isolate Rp3 (SL-CoV Rp3) [12]. The bronchoalveolar lavage fluid (BALF) of a patient with SARS contained the 2019-nCoV. RNA sequencing could reveal about 90% similarity in nucleotides of the novel coronavirus and of SARS-like coronavirus that had previously related to bats [13], [14]. In particular, the S protein of the 2019-nCoV has a high sequence identity of 80–98% with the S protein of bat SARS-like CoVs, such as SARSr-CoV ZXC21 S, ZC45 S, and RaTG13 [3]. Moreover, in phylogenomic trees, branches for the 2019-nCoV are of greater length than those for the 2003 SARS-CoV, and therefore more favorable to bats.
4.1.2.2. Rationale: Bat serum is not able to efficiently neutralize SARS-CoV
The range of bats, belonging to the genus Rhinolophus (horseshoe bats) and the family Rhinolophidae, produce the SARS-CoV antibody [12]. Polymerase chain reaction (PCR) will confirm the presence of SARS-CoV nucleocapsid (N) and polymerase (P) proteins in fecal samples if an individual bat being seropositive for SARS-CoV [12]. There is a significant degree of resemblance of greater than 90% in the nucleotide sequence of the viral genomes between SL-CoV Rp3 [12] and the Tor2 strain of SARS-CoV – which was isolated in Toronto [15]. The differences in the genome sequences of SARS-CoV in the two species occur merely in the S gene – which encodes the S1 domain of the coronavirus spike protein and contains regions with high mutation rates [12]. The coronaviruses commonly possess five open reading frames (ORF) that correlate with the production of the replicase polyprotein (P), the spike (S), envelope (E), and membrane (M) glycoproteins and the nucleocapsid (N) protein. The human SARS-CoV Tor2 and bat SL-CoV Rp3 strains remain more than 90% identical at the proteins P, E, M, and N. the protein S consists of two main domains: 1) the S1 domain conveys the role of receptor binding and 2) the S2 domain assumes the role of the fusion of viral and host-cell membranes. In particular, the human SARS-CoV Tor2 strain shows a noticeable degree of difference in the S1 domain from the bat SL-CoV Rp3 strain. This diversity would suffice to produce functional differences between the species, and is an apparent reason why bat sera having high levels of cross-reactive antibodies not acted efficiently to neutralize SARS-CoV.
4.1.3. Serum from convalescent SARS patients
4.1.3.1. Hypothesis: SARS-CoV and SARS-CoV2 are ideally similar in the structure and the cell entry receptor and protease
SARS-CoV and SARS-CoV2 share absolutely the same cleavage junctions, almost the same sequence (96%) of their main protease, a high degree (76%) of similarity in the amino acid sequence of their S protein, a similar S2′ cleavage site, a similar spectrum of cells they can enter, and the similarity of the most residues essential for binding ACE2 [16], [17], [18]. Also, both of them utilize the same domain of S1B to interact with the ACE2 receptor. However, they differ in proteolytic processing to some degree. Study [16] of the human embryonic kidney (HEK) cell line, 293 T, has shown that a signal for the S2 subunit is present in cells inoculated with SARS-2-S, but not in cells inoculated with SARS-S.
Two main proteases for both SARS-S and SARS-2-S are endosomal cysteine proteases cathepsin B and L (CatB/L) and the transmembrane protease, serine 2TMPRSS2 [16]. In 293 T cells lacking 2TMPRSS2, blocking CatB/L activity through increasing the endosomal pH by ammonium chloride could significantly limit the entry of both SARS-S and SARS-2-S. In TMPRSS2 + Caco-2 cells, the effect of ammonium chloride existed to a lesser extent. A combination of camostat mesylate, a blocker of TMPRSS2, and E-64d, an inhibitor of CatB/L, yielded the complete inhibition of SARS-2-S entry in TMPRSS2 + Caco-2 cells. In both the human lung cancer cell line Calu-3 and the primary human lung cells, there was a reduction of the entry of both SARS-S and SARS-2-S by camostat mesylate, indicating that SARS-S and SARS-2-S partially require TMPRSS2 for a lung infection.
4.1.3.2. Rational: Serum from convalescent SARS patients is able to neutralize SARS-CoV2 efficiently
Antiserum that contains antibodies against human ACE2 could hinder the entry of both SARS-S and SARS-2-S pseudotypes while not affected the entry of VSV-G and MERS-S pseudotypes. It supports the notion that SARS-S and SARS-2-S utilize the same primary entry receptor, i.e., ACE2, which is different from the primary receptors VSV-G and MERS-S engage for cell entry that is LDLR and DPP4, respectively.
Sera from three convalescent SARS patients reduced the SARS-S entry and, to e lesser degree, the SARS-2-S entry. The patient serum effect was in a dose-dependent manner [16].
4.1.4. Serum from rabbits immunized with SARS
4.1.4.1. Hypotheses: Serum from rabbits immunized with SARS is more effective than serum from convalescent SARS patients
Paraoxonases (PON) are mammalian enzymes associated with anti-oxidant and anti-inflammatory effects [19]. Rabbit PON differs from human PON in terms of more activity and more stability under the circumstances [20].
4.1.4.2. Rational: Serum from rabbits immunized with the S1 subunit of SARS-S is able to neutralize SARS-CoV2 very efficiently
Sera from rabbits immunized with the S1 subunit of SARS-S could effectively reduce the entry of both SARS-S and SARS-2-S [16]. When compared with patient serum, rabbit serum revealed to us higher efficiency in inhibition of SARS-2-S entry at the same concentration.
4.2. Intravenous immunoglobulins (IVIG)
4.2.1. Hypothesis: IVIG contains a large pool of human antibodies
IVIG is an immunomodulatory treatment currently useful for a variety of human diseases that share an idiopathic origin, ranging from autoimmune disorders to primary antibody deficiencies. Also, IVIG has shown promising results in case of severe (such as sepsis, Parvovirus B19 infection, West Nile virus encephalitis, HIV, Clostridium difficile infections, Mycobacterium avium, Mycobacterium tuberculosis, and Nocardia infections) and recurrent infections in primary antibodies deficiencies [21].
Most patients develop antibodies against the NP and RBD of 2019-nCoV during the second week after infection onset [22]. Analysis of serum samples collected 14 or more days after symptom onset revealed detection of IgG and IgM antibodies against NP in 94% and 88% and RBD in 100% and 94% among patients with COVID19. Studies consistently show that increased immunoglobulin levels accompany the transition from early to late course of COVID19. It poses the possibility that IVIG therapy might help to accelerate recovery from COVID-19.
4.2.2. Rationale: IVIG might help to improve the outcome of patients with COVID-19
The study [23] included ten patients with COVID-19 who demonstrated worsening symptoms, e.g., decreased lymphocyte count and decreased PaO2/FIO2 ratio and oxygen saturation, following treatment with a short-term moderate-dose corticosteroid (methylprednisolone 80 mg/d) plus immunoglobulin (10 g/d). After switching to the double dose of 1600 mg/d methylprednisolone plus 20 g/d immunoglobulin, all of the patients improved in the clinical, laboratory, and paraclinical outcomes.
Passive immunization protects against disease, and so it should be administered as early as possible when the patient is diagnosed. Studies show that the viral RNA of 2019-nCoV reaches its peak during the first week and then gradually decreases and that IgG and IgM begin to rise from the 10th day so that most patients have anti-viral antibodies by the 14th day.
5. Recombinant type I IFN
5.1. Hypothesis: IFNs play a role in anti-viral immunity
Type I IFNs play a primary role in the inhibition of viral replication through the coordination of anti-viral immune responses and modulation of inflammatory responses [24].
5.2. Rational: Recombinant type I IFN can effectively inhibit SARS-CoV2 replication
In 24–48 h after infection, SARS-CoV2 replication reaches titer that causes the cytopathic effect in Vero E6 cells, which express ACE2 and thus are susceptible to SARS-CoV2 infection [25]. It is similar to the viral replication kinetics for SARS-CoV [25]. Pretreatment with recombinant type I IFN could effectively inhibit SARS-CoV2 replication at both 24 and 48 h after infection [25]. For SARS-CoV, such an effect was present at 24 h but absent at 48 h after infection [25]. The difference between SARS-CoV2 and SARS-CoV in response to type I IFN treatment may be due to differences in structural proteins of these viruses, including NSP3, ORF3b, and ORF6 [25].
6. Development of a human monoclonal antibody (mAb)
6.1. Targeting the S protein
6.1.1. Hypothesis: A mAb against the binding domain of the virus can inhibit SARS-CoV2 infection
Recently, hybridoma supernatants containing antibody repertoires from immunized transgenic mice that express the human immunoglobulin heavy and light chains and rat origin immunoglobulin constant regions were used to detect antibodies that can cross neutralize SARS-S and SARS-2-S [26]. One chimeric 47D11 H2L2 antibody displayed such a cross-neutralizing activity, decreased syncytia formation induced by SARS-S and SARS2-S, and could protect VeroE6 cells against SARS-S and SARS2-S pseudotyped virus [26]. It may lie in its similar affinities for interacting with the same domain of the S1 subunit, i.e., S1B, of each SARS-S and SARS-2-S.
6.1.2. Rational: The chimeric 47D11 H2L2 might not be effective in human lung cells as it is in vitro
47D11 carried a higher affinity for interacting with the S2 subunit of SARS-S than that of SARS-2-S. It is important that for both SARS-S and SARS-2-S, the binding of the 47D11 antibody to the target – the S1B domain – does not block the binding of S1B and S2 to ACE2 receptor [26]. By contrast, neutralizing antibodies that specifically target SARS-S could compete with S1B and S2 for binding to ACE2.
6.2. Targeting pro-inflammatory cytokines
6.2.1. Hypothesis: A mAb against IL6 can attenuate hyper inflammation
Tocilizumab, also known as atlizumab, is a humanized anti-human IL6 receptor antibody approved by FDA for several inflammatory and autoimmune diseases severe, such as cytokine release syndrome, rheumatoid arthritis, giant cell arteritis, polyarticular juvenile idiopathic arthritis, and systematic juvenile idiopathic arthritis. It is safe and effective for both adults and children two years of age and older.
6.2.2. Rationale: Tocilizumab can treat lung injury in patients with critical and severe COVID-19
In the study [27], 21 patients with COVID-19 whose condition was severe or critical received one or two doses of Tocilizumab plus standard therapy. Patients who had a mean IL6 level of more than 100 pg/ml before tocilizumab treatment showed improvement in clinical symptoms and peripheral oxygen saturation and normalization for lymphocyte proportion and CRP levels. Also, lung lesion opacity was absorbed in 90% of patients. Neither serious adverse effects nor deaths occurred with tocilizumab treatment.
There are ongoing clinical trials for tocilizumab treatment in patients with moderate and severe COVID-19. Currently, the use of Tocilizumab is recommended for patients with COVID-19 who have warning signs of hyper inflammation, as can be measured by IL6, ferritin, platelet counts, inflammatory markers, and H score [28].
7. Corticosteroids
7.1. Hypothesis: Corticosteroids can modulate inflammation
Corticosteroids are commonly used for modulation of a variety of inflammatory conditions. In addition to a daily regimen, they can be used in the form of pulse therapy to treat flares of autoimmune diseases. However, caution in the use of corticosteroids is needed due to the potential serious side effects associated with corticosteroid drugs and that corticosteroids generally suppress the immune system. The latter means that corticosteroids modulate hyper inflammation and, on the other hand, inhibit immune responses that are vital for the host defense against the virus [29].
7.2. Rationale: Corticosteroids might help accelerate recovery from COVID-19
The study [30] investigated the effect of inhaled corticosteroids ciclesonide, cortisone, prednisolone, dexamethasone, and fluticasone on the replication of the MERS-CoV. Among the four compounds, the only ciclesonide was capable of inhibiting viral replication. Also, ciclesonide induced a significant inhibition of viral replication of other human coronaviruses, such as HCoV-229E and SARS-CoV, and another positive-strand RNA virus, rubella virus, while not affect the viral replication of negative-strand RNA viruses, e.g., influenza and respiratory syncytial virus. For the MERS-CoV, a nonstructural protein 15 (NSP15) appeared to act as the target of ciclesonide. An amino acid substitution in the NSP15 conferred resistance of the mutated MERS-CoV to ciclesonide. Mometasone could help deal effectively with the mutated MERS-CoV. For the SARS-CoV2, all three ciclesonide, mometasone, and lopinavir were able to inhibit viral replication to a similar degree. Interestingly, their effect was more noticeable than serine protease inhibitors, e.g., nafamostat and camostat in cells that Vero cells that express TMPRSS2. It indicates the tendency of the SARS-CoV2 to enter the cell through the cathepsin/endosomal pathway rather than through the TMPRSS2/cell surface pathway.
The study [31] included 46 patients with severe COVID-19, of these 26 patients received methylprednisolone (1–2 mg/kg/d for 5–7 days), and 20 patients received standard therapy without methylprednisolone. The first group achieved faster improvement in clinical symptoms (fever and peripheral oxygen saturation) and lung lesions detected by CT imaging. However, two deaths occurred in the first group and one death in the second group. Moreover, the two groups did not differ in laboratory parameters, including WBC, lymphocyte count, monocyte count, and cytokines (IL-2, IL-4, IL-6, and IL-10) six days after treatment.
There is a report of the patient with COVID-19 treated with methylprednisolone since day 8 of the disease course. However, his situation worsened and developed respiratory failure and died on day 14 [32].
8. Eggs for increasing ACE2 and copper
Egg ovotransferrin contains an angiotensin-converting enzyme (ACE) inhibitory peptide, known as IRW, that has shown to decrease blood pressure in hypertensive rats [33]. Through the up-regulation of ACE2, E-cadherin, ABCB-1, and IRF-8, IRW can decrease RAS activity, hyperplasia, and vascular inflammation and aid differential regulation of leukocytes. On the other hand, by the down-regulation of pro-inflammatory molecules, e.g., ICAM-1 and VCAM-1, IRW can reduce the recruitment of leukocytes and vascular inflammation.
Copper might help the immune system to combat viral infections. A High-content of copper exists in eggs and eggshells of domestic birds. In particular, the eggshell of pigeon (4 0.29 μg/g) and the egg of quail (4.67 1.08 μg/g) contains high concentrations of copper [34].
9. Conclusion
The novel coronavirus, SARS-CoV2, can cause a potentially fatal disease, COVID-19, in humans. The infection of human cells by SARS-CoV2 includes two sequential steps: attachment of the virus to the surface receptor of target cells and the fusion of viral and host membranes. The former requires at least a receptor-binding domain on the SARS-CoV2 Spike protein that can interact with a cell surface receptor, for example, ACE2, expressed on human cells. The latter requires at least the host protease(s) to mediate proteolytic cleavage of the SARS-CoV2 Spike protein into S1 and S2 subunits and consequently promote the fusion of viral and host membranes. Also, SARS-CoV2 possesses a polybasic cleavage site that can explain the high pathogenicity of SARS-CoV2, N-glycans and O-glycans that make the dense decoration of SARS-CoV2 S protein, and cyclic regions that can interact with cell-surface GRP78. Essential elements that process SARS-CoV2 cell entry and specific characteristics that allow SARS-CoV2 to escape the immune system have the potential as targets for COVID-19 therapy.
Lack of specific treatments for COVID-19 and the very time-consuming process of vaccine development lead us to trust traditional notions of immunization using passive transfer of humoral immunity. Passive immunization can be done using plasma therapy and IVIG therapy. Plasma from patients recovered from COVID-19 that contains antibodies against SARS-CoV2 has shown promising results in patients with severe COVID-19. Also, SARS-CoV and SARS-CoV2 are ideally similar in the structure and the cell entry receptor and protease. Studies show that serum from convalescent SARS patients and serum from rabbits immunized with SARS are both able to neutralize SARS-CoV2 efficiently. However, serum from bats immunized with SARS-lice coronavirus SL-CoV Rp3 could not exert such an effect. It is due to a noticeable degree of difference in the S1 domain in the S1 domain between the bat SL-CoV Rp3 strain and SARS-CoV2. A short-term moderate dose of IVIG combined with moderate-dose of corticosteroids might improve patient outcomes. Studies show that the viral RNA of SARS-CoV2 reaches its peak during the first week and then gradually decreases and that IgG and IgM begin to rise from the 10th day so that most patients have anti-viral antibodies by the 14th day. Passive immunization protects against disease, and so it should be administered as early as possible when the patient is diagnosed.
Evidence links COVID-19 to variable degrees of inflammation. Corticosteroids offer a potent anti-inflammatory option. However, they do not dictate precise actions and might cause suppression of anti-viral immune responses as well. Studies show that the use of corticosteroids might accelerate recovery from COVID-19. There are no controlled clinical trials that show whether the use of corticosteroids can reduce COVID-19-related death. Moreover, the pro-inflammatory cytokine IL6 is the best-documented cytokine in COVID-19 correlated with severity, criticality, viral load, and prognosis of patients with COVID-19. Tocilizumab, a monoclonal antibody against IL6, could confer clinical benefit in patients with high IL6 levels.
References
- 1.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirchdoerfer R.N., Cottrell C.A., Wang N., Pallesen J., Yassine H.M., Turner H.L. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531(7592):118–121. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020 doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao S., Khattar S.K., Subbiah M., Collins P.L., Samal S.K. Mutation of the f-protein cleavage site of avian paramyxovirus type 7 results in furin cleavage, fusion promotion, and increased replication in vitro but not increased replication, tissue tropism, or virulence in chickens. J. Virol. 2012;86(7):3828–3838. doi: 10.1128/JVI.06765-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagdonaite I., Wandall H.H. Global aspects of viral glycosylation. Glycobiology. 2018;28(7):443–467. doi: 10.1093/glycob/cwy021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vigerust D.J., Shepherd V.L. Virus glycosylation: role in virulence and immune interactions. Trends Microbiol. 2007;15(5):211–218. doi: 10.1016/j.tim.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. ARTIC Network. 2020;17 doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibrahim I.M., Abdelmalek D.H., Elshahat M.E., Elfiky A.A. COVID-19 spike-host cell receptor GRP78 binding site prediction. J. Infect. 2020 doi: 10.1016/j.jinf.2020.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet. Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.-M., Lim W.S. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J. Infect. Dis. 2014;211(1):80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.K. Duan, B. Liu, C. Li, H. Zhang, T. Yu, J. Qu, et al., The feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study, medRxiv, 2020.
- 12.Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310(5748):676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 13.Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G. A new coronavirus associated with human respiratory disease in China. Nature. 2020;1–5 doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren L.L., Wang Y.M., Wu Z.Q., Xiang Z.C., Guo L., Xu T. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin. Med. J. (Engl). 2020 doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marra M.A., Jones S.J.M., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S.N. The genome sequence of the SARS-associated coronavirus. Science. 2003;300(5624):1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Y.W. Chen, C.-P.B. Yiu, K.-Y. Wong, Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CL pro) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Research. 9(129) (2020) 129. [DOI] [PMC free article] [PubMed]
- 18.Z. Xu, C. Peng, Y. Shi, Z. Zhu, K. Mu, X. Wang, et al., Nelfinavir was predicted to be a potential inhibitor of 2019-nCov main protease by an integrative approach combining homology modelling, molecular docking and binding free energy calculation, bioRxiv, 2020.
- 19.Litvinov D., Mahini H., Garelnabi M. Antioxidant and anti-inflammatory role of paraoxonase 1: implication in arteriosclerosis diseases. N. Am. J. Med. Sci. 2012;4(11):523–532. doi: 10.4103/1947-2714.103310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo C.L., La Du B.N. Comparison of purified human and rabbit serum paraoxonases. Drug Metab. Dispos. 1995;23(9):935. [PubMed] [Google Scholar]
- 21.Ferrara G., Zumla A., Maeurer M. Intravenous immunoglobulin (IVIg) for refractory and difficult-to-treat infections. Am. J. Med. 2012;125(10):1036–e1. doi: 10.1016/j.amjmed.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 22.K.K.-W. To, O.T.-Y. Tsang, W.-S. Leung, A.R. Tam, T.-C. Wu, D.C. Lung, et al., Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study, The Lancet Infect. Dis. (2020). [DOI] [PMC free article] [PubMed]
- 23.Z.-G. Zhou, S.-M. Xie, J. Zhang, F. Zheng, D.-X. Jiang, K.-Y. Li, et al., Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. 2020.
- 24.Durbin J.E., Fernandez-Sesma A., Lee C.-K., Rao T.D., Frey A.B., Moran T.M. Type I IFN modulates innate and specific anti-viral immunity. J. Immunol. 2000;164(8):4220. doi: 10.4049/jimmunol.164.8.4220. [DOI] [PubMed] [Google Scholar]
- 25.K.G. Lokugamage, C. Schindewolf, V.D. Menachery, SARS-CoV-2 sensitive to type I interferon pretreatment, bioRxiv, 2020.
- 26.C. Wang, W. Li, D. Drabek, N.M.A. Okba, R. van Haperen, A.D.M.E. Osterhaus, et al., A human monoclonal 1 antibody blocking SARS-CoV-2 infection, bioRxiv, 2020. [DOI] [PMC free article] [PubMed]
- 27.X., Xu, M. Han, T. Li, W. Sun, D. Wang, B. Fu, et al., Effective treatment of severe COVID-19 patients with Tocilizumab, ChinaXiV. 2020;202003:v1. [DOI] [PMC free article] [PubMed]
- 28.C. Bergin, N. Conlon, C.N. Choitir, R. Adams, F. King, P. Gilvarry, Interim Recommendations for the use of Tocilizumab in the Management of Patients who have Severe COVID-19 with Suspected Hyperinflammation, 2020.
- 29.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. The Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.S. Matsuyama, M. Kawase, N. Nao, K. Shirato, M. Ujike, W. Kamitani, et al., The inhaled corticosteroid ciclesonide blocks coronavirus RNA replication by targeting viral NSP15, bioRxiv, 2020. [DOI] [PMC free article] [PubMed]
- 31.Y. Wang, W. Jiang, Q. He, C. Wang, B. Wang, P. Zhou, et al., Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan, China, medRxiv, 2020.
- 32.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Majumder K., Liang G., Chen Y., Guan L., Davidge S.T., Wu J. Egg ovotransferrin-derived ACE inhibitory peptide IRW increases ACE2 but decreases pro-inflammatory genes expression in mesenteric artery of spontaneously hypertensive rats. Mol. Nutr. Food Res. 2015;59(9):1735–1744. doi: 10.1002/mnfr.201500050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nisianakis P., Giannenas I., Gavriil A., Kontopidis G., Kyriazakis I. Variation in trace element contents among chicken, turkey, duck, goose, and pigeon eggs analyzed by inductively coupled plasma mass spectrometry (ICP-MS) Biol. Trace Elem. Res. 2009;128(1):62–71. doi: 10.1007/s12011-008-8249-x. [DOI] [PubMed] [Google Scholar]