Abstract
In this research, the bee pollen protein hydrolysate was microencapsulated by spray drying using maltodextrin (MD), whey protein concentrate (WPC) and a mixture of both compounds. For this purpose, the bee pollen was hydrolysed by alcalase (enzyme concentration of 1.5%) at 50 °C and pH 8 during 3.95 h, and then freeze-dried. The hydrolysed protein and wall materials were used in ratio of 1:10 (w/w). The wall materials included maltodextrin 2%, WPC 2%, as well as maltodextrin and WPC mixtures with 3:1 ratio. The resulting capsules were exposed to UV radiation for 48 h to accelerate the oxidation. The results showed that the capsule prepared using maltodextrin and WPC mixture showed the highest DPPH radical scavenging during exposure to UV radiation. Based on the FTIR spectroscopy results, the wall containing maltodextrin and WPC mixture showed the best performance in maintaining the chemical structure of hydrolysed protein. The SEM results indicated that the microcapsules prepared with WPC and maltodextrin mixture as wall material showed uniform and smoother wall than those prepared with maltodextrin alone. Finally, it was found that the maltodextrin and WPC mixture was the best wall with an appropriate protective capability for the microencapsulation of hydrolysed proteins and their protection against UV radiation.
Keywords: Food technology, Pollen protein hydrolysate, Microencapsulation, Spray drying, Maltodextrin, Whey protein concentrate
Food Technology; Pollen protein hydrolysate; microencapsulation; spray drying; maltodextrin; whey protein concentrate
1. Introduction
Protein and peptide compounds are very sensitive to environmental conditions. Most protein compounds, such as medicines and functional foods, are denatured due to the sensitivity to acidic conditions in the stomach and intestines, leading to loss of effectiveness. Various methods can be used to encapsulate the protein and peptide compounds including: solvent gremoval-based encapsulation (Moinard-Che´cot et al., 2008; Borodina et al., 2010), layer-by-layer (LBL) encapsulation (Alamed et al., 2009), emulsion polymerization (EP) (Rao and Geckeler, 2011), emulgels (Iglesias et al., 2013), poly lactic-co-glycolic acid (PLGA) microcapsules and PLGA-zinc complexes (Dai et al., 2005; Mok and Park, 2008), alginate-chitosan microcapsules (Patsialas et al., 2011; Su et al., 2011). Microencapsulation using spray drying is the most commonly used method of preserving active and sensitive food ingredients including proteins and peptides, which increases the stability of the compounds. The materials used as the wall in microencapsulation with the spray drying include gum arabic, maltodextrins, modified starches and mixtures, protein isolates and concentrates such as whey protein and soybean protein (Shen and Quek, 2014). Bee pollen contains 10–40% protein, so it is a valuable source of protein with functional and nutraceutical properties (Estevinho et al., 2012). By performing the hydrolysis and producing bioactive peptides, their functional and health effects can be increased (Maqsoudlou et al., 2018). Researchers have used different wall compounds for the microencapsulation of proteins and peptides and examined the stability and structural properties of final products. Kanbargi et al. (2017) reported that the DPPH radical scavenging capacity of the microencapsulated hydrolysed proteins derived from Ziziphus jujube seed in sodium alginate remained almost constant by increasing the time. The results of FTIR1 spectroscopy showed that the peptides were cross-linked with sodium alginate. They reported that the surface of the produced microcapsules was smooth, spherical and uniform. Molina Ortiz et al. (2009) microencapsulated the hydrolysed casein by soybean protein isolate using spray drying method. Produced microcapsules had a spherical structure without fissures, cracks or disruptions, which protect the inner materials of the microcapsules. Torres-Giner et al. (2017) used the WPC and maltodextrin to microencapsulate aloe vera extract. FTIR results showed that the incorporation of polysaccharide and protein reduced the absorbance related to the C–H stretching band. After exposuring of microcapsules to UV, there was no significant changes in the chemical structure of either WPC or microcapsules containing WPC. They reported that the capsules prepared with the protein wall and the mixture of protein and carbohydrates showed a smooth, disaggregated, and stable surface. Su et al. (2011) reported that the structure of the microcapsules with a mixture of alginate and collagen as the wall material was spherical, and by increasing the protein to carbohydrate ratio in the wall, the number of cracks in the wall was decreased and the capsule structure became more stable. The results of FTIR spectroscopy showed that the location and intensity of the absorbance related to the secondary structure of protein was changed. Gómez-Mascaraque & López-Rubio (2016) reported that microcapsules obtained by microencapsulation of hydrolysed whey protein prepared using spray drying method and gelatin and chitosan, were almost spherical and non-uniform in terms of surface smoothness. In spite of that there are a several literatures about the microencapsulation of bioactive food ingredients; studies about the microencapsulation of hydrolysed proteins and study on their stability using accelerated conditions are very few. On the other hand, by-products of honey bees such as pollen have been less noticed; therefore the aim of this study was the microencapsulation of bioactive bee pollen protein hydrolysate by maltodextrin and whey protein using spray drying method and to study the structural changes and stability of resulted microcapsules treated by UV radiation as an accelerated oxidation model system.
2. Materials and methods
2.1. Preparation of hydrolysed protein from bee pollen
Bee pollen was obtained from an apiary located in naturally preserved area of Gorgan, belongs to Beekeepers Cooperative Company of Golestan province, under the supervision of Animal Science Research Institute (ASRI) of Iran. The powder of pollen was defatted with hexane (1:3) during 24 h using an orbital shaker (Fan, 52E TM Iran, Gostar). The defatted pollen was packed in Ziploc bags after removing residual hexane in oven at 40 °C during 24 h.
In order to prepare enzymatic hydrolysates, the defatted pollen (with 14.5% protein) was suspended in 5 volumes of 0.1 M potassium phosphate buffer (pH 8) and homogenized using an ultrasonic homogenizer (Hielscher, UP100H). The hydrolysis of defatted bee pollen was performed by AlcalaseⓇ (concentration of 1.5%) at 50 °C and pH 8 for 4 h in shaking incubator. Finally, the enzyme reaction was stopped by heating at 85 °C for 10 min. To remove excess compounds, centrifugation was carried out at 4000 g for 30 min, and after collecting, the supernatant was dried by freeze drying (Maqsoudlou et al., 2018).
2.2. Microencapsulation of bee pollen protein hydrolysate
2.2.1. Preparation of hydrolysed protein and wall material mixtures
The solutions of maltodextrin (2% w/w, DE = 17.5), WPC (2% w/w), and solution containing maltodextrin and WPC mixture (with 3:1 ratio and 2% w/w) were prepared in deionised water. Preparation of solutions was carried out by stirring using a magnetic stirrer. For this purpose, the hydrolysed protein powder was first added to deionised water with 1:10 w/w ratio relative to the wall component, and was stirred for 2 h with the magnetic stirrer. This wall to core material ratio was selected according to pre-tests results and previous studies of Kanbargi et al. (2017) and Gómez-Mascaraque & López-Rubio (2016). Then, the wall materials were gradually added and stirred for 4–5 h until a uniform solution was obtained (Gómez-Mascaraque & López-Rubio, 2016; Rosenberg et al., 2016).
2.2.2. Microencapsulation by spray drying
The mixtures of hydrolysed protein and wall materials were converted to powder using a spray dryer (Model B90, Butchi, Switzerland; Dimensions tall setup (W×H×D) 580 × 1500 × 550 mm) with an optimal condition including inlet temperature of 100 °C, outlet temperature of 53 °C, air flow rate of 140 L/min, and pressure of 4 bar obtained from previous studies using this spray dryer (Gómez-Mascaraque & López-Rubio, 2016).
2.3. Exposure to UV radiation
According to Torres-Giner et al. (2017), the UV radiation was used as an accelerator of oxidation to investigate the stability of microcapsules against degrading agents. Based on this method, the produced microcapsules, wall and core materials were separately exposed to UV radiation for 48 h. For this purpose, an Ultra-Vitalux lamp from OSRAM Lighting SL (Madrid, Spain) was used. The radiation of the 300W lamp was similar to the natural sunlight, which was produced using a quartz tube and a tungsten filament (Torres-Giner et al., 2017).
2.4. FTIR spectroscopy
Physicochemical bonds and interactions between hydrolysed protein compounds, maltodextrin and WPC were analyzed and identified using the FTIR spectroscopy. In this system, single spectra of materials were collected with an average 20 scans at 4 cm−1 resolution in the range of 400–4000 cm−1. In order to analyze the chemical structure of the samples, the powder of each sample was cast on the attenuated total reflection (ATR) crystal located on the Bruker Tensor 37 FTIR (Rheinstetten, Germany) system equipped with the golden gate of Specac, Ltd. (Orpington, U.K.) to form a very thin layer of 30 μm thick. Then, the repeated spectroscopy was carried out over time (Torres-Giner et al., 2017).
2.5. Antioxidant properties of microcapsules
The antioxidant properties of microcapsules and hydrolysed protein during the exposure to UV radiation were measured at time intervals of 0, 1, 2, 3, 5, 7, 9, 11, and 48 h, and changes in their antioxidant activity were compared (Torres-Giner et al., 2017). The antioxidant property was evaluated by measurement of DPPH radical scavenging activity according to Hmidet et al. (2011).
2.6. Morphological characteristics of microcapsules
The morphology of the microcapsules was investigated according to Torres-Giner et al. (2017), using the SEM2 (S-4800, Hitachi, Japan). For this purpose, the microcapsules powder was stabilized using a gold-palladium mixture under vacuum. Particle measurements were performed using Apple Aperture (Cupertino, CA, U.S.A) software.
2.7. Analysis of encapsulation efficiency (%EE)
EE was evaluated according to Muangrat et al. (2019) with some modifications. 100 mg of encapsulated powder was mixed with 1 mL of 0.1 M potassium phosphate buffer (pH 8) and stirred for 15 min using a vortex. Bradford protein assay method was used for measuring of Surface protein content (SPC). Calculation of encapsulation efficiency (%EE) was done using the Eq. (1) at below:
| (1) |
TPC in feed solution: the total protein content in the feed solution before encapsulation process (μg/mL).
SPC in encapsulated powder: the protein content on the surface of powder after encapsulation (μg/mL).
2.8. Physical properties
Measuring of water activity of powder was done by an Aqualab analyzer (Decagon Devices, USA, using samples stabilized at 50 °C for 40 min. The ratio of mass of the powder and the volume occupied in the cylinder was used to determine the bulk density (g/mL). For measuring the tap density of powders, first an average of 300 beats was continuously applied to the cylinder using tapped densitometer, and tapped density obtained when the volume changes of powder stopped (Jangam and Thorat, 2010). To determine the solubility of the spray dried powders the proposed methods by Sarabandi et al. (2018) was applied.
2.9. Statistical analysis
The data for antioxidant test were analysed by one-way analysis of variance for HP, Maltodextrin + HP, Maltodextrin + WPC + HP and WPC + HP in each time and over time for each treatment (HP, Maltodextrin + HP, Maltodextrin + WPC + HP and WPC + HP) separately.When the ANOVA indicated a significant effect, Duncan's multiple range test was used for mean comparison. All trials were conducted in three replicates.
The antioxidant test was performed on a completely randomized design with 0.05 significance level using SPSS software, version 25. The mean values of the obtained data were compared at 95% confidence level. Data is presented as the mean ± the Standard deviation (SD).
3. Results and discussion
3.1. DPPH radical scavenging activity of microcapsules
The results of DPPH radical scavenging activity of the produced microcapsules and the changes during the exposure to UV radiation are shown in Table 1.
Table 1.
DPPH Scavenging activity (%) in the different microcapsules (1.5–5.57 μm) exposed to UV over time.
| Time | HP | Maltodextrin + HP | Maltodextrin + WPC + HP | WPC + HP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| h0 | 88.29 ± 0.051 | b | A | 84.59 ± 0.113 | d | B | 89.45 ± 0.31 | a | A | 88 ± 0.028 | c | B |
| h1 | 80.76 ± 0.062 | d | D | 82.68 ± 0.208 | c | E | 87.71 ± 0.101 | a | D | 85.68 ± 0.077 | b | e |
| h2 | 82.26 ± 0.11 | d | B | 82.69 ± 0.078 | c | E | 89.32 ± 0.271 | a | AB | 87.56 ± 0.086 | b | C |
| h3 | 80.57 ± 0.138 | d | D | 82.68 ± 0.334 | c | E | 88.31 ± 0.144 | a | C | 87.65 ± 0.111 | b | C |
| h5 | 81.57 ± 0.164 | d | C | 84.86 ± 0.086 | c | B | 88.62 ± 0.061 | a | C | 88.34 ± 0.089 | b | A |
| h7 | 80.58 ± 0.092 | d | D | 84.12 ± 0.116 | c | C | 89.11 ± 0.133 | a | B | 87.51 ± 0.141 | b | C |
| h9 | 81.57 ± 0.051 | d | C | 81.99 ± 0.220 | c | F | 86.29 ± 0.109 | b | E | 86.99 ± 0.099 | a | D |
| h11 | 77.68 ± 0.043 | d | E | 83.58 ± 0.058 | c | D | 88.55 ± 0.092 | a | C | 85.76 ± 0.072 | b | F |
| h48 | 65.53 ± 0.11 | d | F | 87.4 ± 0.059 | a | A | 86.30 ± 0.061 | b | E | 86.04 ± 0.112 | c | E |
Means with the same lower case letter in each row are not significantly different (P < 0.05).
Means with the same upper case letter in each column are not significantly different (P < 0.05).
DPPH Scavenging activity of WPC and Maltodextrin at h0 were 21% and 0 respectively.
HP: Hydrolysed Protein.
Maltodextrin + WPC + HP: Capsules with wall of maltodextrin and whey protein concentrate.
WPC + HP: Capsules with wall of whey protein concentrate.
Maltodextrin + HP: Capsules with wall of maltodextrin.
h0: zero time.
h1: first hour.
h2: second hour.
h3: third hour.
h5: fifth hour.
h7: seventh hour.
h9: ninth hour.
h11: eleventh hour.
h48: Forty eighth hour.
At all the assayed times, except h9, the capsule prepared with maltodextrin and WPC mixtures as wall material showed the highest levels of radical scavenging activity (P < 0.05), followed by the capsule prepared with WPC as wall material. This can be attributed to the DPPH radical scavenging capability of the WPC. Before conducting the main tests, the scavenging activity of the wall compounds was investigated. The results showed that none of the wall materials showed DPPH radical scavenging capability except WPC which showed 21% DPPH radical scavenging activity (Table 1).
The proper protection of the core material against UV degradation effect was provided using a wall-to-core ratio of 10:1. In the study of Kanbargi et al. (2017) it was shown that the higher ratio of the wall to core material, make a more efficient capsule for maintaining core material.
Except a capsule prepared with maltodextrin as wall material whose antioxidant activity was less than the hydrolysed protein at initial treatment period (0 h), the drying operation in the microencapsulation process had no significant effect on the antioxidant activity of the hydrolysed protein present in the core of the remaining capsules.
According to the results, combination of WPC with maltodextrin could increase the efficiency of protection of core material. In this regards Domian et al. (2017) stated that the carrier material including combination of carbohydrate/protein, supports better oxidative stability, protection and drying properties in microcapsules. In previous studies, it was found that the presence of whey protein in the wall and core composition serves as an emulsifier and produce high-performance capsules with adequate protection against oxidizing agents (Shen and Quek, 2014; Chen et al., 2013). In this regards Fernandes et al. (2016) stated that short chains carbohydrates such as maltodextrin act as matrix formers and filling agents. Low emulsifying capacity is the main detriment of most carbohydrates as encapsulating materials; hence using of compounds with higher emulsifying capacityit is superior. However the effect of WPI on the stability of the core material is different depending on the core material. In this regard, Stănciuc et al. (2017) microencapsulated the anthocyanins obtained from grape skins in WPI and two different matrices of WPI-polysaccharide. A decrease in the DPPH radical scavenging activity of anthocyanins extract encapsulated in WPI was observed. Since wall material and core material had a different chemical structures and the microencapsulation process probably affected the bioactivity of the core material. The results of molecular docking tests and quenching experiments of research done by Stănciuc et al. (2017) showed the binding of β-lactoglobulin and α-lactalbumin of WPI with anthocyanins. However, anthocyanins were successfully loaded into the matrices of WPI and carbohydrate.
In the present study, examining the antioxidant properties of capsules over a 10-day period, we found that the capsules containing whey protein as wall material retain their antioxidant properties at higher values compared to other capsules. Also, as is shown in Table 1, during 5h of UV exposure, the lowest DPPH radical scavenging activity was attributed to the hydrolysed protein. During the test, BHT was considered as a positive control and its DPPH scavenging activity was always measured above 90%, without clear changes in DPPH radical scavenging activity during exposure to UV radiation. However, at 11 and 48 h of exposure to UV radiation, there was a significant decrease in the DPPH radical scavenging activity of the hydrolysed protein.
By observing the differences between the DPPH radical scavenging activity of the encapsulated and non-encapsulated protein hydrolysates, the efficient protective effect of the wall materials can be considered as a suitable method for maintaining the structure of the protein hydrolysates and thus, maintaining their functional characteristics during exposure to UV radation. These results were consistent with the findings of Kanbargi et al. (2017), Mozafari et al. (2006) and Zavareze et al. (2014).
3.2. Structural analysis of capsules using FTIR
Changes in the chemical structure of the hydrolysed protein and the microcapsules before and after exposure to UV radiation were evaluated using FTIR spectroscopy (Figures 1, 2, 3, and 4).
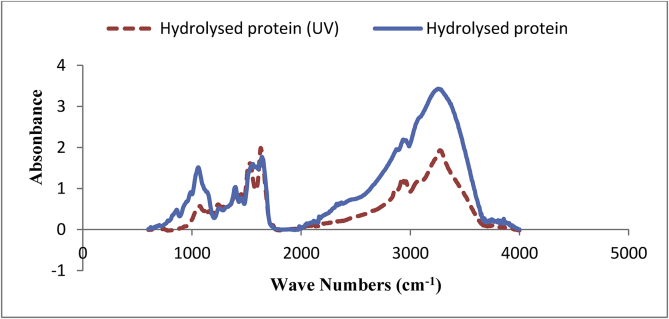
Figure 1.
FTIR spectra of hydrolysed protein before and after exposure to UV radiation.
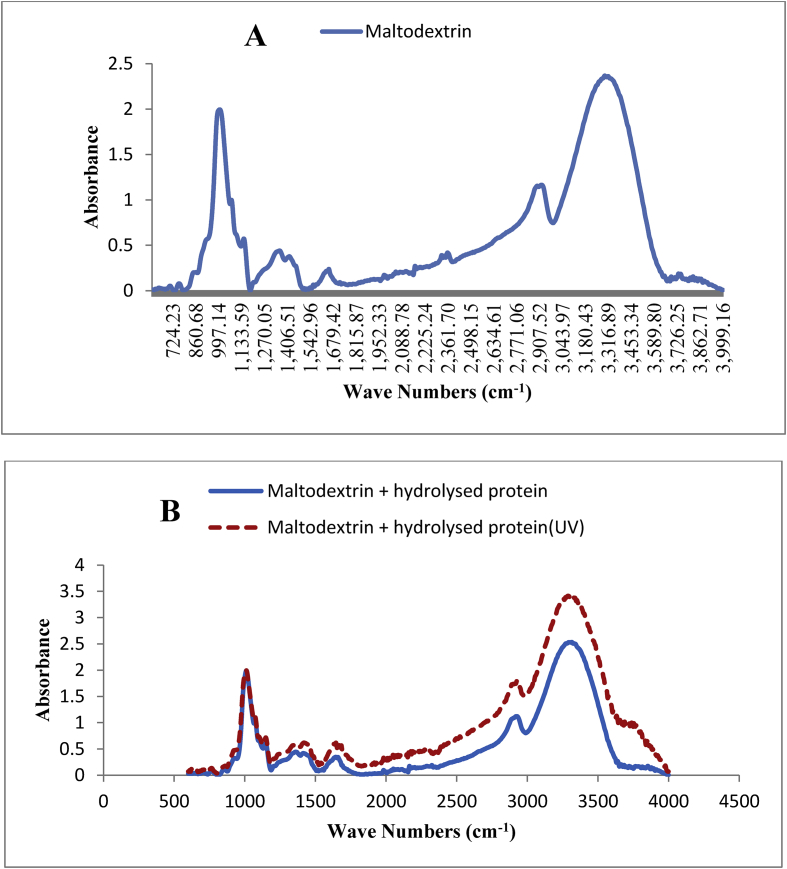
Figure 2.
FTIR spectra of maltodextrin (A) and hydrolysed proteins microencapsulated in maltodextrin after exposure to UV radiation (B).
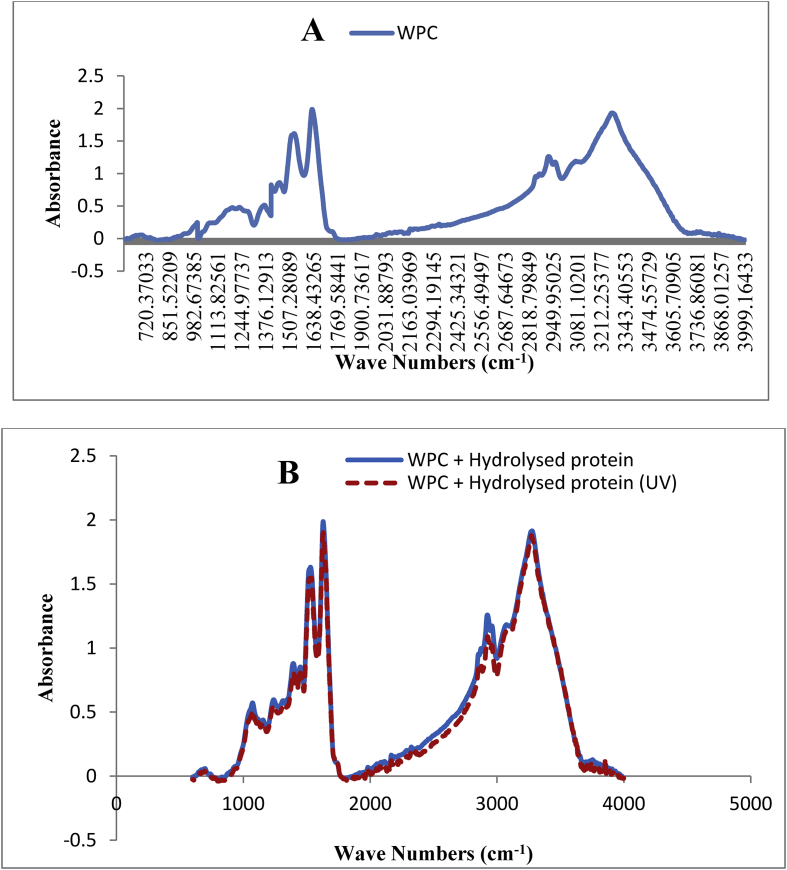
Figure 3.
FTIR spectra for WPC (A) and hydrolysed proteins microencapsulated in WPC after exposure to UV radiation (B).
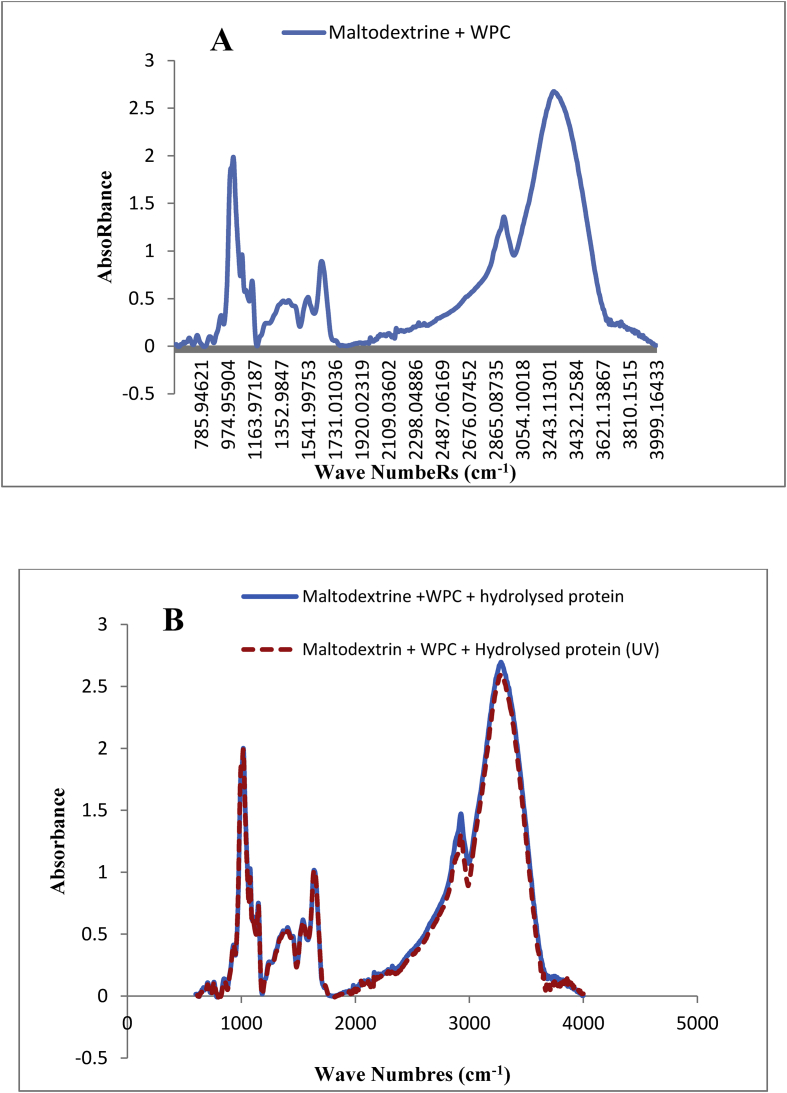
Figure 4.
FTIR spectra for mixture of WPC and maltodextrin (A) and microencapsulated hydrolysed proteins in the mixture of WPC and maltodextrin before and after exposure to UV radiation (B).
FTIR spectra of hydrolysed protein can be seen in Figure 1. In the range of 850 cm−1, there was a small peak with the absorbance level of 0.5, which is related to amide bonds, including N–H, C–N and C=O. Any absorbance in the 800-1800 cm−1 range defines the amide group bonds (Van der Ven et al., 2002).
In the range of 1000–1050 cm−1, there was a peak with the absorbance level of 1.5, which according to Pe´rez-Masia´ et al. (2015), corresponds to the amide group or probable deformation in the NH3 group of the free amino acid of lysine, which is possibly present in the protein hydrolysates.
In the range of 1400–1450 cm−1, a peak was observed with the absorbance level of about 1. According to Pavia et al. (2002), the probable presence of one of the three asymmetrical CH3 bending, symmetrical CO3– stretching, and ring vibrations attributed to free amino acids valine, glutamic acid and phenylalanine, respectively, can cause the peak found in this region, since the presence of free amino acids in the protein hydrolysates was expected.
In the range of 1500–1700 cm−1, a peak with absorbance of 1.5–2 was observed, which is related to two important amide vibration modes. The absorbance in the range of 1600–1700 cm−1 was related to the vibration of amide I (C=O bond) and the 1500-1600 cm−1 range was related to the vibration of amide II (N–H and C–N bonds), which is consistant with the findings by Wang et al. (2013), Assadpour et al. (2016), Torres-Giner et al. (2017) and Kanbargi et al. (2017). Pavia et al. (2002) also identified the absorbance in this region as representing the β-sheet and α-helix structures conforming the secondary structure of proteins, which is related to the C–H stretching.
A large, wide peak was observed with an absorbance of 3.5 in the range of 3000–3500 cm−1. According to Kanbargi et al. (2017) and Torres-Giner et al. (2017), this range belongs to the hydrogen bond in the N–H stretching and bending C–H groups.
Once the hydrolysed protein is exposed to UV radiation, a significant change was made in the FTIR spectroscopy (Figure 1). The decrease in the absorbance at 1050 cm−1 can be due to the degradation of amide bonds. In the region of 1500–1700 cm−1, a dip was observed in the peak center for hydrolysed protein, so two small peaks can be observed in that region. The loss of hydrogen bonds in the secondary structure of proteins, such as the opening of the α helix or the separation of two polypeptide chains and the loss of the β-sheet structure, may be regarded as the possible reasons for this reduction in the absorbance level, which is consistent with the findings of Sullivan et al. (2014) and Alp Erbay et al. (2017).
As shown in Figure 1, the largest peak (2900-3500 cm−1 region) was significantly decreased after exposure to UV due to the breaking and degradation of the hydrogen bonds present in the amide groups such as the N–H stretching and the C–H bends bands.
The FTIR spectra for maltodextrin and the hydrolysed proteins microencapsulated in maltodextrin, before and after exposure to UV radiation are shown in Figure 2 (A and B). The first peak, which showed an absorbance of about 2 in the region of 850–1170 cm−1, was related to the stretching vibrations of anhydroglucose (Figure 2A). At the bonding point of glucose molecules in the maltodextrin structure, a molecule of water is released and creates the structure of anhydroglucose. The stretching vibrations between the bonded glucose units exhibit absorbance in the region lower than 1000 cm−1. These results were consistent with the findings of Cabado et al. (2015).
In the region of 1100–1500 cm−1, a wide peak was absorbed with an absorbance of 0.5, which was attributed to the C–O stretching band in the glucose units.
In the region of 2850–3000 cm−1, a peak was observed with an absorbance of 1, which was attributed to the deformation of C–H group. In this regard Cabado et al. (2015) and Torres-Giner et al. (2017) have reached the same results.
A large peak was observed with the absorbance level of 2.5 in the 3000-3500 cm−1 region. Pavia et al. (2002), Cabado et al. (2015), Torres-Giner et al. (2017) and Assadpour et al. (2016) attributed the absorbance level in this region to the O–H group.
The comparison of the FTIR results for maltodextrin and the hydrolysed protein capsulated by maltodextrin (Figure 2B) showed that the peaks in the range of 850–1700 cm−1 were almost similar. However, the peak absorbance in the region of 2000–3000 cm−1 was slightly reduced for the capsulated hydrolysed protein due to the formation of a complex between the polysaccharide compound of the wall and the protein compound of the core, which was consistent with Torres-Giner et al. (2017). They expressed that the incorporation of polysaccharide and protein reduced the absorbance of the stretching band of C–H.
As is shown in Figure 2B the absorbance of the largest peak in the region of 3000–3500 cm−1 for hydrolysed protein capsulated by maltodextrin was slightly increased compared to that of maltodextrin due to the presence of the O–H group of maltodextrin in the wall and the N–H stretching and C–H bending bands of the amide groups in the hydrolysed protein. López-Rubio and Lagaron (2012) also stated that the simultaneous presence of OH, NH, and CH groups in the protein and polysaccharide compounds increased the intra-molecular and inter-molecular hydrogen bonds and increased the absorbance in the 3340-3360 cm−1 region.
The FTIR results for hydrolysed protein microencapsulated with maltodextrin before and after exposure to UV radiation (Figure 2B) showed that after exposure to UV, the absorbance was increased, especially in the region of 1500–3500 cm−1. According to Khandal et al. (2013), exposure to the radiation provokes cross-linking in maltodextrin and further incorporates the molecules of maltodextrin to protein ones.
The FTIR spectra of the WPC and the hydrolysed proteins microencapsulated in the WPC before and after exposure to UV radiation is shown in Figure 3 (A and B). For WPC, some peaks with different absorbance were observed in the 850-1700 cm−1 region, which determined the bands of the amide group, including the bending bands of N–H, C–N, and C=O groups (Figure 3A).
The peak in the range of 980–1000 cm−1 is probably related to the NH3 group in the side chain of lysine amino acid, the peak in the region of 1310–1390 cm−1 would correspond to the deformation of the O–H group in the serine amino acid or the symmetric bending group in the leucine amino acid. The peak in the 1400-1450 cm−1 region can be attributed to the asymmetric CH3 bending band in the valine amino acid, the symmetric CO3– stretching band present in the glutamic acid amino acid or the ring vibrations in the phenylalanine amino acid. Two peaks in the 1500-1600 cm−1 and 1600-1700 cm−1 region showed two important amide vibration modes in this absorbance range (Figure 3A).
The vibration of amide I, which is related to the C=O bond, has absorbance in the range of 1600–1700 cm−1, and the amide II vibration related to the N–H and C–N bonds has absorbance in the range of 1500–1600 cm−1. In this regard some researcher stated that the absorbance in this region represents the secondary structure of proteins, the most important of which is the β-sheet and α-helix structure (Wang et al., 2013; López-Rubio and Lagaron, 2012; Assadpour et al., 2016; Kanbargi et al., 2017).
A fairly small peak was observed in the range of 2800–3000 cm−1, which was related to the stretching band of C–H. A wide peak was observed in the range of 3000–3500 cm−1, which according to Kanbargi et al. (2017); Torres-Giner et al. (2017); López-Rubio and Lagaron (2012) and Pavia et al. (2002), is associated with the hydrogen bond in the N–H stretching and the C–H bending band.
The unique difference in the FTIR spectra of WPC and the hydrolysed protein encapsulated with WPC (Figure 3B) was the increase in the number and absorbance of peaks in the 1310-1390 cm−1 the spectra region of hydrolysed proteins microencapsulated in WPC. This can be due to the presence of the NH3 group in the side chain of lysine amino acid, the O–H group in the serine amino acid or the symmetric bending bands in the leucine amino acid, which were placed at the end of peptide chain or freely in the protein hydrolysates. This result was consistent with the result obtained by Pavia et al. (2002).
In the FTIR spectra of hydrolysed protein encapsulated with WPC after exposure to UV (Figure 3B), except for a slight decrease in the absorbance level in the region of 2800–3000 cm−1, there was no significant difference before and after exposure to UV. Therefore, it can be concluded that WPC is a suitable biopolymer for the microencapsulation of protein hydrolysates and protects them against the UV radiation as a degrading agent. This result was consistent with those of Torres-Giner et al. (2017) and López-Rubio and Lagaron (2012).
As shown in the FTIR spectra of hydrolysed protein microencapsulated with the mixture of WPC and maltodextrin (Figure 4A), in the 850-1170 cm−1 region, the absorbance was about 2, which was slightly lower than that of maltodextrin and higher than that of WPC. In the region of 1500–1700 cm−1, two distinct peaks were observed with an absorbance of close to 1. The peaks exhibited higher absorbance level than maltodextrin and lower than WPC. In the region of 2850–3000 cm−1, the absorbance was increased in comparison with maltodextrin and WPC in the same region. In the region of 3000–3500 cm−1, a peak with an absorbance level of about 3 was observed, which was increased in comparison with the maltodextrin and WPC peaks in the same region.
The reason for changes in the regions and intensity of absorbance could be due to the establishment of hydrogen bonds and electrostatic forces between the maltodextrin polysaccharide and WPC which increase the protecting effect and stability of wall material. In this regard Fernandes et al. (2016) reported that the FTIR absorption bands were certainly different when maltodextrin were incorporated to WPI. Jain et al. (2015) also reported that by combination of protein and carbohydrates, the C=O stretching vibration and COOH incurvature vibration made a change significantly at 1746 cm−1 and 3286 cm−1 respectively. The bond formed between the carbonyl group of protein carbonyl amide and glucosidal group of carbohydrates were suggested by these changes.
Su et al. (2011) also stated that the hydrogen bonds and electrostatic force alter the frequency of O–H, N–H, C=O, C=N, and C–H bonds. Liu and Zhong (2012) and Wang et al. (2013) also reported that the glycation of whey protein increases or decreases α-helix, β-sheet, β-turn and random coil structures.
The FTIR spectra of the hydrolysed proteins microencapsulated in the mixture of WPC and maltodextrin did not change after exposure to UV radiation (Figure 4B). Therefore, it can be concluded that the mixture of WPC and maltodextrin is a proper mixture of biopolymers for the microencapsulation of the protein hydrolysates and protects them against degrading action of UV radiation. This result was consistent with the result obtained by Rosenberg et al. (2016) that stated that the complex of protein and maltodextrin was a suitable compound for use in the microcapsule wall.
3.3. Scanning electron microscopy (SEM)
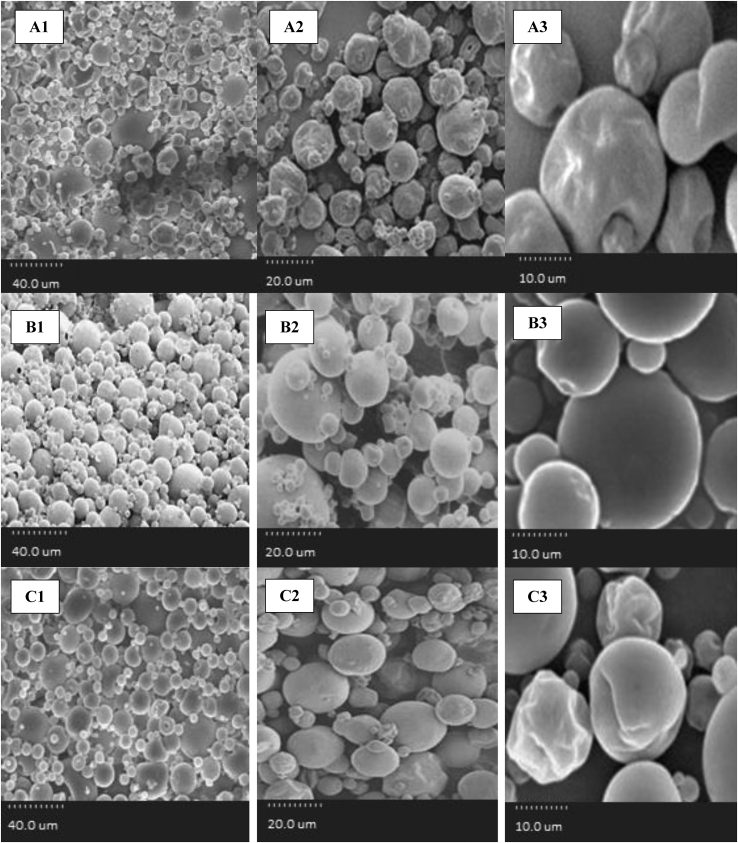
The diameter of all microcapsules with different walls produced in this study was measured using the SEM ranging 1.5–5.57 μm (Figure 5). Various factors affect particle size. The ratio of wall material to core is among the factors affecting the size of the microcapsule particles. The high concentration of wall material causes the large particle size exited from the atomizer and the large size of the final microcapsules which can protect core material properly against accumulation and agglomeration during the microencapsulation process (Jafari et al., 2007; Frascareli et al., 2012). In addition, the composition of the material used in the wall also affects the size of the microcapsules. In this regard Rosenberg et al. (2016) found that the size of microcapsules was reduced by increasing the carbohydrate/protein ratio in the wall composition. As shown in Figure 5, A1, B1 and C1, in general the smaller particles have a greater degree of wrinkle and rigidity than the larger particles, which can be attributed to the high drying rate in the smaller particles. This leads to the rapid hardening and rigidity of the wall (Sheu and Rosenberg, 1998). However, the wide size distribution/range is a typical characteristic of particles produced by spray drying (Aberkan et al., 2014).
Figure 5.
SEM images of produced microcapsules. A1, A2 and A3: Maltodextrin microcapsules containing hydrolysed protein. B1, B2 and B3: WPC microcapsules containing hydrolysed protein. C1, C2, and C3: Microcapsules of maltodextrin and WPC mixture containing hydrolysed protein.
The microcapsules prepared with maltodextrin wall were spherical and some of them have pores and wrinkles on the surface (Figure 5, A1, A2 & A3). Drusch and Berg (2008) considered high temperature of the dryer inlet and the surface evaporation as a reason for wrinkles at the surface of microcapsules. The presence of pores is one of the characteristics of microcapsules prepared by spray drying using carbohydrate wall, which are created by increasing particle temperature and vapor pressure (Rosenberg et al., 1993; Rosenberg et al., 2016; Nijdam and langrish, 2006). The accumulation of microcapsules with the maltodextrin wall was higher than the other two walls, which, according to Mok and Park (2008), was due to the melting of the wall and the agglomeration of dried powders.
The microcapsules prepared with WPC (Figure 5, B1, B2 & B3) had a smooth surface without of wrinkles and cracks, which is consistent with finding of Molina Ortiz et al. (2009), Rosenberg et al. (2016), and Assadpour et al. (2016). Smooth surface without wrinkles and cracks prevents the penetration of gases and moisture into the microcapsules and confirms its high protection capability against degrading agents. Oliveira et al. (2007) also stated that the protein and the mixture of protein and various carbohydrates used as a wall material produces high-strength capsules against degrading agents.
The microcapsules prepared with the mixture of maltodextrin and WPC had a smoother, less wrinkled wall comparing the microcapsules prepared with the maltodextrin wall, which was an advantage since regarding to Aberkan et al. (2014) it implies that capsules would have lower permeability to gases, increasing protection and retention of active material (Figure 5, C1, C2 & C3). The composition of the different wall materials influenced microparticles morphology (Aberkan et al., 2014). It was found that the combination of carbohydrates and proteins in the wall structure increases the efficiency of capsules and better maintains the physical structure by increasing the thermal resistance of proteins and decreasing the protein denaturation (Avadi et al., 2010). Koc et al. (2015) used a mixture of maltodextrin and whey protein isolate as wall materials to optimize the microencapsulation of olive oil. Microstructure of powders was generally non-homogeneous, spherical in shape, and showed a smooth surface. Koc et al. (2015) found that the use of protein and polysaccharides combination as wall materials was superior to achieve the best result in microencapsulation of olive oil. In this regard, Su et al. (2011) also stated that by increasing the protein/carbohydrate ratio in the wall, the size and number of cracks in the wall was decreased and the microstructure became stronger. According to Fernandes et al. (2016) the encapsulation efficiency was significantly changed when the partial substitution of WPI by maltodextrin was done in the wall material. In addition, capsules with the wall material containing combination of maltodextrin and WPI, had amorphous structures and did not have any gap on the surface. Aberkan et al. (2014) used pea protein/maltodextrin and pea protein/pectin/maltodextrins as wall materials to microencapsulate the fish oil. Microcapsules were smooth with some wrinkles or scars on the surface with no apparent cracks or fissures.
3.4. Encapsulation efficiency
Encapsulation efficiency of microcapsules prepared with the wall material including mixture of maltodextrin and WPC, WPC and maltodextrin were measured 82.51 ± 1.9, 78.2 ± 2.3 and 67.84 ± 3.35%, respectively. Capsuls with different wall material showed significant differences in the EE, as the combination of maltodextrin and WPC showed the maximum EE and maltodextrin showed the minimum EE. The low SPC measured in WPI and mixture of WPI and maltodextrin particles is important for preparing storage stability of the encapsulated compounds (Muangrat et al., 2019) which is consistent with the antioxidant results observed in this work.
3.5. Physical properties
Physical properties of different spray dried powders are presented in Table 2. The water activity and moisture content of spray dried powders can be affected by different factors like feed flow rate, inlet air temperature and carrier type and concentration. According to results presented in Table 2, there was no significant difference between moisture content and water activity of different powders (p < 0.05). This could be due to using similar drying condition and similar moisture content of starting materials. The samples with low water activity (lower than 0.6) are stable microbiologicaly, therefore they are suitable for long time storage (Quek et al., 2007). The bulk density and tapped density of samples ranged from 0.282-0.287 g/mL and 0.339–0.348 g/mL, respectively. Generally there was no significant difference between tapped density and bulk density of samples. Therefore, there is slight difference in porosity and particle size distribution of samples. The samples showed high solubility ranged between 96.43 to 97.32% with no significant differences. The solubility is one of the most important functional properties of food powders. Food powders with high solubility reconstitute quickly in water when used in food formulations (Sarabandi et al., 2018).
Table 2.
Physical properties of spray dried powder.
| Moisture content (%) | Water activity | Bulk density (g/mL) | Tapped density (g/mL) | Solubility (%) | |
|---|---|---|---|---|---|
| Maltodextrin + HP | 3.5 ± 0.17a | 0.265 ± 0.01a | 0.287 ± 0.02a | 0.348 ± 0.01a | 97.32 ± 0.45a |
| Maltodextrin + WPC + HP | 3.32 ± 0.11a | 0.255 ± 0.01a | 0.285 ± 0.01a | 0.345 ± 0.01a | 96.68 ± 0.38a |
| WPC + HP | 3.42 ± 0.08a | 0.259 ± 0.02a | 0.282 ± 0.02a | 0.339 ± 0.01a | 96.43 ± 0.52a |
Different letters in the same column indicate statistical significant differences (P < 0.05).
Values are mean ± SD of three replications.
(Capsules diameter ranged between 1.5-5.57 μm).
4. Conclusion
In the present study, the microencapsulation of bee pollen protein hydrolysate increased their resistance to degrading agents. Based on the results from the evaluation of DPPH radical scavenging activity, SEM images, FTIR spectroscopy of microcapsules during the exposure to UV and EE, it can be said that among WPC, maltodextrin (MD) and a mixture of the WPC and MD, the mixture of the WPC and MD was the best wall material with suitable protective capability for the microencapsulation of hydrolysed bee pollen proteins with protection potential against the UV (as a degrading agent). These microcapsules showed reasonable stability and minimal degradation, and also retained a lot of their antioxidant activity during exposure to UV radiation; therefore the biological function of bee pollen protein hydrolysate could be preserved by this method. All wall materials showed good physical property especially high water solubility.
Declarations
Author contribution statement
Atefe Maqsoudlou: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Alireza S. Mahoonak: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Hossein Mohebodini: Contributed reagents, materials, analysis tools or data.
Vahid Koushki: Performed the experiments.
Funding statement
This work was supported by the Ministry of Science, Research and Technology of Iran.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Footnotes
Fourier-transform infrared.
Scanning Electron Microscopy.
References
- Aberkane L., Roudaut G., Saurel R. Encapsulation and oxidative stability of PUFA-rich oil microencapsulated by spray drying using pea protein and pectin. Food Bioprocess Technol. 2014;7:1505–1517. [Google Scholar]
- Alamed J., Chaiyasit W., McClements D.J., Decker E.A. Relationships between free radical scavenging and antioxidant activity in foods. J. Agric. Food Chem. 2009;57:2969–2976. doi: 10.1021/jf803436c. [DOI] [PubMed] [Google Scholar]
- Alp Erbay E., Dağtekin B.B.G., Türe M., Yeş ilsu A.F., Torres-Giner S. Quality improvement of rainbow trout fillets by whey protein isolate coatings containing electrospun poly (ε-caprolactone) nanofibers withUrtica dioica L. extract during storage. LWT Food Sci. Technol. 2017;78:340–351. [Google Scholar]
- Assadpour E., Jafari S.M., Maghsoudlou Y. Evaluation of folic acid release from spray dried powder particles of pectin whey protein nano-capsules. Int. J. Biol. Macromol. 2016 doi: 10.1016/j.ijbiomac.2016.11.023. [DOI] [PubMed] [Google Scholar]
- Avadi M.R., Mir Mohammad Sadeghi A., Mohammadpour N., Abedin S., Atyabi F., Dinarvand R., Rafiee-Tehrani M. Preparation and characterization of insulin nanoparticles using chitosan and Arabic gum with ionic gelation method. Nanomed. Nanotechnol. Biol. Med. 2010;6(1):58–63. doi: 10.1016/j.nano.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Borodina T., Grigoriev D., Moehwald H., Shchukin D. Hydrogen storage materials protected by a polymer shell. J. Mater. Chem. 2010;20:1452–1456. [Google Scholar]
- Cabado M.C., Parra-Ruiz F.J., Casado A.L., Román S. Thermal crosslinking of maltodextrin and citric acid. Methodology to control the polycondensation reaction under processing conditions. Polym. Polym. Compos. 2015;24(8):643–654. [Google Scholar]
- Chen J., Liu S., Ye R., Cai G., J.B., Wu Y. Angiotensin-I converting enzyme (ACE) inhibitory tripeptides from rice protein hydrolysate: purification and characterization. J. Funct. Foods. 2013;5(4):1684–1692. [Google Scholar]
- Dai C., Wang B., Zhao H. Microencapsulation peptide and protein drugs delivery system. Colloids Surf. B Biointerfaces. 2005;41:117–120. doi: 10.1016/j.colsurfb.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Domian E., Kopytowska A.B., Marzec A. Functional properties and sxidative stability of flaxseed oil microencapsulated by spray drying using legume proteins in combination with soluble fiber or trehalose. Food Bioprocess Technol. 2017;10(7):1374–1386. [Google Scholar]
- Drusch S., Berg S. Extractable oil in microcapsules prepared by spray-drying, determination and impact on oxidatve stability. Food Chem. 2008;109:17–24. doi: 10.1016/j.foodchem.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Estevinho L.M., Rodrigues S., Pereira A.P., Feás X. Portuguese bee pollen: palynological study nutritional and microbiological evaluation. Int. J. Food Sci. Technol. 2012;47:429–435. [Google Scholar]
- Fernandes R.V.B., Silva E.K., Borges S.V., Oliveira C.R., Yoshida M.I., Silva Y.F., Carmo E.L., Azevedo V.M., Botrel D.A. Proposing novel encapsulating matrices for spray-dried ginger essential oil from the whey protein isolate-Inulin/maltodextrin blends. Food Bioprocess Technol. 2016;10(1):115–130. [Google Scholar]
- Frascareli E., Silvaa V., Tonona R., Hubingera M. Effect of process conditions on the microencapsulation of coffee oil by spray drying. Food Bioprod. Process. 2012;90:413–424. [Google Scholar]
- Gómez-Mascaraque L.G., López-Rubio A. Protein-based emulsion electrosprayed micro- and submicroparticles for the encapsulation and stabilization of thermosensitive hydrophobic bioactives. J. Colloid Interface Sci. 2016;465:259–270. doi: 10.1016/j.jcis.2015.11.061. [DOI] [PubMed] [Google Scholar]
- Hmidet N., Balti R., Nasri R., Sila A., Bougatef A., Nasri M. Improvement of functional properties and antioxidant activities of cuttlefish (Sepia officinalis) muscle proteins hydrolysed by Bacillus mojavensis A21 proteases. Food Res. Int. 2011;44:2703–2711. [Google Scholar]
- Iglesias G.R., Pirolt F., Sadeghpour A., Tomsic M., Glatter O. Lipid transfer in oil-in-water isasome emulsions: influence of arrested dynamics of the emulsion droplets entrapped in a hydrogel. Langmuir. 2013;29:15496–15502. doi: 10.1021/la4032255. [DOI] [PubMed] [Google Scholar]
- Jafari S.M., He T., Bhandari B. Encapsulation of nanoparticles of D-limoneneby spray drying: role of emulsifiers and emulsifying techniques. Dry. Technol. 2007;25:1079–1089. [Google Scholar]
- Jangam S.V., Thorat B.N. Optimization of spray-drying of ginger extract. Dry. Technol. 2010;28:1426–1434. [Google Scholar]
- Jain A., Thakur D., Ghoshal G., Katare O.P., Shivhare U.S. Microencapsulation by complex coacervation using whey protein isolates and gum Acacia: an approach to preserve the functionality and controlled release of β-Carotene. Food Bioprocess Technol. 2015;8:1635–1644. [Google Scholar]
- Kanbargi K.D., Sachin K., Sonawane S., Shalini S., Arya A. Encapsulation characteristics of protein hydrolysate extracted from Ziziphus jujube seed. Int. J. Food Prop. 2017 [Google Scholar]
- Khandal D., Mikus P.Y., Dole P., Coqueret X. Radiation processing of thermoplastic starch by blending aromatic additives: effect of blend composition and radiation parameters. Radiat. Phys. Chem. 2013;84:218–222. [Google Scholar]
- Koç M., Güngör O., Zungur A., Yalçın B., Selek I., Ertekin F.K., Ötles S. Microencapsulation of extra virgin olive oil by spray drying: effect of wall materials composition, process conditions, and emulsification method. Food Bioprocess Technol. 2015;8:301–318. [Google Scholar]
- Liu G., Zhong Q. Glycation of whey protein to provide steric hindrance against thermal aggregation. J. Agric. Food Chem. 2012;60(38):9754–9762. doi: 10.1021/jf302883b. [DOI] [PubMed] [Google Scholar]
- López-Rubio A., Lagaron J.M. Whey protein capsules obtained through electrospraying for the encapsulation of bioactives. Innovat. Food Sci. Emerg. Technol. 2012;13:200–206. [Google Scholar]
- Maqsoudlou A., Sadeghi Mahoonak A., Mora L., Mohebodini H., Toldrá F., Ghorbani M. Peptide identification in alcalase hydrolysated pollen and comparison of its bioactivity with royal jelly. Food Res. Int. 2018 doi: 10.1016/j.foodres.2018.09.027. [DOI] [PubMed] [Google Scholar]
- Moinard-Checot D., Chevalier Y., Briancon S., Beney L., Fessi H. Mechanism of nanocapsules formation by the emulsion–diffusion process. J. Colloid Interface Sci. 2008;317:458–468. doi: 10.1016/j.jcis.2007.09.081. [DOI] [PubMed] [Google Scholar]
- Mok H., Park T.G. Water-free microencapsulation of proteins within PLGA microparticles by spray drying using PEG-assisted protein solubilization technique in organic solvent. Eur. J. Pharm. Biopharm. 2008;70:137–144. doi: 10.1016/j.ejpb.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Molina Ortiz S.E., Mauri A., Monterrey-Quintero S.E., Trindade M.A., Santana A.S., Favaro-Trindade S.C. Production and properties of casein hydrolysate microencapsulated by spray drying with soybean protein isolate. LWT Food Sci. Technol. 2009;42:919–923. [Google Scholar]
- Mozafari M.R., Flanagan J., Matia-Merino L., Awati A., Omri A., Suntres Z.E., Singh H. Review: recent trends in the lipid-based nanoencapsulation of antioxidants and their role in foods. J. Sci. Food Agric. 2006;86:2038–2045. [Google Scholar]
- Muangrat R., Ravichai K., Jirarattanarangsri W. Encapsulation of polyphenols from fermented wastewater of Miang processing by freeze drying using a maltodextrin/gum Arabic mixture as coating material. J. Food Process. Preserv. 2019 [Google Scholar]
- Nijdam J.J., langrish T.A. The effect of surface composition on the functional properties of milk powders. J. Food Eng. 2006;77(4):919–925. [Google Scholar]
- Oliveira A.C., Moretti T.S., Boschini C., Baliero J.C.C., Freitas L.A.P., Freitas O., Favaro-Trindade C.S. Microencapsulation of B. lactis (BI 01) and L.acidophilus (LAC 4) by complex coacervation followed by spouted-bed drying. Dry. Technol. 2007;25(10):1687–1693. doi: 10.1080/02652040701532908. [DOI] [PubMed] [Google Scholar]
- Patsialas K., Papaioannou E.H., Liakopoulou-Kyriakides M. Encapsulation of the peptide Ac–Glu–Thr–Lys–Thr–Tyr–Phe–Trp–Lys–NH2into polyvinyl alcohol biodegradable formulations—effect of calcium alginate. Carbohydr. Polym. 2011;87:1112–1118. [Google Scholar]
- Pavia D.L., Lampman G.M., Kriz G.S., Vyvyan J.A. 3th Edition. Books/Colne; 2002. Introduction to Spectroscopy; p. 579p. [Google Scholar]
- Pérez-Masiá R., López-Nicolás R., Periago M.J., Ros G., Lagaron J.M., López-Rubio A. Encapsulation of folic acid in food hydrocolloids through nanospray drying and electrospraying for nutraceutical applications. Food Chem. 2015;168:124–133. doi: 10.1016/j.foodchem.2014.07.051. [DOI] [PubMed] [Google Scholar]
- Quek S.Y., Chok N.K., Swedlund P. The physicochemical properties of spraydried watermelon powders. Chem. Eng. Process. Process Intensif. 2007;46:386–392. [Google Scholar]
- Rao J.P., Geckeler K.E. Polymer nanoparticles: preparation techniques and size- control parameters. Prog. Polym. Sci. 2011;36:887–913. [Google Scholar]
- Rosenberg M., Rosenberg Y., Frenkel F. Microencapsulation of model oil in wall matrices consisting of SPI and maltodextrins. AIMS Agric. Food. 2016;1(1):33–51. [Google Scholar]
- Rosenberg M., Young S.L., Brooker & B.E., Colombo V.E. Whey proteins as microencapsulating agents- microencapsulation of anhydrous milkfat- structure evaluation. Food Struct. 1993;12(1):31–41. [Google Scholar]
- Sarabandi k., Sadeghi Mahoonak A.R., Hamishekar H., Jafari S.M., Ghorbani M. Microencapsulation of casein hydrolysates: physicochemical, antioxidant and microstructure properties. J. Food Eng. 2018;237:86–95. [Google Scholar]
- Shen Q., Quek S.Y. Microencapsulation of astaxanthin with blends of milk protein and fiber by spray drying. J. Food Eng. 2014;123:165–171. [Google Scholar]
- Sheu T.Y., Rosenberg M. Microstructure of microcapsules consisting of whey proteins and carbohydrates. J. Food Sci. 1998;63:491–494. [Google Scholar]
- Stănciuc N., Turturică M., Oancea A.M., Barbu V., Ioniţă E., Aprodu I., Râpeanu G. Microencapsulation of anthocyanins from grape skins by whey protein isolates and different polymers. Food Bioprocess Technol. 2017;10(9):1715–1726. [Google Scholar]
- Su R., Zhu X., Fan D., Mia Y., Yang C.Y., Jia X. Encapsulation of probiotic Bifidobacterium longum BIOMA 5920 with alginate–human-like collagen and evaluation of survival in simulated gastrointestinal. Int. J. Biol. Macromol. 2011;49:979–984. doi: 10.1016/j.ijbiomac.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Sullivan S.T., Tang C., Kennedy A., Talwar S., Khan S.A. Electrospinning and heat treatment of whey protein nanofibers. Food Hydrocolloids. 2014;35:36–50. [Google Scholar]
- Torres-Giner S., Wilkanowicz S., Melendez-Rodriguez B., Lagaron J.M. Nanoencapsulation of aloe vera in synthetic and naturally occurring polymers by electrohydrodynamic processing of interest in food Technology and bioactive packaging. J. Agric. Food Chem. 2017;65:4439–4448. doi: 10.1021/acs.jafc.7b01393. [DOI] [PubMed] [Google Scholar]
- Van der Ven C., Muresan S., Gruppen H., Bont D., Merck K.B., Voragen A.G.J. FTIR spectra of whey and casein hydrolysates in relation to their functional properties. J. Agric. Food Chem. 2002;50(24):6943–6950. doi: 10.1021/jf020387k. [DOI] [PubMed] [Google Scholar]
- Wang J., Su Y., Jia F., Jin H. Characterization of casein hydrolysates derived from enzymatic hydrolysis. Chem. Cent. J. 2013;7(62):212–220. doi: 10.1186/1752-153X-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavareze E., Campello Telles A., Lisie S., Rocha M.D., Colussi R., Marques de Assis L., Suita de Castro L.A., Guerra Dias A.R., Prentice-Hernández C. Production and characterization of encapsulated antioxidative protein hydrolysates from Whitemouth croaker (Micropogonias furnieri) muscle and byproduct. LWT Food Sci. Technol. 2014 [Google Scholar]