Abstract
Background
A clinical feasibility study was undertaken at a single center of long-term intra-cerebroventricular drug delivery of the anti-seizure medication valproic acid, into the cerebrospinal fluid (CSF) in order to treat drug resistant focal seizures, using an implantable infusion system. The primary objective was to establish the dose range of VPA administered in this manner. Secondarily, safety, pharmacokinetics (PK) and a preliminary estimate of effectiveness were evaluated.
Methods
In this single arm study, five adult subjects, with 29–234 focal onset seizures per month from a seizure focus involving the mesial temporal lobe were implanted with the system (clinicaltrials.gov identifier NCT02899611). Oral valproic acid (VPA) had previously been ineffective in all subjects. Post-surgery, pharmacokinetic studies of CSF infused VPA were performed. Valproic acid doses were increased stepwise in a standardised protocol.
Findings
The procedure and implantation were well-tolerated by all subjects. Four subjects responded with > 50% seizure reduction at the highest tested dose of 160 mg/day. Two subjects experienced extended periods of complete seizure freedom. All five subjects reported significant quality of life improvement. No clinical dose limiting side effects were encountered and there was no evidence of local periventricular toxicity in three subjects who were evaluated with imaging (T2 MRI). Side effects included nausea and appetite loss but were not dose-limiting. Mean CSF valproic acid levels were 45 μg per ml (range 20–120 μg per ml), with corresponding serum levels of 4–14 μg per ml. Subjects have received therapy for up to 2.5 years in total . The efficacy analysis presented focuses on the period of time with the current pump with a mean 12.5 months, range 11.5–15 months. Pump failure requiring reimplantation was a significant initial issue in all subjects but resolved with use of pumps suitably compatible with long-term exposure to valproic acid.
Interpretation
The study demonstrated that chronic intraventricular administration of valproic acid is safe and effective in subjects with medically refractory epilepsy over many months. The procedure for implanting the infusion system is safe and well-tolerated. High CSF levels are achieved with corresponding low serum levels and this therapy is shown to be effective despite unsuccessful earlier use of oral valproate preparations. Drug side effects were minimal.
Funding
The study was funded by Cerebral Therapeutics Inc., Suite 137 12635 East Montview Blvd Aurora CO 80045.
Research in context.
Evidence before this study
We searched PubMed, Google Scholar, and IEEExplore with the terms “intra-cerebroventricular” or “ventricular” or “intraventricular” with “infusion” or “injection” or “drug” or “therapy” or “treatment” or “delivery” and “epilepsy” for human and animal studies published between Jan 1, 1965, and February 2019. We did not restrict publications by language. Whilst there was some evidence for efficacy of anti-seizure drugs administered using this approach in animal models, we could identify no human studies examining this. There were numerous studies utilizing this means of administration for a variety of other medication however, chiefly anti-neoplastic agents.
Added value of this study
This study is the first in humans that provides continuous administration of an anti-seizure medication via this route. The pharmacokinetics of intraventricular valproate were established, and the degree to which the drug crosses into the peripheral circulation. The tolerability of relatively high cerebrospinal fluid levels of valproate achieved was clarified.
Implications of all the available evidence
This proof-of-concept study shows that intraventricular anti-seizure administration is possible and effective in humans. The study provides an important first step towards the development of new therapeutic strategies as well as new insights into the mechanisms of action and side effects of anti-seizure drugs.
Alt-text: Unlabelled box
Introduction
Epilepsy is a common and serious neurological problem affecting over 60 million people worldwide [1]. At least 30% of affected individuals are resistant to current drug therapies, a proportion that has not diminished despite the increasing number of available agents [2], [3], [4]. Additionally, patients often experience significant side effects, both acute and chronic, in part through the systemic effects of anti-seizure drug (ASD) use [5]. Drug resistant epilepsy is a major therapeutic challenge, leading to the development of several novel approaches in recent years including thalamic stimulation [6,7], responsive neurostimulation [8], and laser interstitial thermal therapy [9], chiefly as alternatives to resective surgery.
Improving delivery of anti-seizure medications is another area of exploration. Application of anti-seizure drugs to a restricted brain region can produce high concentrations of the drug in the region of seizure onset and spread, which may provide control of seizures while potentially avoiding the peripheral and central side effects that limit oral drug administration. Focal drug delivery is a theoretically appealing treatment alternative for patients with medically refractory epilepsy. Treatment options such as the intracranial implantation of polymer-based drug delivery systems have been explored extensively in animal models [10]. These implants slowly break down following implantation, releasing drug locally to the implant site, or by spreading through the CSF if implanted into CSF spaces. Recent studies have demonstrated some success at using similar biodegradable implants to treat animal models of several other neurological disorders with focal pathologies such as Parkinson's disease, Huntington's disease and Alzheimer's disease [11]. Studies in animal models of epilepsy to date have been less successful however, chiefly as it is difficult to provide an implant with a sufficiently long lifespan. A range of alternative methods of ASD delivery directly to the seizure focus has been described [12].
Delivery of medications directly to the brain via an intra-cerebroventricular route (ICV Infusion) has been used in a variety of short-term clinical scenarios, particularly leptomeningeal cancer and meningitis. This method has been shown to be safe, with complications relating to the device and its implantation, specifically in regard to infective complications [13].
Valproic acid is a broad-spectrum anti-seizure medication known to be effective for tonic–clonic, myoclonic, tonic and absence seizures, as well as focal seizures [14,15]. VPA was chosen for this study as a suitable formulation could be prepared and because prior animal studies suggested possible efficacy [16,17].
Generally, valproic acid is well tolerated although significant side effects including nausea, weight gain, tremor, hair change and sedation limit its systemic usage. Hepatotoxicity is rare. Teratogenicity in particular has emerged as a significant problem limiting use in women of child-bearing potential, and this risk seems to be dose-related [18]. Intra-cerebroventricular administration of VPA could potentially avoid VPA systemic toxicity, producing a high concentration of the drug in the brain, but with low serum levels, and consequently less peripheral toxicity. This direct to CSF mode of administration has the potential to increase central nervous system toxicity however, as many of these adverse effects are presumed to be the result of cerebral toxicity which could vary depending upon the distribution of valproic acid in the brain with ICV administration.
Little is known of human CSF levels of VPA. The therapeutic range for valproic acid (total) in serum is 50–125 µg/mL, and the toxic level is greater than 150 µg/mL. The therapeutic range for unbound valproic acid is 6–22 µg/mL in humans. Primate average CSF levels after loading with oral doses targeting 2× human blood levels ranged from 18 to 20 µg/mL [19]. Human average CSF Levels steady state concentrations have been recorded in the range of 3 − 5 µg/mL, though from very limited data [20], [21], [22].
We describe dose-escalation of a continuous infusion of valproic acid after implantation of a pump infusion system directly into one lateral cerebral ventricle in proximity to the presumed seizure focus in subjects with mesial temporal onset of seizure activity. We describe the results and side effects experienced to date.
The primary objective of this study was to establish the dose range of ICV administration of valproic acid. Secondarily, safety, pharmacokinetics and a preliminary estimate of effectiveness were evaluated.
The anatomical target for valproic acid drug delivery in this clinical study was the hippocampus, which is immediately adjacent to the temporal horn of the lateral ventricle. Based on the rate of diffusion within fluid spaces and from fluid spaces into the brain, the anatomic continuity of the lateral ventricle and what is known about the rate of CSF emptying from the ventricle [23], placing the catheter tip in the frontal horn was thought to potentially accomplish high doses of medication in the periventricular areas of the lateral ventricle including the temporal horn because of the lack of barriers between the different segments within the lateral ventricle, relatively slow movement of CSF in the ventricular system and the rapid movement and mixing in the ventricle caused by cerebral vascular pulsations.
Methods
Study design and subjects
This was a dose-ranging study to establish the dose range of ICV valproic acid delivery, with a brief period of blinding during dose escalation for the initial four weeks, designed mainly to obtain an unbiased report of side effects.
We recruited adult subjects (18–65 years of age) with medically refractory focal seizures with or without secondary generalization who averaged four or more disabling focal seizures per month for the 30 days over the three most recent consecutive 30-day periods, with no 30-day period with less than two seizures. Current use of valproate was an exclusion. The study was approved by the Human Research Ethics Committee, St. Vincent's Hospital, Melbourne (approval number HREC 014/16). All subjects were recruited from a single center. The study was registered on clinicaltrials.gov, identifier NCT02899611.
Valproic acid was administered continuously via ICV delivery for 50 days during a blinded evaluation period in order to establish maximum tolerated dose (MTD), after which the dose was escalated further according to a predefined schedule.
The primary objective was to establish the dose range of VPA administered in this manner. The dose range was to be limited by the MTD, which is the highest dose tolerated without experiencing a dose-limiting adverse event, or alternatively the highest dose in a provided dose escalation table. Secondarily, safety, pharmacokinetics (PK) and a preliminary estimate of effectiveness were evaluated.
After providing informed consent, subjects were screened for entrance criteria and all subjects continued their routinely prescribed orally administered ASDs throughout the study duration, except when adjustment was required on clinical grounds. Clinical assessments, adverse events (AEs), seizure diary, concomitant medications, blood samples and CSF were collected and reviewed at designated time points. MRI Scan, EEG, ambulatory video-EEG and ECG studies were also performed. Subjects had their surgery, dose changes and PK performed in an inpatient or infusion clinic setting. 6 subjects were assessed and considered eligible. 1 subject declined further participation as it proved too difficult for them to access the hospital facility regularly from an interstate location.
In order to identify a starting dose, we extrapolated from the literature dosing ratios of systemic versus intrathecal (IT) or intra-cerebroventricular (ICV) drug administration for conditions other than epilepsy. There was considerable variation between the dose administered systemically and intrathecally or ICV, with intrathecal concentration ranges between 1/500th and 1/10th of systemic dose [24]. Further, based on data in Appendix V and VI, as well as previous experience with ICV morphine and baclofen chemotherapy, we created a schedule for increasing ICV doses from 1/400th of the oral dose in the standard range of valproate, monitored by careful observation for potential side effects, gradually to a dose of up to 1/10th of the oral dose of valproate. The starting dose was based on pre-clinical data, clinically established CSF valproic acid levels from oral and IV administration as well as extrapolating from the experience with intrathecal morphine and baclofen delivery [25].
The ICV valproate dose was initially escalated stepwise through Day 50, if tolerated or stopped earlier upon establishment of a subject's maximum tolerated dose (MTD). Dose escalation is described in detail in Table 1. After the initial escalation, subjects were then further escalated stepwise from 60 mg/day up to 160 mg/day. The devices were refilled at varying intervals according to the dose escalation and response, but all were maintained on approximately monthly cycles of refills when optimal doses were reached.
Table 1.
Drug titration schedule.
| Study week | Dosing | Study day(±3 days) | Estimated Fraction of CPTD for a 60 kg person | ICV valproate |
|
|---|---|---|---|---|---|
| IDaily Dose | Concentration (mg/ml) | ||||
| 1 | Level 0 | Days 1–7 | Vehicle (preservative-free normal saline) | Vehicle (programmed as 0.6 mg/day) | Vehicle (programmed as 1 mg/mL) |
| 2 | Level 1 | Day 8 | 5% of CPTD | 3.0 mg/day | 5 mg/mL |
| 3 | Level 2 | Day 15 | 10% of CPTD | 6 mg/day or MTD | 10 mg/mL |
| 4 | Level 3 | Day 22 | 25% of CPTD | 15 mg/day or MTD | 25 mg/mL |
| 5 | Level 4 | Day 29 | 50% of CPTD | 30 mg/day or MTD | 50 mg/mL |
| 6 | Level 5 | Day 36 | 60% of CPTD | 36 mg/day or MTD | 60 mg/mL |
| 7 | Level 6 | Day 42 | 75% of CPTD | 45 mg/day or MTD | 75 mg/mL |
| 8 | Level 7 | Day 49 | 100% of CPTD | 60 mg/day or MTD | 100 mg/mL |
| X | Placebo week randomly inserted | Day 56 + (8 weeks plus) | Placebo (preservative-free normal saline) | Placebo (programmed as 0.6 mg/day) | Placebo (programmed as 1 mg/mL) |
| Last Day | Day 64 or 9 weeks | ||||
Both the subjects and the assessing physician remained blinded to the treatment dose during the Blinded Evaluation Period. Subjects were able to continue in an open-label evaluation period for 52 weeks following the Blinded Evaluation Period. Subjects who completed the Open-Label Evaluation Period and responded well to treatment were able to continue in the Open-Label Extension Period for up to two years (approximately 104 weeks).
The primary safety outcomes were measured via the serious adverse event (SAE) rate. Safety was also assessed via adverse events, vital signs, physical exams, changes in concomitant medications, use of rescue medications, neuropsychological testing, and laboratory tests.
The primary efficacy outcome was measurement of change in the number of seizures. Efficacy was also assessed via seizure type and severity from seizure diary data, measured EEG readings, use of rescue medication, neuropsychological testing, and decrease in oral ASD use.
Statistical analysis
Given the small number of subjects it was not considered appropriate or practical to perform statistical analysis.
Drugs
Epilim® (Sanofi-Aventis) was compounded with 0.4% citrate (Medical Components, Inc., PA) to achieve the desired sodium valproate concentrations (pH 7.6) and used to fill the implanted reservoir of the intrabdominal drug infusion pump for CSF delivery (see below).
Procedures
Screening verified seizure frequency and anatomic localization of seizure onset, blood tests for a complete blood count, liver and kidney function, assay for HIV infection urinalysis, pregnancy test for females were performed and a recent MRI was performed if not done in the prior three years. Neuropsychological studies were performed. There was a baseline ECG and EEG as well as a one-week continuous EEG with an ambulatory video-EEG system. Subjects kept a daily validated electronic seizure diary (EpiDiary, Irody).
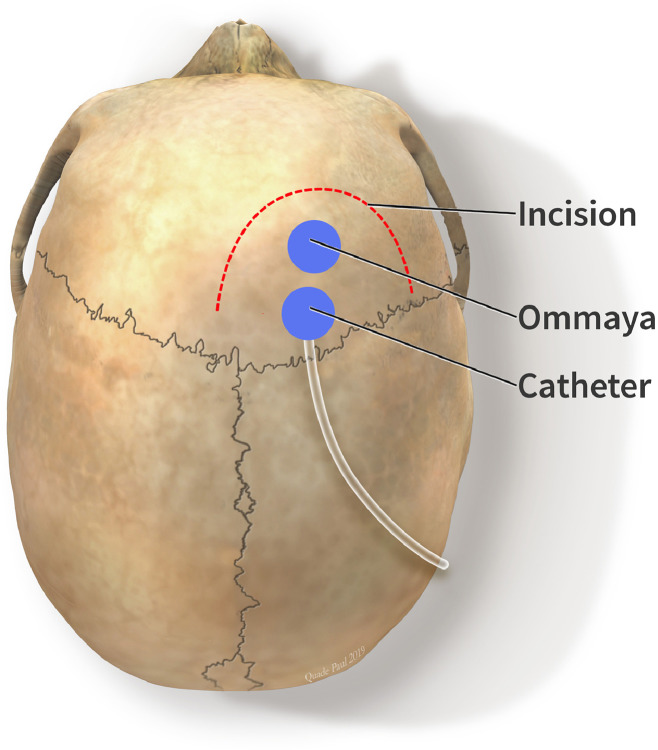
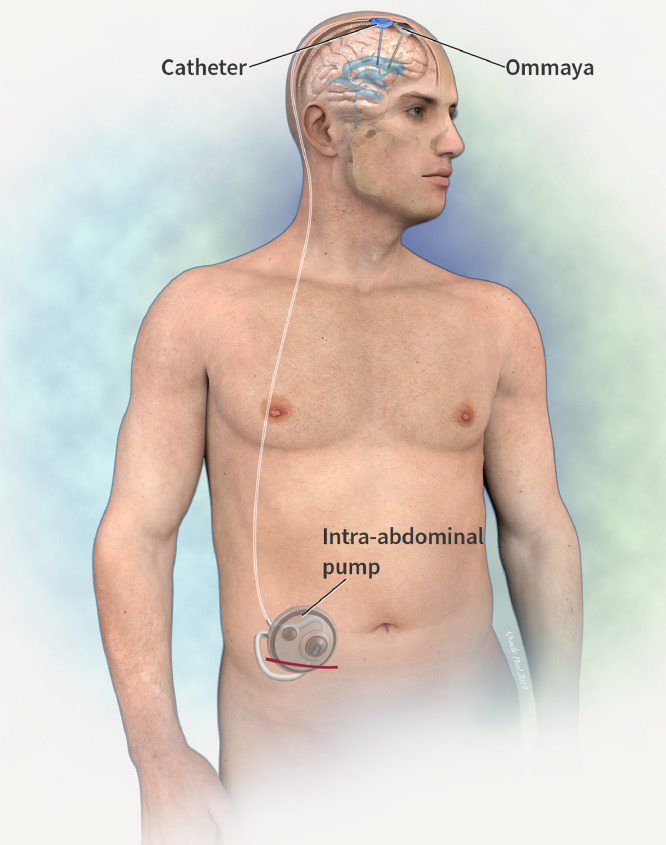
The system was comprised of an abdominal CSF infusion pump (Flowonix, Medtronic, or Tricumed®) with an intraventricular catheter, and an Ommaya® reservoir connected to an intraventricular catheter. Frameless stereotaxy was used (Axium® Medtronic) to plan a right frontal curve incision. Two burr-holes were made at Kocker's point in the sagittal plane to access the frontal horn of the right lateral ventricle. The Ommaya reservoir and catheter were placed first in the anterior burr-hole (Fig. 1). Using a Stimloc Burr-hole cover (Medtronic®) the drug delivery catheter was placed and secured in the burr-hole posterior to the Ommaya device to avoid inadvertent puncture when sampling CSF. An incision and pocket were made above the abdominal fascia in the right lower abdomen to contain the pump (Fig. 2).
Fig. 1.
Location of Intraventricular Catheter and Ommaya reservoir.
Fig. 2.
Location of intra-abdominal pump and subcutaneous catheter.
For the first post-operative week, the pump infused normal saline. The blinded study period ran from Day 8 to 50 with weekly visits, during which blood and CSF samples were taken. Follow-up neurological exams, quality of life questionnaires, collection of seizure diaries, reports of any side effects, and EEGs were performed during the study. At 52 weeks, the subject entered an open-label phase.
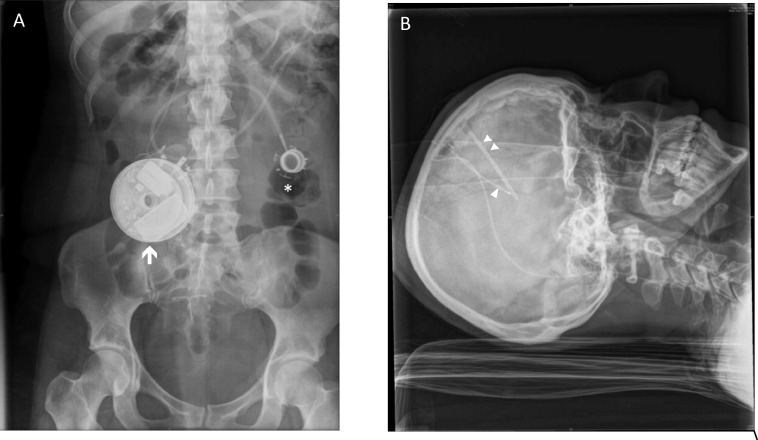
The catheter was passed subcutaneously from the frontal area to the abdomen with a relieving incision over the right mastoid. All implantations were right-sided. Post-operatively a contrast CT study was performed to confirm correct location of the catheter systems and patency (Fig. 3).
Fig. 3.
X-Rays of abdominal pump and catheter location. Shown in A is the location of the abdominal pump (arrow), with a surgical device previously implanted for obesity therapy indicated (asterisk). In B the location of the intraventricular delivery catheter (single arrowhead) and Ommaya reservoir (double arrowhead) are shown.
The drug titration schedule is shown in Table 1. A placebo week was inserted at a random week during the protocol, primarily to validate side effect reporting.
Role of funding body
The sponsor had roles in study design; data collection, analysis, and interpretation; and writing of the article. DA is the CEO and founder; ED is the VP of Clinical Development and SP is a Research Manager of the sponsoring body. RF holds stock options in the sponsoring company. No other authors received compensation. All authors had full access to all study data. The corresponding author made the decision to submit the paper for publication and produced the final draft.
Subjects
Five adult (four females, one male) subjects (age 25–38 years), with severe long-standing focal epilepsy were refractory to more than six ASDs for many years (including valproate greater than 1000 mgs per day). Valproate had been discontinued in all primarily because of lack of efficacy. When previously taking oral valproate, all noted minor sedation, 4 subjects had also complained of tremor, and 2 subjects significant weight gain. Subjects, who were implanted experienced 29–234 complex focal seizures monthly in the month prior to trial commencement, were evaluated by subject-reported seizures using an electronic diary, had undergone extensive clinical and radiological assessment including video-EEG, MRI and EEG-MEG (clinical and demographic features are shown in Table 2), and had electro-clinical and/or imaging features consistent with seizures involving (although, not necessarily originating in) the mesial temporal region. CSF samples were taken via the Ommaya reservoir and used to establish CSF drug levels and kinetics. Peripheral blood samples were taken for systemic ASD levels. Doses were increased stepwise to a predetermined level according to a protocol against suitable clinical parameters. Seizure records were kept using an electronic diary system.
Table 2.
Patient characteristics.
| Age @ dx/ yrs | Localization/Imaging Etiology | Semiology | Presumed Epileptogenic zone | Sz freq | Failed Medications | |
|---|---|---|---|---|---|---|
| 28/f | 12 | Band heterotopia. Bilateral. PET left temporal hypometabolism | Deja vu, nausea, occasionally visual field disturbance. Loss of contact. Posturing right arm, frequent GTCS | MEG and ictal scalp EEG strongly localizing to left mesial temporal lobe | Several/week | VPA, LEV, PMP, ZNS, LCM, PHB, CLZ |
| 41/f | 24 | Periventricular nodular heterotopia | Olfactory aura, loss of contact, left sided weakness | Mesial temporal clinically and on EEG/MEG | Several/week | VPA, LEV, CBZ, CLB, ZNS, PMP, OCZ |
| 40/f | 20 | Encephalitis, clear bilat MTS on MRI, Bilateral mesial temporal hypometabolism on PET | Déjà vu, olfactory or gustatory aura, loss of contact. Occasional GTCS | Bilateral MTS with bilateral onset on scalp VEEG. Interictal independent bitemporal spikes | Several/week | VPA, TPM, PMP, CBZ, LMG, CLZ, PHT |
| 37/m | 30 | Post traumatic? | Olfactory aura, Deja vu oral and manual automatisms, vocalization, loss of awareness, frequent GTCS | Felt mesial temporal on clinical grounds | Several/month | PHT, VPA (cognitive issues), LMG, LEV, CBZ, ZNS, PMP, TPM, LCM, PHB |
| PET normal | ||||||
| MRI normal | ||||||
| 53/f | 48 | Non-lesional on MRI and PET. MEG left mesial temporal | Epigastric aura, confusion, loss of contact | L Mesial temporal on MEG and bilateral HC depth | Several/week | LEV, VPA, TPM, CBZ, LCM, PMP |
Abbreviations: CBZ – carbamazepine, CLZ – clonazepam, LCM – lacosamide, LEV – levetiracetam, LTG – lamotrigine, OXC – oxcarbazepine, PHT – phenytoin, PRP – perampanel, RTG – retigabine, TPM – topiramate, VPA – valproic acid, ZNS – zonisamide, LOA - loss of awareness, GTCS – generalized tonic-clonic seizure.
Results
Safety
The procedure, implantation and intra-cerebroventricular VPA were well-tolerated by all subjects.
No clinical dose-limiting side effects were encountered, and no evidence of local toxicity was detected in three subjects evaluated with MRI T2 imaging. Subjects often noted a persistent central ‘cold’ feeling, often as the dose reached an effective level. Mild nausea and appetite loss were often reported. Insomnia was a frequent but transient phenomenon at dose increases. A single female patient developed minor hair loss at the upper limit of the VPA dose. Other familiar side-effects of VPA, such as tremor, sedation, and appetite increase were not observed in any subjects. No changes were detected otherwise on physical examination, subjects remained alert and comfortable and wished to continue the therapy despite these side-effects.
We performed T2 weighted non-contrast MRI scans to screen for signs of periventricular T2 weighted changes which could suggest local effect of ICV infusion. We were able to demonstrate in these three subjects that there was no peri ventricular T2 changes or the signs of edema associated with ICV therapy exposure. The range of exposure was 4–8 months for dose ranges of 40–100 mg/day during which the MRI scans were conducted.
Subjects have had ICV therapy for up to 2.5 years at the time of submission, and all patients continue to receive ICV VPA treatment. Pump failure was the major complication and occurred with the first pump chosen for the study in all subjects after approximately five months of use, necessitating removal and reimplantation of a new device. The clinical benefits of the study were by this time sufficiently clear to justify re-implantation. Pump failure was quickly identified clinically as seizures promptly returned to pre-trial levels and confirmed with estimation of residual pump volumes. The second pump chosen for the study also failed after approximately five months and again required removal and reimplantation. The reasons for failure were not clear despite engineering review of the devices, though with later pumps it was noted the silicone components of the system had been affected, likely due to the drug formulation.
One patient experienced skin erosion over the Ommaya reservoir leading to removal of the Ommaya reservoir, catheter, and drug delivery catheter. It was later successfully re-implanted, although without the Ommaya reservoir. In another patient, an intra-abdominal pump device became infected after six months. It was removed and re-implanted. Other complications included pain and neck discomfort related to the catheter. An Ommaya reservoir was removed from another patient after returning persistently positive CSF cultures (Propionibacterium acnes), with a pleocytosis (6 polymorphonuclear cells) though the patient was well. After normal lumbar CSF studies, it was elected to remove the Ommaya and catheter alone from this patient and clinically monitor, but to continue delivery of ICV valproate because of the marked clinical improvement. The patient remains well and there are no signs of systemic or CNS infection.
In terms of device adverse events, a total of nine were serious and required intervention to resolve. The majority (7 of 9) were primarily related to pump failure and the rest (2 of 9) were related to infection primarily related to repeated transcutaneous access for pharmacokinetic drug levels. A new pump was chosen free of silicone components. These specialized systems have been functioning for more than 15 months (mean 12.5 months, range 11.5–15 months) with a total of 62-months of on-therapy across all subjects. In addition, drug sampling from the Ommaya was done in a more limited and modified way. The combination of these modifications has resulted in no subsequent device-related adverse events with the current pump system being used by the subjects.
Effectiveness
There are currently five subjects implanted with the new pump and receiving therapy. Across these five subjects there is a total of 62 months of active therapy (mean 12.5 months, range 11.5–15 months). We performed an analysis of seizure frequency compared to baseline for these subjects.
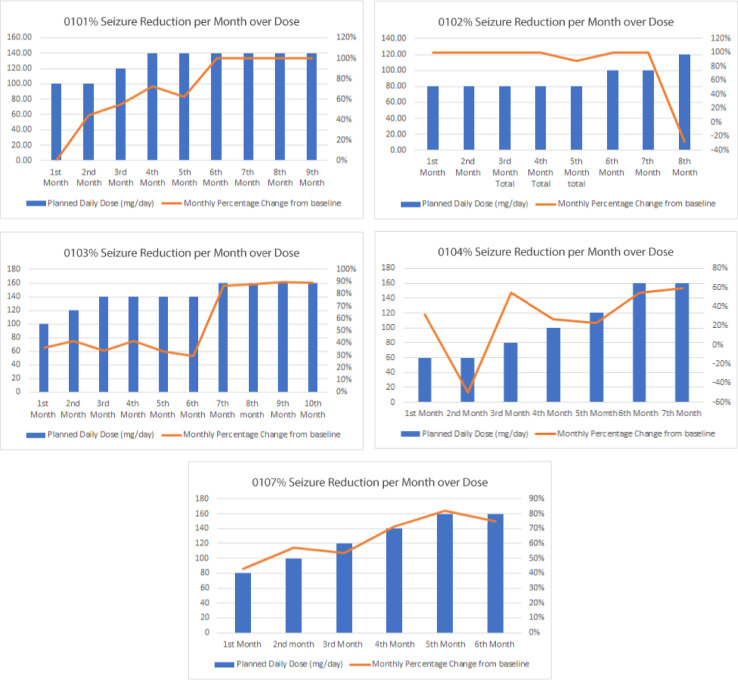
As of data-cutoff at 30 June 2019, mean monthly seizure reduction was 70% (range 45–94%, n = 5). Responder rate of at least a 50% seizure frequency reduction was 80% with maximum doses of 80–160 mg/day (Table 3 & Fig. 4). When looking at the most recent three months, representative of all subjects at their highest doses mean seizure reduction was 80% (range 46–100%, n = 5). Seizure freedom has been achieved in 60% of the subjects with one subject now seven months seizure free and two other subjects having achieved seizure freedom periods of greater than four weeks. Improvement was dose-dependent, such that some subjects demonstrated reduction or elimination of seizures as dosing approached 160 mg per day.
Table 3.
Monthly seizure reduction results for all subjects.
| Subject | Monthly% Change based on Average (n) as of 15 Jan 2019 | Monthly% Change Through March based on Average (n) | Monthly% Change Through March based on Median | Last 3-month% Change based on Average (Jan15-Apr15) | Last 3-month% Change based on Median (Jan15-Apr15) |
|---|---|---|---|---|---|
| 0101 | −69% (6) | −75% (9.5) | −75% | −100% | −100% |
| 0102 | −97% (4) | −94% (7.5) | −100% | −65% | −65% |
| 0103 | −32% (7) | −57% (10) | −42% | −92% | −91% |
| 0104 | −55% (5) | −27% (8) | −31% | −59% | −55% |
| 0107 | −61% (2) | −64% (6.5) | −64% | −71% | −78% |
| Average | −63% | −63% | −62% | −77% | −78% |
Fig. 4.
Dose response curves for individual patients. Monthly percentage changes are shown.
Patient reported outcomes
As part of our approach to patient outcomes beyond seizure reduction, we also measured the Quality of Life in Epilepsy 10 questions (QOLIE 10) and the Beck Depression Index (BDI). Due to patient flow and early pump failures, this was not done on all subjects. Baseline and follow-up QOLIE-10 and BDI scores were obtained on three of the five subjects. Across those three subjects we found improvements in two of three with QOLIE 10 and all three subjects with BDI.
Of the three subjects QOLIE 10 data was collected from, we saw an average improvement of 26% in scores (37%, 33%, 7% respectively with mean point reduction of 8; range 4–16). Most notably were marked improvements in energy (average three-point improvement), social limitations (average three-point improvement) and fear of seizures (average two-point improvement). We also saw clinically meaningful BDI improvements. All three tested subjects dropped at least one depression classification and average of 55% score reduction (12 points).
Valproate pharmacokinetics
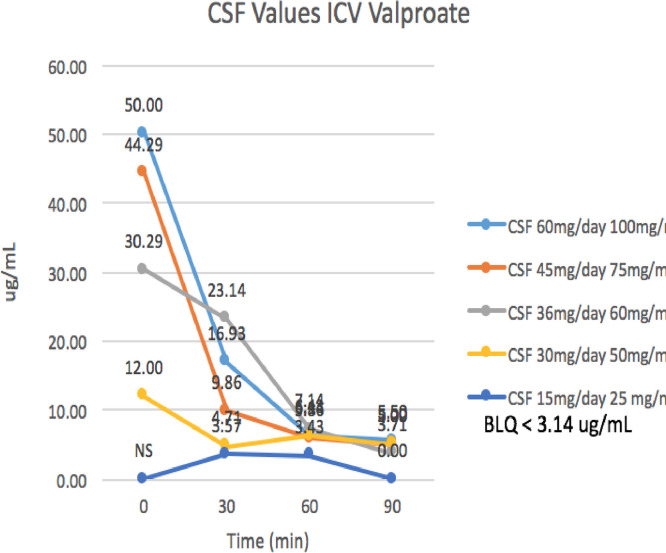
During the dose-escalation phase of the study from 2.5 mg to 60 mgs per day, valproate CSF and serum levels were sampled from zero to 90 min via the Ommaya reservoir, and for serum at intervals up to 24 h. During the open-label phase when the drug was further escalated, zero time point CSF samples were obtained. This data allowed us to understand relative CSF levels and serum levels for given ICV doses, as well as CSF half-life of valproic acid when administered ICV (Figs. 5 & 6).
Fig. 5.
CSF Valproate PK Levels versus Dose for Subject 0101.
Fig. 6.
CSF and Serum Valproate Pharmacokinetics. Valproate dose 45 mg/day, CSF levels free valproate, serum levels protein bound valproate.
In the initial dose escalation phase, CSF valproate levels averaged 45 micrograms/ml (range 20–70), with corresponding serum levels of 4 micrograms/ml. At the later dose escalation phase, the CSF levels increased to 160 and serum levels to 17 micrograms per ml. Pharmacokinetic modeling demonstrated that the CSF half-life was 20 min. The maximum CSF valproate level achieved was 280 for a prescribed daily dose of 160 mg/day. High Peak CSF levels were observed 2–20 times that predicted based on serum free and highest levels seen with animal and human studies. Low serum levels were seen, with free levels 10% of CSF levels, likely accounting for fewer observed systemic side effects. At 45 mg/day doses of valproate, the CSF/Bound plasma valproate ratio was 10:1 and the CSF/plasma free valproate was closer to 100:1, an inversion of the usual CSF/Serum ratio.
Discussion
This pilot study is the first demonstration of chronic ICV therapy as a potentially effective strategy in medically refractory epilepsy. Chronic intraventricular administration of valproic acid appears to safe and effective, and the procedure is well tolerated. Problems related to the pump reliability and infections were the major complications. High CSF levels are achieved with low serum levels and are shown to be effective despite unsuccessful earlier use of oral valproate and other ASD preparations.
The therapy was clearly efficacious, despite the very refractory nature of the epilepsy in these subjects. Pump failure provided a striking example of this, with prompt resumption of seizures to pre-study severity, with control rapidly being re-established on recommencement of ICV infusion.
We experienced a significant number of complications, primarily related to mechanical failure of the pump systems. Potential causes included unanticipated interactions between the valproate and pump components (despite initial compatibility testing for one month) and resolving these has led to re-engineered pumps that have satisfactory performance and lifespan. Other relevant factors may include changes of the viscosity of the valproate at higher concentrations and pump temperature. The infection rate is comparable to other published data for Ommaya reservoirs, with rates of 15–25% previously described [26,27]. Similarly, the rate of pump complications is similar to that seen in studies examining complication rates for devices implanted for spinal cord complaints [28], [29], [30].
Very high peak CSF levels of valproic acid were observed, but significant valproate toxicity was not encountered. It was particularly surprising that tremor and sedation were not observed indicating that the mechanism of these side effects may not be through direct valproate CNS toxicity as previously assumed. Alternatively, the CNS toxicity may be mediated in brain regions that are not exposed to the very high levels encountered in the periventricular regions, such as the brainstem or cerebellum. Kinetics of brain tissue penetration, uptake into the brain substance and half-life might limit exposure of other cerebral structures to valproate, but this hypothesis will require future testing. Tremor is reported to occur in up to 50% of patients taking valproate in other studies [31], and appears to be dose-related. Interestingly, mild reversible alopecia developed in a single patient at the maximum dose of valproate after two years of use after a dose escalation. This side effect is recognized in 11% of those administered valproate [32] and is regarded as unrelated to dose and occurring within the initial six months of use. Weight gain, a common and often limiting side effect of valproate in clinical practice, was conspicuously absent despite having been a problem for these same subjects when given as an oral preparation prior to this study. This is often also presumed to have a central basis, though the pathogenetic mechanism underlying this is uncertain. Verotti [33] notes that medication-induced weight gain is most likely complex and multifactorial with regulation at peripheral and central levels by various appetite-regulating neuropeptides and cytokines that act within the hypothalamus to affect food intake and energy expenditure. Other explanations have included dysregulation of the hypothalamic system, effect on adipokine levels, hyperinsulinaemia, insulin resistance and genetic susceptibility.
We observed steady and sustained peak CSF levels of valproate and widening of the therapeutic window through the combination of high steady state CSF levels, and low systemic levels for these subjects with medically refractory focal onset epilepsy.
Duration of therapeutic benefit depends, not so much on CSF kinetics, as it does on the effect on brain tissue. There has long been a belief that VPA has long-lasting effects [34] despite little detectable binding to brain tissue [35] The mechanism of this possible long-acting effect is unknown, as is its relevance to our current results.
This was a small study in a group of subjects with exceptionally refractory epilepsy, demonstrating clear efficacy of the drug administered in this manner, with relatively few drug-related side effects. While significant initial problems with the devices implanted occurred that required explantation and replacement, the data suggest that ICV valproate may be tolerated in the long term and may be effective in patients previously treated unsuccessfully with valproate orally. Potentially, alternative ASDs may be administered by this method including those without suitable oral preparations. This method is a first demonstration of CSF-infused antiepileptic medication in people with epilepsy and it introduces new treatment options. Before adoption of this therapy, larger studies with appropriate controls will be necessary.
Author contributions
MC -literature search, figures, study design, data collection, data analysis, data interpretation, writing. MM - literature search, data collection, writing. KB - literature search, data collection, writing. WDS - literature search, data analysis, data interpretation, writing. CP - literature search, data analysis, data interpretation, writing. EP - data collection, data analysis, data interpretation, writing. CW - data collection, data analysis, data interpretation, writing. AS - literature search, study design, data analysis, data interpretation, writing. RF - literature search, figures, study design, data analysis, data interpretation, writing. SP - literature search, figures, study design, data collection, data analysis, data interpretation, writing. ED - literature search, figures, study design, data collection, data analysis, data interpretation, writing. TA - study design, data analysis, data interpretation, writing. DA - literature search, figures, study design, data collection, data analysis, data interpretation, writing
Declaration of Competing Interest
SP is a founder and was a part time employee of the sponsor Cerebral Therapeutics and has salary and ownership. TA owns stock in this company. AS reports personal fees from Cerebral Therapeutics, during the conduct of the study. RF reports personal fees and other from Cerebral Therapeutics, personal fees from Medtronic, Eysz, Smart-Monitor, other from Irody, Avails Medical, and Zeto, outside the submitted work. ED is an employee of the sponsor Cerebral Therapeutics. DA is founder and CEO of the sponsor Cerebral Therapeutics and has salary and ownership. All other authors have no conflicts to report.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100326.
Appendix. Supplementary materials
References
- 1.Sander J.W. The epidemiology of epilepsy revisited. Curr Opin Neurol. 2003;16:165–170. doi: 10.1097/01.wco.0000063766.15877.8e. [DOI] [PubMed] [Google Scholar]
- 2.Shorvon S.D. Blackwell Publishing Ltd; 2009. Drug treatment of epilepsy in the century of the ILAE: the second 50 years, 1959–2009. [DOI] [PubMed] [Google Scholar]
- 3.Löscher W., Schmidt D. Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia. 2011;52:657–678. doi: 10.1111/j.1528-1167.2011.03024.x. [DOI] [PubMed] [Google Scholar]
- 4.Golyala A., Kwan P. Drug development for refractory epilepsy: the past 25 years and beyond. Seizure. 2017;44:147–156. doi: 10.1016/j.seizure.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 5.LaPenna P., Tormoehlen L.M. The pharmacology and toxicology of third-generation anticonvulsant drugs. J Med Toxicol. 2017;13:329–342. doi: 10.1007/s13181-017-0626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher R., Salanova V., Witt T. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 7.Salanova V., Witt T., Worth R. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84:1017–1025. doi: 10.1212/WNL.0000000000001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrell M.J. RNS system in epilepsy study group. responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 9.Medvid R., Ruiz A., Komotar R.J. Current applications of MRI-Guided laser interstitial thermal therapy in the treatment of brain neoplasms and epilepsy: a radiologic and neurosurgical overview. AJNR Am J Neuroradiol. 2015;36:1998–2006. doi: 10.3174/ajnr.A4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halliday A.J., Moulton S.E., Wallace G.G., Cook M.J. Novel methods of antiepileptic drug delivery – polymer-based implants. Adv Drug Deliv Rev. 2012;64(10):953–964. doi: 10.1016/j.addr.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Halliday A., Cook Polymer-based drug delivery devices for neurological disorders. CNS Neurol Disord Drug Targets. 2009;8(3):205–221. doi: 10.2174/187152709788680698. [DOI] [PubMed] [Google Scholar]
- 12.Fisher R.S., Ho J. Potential new methods for antiepileptic drug delivery. CNS Drugs. 2002;16:579–593. doi: 10.2165/00023210-200216090-00001. [DOI] [PubMed] [Google Scholar]
- 13.Cohen-Pfeffer J.L., Gururangan S., Lester T. Intracerebroventricular delivery as a safe, long-term route of drug administration. Pediatr Neurol. 2017;67:23–35. doi: 10.1016/j.pediatrneurol.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Löscher W. Basic pharmacology of valproate. CNS Drugs. 2002;16:669–694. doi: 10.2165/00023210-200216100-00003. [DOI] [PubMed] [Google Scholar]
- 15.Perucca E. Pharmacological and therapeutic properties of valproate. CNS Drugs. 2002;16:695–714. doi: 10.2165/00023210-200216100-00004. [DOI] [PubMed] [Google Scholar]
- 16.González-Darder J.M., García-Teno M. Anticonvulsant effect of intraventricular antiepileptic drugs. experimental study. Neurol Res. 2016;17:190–192. doi: 10.1080/01616412.1995.11740311. [DOI] [PubMed] [Google Scholar]
- 17.Serralta A., Barcia J.A., Ortiz P., Durán C., Hernández M.E., Alós M. Effect of intracerebroventricular continuous infusion of valproic acid versus single i.p. and i.c.v. injections in the amygdala kindling epilepsy model. Epilepsy Res. 2006;70:15–26. doi: 10.1016/j.eplepsyres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Vajda F., Lander C., O'Brien T. Australian pregnancy registry of women taking antiepileptic drugs. Epilepsia. 2004;45:1466. doi: 10.1111/j.0013-9580.2004.451103.x. –6. [DOI] [PubMed] [Google Scholar]
- 19.Stapleton S.L., Thompson P.A., Ou C.-.N. Plasma and cerebrospinal fluid pharmacokinetics of valproic acid after oral administration in non-human primates. Cancer Chemother Pharmacol. 2007;61:647–652. doi: 10.1007/s00280-007-0519-3. [DOI] [PubMed] [Google Scholar]
- 20.Morikawa N., Mori T., Abe T., Kawashima H., Takeyama M., Hori S. Pharmacokinetics of cytosine arabinoside, methotrexate, nimustine and valproic acid in cerebrospinal fluid during cerebrospinal fluid perfusion chemotherapy. Biol Pharm Bull. 2000;23:784–787. doi: 10.1248/bpb.23.784. [DOI] [PubMed] [Google Scholar]
- 21.Shen D.D., Ojemann G.A., Rapport R.L., Dills R.L., Friel P.N., Levy R.H. Low and variable presence of valproic acid in human brain. Am Acad Neurol. 1992;42:582–585. doi: 10.1212/wnl.42.3.582. [DOI] [PubMed] [Google Scholar]
- 22.Vajda F., Donnan G.A., Phillips J., Bladin P.F. Human-Brain, plasma, and cerebrospinal-fluid concentration of sodium valproate after 72 H of therapy. Am Acad Neurol. 1981;31:486–487. doi: 10.1212/wnl.31.4.486. [DOI] [PubMed] [Google Scholar]
- 23.Blasberg R.G. Methotrexate, cytosine-arabinoside, and bcnu concentration in brain after ventriculocisternal perfusion. Cancer Treat Rep. 1977;61:625–631. [PubMed] [Google Scholar]
- 24.Krames E.S. Intraspinal opioid therapy for chronic nonmalignant pain: current practice and clinical guidelines. J Pain Symptom Manag. 1996;11:333–352. doi: 10.1016/0885-3924(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 25.Krames E.S., Harb M. Neuromodulation. Academic Press; 2009. The rational use of intrathecal opioid analgesics; pp. 441–455. [Google Scholar]
- 26.Bosse R., Doonan B., Ali A. A retrospective review of complication rates of ommaya reservoir placement for intrathecal medication administration. J Clin Oncol. 2018;36:e18532. –2. [Google Scholar]
- 27.Lishner M., Perrin R.G., Feld R. Complications associated with ommaya reservoirs in patients with cancer - the Princess margaret hospital experience and a review of the literature. Arch Intern Med. 1990;150:173–176. [PubMed] [Google Scholar]
- 28.Taira T., Ueta T., Katayama Y. Rate of complications among the recipients of intrathecal baclofen pump in japan: a multicenter study. Neuromodulation. 2013;16:266–272. doi: 10.1111/ner.12010. [DOI] [PubMed] [Google Scholar]
- 29.Flueckiger B., Knecht H., Grossmann S., Felleiter P. Device-related complications of long-term intrathecal drug therapy via implanted pumps. Spinal Cord. 2008;46:639–643. doi: 10.1038/sc.2008.24. [DOI] [PubMed] [Google Scholar]
- 30.Stetkarova I., Yablon S.A., Kofler M., Stokic D.S. Procedure- and Device-Related complications of intrathecal baclofen administration for management of adult muscle hypertonia: a review. Neurorehabil Neural Repair. 2010;24:609–619. doi: 10.1177/1545968310363585. [DOI] [PubMed] [Google Scholar]
- 31.Alonso-Juarez M., Torres-Russotto D., Crespo-Morfin P., Fidel Baizabal-Carvallo J. The clinical features and functional impact of valproate-induced tremor. Parkins Relat Disord. 2017;44:147–150. doi: 10.1016/j.parkreldis.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Wang X., Wang H., Xu D., Zhu L., Liu L. Risk of valproic acid-related alopecia: a systematic review and meta analysis. Seizure. 2019;69:61–69. doi: 10.1016/j.seizure.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Verrotti A., D'Egidio C., Mohn A., Coppola G., Chiarelli F. Weight gain following treatment with valproic acid: pathogenetic mechanisms and clinical implications. Obes Rev. 2011;12:e32–e43. doi: 10.1111/j.1467-789X.2010.00800.x. [DOI] [PubMed] [Google Scholar]
- 34.Lockard J.S., Levy R.H. Valproic acid - reversibly acting drug. Epilepsia. 1976;17:477–479. doi: 10.1111/j.1528-1157.1976.tb04459.x. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg M.A., Todoroff T. Brain binding of anticonvulsants - Carbamazepine and valproic acid. Am. Acad. Neurol. 1980;30:826–831. doi: 10.1212/wnl.30.8.826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.