Abstract
In response to the energy demand triggered by developmental signals and environmental stressors, the cells launch the mitochondrial biogenesis process. This is a self‐renewal route, by which new mitochondria are generated from the ones already existing. Recently, considerable progress has been made in deciphering mitochondrial biogenesis‐related proteins and genes that function in health and in pathology‐related circumstances. However, an outlook on the intracellular mechanisms shared by the main players that drive mitochondrial biogenesis machinery is still missing. Here, we provide such a view by focusing on the following issues: (a) the role of mitochondrial biogenesis in homeostasis of the mitochondrial mass and function, (b) the signalling pathways beyond the induction/promotion, stimulation and inhibition of mitochondrial biogenesis and (c) the therapeutic applications aiming the repair and regeneration of defective mitochondrial biogenesis (in ageing, metabolic diseases, neurodegeneration and cancer). The review is concluded by the perspectives of mitochondrial medicine and research.
Keywords: ageing, cancer, metabolic diseases, mtDNA, neurodegeneration, nuclear respiratory factors, PGC‐1α, transcription factor A
1. MITOCHONDRIAL HOMEOSTASIS
Mitochondria are the major source of energy for the cellular activity, by ATP generation via oxidative phosphorylation. Emerging evidence of the last decade indicates that mitochondria form a highly dynamic intracellular network that executes the “quality control” of the organelle's population in a process that implies their fusion, fission and autophagic degradation (known as ‘mitophagy’). Mitochondria regulate the operation of intracellular signalling cascades, generate reactive oxygen species (ROS), execute fatty acids β‐oxidation, participate in aminoacid metabolism, pyridine synthesis, phospholipid modifications, calcium regulation and cells survival, senescence and death. The homeostasis of any healthy cell implies also a controlled regulation of mitochondrial mass and function, as an adaptive response to safeguard the mitochondrial (mt) DNA and to meet the energy demands vital for cellular function.
Mitochondrial homeostasis is preserved by the fine co‐ordination between two opposing processes: generation of new mitochondria, by mitochondrial biogenesis, and the removal of damaged mitochondria, by mitophagy. 1 , 2 Among the specific molecules involved in this fine‐tuning, the recent data highlight the peroxisome proliferator‐activated receptor‐γ coactivator (PGC)‐1α, the main regulator of mitochondrial biogenesis, 3 , 4 , 5 the PTEN‐induced putative kinase 1 (PINK1)‐Pakin, 6 that activates protein synthesis in damaged mitochondria, and the ligand‐activated transcription factor aryl hydrocarbon receptor, that functions also as protector from oxidative stress. 7
In examining mitochondrial homeostasis, one should consider the particular traits of these organelles in eukaryotic cells: (a) they have a prokaryotic origin and were acquired by eukaryotic cells via an endosymbiotic event, (b) are semi‐autonomous organelles: synthesize a rather small number of proteins by transcription and replication of mtDNA‐encoded genes, while the larger proportion of mitochondrial proteome (~60%‐70% 8 or more than 95% 9 ) is nuclear‐encoded, synthesized on cytosolic ribosomes, and finally, sorted and imported to the appropriate intra‐mitochondrial location. The encoding factors for nuclear genes identified so far are as follows: PGC‐1α, the transcription factor A (TFAM), the uncoupling proteins 2 (UCP2) and the uncoupling proteins 3 (UCP3), 10 (c) mitochondria biogenesis implies a specific route consisting in the recruitment of the novel proteins by the pre‐existing mitochondria, followed by their fragmentation, via fission. Associated with the rapid cell growth and proliferation, these events ensure the constant renewal of the mitochondrial population. 6 Uncovering the complexity of mitochondrial biogenesis operation is an exciting ongoing topic, and its main features are briefly examined next.
2. MITOCHONDRIAL BIOGENESIS MACHINERY‐ THE ASSOCIATED SIGNALLING PATHWAYS
The process of mitochondrial biogenesis takes place mainly in healthy cells. Interesting, in cancerous cells enhanced oxidative phosphorylation and mitochondrial biogenesis were correlated with invasion and metastasis. 11 It engages co‐ordination between the mitochondrial and the nuclear genomes, in a complex and multistep process (Figure 1) that involves:
Figure 1.
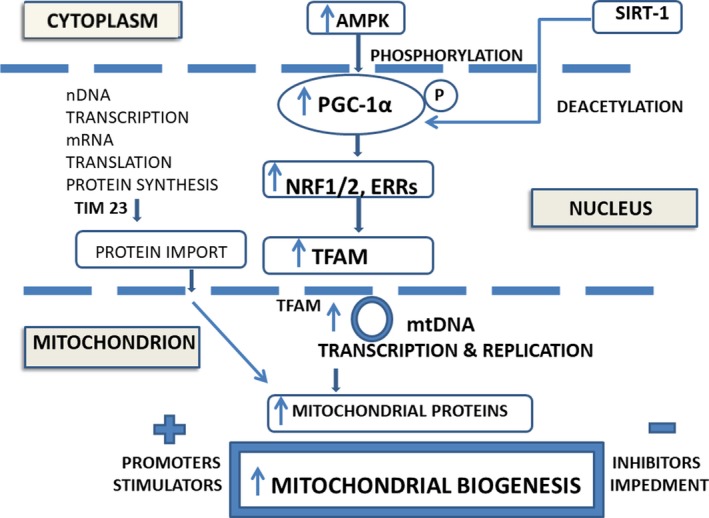
Mitochondrial biogenesis in brief: the central role of PGC‐1α activation and the contribution of proteins encoded by both nuclear and mitochondrial genomes (nDNA and mtDNA) to enhance the mitochondrial proteins content.
AMPK, 5' adenosine monophosphate‐activated protein kinase; ERR, the oestrogen‐related receptor; NRF, the nuclear respiratory factor; PGC‐1α, the peroxisome proliferator‐activated receptor‐γ coactivator‐1α; SIRT‐1, the silent information regulator‐1; TFAM, the transcription factor α; TIM 23, translocase
-
(i)
mtDNA transcription and translation mtDNA transcription is activated by the family of PGC‐1 proteins (PGC‐1α, PGC‐1β and PGC‐1), from which PGC‐1α is considered the master regulator of mitochondrial biogenesis. The pathway is initiated by PGC‐1α activation (by either phosphorylation or deacetylation), followed by stimulation of a series of nuclear transcription factors, that is the nuclear respiratory factor‐1(NRF‐1), NRF‐2 and oestrogen‐related receptor‐α (ERR‐α), and by the increase in expression of TFAM, the final effector of mtDNA transcription and replication. 12 , 13 , 14 Next, translation of the mtDNA‐encoded genes into proteins takes place with the assistance of specific translation factors (encoded by nuclear DNA, nDNA), such as the initiation factor 2 and 3 (mtIF2 and mtIF3), the elongation factors Tu, Ts and G1 (mtEFTu, mtEFTs and mtEFG1), the translational release factor1‐like (mtRF1L) and the recycling factors (mtRRF1 and mtRRF2); furthermore, the levels of mitochondrial proteins are regulated by the translational activator of cytochrome c oxidase 1 (TACO1) that binds the mitochondrial RNA (mRNA). 15
-
(ii)
Synthesis, import and assembly of mitochondrial proteins encoded by nDNA These mitochondrial proteins originate from the preproteins synthesized within the cytosol and provided with an amino‐terminal cleavable targeting signal. The translocase TIM23 directs the signal of preproteins towards the mitochondrial matrix, where they assemble, and are sorted to a precise intra‐mitochondrial location, that is the matrix or the inner mitochondrial membrane (IMM). The energy required for driving this import pathway is provided by the mitochondrial membrane potential and the ATP (by oxidative phosphorylation). 8 , 16 The biogenesis of the outer mitochondrial membrane (OMM) proteins has been studied so far in unicellular organisms, such as the yeast Saccharomyces cerevisiae; the OMM functions as an interface with the cytosol and is particularly important for mitochondrial dynamic changes (fission, fusion) and interaction with other intracellular organelles. 9
The mitochondrial biogenesis markers are the mtDNA copy numbers, the elevated mtDNA:nDNA ratio and the level of mitochondrial gene expression. 1 , 17 In cancer cells, 18 an augmented expression of PGC‐1α, 19 NRF1 and TFAM was reported, although these cells have a reduced number of mitochondria. 20 Moreover, a recent report underlines that the use of TFAM level as a biogenesis marker is questionable, as it does not always match the mtDNA copy number, and the expression of mtDNA‐encoded polypeptides. 21
What are the consequences of mitochondrial biogenesis? The current data acknowledge the increase in the oxidative phosphorylation capacity, the diminishment of pathologic oxidative stress and the repair of mitochondrial‐associated dysfunctions. 13
How can be measured mitochondrial biogenesis? Reliable strategies are based on the magnitude of mtDNA synthesis and of mitochondrial membrane phospholipids. In this context, two cautions are noted: (a) a change in the number of mitochondria is not indicative of biogenesis, as their amount is not exclusively due to synthesis, 22 and (b) mitochondrial biogenesis may conduct to detrimental effects, such as the import of misfolded proteins into the organelle, and the silencing of the unfolded protein response in the endoplasmic reticulum. 23
2.1. Mitochondrial biogenesis inductors/promoters
Mitochondrial biogenesis induction is associated with activation of transcription factors that act on mitochondrial genes and with up‐regulation of local translation of mitochondrial proteins. These effects are produced in response to several natural products, such as 6‐gingerol (the main active component of the ginger extracts) 24 and Ursolic acid (a natural triterpene). 25 In contradistinction, relatively few synthetic drugs have been identified as mitochondrial biogenesis inductors. 12
Reportedly, the following signalling pathways sustain transcription activation during mitochondrial biogenesis:
the AMPK/ PGC‐1α pathway used by C1q/tumour necrosis factor‐related protein‐3 (CTRP3) to promote biogenesis in cardiomyocytes, 26 and by the ginger extract, in both mice and HepG2 cells. 24 Furthermore, AMPK phosphorylates and activates histone acetyltransferase 1 (HAT1), creating a more relaxed chromatin‐DNA structure that favours transcription; AMPK phosphorylates also the epigenetic factor DNA methyltransferase 1 (DNMT1) that limits transcription factors access to promoters. 10 A recent report shows that the diterpene alkaloid benzoylaconine activates AMPK signalling cascade and stimulates mitochondrial biogenesis 27 ;
the induction of PGC‐1α along with its downstream target genes, NRF1 and TFAM. Such signalling cascade was identified in pancreatic MIN6 β‐cells, after the humanin treatment, 28 and in 3T3‐L1 pre‐adipocytes, after salicylate medication. 29 Activation of PGC‐1α signalling pathway is mediated also by the transcription factor cAMP response element‐binding protein (CREB); it binds to certain DNA sequences (the cAMP response elements) and subsequently increases/decreases genes transcription. In endothelial cells, CREB/ PGC‐1α pathway promotes mitochondrial biogenesis by activation of G protein‐coupled receptor (TGR5), 30 the route operating also after lixisenatide medication, a drug approved by the US Food and Drug Administration for the treatment of type 2 diabetes 31 ;
stimulation of the Gβγ (a component of heterotrimeric G proteins)‐Akt‐eNOS‐sGC (soluble guanylatecyclase) pathway by the β2 adrenergic receptor agonists, such as formoterol, allowing recovery from acute and chronic degenerative diseases, 13 and carvedilol, employed in heart failure 32 ;
the return to normal of the Akt/ transcription factor FoxO3a axis under the influence of dietary β‐hydroxy‐β‐methylbutyrate (HMB), is another condition that improves mitochondrial biogenesis 33 ;
the sirtuins assistance in transcription: it is known that the silent information regulator‐1 (SIRT1) activates the PGC‐1α‐mediated transcription of nuclear and mitochondrial genes encoding for proteins during mitochondria proliferation, oxidative phosphorylation and energy production, while SIRT3 stimulates the proteins important for oxidative phosphorylation, tricarboxylic acid cycle and fatty‐acid oxidation, and indirectly, the PGC‐1α and AMPK.
Taken together the above data, it is obvious that up‐regulation of transcription factors is a key event in mitochondrial biogenesis. However, depending on ligands specificity, unwanted genes may be equally activated, conducting to detrimental (neurological and hyperproliferative) effects. 12
Up‐regulation of mitochondrial proteins translation is associated with exercise‐induced mitochondrial biogenesis (in the plantaris muscle). 15 An interesting mechanism implied in biogenesis of healthy mitochondria was deciphered in Drosphila: the MDI protein of the mitochondrial OM recruits the translational stimulator La‐related protein (Larp) and promotes the synthesis (on mitochondrial surface) of a subset of nuclear‐encoded mitochondrial proteins by cytosolic ribosomes. 6
2.2. Mitochondrial biogenesis stimulators and inhibitors
In physiological conditions, the response of cells to energy demands leads to either up‐ or down‐regulation of the transcription factors that stimulate and/or inhibit mitochondrial biogenesis, respectively. The pathology‐associated disturbances of mitochondrial biogenesis consist in an impeded mitochondrial biogenesis, a condition in which stimulation of the declined process is required, or in abnormal higher levels of this process, when and a diminishment is necessary.
Examples of efficient stimulators of mitochondrial biogenesis are the followings: formoterol, used for treating podocytopathies, 18 resveratrol (a polyphenol), that prevents rotenone‐induced neuronal degeneration, 34 acetylcholine, protector in hypoxia/reoxygenation injury, 35 adiponectin, a cardioprotector in diabetes, 36 and tetrahydrobiopterin, helpful for the cardiac contractility. 37 The cellular mechanism beyond the above stimulatory effects on mitochondrial biogenesis entails the up‐regulated expression of the transcriptional regulator PGC‐1α. Recently, normalization of Akt/FoxO3 axis was reported to be involved in the protective effects of dietary HMB against lipopolysaccharide (LPS)‐induced muscle atrophy. 33 Another regulatory mechanism is based on phosphorylation of GSK‐3β exerted by arachidonyl‐2‐chloroethylamide (ACEA, a selective agonist of cannabinoid receptor1) effective at the beginning of cerebral ischemia. 38
Several natural extracts have been found to stimulate mitochondrial biogenesis. Such regulatory effects were recently reported for the Kaempferia parviflora extracts (containing methoxyflavones), that act through the SIRT1/AMPK/PGC‐1α/PPARδ pathway, 39 for tangeretin (a polymethoxylated flavonoid of mandarin fruits), activator of AMPK/PGC‐1α pathway, 40 for salidroside (isolated from Rhodiola rosea L.), that stimulates the miR22/SIRT1 pathway, 41 for the spice saffron (Crocus Sativus L.), that augmented NRF‐1 gene expression in exercised rats, 42 and for the natural precursor of resveratrol, polydatin that enhances SIRT1 expression. 43
The inhibitors of mitochondrial biogenesis down‐regulate the expression of the associated‐transcription factors, such as PGC‐1α, TFAM and AMPK. The activity of PGC‐1α pathway is reduced by miR‐130b‐p, 44 2‐methoxyestradiol, 45 cyclosporine A, 46 XCT790 (a potent and selective inhibitor of the oestrogen‐related receptor α) 47 and the high glucose high‐fat environment. 36 The down‐regulation of TFAM takes place at the use of the local anaesthetic ropivacaine 48 and at the in vitro treatment of cells with silica nanoparticles. 49 Furthermore, the diminished AMPK expression explains resistin inhibitory effects on mitochondrial biogenesis. 50
It is evident that reduced biogenesis is accompanied by other mitochondrial dysfunctions, such as an impaired ATP synthesis capacity leading to acceleration of mitophagy, critical for cell health. A reduced mtDNA/ nuclear ratio 1 has also been reported to be associated with the impairment of biogenesis/altered biogenesis.
The examination of the two opposite sides of mitochondrial biogenesis, that is the impairment (such as in ageing, metabolic and neurodegenerative diseases) and the abnormal intensification (in some tumours) conducted in the last decade to identification of several strategies adequate for exploitation in therapy. Examples are discussed next.
3. DYSREGULATION OF MITOCHONDRIAL BIOGENESIS; REPAIR STRATEGIES
3.1. Ageing
The cells senescence and the consequent ageing is associated with the impairment of mitochondrial biogenesis and bioenergetic potential, the decrease in mitochondrial dynamics, the altered quality control, the failure in mtDNA repair, the accumulation of mtDNA mutations and the decline in mitophagy. 51 , 52 , 53 , 54 The main factors involved in ageing effects on mitochondrial biogenesis are the reduced activity of AMPKα and the decreased expression of SIRT1, PGC‐1α, TFAM and NRF‐1,2, 55 , 56 along with the regulatory loop that engages PGC‐1α and NRF‐2 interaction. 57 Strategies to prevent/delay age‐associated decline in mitochondrial biogenesis consists in stimulation of PGC‐1α signalling with tetrahydrobiopterin 37 or with resveratrol, 58 modulation of TFAM binding to mtDNA, 59 mitophagy regulation, 60 dietary supplementation with acetyl‐l‐carnitine (ALCAR), 51 , 53 cells exposure to gomisin A (a bio‐active compound isolated from the fruit of Schisandra chinensis), 61 the regular exercise training, and the calorie restriction. 51 The current endeavours aimed to delay/counteract the age‐associated decline of mitochondrial biogenesis may have translational relevance for promotion of a healthy ageing, for protection against age‐related pathologies and for the improvement of the quality of life of the elderly.
3.2. Metabolic diseases
The impairment of mitochondrial biogenesis and function has been linked to metabolic diseases such as type 2 diabetes and obesity. In diabetic kidney, the mechanism beyond the reduced mitochondrial biogenesis implies the decrease of PGC‐1α/AMPK/SIRT‐1 signalling pathway. 62 In placentae of diabetic mothers, impaired mitochondrial biogenesis engages PGC‐1α/TFAM signalling pathway and is mainly present at male offspring; this trait may explain the propensity for development of future metabolic diseases in adult males. 63 In diabetic heart, earlier studies reported that hypoadiponectinemia impaired AMPK‐PGC‐1α signalling 64 ; more recently, in a model for type 2 diabetes (a high glucose/high‐fat medium) adiponectin was found to partial rescue mitochondrial biogenesis in cardiomyocytes, via PGC‐1α‐mediated signalling. 36 This pathway participates in cardioprotection and is evaluated as a novel therapeutic target. 65
Mitochondrion is regarded now as a possible target for the prevention and treatment of chronic metabolic disorders; in this context, the endurance exercise it is routinely used to alleviate the reduced mitochondrial biogenesis. 66 Furthermore, the antidiabetic effect of mitochondrial biogenesis enhancers, such as Spirulina platensis 67 and Alogliptin (a dipeptidyl‐peptidase‐4 inhibitor) 68 were recently reported.
Another ongoing topic is the regulation of mitochondrial biogenesis in adipocytes. 69 , 70 The obesity‐associated signalling entails hyperacetylation of PGC‐1α, 71 and induction of pAMPK, PGC‐1α, NRF‐1 and TFAM (after the salicylate treatment of pre‐adipocytes). 29 Activation of AMPK along with stimulation of mitochondrial gene expression and mtDNA replication explain the beneficial effects of isorhamnetin (3‐O‐methyl quercetin) on adipocytes mitochondrial biogenesis. 72 AMPK activation contributes also to the anti‐obesity effects of zeaxanthin (an oxygenated carotenoid) that promotes mitochondrial biogenesis and expression of brown and beige adipogenesis markers. 73 The regulation of mitochondrial biogenesis in beige adipocytes (in the course of browning) involves PGC‐1α signalling, associated with miR‐494‐3p expression. 74 Other stimulatory factors of mitochondrial biogenesis are NRF‐1 and the mitochondrial transcription factor A that intervene in metformin effect on brown adipocytes. 75 In contradistinction, decreased UCP1 expression explains the reduced mitochondrial biogenesis generated by arsenite in brown adipocytes. 76
The above basic findings may be used as a basis for further clinical approaches in metabolic diseases.
3.3. Neurodegeneration
Mitochondrial biogenesis is a potential novel therapeutic target for neurodegenerative diseases treatment including Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD) and amyotrophic lateral sclerosis (ALS). 1 Although this strategy is based on a plethora of basic and (pre)clinical results, in the present overview only the data on the intracellular pathways beyond mitochondrial biogenesis are mentioned.
In AD and PD, mitochondrial biogenesis is impaired 77 and augmenting this process turned into a therapeutic approach. The intracellular mechanism was uncovered in hippocampal neurons, where amyloid β25‐35 inhibits AMPK‐SIRT‐1, PGC‐1α pathway. 78 Recent reports indicate melatonin, as a promoter of mitochondrial biogenesis, 77 along with resveratrol, that induced PGC‐1α and mtTFA expression, 34 berberine (a natural AMPK activator), that stimulates PGC‐1α and NRF‐2 in neuronal cells, 79 and rotenone, an inhibitor of Complex I. 80 Moreover, necdin (a melanoma antigen) prevents mitochondria‐associated neurodegeneration by binding to PGC‐1α and suppressing its proteolytic degradation in the ubiquitin‐proteasomal system. 81 , 82 Interestingly, mtDNA replication appears to be an early response to neurodegeneration‐associated stress and a precursor for mitochondrial biogenesis in axons. 80
Distinctly, the neurotoxic effect of some medicines is accompanied by reduced mitochondrial biogenesis. An example is the local anaesthetic ropivacaine (employed in medical and dental care) that reduces expression of mitochondrial regulators PGC‐1α, NRF‐1 and TFAM. 48
3.4. Cancer
It is known that mitochondrial biogenesis targeted therapies are efficient for the prevention and treatment of relapsed and resistant cancers. 47 The pointed intracellular pathways are PGC‐1α (important also for the cells adaptive response against chemotherapeutic stress), 83 AMPK (a proximal signalling step for mitochondrial biogenesis) 84 and dynamin‐related protein‐1 (Drp1) up‐regulation, accompanied by augmented levels of PGC‐1α, NRF‐1 and TFAM. 19 Among the modulators of mitochondrial biogenesis, sulforaphane (a sulphur‐rich compound found in cruciferous vegetables) is considered a potential antineoplastic agent; in prostate cancer cells, it stabilizes NRF‐2, increases the expression of PGC‐1α and decreases the level of hypoxia‐inducible factor‐1α (HIF‐1α). 85 Chemotherapy medication with cisplatin stimulates PGC‐1α expression and up‐regulates mitochondrial biogenesis. 83
Mitochondrial biogenesis is increased in some invasive cancer cells, such as osteosarcoma; the use of 2‐methoxyestradiol inhibits biogenesis, via regulation of PGC‐1α, COX1 and SIRT‐3. 45 In this circumstance, the strategy to stop the increased propagation of cancer stem cells consists in doxycycline inhibition of mitochondrial biogenesis. 86
4. CONCLUSION AND PERSPECTIVES
The mitochondrial biogenesis is a complex biological process (Figure 1), that controls organelle's self‐renewal and the maintenance of mtDNA, ensuing cell homeostasis. This topic is under intense investigation at present. The intracellular signalling pathways uncovered so far identified PGC‐1α as a master regulator of mitochondrial biogenesis, implicated in the response to several inductors/promoters, stimulators and inhibitors. Dysregulated mitochondrial biogenesis occurs not only in senescence and ageing, but also in metabolic diseases, neurodegeneration and cancer, and is potentially ameliorated by the novel mitochondria‐based therapies. However, there are still several issues that require an answer, such as the association between impaired mitochondrial biogenesis and the early stage of myocardial remodeling, 87 the neuron‐specific mechanism of mitochondrial biogenesis, 81 and the limitation of osteoarthrosis progression in chondrocytes, 55 among others. Challenging topics are the exploitation of mitochondria‐based therapies for the treatment of chronic degenerative diseases 12 , 13 and for the cancer prevention. 88
CONFLICT OF INTEREST
The author confirms that there is no conflict of interest.
Popov L‐D. Mitochondrial biogenesis: An update. J Cell Mol Med. 2020;24:4892–4899. 10.1111/jcmm.15194
DATA AVAILABILITY STATEMENT
Most of the 88 references are associated with the corresponding DOI reference number.
REFERENCES
- 1. Golpich M, Amini E, Mohamed Z, et al. Dysfunction and biogenesis in neurodegenerative diseases: pathogenesis and treatment. CNS Neurosci Ther. 2017;23:5‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ploumi C, Daskalaki I, Tavernarakis N. Mitochondrial biogenesis and clearance: a balancing act. FEBS J. 2017;284:183‐195. [DOI] [PubMed] [Google Scholar]
- 3. Untereiner AA, Fu M, Módis K, et al. Stimulatory effect of CSE‐generated H2S on hepatic mitochondrial biogenesis and the underlying mechanisms. Nitric Oxide. 2016;31(58):67‐76. [DOI] [PubMed] [Google Scholar]
- 4. Wenz T. Regulation of mitochondrial biogenesis and PGC‐1α under cellular stress. Mitochondrion. 2013;13:134‐142. [DOI] [PubMed] [Google Scholar]
- 5. Scarpulla R, Vega R, Kelly D. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab. 2012;23:459‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Y, Xu H. Translational regulation of mitochondrial biogenesis. Biochem Soc Trans. 2016;44:1717‐1724. [DOI] [PubMed] [Google Scholar]
- 7. Wang X, Li S, Liu L, et al. Role of the aryl hydrocarbon receptor signaling pathway in promoting mitochondrial biogenesis against oxidative damage in human melanocytes. J Dermatol Sci. 2019;96:33‐41. [DOI] [PubMed] [Google Scholar]
- 8. Moulin C, Caumont‐Sarcos A, Ieva R. Mitochondrial presequence import: Multiple regulatory knobs fine‐tune mitochondrial biogenesis and homeostasis. Biochim Biophys Acta Mol Cell Res. 2019;1866:930‐944. [DOI] [PubMed] [Google Scholar]
- 9. Bruggisser J, Käser S, Mani J, Schneider A. Biogenesis of a mitochondrial outer membrane protein in Trypanosoma brucei: Targeting signal and dependence on a unique biogenesis factor. J Biol Chem. 2017;292:3400‐3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marin TL, Gongol B, Zhang F, et al. AMPK promotes mitochondrial biogenesis and function by phosphorylating the epigenetic factors DNMT1, RBBP7, and HAT1. Sci Signal. 2017;10(464):eaaf7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. LeBleu VS, O’Connell JT, Gonzalez Herrera KN, et al. PGC‐1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16:992‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cameron RB, Beeson CC, Schnellmann RG. Development of therapeutics that induce mitochondrial biogenesis for the treatment of acute and chronic degenerative diseases. J Med Chem. 2016;59:10411‐10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cameron RB, Peterson YK, Beeson CC, Schnellmann RG. Structural and pharmacological basis for the induction of mitochondrial biogenesis by formoterol but not clenbuterol. Sci Rep. 2017;7:10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. dos Santos TW, Pereira QC, Teixeira L, et al. Effects of polyphenols on thermogenesis and mitochondrial biogenesis. Int J Mol Sci. 2018;19:2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yokokawa T, Kido K, Suga T, et al. Exercise‐induced mitochondrial biogenesis coincides with the expression of mitochondrial translation factors in murine skeletal muscle. Physiol Rep. 2018;6(20):e13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Demishtein‐Zohary K, Azem A. The TIM23 mitochondrial protein import complex: function and dysfunction. Cell Tissue Res. 2017;367:33‐41. [DOI] [PubMed] [Google Scholar]
- 17. Andres AM, Tucker KC, Thomas A, et al. Mitophagy and mitochondrial biogenesis in atrial tissue of patients undergoing heart surgery with cardiopulmonary bypass. JCI Insight. 2017;2(4):e89303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamada S, Asa SL, Kovacs K. Oncocytomas and null cell adenomas of the human pituitary: Morphometric and in vitro functional comparison. Vichows Archiv A Pathol Anat. 1988;413:333‐339. [DOI] [PubMed] [Google Scholar]
- 19. Arif E, Solanki AK, Srivastava P, et al. Mitochondrial biogenesis induced by the β2‐adrenergic receptor agonist formoterol accelerates podocyte recovery from glomerular injury. Kidney Int. 2019;96:656‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zou P, Liu L, Zheng LD, et al. Coordinated upregulation of mitochondrial biogenesis and autophagy in breast cancer cells: The role of Dynamin Related Protein‐1 and implication for breast cancer treatment. Oxid Med Cell Longev. 2016;2016:4085727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kozhukhar N, Alexeyev MF. Limited predictive value of TFAM in mitochondrial biogenesis. Mitochondrion. 2019;49:156‐165. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller BF, Hamilton KL. A perspective on the determination of mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2012;302:E496‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shcherbakov D, Teo Y, Boukari H, et al. Ribosomal mistranslation leads to silencing of the Unfolded Protein Response and increased mitochondrial biogenesis. Commun Biol. 2019;2:381. 2019, eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deng X, Zhang S, Wu J, et al. Promotion of mitochondrial biogenesis via activation of AMPK‐PGC1ɑ signaling pathway by Ginger (Zingiber officinale Roscoe) extract, and its major active component 6‐Gingerol. J Food Sci. 2019;84:2101‐2111. [DOI] [PubMed] [Google Scholar]
- 25. Chen J, Wong HS, Leong PK, et al. Ursolic acid induces mitochondrial biogenesis through the activation of AMPK and PGC‐1 in C2C12 myotubes: a possible mechanism underlying its beneficial effect on exercise endurance. Food Funct. 2017;8:2425‐2436. [DOI] [PubMed] [Google Scholar]
- 26. Zhang CL, Feng H, Li L, et al. CTRP3 promotes mitochondrial biogenesis in cardiomyocytes through AMPK/PGC‐1α pathway. Biochim Biophys Acta Gen Subj. 2017;1861:3085‐3094. [DOI] [PubMed] [Google Scholar]
- 27. Deng XH, Liu JJ, Sun XJ, et al. Benzoylaconine induces mitochondrial biogenesis in mice via activating AMPK signaling cascade. Acta Pharmacol Sin. 2019;40:658‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qin Q, Jin J, He F, et al. Humanin promotes mitochondrial biogenesis in pancreatic MIN6 β‐cells. Biochem Biophys Res Commun. 2018;497:292‐297. [DOI] [PubMed] [Google Scholar]
- 29. Yan Y, Yang X, Zhao T, et al. Salicylates promote mitochondrial biogenesis by regulating the expression of PGC‐1α in murine 3T3‐L1 pre‐adipocytes. Biochem Biophys Res Commun. 2017;49:436‐441. [DOI] [PubMed] [Google Scholar]
- 30. Zhao LJ, Zhang SF. Activation of TGR5 promotes mitochondrial biogenesis in human aortic endothelial cells. Biochem Biophys Res Commun. 2018;500:952‐957. [DOI] [PubMed] [Google Scholar]
- 31. Zhao Z, Pu Y. Lixisenatide enhances mitochondrial biogenesis and function through regulating the CREB/PGC‐1α pathway. Biochem Biophys Res Commun. 2019;508:1120‐1125. [DOI] [PubMed] [Google Scholar]
- 32. Yao K, Zhang WW, Yao L, et al. Carvedilol promotes mitochondrial biogenesis by regulating the PGC‐1/TFAM pathway in human umbilical vein endothelial cells (HUVECs). Biochem Biophys Res Commun. 2016;470:961‐966. [DOI] [PubMed] [Google Scholar]
- 33. Duan Y, Zheng C, Zhong Y, et al. Beta‐hydroxy beta‐methyl butyrate decreases muscle protein degradation via increased Akt/FoxO3a signaling and mitochondrial biogenesis in weanling piglets after lipopolysaccharide challenge. Food Funct. 2019;10:5152‐5165. [DOI] [PubMed] [Google Scholar]
- 34. Peng K, Tao Y, Zhang J, et al. Resveratrol regulates mitochondrial biogenesis and fission/fusion to attenuate rotenone‐induced neurotoxicity. Oxid Med Cell Longev. 2016;2016:6705621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun L, Zhao M, Yu XJ, et al. Cardioprotection by acetylcholine: a novel mechanism via mitochondrial biogenesis and function involving the PGC‐1α pathway. J Cell Physiol. 2013;228:1238‐1248. [DOI] [PubMed] [Google Scholar]
- 36. Wang H, Yan WJ, Zhang JL, et al. Adiponectin partially rescues high glucose/high fat‐induced impairment of mitochondrial biogenesis and function in a PGC‐1α dependent manner. Eur Rev Med Pharmacol Sci. 2017;21:590‐599. [PubMed] [Google Scholar]
- 37. Kim HK, Jeon J, Song IS, et al. Tetrahydrobiopterin enhances mitochondrial biogenesis and cardiac contractility via stimulation of PGC1α signaling. Biochim Biophys Acta Mol Basis Dis. 2019;1865(11):165524. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 38. Bai F, Guo F, Jiang T, et al. Arachidonyl‐2‐Chloroethylamide alleviates cerebral ischemia injury through glycogen synthase kinase‐3β‐mediated mitochondrial biogenesis and functional improvement. Mol Neurobiol. 2017;54:1240‐1253. [DOI] [PubMed] [Google Scholar]
- 39. Kim MB, Kim T, Kim C, Hwang JK. Standardized Kaempferia parviflora extract enhances exercise performance through activation of mitochondrial biogenesis. J Med Food. 2018;21:30‐38. [DOI] [PubMed] [Google Scholar]
- 40. Kou G, Li Z, Wu C, et al. Citrus Tangeretin improves skeletal muscle mitochondrial biogenesis via activating the AMPK‐PGC1‐α pathway in vitro and in vivo: A possible mechanism for its beneficial effect on physical performance. J Agric Food Chem. 2018;66:11917‐11925. [DOI] [PubMed] [Google Scholar]
- 41. Mao GX, Xu XG, Wang SY, et al. Salidroside delays cellular senescence by stimulating mitochondrial biogenesis partly through a miR‐22/SIRT‐1 pathway. Oxid Med Cell Longev. 2019;2019(2019):5276096. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Akbari‐Fakhrabadi M, Najafi M, Mortazavian S, et al. Effect of saffron (Crocus sativus L.) and endurance training on mitochondrial biogenesis, endurance capacity, inflammation, antioxidant, and metabolic biomarkers in Wistar rats. J Food Biochem. 2019;43(8):e12946. [DOI] [PubMed] [Google Scholar]
- 43. Li P, Liu Y, Burns N, et al. SIRT1 is required for mitochondrial biogenesis reprogramming in hypoxic human pulmonary arteriolar smooth muscle cells. Int J Mol Med. 2017;39:1127‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jiang S, Teague AM, Tryggestad JB, Chernausek SD. Role of microRNA‐130b in placental PGC‐1α/TFAM mitochondrial biogenesis pathway. Biochem Biophys Res Commun. 2017;487:607‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gorska‐Ponikowska M, Kuban‐Jankowska A, Eisler SA, et al. 2‐Methoxyestradiol affects mitochondrial biogenesis pathway and succinate dehydrogenase complex flavoprotein subunit A in osteosarcoma cancer cells. Cancer Genomics Proteomics. 2018;15:73‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qi R, Wang D, Xing L, Wu Z. Cyclosporin A inhibits mitochondrial biogenesis in Hep G2 cells. Biochem Biophys Res Commun. 2018;496:941‐946. [DOI] [PubMed] [Google Scholar]
- 47. De Luca A, Fiorillo M, Peiris‐Pagès M, et al. Mitochondrial biogenesis is required for the anchorage‐independent survival and propagation of stem‐like cancer cells. Oncotarget. 2015;6:14777‐14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Niu Z, Tang J, Ren Y, Feng W. Ropivacaine impairs mitochondrial biogenesis by reducing PGC‐1α. Biochem Biophys Res Commun. 2018;504:513‐518. [DOI] [PubMed] [Google Scholar]
- 49. Guo C, Wang J, Jing L, et al. Mitochondrial dysfunction, perturbations of mitochondrial dynamics and biogenesis involved in endothelial injury induced by silica nanoparticles. Environ Pollut. 2018;236:926‐936. [DOI] [PubMed] [Google Scholar]
- 50. Chen Z, Tao S, Li X, Yao Q. Resistin destroys mitochondrial biogenesis by inhibiting the PGC‐1α/ NRF‐1/TFAM signaling pathway. Biochem Biophys Res Commun. 2018;504:13‐18. [DOI] [PubMed] [Google Scholar]
- 51. Chistiakov DA, Sobenin IA, Revin VV, et al. Mitochondrial aging and age‐related dysfunction of mitochondria. Biomed Res Int. 2014;2014:238463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cedikova M, Pitule P, Kripnerova M, et al. Multiple roles of mitochondria in aging processes. Physiol Res. 2016;65(Supplementum 5):S519‐S531. [DOI] [PubMed] [Google Scholar]
- 53. Nicassio L, Fracasso F, Sirago G, et al. Dietary supplementation with acetyl‐l‐carnitine counteracts age‐related alterations of mitochondrial biogenesis, dynamics and antioxidant defenses in brain of old rats. Exp Gerontol. 2017;98:99‐109. [DOI] [PubMed] [Google Scholar]
- 54. Srivastava S. The mitochondrial basis of aging and age‐related disorders. Genes (Basel). 2017;8(12):E398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang Y, Zhao X, Lotz M, et al. Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator‐activated receptor γ coactivator 1α. Arthritis Rheumatol. 2015;67:2141–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yuan Y, Cruzat VF, Newsholme P, et al. Regulation of SIRT1 in aging: Roles in mitochondrial function and biogenesis. Mech Ageing Dev. 2016;155:10‐21. [DOI] [PubMed] [Google Scholar]
- 57. Gureev AP, Shaforostova EA, Popov VN. Regulation of mitochondrial biogenesis as a way for active longevity: Interaction between the NRF‐2 and PGC‐1α signaling pathways. Front Genet. 2019;2019(10):435. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Muhammad MH, Allam MM. Resveratrol and/or exercise training counteract aging‐associated decline of physical endurance in aged mice; targeting mitochondrial biogenesis and function. J Physiol Sci. 2018;68:681‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Picca A, Lezza AM. Regulation of mitochondrial biogenesis through TFAM‐mitochondrial DNA interactions: Useful insights from aging and calorie restriction studies. Mitochondrion. 2015;25:67‐75. [DOI] [PubMed] [Google Scholar]
- 60. Markaki M, Palikaras K, Tavernarakis N. Novel insights into the anti‐aging role of mitophagy. Int Rev Cell Mol Biol. 2018;340:169‐208. [DOI] [PubMed] [Google Scholar]
- 61. Kim JS, Jeong SH, Han SH, Yi HK. Gomisin A modulates aging progress via mitochondrial biogenesis in human diploid fibroblast cells. Clin Exp Pharmacol Physiol. 2018;45:547‐555. [DOI] [PubMed] [Google Scholar]
- 62. Akhtar S, Siragy HM. Pro‐renin receptor suppresses mitochondrial biogenesis and function via AMPK/SIRT‐1/ PGC‐1α pathway in diabetic kidney. PLoS ONE. 2019;14(12):e0225728. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jiang S, Teague AM, Tryggestad JB, et al. Effects of maternal diabetes and fetal sex on human placenta mitochondrial biogenesis. Placenta. 2017;57:26‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yan W, Zhang H, Liu P, et al. Impaired mitochondrial biogenesis due to dysfunctional adiponectin‐AMPK‐PGC‐1α signaling contributing to increased vulnerability in diabetic heart. Basic Res Cardiol. 2013;108:329. [DOI] [PubMed] [Google Scholar]
- 65. Di W, Lv J, Jiang S, et al. PGC‐1: The energetic regulator in cardiac metabolism. Curr Issues Mol Biol. 2018;28:29‐46. [DOI] [PubMed] [Google Scholar]
- 66. Granata C, Jamnick NA, Bishop DJ. Principles of exercise prescription, and how they influence exercise‐induced changes of transcription factors and other regulators of mitochondrial biogenesis. Sports Med. 2018;48:1541‐1559. [DOI] [PubMed] [Google Scholar]
- 67. Oriquat GA, Ali MA, Mahmoud SA, et al. Improving hepatic mitochondrial biogenesis as a postulated mechanism for the antidiabetic effect of Spirulina platensis in comparison with metformin. Appl Physiol Nutr Metab. 2019;44:357‐364. [DOI] [PubMed] [Google Scholar]
- 68. Zhang X, Zhang Z, Zhao Y, et al. Alogliptin, a dipeptidyl peptidase‐4 inhibitor, alleviates atrial remodeling and improves mitochondrial function and biogenesis in diabetic rabbits. J Am Heart Assoc. 2017;6(5):e005945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hey‐Mogensen M, Clausen TR. Targeting mitochondrial biogenesis and mitochondrial substrate utilization to treat obesity and insulin resistance, respectively ‐ Two data‐driven hypotheses. Curr Diabetes Rev. 2017;13:395‐404. [DOI] [PubMed] [Google Scholar]
- 70. Ortega SP, Chouchani ET, Boudina S. Stress turns on the heat: Regulation of mitochondrial biogenesis and UCP1 by ROS in adipocytes. Adipocyte. 2017;6:56‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zamora‐Mendoza R, Rosas‐Vargas H, Ramos‐Cervantes MT, et al. Dysregulation of mitochondrial function and biogenesis modulators in adipose tissue of obese children. Int J Obes (Lond). 2018;42:618‐624. [DOI] [PubMed] [Google Scholar]
- 72. Lee MS, Kim Y. Effects of Isorhamnetin on adipocyte mitochondrial biogenesis and AMPK activation. Molecules. 2018;23(8):E1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liu M, Zheng M, Cai D, et al. Zeaxanthin promotes mitochondrial biogenesis and adipocyte browning via AMPKα1 activation. Food Funct. 2019;10:2221‐2233. [DOI] [PubMed] [Google Scholar]
- 74. Lemecha M, Morino K, Imamura T, et al. MiR‐494‐3p regulates mitochondrial biogenesis and thermogenesis through PGC1‐α signaling in beige adipocytes. Sci Rep. 2018;8:15096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Karise I, Bargut TC, Del Sol M, et al. Metformin enhances mitochondrial biogenesis and thermogenesis in brown adipocytes of mice. Biomed Pharmacother. 2019;111:1156‐1165. [DOI] [PubMed] [Google Scholar]
- 76. Bae J, Jang Y, Kim H, et al. Arsenite exposure suppresses adipogenesis, mitochondrial biogenesis and thermogenesis via autophagy inhibition in brown adipose tissue. Sci Rep. 2019;9:14464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Song C, Li M, Xu L, et al. Mitochondrial biogenesis mediated by melatonin in an APPswe/PS1dE9 transgenic mice model. NeuroReport. 2018;29:1517‐1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dong W, Wang F, Guo W, et al. Aβ25‐35 suppresses mitochondrial biogenesis in primary hippocampal neurons. Cell Mol Neurobiol. 2016;36:83‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yerra VG, Kalvala AK, Sherkhane B, et al. Adenosine monophosphate‐activated protein kinase modulation by berberine attenuates mitochondrial deficits and redox imbalance in experimental diabetic neuropathy. Neuropharmacology. 2018;131:256‐270. [DOI] [PubMed] [Google Scholar]
- 80. Van Laar VS, Arnold B, Howlett EH, et al. Evidence for compartmentalized axonal mitochondrial biogenesis: mitochondrial DNA replication increases in distal axons as an early response to Parkinson's Disease‐relevant stress. J Neurosci. 2018;38:7505‐7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hasegawa K, Yasuda T, Shiraishi C, et al. Promotion of mitochondrial biogenesis by necdin protects neurons against mitochondrial insults. Nat Commun. 2016;7:10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sasaki T, Mochizuki H. Neuroprotective effects of Necdin in the Parkinson's disease. Nihon Rinsho. 2017;75:36‐41. [PubMed] [Google Scholar]
- 83. Shen L, Sun B, Sheng J, et al. PGC1α promotes cisplatin resistance in human ovarian carcinoma cells through upregulation of mitochondrial biogenesis. Int J Oncol. 2018;53:404‐416. [DOI] [PubMed] [Google Scholar]
- 84. Chaube B, Bhat MK. AMPK, a key regulator of metabolic/energy homeostasis and mitochondrial biogenesis in cancer cells. Cell Death Dis. 2016;7:e2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Negrette‐Guzmán M, Huerta‐Yepez S, Vega MI, et al. Sulforaphane induces differential modulation of mitochondrial biogenesis and dynamics in normal cells and tumor cells. Food Chem Toxicol. 2017;100:90‐102. [DOI] [PubMed] [Google Scholar]
- 86. Ozsvari B, Sotgia F, Lisanti MP. A new mutation‐independent approach to cancer therapy: Inhibiting oncogenic RAS and MYC, by targeting mitochondrial biogenesis. Aging (Albany NY). 2017;9:2098‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pisano A, Cerbelli B, Perli E, et al. Impaired mitochondrial biogenesis is a common feature to myocardial hypertrophy and end‐stage ischemic heart failure. Cardiovasc Pathol. 2016;25:103‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jothy SL, Vijayarathna S, Chen Y, et al. Regulating mitochondrial biogenesis: from herbal remedies to phytomedicine for cancer prevention. Asian Pac J Cancer Prev. 2015;16:8015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Most of the 88 references are associated with the corresponding DOI reference number.


