Abstract
Eight G protein‐coupled P2Y receptor subtypes respond to extracellular adenine and uracil mononucleotides and dinucleotides. P2Y receptors belong to the δ group of rhodopsin‐like GPCRs and contain two structurally distinct subfamilies: P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 (principally Gq protein‐coupled P2Y1‐like) and P2Y12–14 (principally Gi protein‐coupled P2Y12‐like) receptors. Brain P2Y receptors occur in neurons, glial cells, and vasculature. Endothelial P2Y1, P2Y2, P2Y4, and P2Y6 receptors induce vasodilation, while smooth muscle P2Y2, P2Y4, and P2Y6 receptor activation leads to vasoconstriction. Pancreatic P2Y1 and P2Y6 receptors stimulate while P2Y13 receptors inhibits insulin secretion. Antagonists of P2Y12 receptors, and potentially P2Y1 receptors, are anti‐thrombotic agents, and a P2Y2/P2Y4 receptor agonist treats dry eye syndrome in Asia. P2Y receptor agonists are generally pro‐inflammatory, and antagonists may eventually treat inflammatory conditions. This article reviews recent developments in P2Y receptor pharmacology (using synthetic agonists and antagonists), structure and biophysical properties (using X‐ray crystallography, mutagenesis and modelling), physiological and pathophysiological roles, and present and potentially future therapeutic targeting.
Abbreviations
- BMD
bone mineral density
- DUSP
dual specificity protein phosphatase
- ECL
extracellular loop
- EPAC
exchange protein activated by cAMP
- KO
knockout
- MSD
musculoskeletal disorder
- SNP
single nucleotide polymorphism
- SS
Sjögren's syndrome
- TM
transmembrane helix
1. INTRODUCTION AND HISTORICAL OVERVIEW
1.1. Initial characterization of P2 receptor subtypes
It is now >90 years since https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1713 and related adenine nucleotides and nucleosides were first isolated and shown to be pharmacologically active (Drury & Szent‐Györgyi, 1929). It took, however, nearly another 50 years before the receptors through which they act were defined on the basis of pharmacological criteria. Burnstock (1978) proposed that P1 purinoceptors, that is, https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=3, had an agonist potency order of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2844 > AMP > ADP > ATP, and methylxanthines, such as theophylline and caffeine, were selective antagonists. In contrast, at P2 purinoceptors, the agonist potency order was ATP > https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1712 > AMP > adenosine, and methylxanthines were inactive. This division received much support and was widely accepted.
Progress, thereafter, was much faster. First, Burnstock and Kennedy (1985) suggested the subdividing of P2 purinoceptors into P2X and P2Y subtypes, based on the relative agonist potency of ATP and several structural analogues in smooth muscle tissues. Shortly afterwards, Gordon (1986) identified P2T purinoceptors on platelets that mediated aggregation in response to ADP, and P2Z purinoceptors, which mediated ATP‐induced degranulation of mast cells. Uracil nucleotides were also known to regulate cellular function and the P2U receptor, activated by https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1734 and/or https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1749, and in some cases, by ATP, was proposed (Abbracchio et al., 2006). P2D purinoceptors sensitive to adenine dinucleotides were also identified (Abbracchio et al., 2006).
1.2. Cloning of P2 receptors
Thus, numerous P2 purinoceptor subtypes had been named by the early 1990s, but their properties, distribution, and physiological roles were largely unclear, as there were no selective antagonists and the endogenous agonists had complex pharmacological profiles. These uncertainties were clarified in the next few years by the cloning of multiple adenine and/or uracil nucleotide‐sensitive receptors. Seven ligand‐gated cation channels, including the P2Z purinoceptor, were all named https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=77, and eight GPCRs, including P2U and P2T purinoceptors, were called https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=52 (Abbracchio et al., 2006, 2019; Jacobson & Müller, 2016). The aim of this article is to review recent developments in our understanding of the pharmacological, biochemical, and biophysical properties of P2Y receptors, their physiological and pathophysiological roles, and how they are targeted clinically, both at present and potentially in the future. Key advances in this field since the last IUPHAR review (Abbracchio et al., 2006) include X‐ray crystallographic structures of two representative subtypes and many new ligand tools.
The gaps in the numbering of P2Y receptors reflects the historical process in which newly cloned receptors were mis‐assigned as purinergic receptors (e.g., P2Y10). Furthermore, several related orphan receptors, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=143 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=88, appear in the literature as “P2Y‐like” receptors. Several but not all reports support GPR17 activation by purinergic agonists and inhibition by antagonists (Burnstock, 2017; Fumagalli, Lecca, & Abbracchio, 2016).
2. SELECTIVE LIGAND TOOLS TO STUDY P2Y RECEPTORS
P2Y receptors are activated by mononucleotides such as ADP (1), ATP (2), UDP (3), or UTP (4), by dinucleotides like https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1732 (Ap4A, 5) or https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1736 (Up4U, 6), and/or by nucleotide sugars such as uridine 5′‐diphosphoglucose (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1783, 7; see Figure 1). Selective agonists and antagonists are important for studying the roles of P2Y receptor subtypes in physiology and pathophysiology (Figures 1 and 2, Table 1, Abbracchio et al., 2019; Conroy, Kindon, Kellam, & Stocks, 2016; Jacobson & Müller, 2016; Rafehi & Müller, 2018). Their structure–activity relationship at each P2Y receptor subtype has been developed in detail through chemical efforts. In most studies, recombinant receptor proteins have been used for the analysis. Differences in native tissues may be due to a mixture of subtypes on cells, receptor oligomerization, differences in receptor density, and the metabolism of ligands by ecto‐nucleotidases, such as https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2888 (NTPDase‐1), https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2889 (NTPDase‐2), and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1232 (NT5E).
FIGURE 1.
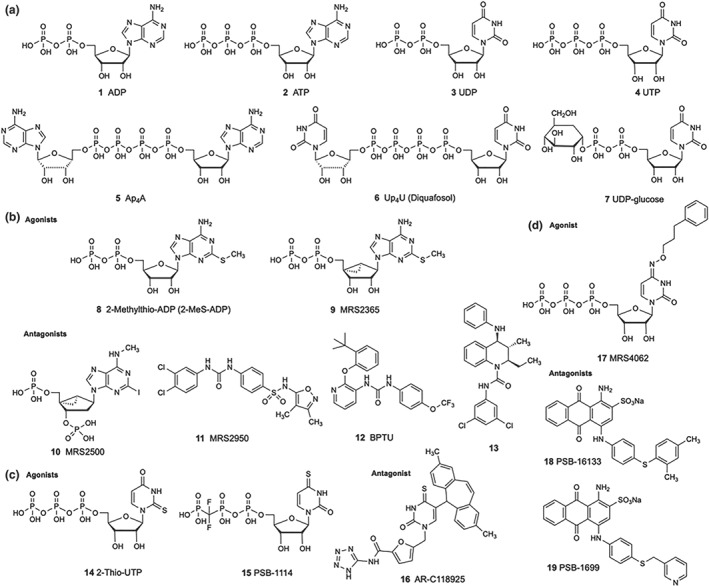
P2Y receptor ligands. (a) Natural agonists of P2Y receptors . (b) P2Y1 receptor agonists and antagonists. (c) P2Y2 receptor agonists and antagonist. (d) P2Y4 receptor agonists and antagonists
FIGURE 2.
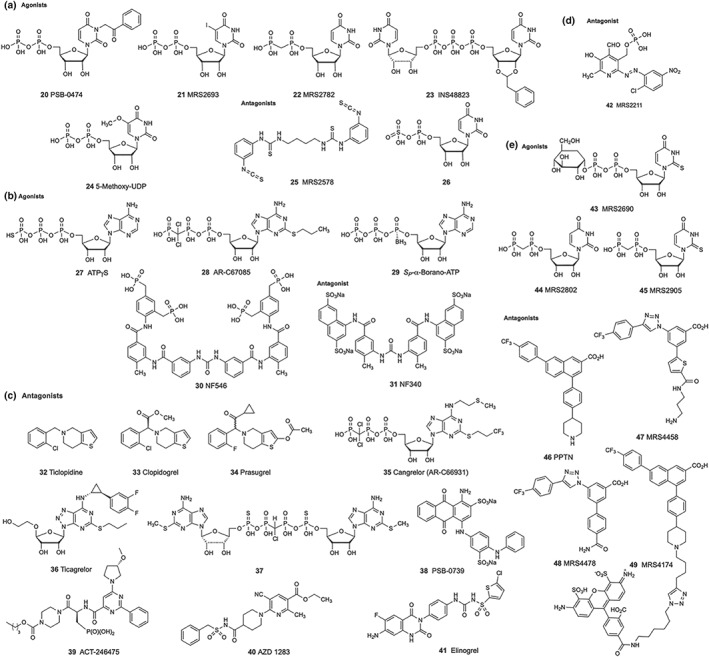
P2Y receptor ligands. (a) P2Y6 receptor agonists and antagonists. (b) P2Y11 receptor agonists and antagonists. (c) P2Y12 receptor agonists and antagonists. (d) P2Y13 receptor antagonist. (e) P2Y14 receptor agonists and antagonists
TABLE 1.
Properties of human P2Y receptors and key agonist/antagonist ligands (Abbracchio et al., 2019; Jacobson et al., 2015; Rafehi & Müller, 2018)
| Group | Subtype, gene symbol, chromosome | Principal G protein coupling; distribution | Ligand agonist/antagonist | pEC50, pIC50, or pKi | Other interactions, Subtype |
|---|---|---|---|---|---|
| P2Y1‐like | P2Y1, P2RY1, 3q25.2 | Gq; platelets, heart, skeletal muscle, brain, intestine | ADP 1 | 5.09 | P2Y12,13 |
| 2‐MeSADP 8 | 5.60 | P2Y12,13 | |||
| MRS2365 9 | 9.40 | Selective | |||
| ADPβS | 5.62 | P2Y12 | |||
| MRS2500 10 d | 9.02 | Selective | |||
| MRS2279 d | 8.10 | Selective | |||
| MRS2179 d | 6.48 | Selective | |||
| MRS2298 | 7.20 | Selective | |||
| MRS2496 | 5.82 | Selective | |||
| P2Y2, P2RY2, 11q13.4 | Gq, Gi; endocrine, reproductive and immune systems, skeletal and cardiac muscle, lung, intestine | UTP 4 | 7.22 | P2Y4 | |
| MRS2698 | 8.10 | Selective | |||
| ATP 2 | 7.07 | P2Y4,11 + others | |||
| Ap4A 5 | 6.1 | P2Y12 and others | |||
| Up4U 6 | 6.68 | P2Y4 (6.89), P2Y6 (5.94) | |||
| PSB‐1114 15 | 6.87 | Selective | |||
| MRS2768 | 5.72 | Selective | |||
| Denufosol | 6.66 | P2Y4 (6.10) | |||
| selective | |||||
| AR‐C118925 16 | 7.24 | ||||
| P2Y4, P2RY4, Xq13.1 | Gq, Gi; placenta, brain, intestine, lung, heart, prostate, fat | UTP 4 | 6.26 | P2Y2 | |
| GTP | 5.18 | Nonselective | |||
| MRS4062 17 | 7.64 | selective | |||
| ATP 2 a | 6.15 | P2Y2,11 + others | |||
| PSB‐16133 18 | 6.63 | Selective | |||
| PSB‐1699 19 | 6.39 | Selective | |||
| P2Y6, P2RY6, 11q13.4 | Gq; lung, heart, spleen, placenta, kidney | UDP 3 | 6.28 | P2Y14 (6.80) | |
| Up3U | 6.57 | P2Y2 (5.88), P2Y4 (6.06) | |||
| MRS2957 | 7.92 | P2Y2 (6.77), P2Y4 (6.10) | |||
| PSB‐0474 20 | 7.15 | Selective | |||
| MRS2693 21 | 7.83 | Selective | |||
| MRS2782 22 | 6.47 | P2Y14 (7.94) | |||
| INS48823 23 | 6.90 | Selective | |||
| 5‐MeO‐UDP 24 | 7.10 | Selective | |||
| MRS2795 | 7.38 | Selective | |||
| MRS2578 25 f | 7.43 | Selective | |||
| Selective | |||||
| U‐phosphosulfate 26 | 3.95 | ||||
| P2Y11, P2RY11, 19p13.2 | Gq, Gs; spleen, intestine, immunocytes | ATP 2 | 4.77 | P2Y2,4 + others | |
| ATPγS 27 | 4.62 | P2Y2,4 + others | |||
| AR‐C67085 28 | 8.5 | P2Y12 | |||
| Sp‐α‐borano‐ATP 29 | 6.47 | P2Y1 (5.92) | |||
| NF546 30 c | 6.27 | Selective | |||
| NF157 | 7.35 | Also blocks P2X1,2,3Rs | |||
| NF340 31 | 7.77 | Selective | |||
| P2Y12‐like | P2Y12, b P2RY12, q25.1 | Gi; platelets, brain, immunocytes | ADP 1 d | 7.22 | P2Y1,13 |
| 2‐MeSADP 8 | 8.3 | P2Y1,13 | |||
| Ap4A 5 | 6.0 | P2Y4 (5.9), P2Y13 (6.7) | |||
| PSB‐0739 38 | 9.8 | Selective | |||
| AZ11931285 d | ~9 | Selective | |||
| AR‐C67085 28 d | 8.2 | P2Y11 | |||
| AR‐C69931MX 35 | 9.40 | P2Y13 (8.3) | |||
| ticagelor 36 | 7.90 | Selective | |||
| Ap4A analogue 37 | 6.66 | P2Y1 (5.67) | |||
| ACT246475 39 | 9.00 | Selective | |||
| AZD1283 40 | 7.50 | Selective | |||
| elinogrel 41 | 7.64 | Selective | |||
| P2Y13, b P2RY13, 3q25.1 |
Gi; spleen, brain, myeloid cells |
ADP 1 | 7.94 | P2Y1,12 | |
| 2‐MeSADP 8 | 7.72 | P2Y1,12 | |||
| MRS2211 42 | 5.97 | Selective | |||
| P2Y14, P2RY14, 3q25.1 | Gi; brain, endocrine and immune systems, muscle, lung, pancreas, intestine, kidney | UDP 3 d | 6.80 | P2Y6 | |
| UDP‐glucose 7 d | 6.45 | P2Y2 | |||
| MRS2690 43 | 7.31 | Selective | |||
| MRS2802 44 | 7.20 | Selective | |||
| MRS2905 45 | 8.70 | Selective | |||
| PPTN 46 | 9.36, 8.22 e | Selective | |||
| MRS4458 47 | 6.77 e | Selective | |||
| MRS4478 48 | 6.57 e | Selective | |||
| MRS4147 49 | 10.10 | Selective |
Note. If no compound number is given, the structure is not shown. The affinity and selectivity in other species is not shown.
ATP acts as antagonist at the human P2Y4 receptor, but an agonist at the rat or mouse P2Y4 receptor.
Selective agonists not yet available.
NF546 activates the P2Y11 receptor, although it belongs to a structural class of antagonists.
Used as a radioligand, when labelled with [3H], [32P], [33P], or [125I], as appropriate.
Fluorescent antagonist 49 binding assay, underestimates affinity in functional assays.
Non‐competitive antagonist.
2.1. P2Y1 receptor ligands
The Gq‐coupled https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=323 is activated by ADP (1) and analogues including the potent agonist https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1710 (8), which is also available as a radioligand https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1763 (Abbracchio et al., 2006, 2019). ATP is an antagonist or partial agonist at the P2Y1 receptor (Waldo & Harden, 2004). The Northern (N)‐methanocarba analogue of 2‐methylthio‐ADP (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3338, 9) is more potent and, in addition, selective for the P2Y1 receptor (Chhatriwala et al., 2004). Selective P2Y1 receptor antagonists have been developed. The bisphosphonate derivative https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1724 (2‐iodo‐N 6‐methyl‐(N)‐methanocarba‐2′‐deoxyadenosine 3′,5′‐bisphosphate, 10) is a potent and selective competitive antagonist (Hechler et al., 2006). The non‐nucleotide compound https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5809 (11) was identified by virtual screening of the modelled receptor orthosteric site (Costanzi, Kumar, Balasubramanian, Harden, & Jacobson, 2012). Another non‐nucleotide compound, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5808 (N‐[2‐[2‐(1,1‐dimethylethyl)phenoxy]‐3‐pyridinyl]‐N′‐[4‐(trifluoromethoxy)phenyl]urea, 12) and its analogues act as an allosteric inhibitors (Gao & Jacobson, 2017; Peng et al., 2018; Wang, Qiao, et al., 2013; Yuan et al., 2016; Zhang, Gao, et al., 2015).
The [32P]‐labelled and [125I]‐labelled analogues of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1726 are radioligands binding to P2Y1 receptors (Ohlmann et al., 2010). An analogue of BPTU has been reported as a novel [18F]‐tracer for imaging of tissue P2Y1 receptor expression using PET (Moldovan et al., 2019). Tetrahydroquinolineamine derivative (13) was reported as a structurally novel P2Y1 receptor antagonist with binding K i 0.5 μM (Morales‐Ramos et al., 2008).
2.2. P2Y2 receptor ligands
The Gq‐coupled https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=324 is activated by both ATP and UTP (Abbracchio et al., 2006, 2019) as well as by dinucleoside polyphosphates including Ap4A and Up4U (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1736, INS365; Xu et al., 2018). Diquafosol is approved in Japan and South Korea for the treatment of dry eye syndrome (Keating, 2015; Yamane et al., 2015). https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3398 (14) and related analogues are selective P2Y2 receptor agonists (El‐Tayeb, Qi, Nicholas, & Müller, 2011; Xu et al., 2018). 4‐Thiouridine‐5′‐O‐(β,γ‐difluoromethylene)triphosphate (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5902, 15) is more than 60‐fold selective for the P2Y2 receptors over P2Y4 and P2Y6 receptors (El‐Tayeb et al., 2011).
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5907 (5‐[[5‐(2,8‐dimethyl‐5H‐dibenzo[a,d]cyclohepten‐5‐yl)‐3,4‐dihydro‐2‐oxo‐4‐thioxo‐1(2H)‐pyrimidinyl]methyl]‐N‐2H‐tetrazol‐5‐yl‐2‐furancarboxamide, 16) is a potent and selective antagonist for P2Y2 receptors (Kindon et al., 2017; Rafehi, Burbiel, Attah, Abdelrahman, & Müller, 2017; Rafehi, Neumann, et al., 2017).
2.3. P2Y4 receptor ligands
The human Gq‐coupled https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=325 receptor is activated by UTP, but not by ATP or nucleoside diphosphates (Abbracchio et al., 2006, 2019). https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4044 (N 4‐(phenylpropoxy)‐CTP, 17), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6199 (a 3′‐deoxy‐3′‐fluoroglucose analogue), and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6200 are potent P2Y4 receptor agonists (Maruoka et al., 2011). PSB‐16133 (18) and PSB‐1699 (19) are novel P2Y4 receptor antagonists acting at submicromolar concentrations (Rafehi, Malik, et al., 2017). PSB‐16133 is somewhat more potent, but PSB‐1699 is more selective for the P2Y4 receptor.
2.4. P2Y6 receptor ligands
The Gq‐coupled https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=326 prefers UDP as an agonist (Abbracchio et al., 2006, 2019). Analogues including https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1745 (PSB‐0474, 20; El‐Tayeb et al., 2011), 5‐iodo‐UDP (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1747, 21; Ko et al., 2008), α,β‐methylene‐UDP (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1748, 22; Ko et al., 2008), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1746 (23, Korcok, Raimundo, Du, Sims, & Dixon, 2005; Rafehi & Müller, 2018), and 5‐methoxy‐UDP (24, Ginsburg‐Shmuel et al., 2012) are much more potent agonists.
The P2Y6 receptor is blocked by https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1753 (N,N″‐1,4‐butanediylbis[N′‐(3‐isothiocyanatophenyl)thiourea, 25; Mamedova, Joshi, Gao, von Kügelgen, & Jacobson, 2004) and uridylyl phosphosulfate (26, Meltzer et al., 2015).
2.5. P2Y11 receptor ligands
The https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=327 couples to both Gq and Gs proteins. ATP activates human P2Y11 receptors, whereas ADP is a canine P2Y11 receptor agonist (Abbracchio et al., 2006, 2019). Human P2Y11 receptors are also activated by https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1714 (27), the P2Y12 receptor antagonist 2‐propylthio‐β,γ‐dichloromethylene‐ATP (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1756, 28) and adenosine‐5′‐O‐(α‐boranotriphosphate) with a preference for the diastereomer that has an S‐configuration at the α‐phosphorus atom (compound 29; Ecke, Tulapurkar, Nahum, Fischer, & Reiser, 2006; Meis et al., 2010). Moreover, the https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1728 analogue https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4046 (4,4′‐(carbonylbis (imino‐3,1‐phenylene‐carbonylimino‐3,1‐(4‐methyl‐phenylene)carbonylimino))‐bis(1,3‐xylene‐α,α′‐diphosphonic acid, 30) has been reported to act as an agonist (Meis et al., 2010). Another suramin analogue, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1762 (4,4′‐(carbonylbis (imino‐3,1‐(4‐methyl‐phenylene)carbonylimino))bis (naphthalene‐2,6‐disulfonic acid, 31), is a potent antagonist with a pA2 value of 8.0 (Meis et al., 2010).
2.6. P2Y12 receptor ligands
The Gi‐coupled https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=328 is activated by ADP and its potent analogue 2‐methylthio‐ADP (8, Abbracchio et al., 2006, 2019; Hollopeter et al., 2001). Many P2Y12 receptor antagonists have been developed (Baqi & Müller, 2019). Some of them are used to reduce platelet aggregation for the prevention or treatment of cardiovascular events such as myocardial infarction or stroke. The thienopyridine compounds https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7307 (32), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7150 (33, Savi et al., 2006), and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7562 (34, Sugidachi, Asai, Ogawa, Inoue, & Koike, 2000; Sugidachi, Mizuno, Ohno, Jakubowski, & Tomizawa, 2016) are liver‐activated prodrugs. Their active metabolites, such as https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1771 (structure not shown, Dansette, Levent, Hessani, & Mansuy, 2015), have been shown to interact in an irreversible manner with the human P2Y12 receptor protein (Algaier, Jakubowski, Asai, & von Kugelgen, 2008; Ding, Bynagari, Mada, Jakubowski, & Kunapuli, 2009; Savi et al., 2006; Zhang, Zhang, Gao, Zhang, et al., 2014). Competitive P2Y12 receptor antagonists include the nucleotide derivatives https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1776 (35, AR‐C69931MX, N 6‐(2‐methylthioethyl)‐2‐(3,3,3‐trifluoropropylthio)‐β,γ‐dichloromethylene‐ATP) and AR‐C67085 (2‐propylthio‐β,γ‐dichloromethylene‐D‐ATP, 28; Ingall et al., 1999) as well as nucleoside derivatives such as the orally active https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1765 (36, AZD6140, Springthorpe et al., 2007; Hoffmann et al., 2009; Hoffmann et al., 2014). Ticagrelor has been reported to act as an inverse agonist under some experimental conditions (Aungraheeta et al., 2016; Garcia et al., 2019). A series of novel analogues of Ap4A blocks P2Y12 receptors at nanomolar concentrations; one of the most potent derivatives was compound 37 (Yanachkov et al., 2016).
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5904 (1‐amino‐9,10‐dihydro‐9,10‐dioxo‐4‐[[4‐(phenylamino)‐3‐sulfophenyl]amino]‐2‐anthracenesulfonic acid, 38) is a potent and competitive non‐nucleotide P2Y12 receptor antagonist with a pA2 value of 9.8 (Baqi & Müller, 2019; Hoffmann et al., 2014). Further novel P2Y12 receptor antagonists include piperazinyl glutamates and derived phosphonates such as ACT‐246475 (39, Caroff et al., 2015), ethyl 6‐aminonicotinate acyl sulfonamides, for example, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7360 (40, Bach et al., 2013; Zhang, Zhang, Gao, Zhang, et al., 2014), and related sulfonamide derivatives such as elinogrel (PRT060128, 41). The reversible and very potent antagonist ACT‐246475 (Caroff et al., 2015) is being tested in clinical trials for use in the prevention or treatment of cardiovascular events (Baldoni et al., 2014). In addition, a variety of moderately potent P2Y12 receptor antagonists have been described, among them 4‐benzhydrylmorpholines (Ahn et al., 2016), flavonolignans (Bijak, Szelenberger, Dziedzic, & Saluk‐Bijak, 2018), and salvianolic acids (Liu et al., 2018). https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6147 is an antagonist radioligand, the tritiated form of AR‐C67085 (28), which binds to P2Y12 receptors with a K D value of 4.6 nM (Ohlmann et al., 2013).
2.7. P2Y13 receptor ligands
ADP and 2‐methylthio‐ADP (8) activate the Gi‐coupled https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=329 (Communi et al., 2001; Abbracchio et al., 2006, 2019). https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1777 (2‐[(2‐chloro‐5‐nitrophenyl)azo]‐5‐hydroxy‐6‐methyl‐3‐[(phosphonooxy)methyl]‐4‐pyridinecarboxaldehyde, 42) is a competitive antagonist with a pA2‐value of 6.3 (Kim et al., 2005). Cangrelor, in contrast, blocks the human and the rat P2Y13 receptors in a non‐competitive mode of action (Fumagalli et al., 2004).
2.8. P2Y14 receptor ligands
UDP and sugar derivatives such as UDP‐glucose and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1782 are Gi‐coupled P2Y14 receptor agonists (Abbracchio et al., 2006, 2019; Carter et al., 2009). The analogues https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3337 (diphosphoric acid 1‐α‐d‐glucopyranosyl ester 2‐[(4′‐methylthio)uridin‐5″‐yl] ester, 43), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5909 (α,β‐difluoromethylene‐UDP, 44), and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5908 (α,β‐methylene‐2‐thio‐UDP, 45) are much more potent agonists (Abbas et al., 2018; Carter et al., 2009; Das et al., 2010).
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5802 (4‐[4‐(4‐piperidinyl)phenyl]‐7‐[4‐(trifluoromethyl)phenyl]‐2‐naphthalenecarboxylic acid, 46) is a potent antagonist acting at the P2Y14 receptor (Barrett et al., 2013). Novel antagonists include https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9998 (3‐{5‐[(3‐aminopropyl)carbamoyl]thiophen‐2‐yl}‐5‐{4‐[4‐(trifluoromethyl)phenyl]‐1H‐1,2,3‐triazol‐1‐yl}benzoic acid, 47) and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9999 (4′‐carbamoyl‐5‐{4‐[4‐(trifluoromethyl)phenyl]‐1H‐1,2,3‐triazol‐1‐yl}‐[1,1′‐biphenyl]‐3‐carboxylic acid, 48; Yu et al., 2018). https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9470 (49) is a high‐affinity fluorescent ligand with antagonistic properties.
3. THE EVOLUTIONARY HISTORY OF P2Y RECEPTORS AND RELATED GPCRS
The availability of genomic sequences of human and other animal species allowed for comprehensive phylogenetic studies and meaningful analysis of the evolutionary history of the GPCR superfamily revealing several major classes of GPCRs—the rhodopsin‐, adhesion/secretin‐, frizzled‐, and glutamate‐like metabotropic receptor classes (Fredriksson & Schiöth, 2005). Based on amino acid sequence similarities, P2Y receptors belong to the rhodopsin‐like receptor class, specifically to the δ group within this class. The δ group does not only include P2Y receptors but also receptors for lipids, hydroxycarboxylic acids, and peptides (Figure 3a).
FIGURE 3.
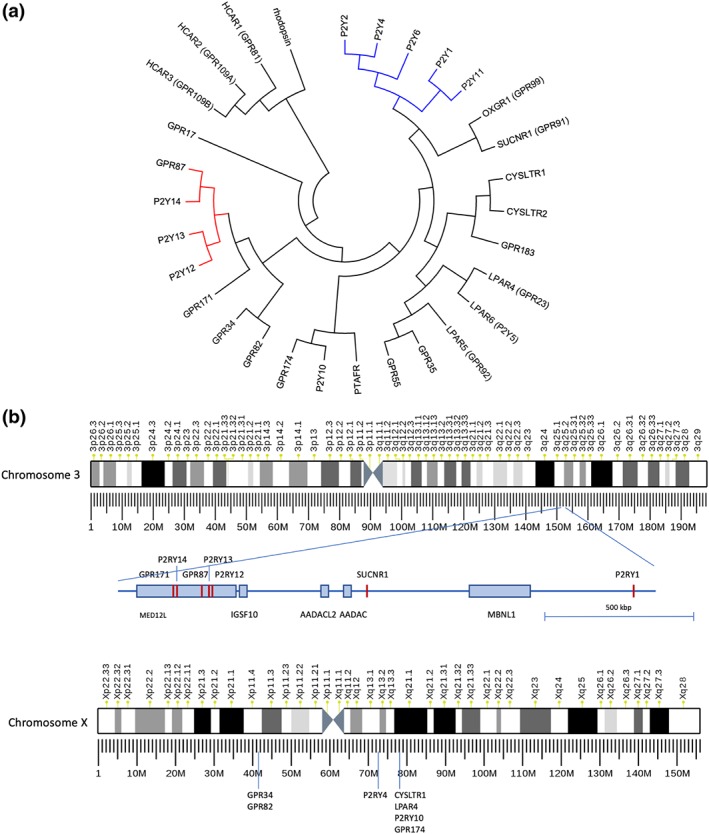
(a) Evolutionary relationships of human P2Y1‐ and P2Y12‐like receptors of the δ group of rhodopsin‐like GPCRs (Fredriksson & Schiöth, 2005). P2Y1‐ and P2Y12‐like receptors form separated clusters of receptors indicating that they evolved independently. The evolutionary history was inferred using the neighbour‐joining method by extending a previous analyses (Le Duc et al., 2017). The optimal tree with the sum of branch length = 14.64350117 is shown. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method and are in the units of the number of amino acid substitutions per site. The analysis involved the amino acid sequences of 30 human receptors which were aligned by using the PAM matrix and default parameters. All positions with less than 95% site coverage were eliminated. That is, fewer than 5% alignment gaps, missing data, and ambiguous bases were allowed at any position. There were a total of 239 positions in the final dataset. Evolutionary analyses were conducted in MEGA7 (Kumar, Stecher, & Tamura, 2016). HCARx, hydroxycarboxylic acid receptors; LPARx, lysophosphatidic acid receptors; OXGR1, 2‐oxoglutarate receptor; SUCNR1, succinate receptor; PTAFR, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=334; P2Yx nucleotide receptors. (b) Genomic clustering of P2Y‐like receptors on chromosome 3 and chromosome X. Several P2Y receptors and other members of the δ group cluster at genomic loci, most prominent clusters at chromosome 3 and chromosome X. The P2Y12 receptor‐like receptors are found in a MED12L gene intron at chromosome 3. Similarly, GPR34 and GPR82 are found in a reverse‐oriented CASK gene intron at Xp11.4. Phylogenetic analysis showed relation of cysteinyl‐LT receptor and lysophosphatidic acid receptors (see (a)). Interestingly, CysLT2 and LPA6 receptors and GPR183 are closely located at chromosome 13 (13q14.2, not shown) whereas CysLT1 receptors genomically cluster with LPA4, P2Y10 receptors, and GPR174 at the X chromosome
The phylogenetic relation of P2Y receptors subdivides them into at least two groups (Figure 3a) that also correspond to their preferential G protein coupling. One group comprises P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 receptors (Gq‐coupled P2Y1 receptor‐like) while the second group contains P2Y12–14 receptors (Gi‐coupled P2Y12 receptor‐like). The diversity of P2Y receptor signalling pathways is discussed below in the context of neural cells. Interestingly, several other structurally related δ group GPCRs, such as the 2‐oxoglutarate receptor (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=162 and GPR99) and the succinate receptor (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=166 and GPR91), as well as the orphan receptors https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=101, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=118, GPR171, and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=122 cluster into one of both groups (Figure 3a).
The phylogenetic clustering of P2Y receptors correlates, at least in part, with the genomic localization of P2Y receptors. Chromosomal clustering in the human genome is found for P2Y12‐14 receptor genes at chromosome 3 (Figure 3b). Here, all genes show the same orientation in a tandem‐like fashion within an intron of the reverse‐oriented Mediator complex subunit 12 like (Med12L) gene. Such condensed genomic localization of related genes most probably arose from multiple rounds of local gene duplications. Interestingly, SUCNR1 and P2Y1 receptors are in close genomic proximity to this P2Y12 receptor‐like receptor gene cluster. However, both genes are in reverse orientation to the P2Y12 receptor cluster (Figure 3b) indicating an independent genomic history. Chromosomal clustering is also found for P2Y2 and P2Y6 receptors at chromosome 11, the https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=48 at chromosome 12, and seven δ group members at chromosome X (Figure 3b).
Several P2Y receptors and other members of the δ group cluster have prominent genomic clusters at chromosome 3 and chromosome X. The P2Y12‐like receptors are found in an intron of the MED12L gene at chromosome 3. Similarly, GPR34 and GPR82 are found in an intron of the reverse‐oriented CASK gene at Xp11.4. Phylogenetic analysis showed relation of cysteinyl‐LT receptor and https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=36 (see Figure 3a). Interestingly, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=270 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=163 receptors and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=81 are closely located at chromosome 13 (13q14.2, not shown), whereas https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=269 genomically clusters with https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=94 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=165 receptors and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=145, at the X chromosome.
In contrast to metabotropic P2Y receptors, the P2X receptors seem to appear in evolution already in invertebrates and plants (Hou & Cao, 2016). Prototypical P2Y receptors, as we know them from vertebrates, seem to be an innovation that occurred first in the Cambrian, because δ group GPCRs do not have orthologous sequences in invertebrates, hemichordates, and cephalochordates (Krishnan, Almen, Fredriksson, & Schiöth, 2013). Unfortunately, the “birth” of P2Y receptors can only be assumed at some time between the cyclostome–gnathostome split because of the lack of other recent genomes covering this evolutionary period. Because almost all P2Y‐ and other δ group‐related sequences are present in cartilaginous and bony fish genomes, these genes must have been present in the common ancestor of both fish lineages.
Most coding regions of P2Y receptors do not contain introns, and splice variants are rare. The genomic structure of these receptors has been highly conserved during evolution. However, introns in the coding regions of several P2Y receptor genes newly appear in some fish species (Schöneberg et al., 2007). In contrast, introns in the 5′UTR seem to be common, for example, in P2Y12 receptor‐like receptors, P2Y2, and P2Y6 receptors. Obviously, human P2Y1 and P2Y4 receptors do not contain introns in their genes. Some P2Y receptors contain cryptic introns in their N terminus‐coding regions (P2Y6, GPR87, and GPR34).
The human P2Y11 receptor, located at chromosome 19, is an exception in many aspects. P2Y11 receptor orthologues are missing in murine and chicken genomes but are present in most other birds, reptiles, and amphibians sequenced to date. In humans, P2Y11 receptor transcripts exist in several variants modifying the N terminus coding region and even producing a fusion protein with an adjacent gene (PPAN; Dreisig & Kornum, 2016). Here, a chimeric transcript, characterized by the first third of PPAN exon 12 joined to P2Y11 receptor exon 2, has been detected. The chromosomal synteny between P2Y11 receptors and PPAN is already established in zebrafish, and fusions at the mRNA level can be found in many birds and mammals.
4. STRUCTURAL CHARACTERIZATION OF P2Y RECEPTORS
The availability of numerous orthologues also allows for comparing the conservation of individual amino acid positions in P2Y receptor proteins. Although P2Y receptors belong to two distinct structural and evolutionary groups (Figure 3a), as confirmed by X‐ray structures (Zhang, Zhang, Gao, Paoletta, et al., 2014; Zhang, Gao, et al., 2015), several members share the same agonist (e.g., ADP). Support for different agonist binding modes between P2Y1 and P2Y12 receptors was observed in mutational analyses. While for P2Y1, positions K6.55, Q7.36, and R7.39 (using standard residue numbering) were shown to be critical for receptor activation following agonist binding (Jiang et al., 1997), positions R6.55, Y6.58, and K7.35 are involved in P2Y12 receptor ligand recognition (Hoffmann et al., 2009; Hoffmann et al., 2014; Schmidt et al., 2013). All these residues are conserved within vertebrate P2Y1 and P2Y12 receptor orthologues but are not shared between both ADP receptors.
Phylogenetic and mutagenesis studies addressing the positions R6.55, Y6.58, and K7.35 of P2Y12 receptors showed that all these residues are 100% conserved among species and that most mutations of these positions interfered with receptor function (Schmidt et al., 2013). However, such residue combination is also present in some GPR87, GPR171, and GPR34 orthologues (all P2Y12 receptor‐like), which are not activated by ADP or ATP. This does not rule out that these residues are involved in nucleotide binding of, for example, P2Y12 receptors, but it implies additional positions which determine ligand specificity.
Structural studies of P2Y receptors have made tremendous progress in recent years. Five structures of two representative receptors, P2Y1 and P2Y12, from two subfamilies were determined in 2014 and 2015, respectively (Zhang, Zhang, Gao, Paoletta, et al., 2014; Zhang, Zhang, Gao, Zhang, et al., 2014; Zhang, Gao, et al., 2015). These structures, in which the receptors are captured in complex with different ligands that vary in chemical structure and binding site, provide essential insights into ligand recognition and activation regulation of P2Y receptors.
The P2Y1 and P2Y12 receptor structures share a canonical seven transmembrane (7TM) helical architecture of GPCRs. Like most other solved class A GPCR structures, the structures of P2Y12 receptors in complex with agonists 2MeSADP and 2MeSATP and P2Y1 receptors bound to inhibitors MRS2500 and BPTU exhibit a conserved disulfide bond connecting TM3 to the second extracellular loop (ECL2). However, this disulfide bond is not observed in the antagonist AZD1283‐bound P2Y12 receptor structure (Zhang, Zhang, Gao, Paoletta, et al., 2014), consistent with previous data suggesting that the ECL2 residue Cys973.25 may act as the covalent binding site for the active metabolites of P2Y12 receptor drugs (Algaier et al., 2008; Ding et al., 2009). In addition to the dynamic disulfide bond, TM5 of the P2Y12 receptor adopts a straight and elongated conformation rather than a bent helix observed in most other GPCR structures, as the highly conserved class A GPCR residue P5.50 that leads to a helical bend in other GPCRs (Zhang, Zhang, Gao, Paoletta, et al., 2014) is substituted by an asparagine (N2015.50) in P2Y12 receptors.
Comparison of agonist‐ and antagonist‐bound P2Y12 receptor structures reveals remarkable differences in the extracellular region (Zhang, Zhang, Gao, Zhang, et al., 2014). Compared to the P2Y12 receptor‐AZD1283 structure, the extracellular tips of TM6 and TM7 shift towards the central axis of the TM bundle by over 10 and 5 Å respectively in the P2Y12 receptor‐2MeSADP structure. The inward movement of TM6 and TM7 allows extensive interactions between the agonist and the extracellular region of the receptor. As a result, the helical bundle, the P2Y12 receptor ECLs, and N terminus undergo conformational changes, and the ligand‐binding pocket shrinks to preclude antagonist binding. In contrast, the antagonist AZD1283's phenyl moiety forms a steric hindrance to impede the inward movement of TM6 in the P2Y12 receptor‐AZD1283 structure. These structural differences suggest that the receptor extracellular region may play a role in modulating receptor activity by cooperating with the bound ligand.
The P2Y12 receptor structures reveal different binding modes for the non‐nucleotide antagonist AZD1283 and the nucleotide agonist 2MeSADP. The binding pockets of these two ligands vary in size and shape, with only partial overlap. AZD1283 binds to the P2Y12 receptor in a large open pocket formed by residues from TM3‐TM7. In contrast, the nucleotide agonist is completely enclosed within the receptor ligand binding pocket. The ligand binding cavity of P2Y12 receptors is separated into two sub‐pockets by a barrier formed by Y1053.33 and K2807.35. Pocket 1 is shaped by TM3–TM7, while pocket 2 is composed of TM1–TM3 and TM7. Although pocket 2 is not occupied in the solved P2Y12 receptor structures, molecular docking studies suggested that the active metabolites of P2Y12 receptor antagonists such as clopidogrel many bind to pocket 2 (Zhang, Zhang, Gao, Paoletta, et al., 2014), providing new clues for the development of allosteric modulators of P2Y12 receptors as anti‐cardiovascular drugs with reduced side effects.
Although P2Y1 and P2Y12 receptors share the same endogenous ligand ADP, the structures of these two receptors reveal (Figure 4) completely different recognition patterns for their nucleotide‐like ligands (Zhang, Gao, et al., 2015). In the P2Y1 receptor structure, the nucleotide‐like antagonist MRS2500 binds to the receptor in a binding pocket bordered by the N terminus, ECL2, and TM6–TM7 within the TM bundle, close to the extracellular surface. The adenine ring of MRS2500 inserts into a sub‐pocket formed by the N terminus, TM6, and TM7, while its two phosphate groups make extensive polar interactions with residues from N terminus, ECL2, TM2, and TM7. In the P2Y12 receptor‐2MeSADP structure, the adenine ring of 2MeSADP reaches deep into the ligand binding pocket, making contacts with TM3 and TM4. The negatively charged diphosphate group of 2MeSADP attracts positively charged residues and hydrogen‐bonding groups from N terminus, ECL2, TM3, TM6, and TM7, forming electrostatic force to stabilize the “closed” extracellular region (Jacobson et al., 2015). These structural differences highlight the diversity of recognition by GPCRs.
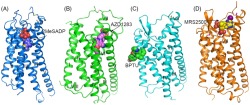
FIGURE 4.
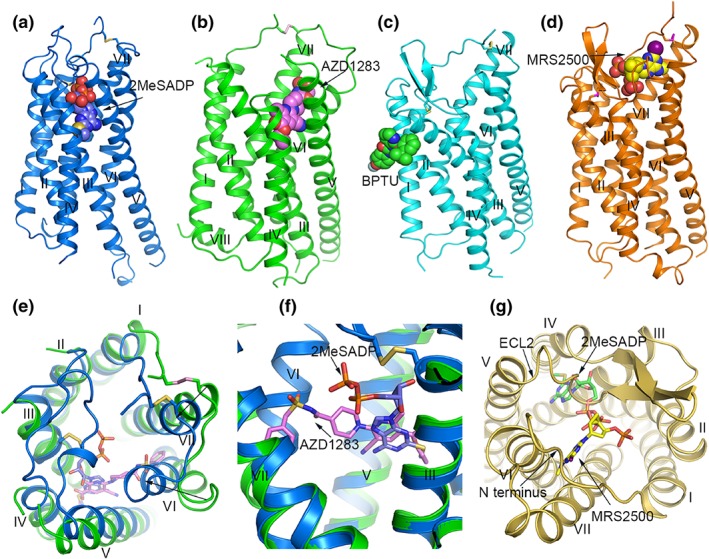
Structures of P2Y12 receptors and P2Y1 receptors. (a) Structure of the P2Y12 receptor‐2MeSADP 8 complex. The P2Y12 receptor is shown in blue cartoon representation. 2MeSADP is shown as blue spheres. The disulphide bonds are shown as yellow sticks. (b) Structure of the P2Y12 receptor‐AZD1283 40 complex. The receptor is coloured green and shown in cartoon representation. AZD1283 is shown as spheres with pink carbons. The disulphide bridge is shown as pink sticks. (c) Structure of the P2Y1 receptor‐BPTU complex. The P2Y1 receptor is coloured cyan and shown as cartoon. The ligand BPTU is shown as spheres with green carbons. The disulphide bonds are shown as yellow sticks. (d) Structure of the P2Y1 receptor‐MRS2500 10 complex. The P2Y1 receptor is shown in orange cartoon representation. MRS2500 is shown as spheres with yellow carbons. The disulphide bonds are show as magenta sticks. (e) Structural comparison of the extracellular region in the P2Y12 receptor‐2MeSADP and P2Y12 receptor‐AZD1283 complexes. In the P2Y12 receptor‐AZD1283 structure, the receptor is shown in green cartoon representation, the ligand AZD1283 is shown as pink sticks. In the P2Y12 receptor‐2MeSADP structure, the receptor is shown in blue cartoon representation and 2MeSADP is shown as blue sticks. The black arrows indicate the movements of helices VI and VII in the 2MeSADP‐bound structure relative to the AZD1283‐bound structure. (f) Comparison of the binding pose of AZD1283 and 2MeSADP in P2Y12 receptors. The colour scheme is the same as that in panel (e). (g) Comparison of the nucleotide binding modes in P2Y1 receptors and P2Y12 receptors. The P2Y1 receptor is shown in yellow cartoon representation. MRS2500 and 2MeSADP are shown as yellow sticks and green carbon respectively
The most striking finding in the recent work on the P2Y receptor structures is that the two P2Y1 receptor inhibitors MRS2500 and BPTU occupy two disparate ligand binding pockets (Figure 4c; Zhang, Gao, et al., 2015). Unlike the nucleotide‐like ligand MRS2500 whose binding site is within the receptor helical bundle, the non‐nucleotide antagonist BPTU binds to the receptor in a shallow binding pocket on the external interface of TM1–TM3 with the lipid bilayer, which accommodates the ligand mainly through hydrophobic interactions. This hydrophobic binding environment of BPTU is consistent with the high hydrophobicity of this ligand, and previous efforts showing that addition of any polar groups to reduce its lipophilicity decreased binding affinity to P2Y1 receptors (Wang, Qiao, et al., 2013). The unique binding site of BPTU indicates that this ligand acts as an allosteric modulator of P2Y1 receptors . It most likely inhibits receptor function by blocking the conformational change of TM1–TM3 to lock the receptor in an inactive state, which is supported by ligand binding assays showing that BPTU can accelerate agonist [35S]2MeSADP dissociation (Zhang, Gao, et al., 2015). BPTU was the first structurally characterized selective GPCR ligand located entirely outside of the canonical ligand binding pocket of GPCRs. As such, the finding offers new opportunities to GPCR drug discovery to target novel sites.
Currently, there is no crystal structure for pyrimidine nucleotide receptors. However, recent mutational and docking studies suggest that the conserved R7.39 in P2Y1‐like receptors is also involved in UDP binding in P2Y6 receptors (Bruser et al., 2017) and agonist binding of P2Y2 receptors (Rafehi, Neumann, et al., 2017) indicating that the binding pocked is very similar at least within the two P2Y receptor groups.
5. SIGNALLING PATHWAYS FOR P2Y RECEPTORS
The cellular sources of nucleotide agonists of P2Y receptors include co‐transmission, endocytosis, release through https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=121#735 hemichannels and lysis in cell damage (Abbracchio et al., 2006; Nishimura et al., 2017). The principal G protein coupling of each of the P2Y receptor subtypes are indicated in Table 1. However, each of the P2Y receptors can be associated with multiple G proteins and other signalling pathways, as was shown for P2Y1 receptors, including both G protein‐dependent and G protein‐independent, for example, β‐arrestin, pathways (Gao & Jacobson, 2017). Some of the P2Y receptors additionally couple to G12/13 (P2Y2 and P2Y6) or Gs (P2Y11).The P2Y receptors regulate https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=288&familyType=ENZYME pathways and consequently affect cell survival and proliferation (Miras Portugal et al., 2019). This review emphasizes the novel contribution of nucleotide receptors to maintain cell homeostasis through the regulation of MAPKs and phosphatases. P2Y receptors also undergo agonist‐induced desensitization and can heterodimerize with other GPCRs, such as the vascular https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=34 and the https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=18 (Nishimura et al., 2017).
6. P2Y RECEPTORS IN THE NERVOUS SYSTEM
The cloning of the different P2Y receptors showed their presence in brain tissue. P2Y receptors are especially abundant in glial cells, astrocytes and microglia, but they are also present in central and peripheral neurons, oligodendrocytes, and cerebral microvasculature. P2Y receptors together with ionotropic P2X receptors mediate neurotransmission, neuron–glia interaction, regulation of cerebral blood flow, and neuroprotection or even contribute to neurodegeneration and pain transmission (Burnstock, 2017; Toth et al., 2015; Weisman et al., 2012). P2Y receptors are also present in adult stem cells, which suggests potential actions in neuroregeneration (Gómez‐Villafuertes et al., 2015; Stefani et al., 2018).
Astroglia from different areas, cortex, hippocampus, striatum, cerebellum, and spinal cord, express a great variety of P2Y receptors: ADP selective receptors, P2Y1 receptors and P2Y13 receptors, the receptor equally activated by UTP and ATP, receptors, and pyrimidine selective receptors P2Y4 and P2Y6 receptors (Franke, Verkhratsky, Burnstock, & Illes, 2012). Astroglial P2Y1 receptors mediate neuron–glia interaction and calcium waves contributing to neuronal activity synchronization (Shigetomi, Hirayama, Ikenaka, Tanaka, & Koizumi, 2018). P2Y1, P2Y2 and P2Y13 receptors are also present in neurons. P2Y1 and P2Y13 receptors together with the ionotrotropic https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=484 regulate neuronal differentiation and are also involved in neuroprotection and neurodegeneration (Miras Portugal et al., 2019; Perez‐Sen et al., 2015). Some P2Y receptors ‐ P2Y1, P2Y2, and P2Y4 ‐ appear to be up‐regulated in pathological conditions such as brain injury, Alzheimer's disease, and epilepsy (Franke et al., 2012). P2Y1 receptor antagonism is associated with cerebroprotection and might improve cognition in Alzheimer's disease (Reichenbach et al., 2018) and glutamate mediated hippocampal neurodegeneration (Simões et al., 2018). P2Y1 receptors could also be novel candidates for the treatment of epilepsy (Alves et al., 2019). However, P2Y1 and P2Y13 receptor agonists could also display neuroprotective actions against oxidative stress and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1369 excitotoxicity as reported in other experimental models (Miras Portugal et al., 2019).
The P2Y12 receptors exhibit a more restricted distribution, mainly located in microglia, where it plays relevant role in inflammation and neuropathic pain (Tozaki‐Saitoh et al., 2008). Microglial P2Y12 and P2Y13 receptors and astroglial P2Y1 receptors are new players in the complex microglia–astrocyte interaction after brain damage. After brain injury, ATP and ADP increase at extracellular space, and ADP acts as a chemotactic signal recruiting microglia to damaged area via P2Y12 receptor activation. Activation of microglial P2Y12 and P2Y13 receptors releases cytokines and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5074‐α, which down‐regulate P2Y1 receptors in astrocytes, avoiding astroglial proliferation. Down‐regulation of astroglial P2Y1 receptors by microglia changes astrocyte phenotype from pro‐inflammatory to neuroprotective phenotype, increasing reactive astrogliosis (enhances glial fibrillary acidic protein expression), glial scar formation, restoring brain blood barrier functions, and suppressing leukocyte infiltration (Shinozaki et al., 2017). P2Y12 receptors are also present in oligodendrocytes. Loss of these receptors could contribute to the demyelination process in multiple sclerosis (Amadio et al., 2010).
As previously mentioned, P2Y and P2X receptors modulate pain transmission (Burnstock, 2017). P2Y1, P2Y2, P2Y12 and P2Y13 receptors are expressed in nociceptive neurons and surrounding glial cells, and both pro‐nociceptive and pro‐analgesic effects have been proposed (Malin & Molliver, 2010). A complex regulatory mechanism for P2Y1 and P2Y13 receptors of glycine transporter function in glutamatergic and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1067ergic synapsis confirms the important role of these receptors as targets to modulate different pain states at the spinal cord (Jiménez et al., 2011).
Concerning the intracellular mechanisms mediating P2Y receptor effects in neurons and glial cells, a great variety of intracellular routes have been unravelled that include the https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=781/https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=285 axis, https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=509, NF‐κB, JAK‐STAT, MAPK, transactivation of growth factor tyrosine‐kinase receptors, and crosstalk with https://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyId=58. Among them, MAPK activation deserves special attention. https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=514 are responsible for astrocyte proliferation and migration (Franke et al., 2012; Paniagua‐Herranz et al., 2017). ERK activation is also required for neuroprotective actions displayed by P2Y13 receptors in cerebellar granule neurons and astrocytes (Miras Portugal et al., 2019). Novel players in P2Y receptor‐activated signalling network encompass dual specificity protein phosphatases (DUSPs), which connect P2Y receptors to the inactivation mechanisms and fine‐tuning of MAPK signalling. P2Y receptor‐mediated DUSP regulation ensures proper intensity and duration of MAPK signalling required to preserve cell survival and to avoid the harmful impact of MAPK over‐activation that can compromise cell viability and promote aberrant proliferation and differentiation rates (Miras Portugal et al., 2019). P2Y13 receptors counteract genotoxic stress by inducing the nuclear DUSP2, which is responsible for the dephosphorylation of https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=519 in the nuclear compartment (Miras Portugal et al., 2019).
7. P2Y RECEPTORS IN ENDOCRINE AND EXOCRINE FUNCTION
Endocrine and exocrine tissues/organs secrete hormones, cytokines, enzymes, and electrolytes and fluid, though in different direction—towards blood (endo‐system) or through ducts to epithelial surfaces (exo‐system). These two systems influence a whole spectrum of functions ranging from metabolism, digestion, to electrolyte/fluid homeostasis, development, growth, and reproduction. The role of purinergic signalling in major endocrine glands has been recently reviewed (Bjelobaba, Janjic, & Stojilkovic, 2015). Here, we focus on tissue/cells regulating metabolism: pancreatic beta‐cells, adipocytes, and the liver.
Extracellular ATP affects insulin secretion from pancreatic beta‐cells, and the positive or negative outcome depends on glucose levels and a particular P2 receptor expressed in a given species (Burnstock & Novak, 2013). ATP originates from nerves, insulin granules, and by transport through Panx1 (Tozzi et al., 2018). In rodent beta‐cells and pancreas, P2Y1 and P2Y6 receptors stimulate insulin secretion (Balasubramanian, Maruoka, Jayasekara, Gao, & Jacobson, 2013), while in mouse, this effect is overshadowed by P2Y13 receptors that inhibit secretion (Amisten et al., 2010). In human beta‐cells, P2X receptors were thought to be more important, but recently, interest in the P2Y1 receptors has been renewed (Khan et al., 2014), not the least because one single nucleotide polymorphism (SNP) in the 3′UTR of P2YR1 is associated with disturbance in glucose homeostasis (Todd et al., 2015). In addition to classical https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=274/Ca2+ signalling stimulating insulin release, one study revealed alternative signalling. P2Y1 receptor agonist https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1755 stimulated https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2352/https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=259, which via PI3K inhibited https://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyId=81, but it is not clear whether this was mediated through the P2Y1 and/or P2Y11 receptors (Zhang, Wang, et al., 2015). Another study showed that the P2Y6 receptors activated calmodulin‐AMPK to increase insulin secretion in murine MIN6 cells (Balasubramanian et al., 2013). Regarding regulation of beta‐cells mass, P2Y6 receptors are cytoprotective, while P2Y13 receptors are pro‐apototic (Burnstock & Novak, 2013). Taken together, targeting P2Y receptors to increase insulin secretion and beta‐cell mass would be a good strategy to ameliorate the course of Type 1 and Type 2 diabetes.
Adipose tissue releases a number of hormones and cytokines, which contribute to the regulation of appetite and satiety, fat distribution, insulin secretion and sensitivity, energy expenditure, and inflammation. White adipocytes express P2Y receptor subtypes that often have opposite effects on adipogenesis, lipogenesis, lipolysis, glucose transport, as well as on leptin and adiponectin production, reflecting adipocyte origin; therefore, the organ or body impact may differ (Tozzi & Novak, 2017). Most illustrative are studies on mice. P2Y1 receptor deletion or its inhibition by MRS2500 reduced leptin production and secretion in lean mice, but this effect disappeared in mice on high‐fat diet (Laplante, Monassier, Freund, Bousquet, & Gachet, 2010). Inhibition or deletion of P2Y4 receptors increased adiponectin secretion in cardiac adipocytes and was cardioprotective (Lemaire et al., 2017). In P2Y2 receptor knockout (KO) mice on high‐fat diet, there was decreased immune cell infiltration and inflammation of adipose tissue, as well as a lower fat and body weight (Merz et al., 2018). In addition, hepatic steatosis was improved, and animals were protected against insulin resistance and hypercholestoremia, all indicating that the P2Y2 receptor is a promising target to combat the initial phases of metabolic syndrome. Brown adipocytes are specialized in energy expenditure, they express P2Y2, P2Y6, and P2Y12 receptors and P2X receptors, but it is not clear yet how they regulate adipocyte function (Tozzi & Novak, 2017).
Recent work shows that plasma uridine levels are influenced by adipocyte‐biliary clearance (Deng et al., 2017). Potentially, uridine levels could affect UDP/UTP levels, which is the case in the hypothalamus, where UDP acts on the P2Y6 receptors of AgRP neurons and promotes feeding behaviour (Steculorum et al., 2015). This offers a challenging opportunity to target this receptor for the treatment of diseases associated with energy balance.
The major exocrine glands include pancreas, salivary glands, liver, sweat, sebaceous and tear glands and associated epithelia. They contribute to digestion and fluid secretion, contain various ions, enzymes, and mucus, and can be affected in a number of diseases, such as cystic fibrosis, Sjögren's syndrome (SS), and cancer. Here, we focus on the first three glands.
Liver, the metabolic hub, also performs endocrine function (secreting, e.g., https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4971) and exocrine function (secreting bile). Not so much is known about the physiological role of purinergic signalling in endocrine function, but the role in liver diseases is well‐reviewed, including the role of P2Y receptors on stellate cells and their relation to liver fibrosis (Lu & Insel, 2014). One key component is the P2Y2 receptor , as KO mice are protected from liver damage and necrosis in experimental liver injury models (Ayata et al., 2012). Cholangiocytes, epithelial cells lining intrahepatic ducts, produce bile containing bile acids/salts and bicarbonate. The bile secretion requires a number of Ca2+‐activated ion channels that are stimulated via P2Y receptors , and one of these, the P2Y12 receptor, resides on the primary cilia (Masyuk et al., 2008).
Pancreatic acini secrete ATP as well as digestive enzymes but do not express active P2 receptors (Haanes et al., 2014). In contrast, pancreatic ducts express various P2 receptors (Novak, 2011), where in particular, the luminal P2Y2 and P2Y4 receptors activate Ca2+‐activated Cl− and K+ channels (https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=130, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=707, and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=384) and thereby potentiate bicarbonate and fluid secretion (Wang, Haanes, & Novak, 2013), thus fine‐tune pancreatic function. In contrast, distension of ducts via ATP release and P2Y2 receptor inhibition of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=380 channels on the basolateral membrane can inhibit secretion (Wang, Haanes, et al., 2013). Potentially, it would be important to increase pancreatic secretion, in, for example, cystic fibrosis or in pancreatitis, but access of drugs to the pancreatic lumen is not feasible.
Salivary gland acini secrete amylase and fluid, and here, P2X receptors are important. Ducts modify saliva to become hypotonic, and P2Y2 receptor agonists increase ductal Cl− reabsorption via cystic fibrosis transmembrane conductance regulator (Novak, 2011). In addition to ion channel regulation via Ca2+ signalling, P2Y2 receptors trans‐stimulate https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1797 that promotes gland repair and regeneration (Ratchford et al., 2010). However, the P2Y2 receptor is up‐regulated in salivary gland inflammation, as in SS, and genetic ablation of P2Y2 receptors in SS mouse models reduced gland inflammation (Woods et al., 2018). Other exocrine glands and epithelia of relevance are the tear glands and corneal epithelium. Secretion of tear glands is mainly regulated via P2X receptors, but minor glands and corneal epithelium express the P2Y2 receptor that stimulates fluid and mucin secretion. This receptor has been a successful target of the drug diquafosol in treating dry eye diseases. A similar approach to target airway epithelia hydration in cystic fibrosis patients with the related drug denufosol (INS37217) did, however, not continue past the second phase III clinical trial (Kellerman et al., 2008).
8. P2Y RECEPTORS IN THE CARDIOVASCULAR SYSTEM
P2Y receptors regulate or participate in all the physiological functions of the cardiovascular system, including heart contractility and cardiac frequency, vascular tone, release of endothelial factors, angiogenesis, smooth muscle cell proliferation, haemostasis, and immunity. As such, they are involved in pathological processes such as acute vascular inflammation, atherosclerosis, arterial thrombosis including myocardial infarction, and cerebrovascular stroke. All the eight P2Y receptor subtypes have been found in the cardiovascular system, that is, in the heart, the vasculature, including smooth muscle cells and endothelial cells, or in blood cells (Nishimura et al., 2017). Studies using combined techniques, such as selective antagonists, or tissue selective or global P2Y receptor subtype deficient mice, helped to elucidate the role of these receptors in most aspects of the cardiovascular system. Also, the role of P2YRs in renal function was recently reviewed (Vallon, Unwin, Inscho, Leipziger, & Kishore, 2019).
The earliest papers on the role of adenine compounds by Drury and Szent‐Györgyi in 1929 reported on their effect on the mammalian heart. P2Y receptors , namely, P2Y1 and P2Y11 receptors, among other P2 receptor subtypes, including P2X receptors, are involved in cardiac function both indirectly via blood vessels and nerves and directly via the cardiomyocytes (Nishimura et al., 2017). These receptors have been reported to exert positive and negative inotropic effects as well as positive and negative chronotropic effects. The nucleotides also play a role in ischaemic preconditioning. All known receptor subtypes have been found, by RT‐PCR, in cardiomyocytes. However, the cardiac function and vascular tone were evaluated in P2Y1 receptor‐deficient mice without detectable differences from those in wild‐type mice (Gachet, unpublished work). These data do not mean that these receptors are not involved in these functions, but clearly, they either only participate in the regulation of these functions or the deficient mice compensated for receptor deficiency. Studies on isolated tissues from these mice indicate a role for P2Y1 and P2Y11 receptors in calcium signalling in the cardiomyocytes (Nishimura et al., 2017).
More is known concerning the vascular function, and one has to subdivide it in endothelial function and smooth muscle cell function. In the endothelium, P2Y1, P2Y2, P2Y4, and P2Y6 receptors are responsible for nucleotide‐induced vasodilation through release of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2509 and prostacyclin (Nishimura et al., 2017). In smooth muscle cells, P2Y2, P2Y4, and P2Y6 receptors are responsible for vasoconstriction. P2Y12 receptors are found in rat capillary endothelial cells (Simon et al., 2002), while other researchers found these receptors on smooth muscle cells where it may trigger vasoconstriction (Wihlborg et al., 2004) and smooth muscle cell proliferation during atherosclerosis (Rauch et al., 2010).
Indeed, in addition to their short‐term effects on vascular tone, nucleotides and P2 receptors are also involved in long‐term trophic effects on cell growth, proliferation, and death which has great implications for diseases such as atherosclerosis and restenosis (Nishimura et al., 2017). The P2Y1 receptors especially play a key role during acute vascular inflammation (Zerr et al., 2011) and in atherosclerosis in apolipoprotein E‐deficient mice (Hechler et al., 2008). Similar results have been reported concerning the P2Y2 (Stachon et al., 2014) and P2Y6 receptors (Garcia et al., 2014) in vascular inflammation.
P2Y receptors are expressed in all blood cells including monocytes, granulocytes, dendritic cells, red blood cells, and platelets. ATP and ADP are major agonists for platelets where they act on https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=478, P2Y1, and P2Y12 receptors in a coordinated fashion (Gachet, 2006). The main role of blood platelets is to ensure primary haemostasis, which is the maintenance of vessel integrity and cessation of bleeding upon injury. While playing a major part in acute arterial thrombosis, platelets are also involved in inflammation, atherosclerosis, and angiogenesis. ADP and ATP play a crucial role in platelet activation, and their receptors are potential targets for antithrombotic drugs. P2Y1 and P2Y12 receptors selectively contribute to platelet aggregation and formation of a thrombus. Owing to its central role in the growth and stabilization of a thrombus, the P2Y12 receptor is an established target of anti‐thrombotic drugs such as clopidogrel, prasugrel, and ticagrelor (Nishimura et al., 2017).
9. P2Y RECEPTORS IN IMMUNE AND PULMONARY FUNCTION
As with P2X receptors, nucleotides acting at P2Y receptors are generally pro‐inflammatory, and their antagonists may eventually be used to treat chronic inflammatory and painful conditions. However, a duality has been noted in which the same P2Y receptor subtype is associated with both damaging (or proinflammatory) and beneficial effects.
Immune cells all express various subtypes of P2Y receptors, which have been shown to be involved in the modulation of inflammation and in immune responses (Idzko, Ferrari, & Eltzschig, 2014). P2Y2 receptor antagonists might be useful in treating pulmonary diseases, other chronic inflammations, or cancer. The P2Y2 receptor contributes to inflammatory responses and fibrotic remodelling in allergic and inflammatory diseases, and thus, P2Y2 receptor antagonists might prove useful in pulmonary and other chronic inflammatoroy diseases. P2Y2 receptor activation on neutrophils and eosinophils induces the release of IL8 and other inflammatory cytokines. P2Y2 receptors on macrophages and neutrophils, activated by damaged cells, induces phagocytosis of bacteria and apoptotic cells to promote wound healing. Migrating neutrophils release ATP from the leading edge, which can induce chemotaxis of neutrophils, dendritic cells, and other immune cells. Platelets that are activated by cancerous tumours release ATP which can open the endothelial barrier to tumour cell extravasation and thus enabling metastasis, by activating an endothelial P2Y2 receptor (Schumacher, Strilic, Sivaraj, Wettschureck, & Offermanns, 2013).
However, there is also reason to assess P2Y2 receptor agonists for therapeutic application. P2Y2 receptors on the lung epithelial surface induce Cl− secretion and improve mucociliary clearance, raising the possibility of using an inhaled P2Y2 receptor agonist for treating cystic fibrosis. Unfortunately, efforts to gain approval of denufosol failed due to the lack of demonstrating long‐term efficacy in patients, possibly due to the simultaneous pro‐inflammatory effects of activated P2Y2 receptors (Kellerman et al., 2008).
On one hand, P2Y6 receptor activation causes release of inflammatory chemokines from immune cells and from epithelial and endothelial cells. On the other hand, P2Y6 receptors induce phagocytosis by microglial cells in the brain, which is beneficial in the removal of dying cell debris. Thus, like the P2Y2, P2Y6 receptors are associated with both beneficial and detrimental effects in disease conditions.
Microglial P2Y12 receptors respond to ADP as a “find me” signal and induces activation and chemotaxis, suggesting a role of this receptor in neurodegeneration and pain signalling (Förster & Reiser, 2015; Tozaki‐Saitoh et al., 2008). Platelets, which express P2Y1 and P2Y12 receptors, themselves play an important role not only in thrombosis but also in modulating inflammatory responses through release of inflammatory mediators or compounds with trophic activity and exposure of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=10014, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1874, and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5077. These molecules allow interaction of platelets with immune cells and their subsequent activation with release of a range of inflammatory cytokines and exposure of platelet tissue factor (Morrell, Aggrey, Chapman, & Modjeski, 2014). Therefore, in addition to acting as antithrombotics, antagonists and inhibitors of platelet P2Y receptors could have anti‐inflammatory effects.
P2Y14 receptors activation promotes chemotaxis in human neutrophils, as studied in cystic fibrosis (Sesma et al., 2016), the release of proinflammatory cytokines from renal intercalated cells in sterile inflammation (Azroyan et al., 2015), and the degranulation of mast cells (Gao, Wei, Jayasekara, & Jacobson, 2013). Thus, P2Y14 receptor antagonists are being developed for anti‐inflammatory applications (Junker et al., 2016).
10. P2Y RECEPTORS IN THE MUSCULOSKELETAL SYSTEM
P2Y receptors are expressed throughout the musculoskeletal system and mediate a variety of pharmacological responses (Burnstock, Arnett, & Orriss, 2013). Their physiological and pathophysiological functions in skeletal muscle cells and cartilage chondrocytes, and how they might be targeted therapeutically, are still unclear, so the focus here is on bone, which not only provides support and protection for the body but also plays a central role in Ca2+ homeostasis. It is a dynamic tissue that turns over continually throughout life, and so requires a balance, termed bone remodelling, between formation, controlled by osteoblasts, and resorption, controlled by osteoclasts, and coordinated by osteocytes and stem cells (Burnstock et al., 2013; Wang & Gartland, 2014).
Musculoskeletal disorders (MSDs) encompass numerous conditions that are characterized by pain and reduced mobility (Burnstock et al., 2013). Furthermore, their incidence is increasing worldwide. MSDs are also a huge financial burden on health care systems, and many treatments are only moderately efficacious and/or expensive, so MSDs are an unmet health need. Thus, P2Y receptors represent potentially novel therapeutic targets for treating MSDs.
10.1. P2Y receptors in osteoporosis
ATP, the main P2Y receptor agonist released in bone, has been implicated in osteoarthritis, rheumatoid arthritis, and cancer‐induced bone diseases, but the P2X7 receptor is the main site of action studied (Wang & Gartland, 2014). In contrast, there is evidence for the involvement of multiple P2Y receptor subtypes in osteoporosis. This is the most common MSD, particularly in post‐menopausal women. Oestrogen normally inhibits bone resorption by osteoclasts, and when this action is lost post‐menopause, bone remodelling becomes unbalanced. Resorption exceeds formation, making bones fragile and more likely to break. As well as stimulating P2Y receptors per se, ATP is dephosphorylated to ADP in the extracellular space by ectonucleotidases, and together, the two agonists stimulate most of the eight P2Y receptor subtypes (Abbracchio et al., 2006, 2019). The limited availability of potent, subtype‐selective antagonists has hindered progress in determining which P2Y receptor subtype(s) are stimulated in bone. Consequently, most advances have arisen from mouse P2Y receptor gene KO studies.
10.2. P2Y2 receptor
The P2YR2 genotype might be a useful prognostic marker for osteoporosis. Analysis of SNPs in Danish post‐menopausal women found an association between the Arg312Ser SNP, which causes a P2Y2 receptor gain‐of‐function, and higher bone mineral density (BMD) and lower rates of bone loss (Wesselius et al., 2011). A subsequent study in Dutch female fracture patients identified a higher incidence of another SNP, Leu46Pro (Wesselius et al., 2013). It is not known yet, however, how this SNP affects P2Y2 receptor function.
Deleting the mouse P2Y2 receptor gene has produced conflicting results. Higher bone mineral content and BMD compared to wild‐type animals was seen by Orriss et al. (2017), but Xing et al. (2014) reported lower bone volume and strength. Consistent with the latter, overexpression of P2Y2 receptors in female rats increased femoral length and strength of the femoral neck but had no effect on BMD (Ellegaard et al., 2017). That these disparities are due to differences in the mouse genetic background or in methodology used cannot, at present, be discounted. The recent commercial availability of AR‐C118925XX, a selective, potent, and competitive P2Y2 receptor antagonist (Rafehi, Neumann, et al., 2017), may help clarify these issues.
10.3. P2Y12 receptor
ADP, but not ATP, stimulates P2Y12 and P2Y13 receptors, and both subtypes appear to modulate bone remodelling. P2Y12 receptor‐KO mice showed lower pathological and age‐related bone loss (Su et al., 2012). Consistent with this, treating wild‐type mice with the selective P2Y12 receptor antagonist, clopidogrel, which is widely prescribed as an antithrombotic agent, inhibited osteoclast formation and increased bone mass. However, a contemporaneous cohort study of osteoporotic fracture in Danish patients prescribed clopidogrel found a dual effect. Those receiving the clinically recommended high dose had an increased risk of fracture, whereas a low dose was associated with a lower risk than people who had not been exposed to clopidogrel (Jørgensen, Schwarz, Iversen, & Vestergaard, 2017). Thus, clopidogrel may potentially increase the risk of osteoporosis, though it should be noted that, while patients with stroke have an increased risk of osteoporotic fractures, clopidogrel does not appear to increase the fracture risk (Jørgensen et al., 2017).
10.4. P2Y13 receptor
Several studies using P2Y13 receptor‐KO mice indicate that this receptor may be protective towards bone growth. First, the mice showed reduced bone trabecular volume and rate of formation, reduced number and activity of trabecular osteoblasts, and less bone loss due to lack of oestrogen after ovariectomy (Wang, Rumney, et al., 2013). In addition, there was greater bone formation in response to mechanical loading, possibly because the receptor mediates negative feedback of the osteogenic agonist, ATP, release from osteoblasts (Wang, Rumney, et al., 2013). Finally, mesenchymal stem cells tended to differentiate towards adipocytes rather than osteoblasts (Biver et al., 2013). Thus, exercise combined with a P2Y13 receptor antagonist is potentially a novel treatment for osteoporosis.
10.5. P2Y6 receptor
P2Y6 receptors are activated by UDP, which appears to be released endogenously, as P2Y6 receptor‐KO mice had increased bone mineral content, cortical bone volume, and cortical thickness in the long bones and spine, but trabecular bone was unaffected (Orriss et al., 2011). Thus, P2Y6 receptor antagonists have potential for treating osteoporosis.
11. CONCLUSIONS
Discovering biomedical applications of agonists or antagonists of P2Y receptors has proven challenging, due to difficulties in using inherently unstable mononucleotides and dinucleotides as pharmacological probes and the complex biological nature of ubiquitous P2Y receptors. Purinergic signalling pioneered by Burnstock and colleagues includes the involvement of P2Y receptors in all physiological systems, including (but not limited to) the following systems: CNS and peripheral nervous, endocrine and exocrine, cardiovascular, immune, and musculoskeletal. Selective agonists and antagonists are important for studying the roles of P2Y receptor subtypes in physiology and pathophysiology, and their structure–activity relationship has been developed in detail through extensive chemical efforts. Nevertheless, there are not yet selective and versatile agonists and antagonists for all of the P2Y receptor subtypes.
Phylogenetic studies have analysed the evolutionary history of P2Y receptors. To summarize, P2Y receptors evolved in early vertebrate evolution, and an expansion of P2Y receptors and P2Y receptor‐related sequences is found in vertebrates. X‐ray crystallographic structures have been determined for members of the two P2Y receptor subfamilies: the Gq‐coupled P2Y1 receptor (with orthosteric and allosteric antagonists) and the Gi‐coupled P2Y12 receptor (with orthosteric agonists and antagonists), and these structures are surprisingly structurally distinct. Phylogenetic, mutational, and crystallographic studies suggest that P2Y1 and P2Y12 receptors evolved independently by convergent evolution presenting the same agonist specificity but structurally different binding sites.
Nucleotides acting at P2Y receptors are generally pro‐inflammatory, and their antagonists may eventually be used to treat chronic inflammatory conditions. However, a duality has been noted in which the same P2Y receptor subtype is associated with both damaging (or proinflammatory) and beneficial effects. Targeting of specific P2Y receptor subtypes has already been shown to be therapeutically useful in case of P2Y12 receptor antagonists as anti‐thrombotics, and P2Y2 receptor agonists for dry eye disease. Current research data indicate that both the clinically validated P2Y receptor subtypes and the other six subtypes have great potential for the development of new pharmacotherapeutic strategies and novel future drugs.
12. NOMENCLATURE OF TARGETS AND LIGANDS
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org/, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos et al., 2019; Alexander, Fabbro et al., 2019a, 2019b; Alexander, Mathie et al., 2019).
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
ACKNOWLEDGEMENTS
The research of Doreen Thor and Torsten Schöneberg was mainly supported by the German Research Foundation (DFG) in SFB 1052, the Integrated Research and Treatment Center (IFB) AdiposityDiseases (BMBF), and intramural funding of the State of Saxony, Germany. Ivana Novak acknowledges support by the Independent Research Fund Denmark (DFF‐4002‐00162). We thank the NIDDK Intramural Research Program (K.A.J.) for support. Christa Müller is grateful for support by the DFG (GRK1873 and FOR2372).
Jacobson KA, Delicado EG, Gachet C, et al. Update of P2Y receptor pharmacology: IUPHAR Review 27. Br J Pharmacol. 2020;177:2413–2433. 10.1111/bph.15005
This article has been contributed to by US Government employees and their work is in the public domain in the USA.
This article, contributed by members of the Nomenclature and Standards Committee of the International Union of Basic and Clinical Pharmacology (NC‐IUPHAR) subcommittee for the P2Y receptors, confirms the existing nomenclature for these receptors, and reviews our current understanding of their structure, pharmacology and functions, and likely physiological roles in health and disease. More information on these receptors can be found in the Concise Guide to PHARMACOLOGY (http://onlinelibrary.wiley.com/doi/10.1111/bph.14748), and in the corresponding open access IUPHAR/BPS Guide to PHARMACOLOGY database (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=52).
REFERENCES
- Abbas, Z. S. B. , Latif, M. L. , Dovlatova, N. , Fox, S. C. , Heptinstall, S. , Dunn, W. R. , & Ralevic, V. (2018). UDP‐sugars activate P2Y14 receptors to mediate vasoconstriction of the porcine coronary artery. Vascular Pharmacology, 103‐105, 36–46. 10.1016/j.vph.2017.12.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbracchio, M. P. , Boeynaems, J. M. , Boyer, J. L. , Burnstock, G. , Ceruti, S. , Fumagalli, M. , … Weisman, G. A. (2019). P2Y receptors (version 2019.4) in the IUPHAR/BPS Guide to Pharmacology Database. IUPHAR/BPS Guide to Pharmacology CITE. 2019(4). Available from: 10.2218/gtopdb/F52/2019.4. [DOI]
- Abbracchio, M. P. , Burnstock, G. , Boeynaems, J. M. , Barnard, E. A. , Boyer, J. L. , Kennedy, C. , … Weisman, G. A. (2006). International Union of Pharmacology LVIII. Update on the P2Y G protein‐coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacological Reviews, 58, 281–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, Y. H. , Lee, J. Y. , Park, H. D. , Kim, T. H. , Park, M. C. , Choi, G. , & Kim, S. (2016). Identification of a new morpholine scaffold as a P2Y12 receptor antagonist. Molecules, 21, E1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … Collaborators, C. G. T. P. (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … Collaborators, C. G. T. P. (2019a). The Concise Guide to PHARMACOLOGY 2019/20: Catalytic receptors. British Journal of Pharmacology, 176, S247–S296. 10.1111/bph.14751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … Collaborators, C. G. T. P. (2019b). The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , … Collaborators, C. G. T. P. (2019). The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176, S142–S228. 10.1111/bph.14749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algaier, I. , Jakubowski, J. A. , Asai, F. , & von Kügelgen, I. (2008). Interaction of the active metabolite of prasugrel, R‐138727, with cysteine 97 and cysteine 175 of the human P2Y12 receptor. Journal of Thrombosis and Haemostasis, 6, 1908–1914. [DOI] [PubMed] [Google Scholar]
- Alves, M. , De Diego, G. L. , Conte, G. , Jimenez‐Mateos, E. M. , D'Orsi, B. , … Engel, T. (2019). Context‐specific switch from anti‐ to pro‐epileptogenic function of the P2Y1 receptor in experimental epilepsy. The Journal of Neuroscience, 39(27), 5377–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadio, S. , Montilli, C. , Magliozzi, R. , Bernardi, G. , Reynolds, R. , & Volonté, C. (2010). P2Y12 receptor protein in cortical gray matter lesions in multiple sclerosis. Cerebral Cortex, 20, 1263–1273. [DOI] [PubMed] [Google Scholar]
- Amisten, S. , Meidute‐Abaraviciene, S. , Tan, C. , Olde, B. , Lundquist, I. , Salehi, A. , & Erlinge, D. (2010). ADP mediates inhibition of insulin secretion by activation of P2Y13 receptors in mice. Diabetologia, 53, 1927–1934. [DOI] [PubMed] [Google Scholar]
- Aungraheeta, R. , Conibear, A. , Butler, M. , Kelly, E. , Nylander, S. , Mumford, A. , & Mundell, S. J. (2016). Inverse agonism at the P2Y12 receptor and ENT1 transporter blockade contribute to platelet inhibition by ticagrelor. Blood, 128, 2717–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayata, C. K. , Ganal, S. C. , Hockenjos, B. , Willim, K. , Vieira, R. P. , Grimm, M. , … Hasselblatt, P. (2012). Purinergic P2Y2 receptors promote neutrophil infiltration and hepatocyte death in mice with acute liver injury. Gastroenterology, 143, 1620–1629. [DOI] [PubMed] [Google Scholar]
- Azroyan, A. , Cortez‐Retamozo, V. , Bouley, R. , Liberman, R. , Ruan, Y. C. , Kiselev, E. , … Breton, S. (2015). Renal intercalated cells sense and mediate sterile inflammation via the P2Y14 receptor. PLoS ONE, 10(3), e0121419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach, P. , Boström, J. , Brickmann, K. , van Giezen, J. J. , Groneberg, R. D. , Harvey, D. M. , … Zetterberg, F. (2013). Synthesis, structure‐property relationships and pharmacokinetic evaluation of ethyl 6‐aminonicotinate sulfonylureas as antagonists of the P2Y12 receptor. European Journal of Medicinal Chemistry, 65, 360–375. [DOI] [PubMed] [Google Scholar]
- Balasubramanian, R. , Maruoka, H. , Jayasekara, P. S. , Gao, Z. G. , & Jacobson, K. A. (2013). AMP‐activated protein kinase as regulator of P2Y6 receptor‐induced insulin secretion in mouse pancreatic β‐cells. Biochemical Pharmacology, 85, 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldoni, D. , Bruderer, S. , Krause, A. , Gutierrez, M. , Gueret, P. , Astruc, B. , & Dingemanse, J. (2014). A new reversible and potent P2Y12 receptor antagonist (ACT‐246475): Tolerability, pharmacokinetics, and pharmacodynamics in a first‐in‐man trial. Clinical Drug Investigation, 34, 807–818. [DOI] [PubMed] [Google Scholar]
- Baqi, Y. , & Müller, C. E. (2019). Antithrombotic P2Y12 receptor antagonists: Recent developments in drug discovery. Drug Discovery Today, 24(1), 325–333. 10.1016/j.drudis.2018.09.021 [DOI] [PubMed] [Google Scholar]
- Barrett, M. O. , Sesma, J. I. , Ball, C. B. , Jayasekara, P. S. , Jacobson, K. A. , Lazarowski, E. R. , & Harden, T. K. (2013). A selective high‐affinity antagonist of the P2Y14 receptor inhibits UDP‐glucose‐stimulated chemotaxis of human neutrophils. Molecular Pharmacology, 84, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijak, M. , Szelenberger, R. , Dziedzic, A. , & Saluk‐Bijak, J. (2018). Inhibitory effect of flavonolignans on the P2Y12 pathway in blood platelets. Molecules, 23, 374 10.3390/molecules23020374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biver, G. , Wang, N. , Gartland, A. , Orriss, I. , Arnett, T. R. , Boeynaems, J. M. , & Robaye, B. (2013). Role of the P2Y13 receptor in the differentiation of bone marrow stromal cells into osteoblasts and adipocytes. Stem Cells, 31, 2747–2758. [DOI] [PubMed] [Google Scholar]
- Bjelobaba, I. , Janjic, M. M. , & Stojilkovic, S. S. (2015). Purinergic signaling pathways in endocrine system. Autonomic Neuroscience, 191, 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruser, A. , Zimmermann, A. , Crews, B. C. , Sliwoski, G. , Meiler, J. , Konig, G. M. , … Schöneberg, T. (2017). Prostaglandin E2 glyceryl ester is an endogenous agonist of the nucleotide receptor P2Y6 . Scientific Reports, 7, 2380 10.1098/s41598-017-02414-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock, G. (1978). A basis for distinguishing two types of purinergic receptor In Straub R. W. (Ed.), Cell membrane receptors for drugs and hormones: A multidisciplinary approach (pp. 107–118). New York: Raven Press. [Google Scholar]
- Burnstock, G. (2017). Purinergic signalling and neurological diseases: An update. CNS & Neurological Disorders ‐ Drug Targets, 16, 257–265. 10.2174/1871527315666160922104848 [DOI] [PubMed] [Google Scholar]
- Burnstock, G. , Arnett, T. R. , & Orriss, I. R. (2013). Purinergic signalling in the musculoskeletal system. Purinergic Signal, 9, 541–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock, G. , & Kennedy, C. (1985). Is there a basis for distinguishing two types of P2‐purinoceptor? General Pharmacology, 5, 433–440. [DOI] [PubMed] [Google Scholar]
- Burnstock, G. , & Novak, I. (2013). Purinergic signalling and diabetes. Purinergic Signal, 9, 307–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroff, E. , Hubler, F. , Meyer, E. , Renneberg, D. , Gnerre, C. , Treiber, A. , … Riederer, M. A. (2015). 4‐((R)‐2‐{[6‐((S)‐3‐Methoxypyrrolidin‐1‐yl)‐2‐phenylpyrimidine‐4‐carbonyl]amino}‐3‐phosphonopropionyl)piperazine‐1‐carboxylic acid butyl ester (ACT‐246475) and its prodrug (ACT‐281959), a novel P2Y12 receptor antagonist with a wider therapeutic window in the rat than clopidogrel. Journal of Medicinal Chemistry, 58, 9133–9153. [DOI] [PubMed] [Google Scholar]
- Carter, R. L. , Fricks, I. P. , Barrett, M. O. , Burianek, L. E. , Zhou, Y. , Ko, H. , … Harden, T. K. (2009). Quantification of Gi‐mediated inhibition of adenylyl cyclase activity reveals that UDP is a potent agonist of the human P2Y14 receptor. Molecular Pharmacology, 76, 1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatriwala, M. , Ravi, R. G. , Patel, R. I. , Boyer, J. L. , Jacobson, K. A. , & Harden, T. K. (2004). Induction of novel agonist selectivity for the ADP‐activated P2Y1 receptor versus the ADP‐activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analog. Journal of Pharmacology and Experimental Therapeutics, 311, 1038–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Communi, D. , Gonzalez, N. S. , Detheux, M. , Brezillon, S. , Lannoy, V. , Parmentier, M. , & Boeynaems, J. M. (2001). Identification of anovel human ADP receptor coupled to Gi. Journal of Biological Chemistry, 276, 41479–41485. [DOI] [PubMed] [Google Scholar]
- Conroy, S. , Kindon, N. , Kellam, B. , & Stocks, M. J. (2016). Drug‐like antagonists of P2Y receptors—From lead identification to drug development. Journal of Medicinal Chemistry, 59(22), 9981–10005. [DOI] [PubMed] [Google Scholar]
- Costanzi, S. , Kumar, T. S. , Balasubramanian, R. , Harden, T. K. , & Jacobson, K. A. (2012). Virtual screening leads to the discovery of novel non‐nucleotide P2Y1 receptor antagonists. Bioorganic & Medicinal Chemistry, 20, 5254–5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansette, P. M. , Levent, D. , Hessani, A. , & Mansuy, D. (2015). Bioactivation of Clopidogrel and Prasugrel: Factors determining the stereochemistry of the thiol metabolite double bond. Chemical Research in Toxicology, 28, 1338–1345. [DOI] [PubMed] [Google Scholar]
- Das, A. , Ko, H. , Burianek, L. E. , Barrett, M. O. , Harden, T. K. , & Jacobson, K. A. (2010). Human P2Y14 receptor agonists: Truncation of the hexose moiety of uridine‐5′‐diphosphoglucose and its replacement with alkyl and aryl groups. Journal of Medicinal Chemistry, 53, 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Y. , Wang, Z. V. , Gordillo, R. , An, Y. , Zhang, C. , Liang, Q. , … Scherer, P. E. (2017). An adipo‐biliary‐uridine axis that regulates energy homeostasis. Science, 355(6330), eaaf5375 10.1126/science.aaf5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, Z. , Bynagari, Y. S. , Mada, S. R. , Jakubowski, J. A. , & Kunapuli, S. P. (2009). Studies on the role of the extracellular cysteines and oligomeric structures of the P2Y12 receptor when interacting with antagonists. Journal of Thrombosis and Haemostasis, 7, 232–234. [DOI] [PubMed] [Google Scholar]
- Dreisig, K. , & Kornum, B. R. (2016). A critical look at the function of the P2Y11 receptor. Purinergic Signal, 12, 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury, A. N. , & Szent‐Györgyi, A. (1929). The physiological activity of adenine compounds with special reference to their action upon the mammalian heart. The Journal of Physiology, 68, 213–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecke, D. , Tulapurkar, M. E. , Nahum, V. , Fischer, B. , & Reiser, G. (2006). Opposite diastereoselective activation of P2Y1 and P2Y11 nucleotide receptors by adenosine 5′‐O‐(α‐boranotriphosphate) analogues. British Journal of Pharmacology, 149, 416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegaard, M. , Agca, C. , Petersen, S. , Agrawal, A. , Kruse, L. S. , Wang, N. , … Agca, Y. (2017). Bone turnover is altered in transgenic rats overexpressing the P2Y2 purinergic receptor. Purinergic Signal, 13, 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Tayeb, A. , Qi, A. , Nicholas, R. A. , & Müller, C. E. (2011). Structural modifications of UMP, UDP, and UTP leading to subtype‐selective agonists for P2Y2, P2Y4, and P2Y6 receptors. Journal of Medicinal Chemistry, 54, 2878–2890. [DOI] [PubMed] [Google Scholar]
- Förster, D. , & Reiser, G. (2015). Supportive or detrimental roles of P2Y receptors in brain pathology?—The two faces of P2Y receptors in stroke and neurodegeneration detected in neural cell and in animal model studies. Purinergic Signal, 11(4), 441–454. 10.1007/s11302-015-9471-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke, H. , Verkhratsky, A. , Burnstock, G. , & Illes, P. (2012). Pathophysiology of astroglial purinergic signalling. Purinergic Signal, 8, 629–657. 10.1007/s11302-012-9300-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson, R. , & Schiöth, H. B. (2005). The repertoire of G‐protein‐coupled receptors in fully sequenced genomes. Molecular Pharmacology, 67, 1414–1425. [DOI] [PubMed] [Google Scholar]
- Fumagalli, M. , Lecca, D. , & Abbracchio, M. P. (2016). CNS remyelination as a novel reparative approach to neurodegenerative diseases: The roles of purinergic signaling and the P2Y‐like receptor GPR17. Neuropharmacology, 104, 82–93. [DOI] [PubMed] [Google Scholar]
- Fumagalli, M. , Trincavelli, L. , Lecca, D. , Martini, C. , Ciana, P. , & Abbracchio, M. P. (2004). Cloning, pharmacological characterisation and distribution of the rat G‐protein‐coupled P2Y13 receptor. Biochemical Pharmacology, 68, 113–124. [DOI] [PubMed] [Google Scholar]
- Gachet, C. (2006). Regulation of platelet functions by P2 receptors. Annual Review of Pharmacology and Toxicology, 46, 277–300. [DOI] [PubMed] [Google Scholar]
- Gao, Z. G. , & Jacobson, K. A. (2017). Distinct signaling patterns of allosteric antagonism at the P2Y1 receptor. Molecular Pharmacology, 92, 613–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Z. G. , Wei, Q. , Jayasekara, M. P. S. , & Jacobson, K. A. (2013). The role of P2Y14 and other P2Y receptors in degranulation of human LAD2 mast cells. Purinergic Signal, 9, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, C. , Maurel‐Ribes, A. , Nauze, M. , N'Guyen, D. , Martinez, L. O. , Payrastre, B. , … Pons, V. (2019). Deciphering biased inverse agonism of cangrelor and ticagrelor at P2Y12 receptor. Cellular and Molecular Life Sciences, 76, 561–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, R. A. , Yan, M. , Search, D. , Zhang, R. , Carson, N. L. , Ryan, C. S. , … Gargalovic, P. S. (2014). P2Y6 receptor potentiates pro‐inflammatory responses in macrophages and exhibits differential roles in atherosclerotic lesion development. PLoS ONE, 9, e111385 10.1371/journal.pone.0111385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg‐Shmuel, T. , Haas, M. , Grbic, D. , Arguin, G. , Nadel, Y. , Gendron, F. P. , … Fischer, B. (2012). UDP made a highly promising stable, potent, and selective P2Y6‐receptor agonist upon introduction of a boranophosphate moiety. Bioorganic & Medicinal Chemistry, 20, 5483–5495. [DOI] [PubMed] [Google Scholar]
- Gómez‐Villafuertes, R. , Rodríguez‐Jiménez, F. J. , Alastrue‐Agudo, A. , Stojkovic, M. , Miras‐Portugal, M. T. , & Moreno‐Manzano, V. (2015). Purinergic receptors in spinal cord‐derived ependymal stem/progenitor cells and their potential role in cell‐based therapy for spinal cord injury. Cell Transplantation, 24(8), 1493–1509. 10.3727/096368914X682828 [DOI] [PubMed] [Google Scholar]
- Gordon, J. L. (1986). Extracellular ATP: Effects, sources and fate. The Biochemical Journal, 233, 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haanes, K. A. , Kowal, J. M. , Arpino, G. , Lange, S. C. , Moriyama, Y. , Pedersen, P. A. , & Novak, I. (2014). Role of vesicular nucleotide transporter VNUT (SLC17A9) in release of ATP from AR42J cells and mouse pancreatic acinar cells. Purinergic Signal, 10, 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res., 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechler, B. , Freund, M. , Ravanat, C. , Magnenat, S. , Cazenave, J. P. , & Gachet, C. (2008). Reduced atherosclerotic lesions in P2Y1/apolipoprotein E double‐knockout mice: The contribution of non‐hematopoietic‐derived P2Y1 receptors. Circulation, 118, 754–763. [DOI] [PubMed] [Google Scholar]
- Hechler, B. , Nonne, C. , Roh, E. J. , Cattaneo, M. , Cazenave, J. P. , Lanza, F. , … Gachet, C. (2006). MRS2500 [2‐iodo‐N6‐methyl‐(N)‐methanocarba‐2′‐deoxyadenosine‐3′,5′‐bisphosphate], a potent, selective, and stable antagonist of the platelet P2Y1 receptor with strong antithrombotic activity in mice. The Journal of Pharmacology and Experimental Therapeutics, 31, 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, K. , Baqi, Y. , Morena, M. S. , Glänzel, M. , Müller, C. E. , & von Kügelgen, I. (2009). Interaction of new, very potent non‐nucleotide antagonists with Arg256 of the human platelet P2Y12 receptor. Journal of Pharmacology and Experimental Therapeutics, 331, 648–655. [DOI] [PubMed] [Google Scholar]
- Hoffmann, K. , Lutz, D. A. , Straßburger, J. , Baqi, Y. , Müller, C. E. , & von Kügelgen, I. (2014). Competitive mode and site of interaction of ticagrelor at the human platelet P2Y12‐receptor. Journal of Thrombosis and Haemostasis, 12, 1898–1905. [DOI] [PubMed] [Google Scholar]
- Hollopeter, G. , Jantzen, H. M. , Vincent, D. , Li, G. , England, L. , Ramakrishnan, V. , … Conley, P. B. (2001). Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature, 409, 202–207. [DOI] [PubMed] [Google Scholar]
- Hou, Z. , & Cao, J. (2016). Comparative study of the P2X gene family in animals and plants. Purinergic Signal, 12, 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzko, M. , Ferrari, D. , & Eltzschig, H. K. (2014). Nucleotide signalling during inflammation. Nature, 509(7500), 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingall, A. H. , Dixon, J. , Bailey, A. , Coombs, M. E. , Cox, D. , McInally, J. I. , … Tomlinson, W. (1999). Antagonists of the platelet P2T receptor: A novel approach to antithrombotic therapy. Journal of Medicinal Chemistry, 42, 213–220. [DOI] [PubMed] [Google Scholar]
- Jacobson, K. A. , & Müller, C. E. (2016). Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology, 104, 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson, K. A. , Paoletta, S. , Katritch, V. , Wu, B. , Gao, Z. G. , Zhao, Q. , … Kiselev, E. (2015). Nucleotides acting at P2Y receptors: Connecting structure and function. Molecular Pharmacology, 88(2), 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Q. , Guo, D. , Lee, B. X. , van Rhee, A. M. , Kim, Y. C. , Nicholas, R. A. , … Jacobson, K. A. (1997). A mutational analysis of residues essential for ligand recognition at the human P2Y1 receptor. Molecular Pharmacology, 52, 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez, E. , Zafra, F. , Pérez‐Sen, R. , Delicado, E. G. , Miras‐Portugal, M. T. , Aragón, C. , & López‐Corcuera, B. (2011). P2Y purinergic regulation of the glycine An Update on P2Y13 Receptor signalling and function neurotransmitter transporters. The Journal of Biological Chemistry, 286(12), 10712–10724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen, N. R. , Schwarz, P. , Iversen, H. K. , & Vestergaard, P. (2017). P2Y12 receptor antagonist, clopidogrel, does not contribute to risk of osteoporotic fractures in stroke patients. Frontiers in Pharmacology, 8, 821 10.3389/fphar.2017.00821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker, A. , Balasubramanian, R. , Ciancetta, A. , Uliassi, E. , Kiselev, E. , Martiriggiano, C. , … Jacobson, K. A. (2016). Structure‐based design of 3‐(4‐aryl‐1H‐1,2,3‐triazol‐1‐yl)‐biphenyl derivatives as P2Y14 receptor antagonists. Journal of Medicinal Chemistry, 59, 6149–6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating, G. M. (2015). Diquafosol ophthalmic solution 3%: A review of its use in dry eye. Drugs, 75, 911–922. [DOI] [PubMed] [Google Scholar]
- Kellerman, D. , Rossi, M. A. , Engels, J. , Schaberg, A. , Gorden, J. , & Smiley, L. (2008). Denufosol: A review of studies with inhaled P2Y2 agonists that led to Phase 3. Pulmonary Pharmacology & Therapeutics, 21, 600–607. [DOI] [PubMed] [Google Scholar]
- Khan, S. , Yan‐Do, R. , Duong, E. , Wu, X. , Bautista, A. , Cheley, S. , … Braun, M. (2014). Autocrine activation of P2Y1 receptors couples Ca2+ influx to Ca2+ release in human pancreatic beta cells. Diabetologia, 57, 2535–2545. [DOI] [PubMed] [Google Scholar]
- Kim, Y. C. , Lee, J. S. , Sak, K. , Marteau, F. , Mamedova, L. , Boeynaems, J. M. , & Jacobson, K. A. (2005). Synthesis of pyridoxal phosphate derivatives with antagonist activity at the P2Y13 receptor. Biochemical Pharmacology, 70, 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindon, N. , Davis, A. , Dougall, I. , Dixon, J. , Johnson, T. , Walters, I. , … Stocks, M. J. (2017). From UTP to AR‐C118925, the discovery of a potent non nucleotide antagonist of the P2Y2 receptor. Bioorganic & Medicinal Chemistry Letters, 27, 4849–4853. [DOI] [PubMed] [Google Scholar]
- Ko, H. , Carter, R. L. , Cosyn, L. , Petrelli, R. , de Castro, S. , Besada, P. , … Jacobson, K. A. (2008). Synthesis and potency of novel uracil nucleotides and derivatives as P2Y2 and P2Y6 receptor agonists. Bioorganic & Medicinal Chemistry, 16, 6319–6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korcok, J. , Raimundo, L. N. , Du, X. , Sims, S. M. , & Dixon, S. J. (2005). P2Y6 nucleotide receptors activate NF‐κB and increase survival of osteoclasts. The Journal of Biological Chemistry, 280, 16909–16915. [DOI] [PubMed] [Google Scholar]
- Krishnan, A. , Almen, M. S. , Fredriksson, R. , & Schiöth, H. B. (2013). Remarkable similarities between the hemichordate (Saccoglossus kowalevskii) and vertebrate GPCR repertoire. Gene, 526, 122–133. [DOI] [PubMed] [Google Scholar]
- Kumar, S. , Stecher, G. , & Tamura, K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Molecular Biology and Evolution, 33, 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante, M. A. , Monassier, L. , Freund, M. , Bousquet, P. , & Gachet, C. (2010). The purinergic P2Y1 receptor supports leptin secretion in adipose tissue. Endocrinology, 151, 2060–2070. [DOI] [PubMed] [Google Scholar]
- Le Duc, D. , Schulz, A. , Lede, V. , Schulze, A. , Thor, D. , Bruser, A. , & Schöneberg, T. (2017). P2Y receptors in immune response and inflammation. Advances in Immunology, 136, 85–121. [DOI] [PubMed] [Google Scholar]
- Lemaire, A. , Vanorle, M. , Horckmans, M. , di Pietrantonio, L. , Clouet, S. , Robaye, B. , … Communi, D. (2017). Mouse P2Y4 nucleotide receptor is a negative regulator of cardiac adipose‐derived stem cell differentiation and cardiac fat formation. Stem Cells and Development, 26, 363–373. [DOI] [PubMed] [Google Scholar]
- Liu, X. , Gao, Z. G. , Wu, Y. , Stevens, R. C. , Jacobson, K. A. , & Zhao, S. (2018). Salvianolic acids from antithrombotic traditional chinese medicine danshen are antagonists of human P2Y1 and P2Y12 receptors. Scientific Reports, 8, 8084 10.1038/s41598-018-26577-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, D. , & Insel, P. A. (2014). Cellular mechanisms of tissue fibrosis. 6. Purinergic signaling and response in fibroblasts and tissue fibrosis. American Journal of Physiology. Cell Physiology, 306, C779–C788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin, S. A. , & Molliver, D. C. (2010). Gi‐ and Gq‐coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Molecular Pain, 6, 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamedova, L. K. , Joshi, B. V. , Gao, Z. G. , von Kügelgen, I. , & Jacobson, K. A. (2004). Diisothiocyanate derivatives as potent, insurmountable antagonists of P2Y6 nucleotide receptors. Biochemical Pharmacology, 67, 1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruoka, H. , Jayasekara, M. P. , Barrett, M. O. , Franklin, D. A. , de Castro, S. , Kim, N. , … Jacobson, K. A. (2011). Pyrimidine nucleotides with 4‐alkyloxyimino and terminal tetraphosphate δ‐ester modifications as selective agonists of the P2Y4 receptor. Journal of Medicinal Chemistry, 54, 4018–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyuk, A. I. , Gradilone, S. A. , Banales, J. M. , Huang, B. Q. , Masyuk, T. V. , Lee, S. O. , … LaRusso, N. F. (2008). Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. American Journal of Physiology. Gastrointestinal and Liver Physiology, 295, G725–G734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meis, S. , Hamacher, A. , Hongwiset, D. , Marzian, C. , Wiese, M. , Eckstein, N. , … Kassack, M. U. (2010). NF546 [4,4′‐(carbonylbis (imino‐3,1‐phenylene‐carbonylimino‐3,1‐(4‐methyl‐phenylene)‐carbonylimino))‐bis(1,3‐xylene‐α,α′‐diphosphonic acid) tetrasodium salt] is a non‐nucleotide P2Y11 agonist and stimulates release of interleukin‐8 from human monocyte‐derived dendritic cells. The Journal of Pharmacology and Experimental Therapeutics, 332, 238–247. [DOI] [PubMed] [Google Scholar]
- Meltzer, D. , Ethan, O. , Arguin, G. , Nadel, Y. , Danino, O. , Lecka, J. , … Fischer, B. (2015). Synthesis and structure‐activity relationship of uracil nucleotide derivatives towards the identification of human P2Y6 receptor antagonists. Bioorganic & Medicinal Chemistry, 23, 5764–5773. [DOI] [PubMed] [Google Scholar]
- Merz, J. , Albrecht, P. , von Garlen, S. , Ahmed, I. , Dimanski, D. , Wolf, D. , … Stachon, P. (2018). Purinergic receptor Y2 (P2Y2)‐ dependent VCAM‐1 expression promotes immune cell infiltration in metabolic syndrome. Basic Research in Cardiology, 113(6), 45 10.1007/s00395-018-0702-1 [DOI] [PubMed] [Google Scholar]
- Miras Portugal, M. T. , Queipo, M. J. , Gil‐Redondo, J. C. , Ortega, F. , Gómez‐Villafuertes, R. , Gualix, J. , … Pérez‐Sen, R. (2019). P2 receptor interaction and signalling cascades in neuroprotection. Brain Research Bulletin, 151, 74–83. [DOI] [PubMed] [Google Scholar]
- Moldovan, R. P. , Wenzel, B. , Teodoro, R. , Neumann, W. , Dukic‐Stefanovic, S. , Kraus, W. , … Brust, P. (2019). Studies towards the development of a PET radiotracer for imaging of the P2Y1 receptors in the brain: synthesis, 18F‐labeling and preliminary biological evaluation. European Journal of Medicinal Chemistry, 165, 142–159. [DOI] [PubMed] [Google Scholar]
- Morales‐Ramos, A. I. , Mecom, J. S. , Kiesow, T. J. , Graybill, T. L. , Brown, G. D. , Aiyar, N. V. , … Marino, J. P. Jr. (2008). Tetrahydro‐4‐quinolinamines identified as novel P2Y1 receptor antagonists. Bioorganic & Medicinal Chemistry Letters, 18, 6222–6226. [DOI] [PubMed] [Google Scholar]
- Morrell, C. N. , Aggrey, A. A. , Chapman, L. M. , & Modjeski, K. L. (2014). Emerging roles for platelets as immune and inflammatory cells. Blood, 123(18), 2759–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, A. , Sunggip, C. , Oda, S. , Numaga‐Tomita, T. , Tsuda, M. , & Nishida, M. (2017). Purinergic P2Y receptors: Molecular diversity and implications for treatment of cardiovascular diseases. Pharmacology & Therapeutics, 180, 113–128. [DOI] [PubMed] [Google Scholar]
- Novak, I. (2011). Purinergic signalling in epithelial ion transport ‐ regulation of secretion and absorption. Acta Physiologica, 202, 501–522. [DOI] [PubMed] [Google Scholar]
- Ohlmann, P. , de Castro, S. , Brown, G. G. , Gachet, C. , Jacobson, K. A. , & Harden, T. K. (2010). Quantification of recombinant and platelet P2Y1 receptors utilizing a [125I]‐labeled high‐affinity antagonist 2‐iodo‐N6‐methyl‐(N)‐methanocarba‐2′‐deoxyadenosine‐3′,5′‐bisphosphate ([125I]MRS2500). Pharmacological Research, 62, 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlmann, P. , Lecchi, A. , El‐Tayeb, A. , Müller, C. E. , Cattaneo, M. , & Gachet, C. (2013). The platelet P2Y12 receptor under normal and pathological conditions. Assessment with the radiolabeled selective antagonist [3H]PSB‐0413. Purinergic Signal, 9, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orriss, I. R. , Guneri, D. , Hajjawi, M. O. R. , Shaw, K. , Patel, J. J. , & Arnett, T. R. (2017). Activation of the P2Y2 receptor regulates bone cell function by enhancing ATP release. The Journal of Endocrinology, 233, 341–356. [DOI] [PubMed] [Google Scholar]
- Orriss, I. R. , Wang, N. , Burnstock, G. , Arnett, T. R. , Gartland, A. , Robaye, B. , & Boeynaems, J. M. (2011). The P2Y6 receptor stimulates bone resorption by osteoclasts. Endocrinology, 152, 3706–3716. [DOI] [PubMed] [Google Scholar]
- Paniagua‐Herranz, L. , Gil‐Redondo, J. C. , Queipo, M. J. , González‐Ramos, S. , Boscá, L. , Pérez‐Sen, R. , … Delicado, E. G. (2017). Prostaglandin E2 impairs P2Y2/P2Y4 receptor signaling in cerebellar astrocytes via EP3 receptors. Frontiers in Pharmacology, 8, 937 10.3389/fphar.2017.00937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, J. , Zhao, L. , Wang, L. , Chen, H. , Qiu, Y. , Wang, J. , … Liu, H. (2018). Design, synthesis, and biological evaluation of 2‐(phenoxyaryl)‐3‐urea derivatives as novel P2Y1 receptor antagonists. European Journal of Medicinal Chemistry, 158, 302–310. [DOI] [PubMed] [Google Scholar]
- Pérez‐Sen, R. , Queipo, M. J. , Morente, V. , Ortega, F. , Delicado, E. G. , & Miras‐Portugal, M. T. (2015). Neuroprotection mediated by P2Y13 nucleotide receptors in neurons. Computational and Structural Biotechnology Journal, 13, 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafehi, M. , Burbiel, J. C. , Attah, I. Y. , Abdelrahman, A. , & Müller, C. E. (2017). Synthesis, characterization, and in vitro evaluation of the selective P2Y2 receptor antagonist AR‐C118925. Purinergic Signal, 13, 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafehi, M. , Malik, E. M. , Neumann, A. , Abdelrahman, A. , Hanck, T. , Namasivayam, V. , … Baqi, Y. (2017). Development of potent and selective antagonists for the UTP‐activated P2Y4 receptor. Journal of Medicinal Chemistry, 60, 3020–3038. [DOI] [PubMed] [Google Scholar]
- Rafehi, M. , & Müller, C. E. (2018). Tools and drugs for uracil nucleotide‐activated P2Y receptors. Pharmacology & Therapeutics, 190, 24–80. [DOI] [PubMed] [Google Scholar]
- Rafehi, M. , Neumann, A. , Baqi, Y. , Malik, E. M. , Wiese, M. , Namasivayam, V. , & Müller, C. E. (2017). Molecular recognition of agonists and antagonists by the nucleotide‐activated G protein‐coupled P2Y2 receptor. Journal of Medicinal Chemistry, 60, 8425–8440. [DOI] [PubMed] [Google Scholar]
- Ratchford, A. M. , Baker, O. J. , Camden, J. M. , Rikka, S. , Petris, M. J. , Seye, C. I. , … Weisman, G. A. (2010). P2Y2 nucleotide receptors mediate metalloprotease‐dependent phosphorylation of epidermal growth factor receptor and ErbB3 in human salivary gland cells. The Journal of Biological Chemistry, 285, 7545–7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch, B. H. , Rosenkrantz, A. C. , Ermler, S. , Böhm, A. , Driessen, J. , Fischer, J. W. , … Schrör, K. (2010). Regulation of functionally active P2Y12 adp receptors by thrombin in human smooth muscle cells and the presence of P2Y12 in carotid artery lesions. Arteriosclerosis, Thrombosis, and Vascular Biology, 30(12), 2434–2442. [DOI] [PubMed] [Google Scholar]
- Reichenbach, N. , Delekate, A. , Breithausen, B. , Keppler, K. , Poll, S. , Schulte, T. , … Petzold, G. C. (2018). P2Y1 receptor blockade normalizes network dysfunction and cognition in an Alzheimer's disease model. The Journal of Experimental Medicine, 215(6), 1649–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savi, P. , Zachayus, J. L. , Delesque‐Touchard, N. , Labouret, C. , Hervé, C. , Uzabiaga, M. F. , … Herbert, J. M. (2006). The active metabolite of clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts. Proceedings of the National Academy of Sciences of the United States of America, 103, 11069–11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, P. , Ritscher, L. , Dong, E. N. , Hermsdorf, T. , Coster, M. , Wittkopf, D. , … Schöneberg, T. (2013). Identification of determinants required for agonistic and inverse agonistic ligand properties at the ADP receptor P2Y12 . Molecular Pharmacology, 83, 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöneberg, T. , Hermsdorf, T. , Engemaier, E. , Engel, K. , Liebscher, I. , Thor, D. , … Schultz, A. (2007). Structural and functional evolution of the P2Y12‐like receptor group. Purinergic Signal, 3, 255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, D. , Strilic, B. , Sivaraj, K. K. , Wettschureck, N. , & Offermanns, S. (2013). Platelet‐derived nucleotides promote tumor‐cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell, 24, 130–137. [DOI] [PubMed] [Google Scholar]
- Sesma, J. L. , Weitzer, C. D. , Livraghi‐Butrico, A. , Dang, H. , Donaldson, S. , Alexis, N. E. , … Lazarowski, E. R. (2016). UDP‐glucose promotes neutrophil recruitment in the lung. Purinergic Signalling, 12, 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi, E. , Hirayama, Y. J. , Ikenaka, K. , Tanaka, K. F. , & Koizumi, S. (2018). Role of purinergic receptor P2Y1 in spatiotemporal Ca2+ dynamics in astrocytes. The Journal of Neuroscience, 38(6), 1383–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki, Y. , Shibata, K. , Yoshida, K. , Shigetomi, E. , Gachet, C. , Ikenaka, K. , … Koizumi, S. (2017). Transformation of astrocytes to a neuroprotective phenotype by microglia via P2Y1 receptor downregulation. Cell Reports, 19, 1151–1164. [DOI] [PubMed] [Google Scholar]
- Simões, A. P. , Silva, C. G. , Marques, J. M. , Pochmann, D. , Porciúncula, L. O. , Ferreira, S. , … Rodrigues, R. J. (2018). Glutamate‐induced and NMDA receptor‐mediated neurodegeneration entails P2Y1 receptor activation. Cell Death & Disease, 9(3), 297 10.1038/s41419-018-0351-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, J. , Filippov, A. K. , Göransson, S. , Wong, Y. H. , Frelin, C. , Michel, A. D. , … Barnard, E. A. (2002). Characterization and channel coupling of the P2Y12 nucleotide receptor of brain capillary endothelial cells. The Journal of Biological Chemistry, 277, 31390–31400. [DOI] [PubMed] [Google Scholar]
- Springthorpe, B. , Bailey, A. , Barton, P. , Birkinshaw, T. N. , Bonnert, R. V. , Brown, R. C. , … Willis, P. A. (2007). From ATP to AZD6140: The discovery of an orally active reversible P2Y12 receptor antagonist for the prevention of thrombosis. Bioorganic & Medicinal Chemistry Letters, 17, 6013–6018. [DOI] [PubMed] [Google Scholar]
- Stachon, P. , Peikert, A. , Michel, A. , Wolf, D. , Hoppe, N. , Dufner, B. , … Zirlik, A. (2014). Extracellular ATP induces atherosclerosis and vascular inflammation via purinergic receptor 2 (P2Y2) in mice. Cardiovascular Research, 103, S97 10.1093/cvr/cvu093.1 [DOI] [Google Scholar]
- Steculorum, S. M. , Paeger, L. , Bremser, S. , Evers, N. , Hinze, Y. , Idzko, M. , … Bruning, J. C. (2015). Hypothalamic UDP increases in obesity and promotes feeding via P2Y6‐dependent activation of AgRP neurons. Cell, 162, 1404–1417. [DOI] [PubMed] [Google Scholar]
- Stefani, J. , Tschesnokowa, O. , Parrilla, M. , Robaye, B. , Boeynaems, J. M. , Acker‐Palmer, A. , … Gampe, K. (2018). Disruption of the microglial ADP receptor P2Y13 enhances adult hippocampal neurogenesis. Frontiers in Cellular Neuroscience, 12, 134 10.3389/fncel.2018.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, X. , Floyd, D. H. , Hughes, A. , Xiang, J. , Schneider, J. G. , Uluckan, O. , … Weilbaecher, K. N. (2012). The ADP receptor P2RY12 regulates osteoclast function and pathologic bone remodeling. The Journal of Clinical Investigation, 122, 3579–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugidachi, A. , Asai, F. , Ogawa, T. , Inoue, T. , & Koike, H. (2000). The in vivo pharmacological profile of CS‐747, a novel antiplatelet agent with platelet ADP receptor antagonist properties. British Journal of Pharmacology, 129, 1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugidachi, A. , Mizuno, M. , Ohno, K. , Jakubowski, J. A. , & Tomizawa, A. (2016). The active metabolite of prasugrel, R‐138727, improves cerebral blood flow and reduces cerebral infarction and neurologic deficits in a non‐human primate model of acute ischaemic stroke. European Journal of Pharmacology, 788, 132–139. [DOI] [PubMed] [Google Scholar]
- Todd, J. N. , Poon, W. , Lyssenko, V. , Groop, L. , Nichols, B. , Wilmot, M. , … Friedman, D. J. (2015). Variation in glucose homeostasis traits associated with P2RX7 polymorphisms in mice and humans. The Journal of Clinical Endocrinology and Metabolism, 100, E688–E696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth, P. , Tarantini, S. , Davila, A. , Valcarcel‐Ares, M. N. , Tucsek, Z. , Varamini, B. , … Ungvari, Z. (2015). Purinergic glio‐endothelial coupling during neuronal activity: Role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. American Journal of Physiology. Heart and Circulatory Physiology, 309(11), H1837–H1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozaki‐Saitoh, H. , Tsuda, M. , Miyata, H. , Ueda, K. , Kohsaka, S. , & Inoue, K. (2008). P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. The Journal of Neuroscience, 28, 4949–4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi, M. , Larsen, A. T. , Lange, S. C. , Giannuzzo, A. , Andersen, M. N. , & Novak, I. (2018). The P2X7 receptor and pannexin‐1 are involved in glucose‐induced autocrine regulation in β‐cells. Scientific Reports, 8, 8926 10.1038/s41598-018-27281-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi, M. , & Novak, I. (2017). Purinergic receptors in adipose tissue as potential targets in metabolic disorders. Frontiers in Pharmacology, 8, 878. 10.3389/fphar.2017.00878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon, V. , Unwin, R. , Inscho, E. W. , Leipziger, J. , & Kishore, B. K. (2019). Extracellular Nucleotides and P2 Receptors in Renal Function. Physiological Reviews, 100(1), 211–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo, G. L. , & Harden, T. K. (2004). Agonist binding and Gq‐stimulating activities of the purified human P2Y1 receptor. Molecular Pharmacology, 65, 426–436. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Haanes, K. A. , & Novak, I. (2013). Purinergic regulation of CFTR and Ca2+‐activated Cl‐ channels and K+ channels in human pancreatic duct epithelium. American Journal of Physiology. Cell Physiology, 304, C673–C684. [DOI] [PubMed] [Google Scholar]
- Wang, N. , & Gartland, A. (2014). Targeting P2 receptors‐current progress in treating musculoskeletal diseases. Current Opinion in Pharmacology, 16, 122–126. [DOI] [PubMed] [Google Scholar]
- Wang, N. , Rumney, R. M. , Yang, L. , Robaye, B. , Boeynaems, J. M. , Skerry, T. M. , & Gartland, A. (2013). The P2Y13 receptor regulates extracellular ATP metabolism and the osteogenic response to mechanical loading. Journal of Bone and Mineral Research, 28, 1446–1456. [DOI] [PubMed] [Google Scholar]
- Wang, T. C. , Qiao, J. X. , Clark, C. G. , Jua, J. , Price, L. A. , Wu, Q. , … Lam, P. Y. (2013). Discovery of diarylurea P2Y1 antagonists with improved aqueous solubility. Bioorganic & Medicinal Chemistry Letters, 23(11), 3239–3243. [DOI] [PubMed] [Google Scholar]
- Weisman, G. A. , Camden, J. M. , Peterson, T. S. , Ajit, D. V. , Woods, L. T. , & Erb, L. (2012). P2 Receptors for extracellular nucleotides in the central nervous system: Role of P2X7 and P2Y2 receptor interactions in neuroinflammation. Molecular Neurobiology, 46(1), 96–113. 10.1007/s12035-012-8263-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselius, A. , Bours, M. J. , Agrawal, A. , Gartland, A. , Dagnelie, P. C. , Schwarz, P. , & Jorgensen, N. R. (2011). Role of purinergic receptor polymorphisms in human bone. Frontiers in Bioscience (Landmark Edition), 16, 2572–2585. [DOI] [PubMed] [Google Scholar]
- Wesselius, A. , Bours, M. J. , Henriksen, Z. , Syberg, S. , Petersen, S. , Schwarz, P. , … Dagnelie, P. C. (2013). Association of P2Y2 receptor SNPS with bone mineral density and osteoporosis risk in a cohort of Dutch fracture patients. Purinergic Signal, 9, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wihlborg, A. K. , Wang, L. , Braun, O. O. , Eyjolfsson, A. , Gustafsson, R. , Gudbjartsson, T. , & Erlinge, D. (2004). ADP receptor P2Y12 is expressed in vascular smooth muscle cells and stimulates contraction in human blood vessels. Arteriosclerosis, Thrombosis, and Vascular Biology, 24, 1810–1815. [DOI] [PubMed] [Google Scholar]
- Woods, L. T. , Camden, J. M. , Khalafalla, M. G. , Petris, M. J. , Erb, L. , Ambrus, J. L. Jr. , & Weisman, G. A. (2018). P2Y2 R deletion ameliorates sialadenitis in IL‐14α‐transgenic mice. Oral Diseases, 24, 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, Y. , Gu, Y. , Bresnahan, J. J. , Paul, E. M. , Donahue, H. J. , & You, J. (2014). The roles of P2Y2 purinergic receptors in osteoblasts and mechanotransduction. PLoS ONE, 9, e108417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, P. , Feng, X. , Luan, H. , Wang, J. , Ge, R. , Li, Z. , & Bian, J. (2018). Current knowledge on the nucleotide agonists for the P2Y2 receptor. Bioorganic & Medicinal Chemistry, 26, 366–375. [DOI] [PubMed] [Google Scholar]
- Yamane, M. , Ogawa, Y. , Fukui, M. , Kamoi, M. , Saijo‐Ban, Y. , Yaguchi, S. , … Tsubota, K. (2015). Long‐term rebamipide and diquafosol in two cases of immune‐mediated dry eye. Optometry and Vision Science, 92, S25–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanachkov, I. B. , Chang, H. , Yanachkova, M. I. , Dix, E. J. , Berny‐Lang, M. A. , Gremmel, T. , … Frelinger, A. L. (2016). New highly active antiplatelet agents with dual specificity for platelet P2Y1 and P2Y12 adenosine diphosphate receptors. European Journal of Medicinal Chemistry, 107, 204–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J. , Ciancetta, A. , Dudas, S. , Duca, S. , Lottermoser, J. , & Jacobson, K. A. (2018). Structure‐guided modification of heterocyclic antagonists of the P2Y14 receptor. Journal of Medicinal Chemistry, 61, 4860–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, S. , Chan, H. C. , Vogel, H. , Filipek, S. , Stevens, R. C. , & Palczewski, K. (2016). The molecular mechanism of P2Y1 receptor activation. Angewandte Chemie (International Ed. in English), 55, 10331–10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerr, M. , Hechler, B. , Freund, M. , Magnenat, S. , Lanois, I. , Cazenave, J. P. , … Gachet, C. (2011). Major contribution of the P2Y1 receptor in purinergic regulation of TNFα‐induced vascular inflammation. Circulation, 123, 2404–2413. [DOI] [PubMed] [Google Scholar]
- Zhang, D. , Gao, Z. G. , Zhang, K. , Kiselev, E. , Crane, S. , Wang, J. , … Wu, B. (2015). Two disparate ligand‐binding sites in the human P2Y1 receptor. Nature, 520, 317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Zhang, K. , Gao, Z. G. , Paoletta, S. , Zhang, D. , Han, G. W. , … Zhao, Q. (2014). Agonist‐bound structure of the human P2Y12 receptor. Nature, 509, 119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, K. , Zhang, J. , Gao, Z. G. , Zhang, D. , Zhu, L. , Han, G. W. , … Zhao, Q. (2014). Structure of the human P2Y12 receptor in complex with an antithrombotic drug. Nature, 509, 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Wang, H. , Guo, Q. , Li, X. , Gao, J. , Liu, Y. , … Yang, J. (2015). PI3K is involved in P2Y receptor‐regulated cAMP/Epac/Kv channel signaling pathway in pancreatic β cells. Biochemical and Biophysical Research Communications, 465, 714–718. [DOI] [PubMed] [Google Scholar]


