Abstract
A phenotype of indefinite growth arrest acquired in response to sublethal damage, cellular senescence affects normal aging and age-related disease. Mitogen-activated protein kinases (MAPKs) are capable of sensing changes in cellular conditions, and in turn elicit adaptive responses including cell senescence. MAPKs modulate the levels and function of many proteins, including proinflammatory factors and factors in the p21/p53 and p16/RB pathways, the main senescence-regulatory axes. Through these actions, MAPKs implement key traits of senescence—growth arrest, cell survival, and the senescence-associated secretory phenotype (SASP). In this review, we summarize and discuss our current knowledge of the impact of MAPKs in senescence. In addition, given that eliminating or suppressing senescent cells can improve health span, we discuss the function and possible exploitation of MAPKs in the elimination (senolysis) or suppression (senostasis) of senescent cells.
Keywords: ERK, JNK, p38, SASP, Gene expression programs, Senescence
Introduction
Cellular senescence is a program implemented by cells responding to a variety of stresses that cause macromolecular damage. In turn, cells that become senescent exhibit long-term growth arrest and the senescence-associated secretory phenotype (SASP), through which cells secrete proinflammatory and tissue-remodeling factors that have local and systemic impacts (Gorgoulis et al. 2019). Senescence has been found to be both beneficial and detrimental for organ homeostasis (He and Sharpless 2017). Among its benefits, senescence contributes to embryonic development, wound healing, and tumor suppression in young persons (Collado and Serrano 2010; Munoz-Espin et al. 2013; Storer et al. 2013; Demaria et al. 2014). On the other hand, the adverse effects of senescent cells accumulating in tissues are often apparent with advancing age, as they exacerbate age-related pathologies including cancer, sarcopenia, diabetes, and Alzheimer’s disease (Campisi 2013; Lopez-Otin et al. 2013; van Deursen 2014; McHugh and Gil 2018). Given the harmful influence of senescent cells during aging, there is much interest in clearing senescent cells therapeutically through genetic and pharmacologic approaches (Demaria et al. 2014; Baker et al. 2016; Chang et al. 2016). While the clinical usefulness of current genetic approaches to intervene in senescence (discussed by Soto-Gamez and Demaria 2017; McHugh and Gil 2018) are limited, there has been an escalation of efforts to identify chemical senolytic and senomorphic/senostatic interventions to combat age-associated diseases (Childs et al. 2014; Zhu et al. 2015; Yosef et al. 2016; Kirkland and Tchkonia 2017).
To develop rational approaches directed at senescent cells, it is essential to understand the molecular programs that enable the various senescence traits. The best-studied senescence-associated signaling pathways are triggered by damage to DNA and other cellular components that activate the p53 (TP53)-p21 (CDKN1A) axis, the p16 (CDKN2A)-retinoblastoma (RB) axis, and the secretion of SASP factors (Lujambio 2016; Soto-Gamez and Demaria 2017; Herranz and Gil 2018). While these pathways are robustly influenced by the PI3K-mTOR (phosphoinositide 3-kinase-mammalian target of rapamycin) signaling kinases, the regulation of senescent traits by MAPK (mitogen-activated protein kinase) cascades is becoming increasingly apparent (Xu et al. 2014; Martinez-Zamudio et al. 2017). The three main classes of MAPKs include ERK1/2 (extracellular signal-regulated kinase), JNK (c-Jun N-terminal kinase), and p38; among these, ERK1/2 and p38 are most closely linked to cellular senescence (Sun et al. 2007; Debacq-Chainiaux et al. 2010; Passos et al. 2010; Freund et al. 2011; Deschenes-Simard et al. 2013; Storer et al. 2013). Although JNK appears to have a less prominent role in senescence, recent reports have implicated this MAPK in senescent traits (Lee et al. 2010; Spallarossa et al. 2010; Yosef et al. 2017; Vizioli et al. 2020). Key aspects of the influence of MAPKs on growth suppression, resistance to apoptosis, and other traits of senescent cells have only emerged in recent years (Fig. 1) (Ziaei et al. 2012; Herranz et al. 2015; Culerrier et al. 2016; Slobodnyuk et al. 2019). Here, we review our knowledge and discuss the role of MAPKs on senescence traits.
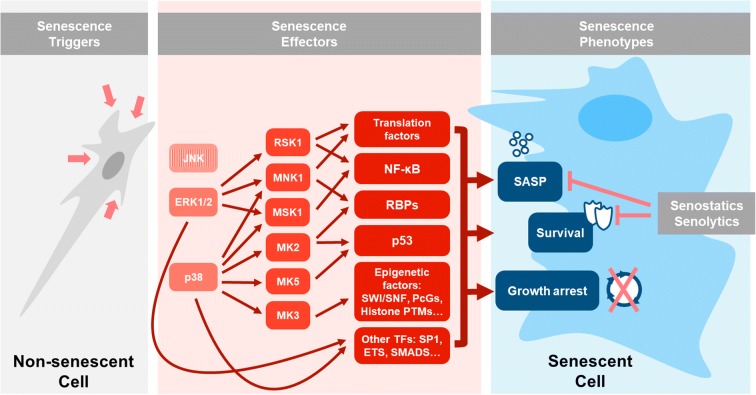
Fig. 1.
Known implications of MAPK pathways in cellular senescence traits. After exposure to senescence-inducing stimuli, MAPK pathways (mainly p38 and ERK1/2) drive the implementation of the senescence phenotype. MAPKs exert direct control over the main senescence traits—cell survival, cell cycle arrest, and the senescence-associated secretory phenotype (SASP). The main MAPK regulatory events thus far implicated in senescence are indicated.
MAPK networks in cellular senescence
Discovered in the early 1990s, MAPKs represent major signaling cascades in cell biology (Pearson et al. 2001). This superfamily of proteins is mainly comprised of kinases that mediate chains of phosphorylation events. Using simplified terminology, membrane receptors activated by mitogens, cytokines, and stress agents activate MAPKKKKs, which phosphorylate MAPKKKs, which in turn phosphorylate MAPKKs, and these then phosphorylate MAPKs. Downstream effectors of MAPKs include several proteins such as kinases and transcription factors, among others, that control cell proliferation, differentiation, survival, and motility (Cargnello and Roux 2011). In physiologic conditions, these phenotypes are under tight molecular control by MAPKs, but in pathologic conditions such as cancer, cardiovascular disease, and neurodegeneration, MAPK signaling is often aberrant.
Although MAPKs encompass a large number of kinases, the best known MAPKs are ERKs (1 and 2), p38s (α, β, γ, and δ), and JNKs (1, 2, and 3). MAPKs control cell response programs by phosphorylating and thereby regulating the activity of many proteins implicated in senescence. In particular, ERK1/2 regulates senescence-associated proteins including RSKs, Sprouty, and MYC (Campaner et al. 2010; Macia et al. 2014; Munoz-Espin and Serrano 2014; Sun et al. 2018) and p38 regulates ATF6, ZNHIT1, HBP1, p53, MK2, and MK5 (Zhang et al. 2006; Sun et al. 2007; Debacq-Chainiaux et al. 2010; Druelle et al. 2016; Macedo et al. 2018). Besides regulating transcription of senescence-associated genes, MAPKs and their effectors (e.g., MNK1, MK2, RSKs) can also control gene expression programs post-transcriptionally by phosphorylating and thereby modulating the activity of RNA-binding proteins (RBPs) implicated in senescence, such as HuR, AUF1, PTBP1, TTP, GRSF1, and hnRNPA1 (Wang et al. 2005; Wang et al. 2016; Ziaei et al. 2012; Alspach et al. 2014; Wiley and Campisi 2016; Georgilis et al. 2018; Noh et al. 2018; Noh et al. 2019). Phosphorylation by MAPKs often alters the ability of RBPs to bind target mRNAs, as shown for HuR, TTP, and AUF1, and modulates the fate of these mRNAs (Grammatikakis et al. 2017; Soni et al. 2019). In addition, the MAPK substrates MK2, MNK1, and RSK appear to be essential for the translation of SASP factors (Herranz et al. 2015; Roux and Topisirovic 2018; Sun et al. 2018) and link MAPKs with the mTOR pathway, which is activated in senescent cells (Tomimatsu and Narita 2015). In short, MAPK signaling governs transcriptional and translational programs in senescent cells.
MAPKs as sensing elements in senescence
As a cellular response to sublethal damaging or oncogenic stressors, senescence relies on MAPKs to implement robust and specific molecular programs. Traditionally, activation of ERK1/2 has been associated with mitogenic signaling, while p38 and JNK are generally implicated in stress signaling (Dhillon et al. 2007; Pimienta and Pascual 2007). In senescence, the ERK1/2 pathway is activated by aberrant RAS/RAF signaling, DNA damage, and oxidative stress (Pearson et al. 2001; Kim et al. 2003; Khalil et al. 2011). RAS activates the RAF-MEK axis to induce ERK1/2 phosphorylation (Mebratu and Tesfaigzi 2009), while sublethal genotoxic damage signal through ATM-AKT to activate ERK1/2 and implement an anti-apoptotic, pro-senescence program (Viniegra et al. 2005; Bozulic et al. 2008; Khalil et al. 2011). Of note, senescence-associated oxidative damage has been shown to activate ERK1/2 through the inhibition of MAPK phosphatases (Kim et al. 2003) as well as by the induction of signaling of receptor tyrosine kinases (RTKs) by reactive oxygen species (Lei and Kazlauskas 2009).
Oxidative stress is traditionally linked to p38 activation (Xu et al. 2014). In this regard, ERK1/2 signaling triggered by oncogenes (e.g., RAF(V600E) or HRAS(V12G)) induces senescence at least partly through a rise in oxidative stress leading to the activation of p38 (Wang et al. 2002). However, only a few mechanisms by which oxidative stress directly activates p38 to trigger cell senescence have been proposed (Passos et al. 2010; Kodama et al. 2013), and other pathways, such as DNA damage-induced senescence through increased GADD45A expression by p53 have implicated p38 as a contributor to the response (Moskalev et al. 2012).
Senescence cell cycle arrest by MAPKs
The indefinite growth arrest of senescent cells is established and maintained primarily by the cyclin-dependent kinase (CDK) inhibitors p21 (CDKN1A), p16 (CDKN2A), and p15 (CDKN2B) (Hernandez-Segura et al. 2018; Casella et al. 2019). The abundance of these CDK inhibitors is under the direct control of MAPKs, particularly ERK1/2 and p38. Paradoxically, while ERK1/2 signaling promotes cell proliferation in dividing cells, it promotes growth arrest in senescent cells; as reported, altered translocation to the nucleus as well as magnitude and duration of activation redirected ERK1/2 function towards cell cycle arrest instead of proliferation (Ebisuya et al. 2005; Zou et al. 2019).
In senescence, p21 is induced transcriptionally by p53 following DNA damage (d’Adda di Fagagna 2008). The transcriptional status of p53 is regulated post-translationally in several ways, including phosphorylation, altered protein levels, and subcellular localization (Baar et al. 2017; Hafner et al. 2019). p38 directly influences p53 activity in senescent cells by phosphorylating p53 at Ser15 and thus stabilizing it (Xu et al. 2014), while the p38-effector MK5 phosphorylates p53 and enhances transcription of p21 mRNA (Sun et al. 2007). ERK1/2 also modulates p53 post-translationally (Wu 2004), but these effects are not well studied in senescence. MAPKs enhance p21 mRNA transcription through transcription factors activated by stimuli other than DNA damage (Abbas and Dutta 2009); for instance, both p38 and ERK1/2 activate ELK1 and SP1, which drive transcription of p21 mRNA (Shin et al. 2011; Kim et al. 2014b). In addition, ERK1/2 activation promotes p21 transcription by SP1 and SMAD proteins (Pardali et al. 2000; Kim et al. 2006; Luo 2017). Thus, even in the absence of DNA damage, MAPKs strongly elevate p21 abundance. Accordingly, ERK1/2 activation contributes to developmental senescence, a senescence phenotype that relies mainly on DNA damage-independent induction of p21 (Munoz-Espin et al. 2013; Storer et al. 2013).
The senescence proteins p16 (CDKN2A) and p14 (ARF) are expressed from the CDKN2A locus (Munoz-Espin and Serrano 2014); p16 inhibits CDKs that phosphorylate RB, while p14 helps stabilize p53 (Kim and Sharpless 2006). Transcription of the CDKN2A locus is repressed epigenetically through Polycomb group (PcG) proteins (Bracken et al. 2007; Ito et al. 2018). In this paradigm, the MAPK effector MK3 phosphorylates and reduces the levels of PcG protein BMI1, thus promoting senescence (Voncken et al. 2005; Lee et al. 2016). Additionally, transcription from the CDKN2A locus is controlled by SWI/SNF protein complexes (Kia et al. 2008), which evict PcG proteins and enhance p16 transcription. In this context, MAPK p38 positively regulates the function of the SWI/SNF protein BAF60 (Simone et al. 2004). Furthermore, p38 facilitates the transcription of p16 mRNA by activating the histone acetyltransferase P300 (Li et al. 2010; Wang et al. 2012). Finally, transcription of p16 mRNA is further promoted by MAPKs that activate ETS, SP1, and MSK1 (Ohtani et al. 2001; Wu et al. 2007; Shin et al. 2011; Culerrier et al. 2016).
MAPKs also modulate the activity of RBPs that control the stability and/or translation of mRNAs encoding senescence-associated CDK inhibitors. In this context, MNK1 phosphorylates hnRNPA1 and dissociates it from p16 and p14 mRNAs, rendering them more stable and enabling increases in p16 and p14 protein levels (Zhu et al. 2002; Ziaei et al. 2012). In another example, phosphorylation of HuR by p38 increases HuR binding to p21 mRNA, increasing p21 mRNA stability and elevating p21 levels (Wang et al. 2000; Lafarga et al. 2009), even though HuR levels decline overall in senescent cells (Wang et al. 2001; Lee et al. 2018). TTP phosphorylation by the MAPK effector MK2 leads to dissociation of TTP from p21 mRNA and increases p21 mRNA stability and p21 production (Al-Haj et al. 2012). Finally, degradation of the RBP AUF1 by the proteasome in an MK2-regulated manner might contribute to the stabilization of target p21 and p16 mRNAs and the reduction in telomerase transcription seen in senescent cells (Wang et al. 2005; Chang et al. 2010; Pont et al. 2012; Li et al. 2013).
Regulation of SASP by MAPKs
The SASP is a complex trait believed to be responsible for many of the pathophysiologic effects of senescent cells (Gorgoulis et al. 2019). SASP factors include many proinflammatory cytokines, growth factors, angiogenic factors, and matrix metalloproteinases.
MAPKs are upstream regulators of NF-κB, a major transcriptional coordinator of the SASP. Upon senescence-inducing stimuli, p38 enhances the DNA damage-driven NF-κB transcriptional activity, which in turn promotes the transcription of SASP genes including IL6, IL8, and GM-CSF (Rodier et al. 2009; Freund et al. 2011; Alimbetov et al. 2016). Although not assessed in senescent cells, MSK1, an effector of p38 and ERK1/2, enhances NF-κB function and increases the transcription of SASP factors IL6 and CXCL8 (Vermeulen et al. 2003; Reber et al. 2009). In senescence induced by oncogenic RAS, elevated ERK1/2 signaling promoted NF-κB-mediated SASP protein production (Catanzaro et al. 2014). Activation of the MAPK substrate RSK1, an enhancer of protein synthesis, elevated IL8 production (Sun et al. 2018), while the MAPK substrate MNK1 phosphorylated eIF4E and thereby enhanced the translation of proteins including SASP factors and MK2 (Wendel et al. 2007; Wu et al. 2013; Herranz et al. 2015). Activated MK2, in turn, phosphorylated ZFP36L1 and thereby suppressed its ability to degrade target mRNAs encoding SASP components (Herranz et al. 2015). Finally, a recent report shows that JNK activation in senescent cells promotes cGas-STING signaling and enhances the SASP (Vizioli et al. 2020).
Among the many SASP factors regulated independently of NF-κB (Davalos et al. 2010), TGFβ, PDGFA, and CTGF were induced by NOTCH signaling in senescent IMR-90 fibroblasts, producing a distinct early wave of the SASP (Hoare et al. 2016). TGFβ promotes senescence by increasing the expression of p15 and p21 (Munoz-Espin et al. 2013), and activates p38 through TGFβ-activated kinase 1 (TAK1) (Yu et al. 2002; Passos et al. 2010). Conversely, p38 can induce TGFβ production by activating NOTCH signaling in RAS-overexpressing fibroblasts or by activating ATF2 (Kim et al. 1992; Weijzen et al. 2002). mTOR is required for the induction of TGFβ in senescent cells (Aarts et al. 2017), but whether MAPKs modulate the early SASP is still unknown.
MAPK-regulated RBPs also play important roles in SASP regulation. Beyond influencing growth arrest (see above), RBPs such as AUF1, HuR, hnRNPA1, GRSF1, and TTP are also linked to the expression of SASP factors (Ross et al. 2012; Hashimoto et al. 2014; Alspach and Stewart 2015; Wang et al. 2016; Noh et al. 2019). For example, MNK1 phosphorylated hnRNPA1 (Ziaei et al. 2012) and enabled NF-κB transcription of proinflammatory SASP mRNAs (Wang et al. 2016), while phosphorylation of ZFP36L1 by MK2 caused dissociation and stabilization of IL8 or IL1B mRNAs, encoding major SASP factors (Herranz et al. 2015). Activated MK2 might also phosphorylate AUF1 to induce its dissociation from target IL6 and IL8 mRNAs in senescent cells (Alspach and Stewart 2015). The reduction of HuR levels in senescence increased the levels of NF-κB-regulated SASP factors (Hashimoto et al. 2014), while other RBPs, such as PTBP1, induced the SASP trait globally (Georgilis et al. 2018). MAPK-regulated RBPs that specifically promote SASP factor production could be promising therapeutic targets.
MAPKs in senescent cell survival
Senescent cells are normally resistant to apoptotic cell death, even though they show activation of apoptotic pathways. To persist within tissues, senescent cells rely on pro-survival nodes, including proteins in the anti-apoptotic programs driven by BCL2, PI3K-AKT, and p53 (Childs et al. 2014; Baar et al. 2017; Kirkland and Tchkonia 2017; Yosef et al. 2017). In light of evidence that clearance of senescent cells from tissues improves age-related pathologies (including fibrotic diseases, sarcopenia, cardiovascular disease, cachexia, diabetes, and Alzheimer’s disease (van Deursen 2014; McHugh and Gil 2018)), there is intense interest in exploiting senolysis in aging, age-related diseases, and situations in which senescent cells accumulate (e.g., cancer therapy).
While elevated levels of cell damage promote apoptosis, sublethal doses lead cells to a senescent state (Childs et al. 2014). In this respect, MAPK signaling together with other signaling pathways such as the DNA damage-p53 axis contribute to implementing an appropriate cellular outcome (Gong et al. 2010). For example, low doses of DNA damage cause ERK1/2 activation, ensuring cell survival, but higher doses fail to activate ERK1/2 and instead result in cell death by apoptosis (Dai et al. 2008; Khalil et al. 2011), and in some cases, ERK1/2 activation may itself promote cell death (Tang et al. 2002). Activation of p38 generally increases with damage severity (Gong et al. 2010; Lumley et al. 2017), but p38 may also be pro-apoptotic or pro-survival depending on the trigger and cell type (Igea and Nebreda 2015); in some cases, the pro-survival effect of p38 in senescent cells may be linked to the induction of autophagy (Slobodnyuk et al. 2019). Additionally, p21 induction opposes apoptosis in senescent cells, and its expression is enhanced by both ERK1/2 and p38 MAPKs (see above) (Yosef et al. 2017). A deeper understanding of how MAPK signaling in senescent cells influences apoptosis is needed.
One of the most frequent approaches to eliminate senescent cells from aged tissues and organs is by treating with a combination of Dasatinib plus Quercetin (D + Q). The senolytic effect of this combination of drugs was first discovered in a screen of proliferative and senescent cells (Zhu et al. 2015). Quercetin, a flavonoid, is a pleiotropic inhibitor of many kinases (Russo et al. 2012; Russo et al. 2017; Bruning 2013). It can promote apoptosis in response to different stresses that activate ERK1/2 or p38 and induce senescence (Kim et al. 2014a; Gong et al. 2018). Dasatinib inhibits many tyrosine kinases (Montero et al. 2011) including SRC, BCR-ABL, C-KIT, PDGFR, and Ephrin receptors (Daulhac et al. 1999; Matsumoto et al. 1999; Kim et al. 2008; Galan-Moya et al. 2008; Abbaspour Babaei et al. 2016; Kania and Klein 2016), and thereby blocks signaling through MAPKs. Interestingly, some ligands linked to the function of these tyrosine kinases are SASP factors, and thus Dasatinib might be suppressing the anti-apoptotic protection engendered by SASP factors. Moreover, the receptor-associated protein Caveolin-1 is highly expressed on the senescent cell plasma membrane (Volonte and Galbiati 2009; Kim et al. 2017) and can help activate receptors by secreted factors such as TGFβ, Ephrins, EGF, and FGFs (Pol et al. 2000; Razani et al. 2001; Vihanto et al. 2006; Katz et al. 2007; Shao et al. 2008; Feng et al. 2012).
Maintenance of senescence by MAPKs
Identifying the mechanisms by which the senescent phenotype is maintained long-term is a major question in aging and cancer. MAPKs are proposed to contribute to this persistent phenotype, as activation of both p38 and ERK1/2 increases over time in senescence (Kim et al. 2003; Freund et al. 2011). Indeed, oxidative stress caused by the persistent activation of ERK1/2 can preclude the function of phosphatases, thus creating a positive feedback loop (Kim et al. 2003; Colavitti and Finkel 2005). Additionally, in senescent cells ERK1/2 and p38 promote the expression of Caveolin-1, which interacts with and inactivates phosphatases PP2A or PP2C, thus retaining active MAPKs and ATM and reinforcing the constitutive signaling through MAPKs and p53 (Meskiene et al. 2003; Dasari et al. 2006; Volonte and Galbiati 2009). The coordinated actions of p53 and p38 may contribute to the enduring growth arrest of senescent cells, since activation of p53 in response to MDM2 antagonists causes irreversible growth inhibition only under atmospheric oxygen (21% O2), which can occur in certain instances of senescence such as during wound repair and in lung disease (Parrinello et al. 2003; Wiley et al. 2018). Given the role of p38 in sensing oxidant stress, persistent p38 signaling could promote a low but prolonged induction of p53 levels in senescence (Sun et al. 2007; Purvis et al. 2012).
MAPKs may also contribute to the maintenance of senescence by alternative ways. For example, senescent cells implement global chromatin rearrangements called senescence-associated heterochromatic foci (SAHF) (Narita et al. 2003). These foci form in cells that are engaged in growth arrest by pRB (Zhang et al. 2005) and require the p38 effector protein HBP1 (Zhang et al. 2013). The p38 MAPK pathway also fuels a DNA damage-dependent activation of NF-κB-STAT3, triggering a positive feedback loop in senescent cells (Kuilman et al. 2010; Freund et al. 2011. Finally, p38 and ERK1/2 may reinforce mTOR signaling for a complete, long-term implementation of the senescence phenotype (Leontieva and Blagosklonny 2010; Gutierrez-Uzquiza et al. 2012; Laberge et al. 2015).
Together, these findings suggest that MAPKs contribute to the maintenance of senescence and the long-term growth arrest phenotype of senescent cells.
MAPKs and senescence in age-associated brain diseases
Recent studies have revealed that cellular senescence plays a key role in many age-associated neurodegenerative pathologies, such as Alzheimer’s disease (AD) (Bussian et al. 2018; Zhang et al. 2019) and Parkinson’s disease (PD) (Chinta et al. 2018). Although MAPKs have not been tightly linked to senescence in neurodegeneration, evidence for their implications is beginning to emerge.
Phosphorylation of p38 is increased in brains from aged mice (Li et al. 2011), and p38 activity is increased in AD when compared with age-matched brains (Hensley et al. 1999). The fact that p38 phosphorylation increases in regions where neurofibrillary tangles are found (Zhu et al. 2000) helps to support a role for p38 in Tau phosphorylation (Hanger et al. 2009; Maphis et al. 2016). Interestingly, senescent astrocytes, which are central players in AD pathogenesis (Salminen et al. 2011; Bhat et al. 2012), are highly dependent on p38 signaling for their phenotype (Mombach et al. 2015). ERK1/2 activity has also been implicated in Tau phosphorylation and in enhancing neurodegeneration in AD (Ferrer et al. 2001; Kirouac et al. 2017; Sun and Nan 2017). Conversely, it was recently found that amyloid oligomers induce ERK1/2 pathway in the hippocampus in the early stages of AD (Faucher et al. 2015).
Cellular senescence has also been found to be detrimental in the pathogenesis of PD, as removal of senescent cells was protective in mice that developed PD after paraquat treatment (Chinta et al. 2018). PD pathogenesis could be exacerbated by SASP factors secreted by senescent astrocytes and microglia in PD brain (Calabrese et al. 2018). Although the role of MAPKs in PD-associated senescence has not been studied directly, MAPKs are central mediators of the cellular response to stress, inflammation, and survival signals, all of which occur in the PD environment. Moreover, the activation of p38 by stress factors in microglia leads to the proinflammatory environment that exacerbates neurodegeneration in PD (He et al. 2018). ERK1/2 activation is required for harmful astrocytic inflammation within the nervous system (Zhuang et al. 2005) and suppression of ERK1/2 improves some side-effects of PD treatment, such as L-DOPA-induced dyskinesia (Santini et al. 2007). In sum, more comprehensive knowledge of MAPKs implicated in neurodegeneration-associated senescence is needed.
Concluding remarks and perspectives
MAPK pathways are integral to senescent traits. In response to stimuli capable of triggering senescence, MAPKs function as sensors to identify the type and extent of damage, and help to decide whether the ensuing response ought to be cell proliferation, differentiation, apoptosis, senescence, or other. If cells adopt a senescent response, MAPKs participate directly in the various traits of senescence. First, MAPKs contribute to implementing the gene expression programs that enable indefinite growth arrest, including increasing production of p21 and p16. Second, as integral mediators of the SASP trait, MAPKs control the production and secretion of SASP factors transcriptionally via NF-κB-dependent and -independent pathways, as well as post-transcriptionally by controlling the activity of RBPs that govern the production of SASP and other senescence-associated factors. Third, MAPKs are central to the anti-apoptotic phenotype that ensures the long-term survival of senescent cells. Therefore, understanding the role of MAPKs in balancing senescence-associated proliferation, gene expression, and survival is essential for the design of effective senotherapies.
As MAPKs control a large number of downstream effectors, in-depth analysis is also needed for identifying the specific contribution of MAPKs to traits like growth arrest, SASP, and resistance to apoptosis. Along with these needs, there are still many aspects of senescence that remain to be fully understood. For example, superior senescence markers must be identified; at present, classical markers such as senescence-associated β-Gal activity, p21 or p16 abundance, and SASP factor levels are not universal markers of senescence, and they lack sufficient specificity and sensitivity. Given that senescence can be detrimental or beneficial depending on the specific senescence paradigm, identifying the contribution of MAPKs to each senescence phenotype could uncover new tools (e.g., MAPK inhibitors) for therapeutic benefit. Improved animal models of senescence must also be developed and studied, and full catalogs of proteins and RNAs driving human senescence programs in cultured cells and in tissues/organs in vivo must be identified systematically. Finally, robust panels of senolytic and senostatic interventions to eliminate or reprogram senescent cells, respectively, must be uncovered. As we gain deeper knowledge of the pleiotropic signaling pathways and gene expression programs driving senescence, including those orchestrated by MAPKs, we will be able to design more rational approaches to modify the senescent cell compartment for therapeutic benefit.
Acknowledgments
We thank J.L. Martindale and R. Munk for their advice.
Funding information
This work was supported in its entirety by the NIA IRP, NIH.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aarts M, Georgilis A, Beniazza M, Beolchi P, Banito A, Carroll T, Kulisic M, Kaemena DF, Dharmalingam G, Martin N, Reik W, Zuber J, Kaji K, Chandra T, Gil J. Coupling shRNA screens with single-cell RNA-seq identifies a dual role for mTOR in reprogramming-induced senescence. Genes Dev. 2017;31:2085–2098. doi: 10.1101/gad.297796.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbaspour Babaei M, Kamalidehghan B, Saleem M, Huri HZ, Ahmadipour F. Receptor tyrosine kinase (c-Kit) inhibitors: a potential therapeutic target in cancer cells. Drug Des Devel Ther. 2016;10:2443–2459. doi: 10.2147/DDDT.S89114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Haj L, Blackshear PJ, Khabar KS. Regulation of p21/CIP1/WAF-1 mediated cell-cycle arrest by RNase L and tristetraprolin, and involvement of AU-rich elements. Nucleic Acids Res. 2012;40:7739–7752. doi: 10.1093/nar/gks545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimbetov D, Davis T, Brook AJ, Cox LS, Faragher RG, Nurgozhin T, Zhumadilov Z, Kipling D. Suppression of the senescence-associated secretory phenotype (SASP) in human fibroblasts using small molecule inhibitors of p38 MAP kinase and MK2. Biogerontology. 2016;17:305–315. doi: 10.1007/s10522-015-9610-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspach E, and Stewart SA. RNA-binding Protein Immunoprecipitation (RIP) to Examine AUF1 Binding to Senescence-Associated Secretory Phenotype (SASP) Factor mRNA. Bio Protoc. 2015; 5. [DOI] [PMC free article] [PubMed]
- Alspach E, Flanagan KC, Luo X, Ruhland MK, Huang H, Pazolli E, Donlin MJ, Marsh T, Piwnica-Worms D, Monahan J, Novack DV, McAllister SS, Stewart SA. p38MAPK plays a crucial role in stromal-mediated tumorigenesis. Cancer Discov. 2014;4:716–729. doi: 10.1158/2159-8290.CD-13-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM, Stryeck S, Rijksen Y, van Willigenburg H, Feijtel DA, van der Pluijm I, Essers J, van Cappellen WA, van IJcken WF, Houtsmuller AB, Pothof J, de Bruin RWF, Madl T, Hoeijmakers JHJ, Campisi J, de Keizer PLJ. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169:132–47 e16. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU, Johnson FB, Trojanowski JQ, Sell C, Torres C. Astrocyte senescence as a component of Alzheimer’s disease. PLoS One. 2012;7:e45069. doi: 10.1371/journal.pone.0045069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBalpha/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol Cell. 2008;30:203–213. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- Bracken AP, Kleine-Kohlbrecher D, Dietrich N, Pasini D, Gargiulo G, Beekman C, Theilgaard-Monch K, Minucci S, Porse BT, Marine JC, Hansen KH, Helin K. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21:525–530. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning A. Inhibition of mTOR signaling by quercetin in cancer treatment and prevention. Anti Cancer Agents Med Chem. 2013;13:1025–1031. doi: 10.2174/18715206113139990114. [DOI] [PubMed] [Google Scholar]
- Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018;562:578–582. doi: 10.1038/s41586-018-0543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Santoro A, Monti D, Crupi R, Di Paola R, Latteri S, Cuzzocrea S, Zappia M, Giordano J, Calabrese EJ, Franceschi C. Aging and Parkinson’s Disease: inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic Biol Med. 2018;115:80–91. doi: 10.1016/j.freeradbiomed.2017.10.379. [DOI] [PubMed] [Google Scholar]
- Campaner S, Doni M, Verrecchia A, Faga G, Bianchi L, Amati B. Myc, Cdk2 and cellular senescence: old players, new game. Cell Cycle. 2010;9:3655–3661. [PubMed] [Google Scholar]
- Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella G, Munk R, Kim KM, Piao Y, De S, Abdelmohsen K, Gorospe M. Transcriptome signature of cellular senescence. Nucleic Acids Res. 2019;47:7294–7305. doi: 10.1093/nar/gkz555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro JM, Sheshadri N, Pan JA, Sun Y, Shi C, Li J, Powers RS, Crawford HC, Zong WX. Oncogenic Ras induces inflammatory cytokine production by upregulating the squamous cell carcinoma antigens SerpinB3/B4. Nat Commun. 2014;5:3729. doi: 10.1038/ncomms4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang N, Yi J, Guo G, Liu X, Shang Y, Tong T, Cui Q, Zhan M, Gorospe M, Wang W. HuR uses AUF1 as a cofactor to promote p16INK4 mRNA decay. Mol Cell Biol. 2010;30:3875–3886. doi: 10.1128/MCB.00169-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep. 2014;15:1139–1153. doi: 10.15252/embr.201439245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta SJ, Woods G, Demaria M, Rane A, Zou Y, McQuade A, Rajagopalan S, Limbad C, Madden DT, Campisi J, Andersen JK. Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to Parkinson’s disease. Cell Rep. 2018;22:930–940. doi: 10.1016/j.celrep.2017.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colavitti R, Finkel T. Reactive oxygen species as mediators of cellular senescence. IUBMB Life. 2005;57:277–281. doi: 10.1080/15216540500091890. [DOI] [PubMed] [Google Scholar]
- Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culerrier R, Carraz M, Mann C, Djabali M. MSK1 triggers the expression of the INK4AB/ARF locus in oncogene-induced senescence. Mol Biol Cell. 2016;27:2726–2734. doi: 10.1091/mbc.E15-11-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Adda di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer. 2008;8:512–522. doi: 10.1038/nrc2440. [DOI] [PubMed] [Google Scholar]
- Dai Y, Chen S, Pei XY, Almenara JA, Kramer LB, Venditti CA, Dent P, Grant S. Interruption of the Ras/MEK/ERK signaling cascade enhances Chk1 inhibitor-induced DNA damage in vitro and in vivo in human multiple myeloma cells. Blood. 2008;112:2439–2449. doi: 10.1182/blood-2008-05-159392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari A, Bartholomew JN, Volonte D, Galbiati F. Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements. Cancer Res. 2006;66:10805–10814. doi: 10.1158/0008-5472.CAN-06-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daulhac L, Kowalski-Chauvel A, Pradayrol L, Vaysse N, Seva C. Src-family tyrosine kinases in activation of ERK-1 and p85/p110-phosphatidylinositol 3-kinase by G/CCKB receptors. J Biol Chem. 1999;274:20657–20663. doi: 10.1074/jbc.274.29.20657. [DOI] [PubMed] [Google Scholar]
- Davalos AR, Coppe JP, Campisi J, Desprez PY. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010;29:273–283. doi: 10.1007/s10555-010-9220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debacq-Chainiaux F, Boilan E, Dedessus Le Moutier J, Weemaels G, Toussaint O. p38(MAPK) in the senescence of human and murine fibroblasts. Adv Exp Med Biol. 2010;694:126–137. doi: 10.1007/978-1-4419-7002-2_10. [DOI] [PubMed] [Google Scholar]
- Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dolle ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes-Simard X, Gaumont-Leclerc MF, Bourdeau V, Lessard F, Moiseeva O, Forest V, Igelmann S, Mallette FA, Saba-El-Leil MK, Meloche S, Saad F, Mes-Masson AM, Ferbeyre G. Tumor suppressor activity of the ERK/MAPK pathway by promoting selective protein degradation. Genes Dev. 2013;27:900–915. doi: 10.1101/gad.203984.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- Druelle C, Drullion C, Desle J, Martin N, Saas L, Cormenier J, Malaquin N, Huot L, Slomianny C, Bouali F, Vercamer C, Hot D, Pourtier A, Chevet E, Abbadie C, Pluquet O. ATF6alpha regulates morphological changes associated with senescence in human fibroblasts. Oncotarget. 2016;7:67699–67715. doi: 10.18632/oncotarget.11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisuya M, Kondoh K, Nishida E. The duration, magnitude and compartmentalization of ERK MAP kinase activity: mechanisms for providing signaling specificity. J Cell Sci. 2005;118:2997–3002. doi: 10.1242/jcs.02505. [DOI] [PubMed] [Google Scholar]
- Faucher P, Mons N, Micheau J, Louis C, Beracochea DJ. Hippocampal injections of oligomeric amyloid beta-peptide (1-42) induce selective working memory deficits and long-lasting alterations of ERK signaling pathway. Front Aging Neurosci. 2015;7:245. doi: 10.3389/fnagi.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Liao WX, Luo Q, Zhang HH, Wang W, Zheng J, Chen DB. Caveolin-1 orchestrates fibroblast growth factor 2 signaling control of angiogenesis in placental artery endothelial cell caveolae. J Cell Physiol. 2012;227:2480–2491. doi: 10.1002/jcp.22984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Blanco R, Carmona M, Ribera R, Goutan E, Puig B, Rey MJ, Cardozo A, Vinals F, Ribalta T. Phosphorylated map kinase (ERK1, ERK2) expression is associated with early tau deposition in neurones and glial cells, but not with increased nuclear DNA vulnerability and cell death, in Alzheimer disease, Pick’s disease, progressive supranuclear palsy and corticobasal degeneration. Brain Pathol. 2001;11:144–158. doi: 10.1111/j.1750-3639.2001.tb00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan-Moya EM, Hernandez-Losa J, Aceves Luquero CI, de la Cruz-Morcillo MA, Ramirez-Castillejo C, Callejas-Valera JL, Arriaga A, Aranburo AF, y Cajal SR, Gutkind JS, Sanchez-Prieto R. c-Abl activates p38 MAPK independently of its tyrosine kinase activity: Implications in cisplatin-based therapy. Int J Cancer. 2008;122:289–297. doi: 10.1002/ijc.23063. [DOI] [PubMed] [Google Scholar]
- Georgilis A, Klotz S, Hanley CJ, Herranz N, Weirich B, Morancho B, Leote AC, D’Artista L, Gallage S, Seehawer M, Carroll T, Dharmalingam G, Wee KB, Mellone M, Pombo J, Heide D, Guccione E, Arribas J, Barbosa-Morais NL, Heikenwalder M, Thomas GJ, Zender L, Gil J. PTBP1-mediated alternative splicing regulates the inflammatory secretome and the pro-tumorigenic effects of senescent cells. Cancer Cell. 2018;34(85-102):e9. doi: 10.1016/j.ccell.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X, Liu A, Ming X, Deng P, Jiang Y. UV-induced interaction between p38 MAPK and p53 serves as a molecular switch in determining cell fate. FEBS Lett. 2010;584:4711–4716. doi: 10.1016/j.febslet.2010.10.057. [DOI] [PubMed] [Google Scholar]
- Gong C, Yang Z, Zhang L, Wang Y, Gong W, Liu Y. Quercetin suppresses DNA double-strand break repair and enhances the radiosensitivity of human ovarian cancer cells via p53-dependent endoplasmic reticulum stress pathway. Onco Targets Ther. 2018;11:17–27. doi: 10.2147/OTT.S147316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, Gil J, Hara E, Krizhanovsky V, Jurk D, Maier AB, Narita M, Niedernhofer L, Passos JF, Robbins PD, Schmitt CA, Sedivy J, Vougas K, von Zglinicki T, Zhou D, Serrano M, Demaria M. Cellular senescence: defining a path forward. Cell. 2019;179:813–827. doi: 10.1016/j.cell.2019.10.005. [DOI] [PubMed] [Google Scholar]
- Grammatikakis I, Abdelmohsen K, and Gorospe M. Posttranslational control of HuR function. Wiley Interdiscip Rev RNA. 2017 Jan; 8(1). [DOI] [PMC free article] [PubMed]
- Gutierrez-Uzquiza A, Arechederra M, Bragado P, Aguirre-Ghiso JA, Porras A. p38alpha mediates cell survival in response to oxidative stress via induction of antioxidant genes: effect on the p70S6K pathway. J Biol Chem. 2012;287:2632–2642. doi: 10.1074/jbc.M111.323709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner A, Bulyk ML, Jambhekar A, Lahav G. The multiple mechanisms that regulate p53 activity and cell fate. Nat Rev Mol Cell Biol. 2019;20:199–210. doi: 10.1038/s41580-019-0110-x. [DOI] [PubMed] [Google Scholar]
- Hanger DP, Anderton BH, Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med. 2009;15:112–119. doi: 10.1016/j.molmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Tsugawa T, Kawagishi H, Asai A, Sugimoto M. Loss of HuR leads to senescence-like cytokine induction in rodent fibroblasts by activating NF-kappaB. Biochim Biophys Acta. 2014;1840:3079–3087. doi: 10.1016/j.bbagen.2014.07.005. [DOI] [PubMed] [Google Scholar]
- He S, Sharpless NE. Senescence in health and disease. Cell. 2017;169:1000–1011. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Zhong W, Zhang M, Zhang R, Hu W. P38 mitogen-activated protein kinase and Parkinson’s disease. Transl Neurosci. 2018;9:147–153. doi: 10.1515/tnsci-2018-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley K, Floyd RA, Zheng NY, Nael R, Robinson KA, Nguyen X, Pye QN, Stewart CA, Geddes J, Markesbery WR, Patel E, Johnson GV, Bing G. p38 kinase is activated in the Alzheimer’s disease brain. J Neurochem. 1999;72:2053–2058. doi: 10.1046/j.1471-4159.1999.0722053.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28:436–453. doi: 10.1016/j.tcb.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Herranz N, Gil J. Mechanisms and functions of cellular senescence. J Clin Invest. 2018;128:1238–1246. doi: 10.1172/JCI95148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz N, Gallage S, Mellone M, Wuestefeld T, Klotz S, Hanley CJ, Raguz S, Acosta JC, Innes AJ, Banito A, Georgilis A, Montoya A, Wolter K, Dharmalingam G, Faull P, Carroll T, Martinez-Barbera JP, Cutillas P, Reisinger F, Heikenwalder M, Miller RA, Withers D, Zender L, Thomas GJ, Gil J. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol. 2015;17:1205–1217. doi: 10.1038/ncb3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare M, Ito Y, Kang TW, Weekes MP, Matheson NJ, Patten DA, Shetty S, Parry AJ, Menon S, Salama R, Antrobus R, Tomimatsu K, Howat W, Lehner PJ, Zender L, Narita M. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat Cell Biol. 2016;18:979–992. doi: 10.1038/ncb3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igea A, Nebreda AR. The stress kinase p38alpha as a target for cancer therapy. Cancer Res. 2015;75:3997–4002. doi: 10.1158/0008-5472.CAN-15-0173. [DOI] [PubMed] [Google Scholar]
- Ito T, Teo YV, Evans SA, Neretti N, Sedivy JM. Regulation of cellular senescence by Polycomb chromatin modifiers through distinct DNA damage- and histone methylation-dependent pathways. Cell Rep. 2018;22:3480–3492. doi: 10.1016/j.celrep.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania A, Klein R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat Rev Mol Cell Biol. 2016;17:240–256. doi: 10.1038/nrm.2015.16. [DOI] [PubMed] [Google Scholar]
- Katz M, Amit I, Yarden Y. Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochim Biophys Acta. 2007;1773:1161–1176. doi: 10.1016/j.bbamcr.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil A, Morgan RN, Adams BR, Golding SE, Dever SM, Rosenberg E, Povirk LF, Valerie K. ATM-dependent ERK signaling via AKT in response to DNA double-strand breaks. Cell Cycle. 2011;10:481–491. doi: 10.4161/cc.10.3.14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kia SK, Gorski MM, Giannakopoulos S, Verrijzer CP. SWI/SNF mediates Polycomb eviction and epigenetic reprogramming of the INK4b-ARF-INK4a locus. Mol Cell Biol. 2008;28:3457–3464. doi: 10.1128/MCB.02019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Wagner S, Liu F, O’Reilly MA, Robbins PD, Green MR. Retinoblastoma gene product activates expression of the human TGF-beta 2 gene through transcription factor ATF-2. Nature. 1992;358:331–334. doi: 10.1038/358331a0. [DOI] [PubMed] [Google Scholar]
- Kim HS, Song MC, Kwak IH, Park TJ, Lim IK. Constitutive induction of p-Erk1/2 accompanied by reduced activities of protein phosphatases 1 and 2A and MKP3 due to reactive oxygen species during cellular senescence. J Biol Chem. 2003;278:37497–37510. doi: 10.1074/jbc.M211739200. [DOI] [PubMed] [Google Scholar]
- Kim YK, Bae GU, Kang JK, Park JW, Lee EK, Lee HY, Choi WS, Lee HW, Han JW. Cooperation of H2O2-mediated ERK activation with Smad pathway in TGF-beta1 induction of p21WAF1/Cip1. Cell Signal. 2006;18:236–243. doi: 10.1016/j.cellsig.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Byun JY, Yun CH, Park IC, Lee KH, Lee SJ. c-Src-p38 mitogen-activated protein kinase signaling is required for Akt activation in response to ionizing radiation. Mol Cancer Res. 2008;6:1872–1880. doi: 10.1158/1541-7786.MCR-08-0084. [DOI] [PubMed] [Google Scholar]
- Kim GT, Lee SH, Kim JI, Kim YM. Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway and induces apoptosis by increasing the generation of intracellular ROS in a p53-independent manner. Int J Mol Med. 2014;33:863–869. doi: 10.3892/ijmm.2014.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Heo JI, Park SH, Shin JY, Kang HJ, Kim MJ, Kim SC, Kim J, Park JB, Lee JY. Transcriptional activation of p21(WAF(1)/CIP(1)) is mediated by increased DNA binding activity and increased interaction between p53 and Sp1 via phosphorylation during replicative senescence of human embryonic fibroblasts. Mol Biol Rep. 2014;41:2397–2408. doi: 10.1007/s11033-014-3094-9. [DOI] [PubMed] [Google Scholar]
- Kim KM, Noh JH, Bodogai M, Martindale JL, Yang X, Indig FE, Basu SK, Ohnuma K, Morimoto C, Johnson PF, Biragyn A, Abdelmohsen K, Gorospe M. Identification of senescent cell surface targetable protein DPP4. Genes Dev. 2017;31:1529–1534. doi: 10.1101/gad.302570.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T. Cellular senescence: a translational perspective. EBioMedicine. 2017;21:21–28. doi: 10.1016/j.ebiom.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirouac L, Rajic AJ, Cribbs DH, and Padmanabhan J. Activation of Ras-ERK signaling and GSK-3 by amyloid precursor protein and amyloid beta facilitates neurodegeneration in Alzheimer’s disease. eNeuro. 2017; 4. [DOI] [PMC free article] [PubMed]
- Kodama R, Kato M, Furuta S, Ueno S, Zhang Y, Matsuno K, Yabe-Nishimura C, Tanaka E, Kamata T. ROS-generating oxidases Nox1 and Nox4 contribute to oncogenic Ras-induced premature senescence. Genes Cells. 2013;18:32–41. doi: 10.1111/gtc.12015. [DOI] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge RM, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, Curran SC, Davalos AR, Wilson-Edell KA, Liu S, Limbad C, Demaria M, Li P, Hubbard GB, Ikeno Y, Javors M, Desprez PY, Benz CC, Kapahi P, Nelson PS, Campisi J. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol. 2015;17:1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafarga V, Cuadrado A, Lopez de Silanes I, Bengoechea R, Fernandez-Capetillo O, Nebreda AR. p38 Mitogen-activated protein kinase- and HuR-dependent stabilization of p21(Cip1) mRNA mediates the G(1)/S checkpoint. Mol Cell Biol. 2009;29:4341–4351. doi: 10.1128/MCB.00210-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Lee JH, Ko YG, Hong SI, Lee JS. Prevention of premature senescence requires JNK regulation of Bcl-2 and reactive oxygen species. Oncogene. 2010;29:561–575. doi: 10.1038/onc.2009.355. [DOI] [PubMed] [Google Scholar]
- Lee JY, Yu KR, Kim HS, Kang I, Kim JJ, Lee BC, Choi SW, Shin JH, Seo Y, Kang KS. BMI1 inhibits senescence and enhances the immunomodulatory properties of human mesenchymal stem cells via the direct suppression of MKP-1/DUSP1. Aging (Albany NY) 2016;8:1670–1689. doi: 10.18632/aging.101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Jung M, Hong J, Kim MK, Chung IK. Loss of RNA-binding protein HuR facilitates cellular senescence through posttranscriptional regulation of TIN2 mRNA. Nucleic Acids Res. 2018;46:4271–4285. doi: 10.1093/nar/gky223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Kazlauskas A. Growth factors outside of the platelet-derived growth factor (PDGF) family employ reactive oxygen species/Src family kinases to activate PDGF receptor alpha and thereby promote proliferation and survival of cells. J Biol Chem. 2009;284:6329–6336. doi: 10.1074/jbc.M808426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontieva OV, Blagosklonny MV. DNA damaging agents and p53 do not cause senescence in quiescent cells, while consecutive re-activation of mTOR is associated with conversion to senescence. Aging (Albany NY) 2010;2:924–935. doi: 10.18632/aging.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wang W, Liu X, Paulson KE, Yee AS, Zhang X. Transcriptional factor HBP1 targets P16(INK4A), upregulating its expression and consequently is involved in Ras-induced premature senescence. Oncogene. 2010;29:5083–5094. doi: 10.1038/onc.2010.252. [DOI] [PubMed] [Google Scholar]
- Li Z, Li J, Bu X, Liu X, Tankersley CG, Wang C, Huang K. Age-induced augmentation of p38 MAPK phosphorylation in mouse lung. Exp Gerontol. 2011;46:694–702. doi: 10.1016/j.exger.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Li ML, Defren J, Brewer G. Hsp27 and F-box protein beta-TrCP promote degradation of mRNA decay factor AUF1. Mol Cell Biol. 2013;33:2315–2326. doi: 10.1128/MCB.00931-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujambio A. To clear, or not to clear (senescent cells)? That is the question. Bioessays. 2016;38(Suppl 1):S56–S64. doi: 10.1002/bies.201670910. [DOI] [PubMed] [Google Scholar]
- Lumley EC, Osborn AR, Scott JE, Scholl AG, Mercado V, McMahan YT, Coffman ZG, Brewster JL. Moderate endoplasmic reticulum stress activates a PERK and p38-dependent apoptosis. Cell Stress Chaperones. 2017;22:43–54. doi: 10.1007/s12192-016-0740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K. Signaling cross talk between TGF-beta/Smad and other signaling pathways. Cold Spring Harb Perspect Biol. 2017; 9(1). [DOI] [PMC free article] [PubMed]
- Macedo JC, Vaz S, Bakker B, Ribeiro R, Bakker PL, Escandell JM, Ferreira MG, Medema R, Foijer F, Logarinho E. FoxM1 repression during human aging leads to mitotic decline and aneuploidy-driven full senescence. Nat Commun. 2018;9:2834. doi: 10.1038/s41467-018-05258-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia A, Vaquero M, Gou-Fabregas M, Castelblanco E, Valdivielso JM, Anerillas C, Mauricio D, Matias-Guiu X, Ribera J, Encinas M. Sprouty1 induces a senescence-associated secretory phenotype by regulating NFkappaB activity: implications for tumorigenesis. Cell Death Differ. 2014;21:333–343. doi: 10.1038/cdd.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maphis N, Jiang S, Xu G, Kokiko-Cochran ON, Roy SM, Van Eldik LJ, Watterson DM, Lamb BT, Bhaskar K. Selective suppression of the alpha isoform of p38 MAPK rescues late-stage tau pathology. Alzheimers Res Ther. 2016;8:54. doi: 10.1186/s13195-016-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Zamudio RI, Robinson L, Roux PF, Bischof O. SnapShot: cellular senescence pathways. Cell. 2017;170(816-16):e1. doi: 10.1016/j.cell.2017.07.049. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Yokote K, Tamura K, Takemoto M, Ueno H, Saito Y, Mori S. Platelet-derived growth factor activates p38 mitogen-activated protein kinase through a Ras-dependent pathway that is important for actin reorganization and cell migration. J Biol Chem. 1999;274:13954–13960. doi: 10.1074/jbc.274.20.13954. [DOI] [PubMed] [Google Scholar]
- McHugh D, Gil J. Senescence and aging: causes, consequences, and therapeutic avenues. J Cell Biol. 2018;217:65–77. doi: 10.1083/jcb.201708092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: is subcellular localization the answer? Cell Cycle. 2009;8:1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meskiene I, Baudouin E, Schweighofer A, Liwosz A, Jonak C, Rodriguez PL, Jelinek H, Hirt H. Stress-induced protein phosphatase 2C is a negative regulator of a mitogen-activated protein kinase. J Biol Chem. 2003;278:18945–18952. doi: 10.1074/jbc.M300878200. [DOI] [PubMed] [Google Scholar]
- Mombach JC, Vendrusculo B, Bugs CA. A model for p38MAPK-induced astrocyte senescence. PLoS One. 2015;10:e0125217. doi: 10.1371/journal.pone.0125217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero JC, Seoane S, Ocana A, Pandiella A. Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors. Clin Cancer Res. 2011;17:5546–5552. doi: 10.1158/1078-0432.CCR-10-2616. [DOI] [PubMed] [Google Scholar]
- Moskalev AA, Smit-McBride Z, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Tacutu R, Fraifeld VE. Gadd45 proteins: relevance to aging, longevity and age-related pathologies. Ageing Res Rev. 2012;11:51–66. doi: 10.1016/j.arr.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, Rodriguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Narita M, Nunez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- Noh JH, Kim KM, Idda ML, Martindale JL, Yang X, Abdelmohsen K, Gorospe M. GRSF1 suppresses cell senescence. Aging (Albany NY) 2018;10:1856–1866. doi: 10.18632/aging.101516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh JH, Kim KM, Pandey PR, Noren Hooten N, Munk R, Kundu G, De S, Martindale JL, Yang X, Evans MK, Abdelmohsen K, Gorospe M. Loss of RNA-binding protein GRSF1 activates mTOR to elicit a proinflammatory transcriptional program. Nucleic Acids Res. 2019;47:2472–2486. doi: 10.1093/nar/gkz082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani N, Zebedee Z, Huot TJ, Stinson JA, Sugimoto M, Ohashi Y, Sharrocks AD, Peters G, Hara E. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature. 2001;409:1067–1070. doi: 10.1038/35059131. [DOI] [PubMed] [Google Scholar]
- Pardali K, Kurisaki A, Moren A, ten Dijke P, Kardassis D, Moustakas A. Role of Smad proteins and transcription factor Sp1 in p21(Waf1/Cip1) regulation by transforming growth factor-beta. J Biol Chem. 2000;275:29244–29256. doi: 10.1074/jbc.M909467199. [DOI] [PubMed] [Google Scholar]
- Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, Miwa S, Olijslagers S, Hallinan J, Wipat A, Saretzki G, Rudolph KL, Kirkwood TB, von Zglinicki T. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol. 2010;6:347. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Pimienta G, Pascual J. Canonical and alternative MAPK signaling. Cell Cycle. 2007;6:2628–2632. doi: 10.4161/cc.6.21.4930. [DOI] [PubMed] [Google Scholar]
- Pol A, Lu A, Pons M, Peiro S, Enrich C. Epidermal growth factor-mediated caveolin recruitment to early endosomes and MAPK activation. Role of cholesterol and actin cytoskeleton. J Biol Chem. 2000;275:30566–30572. doi: 10.1074/jbc.M001131200. [DOI] [PubMed] [Google Scholar]
- Pont AR, Sadri N, Hsiao SJ, Smith S, Schneider RJ. mRNA decay factor AUF1 maintains normal aging, telomere maintenance, and suppression of senescence by activation of telomerase transcription. Mol Cell. 2012;47:5–15. doi: 10.1016/j.molcel.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis JE, Karhohs KW, Mock C, Batchelor E, Loewer A, Lahav G. p53 dynamics control cell fate. Science. 2012;336:1440–1444. doi: 10.1126/science.1218351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani B, Zhang XL, Bitzer M, von Gersdorff G, Bottinger EP, Lisanti MP. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem. 2001;276:6727–6738. doi: 10.1074/jbc.M008340200. [DOI] [PubMed] [Google Scholar]
- Reber L, Vermeulen L, Haegeman G, Frossard N. Ser276 phosphorylation of NF-kB p65 by MSK1 controls SCF expression in inflammation. PLoS One. 2009;4:e4393. doi: 10.1371/journal.pone.0004393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CR, Brennan-Laun SE, Wilson GM. Tristetraprolin: roles in cancer and senescence. Ageing Res Rev. 2012;11:473–484. doi: 10.1016/j.arr.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, and Topisirovic I. Signaling pathways involved in the regulation of mRNA translation. Mol Cell Biol. 2018; 38(12). [DOI] [PMC free article] [PubMed]
- Russo M, Spagnuolo C, Tedesco I, Bilotto S, Russo GL. The flavonoid quercetin in disease prevention and therapy: facts and fancies. Biochem Pharmacol. 2012;83:6–15. doi: 10.1016/j.bcp.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Russo M, Milito A, Spagnuolo C, Carbone V, Rosen A, Minasi P, Lauria F, Russo GL. CK2 and PI3K are direct molecular targets of quercetin in chronic lymphocytic leukaemia. Oncotarget. 2017;8:42571–42587. doi: 10.18632/oncotarget.17246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur J Neurosci. 2011;34:3–11. doi: 10.1111/j.1460-9568.2011.07738.x. [DOI] [PubMed] [Google Scholar]
- Santini E, Valjent E, Usiello A, Carta M, Borgkvist A, Girault JA, Herve D, Greengard P, Fisone G. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J Neurosci. 2007;27:6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H, Yi XM, Wells A. Epidermal growth factor protects fibroblasts from apoptosis via PI3 kinase and Rac signaling pathways. Wound Repair Regen. 2008;16:551–558. doi: 10.1111/j.1524-475X.2008.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SY, Kim CG, Lim Y, Lee YH. The ETS family transcription factor ELK-1 regulates induction of the cell cycle-regulatory gene p21(Waf1/Cip1) and the BAX gene in sodium arsenite-exposed human keratinocyte HaCaT cells. J Biol Chem. 2011;286:26860–26872. doi: 10.1074/jbc.M110.216721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone C, Forcales SV, Hill DA, Imbalzano AN, Latella L, Puri PL. p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat Genet. 2004;36:738–743. doi: 10.1038/ng1378. [DOI] [PubMed] [Google Scholar]
- Slobodnyuk K, Radic N, Ivanova S, Llado A, Trempolec N, Zorzano A, Nebreda AR. Autophagy-induced senescence is regulated by p38alpha signaling. Cell Death Dis. 2019;10:376. doi: 10.1038/s41419-019-1607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni S, Anand P, Padwad YS. MAPKAPK2: the master regulator of RNA-binding proteins modulates transcript stability and tumor progression. J Exp Clin Cancer Res. 2019;38:121. doi: 10.1186/s13046-019-1115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Gamez A, Demaria M. Therapeutic interventions for aging: the case of cellular senescence. Drug Discov Today. 2017;22:786–795. doi: 10.1016/j.drudis.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Spallarossa P, Altieri P, Barisione C, Passalacqua M, Aloi C, Fugazza G, Frassoni F, Podesta M, Canepa M, Ghigliotti G, Brunelli C. p38 MAPK and JNK antagonistically control senescence and cytoplasmic p16INK4A expression in doxorubicin-treated endothelial progenitor cells. PLoS One. 2010;5:e15583. doi: 10.1371/journal.pone.0015583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, Keyes WM. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155:1119–1130. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- Sun J, Nan G. The extracellular signal-regulated kinase 1/2 pathway in neurological diseases: a potential therapeutic target (Review) Int J Mol Med. 2017;39:1338–1346. doi: 10.3892/ijmm.2017.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Yoshizuka N, New L, Moser BA, Li Y, Liao R, Xie C, Chen J, Deng Q, Yamout M, Dong MQ, Frangou CG, Yates JR, 3rd, Wright PE, Han J. PRAK is essential for Ras-induced senescence and tumor suppression. Cell. 2007;128:295–308. doi: 10.1016/j.cell.2006.11.050. [DOI] [PubMed] [Google Scholar]
- Sun X, Shi B, Zheng H, Min L, Yang J, Li X, Liao X, Huang W, Zhang M, Xu S, Zhu Z, Cui H, Liu X. Senescence-associated secretory factors induced by cisplatin in melanoma cells promote non-senescent melanoma cell growth through activation of the ERK1/2-RSK1 pathway. Cell Death Dis. 2018;9:260. doi: 10.1038/s41419-018-0303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Wu D, Hirao A, Lahti JM, Liu L, Mazza B, Kidd VJ, Mak TW, Ingram AJ. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J Biol Chem. 2002;277:12710–12717. doi: 10.1074/jbc.M111598200. [DOI] [PubMed] [Google Scholar]
- Tomimatsu K, Narita M. Translating the effects of mTOR on secretory senescence. Nat Cell Biol. 2015;17:1230–1232. doi: 10.1038/ncb3244. [DOI] [PubMed] [Google Scholar]
- van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1) EMBO J. 2003;22:1313–1324. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihanto MM, Vindis C, Djonov V, Cerretti DP, Huynh-Do U. Caveolin-1 is required for signaling and membrane targeting of EphB1 receptor tyrosine kinase. J Cell Sci. 2006;119:2299–2309. doi: 10.1242/jcs.02946. [DOI] [PubMed] [Google Scholar]
- Viniegra JG, Martinez N, Modirassari P, Hernandez Losa J, Parada Cobo C, Sanchez-Arevalo Lobo VJ, Aceves Luquero CI, Alvarez-Vallina L, y Cajal SR, Rojas JM, Sanchez-Prieto R. Full activation of PKB/Akt in response to insulin or ionizing radiation is mediated through ATM. J Biol Chem. 2005;280:4029–4036. doi: 10.1074/jbc.M410344200. [DOI] [PubMed] [Google Scholar]
- Vizioli MG, Liu T, Miller KN, Robertson NA, Gilroy K, Lagnado AB, Perez-Garcia A, Kiourtis C, Dasgupta N, Lei X, Kruger PJ, Nixon C, Clark W, Jurk D, T. G. Bird, J. F. Passos, S. L. Berger, Z. Dou, and P. D. Adams. Mitochondria-to-nucleus retrograde signaling drives formation of cytoplasmic chromatin and inflammation in senescence. Genes Dev. 2020;34:428–45. [DOI] [PMC free article] [PubMed]
- Volonte D, Galbiati F. Caveolin-1, cellular senescence and pulmonary emphysema. Aging (Albany NY) 2009;1:831–835. doi: 10.18632/aging.100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voncken JW, Niessen H, Neufeld B, Rennefahrt U, Dahlmans V, Kubben N, Holzer B, Ludwig S, Rapp UR. MAPKAP kinase 3pK phosphorylates and regulates chromatin association of the Polycomb group protein Bmi1. J Biol Chem. 2005;280:5178–5187. doi: 10.1074/jbc.M407155200. [DOI] [PubMed] [Google Scholar]
- Wang W, Furneaux H, Cheng H, Caldwell MC, Hutter D, Liu Y, Holbrook N, Gorospe M. HuR regulates p21 mRNA stabilization by UV light. Mol Cell Biol. 2000;20:760–769. [Google Scholar]
- Wang W, Yang X, Cristofalo VJ, Holbrook NJ, Gorospe M. Loss of HuR is linked to reduced expression of proliferative genes during replicative senescence. Mol Cell Biol. 2001;21:5889–5898. doi: 10.1128/MCB.21.17.5889-5898.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Chen JX, Liao R, Deng Q, Zhou JJ, Huang S, Sun P. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic Ras-induced premature senescence. Mol Cell Biol. 2002;22:3389–3403. doi: 10.1128/MCB.22.10.3389-3403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Martindale JL, Yang X, Chrest FJ, Gorospe M. Increased stability of the p16 mRNA with replicative senescence. EMBO Rep. 2005;6:158–164. doi: 10.1038/sj.embor.7400346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Pan K, Chen Y, Huang C, Zhang X. The acetylation of transcription factor HBP1 by p300/CBP enhances p16INK4A expression. Nucleic Acids Res. 2012;40:981–995. doi: 10.1093/nar/gkr818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Han L, Zhao G, Shen H, Wang P, Sun Z, Xu C, Su Y, Li G, Tong T, Chen J. hnRNP A1 antagonizes cellular senescence and senescence-associated secretory phenotype via regulation of SIRT1 mRNA stability. Aging Cell. 2016;15:1063–1073. doi: 10.1111/acel.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijzen S, Rizzo P, Braid M, Vaishnav R, Jonkheer SM, Zlobin A, Osborne BA, Gottipati S, Aster JC, Hahn WC, Rudolf M, Siziopikou K, Kast WM, Miele L. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med. 2002;8:979–986. doi: 10.1038/nm754. [DOI] [PubMed] [Google Scholar]
- Wendel HG, Silva RL, Malina A, Mills JR, Zhu H, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Teruya-Feldstein J, Pelletier J, Lowe SW. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007;21:3232–3237. doi: 10.1101/gad.1604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CD, Campisi J. From ancient pathways to aging cells-connecting metabolism and cellular senescence. Cell Metab. 2016;23:1013–1021. doi: 10.1016/j.cmet.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CD, Schaum N, Alimirah F, Lopez-Dominguez JA, Orjalo AV, Scott G, Desprez PY, Benz C, Davalos AR, Campisi J. Small-molecule MDM2 antagonists attenuate the senescence-associated secretory phenotype. Sci Rep. 2018;8:2410. doi: 10.1038/s41598-018-20000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GS. The functional interactions between the p53 and MAPK signaling pathways. Cancer Biol Ther. 2004;3:156–161. doi: 10.4161/cbt.3.2.614. [DOI] [PubMed] [Google Scholar]
- Wu J, Xue L, Weng M, Sun Y, Zhang Z, Wang W, Tong T. Sp1 is essential for p16 expression in human diploid fibroblasts during senescence. PLoS One. 2007;2:e164. doi: 10.1371/journal.pone.0000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YY, Kumar R, Haque MS, Castillejo-Lopez C, Alarcon-Riquelme ME. BANK1 controls CpG-induced IL-6 secretion via a p38 and MNK1/2/eIF4E translation initiation pathway. J Immunol. 2013;191:6110–6116. doi: 10.4049/jimmunol.1301203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Li N, Xiang R, Sun P. Emerging roles of the p38 MAPK and PI3K/AKT/mTOR pathways in oncogene-induced senescence. Trends Biochem Sci. 2014;39:268–276. doi: 10.1016/j.tibs.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, Vadai E, Dassa L, Shahar E, Condiotti R, Ben-Porath I, Krizhanovsky V. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun. 2016;7:11190. doi: 10.1038/ncomms11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosef R, Pilpel N, Papismadov N, Gal H, Ovadya Y, Vadai E, Miller S, Porat Z, Ben-Dor S, Krizhanovsky V. p21 maintains senescent cell viability under persistent DNA damage response by restraining JNK and caspase signaling. EMBO J. 2017;36:2280–2295. doi: 10.15252/embj.201695553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Hebert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002;21:3749–3759. doi: 10.1093/emboj/cdf366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Poustovoitov MV, Ye X, Santos HA, Chen W, Daganzo SM, Erzberger JP, Serebriiskii IG, Canutescu AA, Dunbrack RL, Pehrson JR, Berger JM, Kaufman PD, Adams PD. Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev Cell. 2005;8:19–30. doi: 10.1016/j.devcel.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kim J, Ruthazer R, McDevitt MA, Wazer DE, Paulson KE, Yee AS. The HBP1 transcriptional repressor participates in RAS-induced premature senescence. Mol Cell Biol. 2006;26:8252–8266. doi: 10.1128/MCB.00604-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gao Y, Zhao L, Han L, Lu Y, Hou P, Shi X, Liu X, Tian B, Wang X, Huang B, Lu J. Mitogen-activated protein kinase p38 and retinoblastoma protein signalling is required for DNA damage-mediated formation of senescence-associated heterochromatic foci in tumour cells. FEBS J. 2013;280:4625–4639. doi: 10.1111/febs.12435. [DOI] [PubMed] [Google Scholar]
- Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, Abdelmohsen K, Bohr VA, Misra Sen J, Gorospe M, Mattson MP. Senolytic therapy alleviates Abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci. 2019;22:719–728. doi: 10.1038/s41593-019-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Rottkamp CA, Boux H, Takeda A, Perry G, Smith MA. Activation of p38 kinase links tau phosphorylation, oxidative stress, and cell cycle-related events in Alzheimer disease. J Neuropathol Exp Neurol. 2000;59:880–888. doi: 10.1093/jnen/59.10.880. [DOI] [PubMed] [Google Scholar]
- Zhu D, Xu G, Ghandhi S, Hubbard K. Modulation of the expression of p16INK4a and p14ARF by hnRNP A1 and A2 RNA binding proteins: implications for cellular senescence. J Cell Physiol. 2002;193:19–25. doi: 10.1002/jcp.10147. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Ziaei S, Shimada N, Kucharavy H, Hubbard K. MNK1 expression increases during cellular senescence and modulates the subcellular localization of hnRNP A1. Exp Cell Res. 2012;318:500–508. doi: 10.1016/j.yexcr.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Lei T, Guo P, Yu J, Xu Q, Luo Y, Ke R, Huang D. Mechanisms shaping the role of ERK1/2 in cellular senescence (Review) Mol Med Rep. 2019;19:759–770. doi: 10.3892/mmr.2018.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]