Abstract
Introduction
Anterior cruciate ligament (ACL) tears are common, with a seemingly constant increase in their number, and potentially serious consequences for sports participation and long-term general and musculoskeletal health.
Areas of agreement
Most players are able to return to cutting sport after ACL reconstruction, but some sustain further knee problems needing different approach to their rehabilitation.
Growing points
Neurocognitive tasks, measuring reaction time, processing speed, visual memory and verbal memory, allow indirect assessment of cerebral performance. Situational awareness, arousal, and attentional resources may influence neurocognitive function, affecting the complex integration of vestibular, visual, and somatosensory information needed for neuromuscular control.
Areas of controversy
The underlying reasons for uncoordinated, high-velocity movements observed during non-contact injuries of the knee producing an ACL tear are not well understood. Fundamental neuropsychological characteristics are responsible for situational awareness, sensory integration, motor planning, and coordination, all of which control joint stiffness. There is a strong link between acquisition of motor skills and neuronal plasticity at cortical and subcortical levels in the central nervous system; these links may evolve over time and engage different spatially distributed interconnected brain regions. A cascade of neurophysiological alterations occurs after ACL injury.
Areas timely for developing research
Training can improve function; hence, rehabilitation programmes which include perturbation training, agility training, vision training and sport-specific skill training are essential after ACL injuries and for injury prevention, and to optimize return to play.
Keywords: Anterior cruciate ligament, Injury, Sensory input, Return to play, Neuroplasticity, Recovery
Introduction
Anterior cruciate ligament (ACL) tears are common, with a seemingly constant increase in their number in Major League Soccer [1]. The injury carries potentially serious consequences for sports participation and long-term health [2]. The rate of ligament injuries in general, including medial collateral ligament (MCL) injuries of the knee and ankle sprains, has declined substantially in European professional football during the past decade [3], but the actual rate of development of ACL injuries is not known [4].
Several studies investigate return to play after ACL injury and reconstruction, some of them documenting successful early returns after ACL reconstruction, and others starting to report that non-surgical management is an option even in athletes. Most players do return to cutting sport after ACL reconstruction, but some sustain further knee problems and will need further surgery [5]. From a medical perspective, a subsequent knee injury or the need of further knee surgery occurring in the final phases of the rehabilitation period or early after return to play are treatment failures [6]. The extent of this problem is, however, still unclear. In addition, although most ACL-reconstructed male professional athletes can return to players within 1 year after surgery, their longer term participation rate is unknown. [7].
We report evidence-based concepts on the connection between neural mechanisms and ACL injury. Biomechanical and neuromuscular characteristics are currently the primary focus of research on non-contact knee injury mechanisms, as these risk factors are modifiable [8].
Clinical Implications for Rehabilitation and Prevention from Neuroplasticity Perspective
Neuroplasticity (or neural plasticity) refers to the ability of central nervous system to adapt in response to extrinsic (environmental) or intrinsic factors (e.g. an anatomically defined lesion). These adaptations may involve alterations to overall cognitive strategies, recruitment of different neural circuits, or amplification or reduction of involvement of certain connections or brain areas [9].
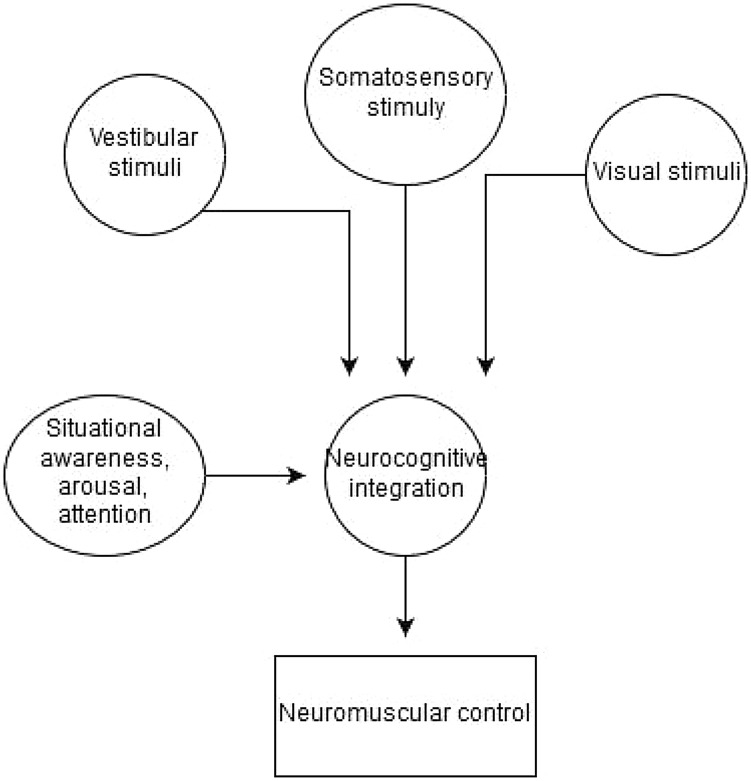
Neurocognitive tasks, such as those measuring reaction time, processing speed, visual memory, and verbal memory, are well established in the neuropsychology literature as indirect measures of cerebral performance [10]. Situational awareness, arousal, and attentional resources of the individual may influence these areas of neuro-cognitive function, affecting the complex integration of vestibular, visual, and somatosensory information needed for neuromuscular control (Fig. 1) [11].
Fig. 1.
Neuromuscular control integration
The viscoelastic properties of muscle are continuously adjusted depending on the anticipated functional demands (e.g., landing, cutting, decelerating) [12, 13]. The neural origin of this ‘fine muscle tuning’ exerts a net effect on muscle contractions that can increase joint stiffness tenfold, maximizing performance while preserving joint equilibrium and stability. To optimize stiffness for each task, the surrounding physical environment must be quickly modeled within the brain before athletic maneuvers are actually executed. This process is largely unconscious, and, in fact, conscious “overthinking” and inordinately high arousal levels may delay or interrupt routine functional maneuvers [3].
Sports activities require situational awareness of a broad attentional field to continuously monitor the surrounding environment, filter irrelevant information, and simultaneously execute complex motor programs [14]. Increased arousal or anxiety changes athletes’ concentration, narrows their attentional field, and alters muscle activity, resulting in poor coordination and inferior performance [15].
The neural computations that generate displayed strength or injury risk movement profile are typically left out of the return to play therapy, limiting our ability to improve the patient’s chance to successfully pass the RTS criteria [16]. Rehabilitators need to better challenge the brain during training to transfer gains from the clinic to sports activity [17].
Following an ACL tear, the central nervous system may increase its reliance on alternative sensory sources, such as visual-feedback and spatial awareness [2]. One previous investigation used neuroimaging to quantify brain activation differences between subjects with ACL deficiency who did not return to previous levels of physical activity and a healthy control group [11]. ACL-deficient subjects exhibited increased activation in the posterior inferior temporal gyrus (visual processing), pre-supplementary motor area (motor planning), and secondary somatosensory area (pain and sensory processing) [13].
The finding of depressed motor cortex excitability suggests that greater motor cortex activation is required to achieve motor drive and/or that motor cortex input from the rest of the brain in the form of structural or functional connectivity must increase to achieve motor drive [18].
Traditional rehabilitation encourages a focus of attention on the knee with increased visual and cognitive knee position control during movement training [17]. It is, therefore, likely that differences in brain activation in part arise from the rehabilitation process. The altered neuromuscular control following ACL injury may induce chronic long-term neuroplastic changes associated with rehabilitation and motor adaptations [19].
Alternatively, a direct approach to alter visual feedback (blindfold, stroboscopic glasses, and virtual reality) during rehabilitation may be beneficial to increase proprioceptive sensory inputs, as opposed to increasing subjects’ reliance on a visual-spatial neural strategy. [20].
Neuromuscular training that incorporates visual or neurocognitive processing, such as ball tracking or engaging other players, task complexity (reaction and decision-making), anticipatory aspects, and cognitive load (dual task) can address the possible sensory re-weighting of visual feedback for motor control [21]. Research on ACL injury pathomechanics has greatly advanced, but the underlying reasons for uncoordinated, high-velocity movements observed during non-contact sprains are not well understood [22]. Fundamental neuropsychological characteristics are responsible for situational awareness, sensory integration, motor planning, and coordination [17], all of which control joint stiffness. Therefore, they may also influence an individual’s injury-avoidance strategy, regardless of sex [23].
The ACL may tear in less than 70 ms [21], but the earliest reflexive activity for dynamic restraint requires at least 35 ms to begin developing muscle tension [17]. Additionally, cognitive appreciation of any coordination errors can take up to 500 ms [24]. Therefore, the high movement velocities and forces associated with athletics require advanced cognitive planning through feed-forward motor control; otherwise, over reliance on reflexive strategies for dynamic stability may be insufficient to protect the ACL. [25].
Increased physiological knee valgus, load reduced neurocognitive function, increased joint laxity, small femoral notch widths, and altered neuromuscular properties have been considered as potential risk factors specific to young females [11, 14, 15]. All these factors have warranted discussion as to potential interventions to target the relevant processes. A further ACL injury following successful reconstruction has been reported in up to 23% in athletes younger than 25 years when returning early to competitive sports involving jumping and cutting activities [6]. Based on the aforementioned continued neuromuscular control deficits, traditional rehabilitation is not capable to restore normal motor function in all patients after ACLR [12]. Components of current rehabilitation programs entail a combination of exercises to increase muscle strength and endurance and improve neuromuscular function. We acknowledge the importance of addressing these factors: there is a clear need for improvement in light of early development of osteoarthritis and second ACL injury risk [22].
Neuroplasticity Alterations After ACL Reconstruction
Altered kinesthesia is common following ACL injuries [11]. Corrigan et al. measured both the ability to reproduce passive positioning and detect passive motion of the knee joint in individuals with torn ACLs and age-matched controls [26]. When compared to controls, those with ACL-deficient knees exhibited significantly diminished ability to reproduce passive positioning and to detect passive motion.
Surgical ACL reconstruction may enhance proprioception and kinesthesia by preserving afferents and regenerating mechanoreceptors [27, 28].
Surgeries around the knee joint should preserve the integrity of the knee’s mechanoreceptors and the afferent nerves of its surrounding structures such as the capsule, collateral ligaments, fat pad, synovium, and perimeniscal tissue [18]. The primary goal during surgery should be to save as much sensory function as possible [29]. With the preservation or restoration of the sensory function of the disrupted ligament, symptoms such as functional instability and muscle weakness may be avoided.
Despite intensive research in this area, the source and the importance of the new population of mechanoreceptors within ACL surgical grafts are currently undetermined. Receptors supplying the ACL graft may be restored by either regrowth, regeneration, growth from the surrounding tissues, dedifferentiation of other cells, or some other mechanism [22]. Also, we do not know yet how these mechanoreceptors actually function. Thus, the enhanced proprioception and kinesthesia after ACL reconstruction may simply result from enhanced functioning of other sensory receptors secondary to the restoration of knee joint osteokinematics [16].
In football, external factors such as possession of the ball and position of team mates and opponents are involved, and are unpredictable [26]. The attentional and environmental components of neuromuscular function are largely not addressed in current ACL rehabilitation programs. More emphasis should be given to integrate sensory–visual–motor control factors during rehabilitation such as reaction time, information processing, and focus of attention, visual–motor control, and complex-task–environmental interaction [30]. This is particularly important in the late stages of the rehabilitation process.
Finally, it should be mentioned that a patient tailored rehabilitation programme is necessary for complete and speedy recovery and return to sport. However, the preliminary stage to well-planned rehabilitation is accurate surgical technique, starting with choice of the appropriate graft for a given patient according to the sport they play. Also, it is extremely important that the surgeon and the physiotherapy team communicate constantly, as the rehabilitation process may need to be adjusted according to the progress of the patient. A goal- and task-oriented approach, instead of a time limited and ‘cook book’ approach is necessary to obtain maximum benefits, and restore full function.
Future Directions
These preliminary ideas may guide researchers to pursue studies in several areas related to ACL injury prevention. More data are needed to establish the precise periods of time when individuals are vulnerable due to cognitive demands such as sensory integration, decision-making, and motor planning [3]. Sport-specific situations that may disrupt situational awareness in athletes can be explored, with particular focus given to visual attention in high-intensity, dynamic, complex environments. Unanticipated events can provoke a universal startle response within the central nervous system [13] resulting in a brief, involuntary, and widespread change in neuromuscular activity. In terms of reliance on visual information, athletes may suffer a brief episode of “inattentional blindness” and fail to recognize important visual cues simply, because they were not expecting them [20].
There is a strong link between acquisition of motor skills and neuronal plasticity at cortical and subcortical levels in the central nervous system that evolves over time and engages different spatially distributed interconnected brain regions [25]. Recent evidence indicates the large cascade of neurophysiological alterations that occur after ACL injury [31]. Although unilateral, an ACL injury induces bilateral lower extremity dysfunction, with sensory information deficits across the whole spectrum of the sensorimotor system, lending further support to the theory of a neurophysiological lesion [24].
Rehabilitation in patients after ACL injury should include sensory challenges to decrease the dependency of patients on visual information and facilitate neuroplasticity [19]. Patients may have ineffective motor-learning strategies and/or motor learning to (re-) acquire motor skills may not be sufficiently stimulated during traditional rehabilitation [18]. Such evidence could help to explain why patients do not always regain motor skills after ACL injury, as the neuroplastic capacities may not be optimally challenged in current rehabilitation programmes. Future research should aim to: (a) evaluate larger samples of prospective ACL patients; (b) include time between testing sessions, as well as other ACL injury risk factors not collected as part of the present study design including mental health challenges, current medications and menstrual cycle as covariates to account for potential confounding effects; (c) investigate changes in connectivity within the S1 and cerebellar lobule XIIB following ACL prevention programs; (d) consider integrating motor behavioral principles into ACL recovery and prevention to explore their relative influence on brain function; and (e) future investigations with larger sample sizes could investigate whether our non-significant connectivity comparisons could provide any further insight on the cerebral central nervous system contributions to ACL injury.
Given the reorganization of the central nervous system that takes place after an ACL injury [12], we need to determine which principles of motor learning could enhance the neuroplastic processes and translate to motor-learning interventions with the goal of optimal function of the patient.
Conclusion
Although its exact neurocircuits are not currently mapped out, the ACL contributes to functional stability of the knee joint by providing sensory feedback to the neuromuscular system [5]. Therefore, functional instability after ACL injuries is likely secondary to both the loss of an important mechanical restraint and a source of proprioception and kinesthesia [25]. Neuromuscular training can improve function; hence, rehabilitation programmes which include perturbation training, agility training, vision training and sport-specific skill training are essential after ACL injuries and for injury prevention.
Future research should quantify musculoskeletal injury-induced neuroplasticity, using more advanced motor-control tasks, such as force or position matching or multi joint movements, to improve the clinical applicability of these results.
Also, future research should focus on which, if any, combinations of the presented novel motor-learning principles yield better clinical outcomes. Motor learning should be applied to support neuroplasticity after ACL injury. Every individual and their brain are different: the optimal solution may require motor-learning principles individually tailored to each injured athletes.
Compliance with Ethical Standards
Conflict of interest
The authors declare that there are no personal or commercial relationships related to this study that would lead to a conflict of interest.
Ethical standard statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
Informed consent
For this type of study informed consent is not required.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regardtojurisdictional claims in published maps and institutional affiliations.
Contributor Information
George Kakavas, Email: info@fysiotek.gr.
Nikolaos Malliaropoulos, Email: contact@sportsmed.gr.
Ricard Pruna, Email: ricard.pruna@fcbarcelona.cat.
David Traster, Email: dr.traster@neurowellnessinstitute.com.
Georgios Bikos, Email: bikosg77@yahoo.gr.
Nicola Maffulli, Email: n.maffulli@qmul.ac.uk.
References
- 1.Mohtadi N, Chan D, Barber R, Paolucci EO. Reruptures, reinjuries, and revisions at a minimum 2-year follow-up: A randomized clinical trial comparing 3 graft types for ACL reconstruction. Clinical Journal of Sport Medicine. 2016;26:96–107. doi: 10.1097/JSM.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 2.Swanik CB, Covassin T, Stearne DJ, Schatz P. The relationship between neurocognitive function and noncontact anterior cruciate ligament injuries. American Journal of Sports Medicine. 2007;35:943–948. doi: 10.1177/0363546507299532. [DOI] [PubMed] [Google Scholar]
- 3.Schutte MJ, Dabezies EJ, Zimny ML, Happel LT. Neural anatomy of the human anterior cruciate ligament. Journal of Bone and Joint Surgery. American Volume. 1987;69:243–247. doi: 10.2106/00004623-198769020-00011. [DOI] [PubMed] [Google Scholar]
- 4.Lephart SM. Proprioceptive considerations for sport rehabilitation. Campaign: Human Kinetics; 1994. [Google Scholar]
- 5.Sherrington CS. On the proprioceptive system, especially in its reflex aspect. Brain. 1907;29:467–482. doi: 10.1093/brain/29.4.467. [DOI] [Google Scholar]
- 6.Barrett DS. Proprioception and function after anterior cruciate reconstruction. Journal of Bone and Joint Surgery. British Volume. 1991;73:833–837. doi: 10.1302/0301-620X.73B5.1894677. [DOI] [PubMed] [Google Scholar]
- 7.Piedade SR, Fabbro IMD, Mischan MM, Piedade C, Maffulli N. Static tensioning promotes hamstring tendons force relaxation more reliably than cycling tensioning. Knee. 2017;24:775–781. doi: 10.1016/j.knee.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Ferrer A, Twycross-Lewis R, Maffulli N. Anterior cruciate ligament deficiency: rotational instability in the transverse plane. A preliminary laboratory in vivo study. Muscle Ligaments and Tendons Journal. 2019;09:55–61. doi: 10.32098/mltj.01.2019.17. [DOI] [Google Scholar]
- 9.Sharma N, Classen J, Cohen LG. Neural plasticity and its contribution to functional recovery. Handbook Clinical Neurology. 2013;110:3–12. doi: 10.1016/B978-0-444-52901-5.00001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy JC, Alexander IJ, Hayes KC. Nerve supply of the human knee and its functional importance. American Journal of Sports Medicine. 1982;10:329–335. doi: 10.1177/036354658201000601. [DOI] [PubMed] [Google Scholar]
- 11.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. Journal of Bone and Joint Surgery. American Volume. 1980;62:259–270. doi: 10.2106/00004623-198062020-00013. [DOI] [PubMed] [Google Scholar]
- 12.Bach LJ, Happe F, Fleminger S, Powell J. Theory of mind: Independence of executive function and the role of the frontal cortex in acquired brain injury. Cognitive Neuropsychiatry. 2000;5:175–192. doi: 10.1080/13546800050083520. [DOI] [Google Scholar]
- 13.Nigg BM, Liu W. The effect of muscle stiffness and damping on simulated impact force peaks during running. Journal of Biomechanics. 1999;32:849–856. doi: 10.1016/S0021-9290(99)00048-2. [DOI] [PubMed] [Google Scholar]
- 14.Kuo CY, Louie JK, Mote CD., Jr Field measurements in snow skiing injury research. Journal of Biomechanics. 1983;16:609–624. doi: 10.1016/0021-9290(83)90111-2. [DOI] [PubMed] [Google Scholar]
- 15.Lephart SM, Pincivero DM, Giraldo JL, Fu FH. The role of proprioception in the management and rehabilitation of athletic injuries. American Journal of Sports Medicine. 1997;25:130–137. doi: 10.1177/036354659702500126. [DOI] [PubMed] [Google Scholar]
- 16.Vasta S, Papalia R, Albo E, Maffulli N, Denaro V. Top orthopedic sports medicine procedures. Journal of Orthopaedic Surgery and Research. 2018;13:190. doi: 10.1186/s13018-018-0889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konishi Y, Konishi H, Fukubayashi T. Gamma loop dysfunction in quadriceps on the contralateral side in patients with ruptured ACL. Medicine and Science in Sports and Exercise. 2003;35:897–900. doi: 10.1249/01.MSS.0000069754.07541.D2. [DOI] [PubMed] [Google Scholar]
- 18.Denti M, Monteleone M, Berardi A, Panni AS. Anterior cruciate ligament mechanoreceptors Histologic studies on lesions and reconstruction. Clinical Orthopaedics and Related Research. 1994;308:29–32. [PubMed] [Google Scholar]
- 19.Grooms D, Appelbaum G, Onate J. Neuroplasticity following anterior cruciate ligament injury: a framework for visual-motor training approaches in rehabilitation. Journal of Orthopaedic and Sports Physical Therapy. 2015;45:381–393. doi: 10.2519/jospt.2015.5549. [DOI] [PubMed] [Google Scholar]
- 20.Kapreli E, Athanasopoulos S. The anterior cruciate ligament deficiency as a model of brain plasticity. Medical Hypotheses. 2006;67:645–650. doi: 10.1016/j.mehy.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 21.Yasuda K, Kondo E, Ichiyama H, Tanabe Y, Tohyama H. Clinical evaluation of anatomic double-bundle anterior cruciate ligament reconstruction procedure using hamstring tendon grafts: comparisons among 3 different procedures. Arthroscopy. 2006;22:240–251. doi: 10.1016/j.arthro.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Gokeler A, Neuhaus D, Benjaminse A, Grooms DR, Baumeister J. Principles of motor learning to support neuroplasticity after ACL injury: Implications for optimizing performance and reducing risk of second ACL injury. Sports Medicine (Auckland, N. Z.) 2019;49:853–865. doi: 10.1007/s40279-019-01058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barber-Westin SD, Noyes FR. Factors used to determine return to unrestricted sports activities after anterior cruciate ligament reconstruction. Arthroscopy. 2011;27:1697–1705. doi: 10.1016/j.arthro.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Safran MR, Seaber AV, Garrett WE., Jr Warm-up and muscular injury prevention. An update. Sports Medicine. 1989;8:239–249. doi: 10.2165/00007256-198908040-00004. [DOI] [PubMed] [Google Scholar]
- 25.Noyes FR, Butler DL, Grood ES, Zernicke RF, Hefzy MS. Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. Journal of Bone and Joint Surgery. American Volume. 1984;66:344–352. doi: 10.2106/00004623-198466030-00005. [DOI] [PubMed] [Google Scholar]
- 26.Corrigan JP, Cashman WF, Brady MP. Proprioception in the cruciate deficient knee. Journal of Bone and Joint Surgery. British Volume. 1992;74:247–250. doi: 10.1302/0301-620X.74B2.1544962. [DOI] [PubMed] [Google Scholar]
- 27.Ellison AE, Berg EE. Embryology, anatomy, and function of the anterior cruciate ligament. Orthopedic Clinics of North America. 1985;16:3–14. [PubMed] [Google Scholar]
- 28.Papalia R, Franceschi F, Tecame A, D’Adamio S, Maffulli N, Denaro V. Anterior cruciate ligament reconstruction and return to sport activity: postural control as the key to success. International Orthopaedics. 2015;39:527–534. doi: 10.1007/s00264-014-2513-9. [DOI] [PubMed] [Google Scholar]
- 29.Hutchison M, Comper P, Mainwaring L, Richards D. The influence of musculoskeletal injury on cognition: implications for concussion research. American Journal of Sports Medicine. 2011;39:2331–2337. doi: 10.1177/0363546511413375. [DOI] [PubMed] [Google Scholar]
- 30.Papalia R, Torre G, Papalia G, Campi S, Maffulli N, Denaro V. Arthroscopic primary repair of the anterior cruciate ligament in adults: a systematic review. British Medical Bulletin. 2019;131:29–42. doi: 10.1093/bmb/ldz019. [DOI] [PubMed] [Google Scholar]
- 31.Papalia R, Maffulli N, Denaro V. The anterior cruciate ligament remnant: to leave it or not? Arthroscopy. 2013;29:1736–1737. doi: 10.1016/j.arthro.2013.08.019. [DOI] [PubMed] [Google Scholar]