Abstract
Moving from the hypothesis that aging processes modulate brain connectivity networks, 170 healthy elderly volunteers were submitted to EEG recordings in order to define age-related normative limits. Graph theory functions were applied to exact low-resolution electromagnetic tomography on cortical sources in order to evaluate the small-world parameter as a representative model of network architecture. The analyses were carried out in the whole brain—as well as for the left and the right hemispheres separately—and in three specific resting state subnetworks defined as follows: attentional network (AN), frontal network (FN), and default mode network (DMN) in the EEG frequency bands (delta, theta, alpha 1, alpha 2, beta 1, beta 2, gamma). To evaluate the stability of the investigated parameters, a subgroup of 32 subjects underwent three separate EEG recording sessions in identical environmental conditions after a few days interval. Results showed that the whole right/left hemispheric evaluation did not present side differences, but when individual subnetworks were considered, AN and DMN presented in general higher SW in low (delta and/or theta) and high (gamma) frequency bands in the left hemisphere, while for FN, the alpha 1 band was lower in the left with respect to the right hemisphere. It was also evident the test-retest reliability and reproducibility of the present methodology when carried out in clinically stable subjects.
Evidences from the present study suggest that graph theory represents a reliable method to address brain connectivity patterns from EEG data and is particularly suitable to study the physiological impact of aging on brain functional connectivity networks.
Keywords: Graph theory; Small world; Functional connectivity; EEG; eLORETA, biotechnical innovation
Introduction
Understanding the relation between structure and function of the brain is one of the basic goals of neuroscience. Considering the brain as a complex matrix of dynamically interacting neuronal assemblies which could be modeled by stable (maintained in time) or unstable (changing in time) networks on the basis of daily experience and individual background, the study of network science offers new insights into higher level brain processes such as memory, planning, problem solving, decision-making, sensorimotor skills, emotions, language, and abstract reasoning as well as various types of brain/mind pathophysiology.
Watts and Strogatz introduced a model—the so-called small world—for brain networks that allows an optimal balance between local specialization (segregation) and global integration (Watts and Strogatz 1998). This novel approach, based on graph theory, is a promising way to characterize brain functional organization (Bassett and Bullmore 2006; Bullmore and Sporns 2009; Ferreri et al. 2014; Stam and Reijneveld 2007). It provides insights to evaluate whether the functional connectivity patterns between brain areas reproduce the organization of theoretically efficient, flexible, or robust networks (based on the strength of synchronization in the time-varying oscillatory electromagnetic activity of different brain regions as measured by EEG or MEG).
The human brain consists of complex inhibitory and excitatory circuits of functionally specialized areas with a continuous interplay which fluctuates in time within a millisecond frame for sharing and integrating information. The white-matter (axonal) fibers provide the anatomical basis for signal transfer and communication; these connections are not random, but are organized in a so-called small-world network topology. The topology of a small-world network is characterized by a high degree of local clustering (segregation) and the presence of long-distance connections (integration) that secures a high level of global communication efficiency. Small-world network organization in brains of healthy humans has been previously described (Bassett et al. 2006; Bullmore and Sporns 2009; Gong et al. 2009; Smit et al. 2008; Sporns and Zwi 2004; Stam et al. 2007; Stam and Reijneveld 2007); however, only few of them have investigated the impact of brain diseases on the small-world architecture (Bartolomei et al. 2006; Micheloyannis et al. 2006; Ponten et al. 2007).
Recent studies (Miraglia et al. 2016; Miraglia et al. 2017; Vecchio et al. 2014a; Vecchio et al. 2014b) investigated brain developmental changes in physiological and pathological aging by analyzing the small-world network through EEG signals. Results were obtained with a method based on estimating the sources of EEG signals (exact low-resolution electromagnetic tomography [eLORETA]; most likely not affected by the ambiguity of localization and reference dependence), and omitted zero phase angle coherences to avoid undue inflation of coherence by volume conduction (Cao and Slobounov 2010; Lehmann et al. 2012). This method is artifact-free, since artifacts such as eye blinking and muscle activity were identified and excluded by the independent component analysis (ICA) composition and directly using the current density obtained from the inverse methods; the weights for the brain network parameters were extracted.
The aim of the present study was to investigate the functioning of three specific brain networks: attentional network, frontal network, default mode network. With the present investigation, we sought to contribute to the estimation of spontaneous brain EEG in human to assess the importance of that effect for clinical examinations of individual subjects. The interest was to determine whether there are small-world attributes within each hemisphere and whether these topological properties show hemispheric-specific network patterns.
To the best of our knowledge, no previous studies either provided normative data in healthy elderly large population or explored the test-retest stability of the measured parameters as a background for clinical application.
Subjects and methods
Participants
A total of 170 healthy human volunteers (mean age = 66.7 ± 0.8 (standard error) years) were recruited. All subjects were right-handed at Handedness Questionnaire (Salmaso and Longoni 1985). Exclusion criteria included a history of neurological, cognitive, or psychiatric disorder; and current treatment with vasoactive or psychotropic medication. The study was approved by the local Ethical Committee. Experimental procedures were conformed to the Declaration of Helsinki and national guidelines.
Furthermore, 32 subjects underwent two recording sessions in nearly identical environmental conditions separated by about 2 days, introducing the factor time (first, second, and third recording sessions).
Data recordings and preprocessing
EEG recordings were carried out with several digital EEG machines from at least 19 electrodes (Fp1, Fp2, F7, F8, F3, F4, T3, T4, C3, C4, T5, T6, P3, P4, O1, O2, Fz, Cz, and Pz) positioned according to the International 10–20 system. The choice of having a standard 19-electrode montage was pursued in order to create a normative database immediately transferable to a clinical scenario where such an EEG montage represents the routine standard. Two separate channels recording vertical and horizontal eye movements (EOGs) were used to monitor blinking and saccades. Impedance was kept below 5 kΩ and the sampling rate frequency was set up at least at 256 Hz. Electroencephalographic signals were measured at rest, in at least 5 min of closed eyes and no task conditions. During the recording, subjects were awake, seated, and relaxed in a sound attenuated and dimly lit room.
The data were processed in MATLAB (MathWorks, Natick, MA) using homemade scripts based on EEGLAB toolbox (Swartz Center for Computational Neurosciences, La Jolla, CA; http://www.sccn.ucsd.edu/eeglab).
The EEG recordings were band-pass-filtered from 0.2 to 47 Hz using a finite impulse response (FIR) filter. Imported data were divided into 2 s. Epochs and visible artifacts in the EEG recordings (i.e., eye movements, cardiac activity, and scalp muscle contraction) were removed using an ICA procedure allowing identification and extraction of ocular artifact components from the EEG data. ICA is a blind source decomposition algorithm that enables the separation of statistically independent sources from multichannel EEG recordings (Hoffmann and Falkenstein 2008; Iriarte et al. 2003; Jung et al. 2000). ICA was performed using the Infomax ICA algorithm (Bell and Sejnowski 1995) as implemented in the EEGLAB. Artifact-free EEG signals were used for further analyses.
Functional connectivity of cortical source analysis
Brain connectivity was computed using exact low-resolution electromagnetic tomography (eLORETA) (Pascual-Marqui et al. 2011) software on regions of interest (ROIs) defined according to the Brodmann areas (Bas): 42 ROIs (BAs 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 13, 17, 18, 19, 20, 21, 22, 23, 24, 25, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47), for the left and for the right hemispheres, and a number of ROIs in three specific resting state subnetworks (Allen et al. 2011; Miraglia et al. 2015) detailed as follows: 13 ROIs in the attentional network (BAs 6, 7, 8, 9, 10, 21, 22, 31, 32, 39, 40, 45, 47); 9 ROIs in the frontal network (BAs 2, 7, 8, 9, 10, 40, 44, 45, 46); 8 ROIs in the default mode network (BAs 7, 8, 10, 23, 31, 32, 39, 46), for the left and the right hemispheres separately (see Fig. 1).
Fig. 1.
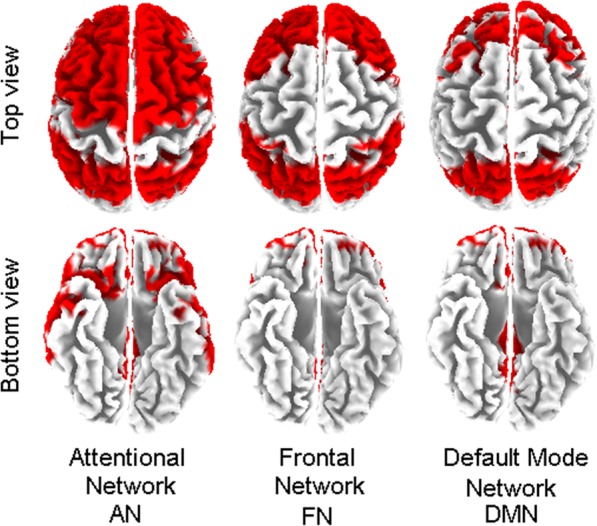
Schematic illustration of considered networks: attentional (AN) Brodmann areas (BA) 6, 7, 8, 9, 10, 21, 22, 31, 32, 39, 40, 45, 47; frontal network (FN) BA 2, 7, 8, 9, 10, 40, 44, 45, 46; default mode network (DMN) BA 7, 8, 10, 23, 31, 32, 39, 46
ROIs are needed for the estimation of electric neuronal activity that is used to analyze brain functional connectivity.
Among the eLORETA current density time series of the ROIs, intracortical lagged linear coherence, extracted by “all nearest voxels” for the 84 ROIs and centered on each BA of interest by a sphere of 19 mm for the resting subnetworks, was computed (Pascual-Marqui 2007; Pascual-Marqui et al. 2011) between all possible pairs of the ROIs for each of the seven independent EEG frequency bands (Kubicki et al. 1979) of delta (2–4 Hz), theta (4–8 Hz), alpha 1 (8–10.5 Hz), alpha 2 (10.5–13 Hz), beta 1 (13–20 Hz), beta 2 (20–30 Hz), and gamma (30–45 Hz), for each subject.
Moving from the definition for the complex valued coherence (Lehmann et al. 2012; Nolte et al. 2004) between time series x and y in the frequency band ω, which is based on the cross-spectrum given by the covariance and variances of the signals, the lagged linear coherence in the frequency band ω is reported on the following equation (Pascual-Marqui 2007; Pascual-Marqui et al. 2011):
where Var and Cov are variances and covariance of the signals, respectively.
This equation was developed to provide a measure of true physiological connectivity not affected by volume conduction and with low spatial resolution.
The values of connectivity computing between all pairs of ROIs for each frequency band and for each subject were used as a measure of weight of the graph in the following graph analyses.
Graph analysis
A network is a mathematical representation of a real-world complex system and is defined by a collection of nodes (vertices) and links (edges) between pairs of nodes. Nodes in large-scale brain networks represent brain regions, while links represent anatomical, functional, or effective connections, depending on the dataset. Anatomical connections typically correspond to white matter fiber tracts between pairs of gray matter relays (cortical areas or subcortical relays). Functional connections correspond to magnitudes of temporal correlations in activity and may occur between pairs of anatomically unconnected regions.
The nature of nodes and links in individual brain networks is determined by combinations of brain mapping methods, anatomical parcellation schemes, and measures of connectivity. Many combinations occur in various experimental settings (Horwitz 2003). Nodes should ideally represent brain regions with coherent patterns of extrinsic anatomical or functional connections (Rubinov and Sporns 2010). Undirected and weighted networks based on the connectivity between different ROIs were then built. The nodes of the network were defined as the ROIs and the edges of the network were weighted by the lagged linear connectivity values (Cao and Slobounov 2010). Two core measures of graph theory were computed: weighted characteristic path length and weighted clustering coefficient, representative of global connectedness and local interconnectedness, respectively (Watts and Strogatz 1998).
Originally described in social networks, the “small-world” property combines high levels of local clustering among nodes of a network (to form families or cliques) and short paths that globally link all nodes of the network. This means that all nodes of a large system are linked through relatively few intermediate steps, despite the fact that most nodes maintain only a few direct connections—mostly within a clique of neighbors. Small-world organization is intermediate between that of random networks, the short overall path length of which is associated with a low level of local clustering, and that of regular networks or lattices, the high-level of clustering of which is accompanied by a long path length.
The measure of network small-worldness (Sw) is defined as the ratio between C and L individually normalized with respect to the frequency bands (Miraglia et al. 2020; Vecchio et al. 2019a; Vecchio et al. 2018a; Vecchio et al. 2018b; Vecchio et al. 2019b)
The Sw coefficient is used to describe the balance between the local connectedness and the global integration of a network. Small-world organization is intermediate between that of random networks, the short overall path length of which is associated with a low level of local clustering, and that of regular networks or lattices, the high-level of clustering of which is accompanied by a long path length. The “small-world” property combines high levels of local clustering among nodes of a network (to form families or cliques) and short paths that globally link all nodes of the network. This means that all nodes of a large system are linked through relatively few intermediate steps, despite the fact that most nodes maintain only a few direct connections—mostly within a clique of neighbors.
In particular, the networks of the present study are designed as undirected and weighted cerebral networks. The nodes are represented by the BAs and the edges are weighted by lagged linear connectivity values. We computed Lw and Cw as a measure of integration and segregation of the network, respectively, and Sw as a measure of global brain network organization.
Statistical evaluation
Analysis of variance (ANOVA) was used between the ROI indices computed in the three populations for all the frequency bands. Although data were normally distributed, ANOVA was chosen since it is known to be robust with respect to the departure of normality and homoscedasticity of data being treated (Zar 1984). Greenhouse and Geisser correction was used for the protection against the possible violation of the sphericity assumption in the repeated measures ANOVA. Besides, post hoc analysis with Duncan’s test and a significance level at 0.05 was performed. All the statistical analyses were performed with the software Statistica (StatSoft Inc., www.statsoft.com).
Furthermore, in order to find differences between the two hemispheres, a two-way ANOVA was evaluated among the factors: hemisphere (left, right) and band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma) for the whole hemisphere and for each of the subnetwork (attentional network (AN); frontal network (FN); default mode network (DMN)).
Finally, in order to evaluate the within-subject test-retest variability, ANOVA was conducted for small world, computed in each frequency band. The significance level was set at p < 0.05 and the ANOVAs were performed between two factors: time (first, second, and third recording sessions) and band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma).
Results
Graph theory based on the EEG cortical sources as estimated by eLORETA
The evaluation of the normalized small world showed a typical trend as reported in Fig. 2; the normative data of this group of subjects and their 5th percentile values are reported in Table 1.
Fig. 2.
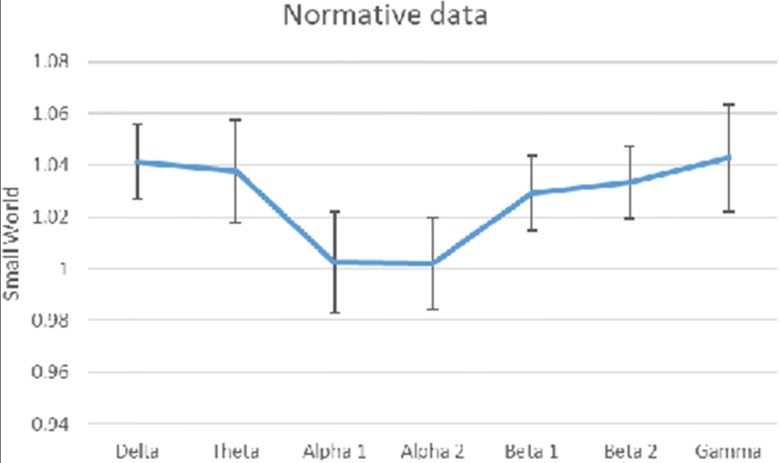
Small world showed a typical trend in the present 170 subjects
Table 1.
Mean small world
| Delta | Theta | Alpha 1 | Alpha 2 | Beta 1 | Beta 2 | Gamma | |
|---|---|---|---|---|---|---|---|
| Mean | 1.041169 | 1.037566 | 1.002419 | 1.001883 | 1.028932 | 1.033532 | 1.04276 |
| 5 percentile | 0.810258 | 0.715102 | 0.6192 | 0.597465 | 0.749131 | 0.766062 | 0.613313 |
In order to find differences between the two hemispheres, a two-way ANOVA was evaluated among the factors: hemisphere (left, right) and band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma). No statistical difference between the left and right SW for each band (F(6, 1002) = 3.2180) was found (see Fig. 3).
Fig. 3.
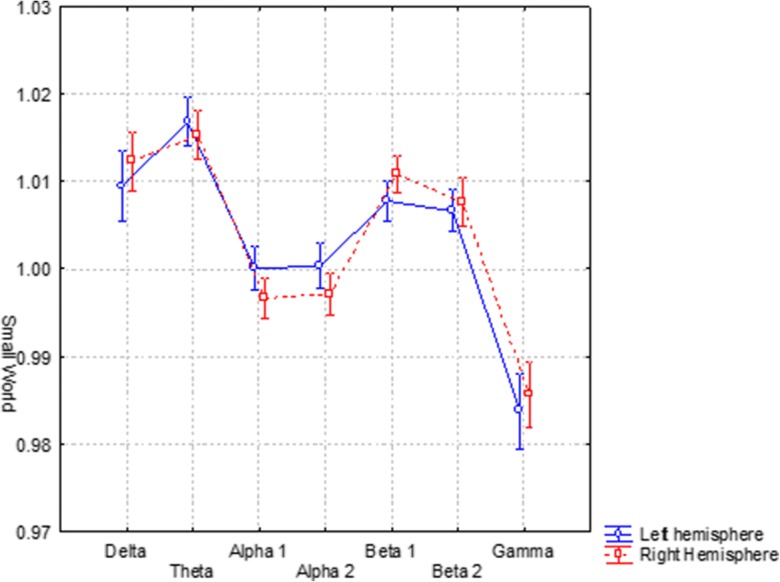
Small world in the left and right hemispheres separately
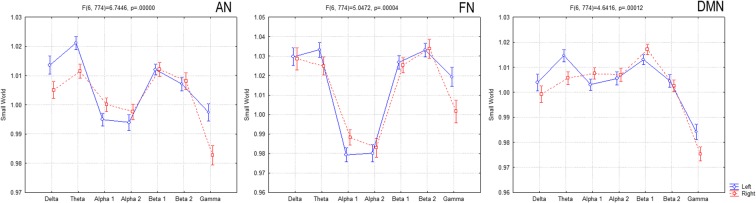
Furthermore, in order to find differences between the right/left hemispheric subnetworks, in each (Fig. 4) of the attentional network (AN), frontal network (FN), and default mode network (DMN), a two-way ANOVA was evaluated among the factors: hemisphere (left, right) and band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma).
Fig. 4.
Small world in the left and right subnetworks: attentional network (AN), frontal network (FN), default mode network (DMN)
In AN (F(6, 774) = 6.7446, p = 0.00000), delta (p < 0.009), theta (p < 0.002), and gamma (p < 0.000004) presented higher SW in the left hemisphere.
In FN (F(6, 774) = 5.0472, p = 0.00004), alpha 1 (p < 0.031) presented higher SW in the right hemisphere, while gamma (p < 0.00001) presented higher SW in the left hemisphere.
In DMN (F(6, 774) = 4.6416, p = 0.00012), theta (p < 0.0015) and gamma (p < 0.0008) presented higher SW in the left hemisphere.
Within-subject test-retest analysis
The ANOVA, performed on the three recordings of the 32 subjects, showed that no interaction including time resulted a significant level (p > 0.5), highlighting the stability of the measured parameters within the present methodology when carried out in clinically stable subjects and in line with a recent study with a higher number of subjects (Vecchio et al. 2019a). As a control analysis, we also performed a nonparametric statistic (Friedman’s ANOVA) for each band and the results confirmed the stability of the measured parameters.
Discussion
Brain connectivity datasets comprise networks of cerebral regions directly connected by anatomical tracts or by functional associations. Brain networks represent an individual “fingerprint” deriving from personal life experience and skills; however, some generalized modules can be identified. They are invariably complex, share a number of common features with networks from other biological and physical systems, and may hence be characterized by using complex network mathematical methods. The concept of functional connectivity is viewed as pivotal for understanding the organized behavior of anatomical regions in the brain during their activity. This organization is probably based on the interaction between different and variably specialized cortical sites. Cortical functional connectivity estimate aims at describing these interactions as connectivity patterns, which reflect strength of the information flow among the involved cortical areas.
Theoretical graph approach can be a very useful tool, intercepting some global and local features in the functional connectivity patterns estimated from the EEG along both physiological and pathological aging (Miraglia et al. 2015; Miraglia et al. 2016; Miraglia et al. 2017; Miraglia et al. 2018; Rossini et al. 2016; Vecchio et al. 2014a; Vecchio et al. 2015; Vecchio et al. 2018a; Vecchio et al. 2017a; Vecchio et al. 2014b; Vecchio et al. 2017b; Vecchio et al. 2016; Vecchio et al. 2018b; Vecchio et al. 2017c). It is worth mentioning that the measured parameters display little within-subject and among-subject variability and that a test-retest variability analysis showed that no interaction including time was significant, highlighting the stability of the present measurements in line with a previous study from our group (Vecchio et al. 2019a).
The results of the present work can be summarizing as follows: for the attentional network, delta, theta, and gamma rhythms presented higher SW in the left hemisphere; for the frontal network, alpha 1 rhythms presented higher SW in the right, while gamma presented higher SW in the left hemisphere; for the default mode network, theta and gamma rhythms presented higher SW in the left hemisphere.
In particular, the mentioned three networks were selected for covering human abilities in the following manner:
Attentional network: cognitive properties such as executive functions such as working memory, language, decision-making, visuospatial abilities, and also alerting, orienting, and executive control (Keehn et al. 2013; Petersen and Posner 2012; Posner and Petersen 1990).
Frontal network: language domain, in particular, functional coupling between frontal areas was found to be related to performance in grammar-learning task in healthy older adults (Antonenko et al. 2013; Antonenko et al. 2012), task-related activity of a semantic fluency paradigm and resting-state functional connectivity (Meinzer et al. 2012).
Default mode network, as a network of intrinsic functional connectivity, well represented in the resting state condition as a sign of mental reorganization and readiness to execute a task. DMN is associated with autobiographical memory and habitual, self-referential thought (Buckner et al. 2005; Greicius et al. 2003; Raichle et al. 2001).
Results showed that while evaluating the whole hemispheres did not present differences but when each subnetworks are considered, in the left hemisphere, AN and DMN presented in general higher SW in low (delta and/or theta) and high (gamma) frequency bands, while in FN, alpha 1 was lower, with respect to the right hemisphere.
These results are in line with previous clinical evidence (Miraglia et al. 2015; Vecchio et al. 2014a; Vecchio et al. 2014b) in which higher levels of delta and theta were accompanied by a lower level of alpha SW in elderly subjects with respect to demented patients. Furthermore, it should be noted that several studies of our group (Vecchio et al. 2017b; Vecchio et al. 2016) demonstrated that small-world characteristics could be modified by age or neurodegeneration in the sense of a reduction of low- and high-frequency rhythmic EEG oscillations (delta and gamma) and an increase in alpha bands.
Keeping in mind the above findings and considering that small-world architecture of hemispheric functional network could represent an optimal organizational pattern according to evolution and development, it could be argued that—as above described of a general better cognitive performance—there is a more specific involvement of left subnetworks with intrinsic characteristics (higher involvement of delta, theta, and gamma bands in AN and DMN, alpha 1 band in FN).
Hemispheric damage in humans suggests that the two cerebral hemispheres have complementary functions: the left hemisphere (LH) is specialized for language and action, and the right hemisphere (RH) for attention and visual spatial perception. The split brain further suggests that each hemisphere is a complete cognitive system. In the normal brain, we can therefore observe asymmetries at all levels of analysis. Anatomical asymmetries show larger perisylvian language areas in the LH. Neurochemical asymmetries suggest LH specialization for activation (dopamine) and RH specialization for arousal (norepinephrine). Physiological asymmetries show greater RH than LH activation for orienting of spatial attention, the perception of faces and musical melodies, spatial imagery, encoding and retrieval of nonverbal information, and pragmatic aspects of language processing. LH activation is greater for perception of words and objects; processing of the sound, grammar, and meaning of words; and encoding and retrieval of verbal semantic memory. There is also evidence for individual differences in emotional processing based on frontal lobe EEG asymmetry. Behavioral asymmetries in the normal brain using hemifield tachistoscopy and dichotic listening show LH specialization for phonetic and grammatical language processing which, however, is not exclusive. The discrepancies between clinical-neurological and normal asymmetries suggest that the damaged hemisphere inhibits residual competence on the other side (Zaidel 2001).
Several evidences also showed frequency-dependent asymmetry in the brain. Regarding alpha band, EEG studies have highlighted that alpha-band activity is relatively reduced over the occipital-parietal areas of the hemisphere contralateral to the direction of spatial attention. Although the inverse problem means that scalp locations cannot be mapped one-to-one to neural sources, source models suggests that, at a gross level, asymmetries observed on the scalp reflect functional asymmetries in underlying neural systems (Pizzagalli et al. 2005; Smith et al. 2018). By far the most commonly studied EEG asymmetry resides in the alpha band (8–13 Hz) over frontal sites (Allen et al. 2018). Frontal alpha asymmetry can be recorded either in the resting state. Alpha is commonly taken to reflect the inverse of cognitive activity (Bazanova and Vernon 2014; Coan and Allen 2003), as alpha suppression is associated with attentional and cognitive engagement (Mazaheri et al. 2014; Ocklenburg et al. 2019). On the other hand, Ocklenburg and colleagues observed significant asymmetries for example in lower and higher frequency bands, in particular in fronto-central electrode sites (Ocklenburg et al. 2019).
Evidences from the present study—by comparing cortical sources of the EEG signals and graph theory approach—confirm the utility to adopt a mathematical analysis to investigate relevant features in real complex brain networks. Results are compatible with the hypothesis that processes as those revealed by changes in functional networks, may represent a facet of normal human adult brain aging. In this sense, graph theory applied to EEG can help the analysis of connectivity patterns particularly in their dynamic properties thanks to the high temporal resolution of the EEG signals.
Although the age-related alteration of the present parameter could be an interesting analysis, the aim of the present study was at this stage to validate a database with the aim to immediately transfer to a clinical scenario of pathological aging people. In future study, collecting a high number of younger subjects, the age-related modulation should be analyzed.
In conclusion, graph analysis tools described here represent an interesting probe to study the distinctive features of physiological aging focusing on functional connectivity networks. Applied to patient data, this technique might provide more insight in the pathophysiological processes underlying age-related brain disconnection as well as for monitoring the impact of eventual pharmacological and rehabilitative treatments.
Funding information
This work was partially supported by the Italian Ministry of Health for Institutional Research (Ricerca corrente) and for the project GR-2013-02358430.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Havlicek M, Rachakonda S, Fries J, Kalyanam R, Michael AM, Caprihan A, Turner JA, Eichele T, Adelsheim S, Bryan AD, Bustillo J, Clark VP, Feldstein Ewing SW, Filbey F, Ford CC, Hutchison K, Jung RE, Kiehl KA, Kodituwakku P, Komesu YM, Mayer AR, Pearlson GD, Phillips JP, Sadek JR, Stevens M, Teuscher U, Thoma RJ, Calhoun VD. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JJB, Keune PM, Schönenberg M and Nusslock R, 2018, Frontal EEG alpha asymmetry and emotion: from neural underpinnings and methodological considerations to psychopathology and social cognition. Psychophysiology, 55. [DOI] [PubMed]
- Antonenko D, Meinzer M, Lindenberg R, Witte AV, Flöel A. Grammar learning in older adults is linked to white matter microstructure and functional connectivity. Neuroimage. 2012;62:1667–1674. doi: 10.1016/j.neuroimage.2012.05.074. [DOI] [PubMed] [Google Scholar]
- Antonenko D, Brauer J, Meinzer M, Fengler A, Kerti L, Friederici AD, Flöel A. Functional and structural syntax networks in aging. Neuroimage. 2013;83:513–523. doi: 10.1016/j.neuroimage.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Bosma I, Klein M, Baayen JC, Reijneveld JC, Postma TJ, Heimans JJ, van Dijk BW, de Munck JC, de Jongh A, Cover KS, Stam CJ. Disturbed functional connectivity in brain tumour patients: evaluation by graph analysis of synchronization matrices. Clin Neurophysiol. 2006;117:2039–2049. doi: 10.1016/j.clinph.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12:512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Meyer-Lindenberg A, Achard S, Duke T, Bullmore E. Adaptive reconfiguration of fractal small-world human brain functional networks. Proc Natl Acad Sci U S A. 2006;103:19518–19523. doi: 10.1073/pnas.0606005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazanova OM, Vernon D. Interpreting EEG alpha activity. Neurosci Biobehav Rev. 2014;44:94–110. doi: 10.1016/j.neubiorev.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Cao C, Slobounov S. Alteration of cortical functional connectivity as a result of traumatic brain injury revealed by graph theory, ICA, and sLORETA analyses of EEG signals. IEEE Trans Neural Syst Rehabil Eng. 2010;18:11–19. doi: 10.1109/TNSRE.2009.2027704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Allen JJ. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology. 2003;40:106–114. doi: 10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Vecchio F, Ponzo D, Pasqualetti P, Rossini PM. Time-varying coupling of EEG oscillations predicts excitability fluctuations in the primary motor cortex as reflected by motor evoked potentials amplitude: an EEG-TMS study. Hum Brain Mapp. 2014;35:1969–1980. doi: 10.1002/hbm.22306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, Beaulieu C. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex. 2009;19:524–536. doi: 10.1093/cercor/bhn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S, Falkenstein M. The correction of eye blink artefacts in the EEG: a comparison of two prominent methods. PLoS One. 2008;3:e3004. doi: 10.1371/journal.pone.0003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B. The elusive concept of brain connectivity. Neuroimage. 2003;19:466–470. doi: 10.1016/s1053-8119(03)00112-5. [DOI] [PubMed] [Google Scholar]
- Iriarte J, Urrestarazu E, Valencia M, Alegre M, Malanda A, Viteri C, Artieda J. Independent component analysis as a tool to eliminate artifacts in EEG: a quantitative study. J Clin Neurophysiol. 2003;20:249–257. doi: 10.1097/00004691-200307000-00004. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, Sejnowski TJ. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37:163–178. [PubMed] [Google Scholar]
- Keehn B, Müller RA, Townsend J. Atypical attentional networks and the emergence of autism. Neurosci Biobehav Rev. 2013;37:164–183. doi: 10.1016/j.neubiorev.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki S, Herrmann WM, Fichte K, Freund G. Reflections on the topics: EEG frequency bands and regulation of vigilance. Pharmakopsychiatr Neuropsychopharmakol. 1979;12:237–245. doi: 10.1055/s-0028-1094615. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Faber PL, Tei S, Pascual-Marqui RD, Milz P, Kochi K. Reduced functional connectivity between cortical sources in five meditation traditions detected with lagged coherence using EEG tomography. Neuroimage. 2012;60:1574–1586. doi: 10.1016/j.neuroimage.2012.01.042. [DOI] [PubMed] [Google Scholar]
- Mazaheri A, van Schouwenburg MR, Dimitrijevic A, Denys D, Cools R, Jensen O. Region-specific modulations in oscillatory alpha activity serve to facilitate processing in the visual and auditory modalities. Neuroimage. 2014;87:356–362. doi: 10.1016/j.neuroimage.2013.10.052. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Antonenko D, Lindenberg R, Hetzer S, Ulm L, Avirame K, Flaisch T, Flöel A. Electrical brain stimulation improves cognitive performance by modulating functional connectivity and task-specific activation. J Neurosci. 2012;32:1859–1866. doi: 10.1523/JNEUROSCI.4812-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheloyannis S, Pachou E, Stam CJ, Breakspear M, Bitsios P, Vourkas M, Erimaki S, Zervakis M. Small-world networks and disturbed functional connectivity in schizophrenia. Schizophr Res. 2006;87:60–66. doi: 10.1016/j.schres.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Miraglia F, Vecchio F, Bramanti P, Rossini PM. Small-worldness characteristics and its gender relation in specific hemispheric networks. Neuroscience. 2015;310:1–11. doi: 10.1016/j.neuroscience.2015.09.028. [DOI] [PubMed] [Google Scholar]
- Miraglia F, Vecchio F, Bramanti P, Rossini PM. EEG characteristics in “eyes-open” versus “eyes-closed” conditions: small-world network architecture in healthy aging and age-related brain degeneration. Clin Neurophysiol. 2016;127:1261–1268. doi: 10.1016/j.clinph.2015.07.040. [DOI] [PubMed] [Google Scholar]
- Miraglia F, Vecchio F, Rossini PM. Searching for signs of aging and dementia in EEG through network analysis. Behav Brain Res. 2017;317:292–300. doi: 10.1016/j.bbr.2016.09.057. [DOI] [PubMed] [Google Scholar]
- Miraglia F, Vecchio F, Rossini PM. Brain electroencephalographic segregation as a biomarker of learning. Neural Netw. 2018;106:168–174. doi: 10.1016/j.neunet.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Miraglia F, Vecchio F, Marra C, Quaranta D, Alù F, Peroni B, Granata G, Judica E, Cotelli M, Rossini PM. Small world index in default mode network predicts progression from mild cognitive impairment to dementia. Int J Neural Syst. 2020;30:2050004. doi: 10.1142/S0129065720500045. [DOI] [PubMed] [Google Scholar]
- Nolte G, Bai O, Wheaton L, Mari Z, Vorbach S, Hallett M. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin Neurophysiol. 2004;115:2292–2307. doi: 10.1016/j.clinph.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Ocklenburg S, Friedrich P, Schmitz J, Schlüter C, Genc E, Güntürkün O, Peterburs J, Grimshaw G. Beyond frontal alpha: investigating hemispheric asymmetries over the EEG frequency spectrum as a function of sex and handedness. Laterality. 2019;24:505–524. doi: 10.1080/1357650X.2018.1543314. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Instantaneous and lagged measurements of linear and nonlinear dependence between groups of multivariate time series: frequency decomposition. eprint arXiv:07111455. 2007;arXiv:0711.1455. [Google Scholar]
- Pascual-Marqui RD, Lehmann D, Koukkou M, Kochi K, Anderer P, Saletu B, Tanaka H, Hirata K, John ER, Prichep L, Biscay-Lirio R, Kinoshita T. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos Trans A Math Phys Eng Sci. 2011;369:3768–3784. doi: 10.1098/rsta.2011.0081. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness: a source-localization study. Psychol Sci. 2005;16:805–813. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Ponten SC, Bartolomei F, Stam CJ. Small-world networks and epilepsy: graph theoretical analysis of intracerebrally recorded mesial temporal lobe seizures. Clin Neurophysiol. 2007;118:918–927. doi: 10.1016/j.clinph.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Di Iorio R, Granata G, Miraglia F, Vecchio F. From mild cognitive impairment to Alzheimer’s disease: a new perspective in the “land” of human brain reactivity and connectivity. J Alzheimers Dis. 2016;53:1389–1393. doi: 10.3233/JAD-160482. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Salmaso D, Longoni AM. Problems in the assessment of hand preference. Cortex. 1985;21:533–549. doi: 10.1016/s0010-9452(58)80003-9. [DOI] [PubMed] [Google Scholar]
- Smit DJ, Stam CJ, Posthuma D, Boomsma DI, de Geus EJ. Heritability of “small-world” networks in the brain: a graph theoretical analysis of resting-state EEG functional connectivity. Hum Brain Mapp. 2008;29:1368–1378. doi: 10.1002/hbm.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Cavanagh JF and Allen JJB, 2018, Intracranial source activity (eLORETA) related to scalp-level asymmetry scores and depression status. Psychophysiology, 55. [DOI] [PMC free article] [PubMed]
- Sporns O, Zwi JD. The small world of the cerebral cortex. Neuroinformatics. 2004;2:145–162. doi: 10.1385/NI:2:2:145. [DOI] [PubMed] [Google Scholar]
- Stam CJ, Jones BF, Nolte G, Breakspear M, Scheltens P. Small-world networks and functional connectivity in Alzheimer’s disease. Cereb Cortex. 2007;17:92–99. doi: 10.1093/cercor/bhj127. [DOI] [PubMed] [Google Scholar]
- Stam CJ, Reijneveld JC. Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed Phys. 2007;1:3. doi: 10.1186/1753-4631-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchio F, Miraglia F, Bramanti P, Rossini PM. Human brain networks in physiological aging: a graph theoretical analysis of cortical connectivity from EEG data. J Alzheimers Dis. 2014;41:1239–1249. doi: 10.3233/JAD-140090. [DOI] [PubMed] [Google Scholar]
- Vecchio F, Miraglia F, Marra C, Quaranta D, Vita MG, Bramanti P, Rossini PM. Human brain networks in cognitive decline: a graph theoretical analysis of cortical connectivity from EEG data. J Alzheimers Dis. 2014;41:113–127. doi: 10.3233/JAD-132087. [DOI] [PubMed] [Google Scholar]
- Vecchio F, Miraglia F, Curcio G, Della Marca G, Vollono C, Mazzucchi E, Bramanti P, Rossini PM. Cortical connectivity in fronto-temporal focal epilepsy from EEG analysis: a study via graph theory. Clin Neurophysiol. 2015;126:1108–1116. doi: 10.1016/j.clinph.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Vecchio F, Miraglia F, Quaranta D, Granata G, Romanello R, Marra C, Bramanti P, Rossini PM. Cortical connectivity and memory performance in cognitive decline: a study via graph theory from EEG data. Neuroscience. 2016;316:143–150. doi: 10.1016/j.neuroscience.2015.12.036. [DOI] [PubMed] [Google Scholar]
- Vecchio F, Miraglia F, Maria Rossini P. Connectome: graph theory application in functional brain network architecture. Clin Neurophysiol Pract. 2017;2:206–213. doi: 10.1016/j.cnp.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchio F, Miraglia F, Piludu F, Granata G, Romanello R, Caulo M, Onofrj V, Bramanti P, Colosimo C, Rossini PM. “Small world” architecture in brain connectivity and hippocampal volume in Alzheimer’s disease: a study via graph theory from EEG data. Brain Imaging Behav. 2017;11:473–485. doi: 10.1007/s11682-016-9528-3. [DOI] [PubMed] [Google Scholar]
- Vecchio F, Miraglia F, Romano A, Bramanti P, Rossini PM. Small world brain network characteristics during EEG Holter recording of a stroke event. Clin Neurophysiol. 2017;128:1–3. doi: 10.1016/j.clinph.2016.10.090. [DOI] [PubMed] [Google Scholar]
- Vecchio F, Miraglia F, Iberite F, Lacidogna G, Guglielmi V, Marra C, Pasqualetti P, Tiziano FD, Rossini PM. Sustainable method for Alzheimer dementia prediction in mild cognitive impairment: electroencephalographic connectivity and graph theory combined with apolipoprotein E. Ann Neurol. 2018;84:302–314. doi: 10.1002/ana.25289. [DOI] [PubMed] [Google Scholar]
- Vecchio F, Miraglia F, Quaranta D, Lacidogna G, Marra C, Rossini PM. Learning processes and brain connectivity in a cognitive-motor task in neurodegeneration: evidence from EEG network analysis. J Alzheimers Dis. 2018;66:471–481. doi: 10.3233/JAD-180342. [DOI] [PubMed] [Google Scholar]
- Vecchio F, Caliandro P, Reale G, Miraglia F, Piludu F, Masi G, Iacovelli C, Simbolotti C, Padua L, Leone E, Alù F, Colosimo C, Rossini PM. Acute cerebellar stroke and middle cerebral artery stroke exert distinctive modifications on functional cortical connectivity: a comparative study via EEG graph theory. Clin Neurophysiol. 2019;130:997–1007. doi: 10.1016/j.clinph.2019.03.017. [DOI] [PubMed] [Google Scholar]
- Vecchio F, Tomino C, Miraglia F, Iodice F, Erra C, Di Iorio R, Judica E, Alù F, Fini M, Rossini PM. Cortical connectivity from EEG data in acute stroke: a study via graph theory as a potential biomarker for functional recovery. Int J Psychophysiol. 2019;146:133–138. doi: 10.1016/j.ijpsycho.2019.09.012. [DOI] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Zaidel E, 2001, Brain Asymmetry. 1321-1329.
- Zar JH. Biostatistical analysis. Englewood Cliffs: Prentice-Hall; 1984. [Google Scholar]