Abstract
Imbalanced mitochondrial dynamics in pancreatic β-cells contributes to β-cell dysfunction in diabetes; however, the molecular mechanisms underlying mitochondrial dynamics in the pathology of diabetes are not fully elucidated. We previously reported the reduction of RNA binding protein HuD in pancreatic β-cells of diabetes. Herein, we demonstrate that HuD plays a novel role in the regulation of mitochondrial dynamics by promoting mitochondrial fusion. We show enhanced mitochondrial fragmentation in the pancreas of db/db mice and HuD KO mice. Downregulation of HuD increases the number of cells with fragmented mitochondria and reduces the mitochondrial activity determined by mitochondrial membrane potential and ATP production in mouse insulinoma βTC6 cells. HuD binds to 3′-untraslated region of mitofusin 2 (Mfn2) mRNA and positively regulates its expression. Ectopic expression of Mfn2 in βTC6 cells stably expressing short hairpin RNA against HuD (shHuD) restores HuD-mediated mitochondrial dysfunction. Taken together, our results suggest that HuD regulates mitochondrial dynamics by regulating Mfn2 level and its reduced expression leads to mitochondrial dysfunction in pancreatic β-cells.
Subject terms: RNA, Metabolic disorders
Introduction
Mitochondria play crucial roles in cellular energy homeostasis and apoptosis by regulating cellular energy production, reactive oxygen species generation, and signal transduction [1–3]. Mitochondria continuously change their morphology through fusion and fission in response to the physiological demands of cells, which is critical to the organelle’s function [4–6]. Abnormal regulation of mitochondrial dynamics has been implicated in the pathogenesis of several human diseases, in particular, metabolic disorders [5, 7–9]. Enhanced fission and reduced fusion were previously reported in obesity and diabetes [10, 11]. The differential expression of mitochondrial dynamics regulating proteins, such as dynamin related protein 1 (Drp1), optic atrophy protein 1 (Opa1), mitofusin 1/2 (Mfn1/2), and mitochondria fission factor (Mff), is responsible for the imbalance between fusion and fission, and contributes to mitochondrial dysfunction [12, 13]. Emerging evidence suggests that the tight regulation of mitochondrial dynamics plays an important role in the maintenance of mitochondrial homeostasis and in the pathogenesis of diseases; however, the underlying mechanisms need to be further elucidated.
Mitofusin 2 (Mfn2), a 757 aa protein containing several well-conserved domains, mediates oligomerization of two adjacent mitochondria via heptad-repeat domains, and regulates mitochondrial distribution, shape, and quality control [14]. Mfn2 has been shown to play a key role in metabolism: Mfn2 depletion leads to reduced mitochondrial membrane potential, cellular oxygen consumption, and mitochondrial proton leak, whereas Mfn2 overexpression promotes oxidative phosphorylation, glucose oxidation, and insulin sensitivity [12, 15–19]. Mfn2 also mediates the interaction between mitochondria and endoplasmic reticulum (ER) and regulates ER stress in response to metabolic stress [20]. Downregulation of Mfn2 expression has been reported in obesity and diabetes, and it suggests the implication of Mfn2 in the pathophysiology of metabolic diseases [12, 14, 15].
Mfn2 expression is regulated at the levels of transcription, posttranscription, and posttranslation. Estrogen-related receptor-α, peroxisome proliferator-activated receptor gamma coactivator 1-beta, and lysocardiolipin acyltransferase 1 regulate Mfn2 transcription [21–23]. microRNAs (miRNAs) including miR-17, −31, −106b, −125a, −195, −214, –497, and −761, suppress, while a long noncoding RNA Plscr4 increases, Mfn2 expression [24–36]. Phosphorylation-dependent ubiquitination by JNK and Wuwe1 also regulates the level of Mfn2 [37]. In addition, Mfn2 activity is affected by protein–protein interactions such as the Smad2-RIN complex [38]. Although several studies have identified the factors regulating the expression or activity of Mfn2, the underlying mechanisms in physiological or pathological conditions need to be further determined.
HuD, one of members of human antigen Hu/ELAVL family, plays an essential role in posttranscriptional regulation of gene expression by controlling mRNA turnover or translation in certain types of cells including neurons and pancreatic β-cells [39–45]. Herein, we identified HuD as a novel factor regulating mitochondrial dynamics and function by promoting Mfn2 expression. Mitochondrial fragmentation is enhanced in pancreatic islets of diabetic db/db mice and HuD knockout (KO) mice. Mitochondrial morphology and activity is regulated in an HuD-dependent manner in the mouse pancreatic β-cell line, βTC6. HuD increases Mfn2 expression by associating with 3′-untranslated region (3′UTR) of Mfn2 mRNA, thereby promoting mitochondrial fusion. Ectopic expression of Mfn2 restores HuD-mediated mitochondrial dysfunction. Our data reveal that HuD plays an essential role in the regulation of mitochondrial homeostasis and its reduction may contribute to impaired mitochondrial function resulting β-cell dysfunction.
Results
Mitochondrial fragmentation is enhanced in diabetic islets
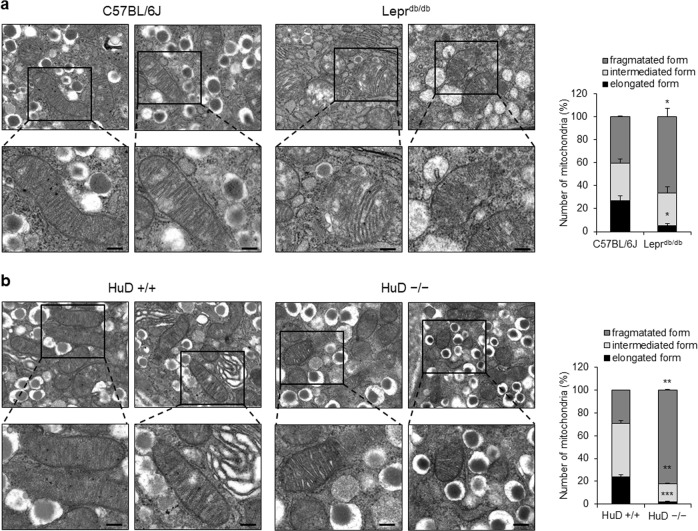
Mitochondria dysfunction has been reported in a diverse diabetes models and abnormal mitochondrial morphology is known to be associated with mitochondrial dysfunction in the pathogenesis of diabetes [11, 46–48]. We investigated the mitochondrial morphology in the pancreas of leptin receptor-deficient mice (db/db mice), one of the diabetes models, compared with normal C57BL/6J mice using an electron microscope. The results show that mitochondria in pancreatic islets of db/db mice were smaller than those of the control mice (Fig. 1a). Our previous study demonstrated that lower HuD expression in pancreatic islets contributed to β-cell dysfunction [44]. To elucidate the relationship between HuD and mitochondrial dynamics, we also analyzed the mitochondrial morphology of pancreatic islets in HuD KO mice. We found that mitochondria in pancreatic islets of KO mice were rounded and smaller than those derived from wildtype mice (Fig. 1b). These observations suggest that mitochondria fragmentation is enhanced in diabetic islets and HuD plays a role in the regulation of mitochondrial dynamics.
Fig. 1.
Mitochondrial morphology in the pancreas of diabetic mice. Mitochondrial morphology was analyzed in the pancreas of normal C57BL/6J and diabetic (Leprdb/db) mice (a) and the pancreas of HuD knockout mice (b) using a transmission electron microscope (TEM). Bar, 0.2 μm. Fifty mitochondria from each group were counted according to their relative length. Images are representative and the values indicate the mean ± SEM obtained from three independent analyses. *p < 0.05; **p < 0.01; ***p < 0.001
HuD functions as an essential regulator in the maintenance of mitochondrial morphology and function
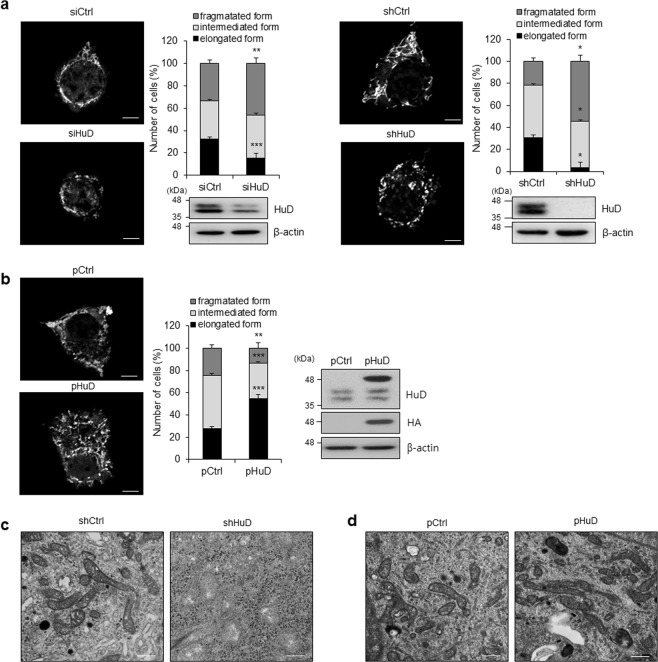
Our previous studies shows HuD plays pivotal roles in pancreatic β-cells and its expression is reduced in the pancreas of db/db mice [42–44]. Figure 1b shows abnormal mitochondrial morphology in the pancreatic islets of HuD KO mice. To determine whether HuD has a potential to regulate mitochondrial dynamics, we analyzed mitochondrial morphology in βTC6 cells transfected with HuD siRNA or in a stable cell line expressing a short hairpin RNA against HuD (shHuD) [44] by staining with MitoTracker® or electron microscopy. Downregulation of HuD resulted in fragmentation of mitochondria in βTC6 cells as shown in Fig. 2a, c. To test the effect of HuD overexpression on mitochondrial dynamics, we transduced βTC6 cells with a retrovirus containing HA-tagged HuD (pHuD) and analyzed mitochondrial morphology. HuD overexpression increased mitochondrial fusion in βTC6 cells (Fig. 2b, d). These results suggest that HuD has a role in the regulation of mitochondrial dynamics by promoting mitochondrial elongation.
Fig. 2.
Regulation of mitochondrial morphology by HuD. a, b HuD knockdowned βTC6 cells using either siRNA (left) or small hairpin RNA (right) (a) and HuD overexpressing βTC6 cells using a retrovirus carrying HA-HuD (pHuD) (b) were incubated with MitoTracker® and the mitochondrial morphology was analyzed using a fluorescence microscope. Bar, 4 μm. The number of mitochondria was assessed by counting at least 300 cells from three independent experiments. Western blot analysis shows relative level of HuD and β-actin. c, d Mitochondrial morphology of βTC6-shHuD cells and βTC6 cells after HuD overexpression (pHuD) was analyzed with a TEM. Bar, 0.5 μm. Images are representative and the values indicate the mean ± SEM from three independent analyses. *p < 0.05; **p < 0.01; ***p < 0.001
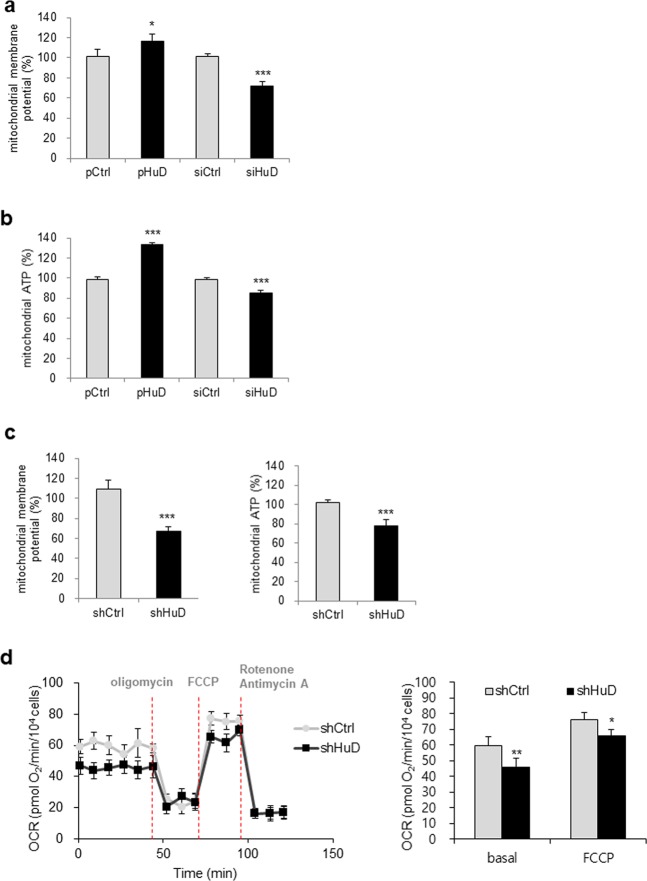
It has been suggested that dynamic changes in mitochondrial morphology are essential for their functions, including cellular respiration and ATP generation. An imbalance of mitochondria dynamics is usually linked to mitochondrial dysfunction and leads to pathogenesis of several diseases such as neurodegenerative diseases and diabetes [2–4, 46]. To address whether HuD affects to the function of mitochondria, we assessed mitochondrial activity by measuring mitochondrial membrane potential after HuD overexpression or knockdown in pancreatic β-cells. HuD overexpression increased, while HuD knockdown decreased mitochondrial membrane potential based on JC-1 staining (Fig. 3a). In addition, the level of mitochondrial ATP was positively regulated by HuD (Fig. 3b). These observations indicate that HuD expression is linked to mitochondrial function. Consistent with these results, a reduction in mitochondrial activity was confirmed in shHuD cells, which indicated that the loss of HuD impaired mitochondrial function (Fig. 3c). In addition, we investigated the impact of HuD on cellular respiration by assessing oxygen consumption rate (OCR) between shHuD cells and shCtrl cells using the Seahorse FX24 Extracellular Flux Analyzer. Basal OCR and maximal OCR assessed by FCCP treatment in shHuD cells were lower than those in shCtrl cell (Fig. 3d), indicating that HuD downregulation impaired basal and maximal rates of mitochondrial respiration. However, no significant change on electron flow through the ETC or nonmitochondrial respiration of both cells was observed. Taken together, these results suggest that HuD plays an essential role in the regulation of mitochondrial morphology and function in pancreatic β-cells.
Fig. 3.
Regulation of mitochondrial activity by HuD. a, b After transfection of βTC6 cells with HuD siRNA or pHuD with appropriate controls, mitochondrial membrane potential (a) and mitochondrial ATP levels (b) were analyzed. Cells were stained with tetraethyl benzimidazoly carbocyanine iodide (JC-1) and the fluorescence was measured at 530 nm (excitation)/590 nm (emission). Cells pretreated with galactose-containing media were incubated with the ATP detection reagent and the luminescence was measured using a microplate reader. c Mitochondrial membrane potential and ATP level were determined in βTC6-shHuD and βTC6-shCtrl cells. d Mitochondrial respiration in βTC6-shCtrl and βTC6-shHuD cells was determined by assessing the oxygen consumption rate. Cells were sequentially incubated with oligomycin, FCCP, rotenone, and antimycin A and OCR was measured using the Seahorse FX24 Extracellular Flux Analyzer. Data represent the mean ± SEM derived from three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001
HuD binds to Mfn2 mRNA and regulates its expression
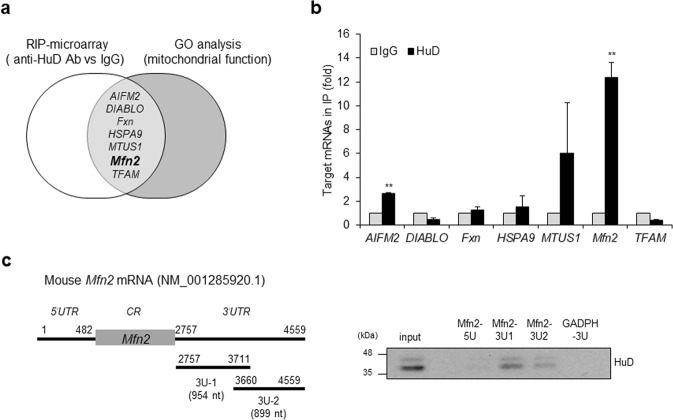
To determine the target mRNAs of HuD involved in the regulation of mitochondrial morphology and function, HuD-associated mRNAs were isolated and identified by HuD-RIP assay followed by microarray. The mRNAs enriched more than fivefold in HuD IP shown in Supplementary Table 1 were further screened by gene ontology-based analysis of genes related to mitochondrial function. This survey identified seven mRNAs including apoptosis inducing factor mitochondria associated 2 (AIFM2), diablo IAP-binding mitochondrial protein (DIABLO), frataxin (Fxn), heat shock 70 KDa Protein 9 (HSPA9, mortalin), mitochondrial tumor suppressor gene 1 (MTUS1), Mfn2, and mitochondrial transcription factor A (TFAM) as putative targets of HuD (Fig. 4a). The association between HuD and these mRNAs was validated by RIP analysis followed by RT-qPCR, and the results showed that Mfn2 mRNA was significantly enriched in HuD-IP compared with normal IgG (Fig. 4b). It suggests that HuD binds to Mfn2 mRNA. HuD is known to regulate mRNA turnover and translation by binding to the 3′UTR of target mRNAs [42, 45, 49, 50]. Therefore, we further assessed the interaction between HuD and Mfn2 mRNA via in vitro pull-down assay using biotin-labeled transcripts corresponding to the 5′UTR (Mfn2 5U) and 3′UTR of Mfn2 mRNA (Mfn2 3U-1 and 3U-2) and streptavidin beads. As shown in Fig. 4c, HuD bound to both transcripts of Mfn2 3U-1 and 3U-2. These results indicate that Mfn2 mRNA is the target of HuD.
Fig. 4.
Identification of target mRNAs of HuD. a Putative targets of HuD identified by both HuD-RIP analysis and GO term analysis (mitochondrial function). b The interaction between HuD and target mRNAs was confirmed by RIP-qPCR using anti-HuD and control IgG antibodies. Gapdh mRNA was used for normalization. c Left: A schematic of mouse Mfn2 mRNA (NM_001285920). The 5′UTR (5U) and 3′UTR of Mfn2 mRNAs (3U-1 and 3U-2) were transcribed in vitro and biotinylated for use in biotin pull-down analysis. Right: The biotinylated transcripts obtained from Mfn2 mRNA were incubated with βTC6 cell lysates. The interaction between the transcripts and HuD was shown by western blot. Biotinylated Gapdh 3′UTR transcript was used as a negative control. Data represent the mean ± SEM derived from three independent experiments. **p < 0.01
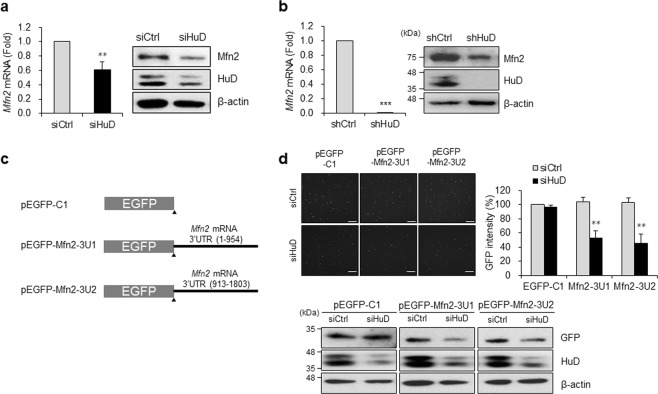
HuD is downregulated in the pancreas of diabetic models [44]. We demonstrate that it binds to Mfn2 mRNA (Fig. 4). To determine whether HuD affects Mfn2 expression, we assessed Mfn2 level using RT-qPCR and western blot analysis after HuD downregulation. Knockdown of HuD using HuD siRNA in βTC6 cells decreased both Mfn2 mRNA and protein level (Fig. 5a), which was further confirmed in shHuD cells (Fig. 5b). In addition, we investigated the HuD-mediated regulation of Mfn2 expression by assessing the EGFP reporter level. Based on the interaction between HuD and the 3′UTR of Mfn2 mRNA (Fig. 4c), we generated EGFP reporters containing the 3′UTR of Mfn2 mRNA (EGFP-Mfn2-3U1 and 3U2) (Fig. 5c) and analyzed EGFP expression after transfection of HuD siRNA. HuD knockdown decreased the EGFP expressions of both Mfn2-3U1 and 3U2 reporters (Fig. 5d), indicating that HuD downregulation decreases Mfn2 expression. Taken together, these results suggest that HuD regulates Mfn2 expression by associating its 3′UTR.
Fig. 5.
Regulation of Mfn2 expression by HuD. Mfn2 mRNA and protein levels were analyzed in βTC6 cells transfected with siHuD (a) or βTC6-shHuD (b) cells by RT-qPCR and western blot analysis, respectively. c Schematic representation of EGFP reporter constructs containing Mfn2 mRNA 3′UTRs (pEGFP-Mfn2-3U1 and -3U2). d Following sequential transfection with a reporter plasmid after HuD knockdown in βTC6 cells, the relative EGFP expression was determined by measuring the fluorescence of EGFP and western blot analysis. Data represent the mean ± SEM from three independent experiments. **p < 0.01; ***p < 0.001
The HuD/Mfn2 axis plays an essential role in the regulation of mitochondrial morphology
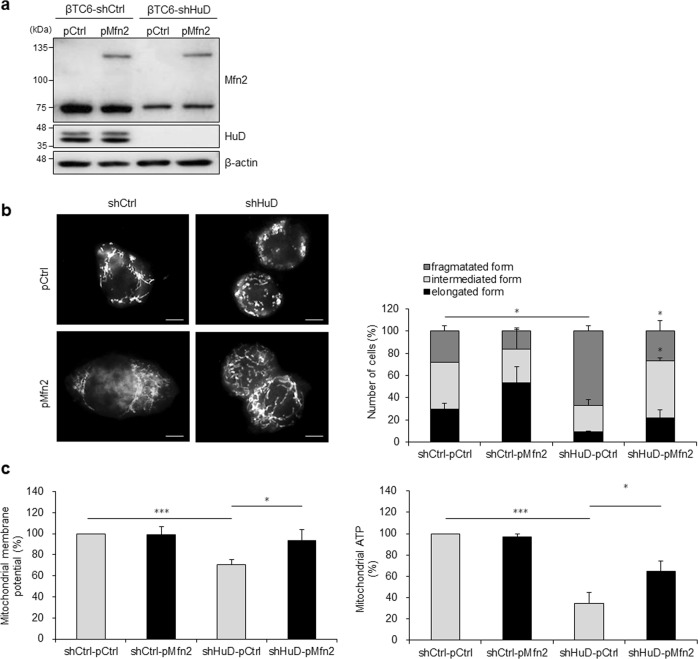
Mfn2 regulates mitochondrial function by controlling mitochondrial elongation [16, 51, 52]. Our observations suggest that HuD acts as a novel factor regulating Mfn2 expression at the posttranscriptional level (Figs. 4 and 5). To evaluate whether the HuD/Mfn2 axis play a role in the regulation of mitochondrial morphology and function, we performed a complementation experiment by overexpressing myc-tagged Mfn2 in shHuD cells (Fig. 6a). As shown in Fig. 6b, ectopic expression of Mfn2 increased the portion of cells with elongated mitochondria in shHuD cells (Fig. 6b). In addition, mitochondrial membrane potential and mitochondrial ATP levels were moderately restored by Mfn2 overexpression (Fig. 6c). These results suggest that the HuD/Mfn2 axis regulates mitochondrial morphology and function, and aberrant expression of HuD in pancreatic β-cells results in mitochondrial dysfunction in diabetes.
Fig. 6.
Regulation of mitochondrial dynamics along the HuD/Mfn2 axis. βTC6-shCtrl and βTC6-shHuD cells were transfected with pMfn2 and pCtrl. a Mfn2 and HuD protein levels were analyzed by western blot analysis. b After transfection with pMfn2, cells were incubated with MitoTracker® and mitochondrial morphology was analyzed using a fluorescence microscope. Bar, 5 μm. c After transfection with pMfn2, mitochondrial membrane potential and ATP level were determined by JC-1 staining and mitochondrial ToxGlo™ assay. Data represent the mean ± SEM derived from three independent experiments. *p < 0.05; ***p < 0.001
Discussion
Tight regulation of mitochondrial structure and activity is critical for cellular homeostasis. Mitochondrial dysfunction is characterized by a reduction in ATP generation, mitochondrial membrane potential, and mitochondrial numbers, and is implicated in the pathology of several human diseases, such as diabetes, neurodegenerative diseases, and cancers. Dynamic regulation of mitochondrial morphology is essential for the normal function of mitochondria and is mediated by several key regulatory proteins including Drp1, Opa1, Fis1, Mff, and Mfn1/2. Herein, we demonstrate that the RNA binding protein HuD plays a novel role in mitochondrial dynamics via posttranscriptional regulation of Mfn2, resulting in mitochondrial dysfunction in pancreatic β-cells. We showed that HuD binds to 3′UTR of Mfn2 mRNA and promotes Mfn2 expression, thereby enhancing mitochondrial elongation. We also demonstrated mitochondrial fragmentation in pancreatic β-cells of diabetic mice, with a lower expression of HuD. Our data reveal that HuD regulates mitochondrial dynamics via posttranscriptional control of Mfn2 expression.
It is still disputed whether mitochondrial dysfunction is a cause or a consequence of diabetes; however, several studies suggest that abnormal mitochondrial function in pancreatic β-cells plays a decisive role in metabolic dysregulation and hyperglycemia [47, 48, 53, 54]. Alteration of mitochondrial morphology in pancreatic β-cells, in particular, fragmentation of mitochondria has been reported in diabetic pancreas [11, 46, 55–57]. Herein, we also report enhanced mitochondrial fission, matrix swelling, and disruption of cristae in pancreatic β-cells of diabetic mice (Fig. 1a). A few key proteins regulating mitochondrial fission and fusion, including Fis1 and Opa1 have been linked to β-cell dysfunction by affecting mitochondrial dynamics [9, 58, 59]. We propose that HuD-mediated Mfn2 regulation also mediates β-cell dysfunction. Because of the complex and possibly synergistic processes involved, elucidation of the molecular relationship between β-cell dysfunction and mitochondrial dynamics is needed.
Our previous report shows that the HuD level is downregulated in the pancreas of diabetic mice [44]. HuD is an RNA binding protein belonging to Hu antigen family and is expressed in certain types of tissues including neurons and pancreatic β-cells [42]. Various target mRNAs of HuD have been identified, while the regulatory mechanism is poorly understood. Posttranslational modifications including phosphorylation by protein kinase C and methylation by coactivator-associated arginine methyltransferase 1 regulate HuD activity [60, 61]. In addition, miRNA-375, one of the enriched miRNAs in the pancreas, acts as a negative regulator of HuD [41]. The miR-375 KO mice show impaired glucose homeostasis by regulating β-cell mass [62]. Therefore, it is worth exploring whether mitochondrial dysfunction occurred in miR-375 KO mice. Further studies exploring the regulation of HuD expression in diabetes may provide the molecular link underlying HuD-mediated β-cell dysfunction.
RIP analysis and in vitro pull-down assay using biotin-labeled transcripts reveal the interaction between HuD and 3′UTR of Mfn2 mRNA (Fig. 4). Tracing the expression of reporters containing 3′UTRs of Mfn2 mRNA may shed additional light on the interactions (Fig. 5d). Both 3U1 and 3U2 regions of 3′UTRs of Mfn2 mRNA are regulated by HuD. The binding of 3U1 region to HuD is relatively strong, while that of 3U2 is moderate, but consistent. Although we could not determine the exact HuD-binding motifs on 3′UTRs of Mfn2 mRNA, 3U1 appears to be subject to dominant regulation by HuD. Crosslinking immunoprecipitation and RNA sequencing analysis in pancreatic β-cells may provide additional information about the sequence responsible for HuD binding.
In conclusion, we propose that HuD is a novel regulatory protein affecting mitochondrial dynamics by enhancing Mfn2 expression. Our results suggest that downregulation of HuD in diabetes may contribute to impaired mitochondrial shape and function. Further studies investigating the differential expression of HuD in pancreatic β-cells and the underlying molecular mechanisms may provide additional insight into the role of HuD-mediated mitochondrial dynamics in the pathogenesis of diabetes.
Materials and methods
Cell culture, transfection of plasmids, and small interfering RNAs
Mouse pancreatic β-cell line, βTC6 cells were cultured in Dulbecco’s Modified Eagle’s Medium (Capricorn scientific, Ebsdorfergrund, Germany) supplemented with 10% fetal bovine serum and 1% antibiotics. βTC6 cell clones stably expressing shHuD plasmid (βTC6-shHuD) was established and maintained with puromycin (Invitrogen, Carlsbad, CA, USA). HuD overexpression plasmid (pHuD) was prepared by cloning CDS of mouse HuD mRNA into a retroviral vector pPGS-HA and viral particles were prepared using HEK293 cells. Enhanced green fluorescent protein (EGFP) reporters were cloned by inserting the 3′UTR of Mfn2 mRNA (2757–4559, 1803 nt) into pEGFP-C1 vector (BD Bioscience, Franklin lake, NJ, USA). Plasmids (pEGFP, pHuD, pMfn2 (Addgene, #23213)) and small interfering RNAs (siRNAs; control siRNA (siCtrl) and HuD siRNA (siHuD)); Genolution Pharmaceuticals, Seoul, Korea) were transiently transfected using LipofectamineTM 2000 (Invitrogen).
Western blot analysis
Whole cell lysates were prepared using RIPA buffer (10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 1 mM EDTA, and 0.1% SDS) containing a 1× protease inhibitor cocktail (Roche, Basel, Switzerland), separated by SDS polyacrylamide gel electrophoresis, and transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). The membranes were incubated with primary antibodies against HuD (Santa Cruz Biotechnology, Dallas, TX, USA), Mfn2 (Abcam, Cambridge, USA), EGFP (Santa Cruz Biotechnology), HA (Biolegend, San Diego, CA, USA), and β-actin (Abcam), and further incubated with the appropriate secondary antibodies conjugated with horseradish peroxidase (Santa Cruz Biotechnology). Protein bands were detected using enhanced chemiluminescence (Bio-Rad, Hercules, CA, USA).
RNA analysis
Total RNA was isolated from whole cells using RNAiso plus Reagent (Takara Bio Inc, Shiga, Japan). After reverse transcription (RT) using random hexamers and reverse transcriptase (Toyobo, Osaka, Japan), the mRNA levels were quantified by real time quantitative PCR analysis using SensiFAST™ SYBR® Hi-ROX Kit (Bioline, London, UK) and gene-specific primer sets (Supplementary Table 2). RT-qPCR analysis was performed using StepOnePlus™ (Applied Biosystems, Foster City, CA, USA).
Ribonucleoprotein complex-immunoprecipitation and qPCR (RIP-qPCR) analysis
RNP complexes were immunoprecipitated using Protein A bead (Invitrogen) coated with anti-HuD or control IgG antibodies (Santa Cruz Biotechnology) at 4 °C overnight [43]. The immunoprecipitated RNP complexes were incubated with DNase I and proteinase K. RNAs were isolated from the complex and further analyzed by RT-qPCR using the primers listed (Supplementary Table 2).
Biotin pull down assay
To synthesize biotinylated transcripts, PCR fragments were prepared using forward primers, including T7 RNA polymerase promoter sequence (T7, 5′-CCAAGCTTCTAATACGACTCACTATAGG-GAGA-3′). Primers used to prepare biotinylated transcripts Mfn2 mRNA (NM_001285920.1) are listed in Supplementary Table 2. After purification of the PCR products, biotinylated transcripts were synthesized using the MaxiScript T7 kit (Ambion, Waltham, MA, USA) and biotin-CTP (Enzo Life Sciences, Farmingdale, NY, USA). Whole-cell lysates (300 μg per sample) were incubated with 1 μg of purified biotinylated transcripts for 30 min at room temperature, and the complexes were isolated using streptavidin-coupled Dynabeads (Invitrogen). Proteins were isolated from the complex and further studied by western blot analysis [43].
Morphological analysis of mitochondria
βTC6 cells were incubated with 100 nM Mitotracker Red CMXRos (Invitrogen) for 30 min at 37 °C. Mitochondria morphology of either βTC6 cells treated with Mitotracker or mt-YFP expressing βTC6 cells was observed using a fluorescence microscope. The fluorescent images were acquired using an Axiovertcam mRM camera attached to an Axiovert 200M (Carl Zeiss, Jena, Germany).
For electron microscopy, tissues or cells were fixed with 1% glutaraldehyde and embedded using Epon 812. Ultrathin sections were observed with a transmission electron microscope JEM 1010 (JEOL, Tokyo, Japan).
Measurement of the mitochondrial membrane potential and mitochondrial ATP level
Mitochondrial membrane potential was measured using the tetraethyl benzimidazoly carbocyanine iodide (JC-1) staining solution (Abcam) according to the manufacturer’s protocol. Cells were incubated with the reagent for 15 min and the fluorescence was measured at 535 nm (excitation)/590 nm (emission) using the Synergy™ H1 multimode microplate reader (BioTek, VT, USA). Mitochondrial ATP level was also measured using the Mitochondrial Toxglo™ assay kit (Promega, Madison, WI, USA). After incubating cells with galactose-containing media, cells were incubated with the ATP detection reagent at 37 °C for 90 min and the luminescence was assessed with the Synergy™ H1 hybrid microplate reader (BioTek, VT, USA).
Analysis of oxygen consumption rate
OCR between βTC6-shCtrl and βTC6-shHuD cells was determined using the Seahorse FX24 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA, USA) with an XF Cell Mito Stress Test Kit (Seahorse Bioscience) according to the manufacturer’s instruction. An ATP synthase blocker, oligomycin (3 μM) and the mitochondrial uncoupler, FCCP (carbonyl cyanide 4‐[trifluoromethoxy] phenylhydrazone, 1 μM) were used to measure the proton leakage and the maximal OCR, respectively. An inhibitor of complex I, rotenone (1 μM) and a complex III blocker, antimycin A (1 μM) were used to determine the nonmitochondrial respiration. The results were analyzed using a Seahorse XF Prep Station [63].
Statistical analysis
Data were expressed as mean ± SEM of three independent experiments. The statistical significances of the data were analyzed by Student’s t test; *p < 0.05; **p < 0.01; ***p < 0.001.
Supplementary information
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (2017R1A2B2009381, 2017R1A2B2005508, and 2019M3E5D5066526).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by D. Rubinsztein
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41418-019-0447-x) contains supplementary material, which is available to authorized users.
References
- 1.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–60. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 2.Johannsen DL, Ravussin E. The role of mitochondria in health and disease. Curr Opin Pharmacol. 2009;9:780–6. doi: 10.1016/j.coph.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duchen MR, Szabadkai G. Roles of mitochondria in human disease. Essays Biochem. 2010;47:115–37. doi: 10.1042/bse0470115. [DOI] [PubMed] [Google Scholar]
- 4.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–5. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy M, Reddy PH, Iijima M, Sesaki H. Mitochondrial division and fusion in metabolism. Curr Opin Cell Biol. 2015;33:111–8. doi: 10.1016/j.ceb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tilokani L, Nagashima S, Paupe V, Prudent J. Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem. 2018;62:341–60. doi: 10.1042/EBC20170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Putti R, Sica R, Migliaccio V, Lionetti L. Diet impact on mitochondrial bioenergetics and dynamics. Front Physiol. 2015;6:109. doi: 10.3389/fphys.2015.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect. 2015;4:R1–R15. doi: 10.1530/EC-14-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baltrusch S. Mitochondrial network regulation and its potential interference with inflammatory signals in pancreatic beta cells. Diabetologia. 2016;59:683–7. doi: 10.1007/s00125-016-3891-x. [DOI] [PubMed] [Google Scholar]
- 10.Holmstrom MH, Iglesias-Gutierrez E, Zierath JR, Garcia-Roves PM. Tissue-specific control of mitochondrial respiration in obesity-related insulin resistance and diabetes. Am J Physiol Endocrinol Metab. 2012;302:E731–9. doi: 10.1152/ajpendo.00159.2011. [DOI] [PubMed] [Google Scholar]
- 11.Anello M, Lupi R, Spampinato D, Piro S, Masini M, Boggi U, et al. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia. 2005;48:282–9. doi: 10.1007/s00125-004-1627-9. [DOI] [PubMed] [Google Scholar]
- 12.Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278:17190–7. doi: 10.1074/jbc.M212754200. [DOI] [PubMed] [Google Scholar]
- 13.Jheng HF, Tsai PJ, Guo SM, Kuo LH, Chang CS, Su IJ, et al. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol. 2012;32:309–19. doi: 10.1128/MCB.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrepfer E, Scorrano L. Mitofusins, from mitochondria to metabolism. Mol Cell. 2016;61:683–94. doi: 10.1016/j.molcel.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 15.Bach D, Naon D, Pich S, Soriano FX, Vega N, Rieusset J, et al. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes. 2005;54:2685–93. doi: 10.2337/diabetes.54.9.2685. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–92. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 17.Pich S, Bach D, Briones P, Liesa M, Camps M, Testar X, et al. The Charcot–Marie–Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum Mol Genet. 2005;14:1405–15. doi: 10.1093/hmg/ddi149. [DOI] [PubMed] [Google Scholar]
- 18.Sebastian D, Hernandez-Alvarez MI, Segales J, Sorianello E, Munoz JP, Sala D, et al. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci USA. 2012;109:5523–8. doi: 10.1073/pnas.1108220109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nie Q, Wang C, Song G, Ma H, Kong D, Zhang X, et al. Mitofusin 2 deficiency leads to oxidative stress that contributes to insulin resistance in rat skeletal muscle cells. Mol Biol Rep. 2014;41:6975–83. doi: 10.1007/s11033-014-3584-9. [DOI] [PubMed] [Google Scholar]
- 20.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–10. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 21.Liesa M, Borda-d’Agua B, Medina-Gomez G, Lelliott CJ, Paz JC, Rojo M, et al. Mitochondrial fusion is increased by the nuclear coactivator PGC-1beta. PLoS One. 2008;3:e3613. doi: 10.1371/journal.pone.0003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zorzano A. Regulation of mitofusin-2 expression in skeletal muscle. Appl Physiol Nutr Metab. 2009;34:433–9. doi: 10.1139/H09-049. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Liu X, Wang H, Zhang W, Chan DC, Shi Y. Lysocardiolipin acyltransferase 1 (ALCAT1) controls mitochondrial DNA fidelity and biogenesis through modulation of MFN2 expression. Proc Natl Acad Sci USA. 2012;109:6975–80. doi: 10.1073/pnas.1120043109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Z, Li SJ, Zhao SX, Fa XN. Upregulated miR-17 regulates hypoxia-mediated human pulmonary artery smooth muscle cell proliferation and apoptosis by targeting mitofusin 2. Med Sci Monit. 2016;22:3301–8. doi: 10.12659/MSM.900487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang S, Chen Z, Wu W, Wang M, Wang R, Cui J, et al. MicroRNA-31 promotes arterial smooth muscle cell proliferation and migration by targeting mitofusin-2 in arteriosclerosis obliterans of the lower extremitie. Exp Ther Med. 2018;15:633–40. doi: 10.3892/etm.2017.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Yang L, Gao YF, Fan ZM, Cai XY, Liu MY, et al. MicroRNA-106b induces mitochondrial dysfunction and insulin resistance in C2C12 myotubes by targeting mitofusin-2. Mol Cell Endocrinol. 2013;381:230–40. doi: 10.1016/j.mce.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, He W, Gao YF, Fan ZM, Gao CL, Xia ZK. MicroRNA-106b regulates skeletal muscle insulin sensitivity and glucose homeostasis by targeting mitofusion-2. Mol Med Rep. 2017;16:6858–63. doi: 10.3892/mmr.2017.7439. [DOI] [PubMed] [Google Scholar]
- 28.Pan L, Zhou L, Yin W, Bai J, Liu R. miR-125a induces apoptosis, metabolism disorder and migrationimpairment in pancreatic cancer cells by targeting Mfn2-related mitochondrial fission. Int J Oncol. 2018;53:124–36. doi: 10.3892/ijo.2018.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang R, Zhou HM, Jiang L, Mao YR, Cui XM, Xie B, et al. MiR-195 dependent roles of mitofusin2 in the mitochondrial dysfunction of hippocampal neurons in SAMP8 mice. Brain Res. 2016;1652:135–43. doi: 10.1016/j.brainres.2016.09.047. [DOI] [PubMed] [Google Scholar]
- 30.Zhang R, Garrett Q, Zhou HM, Wu XX, Mao YR, Cui XM, et al. Upregulation of miR-195 accelerates oxidative stress-induced retinal endothelial cell injury by targeting mitofusin 2 in diabetic rats. Mol Cell Endocrinol. 2017;452:33–43. doi: 10.1016/j.mce.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Sun M, Yu HY, Zhang YY, Li ZJ, Gao W. MicroRNA-214 mediates isoproterenol-induced proliferation and collagen synthesis in cardiac fibroblasts. Sci Rep. 2015;5:18351. [DOI] [PMC free article] [PubMed]
- 32.Wu J, Li J, Chen WK, Liu S, Liu JH, Zhang JS, et al. MicroRNA-214 affects fibroblast differentiation of adipose-derived mesenchymal stem cells by targeting mitofusin-2 during pelvic floor dysfunction in SD rats with birth trauma. Cell Physiol Biochem. 2017;42:1870–87. doi: 10.1159/000479570. [DOI] [PubMed] [Google Scholar]
- 33.Bucha S, Mukhopadhyay D, Bhattacharyya NP. Regulation of mitochondrial morphology and cell cycle by microRNA-214 targeting Mitofusin2. Biochem Bioph Res Commun. 2015;465:797–802. doi: 10.1016/j.bbrc.2015.08.090. [DOI] [PubMed] [Google Scholar]
- 34.Qin L, Yang W, Wang YX, Wang ZJ, Li CC, Li M, et al. MicroRNA-497 promotes proliferation and inhibits apoptosis of cardiomyocytes through the downregulation of Mfn2 in a mouse model of myocardial ischemia-reperfusion injury. Biomed Pharmacother. 2018;105:103–14. doi: 10.1016/j.biopha.2018.04.181. [DOI] [PubMed] [Google Scholar]
- 35.Zhou X, Zhang L, Zheng B, Yan Y, Zhang Y, Xie H, et al. MicroRNA-761 is upregulated in hepatocellular carcinoma and regulates tumorigenesis by targeting Mitofusin-2. Cancer Sci. 2016;107:424–32. doi: 10.1111/cas.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lv L, Li T, Li X, Xu C, Liu Q, Jiang H, et al. The lncRNA Plscr4 controls cardiac hypertrophy by regulating miR-214. Mol Ther Nucleic Acids. 2018;10:387–97. doi: 10.1016/j.omtn.2017.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leboucher GP, Tsai YC, Yang M, Shaw KC, Zhou M, Veenstra TD, et al. Stress-induced phosphorylation and proteasomal degradation of mitofusin 2 facilitates mitochondrial fragmentation and apoptosis. Mol Cell. 2012;47:547–57. doi: 10.1016/j.molcel.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar S, Pan CC, Shah N, Wheeler SE, Hoyt KR, Hempel N, et al. Activation of mitofusin2 by Smad2-RIN1 complex during mitochondrial fusion. Mol Cell. 2016;62:520–31. doi: 10.1016/j.molcel.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinman MN, Lou H. Diverse molecular functions of Hu proteins. Cell Mol Life Sci. 2008;65:3168–81. doi: 10.1007/s00018-008-8252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ratti A, Fallini C, Colombrita C, Pascale A, Laforenza U, Quattrone A, et al. Post-transcriptional regulation of neuro-oncological ventral antigen 1 by the neuronal RNA-binding proteins ELAV. J Biol Chem. 2008;283:7531–41. doi: 10.1074/jbc.M706082200. [DOI] [PubMed] [Google Scholar]
- 41.Abdelmohsen K, Hutchison ER, Lee EK, Kuwano Y, Kim MM, Masuda K, et al. miR-375 inhibits differentiation of neurites by lowering HuD levels. Mol Cell Biol. 2010;30:4197–210. doi: 10.1128/MCB.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee EK, Kim W, Tominaga K, Martindale JL, Yang X, Subaran SS, et al. RNA-binding protein HuD controls insulin translation. Mol Cell. 2012;45:826–35. doi: 10.1016/j.molcel.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim C, Kim W, Lee H, Ji E, Choe YJ, Martindale JL, et al. The RNA-binding protein HuD regulates autophagosome formation in pancreatic beta cells by promoting autophagy-related gene 5 expression. J Biol Chem. 2014;289:112–21. doi: 10.1074/jbc.M113.474700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim C, Lee H, Kang H, Shin JJ, Tak H, Kim W, et al. RNA-binding protein HuD reduces triglyceride production in pancreatic beta cells by enhancing the expression of insulin-induced gene 1. Biochim Biophys Acta. 2016;1859:675–85. doi: 10.1016/j.bbagrm.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim C, Jeong DE, Heo S, Ji E, Rho JG, Jung M, et al. Reduced expression of the RNA-binding protein HuD in pancreatic neuroendocrine tumors correlates with low p27(Kip1) levels and poor prognosis. J Pathol. 2018;246:231–43. doi: 10.1002/path.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu H, Koshkin V, Allister EM, Gyulkhandanyan AV, Wheeler MB. Molecular and metabolic evidence for mitochondrial defects associated with beta-cell dysfunction in a mouse model of type 2 diabetes. Diabetes. 2010;59:448–59. doi: 10.2337/db09-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mulder H, Ling C. Mitochondrial dysfunction in pancreatic beta-cells in Type 2 diabetes. Mol Cell Endocrinol. 2009;297:34–40. doi: 10.1016/j.mce.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 48.Supale S, Li N, Brun T, Maechler P. Mitochondrial dysfunction in pancreatic beta cells. Trends Endocrin Metab. 2012;23:477–87. doi: 10.1016/j.tem.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Allen M, Bird C, Feng W, Liu G, Li W, Perrone-Bizzozero NI, et al. HuD promotes BDNF expression in brain neurons via selective stabilization of the BDNF long 3′UTR mRNA. PLoS One. 2013;8:e55718. doi: 10.1371/journal.pone.0055718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang MJ, Abdelmohsen K, Hutchison ER, Mitchell SJ, Grammatikakis I, Guo R, et al. HuD regulates coding and noncoding RNA to induce APP–>Abeta processing. Cell Rep. 2014;7:1401–9. doi: 10.1016/j.celrep.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franco A, Kitsis RN, Fleischer JA, Gavathiotis E, Kornfeld OS, Gong G, et al. Correcting mitochondrial fusion by manipulating mitofusin conformations. Nature. 2016;540:74–9. doi: 10.1038/nature20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wollheim CB, Maechler P. beta-cell mitochondria and insulin secretion—messenger role of nucleotides and metabolites. Diabetes. 2002;51:S37–42. doi: 10.2337/diabetes.51.2007.s37. [DOI] [PubMed] [Google Scholar]
- 54.Mulder H. Transcribing beta-cell mitochondria in health and disease. Mol Metab. 2017;6:1040–51. doi: 10.1016/j.molmet.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhansali S, Bhansali A, Walia R, Saikia UN, Dhawan V. Alterations in mitochondrial oxidative stress and mitophagy in subjects with prediabetes and Type 2 diabetes mellitus. Front Endocrinol. 2017;8:347. doi: 10.3389/fendo.2017.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanter M, Akpolat M, Aktas C. Protective effects of the volatile oil of Nigella sativa seeds on beta-cell damage in streptozotocin-induced diabetic rats: a light and electron microscopic study. J Mol Histol. 2009;40:379–85. doi: 10.1007/s10735-009-9251-0. [DOI] [PubMed] [Google Scholar]
- 57.Barbosa de Queiroz K, Honorato-Sampaio K, Rossoni Junior JV, Andrade Leal D, Pinto AB, Kappes-Becker L, et al. Physical activity prevents alterations in mitochondrial ultrastructure and glucometabolic parameters in a high-sugar diet model. PLoS One. 2017;12:e0172103. doi: 10.1371/journal.pone.0172103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Z, Wakabayashi N, Wakabayashi J, Tamura Y, Song WJ, Sereda S, et al. The dynamin-related GTPase Opa1 is required for glucose-stimulated ATP production in pancreatic beta cells. Mol Biol Cell. 2011;22:2235–45. [Google Scholar]
- 59.Schultz J, Waterstradt R, Kantowski T, Rickmann A, Reinhardt F, Sharoyko V, et al. Precise expression of Fis1 is important for glucose responsiveness of beta cells. J Endocrinol. 2016;230:81–91. doi: 10.1530/JOE-16-0111. [DOI] [PubMed] [Google Scholar]
- 60.Pascale A, Amadio M, Scapagnini G, Lanni C, Racchi M, Provenzani A, et al. Neuronal ELAV proteins enhance mRNA stability by a PKCalpha-dependent pathway. Proc Natl Acad Sci USA. 2005;102:12065–70. doi: 10.1073/pnas.0504702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fujiwara T, Mori Y, Chu DL, Koyama Y, Miyata S, Tanaka H, et al. CARM1 regulates proliferation of PC12 cells by methylating HuD. Mol Cell Biol. 2006;26:2273–85. doi: 10.1128/MCB.26.6.2273-2285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poy MN, Hausser J, Trajkovski M, Braun M, Collins S, Rorsman P, et al. miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc Natl Acad Sci USA. 2009;106:5813–8. doi: 10.1073/pnas.0810550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today. 2008;13:268–74. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.