Abstract
Defective rhodopsin homeostasis is one of the major causes of retinal degeneration, including the disease Retinitis pigmentosa. To identify cellular factors required for the biosynthesis of rhodopsin, we performed a genome-wide genetic screen in Drosophila for mutants with reduced levels of rhodopsin. We isolated loss-of-function alleles in endoplasmic reticulum membrane protein complex 3 (emc3), emc5, and emc6, each of which exhibited defective phototransduction and photoreceptor cell degeneration. EMC3, EMC5, and EMC6 were essential for rhodopsin synthesis independent of the ER associated degradation (ERAD) pathway, which eliminates misfolded proteins. We generated null mutations for all EMC subunits, and further demonstrated that different EMC subunits play roles in different cellular functions. Conditional knockout of the Emc3 gene in mice led to mislocalization of rhodopsin protein and death of cone and rod photoreceptor cells. These data indicate conserved roles for EMC subunits in maintaining rhodopsin homeostasis and photoreceptor function, and suggest that retinal degeneration may also be caused by defects in early biosynthesis of rhodopsin.
Subject terms: Chaperones, Genetic interaction
Introduction
Retinitis pigmentosa (RP) causes the gradual degeneration of rod and cone photoreceptors and is the most common form of hereditary retinal diseases [1, 2]. Mutations in the light receptor gene rhodopsin account for approximately 25% of all ADRP cases. Over 120 point mutations in the rhodopsin gene have been associated with ADRP, with most disrupting its trafficking from the endoplasmic reticulum (ER) to the plasma membrane [3]. Proper folding and targeting of rhodopsin is crucial for maintaining the structure, function, and homeostasis of photoreceptors [1].
Studying photoreceptor cells in Drosophila has provided insights into the folding and transport of rhodopsin protein [4, 5]. In flies, folding of rhodopsin is tightly regulated by multiple ER chaperons, including NINAA, calnexin, and Xport [6–8]. Without these chaperons the rhodopsin protein fails to properly mature, leading to retinal degeneration, as seen with mutations in rhodopsin itself [7, 9]. RanBP2 is a cyclophilin-related protein that acts as a chaperone for cone opsins, but many other chaperons required for rhodopsin folding are not well conserved in mammals [1, 10]. In fact, no mammalian homologs have been identified for any Drosophila gene involved in rhodopsin biogenesis in the ER that cause retinal degeneration when mutated.
The ER membrane protein complex (EMC) was first identified as a multi-protein transmembrane complex in a genetic screen for the accumulation of misfolded membrane proteins in yeast. Based on a high-content proteomics strategy, this complex was then found to interact with the ER-associated degradation (ERAD) pathway, suggesting a role in the biosynthesis of transmembrane proteins [11, 12]. Recently, it was shown that EMC functions as a transmembrane domain insertase, acting during the translation process to enable biogenesis of its client transmembrane proteins [13, 14]. EMC functions in multiple cellular processes, including autophagy, lipid transfer, viral infection, and lung development [15–22]. Mutation of the zebrafish emc3 gene, partial optokinetic response b (pob), causes degeneration of long-wavelength photoreceptor cells, and Drosophila EMC3 is required for the stable production of rhodopsins [23, 24]. Moreover, a point mutation in the human EMC1 gene has been associated with retinal dystrophy [25]. However, it has not yet been determined which steps in rhodopsin production require EMC components, and whether all EMC subunits are required for maintaining rhodopsin homeostasis and/or photoreceptor cell integrity.
In the present study, we conducted an ethyl methanesulfonate (EMS)-based genetic screen to isolate mutations that affect rhodopsin homeostasis. We identified mutations in 3 EMC subunits, emc3, emc5, and emc6 that each reduced levels of the major rhodopsin Rh1, disrupted phototransduction, and caused gradual photoreceptor degeneration. We found that EMC function was independent of ERAD, most likely regulating rhodopsin levels at an earlier step in the biosynthetic process. Furthermore, knocking out the Emc3 gene in mammalian rod photoreceptor cells led to dysregulated rhodopsin trafficking and retinal degeneration, as seen in flies, suggesting that EMC function is conserved in mammals. Our study highlights the different roles played by EMC proteins in rhodopsin biogenesis.
Materials and methods
EMS mutagenesis
The second chromosome of ey-flp ninaE-Rh1-GFP;FTR40A flies and the third chromosome of ey-flp ninaE-Rh1-GFP;FTR82B flies were isogenized, and young male flies were fed 25 mM ethyl methanesulfonate (EMS) (Sigma, St. Louis, MO) in 2% sucrose for 8 h. Mutagenized flies were mated immediately to ey-flp ninaE-Rh1-GFP;GMR-hid CL FRT40A/Cyo hs-hid or ey-flp ninaE-Rh1-GFP; FRT82B GMR-hid CL/TM3 hs-hid flies, and F1 progenies were screened for loss of GFP by performing the fluorescence deep pseudopupil (DPP) assays 1 day following eclosion [26]. Approximately 10,000 F1 flies of each genotype were screened. The emc3G7, emc3G10, and emc5G2 mutants were isolated by screening ey-flp ninaE-Rh1-GFP;FTR40A flies (looking for changes in Rh1 fluorescence); emc6N10 mutant flies were isolated by screening ey-flp ninaE-Rh1-GFP;FTR82B flies.
Fly stocks
CG3678G4215 (emc2B), CG32441MB09773 (emc10), ninaA2, ninaEP332, M(vas-int.Dm)ZH-2A;M(3xP3-RFP.attP)ZH-86Fb, M(vas-int.Dm)ZH-2A;M(3xP3-RFP.attP) ZH-51C, GMR-Gal4 and dmppee02905 lines were obtained from the Bloomington Stock Center. The emc4RNAi (P{TRiP.HMC06642}attP40) flies were obtained from the TsingHua Fly Center. The emc2ARNAi and emc5RNAi flies were generated using short-hairpin (sh) RNA system. The emc1, emc2A, emc4, emc7, emc8/9, and hrd1 knockout lines were generated using Crispr/Cas9 technology. The ey-flp ninaE-Rh1-GFP;FRT40A, ey-flp ninaE-Rh1-GFP;FRT42D, ey-flp ninaE-Rh1-GFP;FRT2A, ey-flp ninaE-Rh1-GFP;FTR82B, ey-flp ninaE-Rh1-GFP;GMR-hid CL FRT40A/Cyo hs-hid, ey-flp ninaE-Rh1-GFP;FRT42D GMR-hid CL/Cyo hs-hid, ey-flp ninaE-Rh1-GFP;GMR-hid CL FRT2A/TM3 hs-hid, and ey-flp ninaE-Rh1-GFP; FRT82B GMR-hid CL/TM3 hs-hid lines were maintained in the laboratory of T. Wang. Flies were raised in a 12h-light-12h-dark cycle at 25 °C on standard corn food.
Generation of the emc11, emc2A1, emc41, emc71, emc8/91, and hrd11 mutant flies
The emc1, emc2A, emc4, emc7, emc8/9, and hrd1 mutations were generated using the Cas9/sgRNA system, as described [27]. The recognition sequences of the guide RNAs were designed with tools available at the following website: http://www.flyrnai.org/crispr2/.
(emc1, sgRNA1: ACGCGTTCGTTGGTCGCGCG, sgRNA2: CCACCTACAGACACCCAGCG; emc2A, sgRNA1: GTTCCAAATATTCTCAGAAG, sgRNA2: TAGCGACATATTCACTTAGG; emc4, sgRNA: TCTTAAGAGGTCCCAGGGCA; emc7, sgRNA: GATAACCTCGCAGCTGACAA; emc8/9, sgRNA: CGACTACAAGGTCTCGGAGC; hrd1, sgRNA1: TACGGAGACCTGCTTGGCCT, sgRNA2: CTACGACATGCCCAGTACCT). The (nos-Cas9)attP2 flies were used as the Cas9 source, and F1 progeny were screened by PCR and DNA sequencing to identify the mutations. The primers used for genomic PCR were as follows:
emc1-fwd, 5′-CGGAGTTGGAGCAGAGTC-3′
emc1-rev, 5′-GCGGTGTACAGATACATGCC-3′
emc2A-fwd, 5′-TTAGTGTGACCGTCGCTGG-3′
emc2A-rev, 5′-GTAAGCACATGGGCACGAG-3′
emc4-fwd, 5′-CTGGGCTACAATCCATCTGC-3′
emc4-rev, 5′-CCAAGAAGATACTAGACGACTG-3′
emc7-fwd, 5′-ATCATCGGCTGACAATTGG-3′
emc7-rev, 5′-GGGATATGCCACCTGCATAA-3′
emc8/9-fwd, 5′-GATGTGACCAGACTACTGC-3′
emc8/9-rev, 5′-ATGTATCGGACGCCTGTGAG-3′
hrd1-fwd, 5′-ATGCAGCTGCTCTTATCGTC-3′
hrd1-rev, 5′-CGTCTATTCCGCTGTCGTG-3′
Generation of transgenic flies
The emc3, emc5, and emc6 cDNAs were amplified from the cDNA clones LD37839, RE09053, and RE35789, respectively, which were obtained from the Drosophila Genomic Resource Center. To express emc3 and emc5 under control of the ninaE (ninaE: neither inactivation nor afterpotential E) promoter, the cDNAs were subcloned into the pninaE-attB vector between the NotI and XbaI sites, and the constructs were injected into M(vas-int.Dm)ZH-2A;M(3xP3-RFP.attP)ZH-86Fb embryos. To express emc6 in photoreceptor cells, the cDNA was subcloned into the pninaE-attB vector between the NotI and XbaI sites, followed by injected into M(vas-int.Dm)ZH-2A;M(3xP3-RFP.attP) ZH-51C. The transformants were identified on the basis of eye color, and mini-white marker was eliminated by crossing to a Cre-expressing line.
To generate emc2ARNAi and emc5RNAi flies, the following 21-nt sequences were used to generate shRNA constructs, as described.
emc2AshRNA: 5′-GCAAGATTGCCATCCTTAAGG-3′
emc5shRNA: 5′-GCAGGAATGGAATAGCCTTCC-3′
Annealed oligo pairs were cloned into the VALIUM20 vector at the NheI and EcoRI sites [28]. Constructs were injected into M(vas-int.Dm)ZH-2A;M(3xP3-RFP.attP)ZH-86Fb embryo, and transformants were identified on the basis of eye color.
ERG recordings
ERG recordings were performed as described [29]. Briefly, glass microelectrodes filled with Ringer’s solution were placed on the compound eye and the thorax of a fly. After a brief dark adaptation period, white-eyed flies were exposed to 5 s of orange light, followed by two rounds of 5 s of blue light, and two rounds of 5 s of orange light. The time between stimulations was 5 s. ERG signals were amplified with a Warner electrometer IE-210, and recorded with a MacLab/4 s analog-to-digital converter and the clampelx 10.2 program (Warner Instruments, Hamden, USA).
Antibodies
Primary antibodies used for western blotting are listed below: Tubulin (mouse, 1:2000, Developmental Studies Hybridoma Bank), ß-Actin (mouse, 1:2000, Santa Cruz), Rh1 (mouse, 1:2000, Developmental Studies Hybridoma Bank), TRPL (rabbit, 1:500, Fisher, Waltham, USA) [30], TRP (rabbit, 1:2000) [29], INAD(rat, 1:2000) [29]. The TRP and INAD antibodies were a gift form Dr. C. Montell. Secondary antibodies were bought from LI-COR Biosciences, which included IRDye 680 goat anti-mouse-IgG, IRDye 800 goat anti-rabbit-IgG, and IRDye 800 goat anti-rat-IgG antibodies (1:10000).
Primary antibodies used for staining are listed below: Rh1 (mouse, 1:200, Developmental Studies Hybridoma Bank), Cnx99A (mouse, 1:20, Developmental Studies Hybridoma Bank), GM130 (rabbit, 1:200, #ab30637, Abcam) GFP (rabbit, 1:200, Invitrogen), RFP (rat, 1:200, ChromoTek), EMC3 (rabbit, #ab175537, Abcam), NaK ATPase (mouse, #NA163540, Thermo Scientific), M-Opsin opsin (AB5405, Millipore, MA, USA), Rho 1D4 monoclonal antibody against rhodopsin was a gift from Dr. Robert Molday, University of British Columbia, Canada. Secondary antibodies were bought from Invitrogen (anti mouse, rabbit or rat IgG labeled with Alexa Fluor 488, Alexa Fluor 568, Alexa Fluor 594, Alexa Fluor 647, or Alexa Fluor 488), 1:500 dilution.
Immunostaining
Retina were dissected and fixed in 4% freshly made paraformaldehyde in phosphate buffer for 2 h on ice, then dehydrated in a series of ethanol dilutions (10, 25, 40, 55, 75, 90, 100%), 30 min each. Samples were then treated in acetone solution, and embedded in LR white resin (Sigma, St Louis, MO). Sections (1 μm) were then prepared for immunostaining. Samples were blocked using PBST (0.3% Triton X-100) with 5% goat serum for 1 h, then incubated with primary antibodies at 4 °C overnight. This was followed by incubation with secondary antibodies for 1 h. Samples were examined and images were recorded using a Nikon A1-R confocal microscope, and further processed using Photoshop CC2017 software.
Co-immunoprecipitation
Flies were kept in a 12h-light-12h-dark cycle at 25 °C. Approximately 200 flies were collected 5 days after eclosion. The flies were frozen in liquid nitrogen to dissociate the head from the body. Heads were then sieved out and lysed using lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% NP40, 1 × proteasome inhibitor) at 4 °C overnight. They were then incubated with RFP agarose beads (Chromotek) for 2 h at 4 °C. After several washes, the beads were boiled in SDS loading buffer, and supernatant was loaded onto a gel. Sliver staining (Thermo Fisher Scientific) was performed and bands on the gel were cut out for further mass spectra analysis.
Proteomic analysis of the fly retina
Flies were maintained in a 12 h-light/12h-dark cycle at 25 °C. Sixty female flies of each genotype were collected 5 days after eclosion. For each sample, 30 pairs of retinas were dissected in cold phosphate buffer. Two samples were generated for each genotype. Protein extraction was performed as described with several modifications [31]. Briefly, tissues were boiled and sonicated in lysis buffer (6 M guanidinium hydrochloride, 20 mM tris (2-carboxyethyl) phosphine, 50 mM chloroacetamide, 50 mM Tris, pH 8.5), followed by 20 min 18,000 g centrifugation to collect supernatant. About 50 µg protein for each sample was digested with LysC (Promega Corporation, Madison, USA) in an enzyme/protein ratio of 1:100 (w/w) for 4 h. The sample was further digested with trypsin (Promega Corporation, Madison, USA) in an enzyme/protein ratio of 1:50 (w/w) overnight at 37 °C. Finally, the resulting peptides were acidified with trifluoroacetic acid and desalted by C18 column (3 M, Bracknell, UK) as described [32]. The peptide samples were labeled by TMT 10plex™ Isobaric Label Reagent Set label kit (Thermo, USA) according to the instruction. The mixed peptides from 10 samples (two replicates 50 µg each) were fractionated using a reversed-phase C18 column (3 M, Bracknell, UK) as described with some modifications [33]. Briefly, 8 fractions of peptide were eluted with acetonitrile step gradients (7.5, 10, 12.5, 15, 17.5, 20, 22.5, and 50%, pH 10). Then, the 8 fractions were dried in a vacuum centrifuge and stored at −80 °C until LC-MS/MS analysis.
About 2 µg of each high-pH fractionated peptides samples were separated on an in-house packed 75-μm ID × 50 cm capillary column with 2.5 μm Venusil C18 beads (Agela Technologies, China) using an EASY-nLC 1000 system (Thermo Scientific, Odense, Denmark). The column temperature was maintained at 42 °C using an integrated column oven (PRSO-V1, Sonation GmbH, Biberach, Germany). The flow rate was set to 200 nL/min, and a total 240 min or 320 min gradient from 2 to 27% acetonitrile was used. Raw data was collected on Q Exactive mass spectrometer (Thermo Scientific, Bremen, Germany) using Thermo Xcalibur (2.0) control software with a data-dependent MS/MS scans (TopN = 15). MS1 spectra was measured at a resolution of 70,000 at m/z 200, and full scan target was 3 × 106 with a maximum injection time of 50 ms. Mass range was set to 320–1500. The maximum ion injection time for the MS/MS scan was 100 ms with a resolution of 35,000 at m/z 200, and target value for fragment scans was set at 1 × 105. Isolation width was set at 1.2 m/z and normalized collision energy was set at 32. Each fraction was analyzed in twice.
All raw LC−MS/MS data were submitted to Proteome Discoverer (2.2 version, Thermo Science) for TMT quantitation and database analysis using SequestHT. Data were searched against the Drosophila Melanogaster (Fruit fly) Swiss-Prot database (UP000000803, 21,922 sequences) combined with a common contaminants database (247 entries), using default processing and consensus workflow for MS2 TMT quantification method. The standard searching parameters were used: a 10 ppm MS1 error tolerance was used, with 0.02 Da error used for MS2 product ions on the Q Exactive. Trypsin was set as the enzyme, allowing for 2 missed cleavages. Methionine oxidation was set as a variable modification, and carbamidomethyl on cysteines, TMT6 plex on lysine and peptide N-terminus were set as static modifications for all searches. FDR was set to 0.01 for peptide database search.
Cell transfection
S2 cells were transfected by adding 5 µg of plasmid mixed with 2 µl VigoFect agent (Vigorous Biotechnology) into the cell media. Twenty-four hours following transfection, cells were suspended and adhered to a poly-lysine treated coverslip for 1 h and then fixed for 0.5 h in freshly made 4% paraformaldehyde in phosphate buffer. Samples were blocked using PBST (0.3% Triton X-100) with 5% goat serum for 1 h, then incubated with primary antibodies for 1 h. After washing with phosphate buffer, cells were incubated with secondary antibodies for 1 h. Samples were then imaged using a Nikon A1-R confocal microscope. Images were further processed using Photoshop CC2017 software.
Generation of Emc3 knockout mice
All mouse study protocols were approved by the Animal Care and Use Committee of Sichuan Provincial People’s Hospital. All experimental procedures were carried out in accordance with the approved study protocols and relevant regulations. Mice were raised in a 12h-light-12h-dark cycle. The Emc3 conditional knockout allele was generated using the CRISPR/Cas9 system using gRNA sequence CCTTTGTGAAAACCTGTGTGGCA. A DNA template containing two loxp sites flanking exon 2 of Emc3 was used to guide DNA repair: ccttcctcagaaacctttatttgttcagagcccagtcactctggaagactgccttggatccATAACTTCGTATAATGTATGCTATACGAAGTTATggctgtgaaaacctgtgtggcaagttgaatatgtttcattgatttttagggtttttggaaagtttccacacccagcttttgccaaccaggtcctcccttatgtctgagtgggccttctactttacgcttgctttctttgcgtttcacattttaacatctctttgtcttgtgtttttcagTCAGGTCCTAATTCGAAGCAGAGTCCTCAGGGAAAATGGAAAATACATTCCCAAGCAGgtactcactgatattttatttagaggctccaccattcacctgtaagggcagtaaaaacctaaatgtttttttataagaggatgccagagaaaactggaggtggccatccagttattaagctgcagcATAACTTCGTATAATGTATGCTATACGAAGTTATgaattcggtggtggtgtttgctgtcatttgccctcacccttcttaggaaattgtttttaaatgtgactca. Loxp sequences were underlined. Intronic sequences were in lower case letters. Exon 2 was in upper case letters. C57BL/6 J mice were used for embryo injection. Positive founder mice were screened using PCR and DNA sequencing. Primer pair Emc3-screen-F1 and Emc3-screen-R1 was used to identify upstream loxp sequence. Emc3-screen-F1: CCCAGGACGGGGATTATAGTGGTGTG; Emc3-screen-R1: ACTTGCCACACAGGTTTTCACAGCC. A second primer pair Emc3-screen-F1 and Emc3-screen-R2 were used to identify downstream loxp sequence. Emc3-screen-F1: CCCAGGACGGGGATTATAGTGGTGTG; Emc3-screen-R2: CAAATGACAGCAAACACCACCACCG. Cone-specific knockout mice were generated by crossing Emc3loxp/+ to HRGP-Cre mice [34]. The rd1 mutation was bred out before the experiment. Emc3loxp/+ mice were crossed to the broadly expressed inducible CRE CAG-Cre-ER mice (stock Tg(CAG-cre/Esr1*) 5Amc/J, https://www.jax.org/strain/004453) to generate inducible adult knockout mice [35].
Tamoxifen treatment
A total of 100 mg of tamoxifen salt (Sigma, St Louis, MO, USA) was dissolved in 10 ml of ethanol as a stock solution. On the day of injection, a 1 mg/ml working solution was prepared by mixing 10 mg/ml stock solution with corn oil (Sigma-Aldrich, St Louis, MO, USA) and mixed well. On P20, mutant and control mice were intraperitoneally injected with a daily dosage of 25 mg/kg body weight for 3 days [35].
Genotyping by PCR
Genomic DNA extracted from mouse tails was amplified by PCR using the following primer pairs: EMC3-Seq-F1, TGTCTCCCGTCAAATCCAGAAAGG; EMC3-Seq-R1, ATGTGAAACGCAAAGAAAGCAAGC. Amplification was performed using an Invitrogen Platinum SuperFi PCR master mix (Catalog # 12358–050, Invitrogen, Waltham, MA, USA). The first cycle consisted of 95 °C for 2 min, followed by 33 cycles of 94 °C for 15 s, 58 °C for 20 s, and 72 °C for 30 sec. Cre was genotyped using generic Cre primers: Cre-F, TGCCACGACCAAGTGACAGCAATG, and Cre-R, ACCAGAGACGCAAATCCATCGCTC).
Immunostaining of mouse retinas
For immunohistochemistry, enucleated eyes were removed, marked at the nasal side for orientation, and fixed for 3 h in 4% paraformaldehyde in 100 mM phosphate buffer (PB) (pH 7.4) and then cryoprotected in 30% sucrose. Tissues were embedded in optimal cutting temperature solution (OCT) and frozen on dry ice for sectioning. Sections (10 micron) were blocked and permeabilized with 10% normal goat serum and 0.1% Triton X-100 in phosphate buffer for 60 min. Labelling with various antibodies was performed as previously described [36]. Primary antibodies were diluted in phosphate buffer containing 5% normal goat serum and 0.1% Triton X-100. Sections were incubated with primary antibodies overnight. Then, the sections were washed with PB three times and labelled for 1 h with secondary antibody and counterstained with DAPI.
Quantification of mislocalized M-Opsin in the cell bodies of cone cells was performed as follows. P30 and P50 cross-sections of retinas were stained using M-Opsin opsin and DAPI, and higher-magnification images were captured on a Leica SP8 confocal microscope. The number of cones in which M-Opsin mislocalized to the inner segment and to the cell body was counted. Slides were photographed on a Zeiss LSM 800 confocal scanning microscope.
Histology and measurement of outer nuclear layer of the mouse retinas
For H&E staining, eyes from WT and knockout (KO) mice were removed, marked on the nasal side for orientation, and fixed overnight in 1.22% glutaraldehyde and 0.8% paraformaldehyde in 0.08 M phosphate buffer. They were embedded in paraffin, and cut into 5-µm sections. H&E stained sections were used to count the rows of photoreceptors in the outer nuclear layer [37]. Three measurements of the outer nuclear layer were taken every 200 μm from the optic nerve and averaged. The optic nerve was designated as 0 µm.
Results
EMC3, EMC5, and EMC6 are required for rhodopsin expression
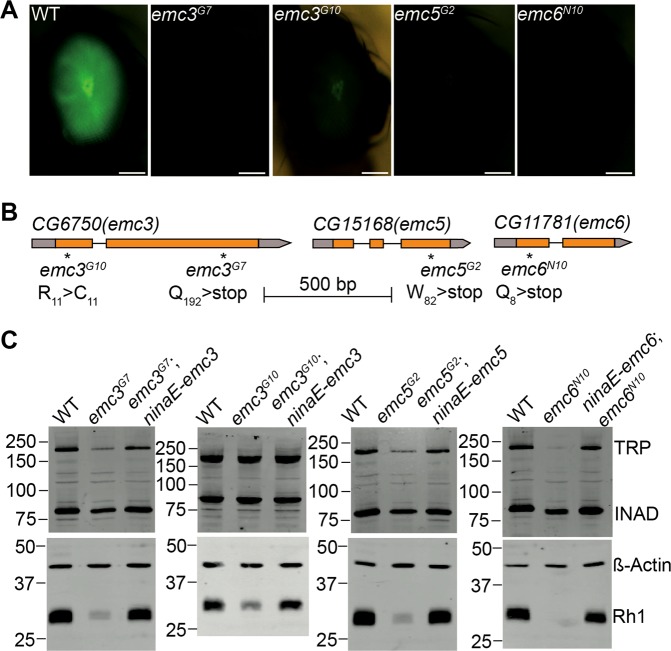
To identify genes involved in rhodopsin homeostasis, we performed EMS mutagenesis and screened chromosome 2 and 3 using the Rh1::GFP ey-flp/hid system, which utilizes the FLP-FRT recombination system to generate eyes homozygous for mutations, while using GFP-tagged Rh1 to track photoreceptor cell integrity [26, 38]. Among the ~20 mutants which displayed low GFP fluorescence (Figure S1), four were mutations in genes encoding different EMC components (Fig. 1a). The emc3G7 and emc3G10 mutations are two new emc3 alleles, containing nonsense and missense mutation, respectively. One new (mutant emc5G2) introduces a premature stop codon in the CG15168 gene, which encodes an EMC5 homologue. The emc6N10 allele introduces a nonsense mutation in the CG11781 gene, which encodes an EMC6 homologue (Fig. 1b and Figure S2).
Fig. 1.
Rhodopsin biosynthesis defects in the emc3, emc5, and emc6 mutants. a Isolation of the emc3G7, emc3G10, emc5G2, and emc6N10 mutations via a forward genetic screen. Rh1-GFP fluorescence in the deep pseudopupil of 1-day-old wild-type (ey-flp rh1-GFP;FRT40A/GMR-hid CL FRT40A), emc3G7 (ey-flp rh1-GFP;emc3G7 FRT40A/GMR-hid CL FRT40A), emc3G10 (ey-flp rh1-GFP;emc3G10 FRT40A/GMR-hid CL FRT40A), emc5G2 (ey-flp rh1-GFP;emc5G2 FRT40A/GMR-hid CL FRT40A), and emc6N10 (ey-flp rh1-GFP; FRT82B emc6N10/FRT82B GMR-hid CL) flies are shown. Scale bar is 100 μm. b The emc3, emc5, and emc6 loci and mutations associated with the emc3G7, emc3G10, emc5G2, and emc6N10 alleles. c Rhodopsin levels were reduced in emc3G7, emc3G10, emc5G2, and emc6N10 flies, and were restored by ninaE-emc3, ninaE-emc5, and ninaE-emc6 transgenes, respectively. Protein extracts from 1/4 head of each genotype (1 d after eclosion) were loaded and probed with antibodies against Rh1, ß-actin, TRP, and INAD
We confirmed via western blotting that endogenous Rh1 protein levels were greatly reduced in emc3G7, emc3G10, emc5G2, and emc6N10 mutant animals. Moreover, restoration of EMC3, EMC5, and EMC6 protein in emc3G7, emc3G10, emc5G2, and emc6N10 photoreceptor cells via the ninaE promoter, completely restored Rh1 levels (Fig. 1c and Figure S3). These results indicate that EMC is essential for rhodopsin biosynthesis. To analyse the specificity of EMC3, EMC5, and EMC6, we measured levels of TRP and INAD, exclusively function in the phototransduction cascade. INAD, a cytosolic scaffold protein has little changes in all 3 mutants, indicating the integrity of photoreceptor cells. However, TRP protein levels were moderately reduced in emc5G2 and emc3G7 mutants, but dramatically downregulated in emc6N10 mutants (Fig. 1c and Figure S3). This suggests that EMC3, EMC5, and EMC6 are involved in regulating the levels of other transmembrane proteins in photoreceptor cells, albeit to different extents.
Loss of Rh1 in emc mutants disrupted phototransduction in flies
Since the major form of rhodopsin Rh1 is essential for photoreceptor function, we asked whether phototransduction was disrupted in emc mutants. Electroretinogram (ERG) is an extracellular recording that measures the summed light responses of all retinal cells (Fig. 2a). After wild-type flies were exposed to blue light, a prolonged depolarization afterpotential (PDA) was detected, reflecting an excessive accumulation of active Rh1 (Fig. 2a) [39]. In mutants with reduced levels of Rh1, PDA was not produced (Fig. 2b) [40, 41]. Similarly, PDA was not detected in emc3G7, emc3G10, emc5G2, and emc6N10 mutants. Restoring EMC3, EMC5, and EMC6 protein levels rescued the PDA response in emc3G7, emc3G10, emc5G2, and emc6N10 mutants, respectively (Figs. 2c–j). Importantly, the ERG phenotypes of emc3G7, emc3G10, emc5G2, and emc6N10 flies were indistinguishable from ninaE mutants, but different from trp mutants [42, 43]. This suggests that EMC affects phototransduction by regulating rhodopsin production.
Fig. 2.
Loss of PDA in emc3, emc5, and emc6 mutants. a ERG recordings in wild-type flies (ey-flp rh1-GFP;FRT40A/GMR-hid CL FRT40A) showed that PDA was induced by blue light and terminated by orange light (arrows). b PDA was eliminated in ninaEP332 flies and in c emc3G7, e emc3G10, g emc5G2, and i emc6N10 flies. PDA was restored in (d) emc3G7;ninaE-emc3, f emc3G10;ninaE-emc3, h emc5G2;ninaE-emc5, and j ninaE-emc6;emc6N10 flies. Flies ~1day after eclosion were dark-adapted for 2 min and subsequently exposed to 5-s pulses of orange (O) or blue (B) light. At least 10 flies of each genotype were tested
Rhodopsin is properly folded and is not degraded through the ERAD pathway in emc mutants
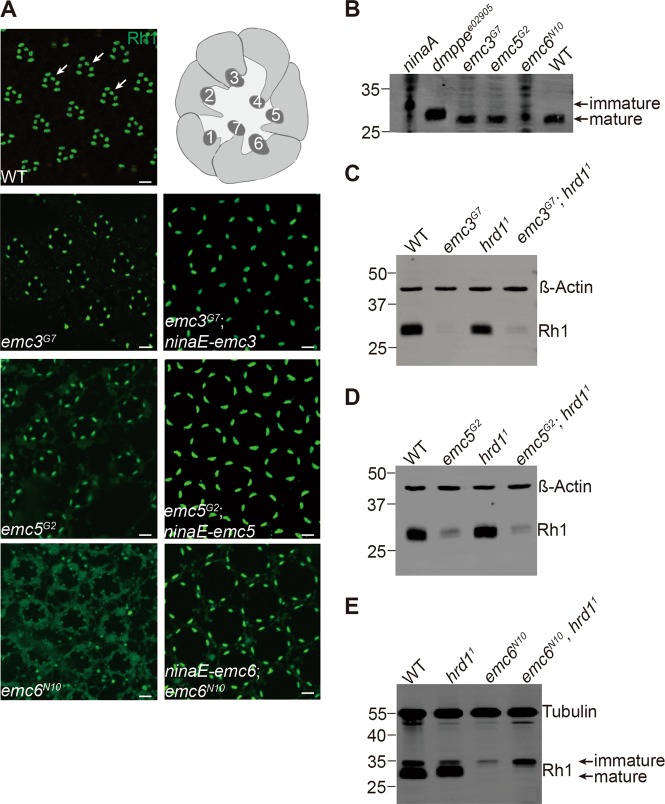
To determine which step of the rhodopsin biosynthetic process requires EMC, we examined the localization of endogenous Rh1 in resin-embedded retina sections from emc mutants. As with wild-type flies, the majority of Rh1 localized to the rhabdomere (the tightly-packed microvilli in which phototransduction occurs) in emc3G7 and emc5G2 flies, although the Rh1 signal was weak compared to rescued flies. This indicates that Rh1 trafficking was normal in both emc3 and emc5 mutants (Fig. 3a). However, Rh1 levels were much lower in emc6N10 mutants, and Rh1 was detected in the cell body (Fig. 3a). Restoring EMC3 and EMC5 protein levels rescued Rh1 signal in emc3G7 and emc5G2 mutants, respectively, and EMC6 restoration rescued both Rh1 signal and localization in emc6N10 flies, albeit not fully mimic wild-type morphology, which may due to different sectioning angle and depth. During biosynthesis, Rh1 is transiently glycosylated in the ER lumen, and this modification is progressively removed as it is transported to the rhabdomere. If Rh1 quality control or trafficking is blocked, such as in ninaA [6] or dmppee02905 [44] mutant flies, immature Rh1, which has a higher molecular weight, can be detected [38, 45]. The major Rh1 protein isolated from emc3G7 and emc5G2 flies was about the same size as in wild-type flies. In emc6N10 flies, the major Rh1 band was the mature form, but immature Rh1 was also detected (Fig. 3b). This is consistent with the detection of Rh1 in the cell body, and suggests that EMC6 may have distinct functions.
Fig. 3.
EMC3, EMC5, and EMC6 were not required for maturation of the Rh1 protein. a Rh1 localized to the rhabdomere region in emc3, emc5, and emc6 mutant photoreceptor cells. Tangential resin-embedded retina sections of compound eyes from ~1-day-old wt, emc3G7, emc3G7;ninaE-emc3, emc5G2, emc5G2;ninaE-emc5, emc6N10, and ninaE-emc6;emc6N10 flies were labeled using antibodies against Rh1. Rhabdomeres are indicated by arrows. Scale bar is 2 μm. Illustration of photoreceptor cells within a single ommatidium is on the top right corner, and rhabdomeres of R1-R7 photoreceptor cells are indicated by numbers. b Deglycosylation of Rh1 is normal in emc3, emc5, and emc6 mutants. Head extracts prepared from 1-day-old wild-type, ninaA, dmppee02905, emc3G7, emc5G2, and emc6N10 flies were probed with antibodies against Rh1, and mature and immature bands are indicated. Extracts from ninaA and emc6N10 flies (10 heads), emc3G7 and emc5G2 flies (5 heads), and wild-type and dmppee02905 flies (0.25 of a head) were loaded. c–e Similar Rh1 levels were seen in (c) emc3G7 and emc3G7;hrd11 flies (ey-flp rh1-GFP;emc3G7 FRT40A/GMR-hid CL FRT40A;FRT82B hrd11/FRT82B GMR-hid CL), d emc5G2 and emc5G2;hrd11 flies (ey-flp rh1-GFP;emc5G2 FRT40A/GMR-hid CL FRT40A;FRT82B hrd11/FRT82B GMR-hid CL), and e emc6N10 and emc6N10 hrd11 (ey-flp rh1-GFP; FRT82B emc6N10 hrd11/FRT82B GMR-hid CL) flies. Protein extracts from 0.25 of a head from ~1-day-old flies were loaded
It has been shown that EMCs functionally interacts with the ERAD pathway, which degrades unassembled polypeptides on the ER [11]. Thus, it is possible that the massive degradation of newly synthesized rhodopsin is responsible for the reduced levels of Rh1 in emc mutants. To test this hypothesis, we generated a deletion mutation for the hrd1 gene (Figure S4), which encodes the major E3 ligase in the ERAD pathway [46]. Eliminating hrd1 did not affect Rh1 levels, and more importantly, loss of hrd1 failed to rescue Rh1 levels in emc3G7, emc5G2, and emc6N10 mutants (Figs. 3c–e). These data suggest that EMCs regulate rhodopsin biosynthesis independent of the ERAD pathway.
Mutating EMC components caused different phenotypes
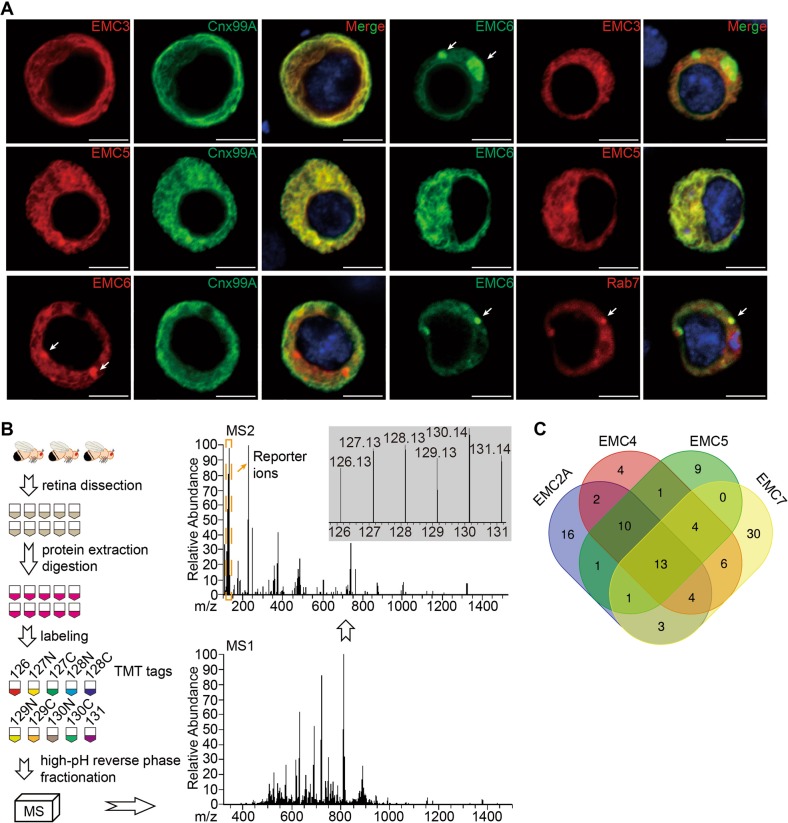
Since the emc5, emc3, and emc6 mutants exhibited similar phenotypes, we assumed that they function through forming the EMC complex. We expressed mCherry-tagged EMC5 in photoreceptor cells, and performed an immunoprecipitation assay with the RFP-Trap followed by mass spectra identification (Figure S5A). We found that most EMC subunits, including EMC3, EMC4, EMC7, EMC8/9, and EMC10, were pulled down (Figure S5B and Supplementary data). This agreed with other mass spectra data indicating that different EMC subunits interact with one another [11, 19].
We next characterized the cellular localization of EMC3, EMC5, and EMC6 in S2 cells. In contrast to EMC5 and EMC3 which co-localized with the ER marker, Cnx99A (calnexin), EMC6 primarily localized to intracellular vesicles that lacked ER markers (Fig. 4a). However, when EMC5 and EMC6 were co-expressed in S2 cells, EMC6 completely colocalized with EMC5, which was not observed when EMC3 and EMC6 were co-expressed (Fig. 4a). To further investigated the compartments in which EMC6 may function independent of the EMC complex in ER, we examined the localization of EMC6 with markers of different intracellular compartments. EMC6 partially co-localized with Rab7, a late endosome marker, but not with Rab5, Lamp1, GM130, or Golgin245, suggesting that EMC6 may also function in the late endosome independent of the EMC complex (Fig. 4a and Figure S6). These results suggest that in addition to its functions as a subunit of the EMC complex, EMC6 may also perform distinct functions.
Fig. 4.
Interaction among EMC subunits. a S2 cells were transiently transfected with emc3-RFP, emc5-mCherry, or emc6-GFP, or co-transfected with emc3-RFP/emc6-GFP, emc5-mCherry/emc6-GFP, emc6-GFP/RFP-rab7. EMC6 puncta are indicated by arrows. Scale bar is 5 μm. b Flowchart of Tandem Mass Tags Labeling coupled with LC-MS/MS for the comparative analysis of protein levels in retina of emc2 (GMR-Gal4/UAS-emc2ARNAi), emc4 (GMR-Gal4/UAS-emc4RNAi), emc5 (GMR-Gal4/UAS-emc5RNAi), and emc7 (FRT42D emc71) mutant flies, as well as wild-type (GMR-gal4) flies. c Venn diagrams showing the overlap of proteins that were downregulated > 50% among the four analyzed genotypes: emc2, emc4, emc5, and emc7
Although EMC subunits form a complex, our genome-wide screen for genes that disrupt rhodopsin biosynthesis only identified emc3, emc5, and emc6. We therefore asked if other EMC components also regulated biosynthesis of rhodopsin. Using the CRISPR/Cas9 system, we generated null mutations for all genes encoding EMC components, namely CG2943 (emc1), CG17556 (emc2A), CG11137 (emc4), CG8397 (emc7), and CG3501 (emc8/9) (Figure S4). We also obtained the transposon insertion lines CG3678G4215 (emc2B) and CG32441MB09773 (emc10), which are considered null mutants (Figure S4). We found that different EMC components displayed different functions and importance. Most emc null mutants were lethal, except for emc7 and emc10 mutants, which had normal levels of Rh1 and normal ERG responses (Table 1 and Figure S7). However, knocking out emc1, emc2A, emc4 or emc8/9 in retinal cells using the Rh1::GFP ey-flp/hid system led to ablation of retinal cells (Figure S7C and Table 1).
Table 1.
Summary of emc mutants phenotypes
| EMC | Length(aa) | TMDa(Y/N) | Lethality | Cell lethality | Rh1 level | PDA | |
|---|---|---|---|---|---|---|---|
| Category 1 | EMC1 | 915 | Yes | Lethal | Lethal | NA | NA |
| EMC2 | 282 | No | Lethal | Lethal | NA | NA | |
| EMC4 | 166 | Yes | Lethal | Lethal | NA | NA | |
| EMC8/9 | 203 | No | Lethal | Lethal | NA | NA | |
| Category 2 | EMC6 | 113 | Yes | Lethal | Viableb | Decrease | No PDA |
| EMC3 | 247 | Yes | Lethal | Viable | Decrease | No PDA | |
| EMC5 | 109 | Yes | Lethal | Viable | Decrease | No PDA | |
| Category 3 | EMC7 | 245 | Yes | Viable | Viable | Normal | Normal |
| EMC10 | 227 | Yes | Viable | Viable | Normal | Normal |
aTransmembrane domain
bemc6 mutation leads to a rapid degeneration in retinal cells
To further demonstrate that different EMC subunits have distinct molecular functions, we performed proteomic analysis of emc mutants from different phenotypic categories. Because null emc2A and emc4 mutations completely lacked retinal cells, we generated weak alleles by expressing RNAis of emc2A, emc4, or emc5 in compound eyes using the GMR-Gal4 driver [28]. The efficiency of emc2A, emc4, and emc5 knockdown was >60% (Figure S8A), and eye morphologies were normal. Importantly, Rh1 levels were significantly reduced in each of these mutants, confirming that these RNAis reduced the function of EMC2A, EMC4, and EMC5, respectively (Figure S8B). These data also demonstrated that EMC2A and EMC4 are required for Rh1 biogenesis. For EMC7, we used null mutants for proteomic analyses. We dissected retinas from control, emc2ARNAi, emc4RNAi, emc5RNAi and emc71 flies for the proteomic assay (Fig. 4b). A total of 6,390 proteins were identified, and compared with controls, 50, 44, 39, and 61 proteins were reduced > 50% in emc2A, emc4, emc5, and emc7 mutants, respectively (Supplementary Tables 1–4 and Supplementary data). Importantly, a majority of these proteins contained transmembrane domain/signal peptide, and Rh1 levels were reduced in emc2A, emc4, and emc5, but not emc7 samples, validating the quantitative proteomic experiments.
Among the reduced proteins, 13 were downregulated in all 4 groups, suggesting that the EMC subunits function cooperatively in the biogenesis of some membrane proteins. In contrast, 16, 4, 9, and 30 downregulated proteins were specific for emc2A, emc4, emc5, and emc7 mutants, respectively, indicating potentially distinct functions for individual EMC subunits. Most proteins were downregulated in 2 or 3 genotypes (emc2A/emc4/emc5: 23, emc2A/emc4/emc7: 17, emc2A/emc5/emc7: 14, emc4/emc5/emc7: 17; emc2A/emc4: 29, emc2A/emc5: 25, emc2A/emc7: 21, emc4/emc5: 28, emc4/emc7: 27, and emc5/emc7: 18) (Fig. 4c and Supplementary Tables 1–4). Together, these data suggest that in addition to functioning as a complex, distinct EMC subunits may be required for the biogenesis of specific membrane proteins.
Mutations in emc3, emc5, and emc6 lead to retinal degeneration
Because mutations in emc3, emc5, and emc6 largely reduced Rh1 protein levels and disrupted phototransduction, we examined if photoreceptor integrity depends on these genes. Wild-type ommatidia consistently exhibit seven photoreceptor cells with intact rhabdomeres (Fig. 5a). Young (~1 day old) emc5G2, emc3G7, and emc6N10 mutants exhibited normal retinal morphology with all 7 rhabdomeres, although the rhabdomeres were smaller in emc6N10 flies, reflecting an early stage of retinal degeneration. Both emc5G2 and emc3G7 mutants contained intact rhabdomeres and photoreceptor cells at 5 days of age, whereas 35-day-old flies exhibited retinal degeneration with prominent vacuoles and obvious loss of rhabdomeres (Figs. 5b, c). Thirty-five-day-old emc5G2 and emc3G7 flies exhibited a decrease in ERG amplitude (Figure S9), also reflecting mild retinal degeneration associated with emc5G2 and emc3G7 flies. Consistent with previous results, more severe photoreceptor cell death was associated with emc6N10 mutant flies, which exhibited disruption of rhabdomeres and the accumulation of abnormal vesicles shortly after eclosion (Fig. 5d). These results demonstrated that EMC3, EMC5, and EMC6 are required for photoreceptor cell survival.
Fig. 5.
Retinal degeneration in emc3, emc5, and emc6 mutant photoreceptors. TEM images of representative ommatidia from (a) wild-type, (b) emc3G7, (c) emc5G2, and (d) emc6N10 flies. Flies were raised in a 12h-light/12h-dark (L/D) cycle for indicated time points. The degenerating photoreceptor cells are indicated by arrows. Scale bars are 2 μm
Loss of Emc3 in mammalian cone cells leads to cone-photoreceptor defects
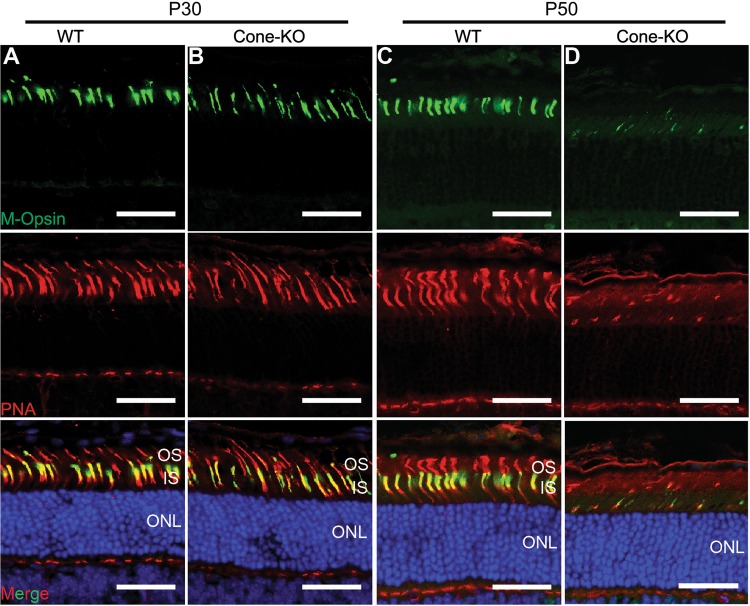
We next assessed the functions of EMC in mammalian photoreceptor cells. A conditional knockout allele of Emc3 was generated using the CRISPR/Cas9 system (Figure S10). In mouse retina, EMC3 is mainly localized to the outer segment (OS), similar to Rho (Figure S11). Weak staining was also observed in the inner segment of photoreceptor cells (Figure S11). One possible explanation of EMC3 localization to OS might be binding of EMC3 to OS targeted membrane proteins, similar to interaction of EMC3 with ABCA3 in the lung [22]. To investigate the role of Emc3 in photoreceptor cells, we generated a cone-photoreceptor knockout line using HRGP-Cre mice (Emc3loxp/loxp HRGP-Cre: Emc3 cone-KO) in which Cre is driven by the human red/green pigment gene promoter [34]. HRGP-Cre mice were used as controls (WT). In Emc3 cone-KO retinas, M-opsin levels were not affected on postnatal day 30 (P30) (Figs. 6a, b). However, by P50 very little M-opsin protein could be detected in Emc3 cone-KO retina sections, suggesting that EMC3 is important for M-opsin stability (Figs. 6c, d).
Fig. 6.
Loss of Emc3 in mammalian cone cells led to cone cell death. a, b Retina cryosections from (a) WT and (b) cone-KO littermate mice at P30. Sections were immunostained for M-Opsin (green) and the cone marker, peanut agglutinin (PNA, red). Cone cell number did not decrease at P30. c, d Retina cryosections from (c) WT and (d) cone-KO mice at P50. Sections were immunostained for M-Opsin (green) and PNA (red). There were almost no cone cells in cone-KO retinas. Nuclei were counterstained with DAPI. ONL outer nuclear layer, OS outer segment, IS inner segment. Scale bar is 25 μm
Deletion of Emc3 in adult animals leads to rod cell degeneration
To further assess the role of EMC3 in mature photoreceptor cells, we used tamoxifen-activated CAG-CreER to remove Emc3 in adult mice. We generated Emc3loxp/loxp; CAG-CreER (named Emc3 iKO) mice and treated the mutant and control (CAG-CreER or Emc3loxp/loxp) animals with tamoxifen at P20 and assessed the retina 7 and 11 days later (P27 and P31). Tamoxifen induction efficiently deleted Emc3 in all retinal cells, and EMC3 levels were reduced by 70% at P27 compared with control retinas (Figure S11 and S12).
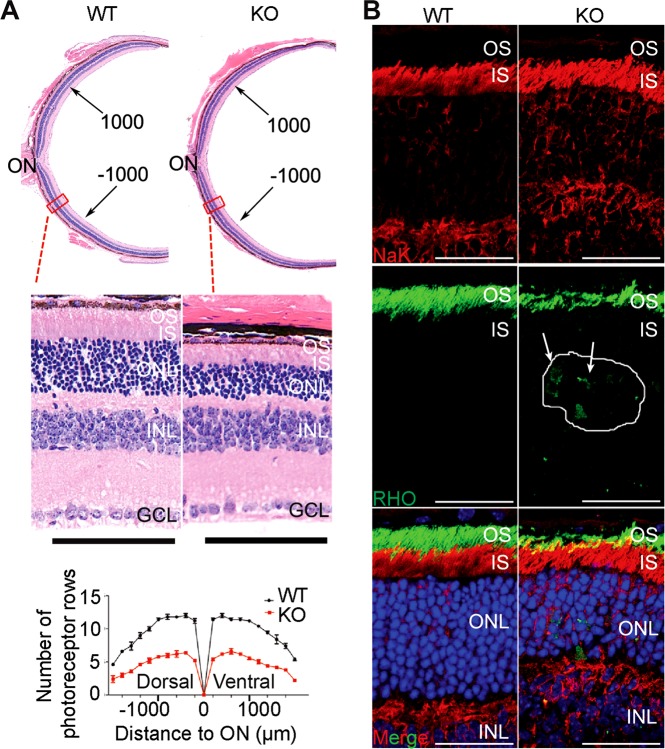
We examined H&E stained retinal sections to assess the morphology of mutant retinas at P27 and P31. At P27, no obvious differences were observed between the mutant and control mice (Figures S13). At P31, however, a thinner outer nuclear layer was seen in the mutant retina (Fig. 7a). The mutant outer nuclear layer (ONL) was reduced by approximately 50% compared with controls, indicating degeneration of Rod photoreceptor cells (Fig. 7a). To investigate if the severe reduction in OS length in Emc3 KO retinas was caused by defective RHO biosynthesis, we examined retina sections via immunohistochemistry using antibodies against RHO. In control retinas, RHO localized exclusively to the OS. In iKO retinas, RHO levels were reduced, and RHO could be detected in the inner segment and in cell bodies at P27 (Figure S13) and P31 (Fig. 7b). These data suggest that RHO trafficking to the OS was compromised in Emc3 iKO retinas.
Fig. 7.
Retinal degeneration and RHO mislocalization in Emc3 inducible KO mice. a Paraffin sections of mice retinas 11 days after induction (P31). Sections were stained with H&E, and quantification of outer nuclear layer is shown on the left. Scale bar is 50 μm. H&E staining of paraffin sections and quantification of outer nuclear layer (ONL) nuclei revealed that the mutant ONL was reduced by ~50% compared to controls 11 days after induction. On top is an illustration of the whole mouse retina. b Immunofluorescence labeling of retina cryosections for control (WT) and mutant (KO) littermates at P31. Sections were labeled using antibodies against RHO (green) and DAPI (blue). NaK ATPase antibodies were used to label the inner segment. RHO was mislocalized to the inner segment and cell bodies in KO retinas (arrows). Scale bar is 25 μm
Discussion
Although EMCs have been implicated in a variety of physiological functions, their molecular function has remained unexplored. EMC components were first identified based on their ability to interact with ERAD components, suggesting that EMC is required for protein folding and/or ER-associated degradation [11, 12]. Later, mutations to EMC subunits were shown to reduce expression of acetylcholine receptors in worms, and rhodopsin in flies, supporting a role for EMC in quality control for a number of multipass transmembrane proteins [24, 47]. Although rhodopsin levels are much lower in emc3, emc5, and emc6 mutant animals, rhodopsin is still transported to rhabdomeres, and the mature, deglycosylated form of rhodopsin is primarily detected, indicating that rhodopsin is folded properly in the ER and transported to the plasma membrane. Our data suggest that EMC functions during the de novo biosynthesis of its client protein, rhodopsin, before the protein-folding procedure.
The ERAD pathway is a specialized protein degradation process that removes proteins that fail to fold appropriately in the ER [48]. If loss of EMC destabilizes unfolded rhodopsin in the ER, and/or EMC is connected to the ERAD pathway, disruption of ERAD would be expected to increase rhodopsin levels. However, loss of hrd1 failed to increase rhodopsin levels in emc3, emc5, and emc6 mutant animals, suggesting that EMC has limited function in the quality control of immature rhodopsin, as reported [24, 49]. Our finding that EMC functions during rhodopsin biosynthesis, rather than during later post-translational modifications, is supported by recent reports that EMC is involved in the initial biogenesis of membrane proteins [13, 14, 50].
Since its first identification, EMC components have been shown to interact with each other in several genetic screens [11, 12, 14, 17], and have been suggested to function together as a unit [19, 21]. However, a genome-wide genetic screen in C. elegans found that EMC6 is required for the expression of acetylcholine receptors, whereas other EMC subunits are not [47]. Consistent with this notion that different EMC components have distinct functions, several other studies have indicated that individual EMC subunits functions in autophagy, viral assembly, and tissue repair [16, 51, 52]. In our genome-wide screen for genes that regulate rhodopsin levels, the only EMC components to emerge were emc3, emc5, and emc6. Moreover, by generating an entire EMC KO library, we were able to measure the function and importance of each EMC subunit in different contexts, ranging from animal viability, cellular viability, rhodopsin levels, and phototransduction. Our data revealed that emc1, emc2A, emc4 and emc8/9 are required to maintain basic cellular functions. This may also explain why emc1, emc2, emc4 and emc8/9 mutants were not identified in our EMS screen. In contrast, no phenotype was observed in emc7 and emc10 null mutants, suggesting a less important role of these two EMC components in cellular functions. The weak effects of emc7 and emc10 mutations may be explained by functional redundancy. However, this is unlikely as no other EMC7 or EMC10 homologues can be found in flies based on protein sequence.
In weak emc2 and emc4 alleles (expressing RNAis), Rh1 levels were significantly decreased as well as applying emc5 RNAi, indicating that rhodopsin is a common substrate of EMC complexes. Whereas EMC7 and EMC10 are not required for the EMC complex to participate in rhodopsin biogenesis. Quantitative proteomic analysis to compare changes in protein levels in emc2, emc4, emc5, and emc7 mutants revealed that 13 proteins were downregulated in all 4 mutants, but most (including rhodopsin) were downregulated in ≤3 emc mutants. These data provide additional support that EMC subunits are differentially required for the biogenesis of distinct membrane proteins.
Different EMC subunits have been shown to function together in a number of cellular processes. Compared with emc5G2 and emc3G7 null mutant animals, emc6N10 null mutants exhibited lower levels of rhodopsin and TRP proteins, more disrupted rhabdomere morphology, and more severe retinal degeneration. Moreover, EMC5 and EMC3 localized to the ER, whereas EMC6 was also concentrated in intracellular vesicles. However, EMC6 localized to the ER when co-expressed with EMC5, indicating that EMC6 is a bona fide component of the EMC complex, while also performing independent functions outside of the ER. The independent functions of EMC6 may explain its role in the biosynthesis of acetylcholine receptors and in autophagy [16, 47]. In S2 cells, EMC6, but not EMC5 and EMC3, partially co-localized with the late endosome maker Rab7, supporting a role for EMC6 in regulating autophagy.
Both rhodopsin and TRP rely on EMC for their biogenesis. However, the emc3, emc5, and emc6 mutant flies only lack the PDA, as seen for ninaE mutants. These are consistent with previous reports that low levels of TRP is enough for maintaining phototransduction, suggesting that loss of rhodopsin is the major cause of the defective visual responses [53]. Rhodopsin also plays structural roles in maintaining the morphology of photoreceptor cells, therefore loss of rhodopsin in most ninaE alleles results in retinal degeneration [54, 55]. Photoreceptor cell degeneration associated with the emc mutants could be explained by a disruption in rhodopsin biosynthesis.
The importance of EMC in various human diseases has emerged in recent years. Mutations in EMC1 are associated with global developmental delay, hypotonia, scoliosis, visual impairment and cerebellar atrophy [56, 57]. In addition, EMC is indispensable for West-Nile-Virus-induced cell death [21], and EMC1-dependent stabilization plays an essential role in membrane penetration of a non-enveloped virus [51]. We showed that removal of Emc3 from photoreceptor cells led to their degeneration. Removal of Emc3 from the adult retina resulted in the accumulation of RHO in the inner segment or in cell bodies, and the death of rods. We did not observe mislocalized cone opsin in Emc3 mutants. One potential explanation is that Emc3 is not essential for cone opsin trafficking. To the best of our knowledge, our study provides the first evidence that an ER membrane protein is essential for mammalian photoreceptor structure.
Supplementary information
Down-regulated proteins identified in emc2A mutant
Down-regulated proteins identified in emc4 mutant
Down-regulated proteins identified in emc5 mutant
Down-regulated proteins identified in emc7 mutant
Acknowledgements
We thank the Bloomington Stock Center, Drosophila Genomic Resource Center, the Developmental Studies Hybridoma Bank, and Drs. H. Bellen, C. Montell, R. Molday, N. Colley, and J. Han for stocks and reagents. We thank Y. Wang, X. Liu, Y. Chen, J. Wang and Dr. Y. Xu for technological assistance. We thank Dr. D. O’Keefe for editing the manuscript. This work was supported by grants from the National Natural Science Foundation of China (81670891 and 81870693) awarded to TW. This study was also supported by grants from the National Natural Science Foundation of China (81770950 and 81470668 to XJZ, and 81700876 to LZ), the National Key Scientific Research Program (2015CB554100 to XJZ) and the Department of Science and Technology of Sichuan Province (2016TD0009 and 2017TJPT0010, 19ZYNLJS to XJZ, and 2018YSZH0020 to LZ). The funders had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript. The authors alone are responsible for the content and writing of the paper.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by E. Baehrecke
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Liangyao Xiong, Lin Zhang
Contributor Information
Xianjun Zhu, Email: xjzhu@uestc.edu.cn.
Tao Wang, Email: wangtao1006@nibs.ac.cn.
Supplementary information
The online version of this article (10.1038/s41418-019-0378-6) contains supplementary material, which is available to authorized users.
References
- 1.Xiong B, Bellen HJ. Rhodopsin homeostasis and retinal degeneration: lessons from the fly. Trends Neurosci. 2013;36:652–60. doi: 10.1016/j.tins.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 3.Nemet I, Ropelewski P, Imanishi Y. Rhodopsin trafficking and mistrafficking: signals, molecular components, and mechanisms. Prog Mol Biol Transl Sci. 2015;132:39–71. doi: 10.1016/bs.pmbts.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Ryoo HD, Domingos PM, Kang MJ, Steller H. Unfolded protein response in a Drosophila model for retinal degeneration. EMBO J. 2007;26:242–52. doi: 10.1038/sj.emboj.7601477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galy A, Roux MJ, Sahel JA, Leveillard T, Giangrande A. Rhodopsin maturation defects induce photoreceptor death by apoptosis: a fly model for RhodopsinPro23His human retinitis pigmentosa. Hum Mol Genet. 2005;14:2547–57. doi: 10.1093/hmg/ddi258. [DOI] [PubMed] [Google Scholar]
- 6.Colley NJ, Baker EK, Stamnes MA, Zuker CS. The cyclophilin homolog ninaA is required in the secretory pathway. Cell. 1991;67:255–63. doi: 10.1016/0092-8674(91)90177-z. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum EE, Hardie RC, Colley NJ. Calnexin Is essential for rhodopsin maturation, Ca2+ regulation, and photoreceptor cell survival. Neuron. 2006;49:229–41. doi: 10.1016/j.neuron.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenbaum Erica E, Brehm Kimberley S, Vasiljevic E, Liu C-H, Hardie Roger C, Colley Nansi J. XPORT-dependent transport of TRP and rhodopsin. Neuron. 2011;72:602–15. doi: 10.1016/j.neuron.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Shi G, Concepcion FA, Xie G, Oprian D, Chen J. Stable rhodopsin/arrestin complex leads to retinal degeneration in a transgenic mouse model of autosomal dominant retinitis pigmentosa. J Neurosci. 2006;26:11929. doi: 10.1523/JNEUROSCI.3212-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira PANT, Pak WL, Travis GH. Cyclophilin-related protein RanBP2 acts as chaperone for red/green opsin. Nature. 1996;383:637–40. doi: 10.1038/383637a0. [DOI] [PubMed] [Google Scholar]
- 11.Christianson JC, Olzmann JA, Shaler TA, Sowa ME, Bennett EJ, Richter CM, et al. Defining human ERAD networks through an integrative mapping strategy. Nat Cell Biol. 2012;14:93–105. doi: 10.1038/ncb2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin C, Jonikas SRC, Denic Vladimir, Oh Eugene, Quan ErinM, Schmid Volker, et al. Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science. 2009;323:1693–7. doi: 10.1126/science.1167983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shurtleff MJ, Itzhak DN, Hussmann JA, Schirle Oakdale NT, Costa EA, Jonikas M, et al. The ER membrane protein complex interacts cotranslationally to enable biogenesis of multipass membrane proteins. eLife. 2018;7:e37018. doi: 10.7554/eLife.37018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guna A, Volkmar N, Christianson JC, Hegde RS. The ER membrane protein complex is a transmembrane domain insertase. Science. 2018;359:470–3. doi: 10.1126/science.aao3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen X, Kan S, Hu J, Li M, Lu G, Zhang M, et al. EMC6/TMEM93 suppresses glioblastoma proliferation by modulating autophagy. Cell Death Dis. 2016;7:e2043. doi: 10.1038/cddis.2015.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Zhao Y, Hu J, Xiao J, Qu L, Wang Z, et al. A novel ER-localized transmembrane protein, EMC6, interacts with RAB5A and regulates cell autophagy. Autophagy. 2013;9:150–63. doi: 10.4161/auto.22742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahiri S, Chao JT, Tavassoli S, Wong AK, Choudhary V, Young BP, et al. A conserved endoplasmic reticulum membrane protein complex (EMC) facilitates phospholipid transfer from the ER to mitochondria. PLoS Biol. 2014;12:e1001969. doi: 10.1371/journal.pbio.1001969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R, Miner JJ, Gorman MJ, Rausch K, Ramage H, White JP, et al. A CRISPR screen defines a signal peptide processing pathway required by flaviviruses. Nature. 2016;535:164–8. doi: 10.1038/nature18625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savidis G, McDougall WM, Meraner P, Perreira JM, Portmann JM, Trincucci G, et al. Identification of Zika virus and dengue virus dependency factors using functional genomics. Cell Rep. 2016;16:232–46. doi: 10.1016/j.celrep.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Marceau CD, Puschnik AS, Majzoub K, Ooi YS, Brewer SM, Fuchs G, et al. Genetic dissection of Flaviviridae host factors through genome-scale CRISPR screens. Nature. 2016;535:159–63. doi: 10.1038/nature18631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma H, Dang Y, Wu Y, Jia G, Anaya E, Zhang J, et al. A CRISPR-based screen identifies genes essential for West-Nile-virus-induced cell death. Cell Rep. 2015;12:673–83. doi: 10.1016/j.celrep.2015.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang X, Snowball JM, Xu Y, Na CL, Weaver TE, Clair G, et al. EMC3 coordinates surfactant protein and lipid homeostasis required for respiration. J Clin Invest. 2017;127:4314–25. doi: 10.1172/JCI94152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor MR, Kikkawa S, Diez-Juan A, Ramamurthy V, Kawakami K, Carmeliet P, et al. The zebrafish pob gene encodes a novel protein required for survival of red cone photoreceptor cells. Genetics. 2005;170:263–73. doi: 10.1534/genetics.104.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takunori Satoh AO, Liu Ziguang, Inagaki Tsuyoshi, Satoh AkikoK. dPob/EMC is essential for biosynthesis of rhodopsin and other multi-pass membrane proteins in Drosophila photoreceptors. eLife. 2015;4:e06306. doi: 10.7554/eLife.06306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abu-Safieh L, Alrashed M, Anazi S, Alkuraya H, Khan AO, Al-Owain M, et al. Autozygome-guided exome sequencing in retinal dystrophy patients reveals pathogenetic mutations and novel candidate disease genes. Genome Res. 2013;23:236–47. doi: 10.1101/gr.144105.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y, Xie J, Wang T. A fluorescence-based genetic screen to study retinal degeneration in Drosophila. PLoS One. 2015;10:e0144925. doi: 10.1371/journal.pone.0144925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han Y, Xiong L, Xu Y, Tian T, Wang T. The β-alanine transporter BalaT is required for visual neurotransmission in Drosophila. eLife. 2017;6:e29146. doi: 10.7554/eLife.29146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni J-Q, Zhou R, Czech B, Liu L-P, Holderbaum L, Yang-Zhou D, et al. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat Methods. 2011;8:405. doi: 10.1038/nmeth.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang T, Wang X, Xie Q, Montell C. The SOCS box protein STOPS is required for phototransduction through its effects on phospholipase C. Neuron. 2008;57:56–68. doi: 10.1016/j.neuron.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Y, Wang T. CULD is required for rhodopsin and TRPL channel endocytic trafficking and survival of photoreceptor cells. J Cell Sci. 2016;129:394. doi: 10.1242/jcs.178764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelstrup CD, Jersie-Christensen RR, Batth TS, Arrey TN, Kuehn A, Kellmann M, et al. Rapid and deep proteomes by faster sequencing on a benchtop quadrupole ultra-high-field orbitrap mass spectrometer. J Proteome Res. 2014;13:6187–95. doi: 10.1021/pr500985w. [DOI] [PubMed] [Google Scholar]
- 32.Lai W, Wang Q, Li L, Hu Z, Chen J, Fang Q. Interaction of gold and silver nanoparticles with human plasma: Analysis of protein corona reveals specific binding patterns. Colloids Surf B Biointerfaces. 2017;152:317–25. doi: 10.1016/j.colsurfb.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 33.Han D, Jin J, Woo J, Min H, Kim Y. Proteomic analysis of mouse astrocytes and their secretome by a combination of FASP and StageTip-based, high pH, reversed-phase fractionation. Proteomics. 2014;14:1604–9. doi: 10.1002/pmic.201300495. [DOI] [PubMed] [Google Scholar]
- 34.Yun-Zheng Le JDA, Muayyad RAl-Ubaidi, Chen Ying, Ma Jian-Xing, Anderson RobertE. Targeted expression of Cre recombinase to cone photoreceptors in transgenic mice. Mol Vis. 2004;10:1011–8. [PubMed] [Google Scholar]
- 35.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–18. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Liu W, Sun K, Jiang L, Zhu X. Tmem30a deficiency leads to retinal rod bipolar cell degeneration. J Neurochem. 2019;148:400–12. doi: 10.1111/jnc.14643. [DOI] [PubMed] [Google Scholar]
- 37.Coleman JA, Zhu X, Djajadi HR, Molday LL, Smith RS, Libby RT, et al. Phospholipid flippase ATP8A2 is required for normal visual and auditory function and photoreceptor and spiral ganglion cell survival. J Cell Sci. 2014;127:1138. doi: 10.1242/jcs.145052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao H, Wang J, Wang T. The V-ATPase V1 subunit A1 is required for rhodopsin anterograde trafficking in Drosophila. Mol Biol Cell. 2018;29:1640–51. doi: 10.1091/mbc.E17-09-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang T, Montell C. Phototransduction and retinal degeneration in Drosophila. Pflug Arch. 2007;454:821–47. doi: 10.1007/s00424-007-0251-1. [DOI] [PubMed] [Google Scholar]
- 40.O’Tousa JE, Baehr W, Martin RL, Hirsh J, Pak WL, Applebury ML. The Drosophila ninaE gene encodes an opsin. Cell. 1985;40:839–50. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- 41.Zuker CS, Cowman AF, Rubin GM. Isolation and structure of a rhodopsin gene from D. melanogaster. Cell. 1985;40:851–8. doi: 10.1016/0092-8674(85)90344-7. [DOI] [PubMed] [Google Scholar]
- 42.Montell C, Jones K, Hafen E, Rubin G. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science. 1985;230:1040. doi: 10.1126/science.3933112. [DOI] [PubMed] [Google Scholar]
- 43.Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–9. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- 44.Cao J, Li Y, Xia W, Reddig K, Hu W, Xie W, et al. A Drosophila metallophosphoesterase mediates deglycosylation of rhodopsin. EMBO J. 2011;30:3701–13. doi: 10.1038/emboj.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenbaum EE, Vasiljevic E, Brehm KS, Colley NJ. Mutations in four glycosyl hydrolases reveal a highly coordinated pathway for rhodopsin biosynthesis and N-glycan trimming in Drosophila melanogaster. PLoS Genet. 2014;10:e1004349. doi: 10.1371/journal.pgen.1004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hampton RY, Gardner RG, Rine J. Role of 26S proteasome and HRD genes in the degradation of 3-hydroxy-3-methylglutaryl-CoA reductase, an integral endoplasmic reticulum membrane protein. Mol Biol Cell. 1996;7:2029–44. doi: 10.1091/mbc.7.12.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richard M, Boulin T, Robert VJ, Richmond JE, Bessereau JL. Biosynthesis of ionotropic acetylcholine receptors requires the evolutionarily conserved ER membrane complex. Proc Natl Acad Sci. 2013;110:E1055–1063. doi: 10.1073/pnas.1216154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol. 2008;9:944–57. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang M-J, Ryoo HD. Suppression of retinal degeneration in Drosophila by stimulation of ER-associated degradation. Proc Natl Acad Sci. 2009;106:17043. doi: 10.1073/pnas.0905566106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chitwood PJ, Juszkiewicz S, Guna A, Shao S, Hegde RS. EMC is required to initiate accurate membrane protein topogenesis. Cell. 2018;175:1507–19. doi: 10.1016/j.cell.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bagchi P, Inoue T, Tsai B. EMC1-dependent stabilization drives membrane penetration of a partially destabilized non-enveloped virus. eLife. 2016;5:e21470. doi: 10.7554/eLife.21470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reboll MR, Korf-Klingebiel M, Klede S, Polten F, Brinkmann E, Reimann I, et al. EMC10 (endoplasmic reticulum membrane protein complex subunit 10) is a bone marrow-derived angiogenic growth factor promoting tissue repair after myocardial infarction. Circulation. 2017;136:1809–23. doi: 10.1161/CIRCULATIONAHA.117.029980. [DOI] [PubMed] [Google Scholar]
- 53.Wang T, Jiao Y, Montell C. Dissecting independent channel and scaffolding roles of the Drosophila transient receptor potential channel. J Cell Biol. 2005;171:685–94. doi: 10.1083/jcb.200508030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar JP, Ready DF. Rhodopsin plays an essential structural role in Drosophila photoreceptor development. Development. 1995;121:4359. doi: 10.1242/dev.121.12.4359. [DOI] [PubMed] [Google Scholar]
- 55.Leonard DS, Bowman VD, Ready DF, Pak WL. Degeneration of photoreceptors in rhodopsin mutants of Drosophila. J Neurobiol. 1992;23:605–26. doi: 10.1002/neu.480230602. [DOI] [PubMed] [Google Scholar]
- 56.Harel T, Yesil G, Bayram Y, Coban-Akdemir Z, Charng WL, Karaca E, et al. Monoallelic and biallelic variants in EMC1 identified in individuals with global developmental delay, hypotonia, scoliosis, and cerebellar atrophy. Am J Hum Genet. 2016;98:562–70. doi: 10.1016/j.ajhg.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geetha TS, Lingappa L, Jain AR, Govindan H, Mandloi N, Murugan S, et al. A novel splice variant in EMC1 is associated with cerebellar atrophy, visual impairment, psychomotor retardation with epilepsy. Mol Genet Genom Med. 2018;6:282–7. doi: 10.1002/mgg3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Down-regulated proteins identified in emc2A mutant
Down-regulated proteins identified in emc4 mutant
Down-regulated proteins identified in emc5 mutant
Down-regulated proteins identified in emc7 mutant