Abstract
The macrophage checkpoint is an anti-phagocytic interaction between signal regulatory protein alpha (SIRPα) on a macrophage and CD47 on all types of cells – ranging from blood cells to cancer cells. This interaction has emerged over the last decade as a potential co-target in cancer when combined with other anti-cancer agents, with antibodies against CD47 and SIRPα currently in preclinical and clinical development for a variety of hematological and solid malignancies. Monotherapy with CD47 blockade is ineffective in human clinical trials against many tumor types tested to date, except for rare cutaneous and peripheral lymphomas. In contrast, pre-clinical results show efficacy in multiple syngeneic mouse models of cancer, suggesting that many of these tumor models are more immunogenic and likely artificial compared to human tumors. However, combination therapies in humans of anti-CD47 with agents such as the anti-tumor antibody rituximab do show efficacy against liquid tumors (lymphoma) and are promising. Here, we review such trials as well as key interaction and structural features of CD47-SIRPα.
Keywords: CD47, SIRPα, immune checkpoint, phagocytosis
Statement of Significance: Immunotherapy with antibodies that block the T cell checkpoint now provide durable cures in some cancer patients, but many solid tumors remain a challenge in the clinic. Because such tumors are often replete with macrophages, the macrophage checkpoint CD47-SIRPα is an attractive target for blockade. This motivates understanding its current status in the clinic as well as structure–function determinants for new vulnerabilities.
INTRODUCTION
Cancer immunotherapy has rapidly expanded into the clinic over the past decade with significant success for therapies that target functionally suppressed immune cells in tumor microenvironments [1]. T cells have been the primary focus of cancer immunotherapy with immune checkpoint inhibitors developed to antagonize either CTLA-4 and PD-1 expressed on T cell membrane proteins, or PD-1’s ligand, PDL-1, which is on the surface of many cells including cancer cells [2,3]. While this receptor–ligand interaction normally inhibits an activated T cell, blocking inhibit is already used in this sentence. this paired receptor interaction with blocking antibodies enables suitably activated T cells to eliminate cancer cells. Dramatic and durable effects are seen in some patients for some malignancies, with tumors having high mutational loads being most likely to activate T cells, but most patients do not respond to this type of immunotherapy, which presents a challenge and an opportunity [4,5].
Macrophages are part of the innate immune response, are often abundant in solid tumors, and have a general ability to clear foreign cells through the activated process of phagocytosis [6,7]. Phagocytosis is modulated by a checkpoint interaction between the surface glycoprotein CD47 found on all cells and the signal regulatory protein alpha (SIRPα) on macrophages. [8,9]. This review focuses on the structure of CD47 and SIRPα, the role of this checkpoint in macrophage function, and therapeutic antibody strategies that target the CD47-SIRPα interaction in cancer clinical trials. We also examine the sequence–structure–function relationships of these paired receptors in efforts to stimulate new therapeutics.
The ubiquitous ‘marker of self’ ligand, CD47
CD47 is an integral membrane glycoprotein that is expressed in all normal and diseased tissues at the RNA and protein levels. This glycoprotein was first discovered as the overexpressed ovarian carcinoma antigen (OA3) [10]. It was also described as associating with β-integrin proteins and thus named integrin associated protein (IAP) [11]. The protein was found on the surface of erythrocytes (which lack integrins) through binding of two different antibodies and was then designated CD47 [12].
CD47 belongs to the immunoglobulin superfamily (IgSF) with a single N-terminal extracellular Ig-like domain, five transmembrane helices, and a C-terminal cytoplasmic tail. Four cytoplasmic tails range in length from four amino acids (Type 1) to 34 amino acids (Type 4), but the 16 amino acid tail isoform (Type 2) is the most abundant and is expressed on the majority of cells in humans and mice [13].
An X-ray crystal structure of CD47 reveals an IgV (variable) topology with α-helical as well as β-sheet secondary structures and a conserved intramolecular disulfide bridge spanning the middle of the β-sandwich [14]. An additional disulfide bridge also forms between the extracellular domain and one of the transmembrane domains, which is unusual for IgSF proteins and some evidence suggests it orients the Ig domain for optimal receptor binding [15]. CD47 interacts primarily with three categories of extracellular receptors: integrins, thrombospondin-1 (TSP-1) protein and SIRPα. Cell adhesion, cell migration, and regulation of inflammation and phagocytosis are among the reported functions of receptor interactions with CD47 [16].
CD47 was first termed a “marker of self” after CD47-deficient red blood cells (RBCs) from a mouse knockout (C57BL/6 strain) were found to be rapidly cleared from the circulation of wildtype mice by splenic macrophages [17]. The in vitro evidence is compelling that the CD47 interaction with SIRPα is a “don’t eat me” anti-phagocytic signal when occurring in parallel with some types of “eat me” signal—most clearly with IgG bound to the phagocytic target (Fig. 1). In principle, the expression of CD47 allows all cells, including cancer cells, to evade macrophage engulfment. Nonetheless, two mysteries continue to persist since this seminal observation: (i) CD47-knockout mice do not exhibit anemia or any evident RBC or platelet deficiencies, and (ii) the in vivo “eat me” signal on RBCs in CD47-knockout mice remains unclear. Some might argue that the clearance cue is the senescence signal that leads to RBC phagocytosis after circulating weeks (in mouse) or months (in human), but CD47-knockout RBCs are all cleared within 1–2 days in the circulation of the wildtype mouse implying that all CD47-knockout RBCs display the senescence signal.
Figure 1.

Phagocytosis is maximized by inhibiting CD47 on ‘self’ cells (the target) or SIRPα on macrophages in combination with antibodies that opsonize the target. CD47 binding to SIRPα signals “don’t eat me” to the macrophage (leftmost). Neither antibody blockade of CD47-SIRPα nor antibody opsonization of a target is sufficient to make target engulfment efficient (middle two), whereas the combination maximizes phagocytosis (rightmost).
The macrophage immune receptor, SIRPα
SIRPα is also an IgSF, integral membrane glycoprotein, and although it is expressed on many if not all cell types, its expression on hematopoietic cells is restricted to myeloid cells: macrophages, monocytes, dendritic cells, and granulocytes (and not T cells, etc.) [18]. SIRPα was first identified on rat fibroblasts as PTPNS1 (protein tyrosine phosphatase, non-receptor type substrate 1) in association with the cytoplasmic tyrosine phosphatase SHP-2 (Src homology region 2 domain containing phosphatase-2) [19]. SIRPα was later found to be expressed on human myeloid cells [20], although expression can vary even within subtypes of macrophages [21].
SIRPα has three IgSF domains, one N-terminal V-like domain (domain-1, D1) and two C1-like domains—which is a structure shared by a larger family of SIRPs [22,23]. One transmembrane helix connects to cytoplasmic tails of varying lengths that govern signaling in the SIRPs. SIRPα’s cytoplasmic tail has four tyrosine residues that conform to an immunoreceptor tyrosine-based inhibitory motif (ITIM), which mediates association with SHP-1 and SHP-2 for inhibitory signaling [19].
Two closely related SIRP members are SIRPβ and SIRPγ. SIRPβ has a short cytoplasmic tail (six amino acids) and lacks phosphatase binding motifs suggesting it lacks inhibitory activity. However, SIRPβ associates with DNAX activation protein 12 (DAP12) and can transmit activating signals [24]. SIRPγ has an even shorter cytoplasmic region (four amino acids) and is also unlikely to signal. Two uncharacterized members of the SIRP family are SIRPβ2 and SIRPδ [1,22].
The extracellular domains of the SIRP members share highly conserved sequence homology with very subtle differences [22,23]. X-ray crystal structures of D1 for each of SIRPα, SIRPβ, SIRPβ2, and SIRPγ closely resemble each other [14]. Additionally, SIRPα is known to be highly polymorphic [25]. Across 10 distinct human SIRPα alleles, 18 amino acids have been identified as polymorphic residues, all located in the N-terminal IgV domain of SIRPα.
While CD47 is the main extracellular ligand for SIRPα and might also weakly bind SIRPγ [8,14,22], additional extracellular ligands that interact with SIRPα include surfactant proteins A and D (Sp-A and Sp-D), found primarily in the lungs [26,27]. Insulin secretion and muscle formation are among some of the functions that somehow involve SIRPα [28]. However, the best characterized function of SIRPα is its role in inhibiting macrophage phagocytosis upon binding CD47 on another cell [1,9,29].
Binding of CD47-SIRPα and their other ligands
CD47 is the main ligand for SIRPα across mouse, rat and human [29], but the interaction is often weak with only sub-micromolar affinity [8,30,31]—as summarized here for various CD47 and SIRPα ligands (Table 1). Differences in reported affinities might reflect differences in methods (such as cell-based measurements, surface immobilized protein, affinity from ratio of rates, etc.) as well as differences in expression constructs (native transmembrane protein versus soluble constructs). It is nonetheless clear that the single N-terminal IgV domain of CD47 interacts with the D1 of SIRPα. The interaction between these paired receptors is species-specific to some extent, with limited cross reactivity across species [30]. The X-ray crystal structure of the human CD47-SIRPα complex reveals three distinct binding sites with the highest density of interactions occurring between the β-strands comprising the FG loop of CD47 and a wide binding pocket made up of SIRPα’s BC, C’D, DE, and FG loops [14]. More than 50% of the interfacial surface between the two proteins occurs at this site. Furthermore, about 45% of CD47’s contact residues with SIRPα consist of the 8 amino acids that make up the loop region between strands F and G. Their binding is mediated mainly by charge complementarity (SIRPα mostly positive and CD47 mostly negative). The FG loop in CD47 is conserved across different species which may explain why human-CD47 binds to some SIRPα polymorphs from different species—such as SIRPα in non-obese diabetic (NOD) mice [31] and also porcine SIRPα [32].
Table 1.
Known affinities of CD47-SIRPα ligands
| Ligand | Receptor | Affinity (μM) | Reference |
|---|---|---|---|
| SIRPαV1 | CD47 | 0.46/0.74 | Rodriguez [39]/Hatherley [38] |
| SIRPαV2 | CD47 | 1.0–2.0 | Brooke [8], Hatherley [104], Hatherley [14] |
| SIRPαV2 | CD47 | 0.44/0.64 | Rodriguez [39]/Hatherley [38] |
| SIRPαV3 | CD47 | 0.84 | Rodriguez [39] |
| SIRPαV4 | CD47 | 0.91 | Rodriguez [39] |
| SIRPαV5 | CD47 | 2.50/0.78 | Rodriguez [39]/Hatherley [38] |
| SIRPαV6 | CD47 | 0.30 | Rodriguez [39] |
| SIRPαV7 | CD47 | 3.21/0.65 | Rodriguez [39]/Hatherley [38] |
| SIRPαV8 | CD47 | 0.65 | Rodriguez [39] |
| SIRPαV9 | CD47 | 1.14 | Rodriguez [39] |
| SIRPαV10 | CD47 | 0.08/0.67 | Rodriguez [39]/Hatherley [38] |
| NOD SIRPα | CD47 | 0.08 | Kwong [31] |
| ‘Self’ peptide | SIRPα | 0.16 | Rodriguez [39] |
| FD6 | CD47 | 4.1  10 -5 10 -5
|
Weiskopf [55] |
| CV1 | CD47 | 1.1  10–5 10–5
|
Weiskopf [55] |
| PKHB1 (peptide) | CD47 | ‘micromolar’ affinity | Martinez-Torres [105] |
| CD47AP | SIRPα | 1.1 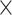 10–2 10–2
|
Lee [94] |
| N3612 (Velcro CD47) | SIRPαV1 | 2.5  10−3 10−3
|
Ho [96] |
| SIRPαV2 | 3.7  10−4 10−4
|
||
| DSP-107 (SIRPα-41BBL) | CD47 | 1.5  10−3 10−3
|
Gozlan [106] |
Binding of CD47 to other ligands such as integrins, TSP-1 and TSP-1-derived peptides, also involves the IgV domain of CD47 [16]. While the precise regions of interaction with integrins have not been determined, binding studies have shown that CD47 can activate integrins in cis independently as well as while bound to TSP-1 derived peptides or SIRPα [33–35], indicating that the binding site for integrins on CD47 is not occupied by either protein. Binding of CD47 to TSP-1, however, inhibits the binding of SIRPα. This finding was further demonstrated with a monoclonal anti-CD47 antibody, B6H12, that inhibited binding of both ligands to CD47 [36]. Although these results suggest that TSP-1 and SIRPα compete for overlapping binding sites on CD47, mutational and biochemical studies have also revealed that post-translational modifications of a critical serine residue on CD47 away from the SIRPα binding site is required for TSP-1 binding [37].
SIRPα also interacts with ligands other than CD47 such as surfactant proteins (in lung) Sp-A and Sp-D, respectively. Sp-D has been shown to bind SIRPα in D3, rather than D1 [27]. CD47 binding to SIRPα in D1 is not impaired in the presence of Sp-D. Sp-A binds SIRPα; however, the binding site is currently unknown. While the binding domain of SIRPα is highly polymorphic in varying between individuals, there has been controversy on whether the polymorphic residues affect CD47 binding. The crystal structure reveals that the 18 polymorphic amino acids all lie outside of the CD47 interaction interface [14,38], although this does not preclude an allosteric effect that is common in protein-protein interactions. Indeed, CD47 affinity to the different SIRPα alleles seems to vary [39]. Separate data suggest SIRPα polymorphism alters post-translational modifications which could also affect CD47 engagement [25].
CD47-SIRPα as an immune checkpoint
Inhibitory immune signaling occurs upon CD47 binding, with phosphorylation of the ITIM motifs in SIRPα that then recruit and activate the cytoplasmic phosphatases SHP-1 and SHP-2 [40–42]. Downstream targets of dephosphorylation include paxillin and nonmuscle myosin IIA, decreasing the efficiency of phagocytosis analogous to direct inhibition of nonmuscle myosin IIA—at least for IgG-opsonized targets [43]. Integrin mediated activation might also lead to the recruitment of the phosphatases and enhanced inhibitory phosphorylation signals [44].
When a target for phagocytosis is IgG opsonized, engulfment begins with the activation of Fc receptors (FcRs) on the surface of the phagocytic cell. This activation leads to the formation of a “phagocytic synapse” with rapid cytoskeletal rearrangement and accumulation of signaling proteins inside the macrophage at its point of contact with the targeted cell, microbe, or particle. The three main events that occur at the synapse are adhesion of the cell or particle with the phagocyte, pseudopod extension of the phagocyte around the target and final internalization [45]. CD47 on the target does not eliminate adhesion, but tends to impede the pseudopod formation and significantly suppresses the internalization.
The initial description of elevated CD47 levels in ovarian cancer followed by the characterization of its role in signaling “don’t eat me” to macrophages eventually inspired investigation of CD47 as a therapeutic target in cancer—particularly because CD47 tends to be modestly elevated in many hematologic and solid malignancies [46–49]. Many proof-of-principle applications have been developed to target the CD47-SIRPα immune checkpoint including fully humanized anti-CD47 antibodies, anti-SIRPα antibodies, SIRPα-fusion IgG proteins, among other protein and peptide antagonists. Table 2 summarizes these antagonists and their in vitro applications against various types of malignancies. Early preclinical studies demonstrated the efficacy of anti-CD47 treatment of various malignancies, with many of these indicating activity as a mono-therapy [46,49–51]. Importantly, however, anti-CD47 is usually species specific, so human tumors are easily targeted as xenografts given no binding to any mouse cells; in addition, anti-CD47 an opsonizing IgG, and so it is difficult with monotherapy to identify the results as (i) the pro-phagocytic effects of opsonizing a cancer cell—which is not novel but potentially useful, and/or (ii) blocking the anti-phagocytic effects of the “do not eat me” signal—which is novel. Antibody-dependent phagocytosis activates the macrophage FcR, which directs the macrophage cytoskeleton towards the target [52,53]. Investigations with FcR-deficient mice and with Fab blocking antibodies (lacking the Fc chain) have suggested the mechanism of antibody-dependent macrophage phagocytosis differs depending on the type of malignancy [54]. For a few cancers, it seems sufficient to interrupt the CD47-SIRPα interaction, but it is ineffective for many cancers, especially solid tumors [55,56].
Table 2.
CD47-SIRPα immune checkpoint inhibitors used for in vitro phagocytosis assays against cancer cells
| Cancer type | CD47 antagonists | SIRPα antagonists |
|---|---|---|
| Acute lymphoblastic leukemia | B6H12 [50,107], BRIC126 [107], TTI-622 [74], ZF1 [108] | anti-mouse SIRPα (not specified) [107] |
| Acute myeloid leukemia | anti-CD47 (not specified) [109], B6H12 [48,50,100], BRIC126 [48], C47B222 [100], DSP-107 (CD47/4-1BB bispecific) [106], Magrolimab [98], NI-1701 (CD47/CD19 bispecific) [110], SIRPα-FC [111], SRF231 [79], TTI-621 [72], TTI-622 [74], ZF1 [108] | P84 [47] |
| B-cell lymphoma | ALX148 [77], B6H12 [50,112–114], BRIC126 [113], CD20-CD47LL (CD47/CD20 bispecific) [115], CD20-CD47SL (CD47/CD20 bispecific) [115], CV1 [55], FD6 [55], DSP-107 [106], Inhibrix [116], NI-1701 [110], SRF231 [79], TG-1801 (CD47/CD20 bispecific) [117], TTI-621 [72], TTI-622 [74] | 040 [118], SE12C3 [118], ADU-1805 [97], KWAR23 [101], N3612 [96] |
| Bladder cancer | anti-CD47 (not specified) [119], B6H12 [46,50] | None |
| Brain cancer | B6H12 [46,50], BRIC126 [46], Magrolimab [49,120,121] | None |
| Breast cancer | B6H12 [46,122], BRIC126 [46], CV1 [55], FD6 [55], RRx-001 [123], TTI-621 [72] | 1.23A [122], 12C4 [122], 040 [118], SE12C3 [118], KWAR23 [101], N3612 [96] |
| Chronic lymphocytic leukemia | PKHB1 [105] | None |
| Chronic myeloid leukemia | B6H12 [50], TTI-621 [72] | None |
| Colon cancer | ALX148 [77], B6H12 [46,124], BRIC126 [46], CV1 [55], FD6 [55], DSP-107 [106], TTI-621 [72] | FAB 119 [83], FAB 136 [83], KWAR23 [101], N3612 [96] |
| Colorectal cancer | TTI-622 [74] | None |
| Endometrial cancer | B6H12 [125] | None |
| Epidermoid cancer | TTI-621 [72] | None |
| Esophageal cancer | ALX148 [77] | FAB 119 [83], FAB 15 [83], FAB 136 [83] |
| Gastric cancer | B6H12 [126] | None |
| Hepatocellular cancer | Ab400 (cross reacts human and mouse CD47) [127], B6H12 [127] | None |
| Leiomyosarcoma | B6H12 [128] | None |
| Lung cancer | CV1 [99], FD6 [99], DSP-107 [106], Magrolimab [99], RRx-001 [123], SIRPαD1-Fc [129], TTI-621 [72], TTI-622 [74] | SE7C2 [21] |
| Melanoma | A4 (anti-mouse CD47) [56], TTI-621 [72] | MY-1 (anti-mouse SIRPα) [102], P84 (anti-mouse SIRPα) [102] |
| Medulloblastoma | Magrolimab [49] | None |
| Myelodysplastic syndrome | TTI-622 [74] | None |
| Myeloma | ALX148 [77], B6H12 [130], TTI-621 [72] | None |
| Osteosarcoma | Ab400 [131], B6H12 [131] | None |
| Ovarian cancer | B6H12 [46], BRIC126 [46], DSP-107 [106], TTI-621 [72] | None |
| Pancreatic cancer | B6H12 [132,133], CV1 [132], FD6 [132], Magrolimab [132] | None |
| Pharynx cancer | DSP-107 [106] | None |
| Renal carcinoma | None | KWAR23 [101], MY-1 [102], P84 [102] |
| Skin cancer | TTI-621 [72] | None |
| T-cell lymphoma | B6H12 [134], SRF231 [134], TTI-621 [72] | None |
| T-cell leukemia | B6H12 [100], C47B157 [100], C47B161 [100], C47B222 [100], TTI-621 [72] | SE7C2 [21] |
Macrophages also express CD47, and recent evidence suggests this interacts in cis with SIRPα. As with the trans interaction, the cis interaction leads to relatively high phosphorylation of SIRPα’s cytoplasmic tail and to relatively low levels of phagocytosis compared to CD47-knockout macrophages [57]. The potency of an anti-CD47 therapy might thus reflect the cumulative effects of inhibiting trans interactions between a macrophage and a cancer cell as well as inhibiting passivating cis interactions on the same macrophage.
Clinical targeting CD47-SIRPα in cancer
Decades ago, one anti-CD47 antibody was injected into ovarian cancer patients in order to image the tumors; the study demonstrated some targetability but provided no insight into therapeutic effects or safety issues [58]. This of course pre-dates by a decade the description in mouse of CD47 as a ‘Marker of Self’. [17] Over the past decade, CD47 has indeed emerged as a potential therapeutic target for macrophage checkpoint blockade in clinical trials against cancer, with monoclonal antibodies being the primary antagonists.
Clinical trials up to Phase 2 have rapidly expanded in numbers, diversity of approach, and targets studied [59–61]. Key strategies and current results from trial reports and conference proceedings are reviewed here (Table 3). A main conclusion is that monotherapy with anti-CD47 shows little to no efficacy across multiple cancer types when administered systemically, and while it often leads to rapid loss of a large fraction of blood cells (consistent with rapid loss of CD47-knockout mouse blood cells upon infusion in normal mice [17]), anti-CD47 can show efficacy in humans in combination therapies. In reviewing the clinical trials (below) with this macrophage checkpoint blockade, it seems that some efforts with anti-CD47 are based on the hope that human tumors would possess macrophage activating activity that could be unleashed by simply preventing the inhibitory signaling from CD47-SIRPα (i.e. a monotherapy). As noted earlier, T cell checkpoint blockade (using antagonists of PD-1’s interaction with PD-L1) succeeds primarily against human tumors with high mutational loads that tend to activate T cells via their T-cell receptor (TCR) [4,5].
Table 3.
Therapeutic CD47-SIRPα antibodies currently being investigated in the clinic
| Drug | Company | Clinical trials | Phase | Status | Targets | Combinations |
|---|---|---|---|---|---|---|
| Magrolimab (Hu5F9-G4) | Forty Seven, Inc. | NCT02678338 | Phase 1 | Completed | Acute myeloid leukemia, myelodysplastic syndrome | Monotherapy |
| NCT02216409 | Phase 1 | Completed | Solid tumors | Monotherapy | ||
| NCT03248479 | Phase 1 | Ongoing | Acute myeloid leukemia, myelodysplastic syndrome | Monotherapy, azacitidine | ||
| NCT02953782 | Phase 1/2 | Ongoing | Colorectal neoplasms, solid tumors | Cetuximab | ||
| NCT02953509 | Phase 1/2 | Ongoing | Lymphoma, Non-Hodgkin lymphoma, Large B-Cell, diffuse indolent lymphoma | Rituximab | ||
| TTI-621 | Trillium Therapeutics, Inc. | NCT02663518 | Phase 1 | Ongoing | Hematologic malignancies, sold tumors | Monotherapy, rituximab, nivolumab |
| NCT02890368 | Phase 1 | Ongoing | Solid tumors, mycosis fungoides | Monotherapy, PD-1/PD-L1 inhibitor, pegylated interferon-α2a, T-Vec, radiation | ||
| TTI-622 | NCT03530683 | Phase 1 | Ongoing | Lymphoma, myeloma | Monotherapy, rituximab, PD-1 inhibitor, proteasome-inhibitor regimen | |
| CC-90002 | Celgene | NCT02367196 | Phase 1 | Ongoing, not recruiting | Hematologic neoplasms | Monotherapy, rituximab |
| ALX 148 | ALX Oncology, Inc. | NCT03013218 | Phase 1 | Ongoing | Solid tumors, Non-Hodgkin lymphoma | Monotherapy, pembrolizumab, trastuzumab, rituximab, ramucirumab + paclitaxel, 5-FU + cisplatin |
| SRF231 | Surface Oncology | NCT03512340 | Phase 1 | Ongoing | Advanced solid cancers, hematologic cancers | Monotherapy |
| AO-176 | Arch Oncology | NCT03834948 | Phase 1 | Ongoing | Solid tumors | Monotherapy |
| BI 765063 (OSE-172) | OSE Immunotherapeutics, Boehringer Ingelheim | NCT03990233 | Phase 1 | Ongoing | Solid tumors | Monotherapy, PD-1 inhibitor |
| HX009 | Waterstone Hanxbio Pty Ltd. | NCT04097769 | Phase 1 | Ongoing | Advanced solid tumors | Monotherapy |
| TJ011133 (TJC4) | I-Mab Biopharma, Co. Ltd. | NCT03934814 | Phase 1 | Ongoing | Solid tumors, lymphoma | Monotherapy, pembrolizumab, rituximab |
| IBI-188 | Innovent Biologics (Suzhou) Co. Ltd. | NCT03763149 | Phase 1 | Ongoing | Advanced malignancies | Monotherapy |
| NCT03717103 | Phase 1 | Ongoing | Advanced malignancies | Monotherapy, rituximab |
Magrolimab, previously known as Hu5F9-G4, is the anti-human-CD47 monoclonal that is most advanced in clinical trials. Two Phase 1 dose-escalation trials have been completed in acute myeloid leukemia (AML) and solid tumors. Magrolimab is a humanized monoclonal IgG4 antibody that was engineered to not only block CD47 signaling but to also minimize engagement of FcRs and thereby limit macrophage activation [49]. This is because the IgG4’s Fc region has weaker affinity for FcRs compared to other IgG subtypes; Magrolimab is therefore more likely to work as an inhibitor and less as an opsonizing antibody. On the other hand, CD47 expression on all cells in the body means that there is a large sink for infused anti-CD47.
First reports of efficacy required a combination treatment of magrolimab and ritixumab (anti-CD20) in relapsed/refractory (r/r) non-Hodgkin’s lymphoma (NHL) patients that were refractory to rituximab alone [62]. Phase 1b results showed 36% complete response rate (CRR) and 50% objective response rate (ORR) for a small cohort of a few dozen patients. Addition of the tumor-specific antibody to activate macrophage effector functions is a growing trend in CD47 blockade trials, reflecting the need for pro-phagocytic cues (e.g. antibody engagement of FcRs) in combination with blockade of ‘don’t eat me’ signals to drive tumor regression. CD24 was recently proposed as another cell-surface ‘do not eat me’ signal and target, although in magrolimab-treated Phase 2 NHL patients, neither CD24 nor CD47 showed prognostic value [63,64]. In another combination Phase 1b trial with the chemotherapeutic azacitidine, ongoing results reported 92% ORR in untreated higher-risk myelodysplastic syndrome (MDS) and 64% ORR in untreated AML patients [65,66]. A tentative mechanism for this combination is that azacitidine results in surface display of pro-phagocytic calreticulin (normally intracellular), which synergizes with CD47 blockade in cancer cell phagocytosis [67]. These latest data contributed to Forty-Seven, Inc’s multi-billion dollar acquisition by the much larger firm, Gilead Sciences, announced in March 2020.
TTI-621 and TTI-622 are SIRPα-Fc fusion proteins in trials against hematologic and solid malignancies [68–71]. Both consist of the CD47-binding domain of human SIRPα fused to a human Fc domain: IgG1 for TTI-621 and IgG4 for TTI-622. The IgG1 domain of TTI-621 contributes to its increased potency, at least in preclinical models [72]. The TTI’s were reported to have no affinity for human RBCs, but a re-analysis of TTI-621 data suggests otherwise. Magrolimab (5F9) and BRIC126 clearly cause hemagglutination by antibody-mediated cross-bridging [72], which is not observed with TTI-621 and other select anti-CD47 agents (Fig. 2A). On the other hand, addition of ‘saturating concentrations’ (~1 μM based on hemagglutination results) to RBCs and then assayed for binding by flow cytometry, TTI-621 (and also TTI-622) gives a signal well above several non-specific antibodies albeit far below several anti-CD47 antibodies; the difference allows one to estimate a weak sub-μM affinity of TTI-621 for RBCs (Fig. 2B). This is only slightly weaker than TTI-621 binding (with ~10 nM to ~1 μM affinities) to fresh white blood cells and platelets as well as to primary hematopoietic tumor samples, and to various human tumor cell lines (Fig. 2C). Curiously, the effective concentrations (EC50) for phagocytosis of the tumor cell lines was ~10–100-fold stronger (~nM) than the above binding affinities, which perhaps relates to dominance of the Fc domain, and it is also curious that RBC phagocytosis results have not been reported. Indeed, tight binding of ~10 nM does not predict efficient phagocytosis (Fig. 2D). Although TTI-621 showed some efficacy when administered intratumorally to patients with cutaneous T-cell lymphoma (mycosis fungoides), intravenous administration showed grade 3 thrombocytopenia in 18% of a varied cohort of leukemia, lymphoma, and other solid tumor patients (25% overall showed some level of thrombocytopenia). It should be noted that platelet measurements are much noisier than RBC counts, and confident measurements of cytopenias/anemias also require measurements of any compensating production (e.g. reticulocytes). Despite potential safety concerns, monotherapies with TTI-621 in B- and T-cell lymphomas produce 18–29% ORR at low doses (0.5 mg/kg) with dose escalation in progress, which is unlike other anti-CD47 monotherapies under clinical study [73]. TTI-622 is being studied in combination with other tumor-specific agents, including rituximab and a PD-1 inhibitor to engage adaptive immune responses with continued claims of preferential tumor cell phagocytosis and no RBC binding [74]. For a deeper understanding of mechanism, future experiments should address RBC phagocytosis effects (i.e. EC50 in vitro) as well as the effect of bivalent/multivalent protein/peptide binding and blocking of SIRPα in the absence of a Fc domain.
Figure 2.

Novel re-analysis of TTI-621 binding and phagocytosis data from Ref. [72]. (A) Molecular partition function (ξ) fitting to the hemagglutination data. K1 and K2 are the association constants, inversely related to dissociation constants or EC50. Schematic of possible binding states of various CD47 affinity agents is shown for two apposed RBC membranes. Magrolimab (5F9) and BRIC126 both exhibit high hemagglutination and show cross-bridging, which can be fit (5F9: K1 = 1.2  10−2 nM−1, K2 = 0.24 nM−1; BRIC126: K1 = 6.6
10−2 nM−1, K2 = 0.24 nM−1; BRIC126: K1 = 6.6 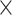 10−2 nM−1; K2 = 1.9 nM−1), whereas TTI-621, B6H12, and 2D3 do not. (B) TTI-621 shows non-zero binding to RBCs, which is weaker than anti-CD47 antibodies but consistent with past reports of sub-μM affinity between CD47 and SIRPα [39]. Inset: same data plotted with y-axis on log scale. Note that the plot follows the same color scheme as in (A). (C) TTI-621 binding data show sub-μM affinity for white blood cells, primary tumor samples, and human tumor cell lines. Phagocytosis of the human tumor lines requires less binding for effective phagocytosis. (D) TTI-621 binding affinities do not predict phagocytic efficiency across various cancer cell types. BR.C: breast cancer, AML: acute myeloid leukemia, BCL: B cell lymphoma, MM: multiple myeloma, and TCL: T-cell lymphoma.
10−2 nM−1; K2 = 1.9 nM−1), whereas TTI-621, B6H12, and 2D3 do not. (B) TTI-621 shows non-zero binding to RBCs, which is weaker than anti-CD47 antibodies but consistent with past reports of sub-μM affinity between CD47 and SIRPα [39]. Inset: same data plotted with y-axis on log scale. Note that the plot follows the same color scheme as in (A). (C) TTI-621 binding data show sub-μM affinity for white blood cells, primary tumor samples, and human tumor cell lines. Phagocytosis of the human tumor lines requires less binding for effective phagocytosis. (D) TTI-621 binding affinities do not predict phagocytic efficiency across various cancer cell types. BR.C: breast cancer, AML: acute myeloid leukemia, BCL: B cell lymphoma, MM: multiple myeloma, and TCL: T-cell lymphoma.
CC-90002 is a humanized, high affinity (sub-nanomolar) monoclonal IgG4 CD47 antibody in Phase 1 trials against advanced solid and hematologic malignancies in combination with rituximab, with an earlier trial terminated due to discouraging safety profiles. In r/r NHL patients of the combined trial, 13% showed a response rate with 25% showing stable disease [75], but 50% showed anemia (of any grade) with 33% showing thrombocytopenia.
ALX 148 is a fusion protein that consists of the CD47 binding domains of SIRPα and a fully inactive Fc domain [76,77]. Notably, its molecular mass is 50% that of a typical antibody, which may enable lower dosing (e.g. 10 mg/kg) to saturate CD47 targets. The most recent reported data show that just 13.3% and 6.7% of patients (n = 30) show thrombocytopenia and anemia, respectively, in a combination cohort with ALX148 and trastuzamab (anti-HER2). Another cohort receiving ALX148 and pembrolizumab (anti-PD1) reported 7.7% in both of the same measures [78]. In a cohort for r/r NHL with rituximab, the maximum tolerated dose was not reached, similar levels of anemia and thrombocytopenia were shown, and ongoing preliminary ORRs varied from 31% to 50% depending on tumor type.
Safety concerns with anti-CD47 remain due to the lack of specificity in targeting a ubiquitously expressed protein. Anemia and thrombocytopenia are widespread in patients and only partially mitigated by priming and dosing strategies [59]. One fully human monoclonal antibody, SRF231, caused blood toxicities at such low doses (12 mg/kg) halting further expansion cohorts in its Phase 1 trial [79]. The addition of tumor-specific agents alongside anti-CD47 may increase efficacy but does not necessarily address safety issues, even in the case of bispecific or Fc-inactive antibodies.
Other current candidates in early trials have yet to report results as they monitor patient safety and dosing profiles such as AO-176, a humanized monoclonal anti-CD47 IgG2 antibody, and HX009, an anti-PD-1/CD47 bispecific antibody [67]. IBI-188 is a CD47 IgG4 monoclonal antibody under Phase 1 trials in the US and China against advanced malignant tumors and lymphomas [80]. TJC4 (also known as TJ011133) is another CD47 monoclonal antibody that recently entered Phase 1 trials in the US for solid tumors and lymphoma in combination with pembrolizumab and rituximab [81,82]. Many other drugs are in active preclinical development by startups and major pharmaceutical companies. The expanding field of candidates indicates an exciting but potentially challenging time in the development of CD47 therapeutics for cancer.
SIRPα is also a target for antibody blockade under preclinical and clinical study in efforts to address the safety and efficacy concerns of early CD47 drugs, especially given CD47’s ubiquitous expression [83]. Several anti-SIRPα antibodies are in active development in efforts to augment anti-tumor responses and overcome the significant off-target toxicities with anti-CD47 [84]. BI 765063/OSE-172 is a monoclonal SIRPα antagonist in a Phase 1 trial that dosed its first patient in June 2019 as a monotherapy and in combination with an anti-PD-1 monoclonal antibody [85].
Sequence–function relationships for CD47-SIRPα
Understanding the residues in CD47 and SIRPα that are key to binding and function will assist in developing new classes of checkpoint blocking proteins and peptides. Antibodies used for blocking are extremely large, glycoprotein complexes with >1000 amino acid residues (~150 kDa). They also possess multiple disulfide bridges that require specialized eukaryotic machinery to faithfully produce the numerous post-translational modifications. For these reasons and more, monoclonal antibodies with specificity for one protein such as CD47 are costly to produce in large quantities even though Good Manufacturing Practice for monoclonals is now a mainstay in biopharma [86]. Indeed, the average annual cost to a patient for a monoclonal antibody treatment is about $100 000, which adds greatly to the rapidly rising costs of drugs and healthcare [87,88].
The co-crystal structure of CD47-SIRPα shows 13 residues in CD47 that contact 12 residues in SIRPα (polymorphic variants 1 and 2) through hydrogen bonding and salt bridges [14,38]. Cross-species interactions, such as between pig CD47 and human SIRPα [30] or between human CD47 and NOD mouse SIRPα [31], have a potential basis in some critical contact residues based on sequence alignments (Fig. 3A). Contact residues in human CD47 are all conserved in pig CD47 except for Lys-6, which is an Ile in pig. From the crystal structure, this residue is outside of the CD47 FG binding loop, and Lys is similar in size to Ile, making it likely that contact is maintained. For similar reasons, monkey CD47 that shares the same contact residues as human CD47, and dog CD47 that shares the same contact residues as pig CD47, should both bind human SIRPα. Mouse and rat CD47 have two non-conserved mutations at human residues Asp-46 and Glu-106, respectively. Mutating Asp to a bulky Tyr residue should interfere with the FG loop in SIRPα and remove an important H-bond. Replacing the negative Glu with a positively charged Lys eliminates a critical salt bridge with Lys-53 in SIRPα’s binding pocket. Likewise, cow, sheep, and chicken all have mutations at critical H-bonding sites, which explain the lack of binding to human SIRPα.
Figure 3.

Sequence alignment and crystal structures reveal constant contact residues critical for cross-species reactivity and ligand binding for both CD47 and SIRPα. (A) Sequence overlays of CD47 and SIRPα, respectively, reveal conserved residues across different species. Green highlighted residues are conserved relative to human wildtype sequence. Blue highlighted residues are non-conserved mutations relative to human wildtype; however, maintain H-bonding. Red highlights are non-conserved mutations. Porcine CD47 binds human SIRPα and this can be seen from the conservation of most of the contact residues. Based on this, monkey CD47, which shares the same contact residues as human CD47, and dog CD47, which shares the same contact residues as pig CD47, should bind to human SIRPα. Likewise, when comparing SIRPα variants across different species, the conservation of contact residues among the sequences of NOD mice and pig SIRPα with human SIRPα provide some rationale as to why human CD47 interacts with these variants. Based on this, human CD47 should interact with monkey and dog SIRPα. Crystal structures of various (B) CD47 and (C) SIRPα bound inhibitors. For all antibody bound structures, only the first 100 residues in each of the heavy and light chains are shown. CD47 and SIRPα contact residues in each complex are highlighted in red. Inset tables list all contact residues in the respective receptors and how many times each contact residue is involved in binding across the various complexes. (B) PDB codes 2JJS (CD47/SIRPαV2), 5IWL (CD47/magrolimab), 5TZ4 (CD47/B6H12), 4KJY (CD47/FD6), 5TZ2 (CD47/C47B222), and 5TZT (CD47/C47B161). (C) PDB codes 2JJS (SIRPαV2/CD47), 6NMR (SIRPαV1/FAB 119), 4CMM (SIRPαV1/CD47), and 6BIT (SIRPαV1/KWAR23).
The 12 contact residues in human SIRPα are conserved across its polymorph variants that all bind human CD47 [39]. NOD-SIRPα reportedly binds human CD47 65-fold more tightly than human SIRPα [31]. Sequence analysis reveals conserved mutations with SIRPαV1 except at residues Gln-52 and Lys-53 (Fig. 3A). From crystal structure analysis, the H-bond formed via Lys-53 is potentially maintained with a Thr mutation found in NOD-SIRPα, a possible explanation for the increased affinity may be due to the increased hydrophobicity of the Q52F mutation. Phe-52 has the propensity to engage in hydrophobic interactions with pyroGlu-1 in SIRPα which might compensate for the loss of the noncritical H-bond. Variance in mouse SIRPα shows that Lys-53 is mutated to aliphatic Ala, eliminating a critical H-bond with Glu-106 in CD47 and perhaps explaining the lack of human CD47 binding. Moreover, two residues in mouse SIRPα (Ser-102 & Glu-103) are absent in NOD-SIRPα and in human SIRPα, which suggests enhanced CD47 affinity for NOD-SIRPα relative to other mouse SIRPα’s.
Interestingly, human CD47 binding to pig SIRPα inhibits phagocytosis, which indicates that the sequence variance between pig and human SIRPα does not prevent signaling [32]. Two contact residue changes between human and pig, Q52F, which is the same mutation found in NOD mouse strains, and G97E, a nonconserved mutation that introduces a salt bridge interaction with Lys37 in CD47 (Fig. 3A). In NOD-SIRPα, the Q52F mutation seemingly enhanced CD47 affinity suggesting the same may be true with pig SIRPα, especially with the addition of a favorable H-bonding interaction at Gly97. However, phagocytosis is inhibited by the interaction of NOD-SIRPα and human CD47, implying that the sequence complementarity of the remaining contact residues, namely Lys53, between the paired receptors is important for signaling regardless of species. When comparing this to the 10 polymorphs of human SIRPα, which all bind human CD47 [25,38,39], the resultant “don’t eat me” signal is dependent on which SIRPα variant CD47 interacts with, even though both are from the same species [89]. When comparing monkey and dog SIRPα sequences, the contact residues are also conserved in the same manner as CD47 (monkey conserved with human sequence and dog conserved with pig sequence except at Gly97). This becomes significant for preclinical safety and efficacy models and modulating engraftment of human cells in other species.
Although both CD47 and SIRPα are glycosylated post-translationally, glycosylation is not a requisite of CD47-SIRPα interaction, with amino acid residues driving the binding [90,91]. Monomeric, recombinant CD47 and SIRPα expressed in E. coli and lacking glycosylation indeed disrupt the CD47-SIRPα interaction in vitro [92]. Glycosylation of SIRPα and of CD47 may sometimes inhibit their binding [93] but otherwise seem important for cis dimerization of SIRPα on the surface of cells [94]. An important post-translational modification, however, is the N-terminal modification of CD47 by glutaminyl-peptide cyclotransferase-like protein (QPCTL) to produce pyroglutamate [95]. This modification has been demonstrated to contribute to SIRPα binding as well as signaling, although the earlier results with CD47 expressed in E. coli did not seem to account for this modification [92]. Nonetheless, inhibiting QPCTL enhanced antibody-mediated phagocytosis [95].
Major advances have been made in engineering high affinity versions of CD47 and SIRPα to function as immune checkpoint inhibitors. The most potent protein CD47 inhibitors developed are FD6 and CV1, which inhibit SIRPα binding at, remarkably, picomolar concentrations [55]. Analysis of the sequence and contact points between wildtype SIRPα and these engineered variants shows that three contact residues are mutated: K53R (conserved), E54Q (non-conserved), and L66T (non-conserved compared to SIRPαV1 but conserved compared to V2). The remaining nine mutations in FD6 (six mutations in CV1) appear to contribute to the stability of the engineered variants and add more hydrophobic contacts with CD47. It is important to note that these engineered variants cross-react with mouse CD47. Notably, an engineered CD47 variant, Velcro-CD47 N3612, potently antagonized SIRPα with no changes made to the binding region [96]. Rather, a three amino acid extension was added (Trp-Gln-Pro) to the N-terminus of CD47 and only a single point mutation made on Gln-1 (pyroGlu) to a Pro residue. Adding additional N-terminus contact residues between CD47 and SIRPαV1 and V2 effectively enhanced CD47 affinity to nanomolar and picomolar concentrations for the SIRPα variants, respectively. A 21-amino acid ‘Self’ peptide derived from the FG binding loop of CD47 was also shown to bind, antagonize SIRPα, and inhibit phagocytosis, suggesting that binding and function primarily converge to this sequence [39].
Pan-allelic anti-SIRPα antibodies that interact with more than one polymorph and/or species of SIRPα have also been engineered in order to overcome limitations that arise in targeting various polymorphs of SIRPα. One study discovered various classes of pan-allelic antibodies against SIRPα variants that antagonized human, mouse and monkey SIRPα [83]. Interestingly, some of these anti-SIRPα antibodies promote phagocytosis without physically blocking the SIRPα binding groove and inhibiting CD47 interaction—although the mechanism remains unknown. A second study reports on ADU-1805, a humanized pan-allelic anti-SIRPα antibody that interacts with SIRPα variants 1, 2, and 8 [97]. ADU-1805 blocks CD47 binding to SIRPα and SIRPγ, and it does not bind to SIRPβ. Pan-allelic agents that bind all SIRPα variants as well as pan-allelic peptides and proteins have yet to be discovered.
Structure–function relationship of the CD47-SIRPα axis
In addition to sequence analysis, crystal structures also assist in determining important structural factors that lead to potent antagonism (Fig. 3B). For the fully humanized antibody magrolimab (Hu5F9-G4) that was made to block CD47 [98], the crystal structure reveals a magrolimab-CD47 binding complex like that of the SIRPα-CD47 complex showing magrolimab competing for the same SIRPα binding site [99]. Crystal structures of the older monoclonal B6H12 as well as hybridoma (C47B161) and phage (C47B222) derived monoclonal anti-CD47 antibodies also show that SIRPα is inhibited due to competitive binding to the same CD47 FG loop binding site [100]. 2D3 is a monoclonal anti-CD47 antibody that binds CD47 but reportedly does not block the interaction with SIRPα nor the inhibitory signal, indicating it interacts at a site away from the CD47 FG binding loop [48].
SIRPα directed antagonists likewise bind and block CD47 by competing for the ligand binding groove in SIRPα (Fig. 3C). KWAR23, an anti-SIRPα blocking antibody, overlaps the same binding region as CD47, revealing a basis for competitive binding [101]. Most recently, a series of blocking and non-blocking anti-SIRPα antibodies have been crystalized in complex with SIRPα [83]. The blocking antibodies all compete for the same binding site in SIRPα as CD47; however, one antibody epitope shares only a single common residue with CD47 in the SIRPα binding groove, but is enough to displace CD47 engagement. These anti-SIRPα blocking and non-blocking antibodies, were potent to different degrees in promoting phagocytosis of colon and esophageal carcinoma cells in vitro. These effects were also observed with monoclonal anti-mouse SIRPα, P84, which does not block CD47 binding, but rather inhibits SIRPα signaling by some other mechanism to promote macrophage phagocytosis [102].
When comparing the crystal structures of bound CD47 and SIRPα, respectively, there are conserved contact residues in both proteins that interact with the bound ligand. In CD47, Thr-102 is involved in binding with all the potent antagonists as seen in the crystal structures (Fig. 3B). Likewise, Lys-96 in SIRPα is a conserved contact residue (Fig. 3C). Considering which residues are conserved in terms of binding can assist in rational design of protein, peptide, and small molecule inhibitors that are reminiscent of the binding interface of either CD47 or SIRPα based on the overall fold and positioning of these conserved contact residues.
It remains unclear whether CD47 binding to SIRPα leads to structural changes in the latter that somehow promotes cytoplasmic signaling. SIRPα is mobile and accumulates at the phagocytic synapse [43]. Interestingly, “forcing” SIRPα into the phagocytic synapse in the absence of CD47 also prevents engulfment of opsonized targets indicating the localization of SIRPα in the synapse is sufficient for signaling “don’t eat me” to the macrophage [103]. Accumulation of SIRPα to the synapse is thus driven by the presence of CD47 and appears to be the main mechanism by which phagocytosis is inhibited.
CONCLUSIONS
A balance of activating and passivating signals in the immune system normally maintains homeostasis but also allows cancer cells to evade clearance and spread. Immune checkpoint blockade of the PD-1/PDL-1 axis on T cells has achieved some success against some cancers as a monotherapy, but current understanding is that T cells in these patients are being activated by an abundance of mutations that can stimulate only upon checkpoint blockade. Although monotherapy against CD47-SIRPα seemed promising based on multiple syngeneic mouse models of cancer that used cancer lines that were known to be immunogenic, monotherapy also seemed unlikely based on minimally immunogenic lines such as B16 melanoma in C57 mice [56]. In this model, even PD-1 blockade is relatively ineffective unless the B16 cells are made more immunogenic with mutations that are also known to favor clinical responses to PD-1 blockade [4,5]. Combination therapies of CD47-SIRPα blockade with tumor-opsonizing antibodies that activate macrophages through the FcR pathway are thus sensible and promising. They also have the theoretical potential for antigenic spread within a patient, if engulfment of the cancer cell by a macrophage or dendritic cell leads to patient-specific antibodies against tumor mutations that otherwise remain hidden behind the macrophage checkpoint.
ACKNOWLEDGEMENTS
J.C.A. was supported under DGE-1845298 by the National Science Foundation Graduate Research Fellowship Program. This work was also supported by DMR-1120901, U54-CA193417-05, and R01-HL124106-06. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of neither the National Institutes of Health nor the National Science Foundation.
CONFLICT OF INTEREST
The authors report no conflicts of interest.
REFERENCES
- 1. Matlung, HL, Szilagyi, K, Barclay, NAet al. The CD47-SIRP alpha signaling axis as an innate immune checkpoint in cancer. Immunol Rev 2017; 276: 145–64. [DOI] [PubMed] [Google Scholar]
- 2. Sharma, P, Allison, JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015; 161: 205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharma, P, Allison, JP. The future of immune checkpoint therapy. Science 2015; 348: 56–61. [DOI] [PubMed] [Google Scholar]
- 4. Mandal, R, Samstein, RM, Lee, Ket al. Genetic diversity of tumors with mismatch repair deficiency influences anti-PD-1 immunotherapy response. Science 2019; 364: 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perumal, D, Imai, N, Lagana, Aet al. Mutation-derived neoantigen-specific T-cell responses in multiple myeloma. Clin Cancer Res 2020; 26: 450–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mantovani, A, Marchesi, F, Malesci, Aet al. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017; 14: 399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alvey, C, Discher, DE. Engineering macrophages to eat cancer: from ``marker of self'' CD47 and phagocytosis to differentiation. J Leukoc Biol 2017; 102: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brooke, G, Holbrook, J, Brown, Met al. Human lymphocytes interact directly with CD47 through a novel member of the signal regulatory protein (SIRP) family. J Immunol 2004; 173: 2562–70. [DOI] [PubMed] [Google Scholar]
- 9. Barclay, AN. Signal regulatory protein alpha (SIRP alpha)/CD47 interaction and function. Curr Opin Immunol 2009; 21: 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campbell, I, Freemonth, P, Foulkes, Wet al. An ovarian tumor-marker with homology to Vaccinia virus contains an Igv-like region and multiple transmembrane domains. Cancer Res 1992; 52: 5416–20. [PubMed] [Google Scholar]
- 11. Brown, E, Hooper, L, Ho, Tet al. Integrin-associated protein - a 50-Kd plasma-membrane antigen physically and functionally associated with integrins. J Cell Biol 1990; 111: 2785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lindberg, F, Lublin, D, Telen, Met al. Rh-related antigen Cd47 is the signal-transducer integrin-associated protein. J Biol Chem 1994; 269: 1567–70. [PubMed] [Google Scholar]
- 13. Reinhold, M, Lindberg, F, Plas, Det al. In-vivo expression of alternatively spliced forms of integrin-associated protein (Cd47). J Cell Sci 1995; 108: 3419–25. [DOI] [PubMed] [Google Scholar]
- 14. Hatherley, D, Graham, SC, Turner, Jet al. Paired receptor specificity explained by structures of signal regulatory proteins alone and complexed with CD47. Mol Cell 2008; 31: 266–77. [DOI] [PubMed] [Google Scholar]
- 15. Rebres, R, Vaz, L, Green, Jet al. Normal ligand binding and signaling by CD47 (integrin-associated protein) requires a long range disulfide bond between the extracellular and membrane-spanning domains. J Biol Chem 2001; 276: 34607–16. [DOI] [PubMed] [Google Scholar]
- 16. Soto-Pantoja, DR, Kaur, S, Roberts, DD. CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit Rev Biochem Mol Biol 2015; 50: 212–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oldenborg, P, Zheleznyak, A, Fang, Yet al. Role of CD47 as a marker of self on red blood cells. Science 2000; 2051-2054: 288. [DOI] [PubMed] [Google Scholar]
- 18. Veillette, A, Thibaudeau, E, Latour, S. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J Biol Chem 1998; 273: 22719–28. [DOI] [PubMed] [Google Scholar]
- 19. Fujioka, Y, Matozaki, T, Noguchi, Tet al. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol 1996; 16: 6887–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seiffert, M, Cant, C, Chen, Zet al. Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood 1999; 94: 3633–43. [PubMed] [Google Scholar]
- 21. Alvey, CM, Spinler, KR, Irianto, Jet al. SIRPα-inhibited, marrow-derived macrophages engorge, accumulate, and differentiate in antibody-targeted regression of solid tumors. Curr Biol 2017; 27: 2065–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Beek, E, Cochrane, F, Barclay, Aet al. Signal regulatory proteins in the immune system. J Immunol 2005; 175: 7781–7. [DOI] [PubMed] [Google Scholar]
- 23. Barclay, A, Brown, M. The SIRP family of receptors and immune regulation. Nat Rev Immunol 2006; 6: 457–64. [DOI] [PubMed] [Google Scholar]
- 24. Dietrich, J, Cella, M, Seiffert, Met al. Cutting edge: signal-regulatory protein beta 1 is a DAP12-associated activating receptor expressed in myeloid cells. J Immunol 2000; 164: 9–12. [DOI] [PubMed] [Google Scholar]
- 25. Takenaka, K, Prasolava, TK, Wang, JCYet al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol 2007; 8: 1313–23. [DOI] [PubMed] [Google Scholar]
- 26. Janssen, WJ, McPhillips, KA, Dickinson, MGet al. Surfactant proteins a and D suppress alveolar macrophage phagocytosis via interaction with SIRP alpha. Am J Respir Crit Care Med 2008; 178: 158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fournier, B, Andargachew, R, Robin, AZet al. Surfactant protein D (Sp-D) binds to membrane-proximal domain (D3) of signal regulatory protein alpha (SIRP alpha), a site distant from binding domain of CD47, while also binding to analogous region on signal regulatory protein beta (SIRP beta). J Biol Chem 2012; 287: 19386–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matozaki, T, Murata, Y, Okazawa, Het al. Functions and molecular mechanisms of the CD47-SIRP alpha signalling pathway. Trends Cell Biol 2009; 19: 72–80. [DOI] [PubMed] [Google Scholar]
- 29. Barclay, AN, van denBerg, TK. The interaction between signal regulatory protein alpha (SIRP alpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol 2014; 32: 25–50. [DOI] [PubMed] [Google Scholar]
- 30. Subramanian, S, Parthasarathy, R, Sen, Set al. Species- and cell type-specific interactions between CD47 and human SIRP alpha. Blood 2006; 107: 2548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kwong, LS, Brown, MH, Barclay, ANet al. Signal-regulatory protein alpha from the NOD mouse binds human CD47 with an exceptionally high affinity - implications for engraftment of human cells. Immunology 2014; 143: 61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boettcher, AN, Cunnick, JE, Powell, EJet al. Porcine signal regulatory protein alpha binds to human CD47 to inhibit phagocytosis: implications for human hematopoietic stem cell transplantation into severe combined immunodeficient pigs. Xenotransplantation 2019; 26: e12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chung, J, Gao, A, Frazier, W. Thrombospondin acts via integrin-associated protein to activate the platelet integrin alpha(IIb)beta(3). J Biol Chem 1997; 272: 14740–6. [DOI] [PubMed] [Google Scholar]
- 34. Fujimoto, T, Katsutani, S, Shimomura, Tet al. Thrombospondin-bound integrin-associated protein (CD47) physically and functionally modifies integrin alpha(IIb)beta(3) by its extracellular domain. J Biol Chem 2003; 278: 26655–65. [DOI] [PubMed] [Google Scholar]
- 35. Barazi, H, Li, Z, Cashel, Jet al. Regulation of integrin function by CD47 ligands - differential effects on alpha(v)beta(3) and alpha(4)beta(1) integrin-mediated adhesion. J Biol Chem 2002; 277: 42859–66. [DOI] [PubMed] [Google Scholar]
- 36. Isenberg, JS, Annis, DS, Pendrak, MLet al. Differential interactions of thrombospondin-1,-2, and-4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem 2009; 284: 1116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaur, S, Kuznetsova, SA, Pendrak, MLet al. Heparan sulfate modification of the transmembrane receptor CD47 is necessary for inhibition of T cell receptor signaling by Thrombospondin-1. J Biol Chem 2011; 286: 14991–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hatherley, D, Lea, SM, Johnson, Set al. Polymorphisms in the human inhibitory signal-regulatory protein alpha do not affect binding to its ligand CD47. J Biol Chem 2014; 289: 10024–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rodriguez, PL, Harada, T, Christian, DAet al. Minimal "self" peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 2013; 339: 971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kant, A, De, P, Peng, Xet al. SHP-1 regulates Fc gamma receptor-mediated phagocytosis and the activation of RAC. Blood 1852–1859; 2002: 100. [PubMed] [Google Scholar]
- 41. Ishikawa-Sekigami, T, Kaneko, Y, Okazawa, Het al. SHPS-1 promotes the survival of circulating erythrocytes through inhibition of phagocytosis by splenic macrophages. Blood 2006; 107: 341–8. [DOI] [PubMed] [Google Scholar]
- 42. Oldenborg, PA, Gresham, HD, Lindberg, FP. CD47-signal regulatory protein alpha (SIRP alpha) regulates fc gamma and complement receptor-mediated phagocytosis. J Exp Med 2001; 193: 855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tsai, RK, Discher, DE. Inhibition of "self" engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol 2008; 180: 989–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Johansen, ML, Brown, EJ. Dual regulation of SIRP alpha phosphorylation by integrins and CD47. J Biol Chem 2007; 282: 24219–30. [DOI] [PubMed] [Google Scholar]
- 45. Richards, DM, Endres, RG. The mechanism of phagocytosis: two stages of engulfment. Biophys J 2014; 107: 1542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Willingham, SB, Volkmer, J, Gentles, AJet al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 2012; 109: 6662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jaiswal, S, Jamieson, CHM, Pang, WWet al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009; 138: 271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Majeti, R, Chao, MP, Alizadeh, AAet al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009; 138: 286–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gholamin, S, Mitra, SS, Feroze, AHet al. Disrupting the CD47-SIRP alpha anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci Transl Med 2017; 9: eaaf2968. [DOI] [PubMed] [Google Scholar]
- 50. Chao, MP, Jaiswal, S, Weissman-Tsukamoto, Ret al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med 2010; 2: 63ra94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sikic, BI, Lakhani, N, Patnaik, Aet al. First-in-human, first-in-class phase I trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. JCO 2019; 37, 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cox, D, Greenberg, S. Phagocytic signaling strategies: fc(gamma) receptor-mediated phagocytosis as a model system. Semin Immunol 2001; 13: 339–45. [DOI] [PubMed] [Google Scholar]
- 53. Bakalar, MH, Joffe, AM, Schmid, EMet al. Size-dependent segregation controls macrophage phagocytosis of antibody-opsonized targets. Cell 2018; 174: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Veillette, A, Chen, J. SIRP alpha-CD47 immune checkpoint blockade in anticancer therapy. Trends Immunol 2018; 39: 173–84. [DOI] [PubMed] [Google Scholar]
- 55. Weiskopf, K, Ring, AM, Ho, CCMet al. Engineered SIRP alpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science 2013; 341: 88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sockolosky, JT, Dougan, M, Ingram, JRet al. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci U S A 2016; 113: E2646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hayes, BH, Tsai, RK, Dooling, LJet al. Macrophages eat more after disruption of cis interactions between CD47 and the checkpoint receptor SIRPa. J Cell Sci 2020; jcs.237800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Massuger, L, Claessens, R, Kenemans, Pet al. Nonantigen-specific tissue localization of monoclonal-antibodies. J Nucl Med 1990; 31: 1438–8. [PubMed] [Google Scholar]
- 59. Andrechak, JC, Dooling, LJ, Discher, DE. The macrophage checkpoint CD47: SIRP alpha for recognition of 'self' cells: from clinical trials of blocking antibodies to mechanobiological fundamentals. Philos Trans R Soc B-Biol Sci 2019; 374: 20180217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu, X, Kwon, H, Li, Zet al. x. Is CD47 an innate immune checkpoint for tumor evasion? J Hematol Oncol 2017; 10: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang, X, Fan, J, Ju, D. Insights into CD47/SIRPa axis-targeting tumor immunotherapy. Antib Ther 2018; 1: 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Advani, R, Flinn, I, Popplewell, Let al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin's lymphoma. N Engl J Med 2018; 379: 1711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Barkal, AA, Brewer, RE, Markovic, Met al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019; 572: 392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maute, RL, Chen, JY, Marjon, KDet al. Translational study of cell surface proteins in non-Hodgkin lymphoma patients treated with the first-in-class anti-CD47 antibody Magrolimab (5F9) in combination with rituximab. Blood 2019; 134: 5229–9. [Google Scholar]
- 65. Sallman, D, Asch, A, Al Malki, Met al. The first-in-class anti-CD47 antibody magrolimab (5F9) in combination with azacitidine is effective in MDS and AML patients: ongoing phase 1b results. Blood 2019; 134: 569–9. [Google Scholar]
- 66. Forty Seven, Inc . Announces updated data from ongoing clinical trial of magrolimab showing robust, durable activity in patients with myelodysplastic syndrome and acute myeloid leukemia. https://www.drugs.com/clinical_trials/forty-seven-inc-announces-updated-data-ongoing-clinical-trial-magrolimab-showing-robust-durable-18389.html (27 March 2020, last accessed).
- 67. Chao, MP, Takimoto, CH, Feng, DDet al. Therapeutic targeting of the macrophage immune checkpoint CD47 in myeloid malignancies. Front Oncol 2020; 9: 1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. A Trial of TTI-621 for Patients With Hematologic Malignancies and Selected Solid Tumors. Trillium Therapeutics Inc. https://clinicaltrials.gov/ct2/show/results/NCT02663518. [Google Scholar]
- 69. Ansell, S, Chen, RW, Flinn, IWet al. A phase 1 study of TTI-621, a novel immune checkpoint inhibitor targeting CD47, in patients with relapsed or refractory hematologic malignancies. Blood 2016; 128: 1812–2. [Google Scholar]
- 70. A Trial of TTI-622 in Patients With Advanced Relapsed or Refractory Lymphoma or Myeloma (TTI-622-01). Trillium Therapeutics Inc. https://clinicaltrials.gov/ct2/show/NCT03530683?id=NCT03530683&draw=2&rank=1. [Google Scholar]
- 71. Trial of Intratumoral Injections of TTI-621 in Subjects With Relapsed and Refractory Solid Tumors and Mycosis Fungoides. Trillium Therapeutics Inc, https://clinicaltrials.gov/ct2/show/NCT02890368 [Google Scholar]
- 72. Petrova, PS, Viller, NN, Wong, Met al. TTI-621 (SIRP alpha fc): a CD47-blocking innate immune checkpoint inhibitor with broad antitumor activity and minimal erythrocyte binding. Clin Cancer Res 2017; 23: 1068–79. [DOI] [PubMed] [Google Scholar]
- 73. Trillium Therapeutics Inc. Trillium Therapeutics Corporate Overview Presentation. https://s22.q4cdn.com/183592819/files/doc_presentations/2020/01/Trillium-Corp-Overview-07Jan2020.pdf (27 March 2020, last accessed). [Google Scholar]
- 74. Lin, GHY, Viller, NN, Chabonneau, Met al. TTI-622 (SIRPα-IgG4 fc), a CD47-blocking innate immune checkpoint inhibitor, suppresses tumor growth and demonstrates enhanced efficacy in combination with antitumor antibodies in both hematologic and solid tumor models. Cancer Res 2018; 78: 2709. [Google Scholar]
- 75. Abrisqueta, P, Sancho, J, Cordoba, Ret al. Anti-CD47 antibody, CC-90002, in combination with rituximab in subjects with relapsed and/or refractory non-Hodgkin lymphoma (R/R NHL). Blood 2019; 134: 4089–9. [Google Scholar]
- 76. Kauder, SE, Kuo, TC, Chen, Aet al. ALX148 is a high affinity Sirpa fusion protein that blocks CD47, enhances the activity of anti-cancer antibodies and checkpoint inhibitors, and has a favorable safety profile in preclinical models. Blood 2017; 130: 112–2. [Google Scholar]
- 77. Kauder, SE, Kuo, TC, Harrabi, Oet al. ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. PLoS One 2018; 13: e0201832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chow, LQM, Gainor, JF, Lakhani, NJet al. A phase I study of ALX148, a CD47 blocker, in combination with established anticancer antibodies in patients with advanced malignancy. J Clin Oncol 2019; 37: 2514–4.31173557 [Google Scholar]
- 79. Holland, PM, Normant, E, Adam, Aet al. CD47 monoclonal antibody SRF231 is a potent inducer of macrophage-mediated tumor cell phagocytosis and reduces tumor burden in murine models of hematologic malignancies. Blood 2016; 128: 1843. [Google Scholar]
- 80. A Study Evaluating the Safety, Tolerability, and Initial Efficacy of Recombinant Human Anti-cluster Differentiation Antigen 47 (CD47) Monoclonal Antibody Injection (IBI188) in Patients With Advanced Malignant Tumors and Lymphomas. Innovent Biologics (Suzhou) Co., Ltd.https://clinicaltrials.gov/ct2/show/NCT03763149. [Google Scholar]
- 81. I-Mab Biopharma Co. sponsor above. study of TJ011133 subjects with relapsed/refractory advanced solid tumors and lymphoma. NCT0393481 2019.
- 82. Meng, Z, Wang, Z, Guo, Bet al. TJC4, a differentiated anti-CD47 antibody with novel epitope and RBC sparing properties. Blood 2019; 134: 4063–3. [Google Scholar]
- 83. Sim, J, Sockolosky, JT, Sangalang, Eet al. Discovery of high affinity, pan-allelic, and pan-mammalian reactive antibodies against the myeloid checkpoint receptor SIRP alpha. MAbs 2019; 11: 1036–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Anonymous 34th Annual Meeting & Pre-Conference Programs of the Society for Immunotherapy of Cancer (SITC 2019): Part 1. Journal for ImmunoTherapy of Cancer 2019, 7, 282–282. DOI: 10.1186/s40425-019-0763-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Immunotherapeutics, O. A trial of BI 765063 monotherapy and in combination with BI 754091 in patients with advanced solid tumours.
- 86. Chames, P, Van Regenmortel, M, Weiss, Eet al. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol 2009; 157: 220–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Verma, V, Sprave, T, Haque, Wet al. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors 11 medical and health sciences 1112 oncology and carcinogenesis. J ImmunoTherapy Cancer 2018; 6: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hernandez, I, Bott, SW, Patel, ASet al. Pricing of monoclonal antibody therapies: higher if used for cancer? Am J Manag Care 2018; 24: 109–12. [PubMed] [Google Scholar]
- 89. Dai, H, Friday, AJ, Abou-Daya, KIet al. Donor SIRP alpha polymorphism modulates the innate immune response to allogeneic grafts. Sci Immunol 2017; 2: eaam6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lee, WY, Weber, DA, Laur, Oet al. Novel structural determinants on SIRP alpha that mediate binding to CD47. J Immunol 2007; 179: 7741–50. [DOI] [PubMed] [Google Scholar]
- 91. Subramanian, S, Boder, ET, Discher, DE. Phylogenetic divergence of CD47 interactions with human signal regulatory protein alpha reveals locus of species specificity - implications for the binding site. J Biol Chem 2007; 282: 1805–18. [DOI] [PubMed] [Google Scholar]
- 92. Lin, Y, Yan, X, Yang, Fet al. Soluble extracellular domains of human SIRP alpha and CD47 expressed in Escherichia coli enhances the phagocytosis of leukemia cells by macrophages in vitro. Protein Expr Purif 2012; 85: 109–16. [DOI] [PubMed] [Google Scholar]
- 93. Ogura, T, Noguchi, T, Murai-Takebe, Ret al. Resistance of B16 melanoma cells to CD47-induced negative regulation of motility as a result of aberrant N-glycosylation of SHPS-1. J Biol Chem 2004; 279: 13711–20. [DOI] [PubMed] [Google Scholar]
- 94. Lee, WY, Weber, DA, Laur, Oet al. The role of cis dimerization of signal regulatory protein alpha (SIRP alpha) in binding to CD47. J Biol Chem 2010; 285: 37953–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Logtenberg, MEW, Jansen, JHM, Raaben, Met al. Glutaminyl cyclase is an enzymatic modifier of the CD47-SIRP alpha axis and a target for cancer immunotherapy. Nat Med 2019; 25: 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ho, CCM, Guo, N, Sockolosky, JTet al. "Velcro" engineering of high affinity CD47 ectodomain as signal regulatory protein alpha(SIRP alpha) antagonists that enhance antibody-dependent cellular phagocytosis. J Biol Chem 2015; 290: 12650–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Voets, E, Paradé, M, Lutje Hulsik, Det al. Functional characterization of the selective pan-allele anti-SIRPa antibody ADU-1805 that blocks the SIRPa-CD47 innate immune checkpoint. J ImmunoTherapy Cancer 2019; 7: 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Liu, J, Wang, L, Zhao, Fet al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS One 2015; 10: e0137345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Weiskopf, K, Jahchan, NS, Schnorr, PJet al. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J Clin Invest 2016; 126: 2610–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pietsch, EC, Dong, J, Cardoso, Ret al. Anti-leukemic activity and tolerability of anti-human CD47 monoclonal antibodies. Blood Cancer J 2017; 7: e536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ring, NG, Herndler-Brandstetter, D, Weiskopf, Ket al. Anti-SIRP alpha antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci U S A 2017; 114: E10578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yanagita, T, Murata, Y, Tanaka, Det al. Anti-SIRPα antibodies as a potential new tool for cancer immunotherapy. JCI Insight 2016; 2: e89140. https://www.biorxiv.org/content/10.1101/752311v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Morrissey, MA, Vale, RD. CD47 suppresses phagocytosis by repositioning SIRPA and preventing integrin activation, 2019; DOI: 10.1101/752311. [DOI]
- 104. Hatherley, D, Harlos, K, Dunlop, DCet al. The structure of the macrophage signal regulatory protein alpha (SIRP alpha) inhibitory receptor reveals a binding face reminiscent of that used by T cell receptors. J Biol Chem 2007; 282: 14567–75. [DOI] [PubMed] [Google Scholar]
- 105. Martinez-Torres, A, Quiney, C, Attout, Tet al. CD47 agonist peptides induce programmed cell death in refractory chronic lymphocytic leukemia B cells via PLC gamma 1 activation: evidence from mice and humans. PLoS Med 2015; 12: e1001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gozlan, YM, Hilgendorf, S, Aronin, Aet al. DSP107-a novel SIRPα-4-1BBL dual signaling protein (DSP) for cancer immunotherapy. Cancer Immunol Res 2019; 7: A076. [Google Scholar]
- 107. Chao, MP, Alizadeh, AA, Tang, Cet al. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res 2011; 71: 1374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zeng, D, Sun, Q, Chen, Aet al. A fully human anti-CD47 blocking antibody with therapeutic potential for cancer. Oncotarget 2016; 7: 83040–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang, Y, Yin, C, Feng, Let al. Ara-C and anti-CD47 antibody combination therapy eliminates acute monocytic leukemia THP-1 cells in vivo and in vitro. Genet Mol Res 2015; 14: 5630–41. [DOI] [PubMed] [Google Scholar]
- 110. Buatois, V, Johnson, Z, Salgado-Pires, Set al. Preclinical development of a bispecific antibody that safely and effectively targets CD19 and CD47 for the treatment of B-cell lymphoma and Leukemia. Mol Cancer Ther 2018; 17: 1739–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Theocharides, APA, Jin, L, Cheng, Pet al. Disruption of SIRP alpha signaling in macrophages eliminates human acute myeloid leukemia stem cells in xenografts. J Exp Med 2012; 209: 1883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Goto, H, Kojima, Y, Matsuda, Ket al. Efficacy of anti-CD47 antibody-mediated phagocytosis with macrophages against primary effusion lymphoma. Eur J Cancer 1836–1846; 2014: 50. [DOI] [PubMed] [Google Scholar]
- 113. Chao, MP, Alizadeh, AA, Tang, Cet al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 2010; 142: 699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chen, J, Zhong, M, Guo, Het al. SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via mac-1 integrin. Nature 2017; 544: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Piccione, EC, Juarez, S, Liu, Jet al. A bispecific antibody targeting CD47 and CD20 selectively binds and eliminates dual antigen expressing lymphoma cells. MAbs 2015; 7: 946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. He, Y, Bouwstra, R, Wiersma, VRet al. Cancer cell-expressed SLAMF7 is not required for CD47-mediated phagocytosis. Nat Commun 2019; 10: 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ribeiro, ML, Normant, E, Garau, RDet al. The novel bispecific CD47-CD19 antibody TG-1801 potentiates the activity of ublituximab-umbralisib (U2) drug combination in preclinical models of B-NHL. HemaSphere 2019; 3: 598. [Google Scholar]
- 118. Murata, Y, Tanaka, D, Hazama, Det al. Anti-human SIRP antibody is a new tool for cancer immunotherapy. Cancer Sci 2018; 109: 1300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Chan, KS, Espinosa, I, Chao, Met al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A 2009; 106: 14016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhang, M, Hutter, G, Kahn, SAet al. Anti-CD47 treatment stimulates phagocytosis of glioblastoma by M1 and M2 polarized macrophages and promotes M1 polarized macrophages in vivo. PLoS One 2016; 11: e0153550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hutter, G, Theruvath, J, Graef, CMet al. Microglia are effector cells of CD47-SIRP alpha antiphagocytic axis disruption against glioblastoma. Proc Natl Acad Sci U S A 2019; 116: 997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zhao, XW, vanBeek, EM, Schornagel, Ket al. CD47-signal regulatory protein-alpha (SIRP alpha) interactions form a barrier for antibody-mediated tumor cell destruction. Proc Natl Acad Sci U S A 2011; 108: 18342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Cabrales, P. RRX-001 acts as a dual small molecule checkpoint inhibitor by downregulating CD47 on cancer cells and SIRP-alpha on monocytes/macrophages. Transl Oncol 2019; 12: 626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tseng, D, Volkmer, J, Willingham, SBet al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A 2013; 110: 11103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Gu, S, Ni, T, Wang, Jet al. CD47 blockade inhibits tumor progression through promoting phagocytosis of tumor cells by M2 polarized macrophages in endometrial cancer. J Immunol Res 2018; 2018: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yoshida, K, Tsujimoto, H, Matsumura, Ket al. CD47 is an adverse prognostic factor and a therapeutic target in gastric cancer. Cancer Med 2015; 4: 1322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Xiao, Z, Chung, H, Banan, Bet al. Antibody mediated therapy targeting CD47 inhibits tumor progression of hepatocellular carcinoma. Cancer Lett 2015; 360: 302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Edris, B, Weiskopf, K, Volkmer, AKet al. Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc Natl Acad Sci U S A 2012; 109: 6656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhang, X, Fan, J, Wang, Set al. Targeting CD47 and autophagy elicited enhanced antitumor effects in non-small cell lung cancer. Cancer Immunol Res 2017; 5: 363–75. [DOI] [PubMed] [Google Scholar]
- 130. Kim, D, Wang, J, Willingham, SBet al. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia 2012; 26: 2538–45. [DOI] [PubMed] [Google Scholar]
- 131. Xu, J, Pan, X, Zhang, Set al. CD47 blockade inhibits tumor progression human osteosarcoma in xenograft models. Oncotarget 2015; 6: 23662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Krampitz, GW, George, BM, Willingham, SBet al. Identification of tumorigenic cells and therapeutic targets in pancreatic neuroendocrine tumors. Proc Natl Acad Sci U S A 2016; 113: 4464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cioffi, M, Trabulo, S, Hidalgo, Met al. Inhibition of CD47 effectively targets pancreatic cancer stem cells via dual mechanisms. Clin Cancer Res 2015; 21: 2325–37. [DOI] [PubMed] [Google Scholar]
- 134. Jain, S, Van Scoyk, A, Morgan, EAet al. Targeted inhibition of CD47-SIRPα requires fc-FcγR interactions to maximize activity in T-cell lymphomas. Blood 2019; 134: 1430–40. [DOI] [PMC free article] [PubMed] [Google Scholar]


