To the editors:
We read with concern the articles that report the presence of coronavirus in kidney based on electron microscopic evidence.1 , 2 Neither article, in fact, demonstrates the presence of coronavirus in the kidney. Su et al. 1 show purported virus particles in the cytoplasm of kidney tubular epithelium and podocytes. These structures are not viral particles, but rather clathrin-coated vesicles, normal cell organelles involved in intracellular transport. The objects in their Figure 2a and b (∼60 nm) are somewhat smaller than coronaviruses (∼80 to 140+ nm), but more importantly, their “spikes” (peplomers) are in contact with the cytosol, as are those on clathrin-coated vesicles; the larger particle in Figure 2d also has spikes that are touching the cytosol and does not have dense dots inside the particles corresponding to the coiled nucleocapsid, cut in cross section. Coronaviruses, on the other hand, have their projections either facing the extracellular space between cells or the space inside vacuoles within the cells.3, 4, 5 This phenomenon is due to the fact that coronaviruses receive their outer covering by budding into or on cellular membranes, thereby forming intracellular vacuoles with the viral projections in contact with the vacuolar content, not the cytosol. During assembly, viral structural proteins are incorporated into the endoplasmic reticulum–Golgi complex of the infected cell, and viral RNA, packaged with another protein, buds into these membranes, forming a membrane-bound sac containing mature virions; the spikes are on the outside of the virion, but inside the vacuole and not in direct contact with the cytosol (Figure 1 ). These virions get out of the cell by exocytosis when the vacuole membrane fuses with the plasma membrane and opens its contents to the outside; thus, complete virions with peplomers are seen within the cell inside the membrane container (sequestered from the cytosol) and outside of cells, frequently still attached to the opened vacuolar membrane that has fused with the plasma membrane. The particles shown in electron micrographs in the article by Su et al. 1 have their spikes in contact with the cytoplasmic fluid, like endocytotic vesicles, that is, clathrin-coated vesicles (see Plate 523, Figures 3–5, pp. 1214–1215 in Ghadially6; Figure 18c and d in Miller7; and Miller8).
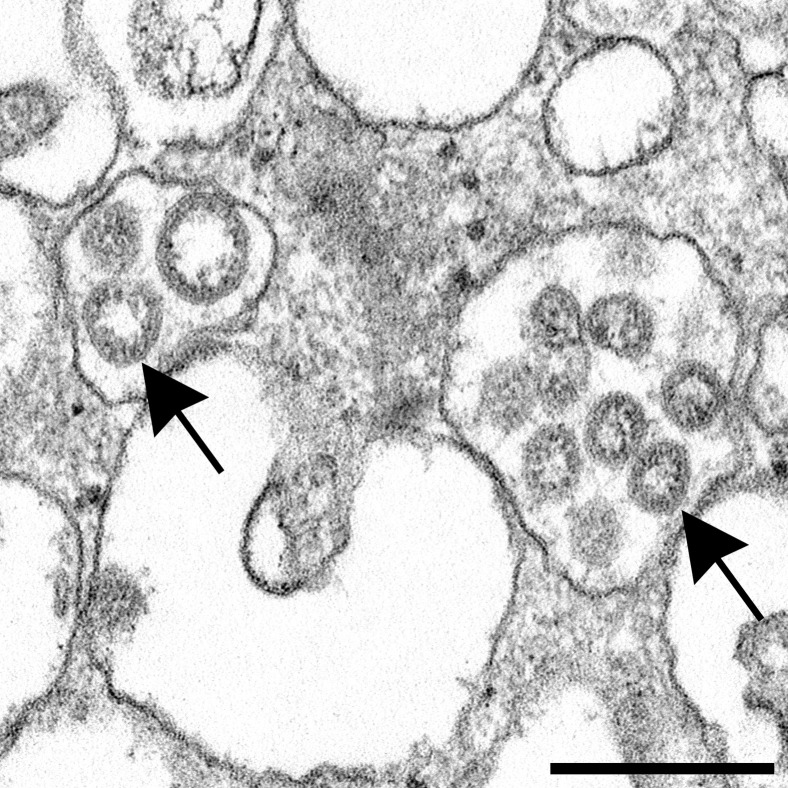
Figure 1.
Electron microscopic image of an isolate of severe acute respiratory syndrome coronavirus 2 seen here inside vacuoles (arrows). Note the dense membrane coat around the viral particles. This micrograph is of viral particles in a cell culture inoculated with infected patient nasopharyngeal and oropharyngeal fluids. Bar = 200 nm. Image provided by Cynthia S. Goldsmith, Centers for Disease Control and Prevention. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Likewise, the particles in Kissling et al. 2 are not coronaviruses. While they are inside a vacuole, have spikes, and are approximately the correct size, they do not have the uniform appearance of virus particles with a membrane outer covering and dots inside indicating the nucleocapsid.3, 4, 5 These objects are inside a vesicle called a multivesicular body (see Plates 277–278, pp. 632–634 in Ghadially6; Calomeni et al.9; and Figure 3, p. 393 in Haguenau10). The article by Kissling et al. 2 is concerning, as electron microscopy is the only alleged evidence presented in support of the suggestion that coronaviruses are actually present in this kidney tissue; all other tests for coronavirus in kidney were negative. These micrographs do not support the statement that the particles are indeed viruses.
Knowledge of virus morphology and morphogenesis, as well as of cellular architecture, is necessary to distinguish viral pathogens from normal subcellular organelles. This distinction is frequently difficult, because numerous cellular components can masquerade as viruses.7, 8, 9, 10, 11
Acknowledgments
We are grateful to Cynthia S. Goldsmith, Centers for Disease Control and Prevention, for critically reviewing this letter, providing helpful suggestions, and supplying the figure of severe acute respiratory syndrome coronavirus 2. We also thank Dr. David N. Howell for reviewing this manuscript.
Contributor Information
Sara E. Miller, Email: saram@duke.edu.
John K. Brealey, Email: john.brealey@sa.gov.au.
References
- 1.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kissling S., Rotman S., Gerber C. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020;98:228–231. doi: 10.1016/j.kint.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldsmith C.S., Tatti K.M., Ksiazek T.G. Ultrastructural characterization of SARS coronavirus. Emerg Infect Dis. 2004;10:320–326. doi: 10.3201/eid1002.030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. In: Maier H.J., Bickerton E., Britton P., editors. Coronaviruses: Methods and Protocols. Vol. 1282. Springer; New York, NY: 2015. pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller S.E. Diagnosis of viral infection by electron microscopy. In: Lennette E.H., Lennette D.A., Lennette E.T., editors. Diagnostic Procedures for Viral, Rickettsial and Chlamydial Infections. American Public Health Association; Washington, DC: 1995. pp. 35–76. [Google Scholar]
- 6.Ghadially F.N. 4th ed. Vol. 2. Butterworth-Heinemann; Boston, MA: 1997. (Ultrastructural Pathology of the Cell and Matrix). [Google Scholar]
- 7.Miller S.E. Detection and identification of viruses by electron microscopy. J Electron Microsc Tech. 1986;4:265–301. doi: 10.1002/jemt.1060040305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller S.E. Problems and pitfalls in diagnostic electron microscopy. Microsc Microanal. 2012;18(suppl 2):172–173. [Google Scholar]
- 9.Calomeni E., Satoskar A., Ayoub I. Multivesicular bodies mimicking SARS-CoV-2 in patients without COVID-19. Kidney Int. 2020;98:233–234. doi: 10.1016/j.kint.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haguenau F. “Viruslike” particles as observed with the electron microscope. In: Dalton A.J., Haguenau F., editors. Ultrastructure of Animal Viruses and Bacteriophages. Academic Press; Waltham, MA: 1973. pp. 391–397. [Google Scholar]
- 11.Goldsmith C.S., Miller S.E., Martines R.B. Electron microscopy of SARS-CoV-2: a challenging task. Lancet. 2020;395:e99. doi: 10.1016/S0140-6736(20)31188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]