Abstract
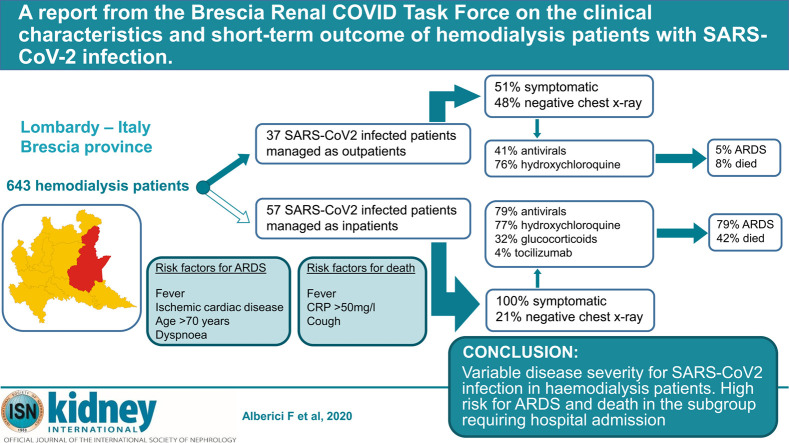
The SARS-CoV-2 epidemic is pressuring healthcare systems worldwide. Disease outcomes in certain subgroups of patients are still scarce, and data are needed. Therefore, we describe here the experience of four dialysis centers of the Brescia Renal COVID Task Force. During March 2020, within an overall population of 643 hemodialysis patients, SARS-CoV-2 RNA positivity was detected in 94 (15%). At disease diagnosis, 37 of the 94 (39%) patients (group 1) were managed on an outpatient basis, whereas the remaining 57 (61%) (group 2) required hospitalization. Choices regarding management strategy were made based on disease severity. In group 1, 41% received antivirals and 76% hydroxychloroquine. Eight percent died and 5% developed acute respiratory distress syndrome (ARDS). In group 2, 79% received antivirals and 77% hydroxychloroquine. Forty two percent died and 79% developed ARDS. Overall mortality rate for the entire cohort was 29%. History of ischemic cardiac disease, fever, older age (over age 70), and dyspnea at presentation were associated with the risk of developing ARDS, whereas fever, cough and a C-reactive protein higher than 50 mg/l at disease presentation were associated with the risk of death. Thus, in our population of hemodialysis patients with SARS-CoV-2 infection, we documented a wide range of disease severity. The risk of ARDS and death is significant for patients requiring hospital admission at disease diagnosis.
Keywords: COVID-19, hemodialysis, SARS-CoV-2
Graphical abstract
The impact of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic in subgroups of patients has yet to be determined. In Brescia, Italy, we have developed a standardized protocol when approaching patients on maintenance hemodialysis (MHD) and kidney transplant recipients, respectively.1 Reports would suggest a more severe disease course in patients with chronic kidney disease,2 although outcomes in MHD patients are still unclear, with earlier small case series suggesting a milder course.3 Management of MHD patients in the context of an epidemic poses several challenges: this group of patients usually requires caregiver assistance and transportation from home to the dialysis units, and they must spend time in crowded waiting areas before and after treatment.4 Moreover, MHD patients are usually old and affected by several comorbidities that are known to be associated with high risk of poor outcomes in patients with coronavirus disease 2019 (COVID-19).
The Lombardy region in general, and Brescia in particular, have been severely hit by the SARS-CoV-2 epidemic, and this has generated several logistical and clinical challenges for the dialysis units of the Brescia Renal COVID Task Force. Here, we describe the characteristics and outcomes of the MHD patients affected by COVID-19 and followed in 4 dialysis units that are part of the Brescia Renal COVID Task Force.
Results
Ninety-four patients tested positive via reverse transcription polymerase chain reaction (RT-PCR) within the overall population of 643 (15%; Table 1 ). Although 3 centers limited the viral testing to only those patients showing symptoms suggestive for SARS-CoV-2 infection, the hemodialysis center of the ASST Spedali Civili of Brescia performed RT-PCR in its entire MHD population (patients with symptoms and patients without symptoms). The positivity rates were not substantially different between these approaches (14% vs. 16%, respectively). Patients testing positive were either triaged to the hospital or back to their dialysis facility based on the clinical judgement of the caring physician, mainly according to the severity of the symptoms shown. The main clinical characteristics of the overall MHD population with SARS-CoV-2 infection, and the subgroups managed as outpatient or in hospital, are shown in Table 2 . The median time from symptom onset to positive RT-PCR was 2 days (interquartile range [IQR], 0–3). Patients that needed to be admitted showed a higher proportion of symptoms compared to the ones not requiring hospitalization (46% vs. 14%).
Table 1.
Hemodialysis patients with SARS-CoV-2 infection in 4 dialysis centers of the Brescia Renal COVID Task Force, Brescia, Italy
| Center | Positive patients admitted | Positive outpatients | Positive overall | Overall population | Percentage of positive patients |
|---|---|---|---|---|---|
| Brescia | 25 | 22 | 49 | 302 | 16 |
| Chiari | 16 | 14 | 30 | 155 | 19 |
| Manerbio | 12 | 0 | 12 | 99 | 12 |
| Montichiari | 4 | 1 | 5 | 87 | 6 |
| TOTAL | 57 | 37 | 94 | 643 | 15 |
COVID, coronavirus disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 2.
Baseline clinical characteristics of 94 hemodialysis patients affected by SARS-CoV-2 infection and followed within 4 centers of the Brescia Renal COVID Task Force, Brescia, Italy
| Characteristics | All patients (94) | Outpatients (37) | Admitted (57) | P valuea |
|---|---|---|---|---|
| Male/female | 62/32 | 24/13 | 39/18 | 0.82 |
| Age (yr) | 72 (62–79) | 67 (60–77) | 73 (64–80) | 0.17 |
| Cause of ESRD | ||||
| Not determined | 40 of 94 (43) | 8 of 37 (22) | 32 of 57 (56) | 0.001 |
| Glomerulonephritis | 19 of 94 (19) | 15 of 37 (41) | 4 of 57 (7) | 0.0001 |
| Genetic diseases | 15 of 94 (16) | 6 of 37 (16) | 9 of 57 (16) | 1 |
| Diabetes | 11 of 94 (12) | 3 of 37 (8) | 8 of 57 (14) | 0.52 |
| Other | 9 of 94 (10) | 5 of 37 (14) | 4 of 57 (7) | 0.31 |
| Comorbidities | ||||
| Hypertension | 93% | 35 of 7 (95) | 52 of 57 (91) | 0.7 |
| Diabetes | 43% | 13 of 37 (35) | 27 of 57 (47) | 0.29 |
| Vascular disease | 23% | 7 of 37 (19) | 15 of 57 (26) | 0.46 |
| Cardiac failure | 18% | 1 of 37 (3) | 16 of 57 (28) | 0.002 |
| Ischemic cardiac disease | 17% | 4 of 37 (11) | 12 of 57 (21) | 0.27 |
| Cancer | 12% | 5 of 37 (14) | 6 of 57 (11) | 0.75 |
| COPD | 11% | 6 of 37 (16) | 4 of 57 (7) | 0.18 |
| Other | 17% | 4 of 37 (11) | 13 of 57 (23) | 0.18 |
| Hemodialysis vintage (yr) | 3 (1–6) | 2 (1–7) | 3.9 (1.6–6.4) | 0.19 |
| Hemodialysis frequency | ||||
| 2 times week | 12 of 94 (13) | 3 of 37 (8) | 9 of 57 (16) | 0.35 |
| 3 times a week | 82 of 94 (87) | 34 of 37 (92) | 48 of 57 (84) | 0.35 |
| Hemodialysis modality | ||||
| HD | 69 of 94 (73) | 26 of 37 (70) | 43 of 57 (75) | 0.64 |
| HDF | 23 of 94 (25) | 11 of 37 (30) | 12 of 57 (21) | 0.46 |
| AFB | 2 of 94 (2) | 0 | 2 of 57 (4) | 0.52 |
| SARS-CoV-2 infection symptoms at disease onset | ||||
| Temperature (>37.5 °C) | 68% | 16 of 37 (43) | 49 of 57 (86) | <0.0001 |
| Cough | 23% | 6 of 37 (16) | 16 of 57 (28) | 0.22 |
| Gastrointestinal symptoms | 6% | 1 of 37 (3) | 5 of 57 (9) | 0.4 |
| Pharyngitis | 2% | 1 of 37 (3) | 1 of 57 (2) | 1 |
| Shortness of breath | 25% | 1 of 37 (3) | 22 of 57 (39) | <0.0001 |
| Myalgia | 17% | 2 of 37 (5) | 14 of 57 (25) | 0.02 |
| Baseline chest X-ray | ||||
| No infiltrates | 30% | 15 of 31 (48) | 11 of 53 (21) | 0.01 |
| Unilateral infiltrates | 25% | 7 of 31 (23) | 13 of 53 (25) | 1 |
| Bilateral infiltrates | 45% | 9 of 31 (29) | 29 of 53 (55) | 0.03 |
| Baseline blood tests | ||||
| WBC (NV 4.00–10.80 × 103/ul) | 5075 (3943–6470) | 5960 (4095–6865) | 4760 (3910–5645) | 0.08 |
| Neutrophils (NV 1.50–8.00 × 103/ul) | 3505 (2688–4770) | 4400 (2760–5330) | 3430 (2680–4080) | 0.1 |
| Lymphocytes (NV 0.90–4.00 × 103/ul) | 745 (550–1085) | 900 (600–1200) | 623 (510–1000) | 0.05 |
| Platelets (NV 130–400 × 103/ul) | 162,000 (126,000–229,500) | 202,000 (124,250–263,750) | 151,000 (127,000–181,000) | 0.02 |
| LDH (NV 135–225 U/l) | 254 (193–354) | 218 (187–303) | 352 (219–404) | 0.004 |
| CPK (NV 39–308 U/l) | 64 (38–138) | 51.5 (33–143) | 96 (49–138) | 0.33 |
| AST (NV 18–54 U/l) | 23 (17–35) | 19 (12–24) | 27 (19–46) | 0.0002 |
| ALT (NV 10–50 U/l) | 17 (11–25) | 14.5 (10–21) | 19 (13–29) | 0.04 |
| Bilirubin (NV < 1.20 mg/dl) | 0.3 (0.28–0.4) | 0.28 (0.21–0.37) | 0.3 (0.3–0.5) | 0.05 |
| CRP (NV < 5.0 mg/l) | 42.9 (10–82) | 12.8 (3.7–33.8) | 60 (19–89) | 0.0004 |
| Time from symptom onset to antiviral therapy initiation | 4 (1–6) | 5 (3–5) | 3 (1–6.5) | 0.05 |
| Time from RT-PCR positivity to antiviral therapy initiation | 2 (0–4) | 3 (2–5) | 1 (0–3) | 0.0003 |
| Antiviral therapy | ||||
| Lopinavir/ritonavir | 19 of 94 (20) | 0 of 37 (0) | 19 of 57 (33) | |
| Darunavir + ritonavir | 41 of 94 (44) | 15 of 37 (41) | 26 of 57 (46) | |
| Hydroxychloroquine | 72 of 94 (77) | 28 of 37 (76) | 44 of 57 (77) |
Data are reported as n (%) for categorical variables and median (interquartile range) for continuous variables. Bold data refer to statistically significant P values.
AFB, acetate-free biofiltration; ALT, alanine aminotransferase; AST, aspartate aminotransferase; COPD, chronic obstructive pulmonary disorder; COVID, coronavirus; CPK, creatine phosphokinase; CRP, C-reactive protein; ESRD, end-stage renal disease; HD, hemodialysis, HDF, hemodiafiltration; LDH, lactate dehydrogenase; NV, normal values; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WBC, white blood cell.
Comparison between the 2 groups—“outpatients” and “admitted.”
Outpatient management
Thirty-seven of 94 (39%) patients were managed as outpatients, for a median follow-up of 8 days (IQR, 6–11). Among the group managed as outpatients, 18 of 37 (49%) were asymptomatic at disease diagnosis, while the remaining patients experienced mild symptoms. Among the asymptomatic patients, in 13 of 18 (72%), a chest X-ray was negative, and unilateral and bilateral infiltrates were detected respectively in 3 of 18 (17%) and 2 of 18 (11%). Detailed patient characteristics are shown in Table 2.
Antiviral therapy and/or hydroxychloroquine were employed in 28 of 37 (76%) patients for a median duration of 4 days (IQR, 3–8). Antibiotics were employed in 25 of 37 (68%): macrolides in 56%, cephalosporins in 48%, carbapenems in 8%, glycopeptides in 8%, aminoglycosides in 8%, beta-lactams in 4%, and fluoroquinolones in 4%. A total of 4 of 37 (11%) received prophylactic heparin, and 3 of 37 (8%) were on angiotensin-converting enzyme inhibitors or angiotensin II receptor antagonists.
One patient had to withdraw from taking hydroxychloroquine due to vomiting. No other adverse event due to the treatment was documented in this patient group.
During follow-up, 7 of 37 (19%) patients experienced a new onset/worsening of the interstitial pneumonia, 3 of 37 (8%) died, 2 of 37 (5%) developed acute respiratory distress syndrome (ARDS), and 2 of 37 (5%) had to be hospitalized. In addition, 5 of 37 patients (14%) developed cough, 5 of 37 (14%) myalgia, 4 of 37 (11%) fever, and 3 of 37 (8%) gastrointestinal symptoms during follow up.
Patients who were asymptomatic at baseline, compared to the symptomatic ones, were less likely to develop ARDS (0 of 18 vs. 2 of 19), develop a new onset or worsening of pneumonia (1 of 18 vs. 6 of 19), and to die (0 of 18 vs. 3 of 19).
Hospitalized patients
Fifty-seven patients were admitted after a median time from symptom onset and from positive RT-PCR test results of 4 (IQR, 1–7) and 2 days (IQR, 1–3), respectively. Median follow-up was 8 days (IQR, 4.8–15). Detailed characteristics of this population are shown in Table 2. Antiviral therapy was employed in 45 of 57 (79%), with 13 of 45 patients (29%) experiencing adverse events: 7 diarrhea, 4 an increase in liver enzymes, 3 prolongation of QTc interval, 2 atrial fibrillation, 1 gastrointestinal bleeding, 1 coagulation alterations, and 1 skin rash. The median duration of lopinavir/ritonavir or darunavir + ritonavir and hydroxychloroquine treatments were 7 days (IQR, 5–12) and 5 days (IQR, 3–7), respectively. Antibiotics were administered in 55 of 57 patients: macrolides in 40%, cephalosporins in 49%, carbapenems in 15%, glycopeptides in 20%, aminoglycosides in 7%, beta-lactams in 25%, and fluoroquinolones in 24%. Thirty-one of 57 (54%) received prophylactic heparin, and 11 of 57 (19%) were on angiotensin-converting enzyme inhibitors or angiotensin II receptor antagonists.
Forty-five of 57 patients (79%) developed ARDS; 24 of 57 (42%) died after a median of 6 days (IQR, 3.8–9.5) from admission and a median of 9 days (IQR, 7–10) from onset of symptoms. Eleven of 57 (19%) patients were discharged after a median of 8 days (IQR, 6.5–13) from admission and 15 days (IQR, 12.5–17.5) from onset of symptoms. Among the patients who died, the cause of death was respiratory failure secondary to ARDS in 15 of 24 (63%), bacterial sepsis in 4 of 9, and sudden death of unknown origin in 5 of 9. Serial chest X-rays were performed in 27 of 57 (47%) patients; among those patients, 20 of 27 (74%) showed chest X-ray worsening compared to baseline.
Dexamethasone was administered to 18 of 57 (32%) patients due to respiratory deterioration; 2 of 18 of these patients also received tocilizumab. In this group, 5 of 18 patients (28%) died. Of 9 patients whose response to glucocorticoids was assessable at the moment of data analyses, 2 of 9 showed stabilization of the arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FIO2 ratio) or chest X-ray improvement, whereas the remaining patients did not improve. Regarding the 2 tocilizumab-treated patients, response was assessable in only one, with no improvements in chest X-ray or PaO2/FIO2.
Factors associated with risk of ARDS and death
In univariate analyses, cardiac failure (odds ratio [OR], 6.22 [95% confidence interval {CI}, 1.85–28.6]; P = 0.007), ischemic heart disease (OR, 5.61 [95% CI, 1.65–25.9]; P = 0.01), fever at disease diagnosis (OR, 18.2 [95% CI, 5.6–82.44]; P = 0.000013), shortness of breath at disease diagnosis (OR, 18.17 [95% CI, 4.8–119.5]; P = 0.0002), myalgia or fatigue at disease diagnosis (OR, 5.6 [95% CI, 1.65–25.9]; P = 0.01), infiltrates at the baseline chest X-ray (OR, 4.4 [95% CI, 1.67–13]; P = 0.004), higher aspartate aminotransferase levels (OR, 2.81 [95% CI, 1.08–7.6]; P = 0.04), and higher C-reactive protein levels (OR, 4.68 [95% CI, 1.83–12.7]; P = 0.002) were associated with the risk of developing ARDS. Ischemic heart disease (OR, 3.11 [95% CI, 1.02–9.6]; P = 0.05), fever at disease diagnosis (OR, 18.7 [95% CI, 3.62–343]; P = 0.005), cough at disease diagnosis (OR, 3.5 [95% CI, 1.28–9.7]; P = 0.01), shortness of breath at disease diagnosis (OR, 5.3 [95% CI, 2–15]; P = 0.001), and higher C-reactive protein level at disease diagnosis (OR 6, [95% CI, 2.1–19]; P = 0.001) were associated with the risk of death (Table 3 ).
Table 3.
Univariate analyses of the association between clinical characteristics and the risk of ARDS or death in hemodialysis patients with SARS-CoV-2 infection
| Variable | Outcome: ARDS |
Outcome: death |
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Sex | 0.68 (0.29–1.61) | 0.39 | 1.05 (0.41–2.78) | 0.93 |
| History of hypertension | 0.37 (0.05–1.83) | 0.25 | 0.27 (0.05–1.31) | 0.1 |
| History of cardiac failure | 6.22 (1.85–28.6) | 0.007 | 1.04 (0.3–3.2) | 0.94 |
| History of diabetes | 1.7 (0.75–3.9) | 0.21 | 1.7 (0.69–4.2) | 0.25 |
| History of peripheral vascular disease | 1.27 (0.49–3.36) | 0.63 | 0.91 (0.29–2.56) | 0.86 |
| History of ischemic cardiac disease | 5.61 (1.65–25.9) | 0.01 | 3.11 (1.02–9.6) | 0.05 |
| History of COPD | 1 (0.26–3.84) | 1 | 1.07 (0.22–4.2) | 0.92 |
| History of cancer | 1.88 (0.53–7.64) | 0.34 | 1.5 (0.36–5.4) | 0.55 |
| Type dialysis (HDF vs. HD) | 0.43 (0.16–1.14) | 0.097 | 1.12 (0.38–3.05) | 0.83 |
| Age (>70 yr vs. ≤70 yr) | 2.18 (0.96–5) | 0.065 | 1.85 (0.75–4.78) | 0.18 |
| Fever at disease diagnosis | 18.2 (5.6–82.4) | 0.000013 | 18.7 (3.62–123) | 0.005 |
| Cough at disease diagnosis | 1.61 (0.62–4.37) | 0.33 | 3.5 (1.28–9.7) | 0.01 |
| Shortness of breath at disease diagnosis | 18.17 (4.8–119.5) | 0.0002 | 5.3 (2–15) | 0.001 |
| Myalgia or fatigue at disease diagnosis | 5.6 (1.65–25.9) | 0.01 | 2.26 (0.72–6.9) | 0.15 |
| Infiltrates at chest X-ray at disease diagnosis | 4.4 (1.67–13) | 0.004 | 2.9 (0.95–11) | 0.08 |
| WBC (≤5 ×103/ul vs. >5 ×103/ul) | 1.2 (0.52–1.8) | 0.66 | 1.82 (0.73–4.6) | 0.2 |
| Lymphocytes (≤0.75 ×103/ul vs. >0.75 ×103/ul) | 1.76 (0.75–4.2) | 0.19 | 1.5 (0.59–3.9) | 0.4 |
| Platelets (≤150 ×103/ul vs. >150 ×103/ul) | 1.43 (0.62–3.37) | 0.41 | 1.9 (0.75–4.8) | 0.17 |
| LDH (>250 U/l vs. ≤250 U/l) | 0.99 (0.32–3.11) | 0.99 | 0.71 (0.18–2.7) | 0.62 |
| AST (>25 U/l vs. ≤25 U/l) | 2.81 (1.08–7.6) | 0.04 | 1.85 (0.66–5.3) | 0.24 |
| ALT (>20 U/l vs. ≤20 U/l) | 1.73 (0.67–4.69) | 0.25 | 2.5 (0.88–7) | 0.085 |
| CRP (>50 mg/l vs. ≤50 mg/l) | 4.68 (1.83–12.7) | 0.002 | 6 (2.1–19) | 0.001 |
| Antiviral therapy | 1.29 (0.48–3.55) | 0.62 | 0.39 (0.14–1.11) | 0.08 |
| Time from symptoms to antiviral commencement (≤5 vs. >5) | 2.09 (0.65–7.51) | 0.23 | 0.55 (0.11–2.1) | 0.41 |
| Hydroxychloroquine | 1.16 (0.44–3.12) | 0.76 | 0.44 (0.16–1.24) | 0.12 |
Bold data refer to statistically significant P values.
ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; CI, confidence interval; COPD, chronic obstructive pulmonary disorder; CRP, C-reactive protein; HD, hemodialysis; HDF, hemodiafiltration; LDH, lactate dehydrogenase; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WBC, white blood cell.
Two multivariate analyses were performed, one for each outcome of interest (ARDS and death). In the first model, the characteristics found to be associated with the risk of ARDS were history of ischemic heart disease (OR, 7.5 [95% CI, 1.6–36.2]; P = 0.04), fever at disease diagnosis (OR, 17 [95% CI, 4.5–64]; P = 0.0009), age at symptom onset (OR, 1.1 [95% CI, 1–1.15]; P = 0.03), and shortness of breath at disease onset (OR, 20 [95% CI, 3.6–79.3]; P = 0.004). In the second model, the characteristics associated with the risk of death were fever at disease diagnosis (OR, 18.7 [95% CI, 2.4–146]; P = 0.02), cough at disease diagnosis (OR, 4 [95% CI, 1.02–17.6]; P = 0.05), and higher serum C-reactive protein at disease diagnosis (OR, 5.6 [95% CI, 1.6–23.5]; P = 0.01; Table 4 ).
Table 4.
Two models of multivariate analyses of the association between clinical characteristics and the risk of ARDS or death in hemodialysis patients with SARS-CoV-2 infection
| Variable | Outcome: ARDS |
|
|---|---|---|
| OR (95% CI) | P value | |
| Model 1 | ||
| History of ischemic cardiac disease | 7.5 (1.6–36.3) | 0.04 |
| Fever at disease onset | 17 (4.5–64) | 0.0009 |
| Age at symptoms (>70 yr vs. ≤70 yr) | 1.1 (1–1.15) | 0.03 |
| Shortness of breath | 20 (3.6–79.3) | 0.004 |
| Myalgia or fatigue | 8.5 (0.83–40.3) | 0.11 |
| Variable | Outcome: death |
|
|---|---|---|
| OR (95% CI) | P value | |
| Model 2 | ||
| History of ischemic cardiac disease | 5 (0.94–32.3) | 0.07 |
| Fever at disease onset | 18.7 (2.4–146) | 0.02 |
| Cough at disease onset | 4 (1.02–17.6) | 0.05 |
| CRP at baseline (>50 mg/l vs. ≤50 mg/l) | 5.6 (1.6–23.5) | 0.01 |
Bold data refer to statistically significant P values.
ARDS, acute respiratory distress syndrome; CI, confidence interval; CRP, C-reactive protein; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Discussion
SARS-CoV-2 infection is challenging healthcare systems around the world. The predictions regarding COVID-19–associated mortality are changing as new information is gathered, although comorbidities such as cardiovascular diseases, diabetes, chronic respiratory diseases, hypertension, and cancer are consistently associated with worse prognoses.5 , 6 We have reported recently7 that hospitalized kidney transplant recipients tended to have a poor prognosis, with a mortality rate of 25%, and another group observed that when such patients did not require hospital admission, they experienced a more favorable outcome.8
The prognosis of hemodialysis patients with COVID-19 is still unclear, and more data are desperately needed. In our cohort, including 4 centers that are part of the Brescia Renal COVID Task Force, we have identified 94 patients with SARS-CoV-2 infection. As expected, infected MHD patients who do not require hospital admission experienced a better disease course compared to patients who required hospitalization. Nevertheless, 5% of the patients treated in the outpatient setting subsequently required admission. There was also substantially less use of antiviral medications in patients managed in the outpatient setting, although the proportion of patients receiving hydroxychloroquine was similar between the 2 groups. These findings should be taken into account when interpreting the results, as it was the managing physician’s decision to start medications. Although the lower rate of antiviral use was associated with a lower incidence of adverse events in the outpatient group, whether this may be the result of less-frequent antiviral use rather than a better overall disease profile needs to be clarified. Finally, in our cohort, only a few patients were treated with glucocorticoids and tocilizumab, which does not allow us to draw any conclusions on the potential efficacy of these treatments.
The disease severity of the SARS-CoV-2 infection is highly variable, and a significant proportion of infected patients appear to experience only mild disease or no symptoms at all in our cohort. Notably, the overall case fatality rate of our population was higher compared to that in the general Italian and Chinese population (28% vs. 7.2% vs. 2.3%).9 , 10 The finding of worse outcome of hemodialysis patients with SARS-CoV-2 infection may be explained by a high prevalence of comorbidities, as well as other risk factors related to end-stage renal disease per se.2
Our study also provides some preliminary information on factors associated with the risk of ARDS and death. The presence of cardiovascular disease and severe inflammation were predictive of worse outcomes. Notably, these factors are not specific for the hemodialysis population, as cardiac comorbidities, fever, and older age have already been described as prognostic factors in the general population with SARS-CoV-2 infection.11 , 12
Our results should be interpreted with some caution. The median follow-up period was 8 days; center bias cannot be ruled out; symptoms severity was not collected; and the relatively small sample size of our cohort may have had an impact on the generalizability of our analyses. The strengths of our study include a shared management approach characterized by relative data homogeneity, and detailed data collection made possible by an unprecedented commitment of the members of our task force.
In conclusion, SARS-CoV-2 infection in maintenance hemodialysis patients presents with a wide range of symptoms. The data represented herein suggest a strikingly higher mortality rate compared to that in the general population, although the risk factors for disease severity are similar. Further data collection and follow-up are necessary to gain a complete picture of the spectrum of COVID-19 in maintenance dialysis patients.
Methods
From March 1, 2020 to April 3, 2020, we enrolled all hemodialysis patients with SARS-CoV-2 infection who were managed within 4 hemodialysis hubs (Table 1) of the Brescia Renal COVID Task Force. Each one of these dialysis centers implemented general prevention measures to safeguard their population. Three of the 4 hemodialysis hubs reserved the RT-PCR test for only those patients showing symptoms suggestive of the disease, after their referral to dedicated clinics. Conversely, starting on March 20th, the Brescia dialysis unit performed RT-PCR analysis on all patients followed.
The therapeutic strategy followed our protocol.1 Antiviral therapy based on lopinavir/ritonavir associated to hydroxychloroquine (with dose adjusted according to kidney function) was considered for all patients, if not contraindicated. In cases of shortage of lopinavir/ritonavir, darunavir and ritonavir were employed. Patients experiencing clinical deterioration after at least 7 days following symptom onset, or no fever for >72 hours, with escalating oxygen requirements, progression of the chest X-ray, and no signs of bacterial infection, were considered for dexamethasone (20 mg/d for 5 days, then 10 mg/d for 5 days) and up to 2 tocilizumab infusions at an interval of 12–24 hours (8 mg/kg of body weight, maximum dose per infusion 800 mg).
Considering the well-known potential of lopinavir/ritonavir and hydroxychloroquine for increasing QTc, a baseline EKG was performed before therapy commencement and afterwards, every 2–3 days; in case of QTc prolongation, a reduction or discontinuation of treatment was considered in a case-by-case manner.
ARDS and cardiac failure have been defined as reported by others.13 , 14 The decision as to whether to admit a patient was made by the attending physician according to symptom severity or signs of respiratory failure.
Ethical approval for this study was obtained according to Italian regulations.
Statistical analyses
Statistical analysis was performed using R software (https://www.r-project.org) and GraphPad Prism 7. Results are expressed as the number and percentage for categorical variables, and the median (IQR) for continuous variables.
Changes in variables were compared by a related sample Wilcoxon test; proportions of patients were compared using a χ2 or Fisher test, as appropriate.
Univariate and multiple logistic regression models were used to assess the ability of some predefined clinical characteristics to predict the risk of ARDS or death. All the statistically significant predictors at univariate analysis were entered in a multivariate model.15 Finally, the best multivariate model was identified by adopting a stepwise selection approach. ORs and their 95% CIs were estimated from logistic regression analysis. P values less than 0.05 (2-tailed) were considered significant.
Disclosure
All the authors declared no competing interests.
References
- 1.Alberici F., Delbarba E., Manenti C. Management of patients on dialysis and with kidney transplantation during the SARS-CoV-2 (COVID-19) pandemic in Brescia, Italy. Kidney Int Rep. 2020;5:580–585. doi: 10.1016/j.ekir.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection [e-pub ahead of print]. Int Urol Nephrol. 10.1007/s11255-020-02451-9. Accessed April 13, 2020. [DOI] [PMC free article] [PubMed]
- 3.Wang R, Liao C, He H, et al. COVID-19 in hemodialysis patients: a report of 5 cases [e-pub ahead of print]. Am J Kidney Dis. 10.1053/j.ajkd.2020.03.009. Accessed April 12, 2020. [DOI] [PMC free article] [PubMed]
- 4.Burgner A., Ikizler T.A., Dwyer J.P. COVID-19 and the inpatient dialysis unit: managing resources during contingency planning pre-crisis. Clin J Am Soc Nephrol. 2020;15:720–722. doi: 10.2215/CJN.03750320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Novel Coronavirus Pneumoniae Emergency Response Epidemiology Team . 2020. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Disease (COVID-19)—China. [press release]. Available at: http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51. Accessed March 22, 2020. [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alberici F., Delbarba E., Manenti C. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;5:1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee D., Popoola J., Shah S. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;5:1076–1082. doi: 10.1016/j.kint.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 10.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy [e-pub ahead of print]. JAMA. 10.1001/jama.2020.4683. Accessed April 14, 2020. [DOI] [PubMed]
- 11.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus disease 2019 pneumonia in Wuhan, China [e-pub ahead of print]. JAMA Intern Med. 10.1001/jamainternmed.2020.0994. Accessed April 14, 2020. [DOI] [PMC free article] [PubMed]
- 12.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Force A.D.T., Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 14.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 15.McMichael T.M., Currie D.W., Clark S. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]