To the editor:
It is now well known that patients with novel coronavirus disease 2019 (COVID-19) due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) commonly have kidney complications, including acute kidney injury, proteinuria, and hematuria. Recent publications in Kidney International used electron microscopy (EM) to detect the virus in autopsy or biopsy specimens of the kidney.1 , 2 Most of the published images depicting the suspected virus are very similar, if not identical, to multivesicular bodies (MVBs). MVBs have been well-known since the 1960s and their appearance and occurrence is detailed in the classic monograph of Feroze Ghadially;3 however, their exact significance and function is unclear. We suspect that the EM images of SARS-CoV-2 published to date are in fact MVBs.
To address this question, we examined the EMs of 11 current consecutive kidney biopsies and 10 kidney biopsies from the pre–COVID-19 era (Table 1 ). Every EM contained renal cortex with 1 to 2 glomeruli. MVBs were found in all 20 kidney biopsies, irrespective of the underlying kidney disease (Figure 1 ). To our surprise, MVBs were always identified in podocytes (1 to 4 podocytes per glomerulus), but we have not seen them in tubular epithelial cells. MVBs were occasionally seen in endothelial cells (mainly arterial or arteriolar) and in a parietal glomerular epithelial cell of 1 biopsy. MVBs theoretically may represent podocyte endocytosis with subsequent formation of intracytoplasmic microvesicles resembling viruses. Seeing an MVB by EM is just a snapshot and we do not know how and when they evolved or how long they remain. MVBs contain microvesicles. However, microvesicles are commonly “free floating” in the cytoplasm of many cell types, including tubular epithelial cells (frequently representing endocytotic vesicles). Su et al. 1 show such cytoplasmic microvesicles in tubular epithelial cells in their Figure 2, but the particles in Figure 2a may have come from an MVB after its membrane dissolved. While these “free floating” cytoplasmic microvesicles could represent viral particles, they are nonspecific. Endocytic vesicles may be coated by proteins, such as clathrin. The presence of coating proteins may cause an electron-dense area around these vesicles giving the appearance of a viral “corona.”4 Su et al. 1 found SARS-CoV nucleoprotein in renal tubules by immunohistochemistry, but the presence of a viral protein does not necessarily mean the presence of complete viral particles. Why MVBs occur so commonly in podocytes and uncommonly in tubular epithelial cells is unclear.
Table 1.
Renal cells with MVB
| Case no. | Year of biopsy | Age (yr) | Sex | Podocyte | Endothelial cell | Parietal epithelial cell | Diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | 2015 | 56 | F | + | + | Membranous glomerulonephritis | |
| 2 | 2015 | 58 | M | + | + | Minimal change disease | |
| 3 | 2015 | 56 | M | + | Focal segmental glomerular sclerosis | ||
| 4 | 2015 | 59 | M | + | Immune complex glomerulonephritis with membranoproliferative pattern | ||
| 5 | 2016 | 60 | M | + | Immune complex glomerulonephritis focal crescents | ||
| 6 | 2016 | 72 | M | + | AL amyloidosis | ||
| 7 | 2016 | 25 | M | + | IgA nephropathy | ||
| 8 | 2016 | 48 | M | + | Cryoglobulinemic glomerulonephritis | ||
| 9 | 2016 | 82 | M | + | + | Oxalate nephropathy | |
| 10 | 2016 | 69 | F | + | Thin basement membrane nephropathy | ||
| 11 | Current | 32 | F | + | Class V+III lupus nephritis (COVID-19–negative) | ||
| 12 | Current | 80 | F | + | Membranous glomerulonephritis | ||
| 13 | Current | 52 | M | + | Diabetic nephropathy | ||
| 14 | Current | 80 | M | + | + | Diabetic nephropathy | |
| 15 | Current | 66 | F | + | Fibrillary glomerulonephritis | ||
| 16 | Current | 79 | M | + | IgA nephropathy | ||
| 17 | Current | 26 | M | + | + | Chronic active antibody-mediated rejection | |
| 18 | Current | 40 | M | + | + | Chronic active antibody-mediated rejection | |
| 19 | Current | 54 | F | + | Thrombotic microangiopathy | ||
| 20 | Current | 86 | F | + | + | Pauci-immune crescentic glomerulonephritis | |
| 21 | Current | 68 | F | + | Acute tubular necrosis |
AL, amyloid λ light chain amyloidosis; COVID-19, coronavirus disease 2019; MVB, multivesicular body.
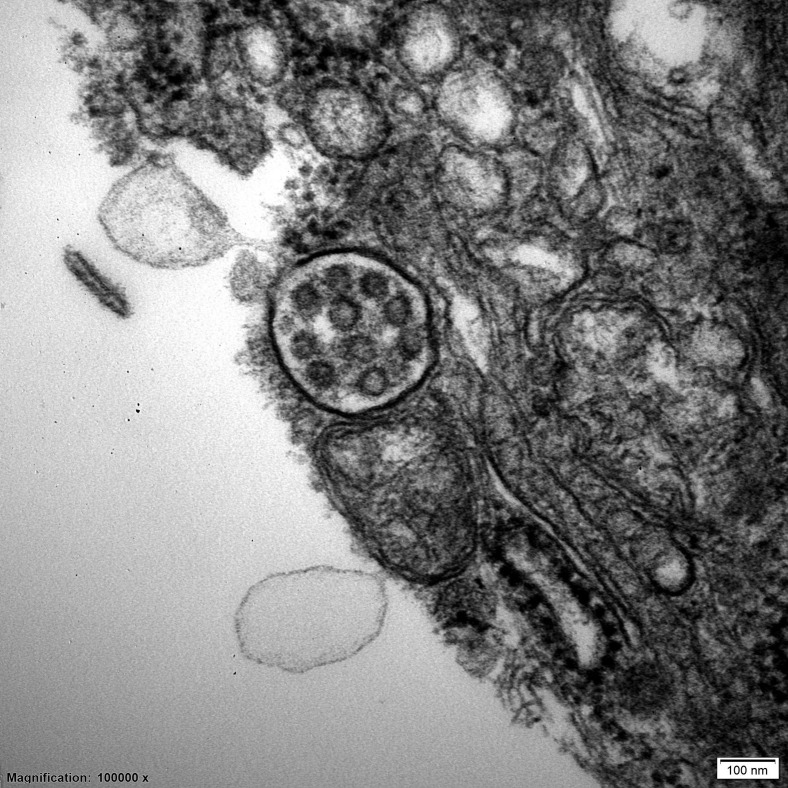
Figure 1.
Multivesicular body in a podocyte of a patient with lupus nephritis who tested negative for coronavirus disease 2019. Uranyl acetate-lead citrate, original magnification ×10,000. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Transmission EM of tissue sections is not a specific or sensitive method for the detection of viral particles; there are numerous structures found by EM that resemble viruses (so-called viral-like particles), such as the well-known endothelial tubuloreticular inclusions (also called myxovirus-like particles). Therefore, caution is suggested when identifying a virus by EM in tissue sections. Immunohistochemistry may also result in nonspecific staining, particularly in renal tubules. Two recent case reports of collapsing glomerulopathy in COVID-19–positive patients failed to identify the virus in the kidney biopsy by in situ RNA analysis.5 , 6 . Another case report describing a patient with collapsing glomerulopathy also failed to find viral RNA in tissue extracted from the biopsy but demonstrated “viral particles” (with the appearance of MVBs) in podocytes.2 Further molecular studies for the presence of the viral genome in renal parenchymal cells would be important in deciding whether SARS-CoV-2 truly infects the kidney.
References
- 1.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kissling S., Rotman S., Gerber C. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020;98:228–231. doi: 10.1016/j.kint.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghadially F.N. Multivesicular bodies and R-bodies. In: Ghadially F.N., editor. 4th ed. Vol. 2. Butterworth-Heinemann; Boston, MA: 1997. pp. 632–639. (Ultrastructural Pathology of the Cell and Matrix). [Google Scholar]
- 4.Marsh M., McMahon H.T. The structural era of endocytosis. Science. 1999;285:215–220. doi: 10.1126/science.285.5425.215. [DOI] [PubMed] [Google Scholar]
- 5.Larsen C.P., Bourne T.D., Wilson J.D. Collapsing glomerulopathy in a patient with COVID-19. Kidney Int Rep. 2020;5:935–939. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peleg Y., Kudose S., D’Agati V. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep. 2020;5:940–945. doi: 10.1016/j.ekir.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]