Abstract
Chronic calorie restriction (CR) improves cardiovascular function and several other physiological markers of healthspan. However, CR is impractical in non-obese older humans due to potential loss of lean mass and bone density, poor adherence, and risk of malnutrition. Time-restricted feeding (TRF), which limits the daily feeding period without requiring a reduction in calorie intake, may be a promising alternative healthspan—extending strategy for midlife and older adults; however, there is limited evidence for its feasibility and efficacy in humans. We conducted a randomized, controlled pilot study to assess the safety, tolerability, and overall feasibility of short-term TRF (eating <8 h day−1 for 6 weeks) without weight loss in healthy non-obese midlife and older adults, while gaining initial insight into potential efficacy for improving cardiovascular function and other indicators of healthspan. TRF was safe and well-tolerated, associated with excellent adherence and reduced hunger, and did not influence lean mass, bone density, or nutrient intake. Cardiovascular function was not enhanced by short-term TRF in this healthy cohort, but functional (endurance) capacity and glucose tolerance were modestly improved. These results provide a foundation for conducting larger clinical studies of TRF in midlife and older adults, including trials with a longer treatment duration.
Electronic supplementary material
The online version of this article (10.1007/s11357-020-00156-6) contains supplementary material, which is available to authorized users.
Keywords: Intermittent fasting, Calorie restriction, Aging, Healthspan
Introduction
Advancing age is the strongest and most common risk factor for chronic diseases and mortality in developed and developing societies (Fries 2015; Olshansky 2018). Regular calorie restriction (CR), characterized by a sustained 10–40% reduction in daily energy intake, extends the lifespan and healthspan of most model organisms and slows many of the basic biological processes of aging (Dean 1990; Masoro 2000; Colman et al. 2009).We and others have shown that chronic CR mitigates cardiovascular aging, including vascular endothelial dysfunction and large elastic artery stiffening (Pierce et al. 2008; Rippe et al. 2010; Donato et al. 2013; Csipo et al. 2018), key antecedents to cardiovascular diseases (CVD), and many other disorders of aging. Moreover, CR improves metabolic regulation and other physiological functions in preclinical models and humans (Colman et al. 2008; Jang et al. 2012; Racette et al. 2017; Matyi et al. 2018). This is important as interventions that slow the progression of aging itself have the potential to reduce the risk of many age-related diseases simultaneously, as opposed to treating individual diseases as they arise (Fries 1980; Butler et al. 2004).
Despite numerous potential benefits, sustained CR is not a feasible public health strategy at the population level because long-term adherence is limited even in highly motivated adults (Moreira et al. 2011; Stewart et al. 2013). Additionally, regular CR is not a pragmatic approach for non-obese older adults because it induces several changes that are contraindicated during aging including weight loss, reductions in skeletal muscle mass and bone mineral density (leading to sarcopenia and increased risk of falls and fractures), reduced immune system function, and reductions in resting metabolic rate and daily energy intake (which increases the challenge of meeting the recommended daily nutrient requirements) (Gardner 2005; Hunt et al. 2012; Normandin et al. 2015; Villareal et al. 2016; Ingram and de Cabo 2017). Therefore, to capitalize on the potential health benefits of CR, while lessening its negative consequences, more practical alternatives to conventional CR are needed to optimize cardiovascular health and other domains of physiological function in non-obese older adults (Martens and Seals 2016).
In this regard, several forms of intermittent fasting involving alternating between periods of unrestricted feeding with shorter periods of restricted energy intake have demonstrated similar improvements in lifespan and healthspan as conventional CR in rodents (Goodrick et al. 1982; Ahmet et al. 2005; Hatori et al. 2012) and have emerged as potential CR-mimicking lifestyle strategies in humans (Varady and Hellerstein 2007; Harvie et al. 2011; Rothschild et al. 2014; Brandhorst et al. 2015). Among these, one form of intermittent fasting known as “time-restricted feeding” (TRF), has the potential to maximize the beneficial effects of CR, while minimizing the numerous potential adverse effects of regular CR in older adults, because unlike all other forms of intermittent fasting, it does not require a reduction in calorie intake (Rothschild et al. 2014). TRF involves consuming one’s normal daily calorie intake within a limited time period (e.g., 8–12 h) and fasting for the remainder of the day. This strategy is likely the most feasible form of intermittent fasting for improving healthspan in non-obese older adults because it theoretically allows individuals to meet daily energy and nutrient intake requirements, maintain lean mass and bone mineral density, and reduce sensations of chronic hunger while still benefiting from the positive physiological effects of increased daily fasting. Consistent with this notion, a recent investigation found that nearly half of the lifespan-extending benefits of CR in mice are actually attributable to TRF as opposed to energy restriction per se, as nearly all CR protocols in rodents also include extended daily fasting (Mitchell et al. 2018).
The results of the first controlled feeding studies on TRF in humans have been promising and suggest that TRF may suppress appetite in overweight adults (Ravussin et al. 2019) and improve cardiovascular and metabolic function in overweight middle-aged men with pre-diabetes (Sutton et al. 2018). Regarding aging, a recent pilot study assessed the effects of 4 weeks of TRF in overweight/obese older adults (Anton et al. 2019); however, the participants lost weight while on the TRF diet. As such, the important question of whether healthy older adults can safely adhere to a TRF eating pattern under free-living conditions without experiencing weight loss has not been addressed. Moreover, the safety, tolerability, and efficacy of TRF for improving cardiovascular function and other indicators of healthspan in non-obese older adults in the absence of weight loss and other adverse consequences associated with conventional CR are not known. To address these critical knowledge gaps, we conducted the first randomized controlled pilot clinical trial to evaluate the feasibility and tolerability of 6 weeks of time-restricted vs. normal feeding (randomized crossover design) for improving cardiovascular function in healthy midlife and older adults under free-living conditions (i.e., self-selected food intake). To gain initial insight into the possible benefits of TRF for improving other indicators of healthspan in this group, we also assessed cardiorespiratory fitness, glucose tolerance, motor function, and cognitive performance.
Results
Subject enrollment and baseline characteristics
The total number of participants who were consented, randomized, and completed this study are presented in Supplementary Fig. 1. Sixty-four healthy midlife and older adult men and women between the ages of 55 and 79 years were recruited for this study and provided their written informed consent. The study was registered on clinicaltrials.gov under the identifier NCT02970188 and was completed between June 2016 and December 2017. On average, the individuals recruited for this study were lean (average BMI = 24.7 ± 0.6 kg m−2) and healthy and were representative of the midlife/older adult population within the greater Boulder County Colorado community. A total of 24 subjects were ultimately enrolled in the study. Of these, 10 subjects were randomized to maintain their normal feeding pattern for the initial 6 weeks of the study before crossing over to a TRF pattern (consuming all meals within an 8-h window; see “Methods” sections for details) for the remaining 6 weeks. The other 14 subjects were randomized to practice TRF during the first 6 weeks and then return to normal feeding for the remaining 6 weeks of the study. Two participants withdrew from the study due to an inability to remain weight stable (N = 1) and the development of GI-distress (N = 1), both of which occurred during the normal feeding arm.
Overall, the 22 individuals who completed the study were non-obese and apparently healthy at baseline, exhibiting normal blood chemistry values for this age range, normotensive blood pressure, and above-average T-scores on composite tests of motor and cognitive function. One individual who qualified for the study based on a fasting blood glucose < 126 mg dl−1 (i.e., the clinical cutoff for diabetes) was later identified as having diabetes based on assessment of glucose tolerance (see below). Complete baseline subject characteristics are presented in Supplementary Table 1.
TRF is safe and well-tolerated in midlife and older adults
No serious adverse events were reported during either condition. A total of two treatment-emergent adverse events were reported by two of the 24 participants enrolled in the study. Both self-reported adverse events were mild-to-moderate in severity and included symptoms of lightheadedness (N = 1) during the TRF condition and gastrointestinal distress (N = 1) during the normal feeding condition. The subject with gastrointestinal distress withdrew from the study during the normal feeding condition; no other participants withdrew from the study due to treatment-emergent adverse events. Clinical laboratory values were obtained from blood samples at the end of each phase of the study for all but one participant. No differences were observed between conditions for measures of hematology (Supplementary Table 2) or blood chemistry, including indicators of renal function and liver enzymes (Supplementary Table 3). However, we did observe a moderate increase in total (NF 178 ± 7 vs. TRF 189 ± 9 mg dL−1; p = 0.009) and LDL-cholesterol (NF 105 ± 7 vs. TRF 116 ± 9 mg dL−1; p = 0.002) following the TRF condition compared with normal feeding (Supplementary Table 4), which was not associated with a change in diet quality (i.e., adopting a less healthy diet during TRF—see below).
TRF is feasible without reducing body mass or bone density
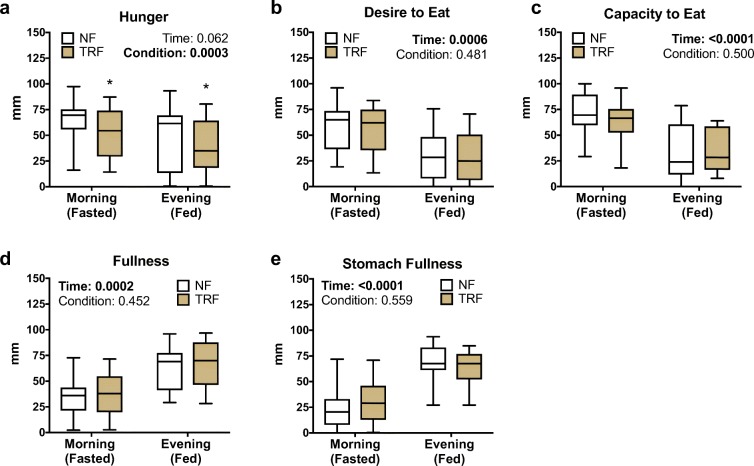
All subjects were successful at reducing the duration of their caloric intake by approximately 4 h/day (Fig. 1a) resulting in an average reduction in their daily feeding period of 33% (p < 0.0001; Fig. 1b). Subjects were able to maintain a daily reduced feeding window during the TRF condition throughout the duration of the 6-week intervention (Fig. 1c). Average total adherence, defined as the proportion of subjects eating within the targeted 8-h daily feeding period throughout the 6-week TRF phase, was 84%, and 95% of all subjects were adherent if the target feeding period is extended by only 30 min (to 8.5-h per day) (Fig. 1d). Importantly, TRF did not adversely influence diet composition as assessed by the healthy eating index (Krebs-Smith et al. 2018) (NF: 63 ± 3 vs. TRF: 61 ± 2 AU; p = 0.46), a composite measure of overall diet quality (Fig. 1e), and total daily energy intake remained isocaloric (Fig. 1f) and was well-matched with estimates of total (resting and non-resting) daily energy expenditure (Fig. 1g) between phases. As such, subjects successfully maintained their body mass (NF 69.3 ± 2.7 vs. TRF 69.4 ± 2.8 kg; p = 0.82) throughout the TRF intervention (Fig. 1h). There were no changes in oxidative fuel source (carbohydrate vs. fat), as indicated by a similar resting respiratory exchange ratio (RER) between conditions (NF 0.83 ± 0.01 vs. TRF 0.83 ± 0.02; p = 0.92). Sensations of hunger were reduced during the TRF condition compared with normal feeding (p = 0.0003) in both the morning (NF 63 ± 5 vs. TRF 52 ± 5 mm) and evening (NF 48 ± 7 vs. TRF 40 ± 6 mm) and tended to be more stable throughout the day (i.e., less difference between fasted and fed states; p = 0.06) (Fig. 2a). TRF did not affect any other measures of appetite (p > 0.05; Fig. 2b–e), and we observed no difference in snacking behavior between conditions (no difference in number of snacks per day, p = 0.25).
Fig. 1.
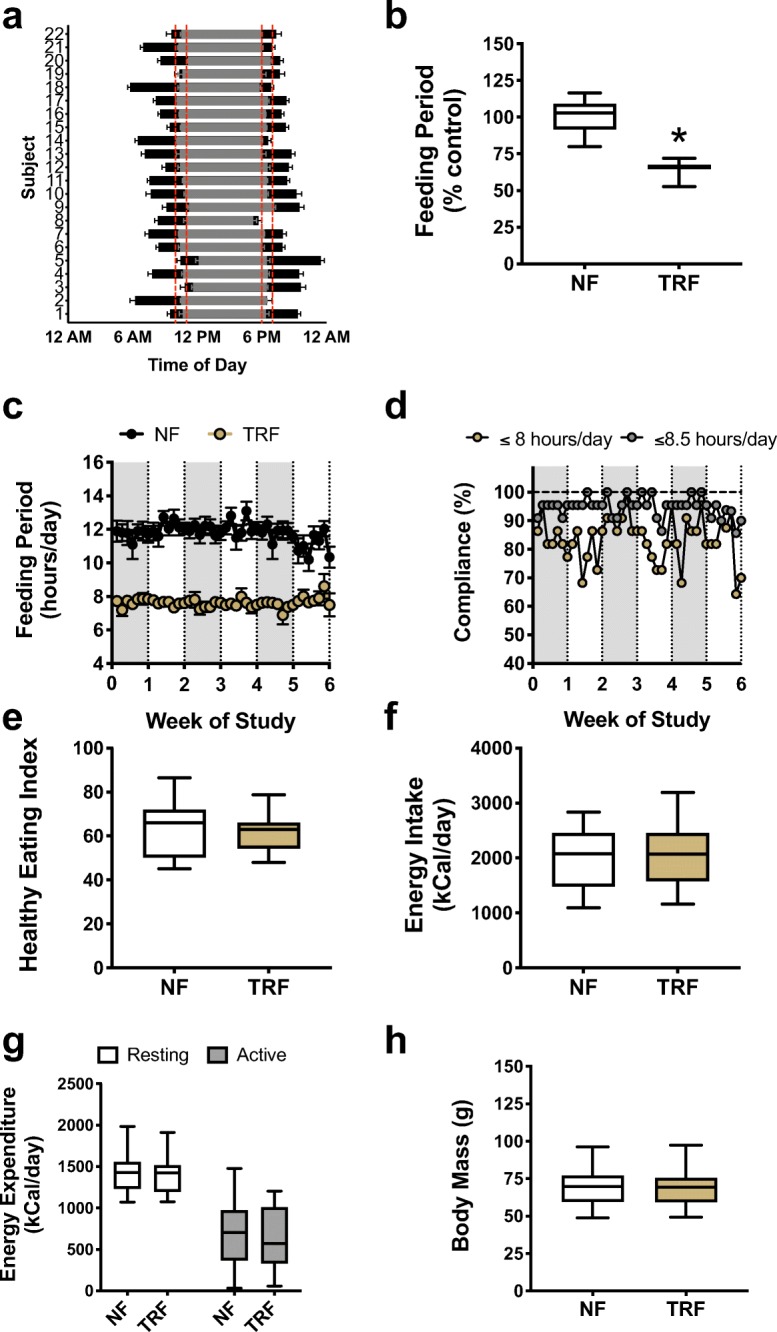
Feasibility of time-restricted feeding. a Compared with normal feeding (NF, black bars), all subjects successfully reduced their feeding window to within the allowed feeding duration (red lines) during time-restricted feeding (TRF, gray bars) resulting in b a reduction in the average daily feeding window. c Subjects were able to maintain a reduction in their daily feeding period throughout the 6-week TRF period with d good overall adherence. There were no adverse changes in e overall diet quality as assessed by the healthy eating index, f energy intake, g estimated energy expenditure, or h body mass. N = 22. Boxes represent median and interquartile range with 95% confidence intervals as whiskers. *p < 0.05. See also Supplementary Table 1, Supplementary Table 2, Supplementary Table 3, and Supplementary Table 4
Fig. 2.
Subjective appetite. Six weeks of time-restricted feeding (TRF) vs. normal feeding (NF) reduced sensations of a hunger but did not alter b desire to eat, c capacity to eat, d fullness, or e stomach fullness despite expected fluctuations between morning (fasted) and evening (fed) conditions. N = 22. Boxes represent median and interquartile range with 95% confidence intervals as whiskers. Data are in millimeters (mm) drawn on a standardized visual-analog scale. *p values < 0.05 based on two-factor repeated measures ANOVA
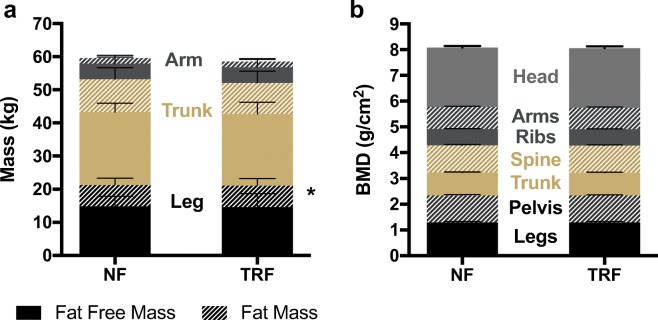
Because conventional CR and alternate-day fasting have been associated with a reduction in body mass that poses a risk to fat-free mass (primarily skeletal muscle) and bone mineral density (Villareal et al. 2016; Stekovic et al. 2019), we next sought to gain initial insight into whether a daily reduction in feeding window by TRF could be achieved in the absence of these deleterious changes in body composition in healthy midlife and older adults. Overall, there was no difference in total body composition between conditions (Fig. 3a). TRF slightly increased leg fat mass by 2.5% compared with normal feeding; however, leg fat-free mass, an indicator of leg skeletal muscle mass, was unaffected by the intervention. Likewise, total and regional bone mineral density were not different between conditions (Fig. 3b), suggesting that 6 weeks of TRF does not result in a loss of bone mass in midlife and older adults.
Fig. 3.
Body composition and bone density. In comparison to normal feeding (NF), time-restricted feeding (TRF) was associated with a a small change in leg fat mass that did not affect total body composition, and b no change in bone mineral density compared with normal feeding (NF). N = 22. Data are mean ± standard error of mean (SEM). *p < 0.05
Efficacy of TRF for improving indicators of healthspan
In addition to determining the safety and feasibility of TRF in a group of healthy midlife and older adults, a key objective of this pilot study was to determine whether TRF improves indicators of healthspan and physiological function. We focused on cardiovascular function based on our laboratory’s experience demonstrating benefits of CR and CR-mimicking strategies on cardiovascular aging (Donato et al. 2013; Martens et al. 2018; de Picciotto et al. 2016; Pierce et al. 2008; Rippe et al. 2010); however, additional measures were included to identify other potential targets for future studies aimed at improving physiological and clinical indicators of healthspan in this population.
Cardiovascular function
There was no effect of TRF on our primary marker of cardiovascular function, endothelium-dependent dilation (Fig. 4a), or secondary markers, including large elastic artery stiffness (Fig. 4b, c) and carotid artery intima-media thickness (Fig. 4d). Likewise, TRF did not influence resting (casual) blood pressure in our cohort of healthy, normotensive midlife and older adults (Fig. 4e, f). These results were unaffected after accounting for differences in plasma LDL-cholesterol, which have been shown to influence vascular function with aging (Walker et al. 2009).
Fig. 4.
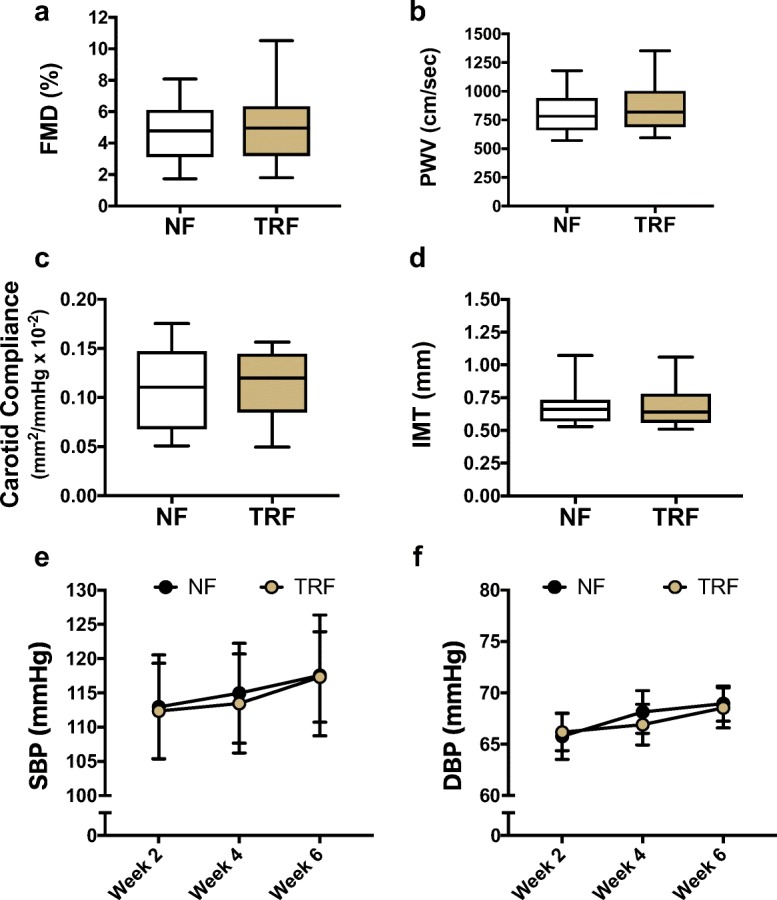
Vascular function and blood pressure at rest. Compared with normal feeding (NF), 6 weeks of TRF did not influence a flow-mediated dilation (FMD), b carotid-femoral pulse wave velocity (PWV), c carotid artery compliance, d carotid intima-media thickness (IMT), e resting systolic blood pressure (SBP), or f resting diastolic blood pressure (DBP). N = 22. Boxes represent median and interquartile range with 95% confidence intervals as whiskers
Cardiorespiratory fitness and functional (endurance) capacity
TRF increased total distance traveled (NF 570 ± 14 vs. TRF 589 ± 15 m; p = 0.02) during a 6-min walk test (Fig. 5a), a well-validated field test of functional (endurance) capacity that is highly influenced by cardiorespiratory fitness (Sperandio et al. 2014). We also observed a small but statistically significant reduction in heart rate during light (NF 98 ± 2 vs. TRF 95 ± 2 beats per minute; p = 0.03) and moderate (NF 118 ± 2 bpm vs. TRF 115 ± 2 bpm; p = 0.03) intensity workloads of a submaximal cycling exercise test during the TRF condition compared with normal feeding (Fig. 5b). Although maximal oxygen consumption (VO2max) was not measured, submaximal exercise oxygen consumption (energy expenditure), the respiratory exchange ratio (carbohydrate vs. fat utilization), and blood pressure were not different during TRF compared with normal feeding (Supplementary Fig. 2).
Fig. 5.
Cardiorespiratory fitness. In comparison to normal feeding (NF), time-restricted feeding (TRF) was associated with a increased distance traveled during a 6-min walk test and b reduced heart rate during light and moderate intensity exercise. N = 22. Boxes represent median and interquartile range with 95% confidence intervals as whiskers. *p < 0.05 based on mixed effects model with repeated measures. See also Supplementary Fig. 2 and Supplementary Table 5
Oral glucose tolerance
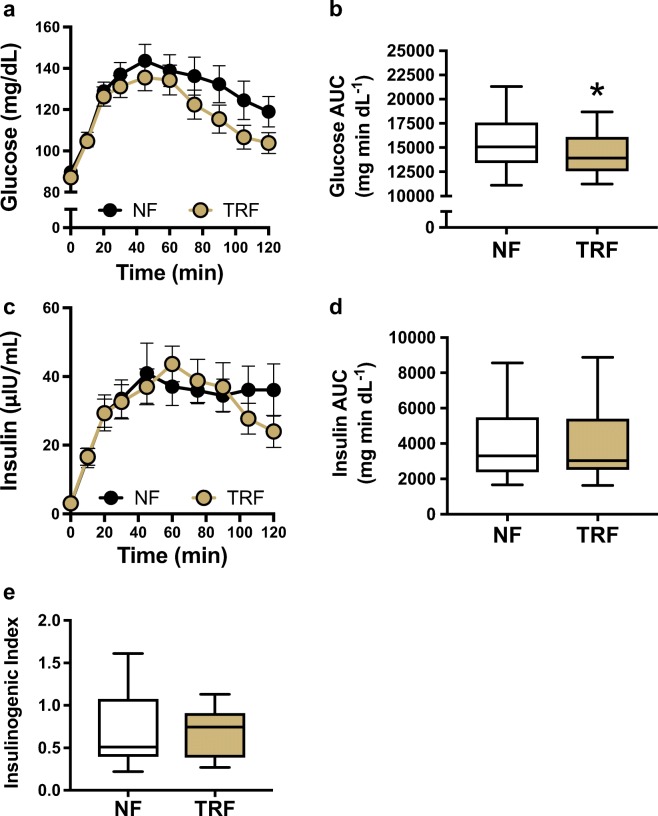
Six weeks of TRF did not affect fasting blood glucose levels in this cohort (Supplementary Table 3). However, compared with the normal feeding condition, TRF tended to lower the glucose area-under-the-curve (AUC) during a 2-h oral glucose tolerance test (OGTT; p = 0.06). One participant who had fasting blood glucose in the normal range at enrollment demonstrated an OGTT response indicative of diabetes. Removal of this subject using a sensitivity analysis strengthened the finding that TRF significantly improved glucose tolerance compared with normal feeding (NF 15,533 vs. TRF 14,387 mg min dl−1; p = 0.03; Fig. 6b). However, TRF did not improve plasma insulin levels at any time point during the OGTT (Fig. 6c) or in the total insulin AUC (Fig. 6d). Likewise, there was no influence of TRF on the insulinogenic index at the 20-min time point (Fig. 6e), which is a marker of pancreatic β-cell function (Phillips et al. 1994).
Fig. 6.
Glucose tolerance. Compared with normal feeding (NF), time-restricted feeding (TRF) was associated with a, b a reduction in glucose area-under-the-curve (AUC) during a 2-h OGTT, c, d no changes in insulin levels and the insulin AUC, and e no change in the insulinogenic index (a marker of pancreatic β-cell function). N = 16 participants. Boxes represent median and interquartile range with 95% confidence intervals as whiskers. *p < 0.05 based on mixed effects model with repeated measures
Other physiological markers
TRF did not significantly influence measures of motor function in these healthy, well-functioning midlife and older men and women (Supplementary Table 6). Likewise, we observed no change in any domain of cognitive function after 6 weeks of TRF vs. normal feeding; however, it should be noted that the participants in this trial had above-average T-scores (> 50) at baseline for all composite measures of cognitive function, which may have created a ceiling effect in response to TRF.
Markers of oxidative stress, inflammation, and energy sensing
Despite increases in plasma total and LDL-cholesterol with TRF, we did not observe corresponding increases in plasma oxidized-LDL (Fig. 7a) or the oxidized-to-total LDL ratio (Fig. 7b), both of which are circulating markers of oxidative stress and atherogenic risk (Berliner and Heinecke 1996). Although CR-induced weight loss may decrease circulating levels of the inflammatory biomarker C-reactive protein in overweight/obese adults (Pierce et al. 2008), we did not observe any changes in C-reactive protein or interleukin-6 (IL-6) with TRF in the healthy older adults studied here. Because alternate-day fasting has been associated with moderate increases in plasma ketones (Johnson et al. 2007; Stekovic et al. 2019), we also assessed whether TRF increased levels of plasma acetoacetate and β-hydroxybutyrate; however, no changes were observed. Finally, we conducted an exploratory analysis of the circulating nicotinamide adenine dinucleotide (NAD+) metabolome and other related metabolites (e.g., AMP, ADP, ATP). Tissue concentrations of these metabolites can be affected during activation of cellular energy sensing and catabolic pathways by CR (Chen et al. 2008) or fasting (Rodgers et al. 2005; Canto et al. 2010) in animal models. We did not observe any changes in plasma concentrations of these metabolites in response to TRF (Supplementary Table 6). These findings are in line with a recent study that reported no changes in plasma NAD+ and related metabolites following prolonged fasting in humans (Bak et al. 2018).
Fig. 7.

Mechanistic biomarkers. Compared with the normal feeding (NF) condition, time-restricted feeding (TRF) was not associated with any changes in a oxidized-LDL levels, b the oxidized-to-total LDL ratio, c interleukin-6 (IL-6), d C-reactive protein (CRP) or plasma ketones including e acetoacetate or f β-hydroxybutyrate. Boxes represent median and interquartile range with 95% confidence intervals as whiskers. See also Supplementary Table 6
Discussion
The main finding of this pilot study is that 6 weeks of TRF is a feasible and well-tolerated dietary strategy for healthy midlife and older adults that can be achieved under free-living conditions in the absence of adverse changes in body weight, lean mass, bone density, energy balance, or diet quality. Moreover, TRF is associated with lower sensations of hunger without corresponding changes in satiety, which may have contributed to the excellent overall adherence that we observed. Our study provides the first direct support for the concept that TRF is a feasible alternative lifestyle strategy to conventional CR in individuals for whom weight loss is not a goal. Although we did not observe an improvement in our primary outcome of vascular endothelial function, our study provides preliminary evidence that TRF may improve functional (endurance) capacity and glucose tolerance, two clinically important indicators of healthspan that decline with normal aging and predict future risk of age-related disease and/or disability. Collectively, these findings create a critical platform for conducting larger, longer-term clinical trials of TRF for improving human healthspan, including older adults with functional limitations, disability, and/or chronic disease.
The benefits of sustained CR for extending lifespan and enhancing physiological function in animal models are well established (see review by Chung et al. 2013). However, in humans, CR is associated with poor overall adherence and risk of adverse health consequences. In the NIH-sponsored CALERIE trial, the largest study of sustained CR in non-obese humans to date, even the highly motivated subjects recruited into the study were unable to maintain the initial target of 25% energy restriction over the entire 2-year intervention period (Rochon et al. 2011; Stewart et al. 2013). Moreover, the ~ 10% energy restriction that was ultimately achieved was associated with a reduction in body weight, fat-free mass, and bone mineral density (Villareal et al. 2016). More recently, 4 weeks of alternate-day fasting was shown to result in a 37% reduction in energy intake and a corresponding reduction in bone mineral density in the lumbar spine of midlife adults compared with baseline levels (Stekovic et al. 2019). Because weight loss is a strong predictor of hip fracture in both lean and overweight/obese older adults (Ricci et al. 2001; Ensrud et al. 2005), it is possible that any potential benefits of sustained CR or alternate-day fasting on physiological function would be outweighed by these adverse effects, particularly among those at greatest risk for sarcopenia/sarcopenic obesity, osteoporosis, and frailty (Schafer 2016).
The present findings suggest that short-term TRF is feasible and well-tolerated in midlife and older adults under free-living conditions. Our participants reported no serious adverse effects related to TRF and were able to complete the intervention without any changes in body composition, bone mineral density, energy intake, or diet quality. Interestingly, our subjects reported reduced feelings of hunger following TRF. Previous studies into the effects of TRF on hunger are mixed, with some reporting either a decrease (Gill and Panda 2015; Ravussin et al. 2019), increase (Stote et al. 2007), or no change (Sutton et al. 2018). Discrepancies among these studies may be due to differences in the time of day of measurement, the feeding window duration (single meal vs. extended window), study design (controlled feeding vs. free-living), and/or subject characteristics (overweight/obese and/or prediabetic vs. lean and healthy), among other factors. In contrast, conventional CR is associated with a greater hunger and desire to eat compared with ad libitum feeding (Anton et al. 2009).
Another goal of the present study was to gain initial insight into the potential efficacy of TRF for improving cardiovascular and other select domains of physiological function. We observed no improvement in vascular endothelial function (primary outcome) but did detect modest effects of TRF on two important indicators of healthspan: functional endurance capacity and blood glucose regulation. In this regard, TRF was associated with an improvement in 6-min walk distance, a reliable measure of functional (endurance) capacity in older adults that is highly linked to cardiorespiratory fitness (Blomquist and Saltin 1977; Carter et al. 2003). We also found a small reduction in heart rate during submaximal exercise following TRF, consistent with the possibility of an improvement in aerobic fitness. As such, these physiological functions should be included as outcomes in larger trials of TRF to further establish the effects of TRF in older adults. Such investigations also may wish to explore the mechanisms for potential enhancement of functional capacity with TRF, including possible improvements in related biomarkers of frailty such as walking effort (perceived difficulty) and efficiency and gait perturbations (LaRoche et al. 2018; McCrum et al. 2019).
Blood glucose regulation in response to an oral glucose challenge also appeared to improve slightly with TRF in the absence of obvious changes in plasma insulin levels or β-cell function compared with normal feeding. Blood glucose control generally is impaired with aging and is associated with an increased risk of type 2 diabetes, cardiovascular disease, and other chronic disorders (Saydah et al. 2001; Crandall et al. 2009; Kalyani and Egan 2013). In middle-aged overweight men with pre-diabetes, TRF reduced insulin levels and enhanced β-cell responsiveness during an oral glucose challenge with no effects on plasma glucose levels (Sutton et al. 2018), suggesting that changes in glucose-insulin function may be influenced by baseline metabolic health status. In any case, both studies support the idea that glucose and/or insulin signaling may be improved following TRF.
It is worth noting that we observed a moderate increase in total and LDL-cholesterol during the TRF condition compared with normal feeding. Increases in LDL-cholesterol have also been reported following 12 months of alternate-day fasting in metabolically healthy obese humans (Trepanowski et al. 2017) and after 3 weeks of TRF in rats (Rivera-Zavala et al. 2017), although reductions in total and LDL-cholesterol also have been reported with shorter-term (4 weeks) alternate-day fasting (Stekovic et al. 2019). Although the mechanisms are not completely understood, fasting is associated with a decreased expression of the liver LDL receptor (Van Der Wal et al. 1998) and an increase in endogenous corticosteroid production (Luna-Moreno et al. 2009), which may reduce the clearance of LDL from the blood. Importantly, the increase in LDL-cholesterol in the present study was not associated with changes in cardiovascular function and there was no increase in oxidized-LDL levels, a circulating marker of oxidative stress and atherogenic risk (Berliner and Heinecke 1996). Expression of the gene encoding the paraoxonase enzyme, which is responsible for preventing the oxidation of LDL-cholesterol, increased following daytime-restricted feeding in rats (Rivera-Zavala et al. 2017) and could explain this observation.
In the present study, we observed no increases in plasma ketone levels, which have been reported to increase following alternate-day fasting in healthy non-obese adults (Stekovic et al. 2019) and in overweight adults with moderate asthma (Johnson et al. 2007). The increase in plasma ketones is believed to contribute to improvements in physiological function during longer duration fasting when liver glycogen stores become more depleted (Longo and Mattson 2014); however, our results suggest that 8 h/day of TRF is not a sufficient stimulus for raising plasma ketones. We also found no differences in blood-cellular NAD+ concentration after TRF compared with normal feeding. NAD+ is an important co-substrate for energy-sensing enzymes that decline with advancing age. Increases in NAD+ levels through administration of precursor molecules (de Picciotto et al. 2016; Mills et al. 2016; Kiss et al. 2019) or in response to fasting (Rodgers et al. 2005; Canto et al. 2010) have been linked to improvements in physiological function in animal models; however, our results suggest that 6 weeks of TRF is not sufficient for boosting NAD+ metabolite levels in humans. It is possible that potential differences in plasma ketones and NAD+ metabolite levels may have been blunted by the overnight fasting condition employed prior to all blood draws. Although this is a standard best-practice procedure for clinical trials, we cannot rule out the possibility that differences may have existed during other time points within the 24 h day (e.g., postprandial).
There are several limitations that may be relevant when designing future clinical trials of TRF in older adults. Although our subjects were able to maintain excellent adherence to 8-h of TRF over a 6-week period, it will be important to assess feasibility over longer treatment durations and with minimal dietary counseling to enhance translation to the public health setting. Because our study was designed to test the feasibility of TRF under free-living conditions, we selected a feeding window that we believed would be most easily integrated into daily life. As such, our study was not designed to assess potential circadian-related feeding effects or the impact of TRF on circadian-influenced behaviors, including sleep and physical activity patterns. Future trials may wish to assess outcomes under a variety of eating schedules (e.g., early morning vs. late afternoon TRF) and/or assess these and other behaviors as outcomes.
More information also is needed regarding the potential effects of the magnitude of change in the feeding window when transitioning from the baseline feeding pattern to TRF. In the present study, we successfully reduced the baseline eating window by ~ 33% (i.e., from ~ 12 to < 8 h/day), whereas in overweight individuals, Gill and Panda (2015) previously reported a 28% reduction in daily feeding period (from 14 to 10 h). Given the significant magnitude of change, we believe that the reduction in the eating window in the present study should represent a significant physiological stimulus to influence function should such effects be possible in this population. Finally, although we report no changes in bone mineral density following 6 weeks of TRF compared with normal feeding, it will be important to assess longer TRF treatment durations and to examine sites with higher bone turnover such as the lumbar spine and femoral neck.
Although we did not observe any significant effects of TRF in cardiovascular health markers or in motor or cognitive performance, it is possible that a longer intervention duration may have induced improvements in one or more of these physiological functions. Sutton et al. (Sutton et al. 2018) recently showed that an even shorter (5-week) period of TRF improved cardio-metabolic function in men with pre-diabetes; however, these subjects likely had greater baseline dysfunction than our cohort. It is also important to emphasize that our study was designed to determine the feasibility and efficacy of TRF (adherence, safety and tolerability) among healthy, independently living midlife and older adults. As a result, our participants were generally active with blood pressure in the normal range (< 120/80 mmHg on average) and no vascular disease. Likewise, they all had above-average scores (adjusted for age, ethnicity, and education status) on both the NIH Toolbox motor and cognitive function batteries, which may have created a ceiling effect that limited the ability to show improvements in these functions in response to the intervention. Our study therefore provides initial evidence supporting the safety of TRF as a potential CR-mimicking lifestyle strategy and provides an investigational platform for larger clinical trials aimed at establishing the efficacy of TRF in groups with more impaired baseline function, such as older adults with elevated cardiovascular risk factors, mobility disorders, and cognitive impairments. Finally, as an exploratory, hypothesis-generating initial trial, we did not adjust our statistical analysis for multiple endpoints as this would have increased the risk of type II error (false negative) and undermined our ability to identify possible endpoints for future, single-endpoint clinical trials of TRF (Schulz and Grimes 2005). Nevertheless, we took steps to ensure a robust and transparent analysis by only testing pre-specified hypotheses using a similar analytical approach on a priori determined outcomes.
In summary, our results provide the first evidence that short-term (6 weeks) TRF is safe and well-tolerated in healthy midlife and older adults. Moreover, adherence was excellent under these free-living conditions and TRF was not associated with any of the potential adverse effects of conventional CR. Our findings also suggest that TRF may improve functional (endurance) capacity and glucose tolerance, two important indicators of healthspan. Taken together, our results provide an essential experimental foundation for conducting larger, longer-term clinical trials of TRF. Such trials might target “signals” from the present study, such as blood glucose regulation, in healthy aging populations, and/or assess a broader set of healthspan markers in groups with more greatly impaired baseline physiological function or age-related clinical disorders.
Methods
All procedures were reviewed and approved by the University of Colorado Boulder Institutional Review Board. The nature, benefits, and risks of the study were explained to all subjects, and their written informed consent was obtained prior to participation. All measurements were performed in the Integrative Physiology of Aging Laboratory. Analysis of all outcomes was conducted by individuals blinded to treatment condition. The study was registered on clinicaltrials.gov under the identifier NCT02970188.
Study participants
Healthy midlife and older men and postmenopausal women aged 55 to 79 years were recruited from Boulder Colorado and surrounding communities. Of the 64 participants recruited for this study, 33 did not meet the inclusion criteria and were excluded from participation in the study. Seven participants dropped out of the study prior to randomization due to the time commitment (N = 5), a stated desire to lose weight (N = 1) or poor adherence to the study restrictions during the 1-week lead-in period (N = 1). All subjects were apparently healthy and free of overt clinical diseases as assessed by medical history and baseline blood chemistries. Subjects were excluded if they exhibited abnormal blood chemistries indicative of impaired renal or liver function (as determined by the study physician) or had elevated fasting blood glucose indicative of type 2 diabetes (i.e., > 126 mg/dl). Subjects were also excluded if they had alcohol dependence, uncontrolled thyroid disease, severe obesity (body mass index ≥40 kg m−2), were not weight stable for at least 3 months prior to enrolling in the study (defined as > 2 kg change in body mass), or expressed a desire to lose weight during the course of the study. Body mass, BMI, and waist and hip circumferences were measured by anthropometry, and total body fat percentage was measured using dual-energy x-ray absorptiometry (Lunar/Prodigy, GE Healthcare, Chicago IL).
Study design, randomization, and intervention
The study design consisted of a 2 × 6 week randomized controlled crossover clinical trial. All participants underwent a 1-week lead-in period during which they were instructed to maintain their normal feeding pattern but were required to submit daily records of their feeding window (start and stop times) and complete a single 24-h dietary recall (see below). Subjects who were non-compliant to the lead-in restrictions were excluded and all remaining participants underwent baseline testing and were assigned to perform 6 weeks of time-restricted feeding (TRF) and 6 weeks of normal feeding in a randomized order. All participants were evaluated by a registered research dietitian by telephone at the start of the study and received instructions on how to maintain their normal dietary pattern (i.e., energy intake, meal frequency and diet composition) throughout both arms of the study.
Adherence to each intervention was monitored by daily surveys of feeding periods, and dietary composition was reviewed by the registered research dietitian via analysis of 24-h dietary records (see below). Subjects successfully maintained their body weight and calorie intake independently during both arms of the study without further dietary consultation. We did not include a formal washout period in this study; however, all outcomes were assessed for potential carry-over effects using linear mixed models and none were identified.
During the TRF arm of the study, subjects were allowed to self-select a starting time between 10:00–11:00 h but were required to maintain the same 8-h feeding window each day for the duration of the TRF arm. For example, if a subject started eating at 10:30 h, they would be required to stop eating by 18:30 h, each day of the study. This particular eating window was selected because we reasoned that it would be the least disruptive to normal daily life (i.e., allow for family dinners) and therefore more translatable to the majority of older adults in terms of adherence. During the TRF phase, no calorie-containing food or beverages (except black coffee or unsweetened tea) were allowed outside of the 8-h feeding window. Subjects were instructed to maintain their normal energy intake within the 8-h feeing window but were free to divide their food intake into as many meals or snacks as they desired. No restrictions were given for the timing or duration of the feeding window during the normal feeding arm; however, subjects were instructed to eat within their regular feeding window reported during the lead-in period. No exclusion criteria were set for prescription medication use; however, subjects refrained from taking any over-the-counter medications for 48 h and prescription medications for 24 h prior to all experimental testing, as previously done during other intervention studies in our laboratory (Kaplon et al. 2016; DeVan et al. 2016; Santos-Parker et al. 2017; Martens et al. 2018). All assessments were performed at the end of each intervention phase after a 16-h overnight fast (regardless of assigned feeding condition) with the exception of motor and cognitive function tests, which were performed 2 h after a standardized light meal or snack in order to ensure that subjects had enough energy to complete the testing battery and also included a baseline measurement at the beginning of the study to control for potential learning effects. All assessments were made in the morning and the timing of each assessment was matched between feeding conditions. As such, all motor and cognitive testing was performed in the late-morning or afternoon to avoid disrupting the fasting window. Subjects refrained from consuming alcohol or engaging in vigorous exercise for 24 h and refrained from caffeine intake for at least 12 h prior to all testing sessions.
Adherence and feasibility
Adherence to each intervention arm was assessed by asking participants to complete a daily electronic survey that was administered by e-mail through a secure electronic data capture system (REDCap). Surveys were sent in the evening at 19:00 h regardless of which dietary condition the subject was currently assigned to. Subjects were asked to report the time of day that they started and stopped eating each day. Once per week, they were also asked to rate their perceptions of hunger and satiety using a visual-analog scale (0–100 mm). Subjects were expected to report their perceptions of appetite at the moment they received the survey, which was administered at a standardized time of day (19:00 h). Twice per week, they were asked to report their body weight using a digital scale that was provided for them to take home at the start of the study.
Safety and tolerability
Subjects reported to the laboratory every 2 weeks to assess body weight and to discuss any issues with adherence or treatment-emergent adverse events. Standard clinical markers of hematology, liver and kidney function and blood lipids were assessed at the end of each treatment period using standardized clinical assays performed in the Boulder Community Hospital clinical laboratory.
Energy balance and dietary intake
Energy intake and diet composition were assessed using the online Automated Self-Administered 24-h Recall (ASA24) software provided by the National Cancer Institute (epi.grants.cancer.gov/asa24/) during the lead-in period and once per week throughout each intervention arm. Dietary records from each feeding condition were analyzed for total energy intake, relative macronutrient composition, and micronutrient content. Energy intake and dietary composition were determined by averaging the values obtained during weeks 3–5 of each intervention phase. Diet quality was determined by calculating the USDA’s Healthy Eating Index, as described elsewhere (Krebs-Smith et al. 2018).
Resting metabolic rate was measured by indirect calorimetry (ParvoMedics TrueOne 2400) as previously described by our laboratory (Bell et al. 2003, 2006; Martens et al. 2018). Subjects rested quietly in the supine position for 45 to 60 min with a ventilated hood placed over their head to measure the concentrations of oxygen (O2) and carbon dioxide (CO2) in their expired breath. Resting energy expenditure and the respiratory exchange ratio (RER; an indicator of oxidative fuel source) were calculated in 1-min segments and averaged from at least 30 min of steady data.
Physical activity was estimated using the Community Healthy Activities Model Program for Seniors (CHAMPS) Physical Activity and Energy Expenditure questionnaire, as previously described (Santos-Parker et al. 2017, 2018).
Primary physiological outcome
Endothelial function
Endothelium-dependent dilation (vascular endothelial function) was measured by brachial artery flow-mediated dilation (FMD) to reactive hyperemia, using high-resolution ultrasonography (PowerVision 6000, Toshiba) as previously described (Eskurza et al. 2004; Jablonski et al. 2013; Walker et al. 2014). FMD was expressed as the percentage change (%Δ) in brachial artery diameter from baseline.
Secondary physiological outcomes
Blood pressure and arterial stiffness
Resting blood pressure was measured in the seated position following 10 min of quiet rest using a semi-automated blood pressure device (Dynamap™ XL, Johnson & Johnson, Arlington, TX, USA) as previously described (Martens et al. 2018). Multiple measurements were obtained from the non-dominant arm, with 2 min of quiet rest between recordings, until three blood pressure values were obtained that were within 5 mmHg of one another. These values were then averaged to determine resting systolic and diastolic blood pressure.
Aortic stiffness was measured using carotid-to-femoral pulse wave velocity (PWV), the gold-standard assessment of aortic stiffness in humans (Laurent et al. 2001, 2006). Simultaneous pressure waveforms were obtained noninvasively from the carotid and femoral arteries using applanation tonometry (Millar SPT-301, Millar Inc., Houston Texas) and the aortic pressure wave transit time was measured as the time-delay between the foot of the carotid and femoral pressure waves, as previously described (Tanaka et al. 1998; Martens et al. 2018). PWV was calculated by dividing the distance between the two measurement sites by the time-delay.
Carotid artery compliance was determined by measuring the change in diameter of the right common carotid artery as assessed using high-resolution ultrasonography (PowerVision 6000, Toshiba, Tokyo Japan) relative to the change in carotid blood pressure as assessed using applanation tonometry across the cardiac cycle. Carotid pressure was normalized to brachial artery pressure obtained using a semi-automated blood pressure cuff (Dynamap™ XL, Johnson & Johnson, Arlington, TX, USA). Compliance was calculated as CC = π × DD2 × (ΔD DD−1) / (2 × PP) where DD is diastolic diameter, ΔD is the change in diameter, and PP is the arterial pulse pressure, as described previously by our laboratory (Moreau et al. 2003; Pierce et al. 2012; Martens et al. 2018). Carotid artery intima-media thickness was also assessed as previously described (Tanaka et al. 2001).
Submaximal exercise, walking endurance, and motor function
Submaximal exercise responses were determined by measuring heart rate during the last minute of a submaximal exercise test on a cycle ergometer. Oxygen consumption (an estimate of energy expenditure), RER, and blood pressure were also measured. Following a brief (~ 5 min) warm up, subjects completed 3 × 4-min stages at workloads that were determined during baseline testing to correspond to 10%, 30%, and 50% of each subject’s heart rate reserve, calculated as the age-predicted maximum heart rate [220-age]—resting heart rate.
Walking endurance was assessed by measuring the distance covered during a 6-min walking task on a 50-ft (out-and-back) indoor course as previously described (Sperandio et al. 2014; Justice et al. 2015; Santos-Parker et al. 2018).
Motor function was assessed using select measures of the NIH Toolbox Motor Battery as previously described (Justice et al. 2015; Santos-Parker et al. 2018). Tests included measures of strength (hand grip strength), manual dexterity (9-hole pegboard test), mobility (4-m walk gait speed test), and dynamic balance (rapid step test). In addition to the NIH Toolbox battery, we also performed additional tests of leg strength (knee extension 1 repetition maximum) and power (peak leg extension power at 40% and 70% of 1 repetition maximum), mobility (stair ascent test, five-repeat sit-to-stand test), and fatigability (heel-rise test) as previously used by our laboratory and others (Callahan et al. 2007; Justice et al. 2015; Santos-Parker et al. 2018).
Oral glucose tolerance test
Glucose tolerance was assessed using an oral glucose tolerance test (OGTT). Following insertion of an intravenous catheter and collection of a baseline blood sample, participants ingested a beverage containing a 75-g load of glucose within a 10-min time frame. Blood was collected at 10, 20, 30, 45, 60, 75, 90, 105, and 120 min after glucose ingestion and was analyzed for glucose and insulin concentrations. Outcomes included the area under the curve (AUC) for glucose and insulin, which was determined using the trapezoidal method. The insulinogenic index, an indicator of pancreatic β-cell responsiveness, was calculated by dividing the change in insulin by the change in glucose during the first 20 min of the test (Phillips et al. 1994).
Cognitive function
Cognitive function was evaluated with the NIH Toolbox Cognition Battery as previously described by our laboratory (Justice et al. 2015; Santos-Parker et al. 2018). Briefly, we administered a short battery consisting of seven computerized tests which assessed five major domains of fluid cognition (inhibitory control and attention, episodic memory, working memory, processing speed, and executive function) and one major domain of crystallized cognition (language), resulting in total, fluid, and composite cognition T-scores, as well as individual sub-domain T-scores, which were adjusted for age, sex, and education status. In addition to the NIH Toolbox, we also administered the Trail Making Test (parts A and B) as another measure of processing speed and executive function.
Serum biomarkers
Plasma levels of oxidized-LDL-cholesterol, C-reactive protein, and interleukin-6 were analyzed by the University of Colorado Denver Clinical and Translational Research Center (CTRC) Core Laboratory, as previously described by our laboratory (Pierce et al. 2008). NAD+ and related metabolites were measured in peripheral blood mononuclear cells using liquid chromatography-mass spectrometry (LC-MS) by the Mass Spectrometry Facility at the University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, as recently described in detail by our laboratory (Martens et al. 2018). Plasma ketone levels were measured using commercially available colorimetric assays for acetoacetate (Abcam, ab180875) and β-hydroxybutryate (Cayman Chemical, no. 700190).
Statistical analyses
A sample size of 19 subjects was determined to be sufficient to observe improvements in our primary outcome measure (endothelium-dependent dilation) at 80% power and an alpha level of 0.05 based on our laboratory’s previous experience studying CR and other lifestyle interventions in midlife and older adults in which we observe effect sizes for brachial artery FMD that range between 0.7 and 0.8 (Pierce et al. 2008; Jablonski et al. 2013). Statistical analyses were conducted by a biostatistician within the Center for Innovative Design & Analysis at University of Colorado Anschutz Medical Campus. All analyses were conducted as two-sided tests with a significance threshold set at α = 0.05. Adherence to the feeding window and all safety-related outcomes (e.g., clinical laboratory values, body composition and energy intake/expenditure) were assessed using two-sided paired t tests. Subjective measures of appetite were assessed by the mixed-effect model with repeated measures with time of day (morning vs. evening), condition (TRF vs. normal feeding), and sequence set as fixed effects and participants as random effect. All physiological outcome variables were assessed as the difference between normal feeding vs. TRF using linear mixed models. Model estimation was based on the restricted maximum likelihood estimation (REML) using either a first-order autoregressive or compound symmetry covariance structure (submaximal exercise only) with period (end of TRF phase vs. end of normal feeding phase) and sequence (order of TRF vs. normal feeding) set as fixed effects and participants as random effect. Missing data were considered missing at random (MAR). NIH Toolbox cognitive function data were adjusted for baseline values to control for learning effects. An exploratory assessment of cardiovascular variables was conducted with adjustment for LDL-cholesterol. A sensitivity analysis was conducted on blood samples from the OGTT to account for one participant that was later determined to have clinical diabetes. All data are presented as raw mean ± standard error of mean (SEM). Statistical analyses were conducted using SAS version 9.4 (SAS, Cary, North Carolina).
Electronic supplementary material
(DOCX 155 kb)
Acknowledgments
We wish to acknowledge Hailey Lynch and Ross Tanick for their technical assistance with data collection and data entry and senior biostatistician David Weitzenkamp from the Center for Innovative Design & Analysis for offering insight and consultation during the statistical analysis.
Author contributions
C.R.M., B.A.D., and D.R.S. conceived and designed the clinical trial with input from C.M.P. C.R.M., M.J.R., M.R.M., L.R.J., and J.J.R., and E.N. collected and analyzed all of the data. S.A.J. performed all dietary counseling and analyses. Y.W. performed the statistical analyses. B.P.Z. conducted and analyzed all plasma ketone assays. M.C. provided medical oversight of all study subjects, evaluated inclusion/exclusion criteria, and reviewed adverse events. C.M., M.J.R, and D.R.S. drafted the manuscript. All the authors helped interpret the data, provided critical revisions of the manuscript, and approved the final version of the manuscript.
Funding information
This work was supported by NIH grants AG000279 and AG053009.
Data availability
The data that support the findings of this study are openly available in Mendeley Data at 10.17632/w83t9s27dx.2
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmet I, Wan RQ, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112:3115–3121. doi: 10.1161/CIRCULATIONAHA.105.563817. [DOI] [PubMed] [Google Scholar]
- Anton SD, Han H, York E, Martin CK, Ravussin E, Williamson DA. Effect of calorie restriction on subjective ratings of appetite. J Hum Nutr Diet. 2009;22:141–147. doi: 10.1111/j.1365-277X.2008.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton SD, Lee SA, Donahoo WT, et al. The effects of time restricted feeding on overweight, older adults: a pilot study. Nutrients. 2019;11:1500. doi: 10.3390/nu11071500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak AM, Vendelbo MH, Christensen B, et al. Prolonged fasting-induced metabolic signatures in human skeletal muscle of lean and obese men. PLoS One. 2018;13:1–19. doi: 10.1371/journal.pone.0200817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C, Jones PP, Seals DR. Oxidative stress does not modulate metabolic rate or skeletal muscle sympathetic activity with primary aging in adult humans. J Clin Endocrinol Metab. 2003;88:4950–4954. doi: 10.1210/jc.2003-030454. [DOI] [PubMed] [Google Scholar]
- Bell C, Stob NR, Seals DR. Thermogenic responsiveness to beta-adrenergic stimulation is augmented in exercising versus sedentary adults: role of oxidative stress. J Physiol. 2006;570:629–635. doi: 10.1113/jphysiol.2005.098756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berliner JA, Heinecke JW (1996) The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med 20:707–727 [DOI] [PubMed]
- Blomquist C, Saltin B. Cardiovascular adaptations to physical training. Annu Rev Physiol. 1977;39:221–231. doi: 10.1146/annurev.ph.39.030177.001253. [DOI] [PubMed] [Google Scholar]
- Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, di Biase S, Mirzaei H, Mirisola MG, Childress P, Ji L, Groshen S, Penna F, Odetti P, Perin L, Conti PS, Ikeno Y, Kennedy BK, Cohen P, Morgan TE, Dorff TB, Longo VD. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 2015;22:86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler RN, Warner HR, Williams TF, et al. The aging factor in health and disease: the promise of basic research on aging. Aging Clin Exp Res. 2004;16:104–111. doi: 10.1007/BF03324538. [DOI] [PubMed] [Google Scholar]
- Callahan D, Phillips E, Carabello R, et al. Assessment of lower extremity muscle power in functionally-limited elders. Aging Clin Exp Res. 2007;19:194–199. doi: 10.1007/BF03324689. [DOI] [PubMed] [Google Scholar]
- Canto C, Jiang LQ, Deshmukh AS, et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JB, Banister EW, Blaber AP. Effect of endurance exercise on autonomic control of heart rate. Sports Med. 2003;33:33–46. doi: 10.2165/00007256-200333010-00003. [DOI] [PubMed] [Google Scholar]
- Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, Guarente L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008;22:1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KW, Kim DH, Park MH, Choi YJ, Kim ND, Lee J, Yu BP, Chung HY. Recent advances in calorie restriction research on aging. Exp Gerontol. 2013;48:1049–1053. doi: 10.1016/j.exger.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(80):201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Allison DB, Weindruch R. Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J Gerontol Ser A-Biol Sci Med Sci. 2008;63:556–559. doi: 10.1093/gerona/63.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall JP, Shamoon H, Cohen HW, et al. Post-challenge hyperglycemia in older adults is associated with increased cardiovascular risk profile. J Clin Endocrinol Metab. 2009;94:1595–1601. doi: 10.1210/jc.2008-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csipo T, Fulop GA, Lipecz A, Tarantini S, Kiss T, Balasubramanian P, Csiszar A, Ungvari Z, Yabluchanskiy A. Short-term weight loss reverses obesity-induced microvascular endothelial dysfunction. GeroScience. 2018;40:337–346. doi: 10.1007/s11357-018-0028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Picciotto NE, Gano LB, Johnson LC, et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15:522. doi: 10.1111/acel1246115:522-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean W. The retardation of aging and diseases of aging by dietary restriction - Weindruch,r, Walford,rl. J Am Geriatr Soc. 1990;38:736. doi: 10.1111/j.1532-5415.1990.tb04441.x. [DOI] [Google Scholar]
- DeVan AE, Johnson LC, Brooks FA, Evans TD, Justice JN, Cruickshank-Quinn C, Reisdorph N, Bryan NS, McQueen M, Santos-Parker JR, Chonchol MB, Bassett CJ, Sindler AL, Giordano T, Seals DR. Effects of sodium nitrite supplementation on vascular function and related small metabolite signatures in middle-aged and older adults. J Appl Physiol. 2016;120:416–425. doi: 10.1152/japplphysiol.00879.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA, Seals DR. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell. 2013;12:772–783. doi: 10.1111/acel.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensrud KE, Fullman RL, Barrett-Connor E, Cauley JA, Stefanick ML, Fink HA, Lewis CE, Orwoll E, Osteoporotic Fractures in Men Study Research Group Voluntary weight reduction in older men increases hip bone loss: the osteoporotic fractures in men study. J Clin Endocrinol Metab. 2005;90:1998–2004. doi: 10.1210/jc.2004-1805. [DOI] [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med. 1980;303:130–135. doi: 10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- Fries JF. Perspectives from masters in rheumatology and autoimmunity: perspectives on the conventional wisdom. Arthritis Rheum. 2015;67:2806–2812. doi: 10.1002/art.39207. [DOI] [PubMed] [Google Scholar]
- Gardner EM. Caloric restriction decreases survival of aged mice in response to primary influenza infection. J Gerontol Ser A Biol Sci Med Sci. 2005;60:688–694. doi: 10.1093/gerona/60.6.688. [DOI] [PubMed] [Google Scholar]
- Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22:789–798. doi: 10.1016/j.cmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Effects of intermittent feeding upon growth and life span in rats. Gerontology. 1982;28:233–241. doi: 10.1159/000212538. [DOI] [PubMed] [Google Scholar]
- Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A, Howell A. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes. 2011;35:714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt ND, Li GD, Zhu M, Miller M, Levette A, Chachich ME, Spangler EL, Allard JS, Hyun DH, Ingram DK, de Cabo R. Effect of calorie restriction and refeeding on skin wound healing in the rat. Age (Omaha) 2012;34:1453–1458. doi: 10.1007/s11357-011-9321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DK, de Cabo R. Calorie restriction in rodents: caveats to consider. Ageing Res Rev. 2017;39:15–28. doi: 10.1016/j.arr.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski KL, Racine ML, Geolfos CJ, et al. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol. 2013;61:335–343. doi: 10.1016/j.jacc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YC, Liu Y, Hayworth CR, Bhattacharya A, Lustgarten MS, Muller FL, Chaudhuri A, Qi W, Li Y, Huang JY, Verdin E, Richardson A, van Remmen H. Dietary restriction attenuates age-associated muscle atrophy by lowering oxidative stress in mice even in complete absence of CuZnSOD. Aging Cell. 2012;11:770–782. doi: 10.1111/j.1474-9726.2012.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Telljohann R, Maudsley S, Carlson O, John S, Laub DR, Mattson MP. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice JN, Johnson LC, DeVan AE, et al. Improved motor and cognitive performance with sodium nitrite supplementation is related to small metabolite signatures: a pilot trial in middle-aged and older adults. Aging-Us. 2015;7:1004–1021. doi: 10.18632/aging.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyani RR, Egan JM. Diabetes and altered glucose metabolism with aging. Endocrinol Metab Clin N Am. 2013;42:333–347. doi: 10.1016/j.ecl.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplon RE, Hill SD, Bispham NZ, et al. Oral trehalose supplementation improves resistance artery endothelial function in healthy middle-aged and older adults. Aging (Albany NY) 2016;8:1167–1183. doi: 10.18632/aging.100962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T, Balasubramanian P, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Csipo T, Lipecz A, Reglodi D, Zhang XA, Bari F, Farkas E, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for the prevention of vascular cognitive impairment. GeroScience. 2019;41:619–630. doi: 10.1007/s11357-019-00074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. 2018;118:1591–1602. doi: 10.1016/j.jand.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRoche DP, Melanson EL, Baumgartner MP, Bozzuto BM, Libby VM, Marshall BN. Physiological determinants of walking effort in older adults: should they be targets for physical activity intervention? GeroScience. 2018;40:305–315. doi: 10.1007/s11357-018-0032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertens. 2001;37:1236–1241. doi: 10.1161/01.HYP.37.5.1236. [DOI] [PubMed] [Google Scholar]
- Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Moreno D, Aguilar-Roblero R, Díaz-Muñoz M. Restricted feeding entrains rhythms of inflammation-related factors without promoting an acute-phase response. Chronobiol Int. 2009;26:1409–1429. doi: 10.3109/07420520903417003. [DOI] [PubMed] [Google Scholar]
- Martens CR, Denman BA, Mazzo MR et al (2018) Chronic nicotinamide riboside supplementation is well-tolerated and effectively elevates NAD+ in healthy middle-aged and older adults. Nat Commun 9. 10.1038/s41467-018-03421-7 [DOI] [PMC free article] [PubMed]
- Martens CR, Seals DR. Practical alternatives to chronic caloric restriction for optimizing vascular function with ageing. J Physiol. 2016;594:7177–7195. doi: 10.1113/JP272348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2000;35:299–305. doi: 10.1016/S0531-5565(00)00084-X. [DOI] [PubMed] [Google Scholar]
- Matyi S, Jackson J, Garrett K, Deepa SS, Unnikrishnan A. The effect of different levels of dietary restriction on glucose homeostasis and metabolic memory. GeroScience. 2018;40:139–149. doi: 10.1007/s11357-018-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrum C, Karamanidis K, Grevendonk L, Zijlstra W, Meijer K (2019) Older adults demonstrate interlimb transfer of reactive gait adaptations to repeated unpredictable gait perturbations. GeroScience.:1–11. 10.1007/s11357-019-00130-x [DOI] [PMC free article] [PubMed]
- Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, Redpath P, Migaud ME, Apte RS, Uchida K, Yoshino J, Imai SI. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 2016;24:795–806. doi: 10.1016/j.cmet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Bernier M, Mattison JA et al (2018) Daily fasting improves health and survival in male mice independent of diet composition and calories. Cell Metab:1–8. 10.1111/j.1096-0031.2010.00328.x [DOI] [PMC free article] [PubMed]
- Moreau KL, Donato AJ, Seals DR, DeSouza C, Tanaka H. Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc Res. 2003;57:861–868. doi: 10.1016/S0008-6363(02)00777-0. [DOI] [PubMed] [Google Scholar]
- Moreira EAM, Most M, Howard J, Ravussin E. Dietary adherence to long-term controlled feeding in a calorie-restriction study in overweight men and women. Nutr Clin Pract. 2011;26:309–315. doi: 10.1177/0884533611405992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normandin E, Houston DK, Nicklas BJ. Caloric restriction for treatment of geriatric obesity: do the benefits outweigh the risks? Curr Nutr Rep. 2015;4(2):143–155. doi: 10.1007/s13668-015-0123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshansky SJ. From lifespan to healthspan from lifespan to HealthspanFrom lifespan to Healthspan. JAMA. 2018;320:1323–1324. doi: 10.1001/jama.2018.12621. [DOI] [PubMed] [Google Scholar]
- Phillips DIW, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11:286–292. doi: 10.1111/j.1464-5491.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension. 2008;52:72–79. doi: 10.1161/HYPERTENSIONAHA.108.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Jablonski KL, Walker AE, Seibert SM, DeVan A, Black SM, Sharma S, Seals DR. Tetrahydrobiopterin supplementation enhances carotid artery compliance in healthy older men: a pilot study. Am J Hypertens. 2012;25:1050–1054. doi: 10.1038/ajh.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette SB, Rochon J, Uhrich ML, Villareal DT, DAS S, Fontana L, Bhapkar M, Martin CK, Redman LM, Fuss PJ, Roberts SB, Kraus WE. Effects of two years of calorie restriction on aerobic capacity and muscle strength. Med Sci Sports Exerc. 2017;49:2240–2249. doi: 10.1249/MSS.0000000000001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity. 2019;27:1244–1254. doi: 10.1002/oby.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci TA, Heymsfield SB, Pierson RN, Stahl T, Chowdhury HA, Shapses SA (2001) Moderate energy restriction increases bone resorption in obese postmenopausal women. Am J Clin Nutr 73:347-52. 10.1093/ajcn/73.2.347 [DOI] [PubMed]
- Rippe C, Lesniewski LA, Connell ML, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9:304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Zavala JB, Molina-Aguilar C, Pérez-Mendoza M, et al. Daytime restricted feeding modifies the daily regulation of fatty acid β-oxidation and the lipoprotein profile in rats. Br J Nutr. 2017;117:930–941. doi: 10.1017/S0007114517000800. [DOI] [PubMed] [Google Scholar]
- Rochon J, Bales CW, Ravussin E, Redman LM, Holloszy JO, Racette SB, Roberts SB, Das SK, Romashkan S, Galan KM, Hadley EC, Kraus WE, CALERIE Study Group Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. J Gerontol Ser A Biol Sci Med Sci. 2011;66(A):97–108. doi: 10.1093/gerona/glq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rothschild J, Hoddy KK, Jambazian P, Varady KA. Time-restricted feeding and risk of metabolic disease: a review of human and animal studies. Nutr Rev. 2014;72:308–318. doi: 10.1111/nure.12104. [DOI] [PubMed] [Google Scholar]
- Santos-Parker JR, Lubieniecki KL, Rossman MJ, van Ark H, Bassett CJ, Strahler TR, Chonchol MB, Justice JN, Seals DR. Curcumin supplementation and motor-cognitive function in healthy middle-aged and older adults. Nutr Heal Aging. 2018;4:323–333. doi: 10.3233/NHA-170029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Parker JR, Strahler TR, Bassett CJ, et al. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging (Albany NY) 2017;9:187–208. doi: 10.18632/aging.101149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saydah SH, Miret M, Sung J, et al. Postchallenge hyperglycemia and mortality in a national sample of US adults. Diabetes Care. 2001;24:1397. doi: 10.2337/diacare.24.8.1397. [DOI] [PubMed] [Google Scholar]
- Schafer AL. Decline in bone mass during weight loss: a cause for concern? J Bone Miner Res. 2016;31:36–39. doi: 10.1002/jbmr.2701. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Grimes DA. Series epidemiology 4 multiplicity in randomised trials I : endpoints and treatments. Lancet. 2005;365:1591–1595. doi: 10.1016/S0140-6736(05)66461-6. [DOI] [PubMed] [Google Scholar]
- Sperandio E, Matheus A, Lauria V et al (2014) Intensity and physiological responses to the six-minute walk test in middle-aged and older adults: a comparison with cardiopulmonary exercise testing. Eur Respir J:44 [DOI] [PMC free article] [PubMed]
- Stekovic S, Hofer SJ, Tripolt N, et al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. 2019;30:462–476.e5. doi: 10.1016/j.cmet.2019.07.016. [DOI] [PubMed] [Google Scholar]
- Stewart TM, Bhapkar M, Das S, Galan K, Martin CK, McAdams L, Pieper C, Redman L, Roberts S, Stein RI, Rochon J, Williamson DA, CALERIE Study Group Comprehensive assessment of long-term effects of reducing intake of energy phase 2 (CALERIE phase 2) screening and recruitment: methods and results. Contemp Clin Trials. 2013;34:10–20. doi: 10.1016/j.cct.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stote K, Baer D, Spears K, et al. A controlled trial of reduced meal frequency without caloric. Am J Clin Nutr. 2007;85:1–8. doi: 10.1093/ajcn/85.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton EF, Beyl R, Early KS, et al. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27:1212–1221.e3. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, DeSouza CA, Seals DR. Arterial stiffness and hormone replacement use in healthy postmenopausal women. J Gerontol Ser A Biol Sci Med Sci. 1998;53:M344–M346. doi: 10.1093/gerona/53A.5.M344. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, et al. Carotid artery wall hypertrophy with age is related to local systolic blood pressure in healthy men. Arterioscler Thromb Vasc Biol. 2001;21:82–87. doi: 10.1161/01.ATV.21.1.82. [DOI] [PubMed] [Google Scholar]
- Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, Gabel K, Freels S, Rigdon J, Rood J, Ravussin E, Varady KA. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930–938. doi: 10.1001/jamainternmed.2017.0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Wal AMG, Bakker O, Wiersinga WM. The decrease of liver LDL receptor mRNA during fasting is related to the decrease in serum T3. Int J Biochem Cell Biol. 1998;30:209–215. doi: 10.1016/S1357-2725(97)00120-9. [DOI] [PubMed] [Google Scholar]
- Varady KA, Hellerstein MK. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr. 2007;86:7–13. doi: 10.1093/ajcn/86.1.7. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Fontana L, Das SK, Redman L, Smith SR, Saltzman E, Bales C, Rochon J, Pieper C, Huang M, Lewis M, Schwartz AV, CALERIE Study Group Effect of two-year caloric restriction on bone metabolism and bone mineral density in non-obese younger adults: a randomized clinical trial. J Bone Miner Res. 2016;31:40–51. doi: 10.1002/jbmr.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE, Eskurza I, Pierce GL, Gates PE, Seals DR. Modulation of vascular endothelial function by low-density lipoprotein cholesterol with aging: influence of habitual exercise. Am J Hypertens. 2009;22:250–256. doi: 10.1038/ajh.2008.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE, Kaplon RE, Pierce GL, Nowlan MJ, Seals DR. Prevention of age-related endothelial dysfunction by habitual aerobic exercise in healthy humans: possible role of nuclear factor kappa B. Clin Sci. 2014;127:645–654. doi: 10.1042/CS20140030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 155 kb)
Data Availability Statement
The data that support the findings of this study are openly available in Mendeley Data at 10.17632/w83t9s27dx.2