Abstract
Aging is the major risk factor for many metabolic chronic diseases. Several metabolic pathways suffer a progressive impairment during aging including body composition and insulin resistance which are associated to autophagy dysfunction and increased inflammation. Many of these alterations are aggravated by non-healthy lifestyle such as obesity and hypercaloric diet which have been shown to accelerate aging. Here, we show that the deleterious effect of hypercaloric diets is reverted by the NLRP3 inflammasome inhibition. NLRP3 deficiency extends mean lifespan of adult mice fed a high-fat diet. This lifespan extension is accompanied by metabolic health benefits including reduced liver steatosis and cardiac damage, improved glucose and lipid metabolism, and improved protein expression profiles of SIRT-1, mTOR, autophagic flux, and apoptosis. These findings suggest that the suppression of NLRP3 prevented many age-associated changes in metabolism impaired by the effect of hypercaloric diets.
Electronic supplementary material
The online version of this article (10.1007/s11357-019-00151-6) contains supplementary material, which is available to authorized users.
Keywords: NLRP3 inflammasome, High-fat diet, Aging, Autophagy, Longevity, Obesity
Introduction
It is assumed that obesity is caused by excessive intake of nutrients and possibly due to lack of exercise; however, this generates metabolic stress that is associated with metabolic dysfunctions (Ren et al., 2018). This condition is accompanied by the development of a progressive impairment of several metabolic pathways that define body composition, insulin resistance, mitochondrial and autophagy dysfunction, and chronic low-grade inflammation that is promoted by expanding adipose tissue (Corica et al., 2015). Consequently, obesity is a risk factor for several diseases, such as cardiovascular disease, metabolic syndrome, or type I diabetes (Corica et al., 2015). Obesity shares several biological similarities with aging, such as chronic inflammation, cell senescence, and immune and autophagy dysfunction, and there is growing evidence that obesity and increased adiposity accelerate the aging process and age-related cardiac and metabolic dysfunction, mainly by promoting inflammation (Chia et al., 2018). Accordingly, understanding the interplay between accelerated aging, obesity, and adipose tissue dysfunction is essential to gain insight into the aging process and the pathophysiology of obesity resulting in insulin resistance.
Recently, the role of NLRP3 inflammasome in obesity, cardiovascular, and metabolic diseases has been studied. Mice deficient in NLRP3 were resistant to the development of obesity induced by a high-fat diet, protected from obesity-induced insulin resistance and protected from cardiac damage (Stienstra et al., 2011; Pavillard et al., 2017). The NLRP3 inflammasome is upregulated after myocardial infarction, atherosclerosis, ischemic heart disease, diabetic cardiomyopathy, chronic heart failure, and hypertension and recently, NLRP3 and IL-1β have also been proposed as new biomarkers of cardiovascular risk (Bullón et al., 2017; Liu et al., 2017). Previous studies have also suggested a role for NLRP3 inflammasome in several events associated with aging. It has been shown that the genetic deletion of NLRP3 in mice improves healthspan by attenuating multiple age-related degenerative changes, such as glycemic control, bone loss, cognitive function, and motor performance (Youm et al., 2013). In addition, the deletion of NLRP3 in older mice increased muscle strength and endurance, and prevented age-related increase in the number of myopathic fibers (McBride et al., 2017). However, the role of the NLRP3 inflammasome in the interaction between obesity and aging and its effect on lifespan and healthspan in obese mice has not been studied. Furthermore, studies in NLRP3 and obesity typically implement a relatively short dietary intervention that lasts between 4 and 16 weeks and the effect of a long-term high-fat diet has not been evaluated. Hence, we seek to determine whether the genetic deletion of NLRP3 can have an effect on lifespan and potentially prevent metabolic aging in mice fed with HFD for the rest of their life.
Material and methods
Ethical statements
Animal studies were performed in accordance with the European Union guidelines (2010/63/EU) and the corresponding Spanish regulations for the use of laboratory animals in chronic experiments (RD 53/2013 on the care of experimental animals). All experiments were approved by the local institutional animal care committee.
Mouse longevity study
For all experiments, only male mice were used. The strain of NLRP3−/− mice was originally generated and characterized in the laboratory of J. Tschopp. Young and old NLRP3−/− transgenic mice (C57BL/6J background) and controls of littermate WT/NLRP3+/+ with a weight of 25–30 g were maintained in a regular light/dark cycle of 12 h. The mice were housed in groups of four to eight same-sex littermates under specific pathogen-free conditions. Two groups of 3 months old WT and NLRP3−/− mice were divided with different nutritional status. These groups correspond to the following dietary regimens: (i) regular chow or standard diet (SD) from Teklad Global 14% Protein Rodent Maintenance Diet, Harlan Laboratories (carbohydrate:protein:fat ratio of 48:14:4% of kcal) and (ii) an HFD consisting of Teklad Global modified to provide 45% of fat calories. Individuals were monitored daily and weighed monthly but otherwise they were not bothered until they died. Survival was assessed using a first cohort of male mice (50 per group), and all animals were dead by the time of this report. Kaplan–Meier survival curves were constructed using known birth and death dates, and differences between groups were evaluated by the log rank test. Body weight and food intake were monitored monthly in this cohort. A second cohort was used under the same conditions for different experimental procediments using between 6 and 8 mice per experiment. All groups had ad libitum access to their prescribed diet and water throughout the study. Body weight and food intake were monitored weekly. The animals’ rooms were kept at 20–22 °C with a relative humidity of 30–70%.
Reagents
Monoclonal antibodies specific for Beclin-1 and p62 were purchased from Sigma-Aldrich (Saint Louis, USA). Anti-GAPDH monoclonal antibody was acquired from Calbiochem-Merck Chemicals Ltd. (Nottingham, UK). Similarly, anti-active caspase-3, anti-SIRT-1, p-mTOR, and mTOR were obtained from Cell Signaling Technology (Beverly, MA, USA). Finally, anti-Bcl-2, anti-Bax, and anti-MAP-LC3 antibodies from (Santa Cruz Biotechnology). A cocktail of protease inhibitors (Complete™ Protease Inhibitor Cocktail) was purchased from Boehringer Mannheim (Indianapolis, IN). The Immun Star HRP substrate kit was obtained from Bio-Rad Laboratories Inc. (Hercules, CA).
Glucose and insulin tolerance test
Glucose tolerance tests were performed in young (3 months) and old (20 months) mice after fasting overnight for 16 h and then injecting glucose (1 g/kg), intraperitoneally. Glucose measurements were performed using a Bayer Contour blood glucose meter and test strips. For ITT, mice were starved for 6 h and injected (i.p.) with 0.75 U/kg of recombinant human insulin (Sigma). Blood was obtained from the tail at the time points for glucose measurement.
Leptin, adiponectin and IGF-1
Serum leptin, adiponectin, and IGF-1 levels were assayed in young (3 months) and old (20 months) mice in duplicate using commercial ELISA kits (R&D Systems, Minneapolis, USA).
TNF-α levels
Serum TNF-α (Biosource, UK, and GenWay, San Diego, USA) were assayed in young (3 months) and old (20 months) mice in duplicate using commercial ELISA kits.
Serum biomarkers
Serum levels of glucose, triglycerides, cholesterol, uric acid, aspartate aminotransferase, alanine aminotransferase, and creatine kinase were assayed using commercial kits (Randox Laboratories, Antrim, UK).
Immunoblotting
The mouse heart was collected and immediately frozen in liquid nitrogen, then tissues were homogenized in liquid nitrogen using pestle and mortar to obtain powdered tissue.Western blotting was performed using standard methods. After protein transfer, the membrane was incubated with various primary antibodies diluted 1:1000, and then with the corresponding secondary antibodies coupled to horseradish peroxidase at a 1:10000 dilution. Specific protein complexes were identified using the Immun Star HRP substrate kit (Biorad Laboratories Inc., Hercules, CA, USA).
Histological study
All data are expressed as means ± SEM. After the normality assessment using the Shapiro–Wilk test, statistical differences between the different groups were measured by an unpaired Student’s t test or a one-way analysis of variance (ANOVA) when appropriate with the Tukey’s post hoc test. A P value of ≤ 0.05 was considered statistically significant. Statistical analyses were performed with Prism software version 5.0a (GraphPad, San Diego, CA). The asterisks in the figures represent the following: *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001.
Statistics
All data are expressed as means ± SEM. After, evaluation of normality using Shapiro–Wilk test, statistical differences among the different groups were measured using either an unpaired Student’s t test or one-way analysis of variance (ANOVA) when appropriate with Tukey’s post hoc test. A P value of ≤ 0.05 was considered statistically significant. Statistical analyses were performed using Prism software version 5.0a (GraphPad, San Diego, CA). Asterisks in the figures represent the following: *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001.
Results
NLRP3 deficiency improved lifespan in aged obese mice
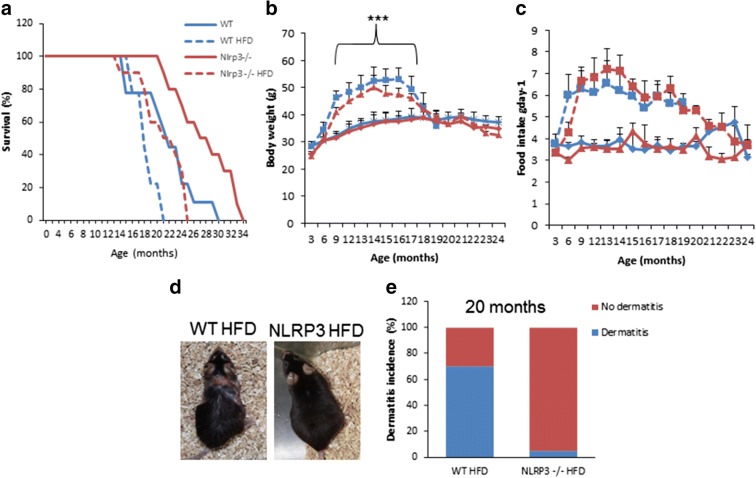
To assess the impact of NLRP3 deletion on survival and metabolic changes during aging in obese mice, we placed 3-month-old NLRP3-deficient (NLRP3−/−) and NLRP3+/+ littermate control (WT) mice on a high-fat diet (HFD) and monitored them for the rest of their lifespan. The survival of NLRP3−/− mice compared to littermate controls using a Kaplan–Meier survival curve was increased with a 27% mean lifespan (Fig. 1a). Survival among the high-fat groups was significantly different by the log rank test, with NLRP3−/− mice fed HFD living longer (16%) than their WT HFD counterparts. Body weight and food intake were not different between groups throughout the observation period with increase in WT and NLRP3−/− mice fed with HFD (Fig. 1b and c). Twenty-month-old WT animals displayed an increase in aged-related alopecia compared to their NLRP3 knockout mice. One of the inflammatory conditions observed in WT mice fed with HFD is severe ulcerating dermatitis that was not presented by NLRP3−/− (Fig. 1d and e). Ulcerating dermatitis is a severe inflammatory skin disorder with an unknown etiology, but associated with aging due to the effect of HFD (Brandhorst et al., 2015; Neuhaus et al., 2012). These observations indicate that NLRP3 ablation protects against inflammation and HFD-induced skin lesions associated with inflammation.
Fig. 1.
NLRP3 signaling suppression in obese mice extended the lifespan and preserved mental health. a Kaplan–Meier graph showing a significant increase in mean and maximum lifespan in obese and non-obese NLRP3 mice (violet and green, respectively) compared to WT mice (blue and red, respectively). b, c Body weights and average daily oral food intake. d, e Incidence of dermatitis in two cohorts in % in obese mice. Representative photographs of 20-month-old mice fed with HFD with dermatitis progression. All data are presented as means ± SEM, n = 50 mice per group; ***P < 0.001 young vs old and obese WT mice
NLRP3 deficiency diminished metabolic impairment induced by HFD during aging
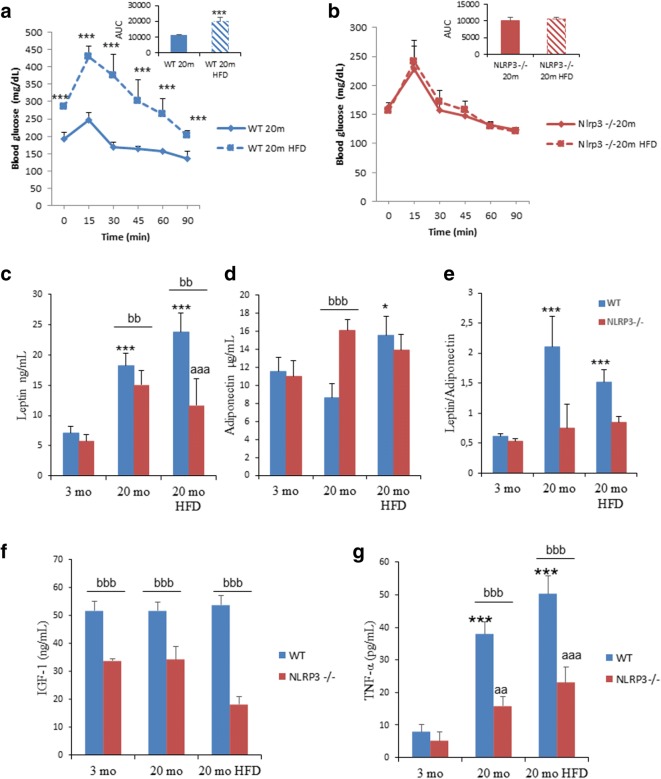
The old NLRP3−/− mice fed with HFD were significantly more glucose tolerant at the OGTT peak (> 15 min) compared to the old WT mice fed with HFD (Fig. 2a and b), indicating a higher glucose tolerance measured as a trend towards unchanged in the values of the area under the curve (AUC) of the glucose tolerance test (insert of Fig. 2a and b), which was corroborated after the insulin tolerance test showing that NLRP3 old and obese mice were more insulin sensitive compared to wild-type animals (Figure S1). Further, leptin is a known regulator of body weight, and dysregulation of the leptin/adiponectin ratio has been associated with cardiovascular disease, metabolic syndrome, and non-alcoholic fatty liver disease (DiNicolantonio et al., 2016). The old NLRP3−/− mice maintained low serum levels of leptin and a low leptin/adiponectin ratio, not only under standard diet but also under HFD, while these parameters increased in WT mice with age (Fig. 2c–e). Fasting blood glucose levels and circulating IGF-1 are predictors of diabetes and short lifespan, and these were reduced in young and old NLRP3−/− mice, including those fed with HFD compared to WT mice (Table S1 and Fig. 2f). Reduced glucose levels and IGF-1 have been associated with stress resistance and anti-aging effect (Brandhorst et al., 2015). Plasma lipid levels were reduced in old NLRP3−/− HFD mice accompanied by a significant reduction of hepatic transaminases, creatine phosphokinase and lactate dehydrogenase (Table S1). However, although increased levels of TNF-α were observed in old and obese WT and NLRP3−/− mice, NLRP3 deficiency resulted in reduced levels compared to WT (Fig. 2g). Together, these results suggest that the absence of NLRP3 improved metabolic homeostasis in obese mice during aging.
Fig. 2.
NLRP3 signaling suppression in obese mice improved metabolic homeostasis. a, b Oral glucose tolerance test with area under the curve of aged mice fed with HFD and standard diet (SD). c–e Leptin, adiponectin levels, and ratio in plasma. Blood samples were collected after overnight fasting. f, g Circulating levels of IGF-1 and TNF-α. All data are presented as means ± SEM, n = 8 mice; ***P < 0.001 young vs old and obese WT mice. aaP < 0.005 and aaaP < 0.001 young vs old and obese NLRP3−/− mice. bbP < 0.005 and bbbP < 0.001, WT vs NLRP3−/− mice
NLRP3 deletion preserved cardiac and liver integrity
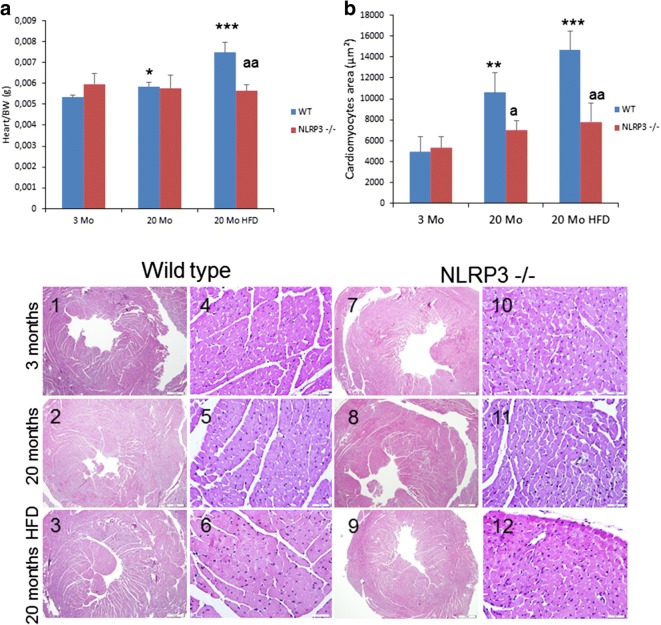
Heart weight normalized to body weight increased in old WT HFD mice, but not in old NLRP3−/− HFD animals (p < 0.001) (Fig. 3a). As a typical pathophysiological feature of cardiac aging (Dutta et al., 2012), cardiac hypertrophy measured by the thickness of the left ventricular wall was increased significantly in old WT mice and even more so in WT HFD mice (Fig. 3 c1–3) compared to NLRP3−/− mice (Fig. 3 c7–9). To assess the impact of aging and cardiac hypertrophy due to HFD on myocardial histology, cardiomyocyte cross-sectional areas were quantified. In the hematoxylin and eosin-stained sections, HFD induced in aged WT mice an increased cardiomyocyte cross-sectional areas but not in NLRP3−/− mice (Fig. 3 b and c4–6 and 10–12).
Fig. 3.
NLRP3 signaling suppression in mice induced cardiac protection. a Heart weight normalized to body weight. b Quantitative analysis of the cross-sectional (transverse) area of cardiomyocytes with measurements of ≈ 100 cardiomyocytes from 3 to 6 mice per group. **P < 0.005 and ***P < 0.001 young vs old and obese WT mice. aP < 0.05 and aaP < 0.005, WT vs NLRP3−/− mice. c Representative images of centripetal concentric LV hypertrophy and hematoxylin and eosin (H&E)-stained section (left) of cardiac tissues from young and old WT and NLRP3−/− mice and fed with HFD. All data are presented as means ± SEM, n = 6 mice
The examination of the livers also revealed that NLRP3-deficient mice fed with HFD exhibited normal liver coloration, which contrasted with the characteristic pale color of old and obese WT mice (Figure S1). Histological analysis of the liver indicated a clear increase in lipid accumulation and a significant steatosis recognized by hematoxylin and eosin staining in obese mice during aging compared to NLRP3−/− mice (Figure S2).
Age-associated metabolic changes were prevented in NLRP3−/− animals
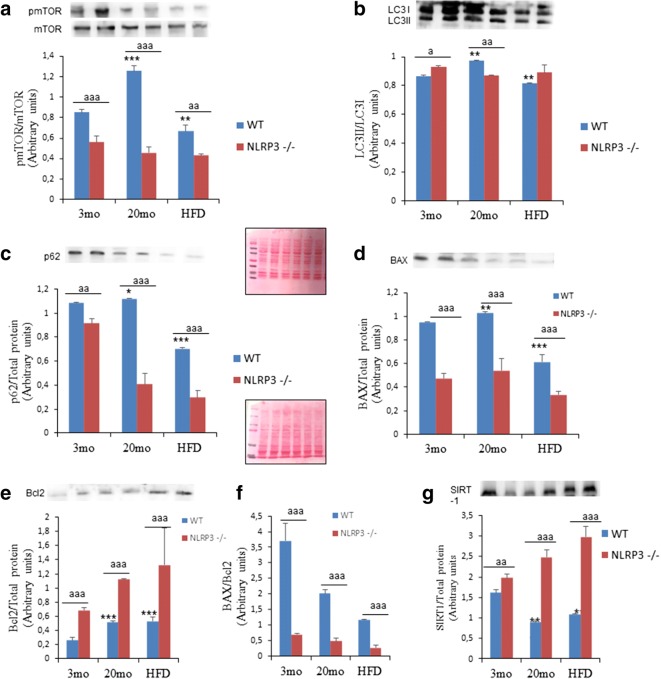
To gain insight on the metabolic pathways “regulating longevity,” we investigated the signaling pathways of mTOR and autophagy in the heart. The phosphorylation of mTOR (Ser2448) (Fig. 4a) decreased in the heart of NLRP3−/− elderly and obese mice. These data are consistent with previous observations of the role of mTOR inhibition for healthspan (Wu et al., 2013a). Furthermore, mTOR is associated with autophagy, which is involved in cell homeostasis through protein degradation and removal of damaged intracellular organelles (Pyo et al., 2013). Autophagic dysfunction has also been linked to aging and obesity with blocked autophagic flux and the accumulation of non-degraded substrates in the form of autophagosome (Ren et al., 2018; Dutta et al., 2012). Interestingly, NLRP3−/− mice showed normal levels of LC3II protein expression in old and obese NLRP3−/− mice, but with a reduction of p62/SQSTM1 showing this a high quality autophagic process (Fig. 4b and c). This could be explained when the NLRP3 inhibition induced an improved quality of autophagy in the heart during aging and is preserved from the effect of the hypercaloric diet.
Fig. 4.
Changes in the SIRT-1, mTOR, and autophagy pathway observed in cardiac tissues of young and old mice and the effect of HFD. Densitometric analysis with representative western blot showing mTOR, SIRT-1, and autophagic flux by LC3 and p62 expression in the heart of aged and obese NLRP3−/− mice compared to WT. Apoptosis levels were determined by Bcl-2 and BAX proteins. Densitometric analysis are presented as means ± SEM, n = 6 mice; *P < 0.05; **P < 0.01; ***P < 0.001 young vs old mice. aaP < 0.01; aaaP < 0.001, WT vs NLRP3−/− mice
Because the high-fat diet and aging are also associated with cardiomyocyte apoptosis, which is combined with oxidative stress and inflammation dysregulation (Ren et al., 2018; Hsu et al., 2016), we determined several apoptotic biomarkers. Aging resulted in a significant increase in protein expression of cardiac BAX, but its reduction under HFD feeding (Fig. 4d) accompanied by a moderate increase in anti-apoptotic Bcl-2 protein levels (Fig. 4e) in WT mice, which resulted in a higher proportion in all groups of BAX/Bcl2 in WT compared to NLRP3−/− mice (Fig. 4f). NLRP3−/− mice showed reduced contents of the pro-apoptotic Bax protein, together with a significant accumulation of the anti-apoptotic Bcl-2 protein (Fig. 4d–f). Interestingly, these observations were also accompanied by an increase in the expression levels of the SIRT-1 protein in elderly and HFD NLRP3−/− mice (Fig. 4g) that has a role in heart protection and metabolic improvement during aging by mTOR inhibition and autophagy induction (North & Sinclair, 2012a).
Again, the examination of the livers also revealed similar data on the reduction of mTOR and improved the quality of autophagy with the reduction of p62 in NLRP3 deficient mice fed with HFD compared to WT mice (Figure S3). However, a loss of consistency was observed in the expression of SIRT-1 in liver compared to heart, which could be associated with previous observation of reduced SIRT-1 expression in liver after caloric restriction, which correlated with the reduced role of this organ in fat synthesis, and the protective role of SIRT-1 in heart (Nogueiras et al., 2012).
Discussion
Aging is the major risk factor for cardiovascular and metabolic diseases. The main molecular pathways impaired during aging comprise nutrient sensing pathways such as glucose metabolism, insulin response, dysregulation of mTOR and SIRT1, and inflammation, which can be a primary and/or secondary event in the metabolic dysfunction (López-Otín et al., 2016). These age-dependent changes are highly associated with lifestyle and may be exacerbated by hypercaloric nutrition and sedentary lifestyle (López-Otín et al., 2016). This study showed that NLRP3 is associated with damage induced by the hypercaloric diet during aging by improving lifespan and healthspan by modifying several of the hallmarks of aging. Our results provide evidence that NLRP3 deficiency can increase longevity and healthspan despite the effect of the hypercloric diet. NLRP3−/− mice fed with HFD showed no weight gain. This observation is difficult to explain; however, this could be associated with the activation of metabolic parameters such as AMPK which, therefore counteracts diabetes, obesity and aging (López-Otín et al., 2016). However, NLRP3−/− mice fed with a normal diet showed a similar weight gain compared to WT mice. These findings could show that the protective effect of NLRP3 deficiency is not related to obesity during aging but with downstream effectors that arise from the intake of HFD and induce IL-1β and IL-18 after NLRP3-inflammasome activation. Therefore, this could also be mediated by several of the metabolic changes associated with obesity and aging such as increased glucose tolerance, reduced leptin, increased adiponectin, and regulation of dyslipidemia typically linked to inflammaging (Latz & Duewell, 2018). Insulin hypersensitivity has been previously described as a key phenotype of the NLRP3-deficient mouse (Haneklaus & O'Neill, 2015). Our study showed that this insulin hypersensitivity is maintained throughout the life of the mouse. Recently, insulin sensitivity was associated with visceral fat and this visceral fat with inflammation (Bennis et al., 2017; Fang et al., 2017). In this regard, long-life visceral fat transplantation has been shown to improve insulin sensitivity (Bennis et al., 2017; Fang et al., 2017). Insulin hypersensitivity of NLRP3 KO mice may be associated with the reduction of inflammasome-related cytokines. Furthermore, despite the differential level of another cytokine such as TNF-α in WT and NLRP3−/−, the NLRP3 ablation also showed an increase in TNF-α. This result supports the known role of low-grade inflammation in obesity and aging (López-Otín et al., 2016).
Our results can also be associated with the observed changes in nutrient sensing pathways such as reduced levels of IGF-1, decreased mTOR phosphorylation, increased expression of SIRT-1, and improved autophagy proteins that are described as metabolic “longevity regulatory” pathways (López-Otín et al., 2016; Finkel, 2015). Despite the contradictory protective role of IGF-1, the data suggests that low serum levels of IGF-1 are the end product of reduced insulin/IGF-1 signaling that prolongs lifespan in both invertebrates and vertebrates, and agree with the demonstrated longevity of animals with reduced IGF pathway activity such as Ames dwarf mice (Finkel, 2015; Fontana et al., 2012). In addition, low levels of IGF-1 in the early period of life are associated with a greater capacity for cell DNA repair (Podlutsky et al., 2017); these levels during the early period will be associated with the improvement of life- and healthspan (Ashpole et al., 2017), which could be associated with the protective effect during aging of the reduced levels of IGF-1 from 3 months age observed in NLRP3 KO mice.
It has been shown that the genetic and pharmacological inhibition of mTOR, a serine-threonine kinase that functions as an intracellular energy sensor, extends the lifespan in a wide range of organisms (Wu et al., 2013b; Cordero et al., 2018). Since it is known that mTOR inhibition induces autophagy, NLRP3 deficiency also showed high quality of autophagy in obese and aged mice, showing a maintained autophagy throughout the life of the mouse despite the hypercaloric diet with respect to our previous data that showed the effect of NLRP3 inhibition on autophagy after short-term hypercaloric diet (Pavillard et al., 2017).
Cardiac aging is characterized by the presence of hypertrophy, fibrosis, dysfunctional mitochondria, and compromised autophagy, and obesity causes premature cardiac aging (North & Sinclair, 2012b). In this sense, because autophagy and autophagic flux decrease in cardiac tissues during aging, a stimulation of autophagy would improve cardiac function by removing accumulated cellular content. Our results show that NLRP3 ablation can reduce p62/SQSTM1 and increase levels of autophagic flux in cardiac tissues in obese and aged mice. This mechanism could be key in improving the longevity and healthspan induced by the inhibition of NLRP3 in obese mice during aging with similar strategies used to improve the extension of lifespan and healthspan by demonstrated anti-aging interventions such as rapamycin, caloric restriction metformin, or resveratrol, which have two common mechanisms: an improvement of autophagy and NLRP3-inflammasome inhibition (Cordero et al., 2018). Furthermore, obesity has also been associated with endothelial dysfunction with a clear inflammatory component (Csipo et al., 2018; Fulop et al., 2018). Weight loss was shown to reverse microvascular endothelial dysfunction (Csipo et al., 2018). Because NLRP3 has previously been related to endothelial dysfunction (Zhang et al., 2017), our data may be consistent with the possibility that NLRP3 inhibition prevents endothelial dysfunction related to age and obesity.
We recognize several limitations of this study. Autophagy has been studied using tissues, so an in vitro model design of the effect of HFD in cells during aging could help study the molecular mechanism of the autophagy response observed by us. In addition, the identification of autophagosome by electron microscopy would also help the interpretation of autophagy. We also recognize the limitations of using only male mice, so the study of female mice will be a very interesting gender effect.
In conclusion, our findings suggest that NLRP3 deficiency attenuates the deleterious effects of obesity during aging by preventing metabolic and cardiac aging and prolongs the lifespan in male obese mice. For this, the ablation of NLRP3 improves the metabolic characteristics related to hypercaloric diets during aging, such as glucose tolerance, lipid metabolism, and leptin/adiponectin, and modulate IGF-1 signaling and mTOR pathway improving autophagic flux. Our study shows a central role of NLRP3 in the modulation of several metabolic effects of a lifestyle associated with a hypercaloric diet during aging that represents a highly prevalent lifestyle on the industrialized countries. Accordingly, NLRP3 offers a promising target for the prevention of the metabolic consequences of an unhealthy diet during aging.
Electronic supplementary material
(DOCX 2547 kb)
Funding information
This study was supported by a grant from the Andalusian regional government (Grupo de Investigacion Junta de Andalucia CTS113). FMA has the benefit of a FPU Fellowship (FPU 13/03173) from The Ministry of Education, Science and Sport. JRC is supported by grants from the Ministerio de Economía, Industria y Competitividad (MEIC) (SAF2017-84494-C2-R), Programa Red Guipuzcoana de Ciencia, Tecnología e Información 2018-CIEN-000058-01 and from the Gobierno Vasco, Dpto. Industria, Innovación, Comercio y Turismo under the ELKARTEK Program (Grant No. KK-2019/bmG19). JRC received funding from the BBVA Foundation (Ayudas a Equipos de investigación científica Biomedicina 2018). CIC biomaGUNE is supported by the Maria de Maeztu Units of Excellence Program from the Spanish State Research Agency – Grant No. MDM-2017-0720.
Compliance with ethical standards
Ethical statements
Animal studies were performed in accordance with the European Union guidelines (2010/63/EU) and the corresponding Spanish regulations for the use of laboratory animals in chronic experiments (RD 53/2013 on the care of experimental animals). All experiments were approved by the local institutional animal care committee.
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience. 2017;39:129–145. doi: 10.1007/s11357-017-9971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennis MT, Schneider A, Victoria B, Do A, Wiesenborn DS, Spinel L, Gesing A, Kopchick JJ, Siddiqi SA, Masternak MM. The role of transplanted visceral fat from the long-lived growth hormone receptor knockout mice on insulin signaling. Geroscience. 2017;39:51–59. doi: 10.1007/s11357-017-9957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, di Biase S, Mirzaei H, Mirisola MG, Childress P, Ji L, Groshen S, Penna F, Odetti P, Perin L, Conti PS, Ikeno Y, Kennedy BK, Cohen P, Morgan TE, Dorff TB, Longo VD. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 2015;22:86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullón P, Cano-García FJ, Alcocer-Gómez E, Varela-López A, Roman-Malo L, Ruiz-Salmerón RJ, Quiles JL, Navarro-Pando JM, Battino M, Ruiz-Cabello J, Jiménez-Borreguero LJ, Cordero MD. Could NLRP3-inflammasome be a cardiovascular risk biomarker in acute myocardial infarction patients? Antioxid Redox Signal. 2017;27:269–275. doi: 10.1089/ars.2016.6970. [DOI] [PubMed] [Google Scholar]
- Chia CW, Egan JM, Ferrucci L. Age-related changes in glucose metabolism, hyperglycemia, and cardiovascular risk. Circ Res. 2018;123(208):886–904. doi: 10.1161/CIRCRESAHA.118.312806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero MD, Williams MR, Ryffel B. AMP-activated protein kinase regulation of the NLRP3 inflammasome during aging. Trends Endocrinol Metab. 2018;29:8–17. doi: 10.1016/j.tem.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Corica F, Bianchi G, Corsonello A, Mazzella N, Lattanzio F, Marchesini G. Obesity in the context of aging: quality of life considerations. Pharmacoeconomics. 2015;33:655–672. doi: 10.1007/s40273-014-0237-8. [DOI] [PubMed] [Google Scholar]
- Csipo T, Fulop GA, Lipecz A, Tarantini S, Kiss T, Balasubramanian P, Csiszar A, Ungvari Z, Yabluchanskiy A. Short-term weight loss reverses obesity-induced microvascular endothelial dysfunction. Geroscience. 2018;40:337–346. doi: 10.1007/s11357-018-0028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNicolantonio JJ, et al. The evidence for saturated fat and for sugar related to coronary heart disease. Prog Cardiovasc Dis. 2016;58:464–472. doi: 10.1016/j.pcad.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D, Calvani R, Bernabei R, Leeuwenburgh C, Marzetti E. Contribution of impaired mitochondrial autophagy to cardiac aging: mechanisms and therapeutic opportunities. Circ Res. 2012;110:1125–1138. doi: 10.1161/CIRCRESAHA.111.246108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, et al. Differential effects of early-life nutrient restriction in long-lived GHR-KO and normal mice. Geroscience. 2017;39:347–356. doi: 10.1007/s11357-017-9978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. The metabolic regulation of aging. Nat Med. 2015;21:1416–1423. doi: 10.1038/nm.3998. [DOI] [PubMed] [Google Scholar]
- Fontana L, Vinciguerra M, Longo VD. Growth factors, nutrient signaling, and cardiovascular aging. Circ Res. 2012;110:1139–1150. doi: 10.1161/CIRCRESAHA.111.246470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience. 2018;40:513–521. doi: 10.1007/s11357-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneklaus M, O'Neill LA. NLRP3 at the interface of metabolism and inflammation. Immunol Rev. 2015;265:53–62. doi: 10.1111/imr.12285. [DOI] [PubMed] [Google Scholar]
- Hsu HC, et al. High-fat diet induces cardiomyocyte apoptosis via the inhibition of autophagy. Eur J Nutr. 2016;55:2245–2254. doi: 10.1007/s00394-015-1034-7. [DOI] [PubMed] [Google Scholar]
- Latz E, Duewell P. NLRP3 inflammasome activation in inflammaging. Semin Immunol. 2018;40:61–73. doi: 10.1016/j.smim.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Liu D, et al. Role of NLRP3 inflammasome in the pathogenesis of cardiovascular diseases. Basic Res Cardiol. 2017;113:5. doi: 10.1007/s00395-017-0663-9. [DOI] [PubMed] [Google Scholar]
- López-Otín C, Galluzzi L, Freije JMP, Madeo F, Kroemer G. Metabolic control of longevity. Cell. 2016;166:802–821. doi: 10.1016/j.cell.2016.07.031. [DOI] [PubMed] [Google Scholar]
- McBride MJ, et al. The NLRP3 inflammasome contributes to sarcopenia and lower muscle glycolytic potential in old mice. Am J Physiol Endocrinol Metab. 2017;313:E222–E232. doi: 10.1152/ajpendo.00060.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus B, Niessen CM, Mesaros A, Withers DJ, Krieg T, Partridge L. Experimental analysis of risk factors for ulcerative dermatitis in mice. Exp Dermatol. 2012;21:712–713. doi: 10.1111/j.1600-0625.2012.01558.x. [DOI] [PubMed] [Google Scholar]
- Nogueiras R, et al. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92(3):1479–1514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavillard LE, Cañadas-Lozano D, Alcocer-Gómez E, Marín-Aguilar F, Pereira S, Robertson AAB, Muntané J, Ryffel B, Cooper MA, Quiles JL, Bullón P, Ruiz-Cabello J, Cordero MD. NLRP3-inflammasome inhibition prevents high fat and high sugar diets-induced heart damage through autophagy induction. Oncotarget. 2017;8:99740–99756. doi: 10.18632/oncotarget.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlutsky A, et al. The GH/IGF-1 axis in a critical period early in life determines cellular DNA repair capacity by altering transcriptional regulation of DNA repair-related genes: implications for the developmental origins of cancer. Geroscience. 2017;39:147–160. doi: 10.1007/s11357-017-9966-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo JO, et al. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat Commun. 2013;4:2300. doi: 10.1038/ncomms3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Sowers JR, Zhang Y. Metabolic stress, autophagy, and cardiovascular aging: from pathophysiology to therapeutics. Trends Endocrinol Metab. 2018;29:699–711. doi: 10.1016/j.tem.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienstra R, van Diepen J, Tack CJ, Zaki MH, van de Veerdonk F, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I, van den Berg S, Romijn J, Rensen PC, Joosten LA, Netea MG, Kanneganti TD. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A. 2011;108:15324–15329. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N, Fergusson MM, Rovira II, Allen M, Springer DA, Lago CU, Zhang S, DuBois W, Ward T, deCabo R, Gavrilova O, Mock B, Finkel T. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep. 2013;4:913–920. doi: 10.1016/j.celrep.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N, Fergusson MM, Rovira II, Allen M, Springer DA, Lago CU, Zhang S, DuBois W, Ward T, deCabo R, Gavrilova O, Mock B, Finkel T. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep. 2013;4:913–920. doi: 10.1016/j.celrep.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youm YH, Grant RW, McCabe L, Albarado DC, Nguyen KY, Ravussin A, Pistell P, Newman S, Carter R, Laque A, Münzberg H, Rosen CJ, Ingram DK, Salbaum JM, Dixit VD. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013;18:519–532. doi: 10.1016/j.cmet.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xia L, Zhang F, Zhu D, Xin C, Wang H, Zhang F, Guo X, Lee Y, Zhang L, Wang S, Guo X, Huang C, Gao F, Liu Y, Tao L. A novel mechanism of diabetic vascular endothelial dysfunction: hypoadiponectinemia-induced NLRP3 inflammasome activation. Biochim Biophys Acta Mol basis Dis. 2017;1863:1556–1567. doi: 10.1016/j.bbadis.2017.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 2547 kb)