Abstract
Aging-induced structural and functional alterations of the neurovascular unit lead to impairment of neurovascular coupling responses, dysregulation of cerebral blood flow, and increased neuroinflammation, all of which contribute importantly to the pathogenesis of age-related vascular cognitive impairment (VCI). There is increasing evidence showing that a decrease in NAD+ availability with age plays a critical role in age-related neurovascular and cerebromicrovascular dysfunction. Our recent studies demonstrate that restoring cellular NAD+ levels in aged mice rescues neurovascular function, increases cerebral blood flow, and improves performance on cognitive tasks. To determine the effects of restoring cellular NAD+ levels on neurovascular gene expression profiles, 24-month-old C57BL/6 mice were treated with nicotinamide mononucleotide (NMN), a key NAD+ intermediate, for 2 weeks. Transcriptome analysis of preparations enriched for cells of the neurovascular unit was performed by RNA-seq. Neurovascular gene expression signatures in NMN-treated aged mice were compared with those in untreated young and aged control mice. We identified 590 genes differentially expressed in the aged neurovascular unit, 204 of which are restored toward youthful expression levels by NMN treatment. The transcriptional footprint of NMN treatment indicates that increased NAD+ levels promote SIRT1 activation in the neurovascular unit, as demonstrated by analysis of upstream regulators of differentially expressed genes as well as analysis of the expression of known SIRT1-dependent genes. Pathway analysis predicts that neurovascular protective effects of NMN are mediated by the induction of genes involved in mitochondrial rejuvenation, anti-inflammatory, and anti-apoptotic pathways. In conclusion, the recently demonstrated protective effects of NMN treatment on neurovascular function can be attributed to multifaceted sirtuin-mediated anti-aging changes in the neurovascular transcriptome. Our present findings taken together with the results of recent studies using mitochondria-targeted interventions suggest that mitochondrial rejuvenation is a critical mechanism to restore neurovascular health and improve cerebral blood flow in aging.
Keywords: Aging, Geroscience, Vascular cognitive impairment, Mitochondria dysfunction, Transcriptomics
Introduction
In recent years, there has been increasing appreciation that the health of the neurovascular unit (NVU) is critical for brain health (Kisler et al. 2017; Zlokovic 2010, 2011; Iadecola 2017; Stanimirovic and Friedman 2012). The extended NVU consists of cerebral microvessels that receive input from neurons via astrocytic endfeet, pericytes, and perivascular microglia (Iadecola 2017; Stanimirovic and Friedman 2012). The NVU is responsible for the tight coupling between neural activity and regional cerebral blood flow (“neurovascular coupling”), which ensures adequate oxygen and nutrient delivery to the brain (Tarantini et al. 2017a; Toth et al. 2017). Endothelial dysfunction and/or impaired astrocytic function results in neurovascular uncoupling contributing to cognitive impairment (Toth et al. 2017; Tarantini et al. 2015, 2018, 2017b). In addition, cells constituting the NVU form and maintain the blood-brain barrier (Zlokovic 2010, 2011; Montagne et al. 2017; Sweeney et al. 2018, 2019a; Zlokovic 2008), regulate transport processes and waste removal, deposit the extracellular matrix, control the structural remodeling of the cerebral microcirculation (including angiogenesis, vessel regression, adaptation to hypertension (Csiszar et al. 2017; Tarantini et al. 2016, 2017c; Tucsek et al. 2014; Warrington et al. 2013; Ungvari et al. 2013, 2018a, 2017; Toth et al. 2015)), form and operate the glymphatic system (Iliff et al. 2013; Jessen et al. 2015; Kress et al. 2014), maintain stem-cell niches (Solano Fonseca et al. 2016), synthesize the glycocalyx, and control the adhesion and extravasation of inflammatory circulating cells that participate in central nervous system immune surveillance (Stanimirovic and Friedman 2012). With age, the phenotype and function of the cells constituting the NVU are altered, which fundamentally affects all of the aforementioned physiological processes (Kisler et al. 2017; Zlokovic 2010, 2011; Tarantini et al. 2017a). Age-related neurovascular dysfunction is now considered as a critical contributing factor to the pathogenesis of both vascular cognitive impairment (VCI) and neurodegenerative diseases, including Alzheimer’s disease (Sweeney et al. 2019b). In order to develop novel methods for prevention and treatment of these diseases and to preserve cognitive function in older adults, it is important to identify therapeutic interventions that can reverse age-related impairment of the NVU. Understanding the role of fundamental cellular and molecular mechanisms of aging in age-related neurovascular impairment is critical in that regard.
Nicotinamide adenine dinucleotide (NAD+) is a coenzyme central to hundreds of redox reactions in eukaryotic cells. NAD+ also has a critical role in the regulation of the activity of NAD+-consuming enzymes, including SIRT1 and other sirtuins (Gomes et al. 2013; Michan et al. 2010; Mitchell et al. 2014; Yang et al. 2007). Sirtuin enzymes are implicated in regulation of cellular processes of aging, mitochondrial function, stress resilience, apoptosis, and inflammation (Das et al. 2018; Csiszar et al. 2009a, b, 2008a). Aging is associated with cellular NAD+ depletion (Gomes et al. 2013; Massudi et al. 2012) (Yoshino, 2018 #10180), which has been proposed to be a critical driving force of aging processes (Imai and Guarente 2014), impairing nuclear and mitochondrial functions and contributing to the genesis of many age-associated pathologies. Accordingly, restoration of cellular NAD+ biosynthesis extends lifespan in model organisms (Anderson et al. 2002) and improves health span and extends lifespan in murine models of aging (Zhang, 2016 #10167) (Mitchell et al. 2018). There is emerging evidence that vascular aging is also characterized by cellular NAD+ depletion (Tarantini et al. 2019a; Csiszar et al. 2019; Kiss et al. 2019a). Importantly, our recent studies showed (Tarantini et al. 2019a) that in murine models of aging restoration of cellular NAD+ levels by chronic treatment with the NAD+ precursor, nicotinamide mononucleotide (NMN) (Yoshino et al. 2018) confers potent anti-aging neurovascular effects, rescuing cerebromicrovascular endothelial dysfunction and neurovascular coupling responses, increasing cerebral blood flow, and improving cognitive performance. In cultured cerebromicrovascular endothelial cells derived from aged rats, 5 days of treatment with NMN restored NAD+ levels and rescued mitochondrial function and attenuated mitochondrial oxidative stress in a sirtuin-dependent manner (Tarantini et al. 2019a).
The present study was designed to test the hypothesis that age-related NAD+ depletion in the NVU is causally linked to dysregulated expression of genes important for normal neurovascular function. A corollary hypothesis is that functional neurovascular rejuvenation in NMN-treated aged mice is associated with SIRT1-mediated restoration of a youthful neurovascular mRNA expression profile. To test these hypotheses, aged mice were treated with NMN for 2 weeks and transcriptomic signatures in cells of the neurovascular unit were compared with those in cells obtained from untreated young and aged control mice. The transcriptomic footprint of SIRT1 activation was analyzed, and the predicted multifaceted protective effects of NMN supplementation on diverse aspects of cerebromicrovascular and neurovascular biology were tested.
Methods
Animals, NMN supplementation
Young (3 months old) and aged (24 months old) male C57BL/6 mice were purchased from the aging colony maintained by the National Institute on Aging at Charles River Laboratories (Wilmington, MA). The biological age of 24-month-old mice corresponds to that of ~ 60-year-old humans. Mice were housed under specific pathogen-free barrier conditions in the Rodent Barrier Facility at University of Oklahoma Health Sciences Center under a controlled photoperiod (12 h light; 12 h dark) with unlimited access to water and were fed a standard AIN-93G diet (ad libitum). Mice in the aged cohort were assigned to two groups. One group of the aged mice was injected daily with NMN (IP injections of 500 mg NMN/kg body weight per day) or the equivalent volume of PBS for 14 consecutive days at 6 PM and 8 AM on day 14 and were sacrificed 4 h after last injection. Similar dosages of NMN has been shown to exert potent anti-aging effects on mouse health span (Csiszar et al. 2019; de Picciotto et al. 2016), including rescue of neurovascular coupling responses, attenuation of vascular oxidative stress, and rescue of gene expression changes in the aorta (Tarantini et al. 2019a; Kiss et al. 2019b). All procedures were approved by the Institutional Animal Use and Care Committees of the University of Oklahoma Health Sciences Center. All animal experiments complied with the ARRIVE guidelines and were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). The effects of NMN treatment on cognitive function, neurovascular coupling responses, and microvascular and aorta endothelial function in a similar cohort of mice have been recently reported (Tarantini et al. 2019a).
Isolation of cells of the neurovascular unit
Animals were killed and transcardially perfused with PBS as previously described (Tarantini et al. 2019a; Kiss et al. 2019b). The brains were quickly removed and rinsed in ice-cold PBS, and minced into ≈ 1 mm2 pieces. The tissue was washed twice in ice-cold 1× PBS by low-speed centrifugation (50g, 3 min). The diced tissue was digested in a buffer solution containing collagenase (800 U/g tissue), hyaluronidase (2.5 U/g tissue), and elastase (3 U/g tissue) in 1 mL PBS/100 mg tissue for 45 min at 37 °C in a rotating humid incubator. The digested tissue was passed through a 100-μm cell strainer. The single-cell lysate was centrifuged for 2 min at 70g. After removing the supernatant, the pellet was washed twice in cold PBS supplemented with 2.5% fetal calf serum (FCS), and the suspension was centrifuged at 300g for 5 min at 4 °C. To create fraction enriched for cells of the neurovascular unit, the cell suspension was centrifuged using an OptiPrep gradient solution (Axi-Shield, PoC, Norway). Briefly, the cell pellet was resuspended in Hanks’ balanced salt solution (HBSS) and mixed with 40% iodixanol thoroughly (final concentration 17% (v/v) iodixanol solution; ρ = 1.096 g/mL). Two milliliters of HBSS was layered on top and centrifuged at 400g for 15 min at 20 °C. Endothelial cells with attached astrocytes and pericytes, which banded at the interface between HBSS and the 17% iodixanol layer, were collected. The neurovascular-enriched fraction was incubated for 30 min at 4 °C in the dark with anti-CD31/PE (BD Biosciences, San Jose, CA, USA) and anti-MCAM/FITC (BD Biosciences, San Jose, CA, USA). After washing the cells twice with MACS buffer (Milltenyi Biotech, Cambridge, MA, USA), anti-FITC and anti-PE magnetic bead–labeled secondary antibodies were used for 15 min at room temperature. The endothelial/neurovascular enriched fraction was collected by magnetic separation using the MACS LD magnetic separation columns according to the manufacturer’s guidelines (Milltenyi Biotech, Cambridge, MA, USA). Our pilot studies indicated that this method using gentle cell dissociation protocols results in enrichment for cerebromicorvascular endothelial cells with astrocytes and pericytes.
RNA isolation, cDNA synthesis, library construction, and next generation sequencing
RNA was isolated from the samples using AllPrep DNA/RNA Mini Kit (Qiagen) as previously described (Imperio et al. 2016; Valcarcel-Ares et al. 2018). RNA quantity and quality (> 8 RNA integrity number) were measured using the RNA 6000 Nano Assay with an Agilent 2100 Bioanalyzer (Agilent, Palo Alto, CA). Using 1 μg RNA, cDNA was synthesized from purified RNA using ABI High-capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) (Imperio et al. 2016; Valcarcel-Ares et al. 2018). Library construction was performed in a stranded manner to retain the directionality of the transcripts for as described (Valcarcel-Ares et al. 2018). In brief, prior to RNA-seq analysis, quality control measures were implemented. Concentration of RNA was ascertained via fluorometric analysis on a Thermo Fisher Qubit Fluorometer. Overall quality of RNA was verified using an Agilent Tapestation instrument. Following initial QC steps, sequencing libraries were generated using the Illumina Truseq Stranded mRNA with library prep kit according to the manufacturers protocol. Briefly, mature mRNA was enriched for via pull down with beads coated with oligo-dT homopolymers. The mRNA molecules were then chemically fragmented, and the first strand of cDNA was generated using random primers. Following RNase digestion, the second strand of cDNA was generated replacing dTTP in the reaction mix with dUTP. Double stranded cDNA then underwent adenylation of 3′ ends following ligation of Illumina-specific adapter sequences. Subsequent PCR enrichment of ligated products was further selected for those strands not incorporating dUTP, leading to strand-specific sequencing libraries. Final libraries for each sample were assayed on the Agilent Tapestation for appropriate size and quantity. These libraries were then pooled in equimolar amounts as ascertained via fluorometric analyses. Final pools were absolutely quantified using qPCR on a Roche LightCycler 480 instrument with Kapa Biosystems Illumina Library Quantification reagents. Sequencing was performed on an Illumina NovaSeq 6000 instrument with paired-end 50 bp reads.
RNA-seq data analysis and visualization
Raw sequencing reads were trimmed of their Illumina TruSeq adapter sequences using Trimmomatic v0.35 (Bolger et al. 2014), then aligned to the mouse genome version GRCm38 using Kallisto v0.43.03 (Bray et al. 2016). Samples were checked for outliers and separation by principle components analysis (PCA) with the R function prcomp. Raw expression counts were summarized at the gene level to transcript-length adjusted, library-size scaled counts per million (CPM) with the R package tximport (Soneson et al. 2015). Differential expression analysis was performed using the empirical Bayes approach implemented in the R/Bioconductor package DESeq2 (Love et al. 2014). Significantly differentially expressed (DE) genes had an absolute log2 fold-change ≥ 0.585 (corresponding to a change of 50% or more in expression) and the false discovery rate (FDR)-adjusted p value ≤ 0.05. Gene annotation was done using biomaRt (Durinck et al. 2009) in R/Bioconductor package. Hierarchical clustering was performed via the R package ComplexHeatmap.
Functional annotation
The org.Mm.eg.db v3.8.2 R/Bioconductor package was used to collect Gene Ontology terms associated with our differentially expressed genes. The hypergeometric test implemented in GOstats v2.51.0 R/Bioconductor package was used to calculate enrichment of the individual terms (Falcon and Gentleman 2007).
We used upstream regulator analysis (URA) algorithm (Kramer et al. 2014) implemented in the Ingenuity Pathway Analysis (QIAGEN) software find upstream regulators that potentially explains the observed gene expression changes in our samples. The IPA uses a manually curated database (Ingenuity Knowledge Base) to calculate “enrichment” score (Fisher’s exact test (FET) p value), measures the overlap of observed and predicted regulated gene sets, and a z-score assessing the match of observed and predicted up/downregulation patterns.
Results
NMN treatment reverses age-related changes in neurovascular mRNA expression profile in mice
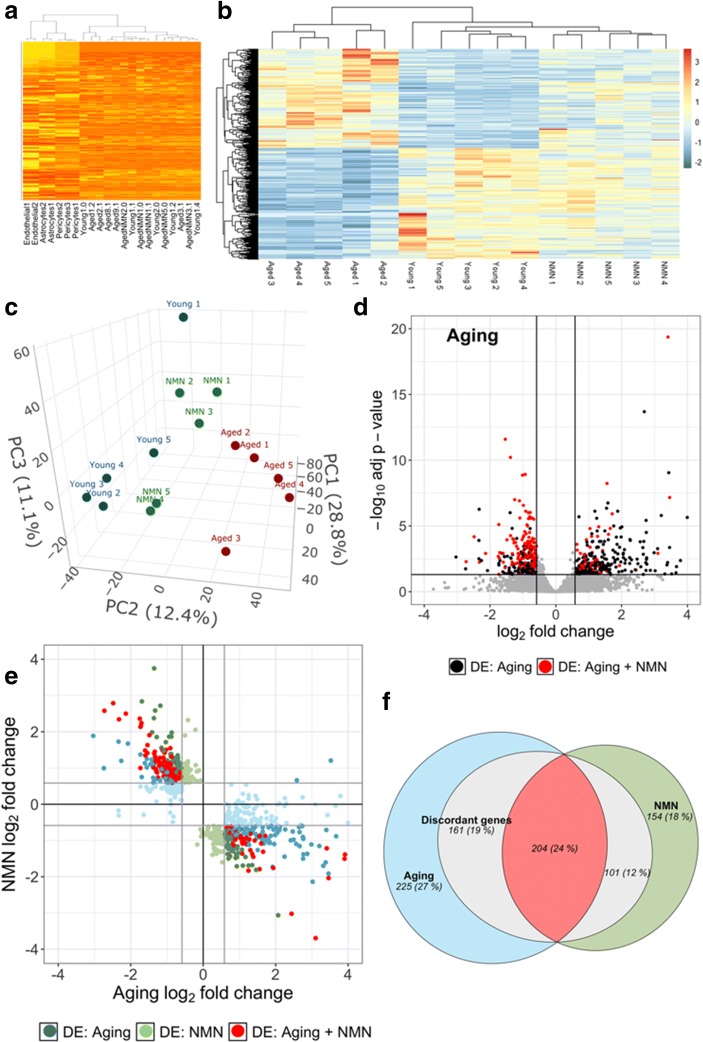
To isolate NVU-enriched mRNA, we employed an endothelial cell isolation-based strategy. Using RNA-seq to sequence the neurovascular transcriptome, we compared normalized mRNA expression values for each sample to that in individual cell types constituting the NVU (endothelial cells, astrocytes, and pericytes). To achieve that goal, we developed a list of cell-specific markers from published RNA-seq data of purified cortical cell types (GEO dataset GSE52564 (Zhang et al. 2014)). Comparison of mRNA levels in the NVU samples with the input shows a significant enrichment for endothelial cell, astrocyte, and pericyte genes (Fig. 1a).
Fig. 1.
NMN treatment reverses age-related changes in neurovascular mRNA expression profile. a Heatmap displaying normalized mRNA expression values for each sample as compared with that in cells of the neurovascular unit (endothelial cells, astrocytes, and pericytes) from the reference datasets by Zhang et al. (2014). Note that neurovascular genes are enriched in the samples. b The heat map is a graphic representation of normalized expression values of differentially expressed genes in neurovascular samples derived from young (3 months old), aged (24 months old), and NMN-treated aged mice. Hierarchical clustering analysis revealed the similarities on neurovascular mRNA expression profiles in young and NMN-treated aged mice. c Principal component analysis (PCA) plot of neurovascular mRNA expression profiles in young, aged control, and NMN-treated aged mice. The profiles from aged mice (red) cluster separately from clusters representing young mice (blue) and NMN-treated aged mice (green). PC1, PC2, and PC3 are principal components 1, 2, and 3, respectively. d Volcano plot depicting differentially expressed genes comparing neurovascular samples derived from young and aged mice. Stratified p values are plotted against expression fold-changes for results obtained in aged samples normalized to young samples. Colored points refer to genes whose expression is significantly altered by NMN treatment. e NMN-induced changes in gene expression plotted against age-related changes in the neurovascular transcriptome. Red symbols indicate discordant differentially expressed genes with youthful shifts, whose expression significantly changes with age and is restored by NMN treatment toward youthful levels. Blue and green symbols denote discordant genes with youthful shifts, whose expression changes in aging and is restored by NMN treatment toward youthful levels, but only the aging (blue) or the NMN effect (green) reaches the cutoff for statistical significance. f Venn diagrams sowing the numbers of differentially expressed mRNAs in each group. The blue circle represents neurovascular genes, which are significantly up or downregulated in aged mice as compared with young mice. The green circle represents neurovascular genes, which are significantly up or downregulated in aged mice by NMN treatment. The red area represents discordant differentially expressed genes. Gray areas represent discordant genes, whose expression is changed by NMN treatment toward youthful levels, but the effect does not reach the cutoff for statistical significance
We assessed transcriptomic changes in the NVU associated with aging and with NMN treatment (Fig. 1b). We performed unsupervised clustering of RNA-seq data from all samples using the topmost variably expressed genes across all samples. This showed that biological replicates from the same group cluster together, and that young samples segregate away from aged ones (Fig. 1b). PCA (Fig. 1c) of the transcriptomic data also showed a clear separation between the young and aged groups. Aged control mice and aged NMN-treated mice were also segregated in the PCA and hierarchical clustering. This finding indicated a clear difference between the transcriptome profiles of the two age groups. In contrast, mRNA expression in young mice and NMN-treated aged mice were similar, and these groups did not separate well in the PCA and hierarchical clustering.
We then determined the number of genes that are significantly upregulated or downregulated (DE, fold-change ≥ 1.5 or < 0.67; p < 0.05 adjusted for multiple comparisons) in the NVU by aging or by NMN treatment. We then filtered for genes that are significantly altered (adjusted p < 0.05), expressed at an appreciable level (fragments per kilobase of transcript per million mapped reads > 1), and are expressed in cells of the NVU. We identified 590 differentially expressed genes in aged animals compared with young controls. We also identified 459 DE genes in the NMN-treated aged mice compared with the untreated aged controls. In Fig. 1d, a volcano plot shows statistical significance (p value) versus magnitude of age-related change in gene expression. Red symbols denote genes, whose expression levels differed in the aged phenotype, but have shifted back toward the young phenotype by NMN treatment (discordant DE genes). The Venn diagram in Fig. 1f shows that neurovascular expression of 204 genes, which are differentially expressed in aged mice, was shifted back toward youthful levels by NMN treatment of aged mice.
We realized that significance cutoffs to identify differentially expressed genes shared between the age-effect and NMN-effect datasets may be too stringent, and the analysis illustrated in Fig. 1d may miss discordant patterns (youthful shifts) of gene expression with important biological relevance for NMN-induced neurovascular rejuvenation. Thus, we also used an approach to detect discordant transcriptional patterns (youthful shifts) by comparing the age-effect and NMN-effect gene expression datasets using combination criteria that took into account the effect direction. Genes were ranked by their effect size direction, and ranked lists were compared to identify overlapping genes across a continuous significance gradient. Our analysis required that discordant genes with youthful shifts (1) are “differentially expressed” based on both p value and fold-change criteria either in aging or the NMN treatment group, (2) satisfy a fold-change criterion with a cutoff of ≥ 1.5 or < 0.67 in the group in which expression did not satisfy the statistical significance p < 0.05, and (3) satisfy the criterion that the effect directions of the age-effect and NMN-effect are opposite. We found that these combination criteria found more biologically meaningful sets of genes than p values alone.
In Fig. 1e, the magnitude of age-related changes in gene expression is plotted against the magnitude of NMN-induced changes in gene expression. Red symbols denote discordant DE genes, whose expression levels shifted back toward the young phenotype by NMN treatment with statistical significance. Genes which are DE only in one group but otherwise satisfy the other criteria are denoted by blue (DE in aging) and green (DE in NMN-treated) symbols. Using this approach, we have identified 466 discordant genes with youthful shifts, which changed in opposite directions between the two datasets (Fig. 1f). These data suggest that NAD+ depletion has a critical role in age-related dysregulation of neurovascular gene expression.
Transcriptional footprint of neurovascular SIRT1 activation in NMN-treated aged mice
Previous studies suggested that restoration of NAD+ levels in aged cells by NMN treatment activates the NAD+-dependent histone deacetylase enzyme SIRT1 (Gomes et al. 2013; Tarantini et al. 2019a). To provide additional evidence that SIRT1 activation contributes to the neurovascular protective effects of NMN, we examined the transcriptional footprint of neurovascular SIRT1 activation in NMN-treated aged mice using three approaches. First, we analyzed age-related and NMN-induced changes in expression of SIRT1-regulated genes identified by IPA. We found that aging is associated with changes in the expression of several known SIRT1-regulated genes and that majority of these transcriptional changes are reversed by NMN treatment (Fig. 2a).
Fig. 2.
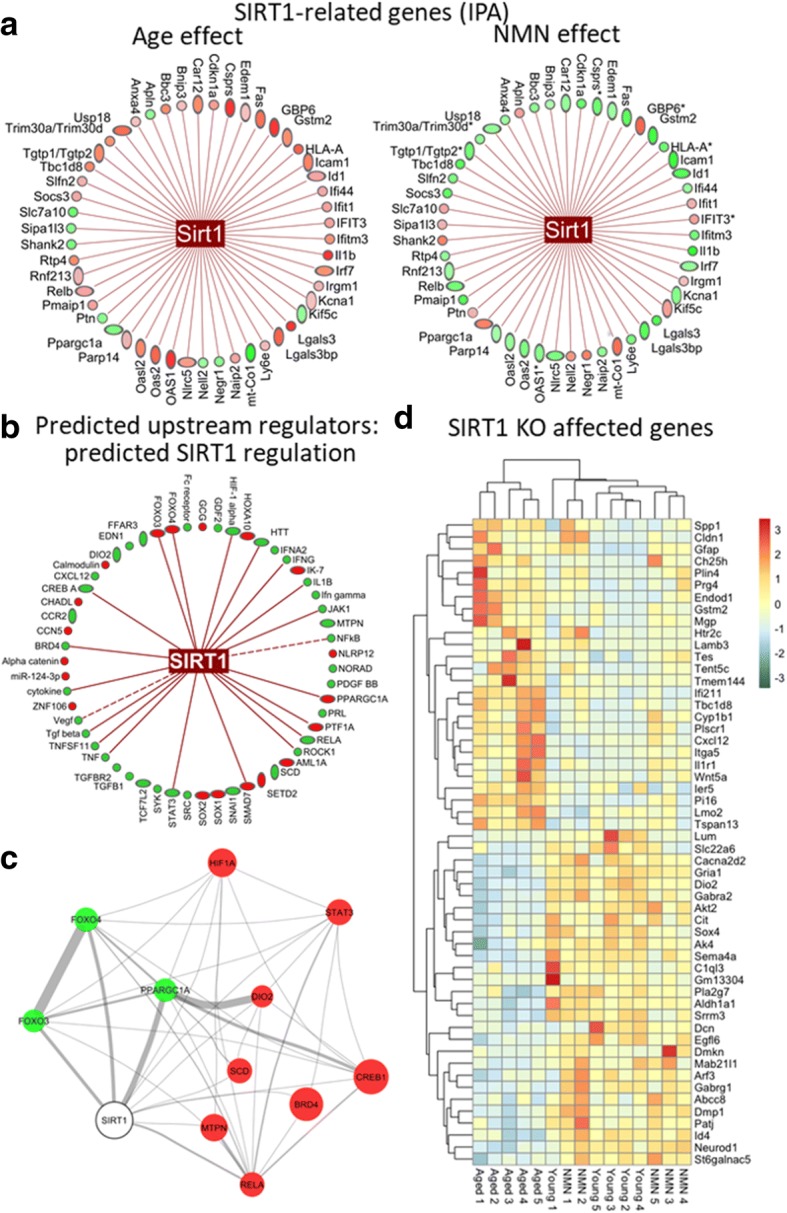
NMN reverses age-related changes in neurovascular expression of SIRT1-regulated genes. a IPA results showing age-related (left) and NMN-induced (right) changes in the expression of SIRT1-regulated genes (classified as such by IPA). Green, downregulation; red, upregulation. b Results of the IPA upstream regulator analysis. Shown are predicted upstream transcriptional regulators that may contribute to the observed NMN-induced transcriptomic changes in our dataset. Known links between the predicted upstream regulators activated by NMN and SIRT1 activity are indicated. c Literature-based relationships with positive mutual information among the predicted upstream regulators. Node size correlates with the activation z-score from IPA (bigger = higher z-score), edge width correlates with the mutual information of the genes within the literature, green marks which are predicted activators and red marks predicted repressors. d The heat map is a graphic representation of normalized expression values of differentially expressed SIRT1-dependent genes in neurovascular samples derived from young (3 months old), aged (24 months old), and NMN-treated aged mice. Hierarchical clustering analysis revealed the similarities on neurovascular expression profiles of SIRT1-dependent genes in young and NMN-treated aged mice. SIRT1-dependent genes were identified based on their differential expression in the brain of SIRT1−/− mice (Libert et al. 2011)
Ingenuity upstream regulator analysis
We have also performed IPA upstream regulator analysis (Kramer et al. 2014) to identify upstream transcriptional regulators that may contribute to the observed transcriptomic changes in our dataset, which can help to identify the mechanism of action of NMN in the aged neurovascular unit. The upstream regulator analysis is based on information in the Ingenuity Knowledge Base (a curated relational database of the available biomedical literature) on the expected effects between transcriptional regulators and their target genes. Using the IPA upstream regulator analysis, it was examined how many known targets of each transcriptional regulator were differentially expressed in our samples, and the direction of these gene expression changes were compared with what is expected from the literature. On the basis of the observed direction of change, a prediction of the activation state of the predicted transcriptional regulators (“activated” or “inhibited”) were made (not shown). For each potential transcriptional regulator, two statistical measures, an overlap p value and an activation z-score, were computed. The overlap p value calls likely upstream regulators based on significant overlap between the differentially expressed genes and known targets regulated by that particular transcriptional regulator. The activation z-score is used to infer the activation state of the predicted transcriptional regulators (“activated” or “inhibited”) based on comparison with a model that assigns random regulation directions. The results of the IPA upstream regulator analysis are visualized in Fig. 2b. We also determined the link between the predicted upstream regulators activated by NMN and SIRT1 using IPA. As indicated in Fig. 2b, we found that ~ 38% of the predicted upstream regulators activated by NMN are known to be regulated by SIRT1-dependent pathways. In particular, the IPA upstream regulator analysis predicts that NMN-induced SIRT1 activation upregulates PGC-1α (PPARGC1A), FOXO3- and FOXO4-mediated pathways, whereas it inhibits HIF-1α-regulated pathways (Fig. 2b). We also attempted to predict NMN-activated, SIRT1-dependent regulatory networks by identifying relationships between SIRT1 and the predicted upstream regulators utilizing the IRIDESCENT (Implicit Relationship IDEntification by in-Silico Construction of an Entity-based Network from Text) system (Wren and Garner 2004). IRIDESCENT processes all available MEDLINE abstracts and uses a statistical model to determine whether each upstream regulator co-occurs with a term of interest more frequently than would be expected by chance, and quantifies this in terms of the mutual information measure. The results of this analysis provide additional support to the view that the predicted NMN-induced SIRT1 activation results in inhibition of HIF-1α29 and activation of PGC-1α- and FOXO3-dependent pathways (Hubbard et al. 2013). PGC-1α and FOXOs are known targets for SIRT1-mediated deacetylation (Fig. 2c).
In addition, we also intersected the list of differentially expressed genes in our dataset with the list of genes differentially expressed in the brains of SIRT1−/− mice (NCBI Gene Expression Omnibus: GSE28790) (Libert et al. 2011). The heat map showing the expression pattern of these SIRT1-sensitive genes is shown in Fig. 2d. Hierarchical clustering of the data showed a clear separation between the young and aged groups. Aged control mice and aged NMN-treated mice were also clearly separated. In contrast, expression of SIRT1-sensitive genes in young mice and NMN-treated aged mice were similar, and these groups did not separate well in the hierarchical clustering, consistent with the idea that aging is associated with dysregulation of SIRT1-sensitive genes, which is rescued by NMN treatment.
NMN-induced neurovascular transcriptomic changes in aged mice predict mitochondrial rejuvenation
We performed GO enrichment analysis to explore potential biological functions of the NMN-regulated discordant differentially expressed genes with youthful shifts. GO enrichment analysis of discordant differentially expressed genes with youthful shifts identified functions in mitochondrial regulation and oxidative stress, apoptosis, inflammation, endothelial activation, and transcriptional regulation (Fig. 3).
Fig. 3.
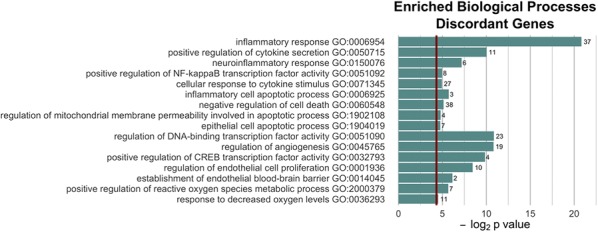
Most significantly enriched Gene Ontology (GO) terms for discordant genes. Note that NMN treatment is associated with transcriptional changes indicating multifaceted anti-inflammatory, anti-apoptotic, mitochondrial protective, and anti-oxidative effects
Our recent studies, demonstrate that aging is associated with mitochondrial dysfunction and oxidative stress in cerebromicrovascular endothelial cells, which play a critical role in dysregulation of cerebral blood flow and impaired neurovascular coupling responses in aged mice (Tarantini et al. 2018, 2019a). To find out whether mitochondria-related gene expression is altered in the aging NVU, we analyzed expression of both nuclear-encoded and mtDNA-encoded mitochondria-related genes. We have used existing databases to compile a list of genes with mitochondrial targeting sequences and known functions related to regulation of mitochondrial processes. We used Gene Set Enrichment Analysis (GSEA) for interpreting expression of mitochondria-related genes (Subramanian et al. 2005). GSEA of mtDNA-encoded genes encoding components of the mitochondrial electron transport chain (ETC) was performed using a pre-ranked gene list based on the magnitude of the fold-change (largest upregulation to most downregulated; Fig. 4a, b). Figure 4a, b depict a running-sum statistic (enrichment score) based on Fig. 4, increasing when a gene is a member of the mtDNA-encoded ETC gene set and decreasing when it is not. Note that in aged mice, GSEA scores increased predominantly on the right indicating downregulation of mtDNA-encoded ETC genes by aging. In contrast, in NMN-treated aged mice GSEA scores increased predominantly on the left indicating upregulation of mtDNA-encoded ETC genes by NMN treatment in aged mice. The heat maps showing the expression pattern of nuclear-encoded and mtDNA-encoded mitochondria-related genes are shown in Fig. 4 c and d, respectively. Hierarchical clustering of the data showed a clear separation between the young and aged groups. Aged control mice and aged NMN-treated mice were also separated. In contrast, expression of mitochondria-related genes in young mice and NMN-treated aged mice were similar, and these groups did not separate well in the hierarchical clustering, consistent with the idea that age-related dysregulation of mitochondria-related genes in the NVU is reversed, at least in part, by NMN treatment.
Fig. 4.
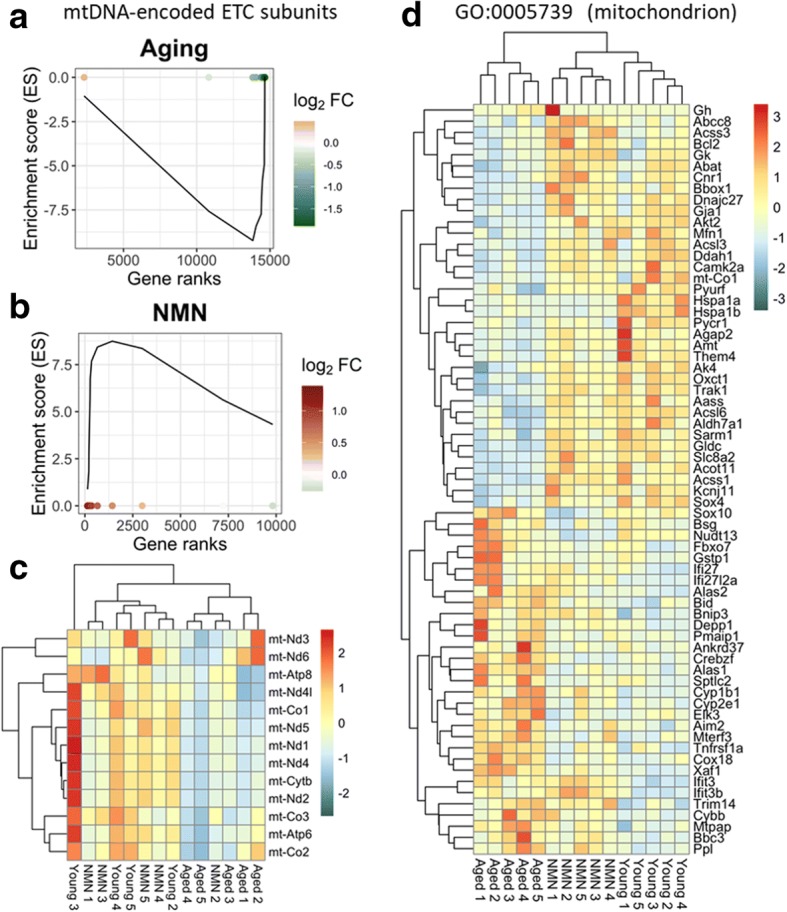
NMN treatment reverses age-related changes in neurovascular expression of mitochondria-related genes. Gene Set Enrichment Analysis (GSEA) to test for enrichment of the set of mtDNA-endcoded subunits of the electron transport chain (ETC) by comparing NVU samples derived from aged (24 months old) mice with NVU samples derived from young (3 months old) mice (a) and NMN-treated aged NVU samples with untreated aged NVU samples (b). Aging-induced gene expression changes were ranked from most upregulated (left, red) to most downregulated (right, green). Dots represent identified mtDNA-encoded ETC genes. Panels a, b depict a running-sum statistic (enrichment score) based on panel c, increasing when a gene is a member of the mtDNA-encoded ETC gene set and decreasing when it is not. Note that in aged mice, GSEA scores increased predominantly on the right indicating downregulation of mtDNA encoded ETC genes by aging. In contrast, in NMN-treated aged mice GSEA scores increased predominantly on the left indicating upregulation of mtDNA-encoded ETC genes by NMN treatment in aged mice. The heat maps are graphic representations of normalized expression values of differentially expressed mtDNA-encoded ETC genes (c) and nuclear-encoded mitochondria-related genes (d). Hierarchical clustering analysis revealed the similarities on neurovascular expression profiles of mitochondria-related genes in young and NMN-treated aged mice. Mitochondria-related genes were identified on the basis of GO classifications (GO:0005739). Note that one young sample was a statistical outlier and was therefore excluded from the mtDNA-encoded gene expression analysis
NMN-induced neurovascular transcriptomic changes in aged mice predict anti-apoptotic effects
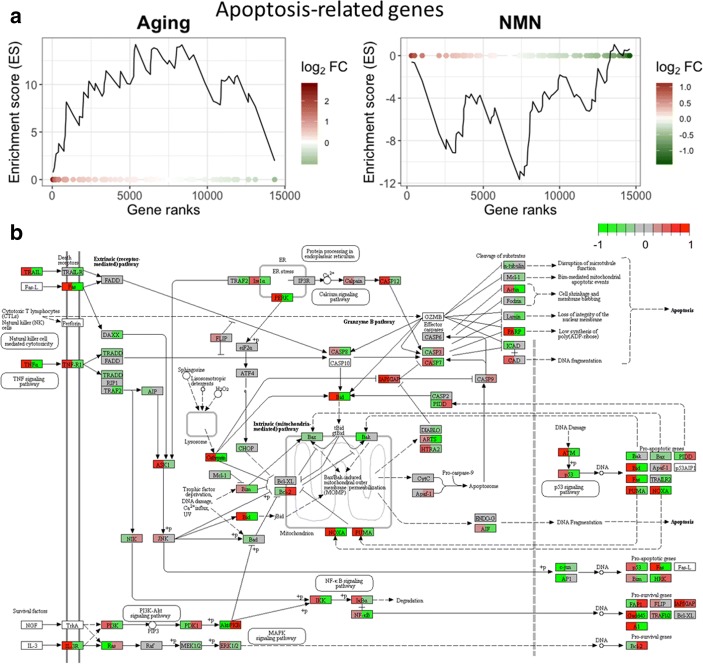
Previous studies suggest that endothelial cell apoptosis plays a critical role in age-related structural remodeling of cerebromicrovascular network by contributing to microvascular rarefaction (Ungvari et al. 2018a, b). To determine how NMN treatment alters apoptosis-related gene expression in the aging NVU, we analyzed expression of genes known to be involved in regulation of programmed cell death. Apoptosis-related genes were identified based on GO classification. GSEA analysis suggested that aging is associated with upregulation of pro-apoptotic genes, which tends to be reversed by NMN treatment (Fig. 5a, b). KEGG pathway map depicting age- and NMN treatment-related changes in the expression of genes in the apoptosis pathways is shown in Fig. 5c.
Fig. 5.
NMN treatment reverses age-related changes in neurovascular expression of apoptosis-related genes. Gene Set Enrichment Analysis (GSEA) to test for enrichment of the set of pro-apoptotic genes by comparing NVU samples derived from aged (24 months old) mice with NVU samples derived from young (3 months old) mice (left, a) and NMN-treated aged NVU samples with untreated aged NVU samples (right, b). Aging-induced gene expression changes were ranked from most upregulated (left, red) to most downregulated (right, green). Dots represent identified pro-apoptotic genes. Panels a and b depict a running-sum statistic (enrichment score) based on panel b, increasing when a gene is a member of the apoptosis-related gene set and decreasing when it is not. Note that in aged mice, GSEA scores increased predominantly on the left indicating upregulation of pro-apoptotic genes by aging. In contrast, in NMN-treated aged mice, GSEA scores increased predominantly on the right indicating downregulation of pro-apoptotic genes by NMN treatment in aged mice. b Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway map depicting age- and NMN treatment-related changes in the expression of genes in the apoptosis pathways. Each rectangle on the map represents a gene product in the apoptosis pathway. The rectangles are set to color by age-related (left side) and NMN treatment-induced (right side) changes in gene expression (fold-change). Red color indicates upregulation, green color indicates downregulation. Genes involved in positive regulation of apoptosis were identified based on GO classification
NMN-induced neurovascular transcriptomic changes predict anti-inflammatory effects, including inhibition of endothelial activation in aged mice
Chronic low-grade inflammation, characterized by endothelial activation, is a hallmark of vascular aging (Ungvari et al. 2018b, 2007a; Csiszar et al. 2007, 2004, 2003). To elucidate the putative anti-inflammatory effects of NMN treatment, we assessed its effect on the expression of endothelial activation–related genes. Endothelial activation–related genes were identified based on published microarray data (GEO database; GSE45880), showing mRNA expression changes after activation of cultured cerebromicrovascular endothelial cells (CMVECs) by 10 ng/mL TNFα and IFNγ (Lopez-Ramirez et al. 2013). GSEA analysis showed that aging is associated with upregulation of endothelial activation–related genes in the NVU (Fig. 6a). We found that NMN treatment exerts significant anti-inflammatory effects, downregulating endothelial activation–related genes in the NVU (Fig. 6b). The heat map shown in Fig. 6c is a graphic representation of normalized expression values of differentially expressed endothelial activation–related genes in NVU samples derived from young, aged, and NMN-treated aged mice. We also found that 17 genes, which are important for blood-brain barrier integrity (Nyul-Toth et al. 2016) were significantly affected by NMN treatment (data not shown) (Fig. 7).
Fig. 6.
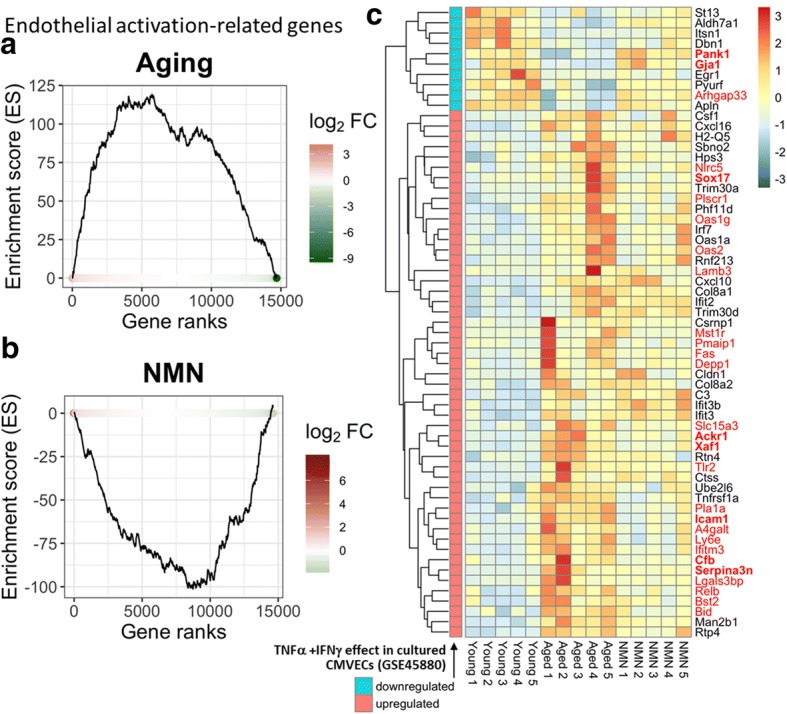
NMN treatment reverses age-related changes in neurovascular expression of endothelial activation-related genes. Gene Set Enrichment Analysis (GSEA) to test for enrichment of the set of endothelial activation-related genes by comparing NVU samples derived from aged (24 months old) mice with NVU samples derived from young (3 months old) mice (a) and NMN-treated aged NVU samples with untreated aged NVU samples (b). Aging-induced gene expression changes were ranked from most upregulated (left, red) to most downregulated (right, green). Dots represent identified endothelial activation–related genes. Panels a, b depict a running-sum statistic (enrichment score) based on the upregulated endothelia activation–related genes in panel c, increasing when a gene is a member of the endothelial activation–related gene set and decreasing when it is not. Note that in aged mice, GSEA scores increased predominantly on the left indicating upregulation of endothelial activation–related genes by aging. In contrast, in NMN-treated aged mice, GSEA scores increased predominantly on the right indicating downregulation of endothelial activation–related genes by NMN treatment in aged mice. c The heat map is a graphic representation of normalized expression values of differentially expressed endothelial activation–related genes in neurovascular samples derived from young, aged, and NMN-treated aged mice. Hierarchical clustering analysis revealed the similarities on neurovascular expression profiles of endothelial activation–related genes in young and NMN-treated aged mice. Endothelial activation–related genes were identified based on published microarray data (GEO database; GSE45880), showing a distinct transcriptional signature of up and downregulated genes after activation of cultured cerebromicrovascular endothelial cells with 10 ng/mL TNFα and IFNγ (Lopez-Ramirez et al. 2013). Included in the figure are genes whose expression in aging changes similarly to the expressional changes observed in vitro upon cytokine stimulation. Discordant genes are shown in red font (bold, DE both in aging and NMN treated groups)
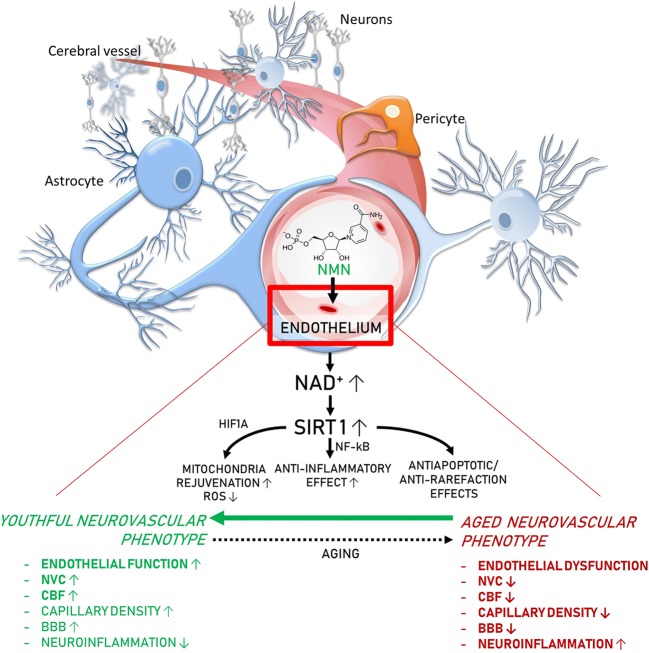
Fig. 7.
Proposed scheme for the mechanisms by which restoration of NAD+ levels in the aged neurovascular unit by NMN supplementation promotes neurovascular rejuvenation. The model, based on our present and previous findings and earlier data from the literature (Das et al. 2018; Tarantini et al. 2019a; Csiszar et al. 2019), predicts that increased NAD+ activates sirtuin-mediated pathways, which leads to anti-aging transcriptomic changes, restores cellular energetics, and attenuates mitochondrial ROS production, rescuing a youthful neurovascular phenotype. These effects are predicted to act to improve endothelial function, increase neurovascular coupling responses, capillary density and cerebral blood flow (CBF), maintain blood-brain barrier (BBB) integrity, and inhibit neuroinflammation, protecting cognitive health (bold font, experimentally validated effects; regular fonts, predicted effects)
Discussion
Our study demonstrates that protective effects of NMN treatment on cerebromicrovascular endothelial function and neurovascular coupling responses are associated with anti-aging changes in the mRNA expression profile in the NVU in a mouse model of aging that recapitulates vascular alterations and deficits present in elderly humans at risk for vascular cognitive impairment.
To our knowledge, this is the first study to demonstrate that NMN treatment in aged mice reverses, at least in part, age-related, pro-inflammatory, pro-oxidative, pro-apoptotic, and endothelial dysfunction-promoting transcriptional alterations in the cerebral microcirculation. The results of the present study extend the findings of earlier investigations showing that treatment with NMN confers potent anti-aging neurovascular effects in aged mice, rescuing cerebromicrovascular endothelial dysfunction and neurovascular coupling responses, increasing cerebral blood flow, and improving cognitive performance (Yoshino et al. 2018). Demonstration of NMN-induced phenotypic and functional changes in the NVU is particularly important as neurovascular alterations (including impaired neurovascular coupling, blood-brain barrier disruption, and pro-inflammatory changes) associated with aging have been causally linked to the development of both neurodegenerative diseases and the entire spectrum of brain pathologies that contribute to vascular cognitive impairment (Iadecola 2017; Toth et al. 2017; Sweeney et al. 2019b). Endothelial protective effects of NMN have also been demonstrated in the peripheral circulation of aged mice (Das et al. 2018; Yoshino et al. 2018; de Picciotto et al. 2016), suggesting that the effects of NMN on endothelial cells in the NVU likely play a key role in NMN-induced neurovascular rejuvenation. Administration of NMN or other NAD+ precursors (e.g., nicotinamide riboside) to aged mice was reported to increase NAD+ levels in homogenates of complex tissues derived from multiple organs (Yoshino et al. 2018; Mills et al. 2016; Zhang et al. 2016), including the aorta (Tarantini et al. 2019a). In vitro treatment with NMN was also demonstrated to restore NAD+ levels in aged cerebromicrovascular endothelial cells (Tarantini et al. 2019a). Future studies should determine how in vivo NMN treatment affects NAD+ levels in each cell type constituting the NVU and elucidate the cell type–specific functional and transcriptomic effects of NMN treatment in aging.
Our study demonstrates that NMN treatment, which augments the vascular NAD+ metabolome (Tarantini et al. 2019a), induces a neurovascular gene expression signature suggestive of SIRT1 activation. Our results expand the findings of previous studies showing that increases in NAD+ levels induced by NMN treatment also activate SIRT1 in skeletal muscle (Gomes et al. 2013). As our recent studies demonstrate that shRNA knockdown of SIRT1 prevents the beneficial effects of NMN on aged cerebromicrovascular endothelial cells in vitro (Tarantini et al. 2019a), we posit that NMN-induced SIRT1 activation plays a critical role also in neurovascular rejuvenation in vivo. Sirtuins are known to mediate beneficial anti-aging (Cohen et al. 2004; Moroz et al. 2014; Wood et al. 2004) and vasoprotective effects (Csiszar et al. 2009a, 2013; 2014a) of caloric restriction as well. Our bioinformatics analysis also revealed a role for Hif1α signaling, confirming earlier findings (Gomes et al. 2013). Further, our recent study demonstrate that NMN treatment reverses age-related changes in miRNA expression in the aged mouse aorta (Kiss et al. 2019b). In that regard, it is significant that dysregulation of miRNA expression has been shown to significantly contribute to age-related phenotypic and functional changes in the cerebromicrovascular endothelial cells as well (Ungvari et al. 2013). These findings raise the possibility that complex changes in transcriptional and/or post-transcriptional control of expression of genes that encode critical factors determining neurovascular health contribute to the beneficial effects of treatment with NAD+ boosters. GO enrichment analysis of discordant differentially expressed neurovascular genes identified functions in mitochondrial regulation, apoptosis, inflammation, and endothelial activation.
Mitochondrial dysfunction and increased mitochondrial oxidative stress have a critical role in the genesis of aging-induced cerebromicrovascular endothelial impairment and neurovascular dysfunction (Tarantini et al. 2018, 2019a). In support of this concept, attenuation of mitochondrial oxidative stress and restoration of mitochondrial energy metabolism in the cerebromicrovascular endothelial cells by treatment with the mitochondria-targeted antioxidants were shown to rescue neurovascular function in aged mice (Tarantini et al. 2018). Here, we report that NMN treatment rescues aging-induced changes in mitochondria-related gene expression in the NVU. Importantly, these NMN-induced changes in the mitochondria-related transcriptome are associated with attenuated mitochondrial oxidative stress and restoration of mitochondrial energy metabolism in aging cerebromicrovascular endothelial cells (Tarantini et al. 2019a; Kiss et al. 2019a). On the basis of previous findings (Gomes et al. 2013), we posit that rescue of vascular mitochondrial function by restoring the expression of ETC subunits contributes to the neurovascular protective effects of NMN. Treatment with NMN was also shown to rescue expression of mitochondrial-encoded ETC subunits in cerebral arteries of aged mice and in aged cerebromicrovascular endothelial cells (Gomes et al. 2013). It is believed that rescue of electron flow through the electron transport chain, due to the restored expression of complex I and complex III (Kwong and Sohal 2000), likely attenuates electron leak, limiting mtROS production. Treatment with NAD+ boosters was also demonstrated to upregulate mitochondrial gene expression in the mouse skeletal muscle (Canto et al. 2012). Our recent studies provide evidence that NMN treatment exerts its mitochondrial protective effects in cerebromicrovascular endothelial cells in a SIRT1-dependent manner (Gomes et al. 2013). Our observations accord with findings from earlier studies showing that many of the health benefits conferred by SIRT1 activation are linked to improved mitochondrial function (Baur et al. 2006). In addition to sirtuin-mediated transcriptomic effects, a mitochondrial ATP production requires NAD+ as an essential cofactor, rescuing normal cellular NAD/NADH ratio per se may also promote efficient mitochondrial function in cells of the NVU.
Analysis of the transcriptomic signature of NMN treatment predicts potent anti-apoptotic effects in the NVU. This is significant, as endothelial cell apoptosis plays a critical role in age-related cerebromicrovascular rarefaction (Ungvari et al. 2018a, b). Thus, future studies should determine how NMN treatment affects the number of apoptotic endothelial cells in the NVU as well as capillary density in the aged brain. Recent studies show that NMN also protects the integrity of the blood-brain barrier in a mouse model of brain ischemia (Wei et al. 2017). On the basis of our findings that NMN upregulates factors controlling barrier integrity, it will be also of great interest to determine whether NMN treatment can also protect against age-related disruption of the blood-brain barrier.
Our studies demonstrate that NMN treatment in aged mice reverses, at least in part, age-related, pro-inflammatory alterations in mRNA expression profile in the NVU. Our findings expand the results of recent studies demonstrating that treatment of aged mice with NMN promotes anti-inflammatory phenotypic changes in the peripheral vasculature as well (Kiss et al. 2019b). Previous studies attributed age-related endothelial activation and chronic sterile microvascular inflammation to oxidative stress-mediated activation of NF-κB and upregulation of pro-inflammatory cytokines in the vascular wall (Csiszar et al. 2003, 2008b, 2014a; Ungvari et al. 2007a). SIRT1 activation is known to attenuate cellular and mitochondrial oxidative stress, inhibit NF-κB, and attenuate microvascular inflammation (Toth et al. 2015; Csiszar et al. 2008a, 2012; Baur et al. 2012; Ungvari et al. 2009; Zhang et al. 2009; Mattison et al. 2014). Thus, it is likely that SIRT1 activation and the previously documented anti-oxidative neurovascular effects contribute significantly to the observed anti-inflammatory effects associated with NMN treatment.
Additional studies are warranted to determine the efficacy of combination treatments with NAD+ boosters (Mitchell et al. 2018; Yoshino et al. 2018; Liu et al. 2018) and compounds that directly activate SIRT1 and/or inhibit NAD+ overutilization for neurovascular protection. Similar to NAD+ boosters, SIRT1-activating compounds (STACs; including resveratrol and SRT1720) were demonstrated to exert important vasoprotective effects in models of aging and accelerated vascular aging (Csiszar et al. 2008a; Ungvari et al. 2007a, b, 2011; Pearson et al. 2008; Zarzuelo et al. 2013; Chen et al. 2015; Gano et al. 2014; Minor et al. 2011). These SIRT1-mediated effects include increased mitochondrial biogenesis (Csiszar et al. 2009b), attenuation of mitochondrial oxidative stress (Ungvari et al. 2009; Csiszar et al. 2012), activation of anti-oxidative defense mechanisms (Csiszar et al. 2014b), and inhibition of apoptosis (Pearson et al. 2008). Treatment with the STAC resveratrol was shown to improve cerebromicrovascular endothelial function and rescue neurovascular coupling responses in aged mice (Toth et al. 2014; Wiedenhoeft et al. 2019). Resveratrol was also shown to increase capillary density (Oomen et al. 2009) and prevent microvascular fragility (Toth et al. 2015) in the aged mouse brain and to exert similar vasoprotective effects in non-human primate models as well (Mattison et al. 2014; Bernier et al. 2016). The molecular mechanisms contributing to age-related decline in NAD+ in cells of the NVU are likely multifaceted. In addition to downregulation of NAMPT (nicotinamide phosphoribosyltransferase/NMN synthase; which catalyzes the rate limiting step in the biosynthesis of NAD+) (Tarantini et al. 2019a), the increased utilization of NAD+ by activated poly(ADP-ribose) polymerase 1 (PARP-1) (Csiszar et al. 2019; Pacher et al. 2002) also likely plays an important role in age-related decline in NAD+ in the NVU. Accordingly, treatment with PJ-34, a potent PARP inhibitor, restores neurovascular coupling responses in aged mice, similar to the neurovascular protective effects of NMN treatment (Tarantini et al. 2019b). Thus, future studies should determine whether combination of NAD+ boosters with STACs, mitochondria-targeted agents, and/or PARP-1 inhibitors confers greater neurovascular and cognitive health benefits as compared with NAD+ booster treatment alone.
Conclusions
In conclusion, rescue of cerebromicrovascular endothelial function and neurovascular coupling responses in NMN-treated aged mice are accompanied by marked anti-aging changes in the neurovascular transcriptome. We hope that our findings will facilitate future endeavors to uncover the mechanistic role of neurovascular NAD+ depletion in brain aging and the pathogenesis of VCI. The recently appreciated complex role of NVU dysfunction (ranging from impaired neurovascular coupling to blood-brain barrier disruption) in neurodegenerative diseases and VCI supports the concept that pharmacological treatments, which maintain neurovascular health, promote brain health (Kisler et al. 2017; Zlokovic 2010, 2011; Csipo et al. 2019a, b; de Montgolfier et al. 2019; Farias Quipildor et al. 2019; Fulop et al. 2019; Sorond et al. 2019; Sagare et al. 2013). Potentially, NAD+ booster treatments (e.g., in combination with STACs) could be harnessed for development of new pharmacological approaches for neurovascular protection for the prevention and treatment of VCI and neurodegenerative diseases in older adults.
Funding information
This work was supported by grants from the American Heart Association (ST), the Oklahoma Center for the Advancement of Science and Technology (to AC, AY, ZU), the National Institute on Aging (R01-AG047879; R01-AG038747; R01-AG055395), the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS056218 to AC, R01-NS100782 to ZU), the National Institute of General Medical Sciences Oklahoma Shared Clinical and Translational Resources (OSCTR) (GM104938, to AY and JW) and Molecular Mechanisms and Genetics of Autoimmunity COBRE (P30-GM110766, to LG), the Presbyterian Health Foundation (to ZU, AC, AY), the NIA-supported Geroscience Training Program in Oklahoma (T32AG052363), the Oklahoma Nathan Shock Center (P30AG050911), and the Cellular and Molecular GeroScience CoBRE (1P20GM125528, sub#5337). The funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Compliance with ethical standards
All procedures were approved by the Institutional Animal Use and Care Committees of the University of Oklahoma Health Sciences Center. All animal experiments complied with the ARRIVE guidelines and were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tamas Kiss, Ádám Nyúl-Tóth, Priya Balasubramanian and Stefano Tarantini contributed equally to this work.
References
- Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Cohen H, Lin SS, Manchester JK, Gordon JI, Sinclair DA. Manipulation of a nuclear NAD+ salvage pathway delays aging without altering steady-state NAD+ levels. J Biol Chem. 2002;277:18881–18890. doi: 10.1074/jbc.M111773200. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov. 2012;11:443–461. doi: 10.1038/nrd3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier M, Wahl D, Ali A, Allard J, Faulkner S, Wnorowski A, Sanghvi M, Moaddel R, Alfaras I, Mattison JA, Tarantini S, Tucsek Z, Ungvari Z, Csiszar A, Pearson KJ, de Cabo R. Resveratrol supplementation confers neuroprotection in cortical brain tissue of nonhuman primates fed a high-fat/sucrose diet. Aging (Albany NY) 2016;8:899–916. doi: 10.18632/aging.100942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YX, Zhang M, Cai Y, Zhao Q, Dai W. The Sirt1 activator SRT1720 attenuates angiotensin II-induced atherosclerosis in apoE(−)/(−) mice through inhibiting vascular inflammatory response. Biochem Biophys Res Commun. 2015;465:732–738. doi: 10.1016/j.bbrc.2015.08.066. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Csipo T, Lipecz A, Fulop GA, Hand RA, Ngo BN, Dzialendzik M, Tarantini S, Balasubramanian P, Kiss T, Yabluchanska V, Silva-Palacios F, Courtney DL, Dasari TW, Sorond F, Sonntag WE, Csiszar A, Ungvari Z, Yabluchanskiy A. Age-related decline in peripheral vascular health predicts cognitive impairment. Geroscience. 2019;41:125–136. doi: 10.1007/s11357-019-00063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csipo T, Mukli P, Lipecz A, Tarantini S, Bahadli D, Abdulhussein O, Owens C, Kiss T, Balasubramanian P, Nyul-Toth A, Hand RA, Yabluchanska V, Sorond FA, Csiszar A, Ungvari Z, Yabluchanskiy A. Assessment of age-related decline of neurovascular coupling responses by functional near-infrared spectroscopy (fNIRS) in humans. Geroscience. 2019;41:495–509. doi: 10.1007/s11357-019-00122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in rat coronary arteries. FASEB J. 2003;17:1183–1185. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-TNFalfa treatment in aging. Am J Pathol. 2007;170:388–698. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Wang M, Lakatta EG, Ungvari ZI. Inflammation and endothelial dysfunction during aging: role of NF-{kappa}B. J Appl Physiol. 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson KJ, de Cabo R, Pacher P, Zhang C, Ungvari ZI (2009b) Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009 Jul;297(1):H13-20. 10.1152/ajpheart.00368.2009 [DOI] [PMC free article] [PubMed]
- Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–820. doi: 10.1093/gerona/glr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Sosnowska D, Tucsek Z, Gautam T, Toth P, Losonczy G, Colman RJ, Weindruch R, Anderson RM, Sonntag WE, Ungvari Z. Circulating factors induced by caloric restriction in the nonhuman primate Macaca mulatta activate angiogenic processes in endothelial cells. J Gerontol A Biol Sci Med Sci. 2013;68:235–249. doi: 10.1093/gerona/gls158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol. 2014;307:H292–H306. doi: 10.1152/ajpheart.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Pinto JT, Gautam T, Kleusch C, Hoffmann B, Tucsek Z, Toth P, Sonntag WE, Ungvari Z (2014b) Resveratrol encapsulated in novel fusogenic liposomes activates Nrf2 and attenuates oxidative stress in cerebromicrovascular endothelial cells from aged rats. J Gerontol A Biol Sci Med Sci. 2015 Mar;70(3):303-13. 10.1093/gerona/glu029 [DOI] [PMC free article] [PubMed]
- Csiszar A, Tarantini S, Fulop GA, Kiss T, Valcarcel-Ares MN, Galvan V, Ungvari Z, Yabluchanskiy A (2017) Hypertension impairs neurovascular coupling and promotes microvascular injury: role in exacerbation of Alzheimer’s disease. GeroScience. 2017 Aug; 39(4): 359–372. [DOI] [PMC free article] [PubMed]
- Csiszar A, Tarantini S, Yabluchanskiy A, Balasubramanian P, Kiss T, Farkas E, Baur JA, Ungvari ZI (2019) Role of endothelial NAD+ deficiency in age-related vascular dysfunction. Am J Physiol Heart Circ Physiol. 2019 Jun 1;316(6):H1253-H1266. 10.1152/ajpheart.00039.2019 [DOI] [PMC free article] [PubMed]
- Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, Trevino-Villarreal JH, Kang MJ, Hung TT, Lee B, Williams EO, Igarashi M, Mitchell JR, Wu LE, Turner N, Arany Z, Guarente L, Sinclair DA. Impairment of an endothelial NAD(+)-H2S signaling network is a reversible cause of vascular aging. Cell. 2018;173:74–89 e20. doi: 10.1016/j.cell.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Montgolfier O, Pouliot P, Gillis MA, Ferland G, Lesage F, Thorin-Trescases N, Thorin E. Systolic hypertension-induced neurovascular unit disruption magnifies vascular cognitive impairment in middle-age atherosclerotic LDLr(−/−):hApoB(+/+) mice. Geroscience. 2019;41:511–532. doi: 10.1007/s11357-019-00070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, Imai S, Seals DR. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15:522–530. doi: 10.1111/acel.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durinck S, Spellman PT, Birney E, Huber W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protoc. 2009;4:1184–1191. doi: 10.1038/nprot.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon S, Gentleman R. Using GOstats to test gene lists for GO term association. Bioinformatics. 2007;23:257–258. doi: 10.1093/bioinformatics/btl567. [DOI] [PubMed] [Google Scholar]
- Farias Quipildor GE, Mao K, Hu Z, Novaj A, Cui MH, Gulinello M, Branch CA, Gubbi S, Patel K, Moellering DR, Tarantini S, Kiss T, Yabluchanskiy A, Ungvari Z, Sonntag WE, Huffman DM. Central IGF-1 protects against features of cognitive and sensorimotor decline with aging in male mice. Geroscience. 2019;41:185–208. doi: 10.1007/s11357-019-00065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop GA, Ahire C, Csipo T, Tarantini S, Kiss T, Balasubramanian P, Yabluchanskiy A, Farkas E, Toth A, Nyul-Toth A, Toth P, Csiszar A, Ungvari Z. Cerebral venous congestion promotes blood-brain barrier disruption and neuroinflammation, impairing cognitive function in mice. Geroscience. 2019;41:575–589. doi: 10.1007/s11357-019-00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano LB, Donato AJ, Pasha HM, Hearon CM, Jr, Sindler AL, Seals DR. The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am J Physiol Heart Circ Physiol. 2014;307:H1754–H1763. doi: 10.1152/ajpheart.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, E SY, Lamming DW, Pentelute BL, Schuman ER, Stevens LA, Ling AJ, Armour SM, Michan S, Zhao H, Jiang Y, Sweitzer SM, Blum CA, Disch JS, Ng PY, Howitz KT, Rolo AP, Hamuro Y, Moss J, Perni RB, Ellis JL, Vlasuk GP, Sinclair DA. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013;339:1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96:17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–1309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24:464–471. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperio CG, McFalls AJ, Colechio EM, Masser DR, Vrana KE, Grigson PS, Freeman WM. Assessment of individual differences in the rat nucleus accumbens transcriptome following taste-heroin extended access. Brain Res Bull. 2016;123:71–80. doi: 10.1016/j.brainresbull.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: a Beginner’s guide. Neurochem Res. 2015;40:2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18:419–434. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T, Balasubramanian P, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Csipo T, Lipecz A, Reglodi D, Zhang XA, Bari F, Farkas E, Csiszar A, Ungvari Z (2019a) Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for prevention of vascular cognitive impairment. GeroScience. in press [DOI] [PMC free article] [PubMed]
- Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, Gautam T, Csipo T, Nyul-Toth A, Lipecz A, Szabo C, Farkas E, Wren JD, Csiszar A, Ungvari Z (2019b) Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. Geroscience. 2019 Aug;41(4):419–439. 10.1007/s11357-019-00095-x. Accessed 28 Aug 2019 [DOI] [PMC free article] [PubMed]
- Kramer A, Green J, Pollard J, Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30:523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76:845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong LK, Sohal RS. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch Biochem Biophys. 2000;373:16–22. doi: 10.1006/abbi.1999.1495. [DOI] [PubMed] [Google Scholar]
- Libert S, Pointer K, Bell EL, Das A, Cohen DE, Asara JM, Kapur K, Bergmann S, Preisig M, Otowa T, Kendler KS, Chen X, Hettema JM, van den Oord EJ, Rubio JP, Guarente L. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell. 2011;147:1459–1472. doi: 10.1016/j.cell.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Su X, Quinn WJ, 3rd, Hui S, Krukenberg K, Frederick DW, Redpath P, Zhan L, Chellappa K, White E, Migaud M, Mitchison TJ, Baur JA, Rabinowitz JD. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab. 2018;27:1067–1080. doi: 10.1016/j.cmet.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Ramirez MA, Male DK, Wang C, Sharrack B, Wu D, Romero IA. Cytokine-induced changes in the gene expression profile of a human cerebral microvascular endothelial cell-line, hCMEC/D3. Fluids Barriers CNS. 2013;10:27. doi: 10.1186/2045-8118-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One. 2012;7:e42357. doi: 10.1371/journal.pone.0042357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Wang M, Bernier M, Zhang J, Park SS, Maudsley S, An SS, Santhanam L, Martin B, Faulkner S, Morrell C, Baur JA, Peshkin L, Sosnowska D, Csiszar A, Herbert RL, Tilmont EM, Ungvari Z, Pearson KJ, Lakatta EG, de Cabo R. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014;20:183–190. doi: 10.1016/j.cmet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michan S, Li Y, Chou MM, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LE, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30:9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, Redpath P, Migaud ME, Apte RS, Uchida K, Yoshino J, Imai SI. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 2016;24:795–806. doi: 10.1016/j.cmet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin YK, Canto C, Scheibye-Knudsen M, Krawczyk M, Irusta PM, Martin-Montalvo A, Hubbard BP, Zhang Y, Lehrmann E, White AA, Price NL, Swindell WR, Pearson KJ, Becker KG, Bohr VA, Gorospe M, Egan JM, Talan MI, Auwerx J, Westphal CH, Ellis JL, Ungvari Z, Vlasuk GP, Elliott PJ, Sinclair DA, de Cabo R (2011) SRT1720 improves survival and healthspan of obese mice. Sci Rep. 1. 10.1038/srep00070 [DOI] [PMC free article] [PubMed]
- Mitchell SJ, Martin-Montalvo A, Mercken EM, Palacios HH, Ward TM, Abulwerdi G, Minor RK, Vlasuk GP, Ellis JL, Sinclair DA, Dawson J, Allison DB, Zhang Y, Becker KG, Bernier M, de Cabo R. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep. 2014;6:836–843. doi: 10.1016/j.celrep.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Bernier M, Aon MA, Cortassa S, Kim EY, Fang EF, Palacios HH, Ali A, Navas-Enamorado I, Di Francesco A, Kaiser TA, Waltz TB, Zhang N, Ellis JL, Elliott PJ, Frederick DW, Bohr VA, Schmidt MS, Brenner C, Sinclair DA, Sauve AA, Baur JA, de Cabo R. Nicotinamide improves aspects of healthspan, but not lifespan, in mice. Cell Metab. 2018;27:667–676 e4. doi: 10.1016/j.cmet.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Zhao Z, Zlokovic BV. Alzheimer’s disease: a matter of blood-brain barrier dysfunction? J Exp Med. 2017;214:3151–3169. doi: 10.1084/jem.20171406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz N, Carmona JJ, Anderson E, Hart AC, Sinclair DA, Blackwell TK. Dietary restriction involves NAD(+)-dependent mechanisms and a shift toward oxidative metabolism. Aging Cell. 2014;13:1075–1085. doi: 10.1111/acel.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyul-Toth A, Suciu M, Molnar J, Fazakas C, Hasko J, Herman H, Farkas AE, Kaszaki J, Hermenean A, Wilhelm I, Krizbai IA. Differences in the molecular structure of the blood-brain barrier in the cerebral cortex and white matter: an in silico, in vitro, and ex vivo study. Am J Physiol Heart Circ Physiol. 2016;310:H1702–H1714. doi: 10.1152/ajpheart.00774.2015. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Farkas E, Roman V, van der Beek EM, Luiten PG, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Aging Neurosci. 2009;1:4. doi: 10.3389/neuro.24.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Mabley JG, Soriano FG, Liaudet L, Komjati K, Szabo C. Endothelial dysfunction in aging animals: the role of poly(ADP-ribose) polymerase activation. Br J Pharmacol. 2002;135:1347–1350. doi: 10.1038/sj.bjp.0704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare AP, Bell RD, Zlokovic BV (2013) Neurovascular defects and faulty amyloid-beta vascular clearance in Alzheimer's disease. J Alzheimers Dis 33(Suppl 1):S87–S100 [DOI] [PMC free article] [PubMed]
- Solano Fonseca R, Mahesula S, Apple DM, Raghunathan R, Dugan A, Cardona A, O'Connor J, Kokovay E. Neurogenic niche microglia undergo positional remodeling and progressive activation contributing to age-associated reductions in neurogenesis. Stem Cells Dev. 2016;25:542–555. doi: 10.1089/scd.2015.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneson C, Love MI, Robinson MD. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res. 2015;4:1521. doi: 10.12688/f1000research.7563.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Whitehead S, Arai K, Arnold D, Carmichael ST, De Carli C, Duering M, Fornage M, Flores-Obando RE, Graff-Radford J, Hamel E, Hess DC, Ihara M, Jensen MK, Markus HS, Montagne A, Rosenberg G, Shih AY, Smith EE, Thiel A, Tse KH, Wilcock D, Barone F (2019) Proceedings from the Albert Charitable Trust Inaugural Workshop on white matter and cognition in aging. Geroscience. 2019 Dec 6. 10.1007/s11357-019-00141-8 [DOI] [PMC free article] [PubMed]
- Stanimirovic DB, Friedman A. Pathophysiology of the neurovascular unit: disease cause or consequence? J Cereb Blood Flow Metab. 2012;32:1207–1221. doi: 10.1038/jcbfm.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14:133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiol Rev. 2019;99:21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, Harrington MG, Pa J, Law M, Wang DJJ, Jacobs RE, Doubal FN, Ramirez J, Black SE, Nedergaard M, Benveniste H, Dichgans M, Iadecola C, Love S, Bath PM, Markus HS, Salman RA, Allan SM, Quinn TJ, Kalaria RN, Werring DJ, Carare RO, Touyz RM, Williams SCR, Moskowitz MA, Katusic ZS, Lutz SE, Lazarov O, Minshall RD, Rehman J, Davis TP, Wellington CL, Gonzalez HM, Yuan C, Lockhart SN, Hughes TM, CLH C, Sachdev P, O’Brien JT, Skoog I, Pantoni L, Gustafson DR, Biessels GJ, Wallin A, Smith EE, Mok V, Wong A, Passmore P, Barkof F, Muller M, MMB B, Roman GC, Hamel E, Seshadri S, Gottesman RF, van Buchem MA, Arvanitakis Z, Schneider JA, Drewes LR, Hachinski V, Finch CE, Toga AW, Wardlaw JM, Zlokovic BV. Vascular dysfunction-the disregarded partner of Alzheimer’s disease. Alzheimers Dement. 2019;15:158–167. doi: 10.1016/j.jalz.2018.07.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges EL, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–1881. doi: 10.1038/jcbfm.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Tucsek Z, Valcarcel-Ares M, Toth P, Gautam T, Giles C, Ballabh P, Wei Y, Wren J, Ashpole N, Sonntag W, Ungvari Z, Csiszar A. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr) 2016;38:273–289. doi: 10.1007/s11357-016-9931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–58. doi: 10.1016/j.exger.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Yabluchanksiy A, Fulop GA, Hertelendy P, Valcarcel-Ares MN, Kiss T, Bagwell JM, O'Connor D, Farkas E, Sorond F, Csiszar A, Ungvari Z. Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. Geroscience. 2017;39:601–614. doi: 10.1007/s11357-017-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A, Ungvari Z. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell. 2017;16:469–479. doi: 10.1111/acel.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, Ungvari Z (2018) Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018 Apr;17(2). 10.1111/acel.12731 [DOI] [PMC free article] [PubMed]
- Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Sule Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. doi: 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Yabluchanskiy A, Csipo T, Fulop G, Kiss T, Balasubramanian P, DelFavero J, Ahire C, Ungvari A, Nyul-Toth A, Farkas E, Benyo Z, Toth A, Csiszar A, Ungvari Z (2019b) Treatment with the poly(ADP-ribose) polymerase inhibitor PJ-34 improves cerebromicrovascular endothelial function, neurovascular coupling responses and cognitive performance in aged mice, supporting the NAD+ depletion hypothesis of neurovascular aging. Geroscience. 2019 Oct;41(5):533–542. 10.1007/s11357-019-00101-2 [DOI] [PMC free article] [PubMed]
- Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari ZI. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and down-regulation of NADPH oxidas. Am J Physiol Heart Circ Physiol. 2014;306:H299–H308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. 2015;14:400–408. doi: 10.1111/acel.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–H20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am J Physiol. 2007;292:H2417–H2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–H1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Sonntag WE, de Cabo R, Baur JA, Csiszar A (2011) Mitochondrial protection by resveratrol. Exerc Sport Sci Rev. 2011 Jul;39(3):128–32. 10.1097/JES.0b013e3182141f80. [DOI] [PMC free article] [PubMed]
- Ungvari Z, Tucsek Z, Sosnowska D, Toth P, Gautam T, Podlutsky A, Csiszar A, Losonczy G, Valcarcel-Ares MN, Sonntag WE. Aging-induced dysregulation of Dicer1-dependent MicroRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci. 2013;68:877–891. doi: 10.1093/gerona/gls242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Kirkpatrick AC, Csiszar A, Prodan CI. Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am J Physiol Heart Circ Physiol. 2017;312:H1128–H1143. doi: 10.1152/ajpheart.00780.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, Murfee WL, Pacher P, Csiszar A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol. 2018;15:555–565. doi: 10.1038/s41569-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcarcel-Ares MN, Tucsek Z, Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, Gautam T, Galvan V, Ballabh P, Richardson A, Freeman WM, Wren JD, Deak F, Ungvari Z, Csiszar A (2018) Obesity in aging exacerbates neuroinflammation, dysregulating synaptic function-related genes and altering eicosanoid synthesis in the mouse hippocampus: potential role in impaired synaptic plasticity and cognitive decline. J Gerontol A Biol Sci Med Sci. 2019 Feb 15;74(3):290–298. 10.1093/gerona/gly127. [DOI] [PMC free article] [PubMed]
- Warrington JP, Ashpole N, Csiszar A, Lee YW, Ungvari Z, Sonntag WE. Whole brain radiation-induced vascular cognitive impairment: mechanisms and implications. J Vasc Res. 2013;50:445–457. doi: 10.1159/000354227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CC, Kong YY, Hua X, Li GQ, Zheng SL, Cheng MH, Wang P, Miao CY. NAD replenishment with nicotinamide mononucleotide protects blood-brain barrier integrity and attenuates delayed tissue plasminogen activator-induced haemorrhagic transformation after cerebral ischaemia. Br J Pharmacol. 2017;174:3823–3836. doi: 10.1111/bph.13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenhoeft T, Tarantini S, Nyúl-Tóth A, Yabluchanskiy A, Csipo T, Balasubramanian P, Lipecz A, Kiss T, Csiszar A, Csiszar A, Ungvari Z (2019) Fusogenic liposomes effectively deliver resveratrol to the cerebral microcirculation and improve endothelium-dependent neurovascular coupling responses in aged mice. Geroscience. 2019 Dec;41(6):711–725. 10.1007/s11357-019-00102-1 [DOI] [PMC free article] [PubMed]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Wren JD, Garner HR. Shared relationship analysis: ranking set cohesion and commonalities within a literature-derived relationship network. Bioinformatics. 2004;20:191–198. doi: 10.1093/bioinformatics/btg390. [DOI] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Baur JA, Imai SI. NAD(+) intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab. 2018;27:513–528. doi: 10.1016/j.cmet.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarzuelo MJ, Lopez-Sepulveda R, Sanchez M, Romero M, Gomez-Guzman M, Ungvary Z, Perez-Vizcaino F, Jimenez R, Duarte J. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem Pharmacol. 2013;85:1288–1296. doi: 10.1016/j.bcp.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNF{alpha} and vascular oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D'Amico D, Ropelle ER, Lutolf MP, Aebersold R, Schoonjans K, Menzies KJ, Auwerx J. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurodegeneration and the neurovascular unit. Nat Med. 2010;16:1370–1371. doi: 10.1038/nm1210-1370. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]