Abstract
Binge eating (BE) is a heritable symptom of eating disorders with an unknown genetic etiology. Rodent models for binge-like eating (BLE) of palatable food permit the study of genetic and biological mechanisms. We previously genetically mapped a coding mutation in Cyfip2 associated with increased BLE of sweetened palatable food in the C57BL/6NJ versus C57BL/6J substrain. The increase in BLE in C57BL/6NJ mice was associated with a decrease in transcription of genes enriched for myelination in the striatum. Here, we tested the hypothesis that decreasing myelin levels with the demyelinating agent cuprizone would enhance BLE. Mice were treated with a 0.3% cuprizone home cage diet for two weeks. Cuprizone induced similar weight loss in both substrains and sexes that recovered within 48 h after removal of cuprizone. Following a three-week recovery period, mice were trained for BLE in an intermittent, limited access procedure. Surprisingly, cuprizone significantly reduced BLE in male but not female C57BL/6NJ mice while having no effect in C57BL/6J mice. Cuprizone also reduced myelin basic protein (MBP) at seven weeks post-cuprizone removal while having no effect on myelin-associated glycoprotein at this time point. C57BL/6NJ mice also showed less MBP than C57BL/6J mice. There were no statistical interactions of Treatment with Sex on MBP levels, indicating that differences in MBP reduction are unlikely to account for sex differences in BLE. To summarize, cuprizone induced an unexpected, significant reduction in BLE in B6NJ males which could indicate genotype-dependent sex differences in the biological mechanisms of BLE.
Keywords: binge eating disorder, demyelination, sex differences, reduced complexity cross, QTL, sex as a biological variable
INTRODUCTION
Binge eating is operationally defined as the consumption of a relatively large amount of food over a short period of time and feelings of loss of control (Amianto et al., 2015). Repeated binge eating that occurs at least once per week for at least three months is termed binge eating disorder (BED). Binge eating has a lifetime prevalence of approximately 4.5% (Hudson et al., 2007) and is comorbid with several conditions, including substance abuse, depression, obesity, and chronic pain (Bulik and Reichborn-Kjennerud, 2003; Hudson et al., 2007; Citrome, 2017). Although there is no sex difference in the lifetime prevalence of binge eating, women are nearly twice as likely as men to progress to more severe BED (3.5% vs. 2.0% respectively (Hudson et al., 2007). However, the etiology of this sex difference is not known.
Numerous neurophysiological and neuroanatomical changes have been identified in eating disorders (ED), including BED, bulimia nervosa (BN) and anorexia nervosa (AN), including alterations in both dopaminergic and serotonergic signaling in BN and AN (Tauscher et al., 2001; Frank et al., 2005; Kaye, 2008), reduced cerebral glucose metabolism in the anterior cingulate (Naruo et al., 2001; Kojima et al., 2005), and enhanced cerebral perfusion in the thalamus and amygdala-hippocampus complex in AN, and greater frontal cortical response to food stimuli in BED (Geliebter et al., 2006). Another important neuroanatomical change common to multiple ED involves changes in myelin which is the lipid-rich, membranous insulation sheath that extends from oligodendrocytes to the axons and is involved in axon maintenance (via oligodendrocyte signaling) and function, including rapid saltatory conduction of action potentials. BN is associated with a reduction in fractional anisotropy, a brain imaging measure of white matter integrity, in the corona radiata and corpus callosum (Mettler et al., 2013) as well as in forceps major and minor, inferior fronto-occipital, superior longitudinal, and uncinate fasciculi, corticospinal tract, and cingulum (He et al., 2016). In women with AN, a reduced fractional anisotropy relative to controls was reported in the left superior longitudinal fasciculus (Via et al., 2014). Importantly, previous results found no differences in white matter integrity between healthy controls and women who have recovered from AN (Yau et al., 2013), indicating that decreased myelination is a reversible consequence rather than a pre-existing cause of subsequent aberrant eating behavior in ED. Additionally, both women and men with obesity show a decrease in myelination which, in women, correlated with BMI and leptin levels (Mueller et al., 2011). In summary, demyelination is a consequence of ED that, once established, could help to sustain maladaptive eating behaviors.
Using transcriptome analysis via mRNA sequencing (RNA-seq), we previously showed that binge-like eating (BLE) of sweetened palatable food (PF) relative to chow controls induced a downregulation of several genes in the striatum (other brain regions were not tested) that were enriched for myelination, axon ensheathment, and oligodendrocyte formation and differentiation, providing evidence that, similar to BN and AN, decreased myelination is a consequence of BLE in our preclinical model (Kirkpatrick et al., 2017). Because transcriptome analysis is conducted at the mRNA level, it is not known whether the BLE-induced decrease in myelin-associated transcripts translates to a decrease in myelin proteins. Furthermore, whether decreasing myelin levels could induce or enhance BLE has not been tested. Finally, potential sex differences in BLE-associated changes in myelin proteins have not been addressed.
In the present study, we tested the hypothesis that administration of the demyelinating agent cuprizone (cyclohexylidene hydrazide) in C57BL/6 mice (Hiremath et al., 1998) would mimic the enhanced BLE previously observed in B6NJ mice that showed a downregulation of genes involved in myelination BLE training. Specifically, we predicted that cuprizone would induce BLE in the BLE-resistant C57BL/6J (B6J) substrain of both sexes and would further enhance BLE in the BLE-prone C57BL/6NJ (B6NJ) substrain of both sexes (Kirkpatrick et al., 2017). When referring to “prone” and “resistant”, it is important to note that we are referring to the behaviors of these substrains in our specific BLE paradigm comprising intermittent, limited access of sweetened chow that is provided in a conditioned place preference paradigm (Goldberg et al., 2017; Kirkpatrick et al., 2017; Babbs et al., 2018, 2019) and acknowledge that under different diets (for example, high fat diet), different regimens (for example, extended access or operant access), or different environmental history (for example, stress or food restriction), the behaviors of the substrains could be different. Our hypothesis that cuprizone would increase BLE in both sexes is based on our previous observation of enhanced BLE in both female and male B6NJ mice versus B6J mice that was accompanied by a downregulation of striatal genes that are involved in myelination. To summarize, we predicted that the demyelinating agent cuprizone would be sufficient to induce BLE in female and male B6J mice and would further enhance BLE in female and male B6NJ mice.
Laboratory chow containing 0.3% cuprizone induces a significant decrease in myelination of the corpus callosum at 4 weeks and complete demyelination after six weeks (Hiremath et al., 1998) and two-week exposure of 0.2% cuprizone has been shown to be sufficient to induce nearly complete demyelination in mice (Doan et al., 2013). At the completion of the study, we harvested brain tissue and quantified two myelin proteins that are downregulated in the mouse brain following cuprizone treatment, including myelin-associated glycoprotein (MAG) and myelin basic protein (MBP) (Ludwin and Sternberger, 1984).
METHODS
Mice
All experiments were conducted in accordance with the NIH Guidelines for the Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Boston University (AN-15403). Mice were maintained on a 12 h/12 h light/dark cycle (lights on at 0630 h) and housed four animals per cage in same-sex cages. Laboratory chow (Teklad 18% Protein Diet, Envigo, Indianapolis, IN, USA) and tap water were available ad libitum in home cages prior to the experiment. Testing was conducted in the a.m. of the light phase (0800 h to 1200 h) on a 12 h light/dark cycle (lights on at 0630 h). 32 C57BL/6J (B6J) and 32 C57BL/6NJ (B6NJ) mice (7 weeks old) were purchased from the Jackson Laboratory (JAX; Bar Harbor, ME). Mice were habituated to the vivarium for one week prior to cuprizone treatment in a room separate from the testing room. Mice were 56 days old at the beginning of cuprizone treatment and 91 days old on the first day of BE training.
Power Analysis
Because we do not have any prior datasets examining the effect of cuprizone on PF intake and because we hypothesized that cuprizone would mimic the effect of the binge-prone B6NJ substrain to increase PF intake, we used a historical dataset comprising total PF intake from Day 2 to Day 18 from 75 B6J × B6NJ-F2 mice. Each of the F2 mice used in the power analysis was specifically selected to be homozygous for either the B6NJ allele (n=40; 19 females and 21 males) or the B6J allele (n=35; 18 females and 17 males) at the Cyfip2 locus – a major genetic locus explaining a large portion of the variance in PF intake between the parental strains (Kirkpatrick et al., 2017). We chose to use this historical F2 dataset rather than the dataset comprising the parental strains (n=15, sex-combined per substrain) because it contained more than double the sample size and thus, could provide a more accurate estimate of the effect size and thus, the required sample size to achieve 80% power (p<0.05).
We used the freely available G*Power 3 program (http://www.gpower.hhu.de/en.html) to conduct power analyses. A complete description of the statistical procedures used in G*Power is published (Faul et al., 2007). We selected the option, “a priori: compute required sample size – given alpha, power, and effect size”, to calculate the required sample size to achieve 80% power (p < 0.05; two-tailed test). In considering total PF intake from D2-D18, the effect size was Cohen’s d = 1.15. Using G*Power and with an input of 80% power and p < 0.05, a sample size of n=13 per genotype was required. In this study, we employed a sample size of n=16 per Genotype (Substrain) per Treatment which was predicted to provide 88% power based on the assumed effect size of Cohen’s d = 1.15.
Cuprizone diet
Sixty-four mice were assigned to a treatment group by cage (four mice per cage) in a factorial design with n = 15–16 (8 females, 7 or 8 males) per Substrain (B6J, B6NJ) per Treatment (0.3% Cuprizone-treated Chow, Untreated Chow). A single cuprizone-assigned male B6J mouse died early on during the study. Therefore, the results are presented for 63 mice instead of 64 mice. Untreated, control Chow mice continued to have ad libitum access to water and standard laboratory chow described above throughout the entire study. On D1 of the study, the remaining 32 mice had the standard chow replaced with a chow that was structurally and nutritionally identical, but also contained 0.3% cuprizone (Envigo, Indianapolis, IN, USA), a copper chelating agent that induces demyelination in C57BL/6 mice by causing mitochondrial and endoplasmic reticulum stress and apoptosis in mature oligodendrocytes that in turn generates myelin debris and activation of astrocytes and microglia (Hiremath et al., 1998; Gudi et al., 2014; Sen et al., 2019). Both the cuprizone-treated and control food were refrigerated and provided fresh every other day from a single food lot (Envigo) on Monday, Wednesday, and Friday for two weeks.
Mice were maintained on this diet for two weeks, and on D14 the cuprizone diet was replaced with standard laboratory chow as described above. Mice were allowed to recover for 21 days before binge-like eating (BLE) training commenced. A nearly identical abbreviated, two-week cuprizone treatment protocol (0.2% rather than 0.3% cuprizone) was previously shown to be sufficient to induce robust demyelination in the corpus callosum at three weeks post-cuprizone treatment C57BL/6 (substrain not specified) mice (Doan et al., 2013). Specifically, the two-week 0.2% cuprizone regimen resulted in hallmark effects, including a marked depletion of mature oligodendrocytes and a significant accumulation of microglia and astrogliosis in the corpus callosum that peaked at the fifth week (3 weeks post-cuprizone termination) (Doan et al., 2013). The five week time point after 2 or 3 weeks of cuprizone diet is considered peak demyelination, after which remyelination begins and the levels of microglia and astrocytes decrease (Doan et al., 2013).
BLE procedure
Mice were trained in an intermittent, limited access BLE procedure as described (Kirkpatrick et al., 2017; Babbs et al., 2018, 2019). BLE was operationally defined in this study as a significant, positive slope in the escalation of PF intake over time (Babbs et al., 2012). We used a two-chambered conditioned place preference (CPP) apparatus, with differently textured floors in each chamber. The right and left sides were designated the food-paired and no-food-paired sides, respectively. Mice were trained and video recorded in unlit sound-attenuating chambers (MedAssociates, St. Albans, VT USA). On Day 1, initial side preference was determined by placing each mouse on the left, no-food-paired side with the divider containing an open entryway that provided free access to the both sides for 30 min. Clean, empty food bowls were placed in the far corners of each side. On Days 2, 4, 9, 11, 16, and 18, mice were confined to the food-paired side with a closed divider that prevented access to the no-food-paired side. Mice were provided forty, 20 mg sweetened palatable food pellets (PF; TestDiet 5-TUL, St. Louis, MO, USA) in a non-porous porcelain dish in the far corner of the chamber. On Days 3, 5, 10, 12, 17, and 19, mice were confined to the no-food-paired side with no access to the FP side. A clean, empty, and non-porous porcelain dish was placed in the far corner of the chamber during this time. On Day 22, side preference was again assessed with open access to both sides. No food was present in either bowl at this time. The experimenter was blinded to Genotype throughout data collection, video tracking, and analysis. Video-recorded data were tracked using AnyMaze (Stoelting Co., Wood Dale, IL USA). Locomotor activity was concomitantly video-recorded (Swann Communications USA Inc., Santa Fe Springs, CA) during the CPP (open entryway) and PF training sessions (closed entryway) and distance traveled (m) was calculated using AnyMaze video tracking analysis.
Light/dark conflict test of compulsive-like eating
Following BE training and assessment of PF-CPP, on Day 23, we subsequently assessed compulsive-like eating and concomitant behaviors in the anxiety-provoking light/dark conflict test (Kirkpatrick et al., 2017; Babbs et al., 2018, 2019) in which rodents will normally avoid the aversive, light side. The light/dark box consists of a dark side, which is an enclosed black, opaque Plexiglas chamber, and a light side with clear Plexiglas. An open doorway allowed free access to both sides. The light source originated from the ceiling lights in the procedure room. The lux values for the eight light sides were 154.8, 160.3, 141.3, 122.6, 136, 140.7, 143.6, and 137.5. The lux values for the eight dark sides of the same boxes were 0.5, 0.5, 0.5, 0.4, 0.5, 0.6, 0.4, and 0.5. A non-porous ceramic bowl containing forty, 20 mg PF pellets was placed in the center of the light side. Mice were initially placed on the light side facing both the food and the doorway and were video recorded for 30 min. Because the light side is aversive, increased behaviors in this environment were operationalized as compulsive-like, including compulsive-like eating.
On D24, brains were extracted and punches of the left and right striatum were harvested and combined as previously described (Yazdani et al., 2015; Kirkpatrick et al., 2017), flash frozen on acetone and dry ice, and stored at −80°C until western blotting procedures commenced. We focused on the striatum to examine myelin proteins because this is the region where we previously identified a set of downregulated genes involved in myelination following BLE training (Kirkpatrick et al., 2017). Cuprizone treatment has been shown to induce multiple signs of demyelination in the striatum, including the hallmark cuprizone effects of a decrease in MBP and other myelin proteins as well as an increase in astrogliosis (via GFAP) and microgliosis (via IBA1) in female C57BL/6N mice (Beckmann et al., 2018; Mandolesi et al., 2019). We recognize that there are multiple other brain regions that show varying degrees and rates of demyelination and remyelination that could be relevant to BLE (Skripuletz et al., 2011). Bilateral punches of the dorsal, medial, and lateral striatum (2.5 mm diameter, 2 mm thick) were collected on D24 – 24 h after assessment of compulsive-like PF intake in the light/dark box. Brains were quickly dissected, placed into the mouse brain matrix, and razors were placed into the third and fifth slots, thus yielding sections that were 2 mm thick. The sections were transferred to an ice cold petri dish. The anterior commissure served as the dorsal landmark and the cortex served as the lateral and ventral landmarks for the punches. The sections were laid out flat on the petri dish and punches were collected at a right angle relative to the dish surface. Left and right punches were pooled, transferred to 1.5 ml Eppendorf tubes, and flash frozen on dry ice.
Immunoblotting for striatal MAG and MBP
Striatal tissue was homogenized in RIPA buffer (Thermo Scientific, Waltham, MA USA, #89901) containing 1x HALT protease/phosphatase inhibitor cocktail (Thermo Scientific, #1861284) with an ultrasonic homogenizer and then spun at 17200 RCF for 20 min at 4°C to collect supernatant. Protein concentration was quantified by BCA protein estimation (Thermo Scientific, #23225). 30 μg of sample protein and loading buffer (BioRad, Hercules, CA #161–0747) was loaded into 4–15% Criterion TGX gels (BioRad, #5671085) and run at 200 V for 50 min. Gels were transferred onto nitrocellulose membranes (General Electric, Boston, MA, USA, #10600002) for 1 h at 90 V in Towbins buffer containing 20% methanol. Blots were then blocked with 5% BSA in TBST (0.5% Tween 20 in tris-buffered saline) for 1 h.
For MAG detection, blots were incubated in a 1: 5,000 dilution of anti-MAG antibody (EMD Millipore, Burlington, MA, USA, #MAB1567) in 5% BSA in TBST overnight at 4°C, then a 1: 10,000 dilution of peroxidase conjugated donkey anti mouse antibody (Jackson Immunoresearch, West Grove, PA, USA, #715-035-151). For MBP detection, blots were incubated in a 1: 10,000 dilution of anti-MBP antibody (EDM Millipore, #MAB 386) in 5% BSA in TBST overnight at 4°C, then a 1: 10,000 dilution of peroxidase conjugated donkey anti rat antibody (Jackson Immunoresearch #712-035-153) for one hour. After probing, all blots were imaged using Clarity ECL (BioRad, #170–5061) and a c300 imager (Dublin, CA, USA).
Blots were then stripped (Thermo Scientific, 46430) at 55°C for 30 min, reblocked with 5% BSA in TBST, incubated in 1:50,000 Beta-actin antibody (Sigma Aldrich, St, Louis, MO, USA, #A2228) in 5% BSA in TBST for 1 h, and then a 1:10,000 dilution of peroxidase conjugated donkey anti mouse antibody for 1 h. Blots were then reimaged for beta actin. Densitometry was conducted using ImageJ, and each lane was normalized to its respective densitometry value for beta actin. ImageJ is imaging analysis software that is provided by the National Institutes of Health (https://imagej.nih.gov/ij/). Each lane was then normalized to the average wild-type value of that particular blot and then finally, data were combined across blots and statistical analysis was run in R as described below.
Statistical analysis
Data were analyzed in R (https://www.r-project.org/). We tested for normality of the distribution of the residuals for each phenotype that were derived from a three-way ANOVA model (Substrain, Treatment, and Sex as factors) using the Kolmogorov-Smirnov test. Small but statistically significant deviations from normality (p < 0.05) were detected for a subset of the BLE training days for PF intake (D2, D9, and D18; p = 0.028, 0.01, 0.02). Visual inspection of the histograms for the residuals of the 63 mice included in the three-way ANOVA model as well as the quantile-quantile (q-q) plots of the observed sample quantiles versus the theoretical quantiles confirmed that there were no major deviations from normality, especially given the sample size (see Fig.S1). Thus, to retain all of the data within the same scale, we conducted parametric analysis on the existing datasets rather than conducting a normalization procedure across all BLE training days.
Data were analyzed using mixed-effects ANOVAs with Genotype, Sex, and Treatment as independent variables, and Day as a repeated measure. We then analyzed females and males separately using mixed-effect ANOVAs with Genotype and Treatment as independent variables, and Day as a repeated measure. Bonferroni-adjusted post hoc pairwise unpaired t-tests were used to determine whether group differences were significant on individual days. The number of corrections and adjusted p-values are indicated when reporting the statistical results. Slope analyses were conducted as previously described (Babbs et al., 2012, 2019) using regression analysis in GraphPad Prism 7 (GraphPad Software, La Jolla, CA USA) and are presented with regression lines for PF intake during BLE across training days for each group.
RESULTS
The complete set of statistical results are provided in the figure legends.
Cuprizone treatment induces significant weight loss in female and male B6J and B6NJ mice
We employed a regimen consisting of one week of habituation to the colony, two weeks of the cuprizone diet, three weeks of recovery from cuprizone, and three weeks of training for BLE of PF (Fig.1). A single cuprizone-assigned male B6J mouse died early on during the study. Therefore, the results are presented for 63 mice instead of 64 mice.
Figure 1. Study protocol and timeline.

This schematic illustrates the colony habituation week, the cuprizone treatment weeks, the recovery weeks, and the BLE training weeks. Mice were habituated for one week after their arrival. Mice then received either ad libitum standard laboratory chow or ad libitum cuprizone-treated lab diet for two weeks. After removal of cuprizone treatment, all mice were given ad libitum standard laboratory chow for three weeks. Finally, in weeks 7–10, training for binge-like eating (BLE) commenced with continued ad libitum access to normal chow minus the 30 min training sessions with palatable food (PF). Mice were tested for side preference in the CPP chamber before and after training (D1 and D22) for BE of PF. On D2, 4, 9, 11, 16, and 18, mice were confined to the right side of the chamber with access to PF. On D3, 5, 10, 12, 17, and 19, mice were confined to the left side of the chamber with an empty food bowl. On D23, the light/dark conflict test was conducted for compulsive-like PF consumption. On D24, brains were harvested, flash frozen, and stored at −80°C until further processing for immunoblot analysis.
In examining cuprizone-induced weight loss, mixed-effects ANOVA with Substrain, Sex, and Treatment as factors and Day as a repeated measure indicated a main effect of Sex (p < 2 × 10−16) and multiple interactions with Sex, including a Sex × Substrain (p = 0.01), Sex × Day (p = 3.4 × 10−5), and a Sex × Treatment × Day interaction (p = 3.9 × 10−15). These interactions provided a rationale to analyze the sexes separately.
For females, cuprizone-treated females from both substrains showed a significant weight loss from D4-D14 (*p < αadjusted = 0.05/15 = 0.0031; Fig.2A). For males, cuprizone-treated B6J males from both substrains weighed less than control B6J males on D8-D14 (*p < αadjusted = 0.0031; Fig.2B). Both sexes showed recovery of weight loss within 24 following removal of the cuprizone diet and replacement with normal home cage chow (Fig.2A,B).
Figure 2. Changes in body weight over the course of cuprizone treatment and the week following termination of cuprizone treatment.
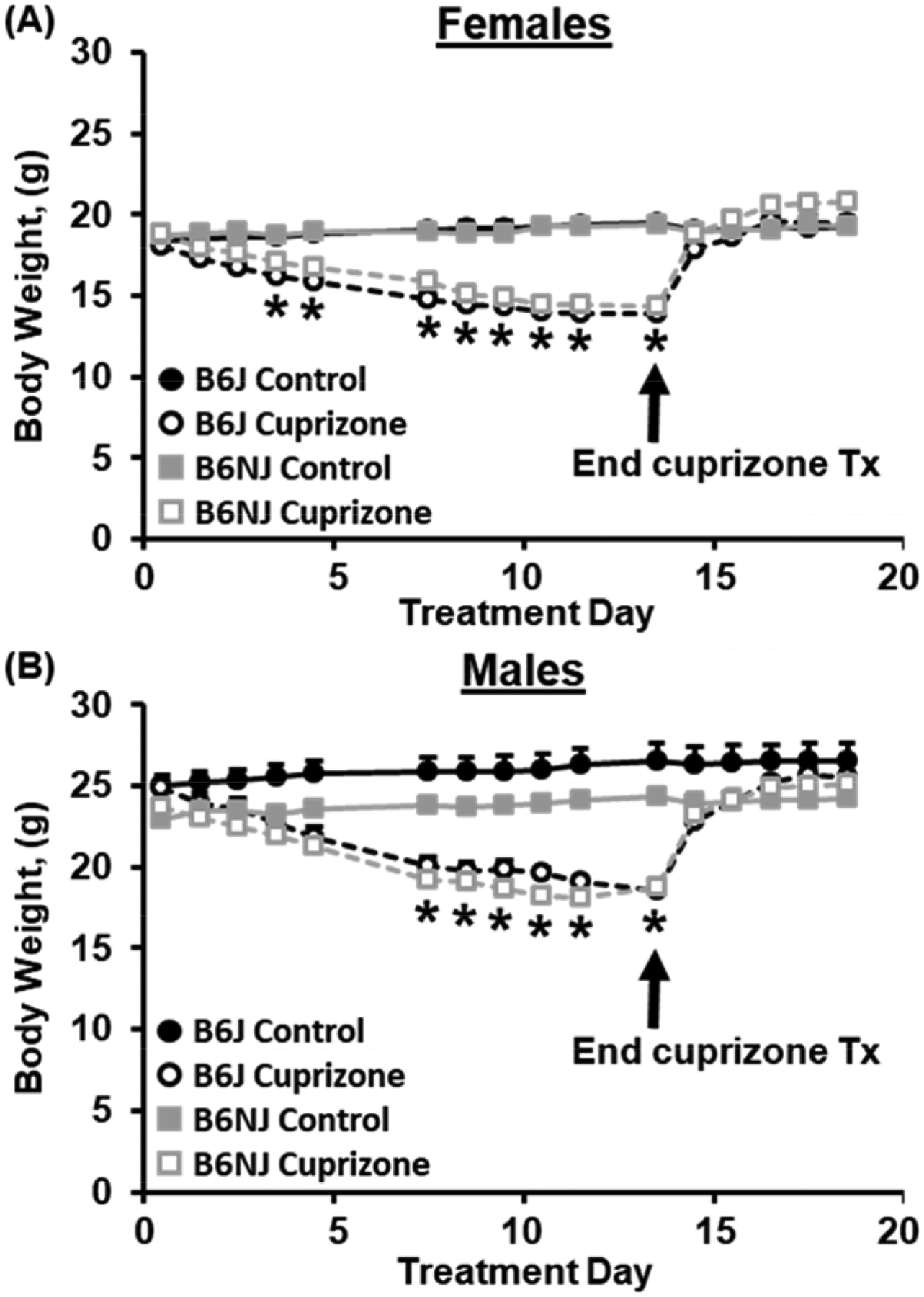
Both substrains and sexes demonstrated significant cuprizone-induced weight loss and rapid recovery of weight loss following replacement of the cuprizone diet with the normal chow diet. Mixed-effects ANOVA with Substrain, Sex, and Treatment as factors and Day as a repeated measure indicated an effect of −9, Treatment, Sex, and a Substrain × Sex interaction [F (1,55) = 54.04, 245.41, 7.07; p = 1.0 × 10< 2 × 10–16, 0.01]. There was also a main effect of Day as well as a Substrain × Day, Treatment × Day, Sex × Day, Substrain × Treatment × Day, and Treatment × Sex × Day interaction [F (15,825) = 342.62, 2.02, 365.81, 3.21, 2.73, 7.24; p < 2 × 10−16, 0.012, <2 × 10−16, 3.4 × 10−5, × 10−4, 3.9 × 10−15]. (A): In females, there was an effect of Treatment [F (1,28) = 47.21; p = 4.2 1.8 × 10−7], Day [F (15,420) = 226.0; p < 2 × 10−16], a nonsignificant Substrain × Day interaction 23 [F(15,420) = 1.68; p = 0.052], and a Treatment × Day interaction [F(15,420) = 239.03; p < 2 × 10−16]. Cuprizone-treated B6J females weighed less than control B6J females on D4-D14 [t (14) = 5.34–12.42, *p < αadjusted = 0.0031]. Similarly, cuprizone-treated B6NJ females also weighed less than control B6NJ females on D4-D14 [t (14) = 4.46–11.02; *p < αadjusted = 0.0031]. (B): In males, there was a main effect of Substrain [F (1,488) = 48.5; p < 1.0 × 10−11], Treatment [F(1,488) = 170.3; p < 2 × 10−16], and Day [F(1,488) = 88.0; p = 0.0002] and a Substrain × Treatment interaction [F(1,488) = 12.4; p = 0.0005]. Cuprizone-treated B6J males weighed less than control B6J males on D8-D14 [t (13) = 5.29–7.1; *p < αadjusted = 0.0031]. Similarly, cuprizone-treated B6NJ males also weighed less than control B6NJ males on D8-D14 [t (14)= 6.55–8.59; *p < αadjusted = 0.0031].
Changes in body weight over the course of BLE training and testing for CLE in the light/dark conflict test
In examining changes in body weight during BLE training and CLE assessment, mixed-effects ANOVA identified several statistical interactions, including Sex × Substrain (p = 0.0013), Substrain × Day (p = 0.00012), Sex × Treatment × Day (p = 2.4 × 10−8), and Sex × Genotype × Treatment × Day interactions (p = 9.3 × 10−8; Fig.3A–D). Notably, there was a more rapid increase in body weight in cuprizone-treated B6NJ males compared to control B6NJ males (Fig.3D) in that they gained a significant increase on D16 through D23 relative to their own body weitght on D1 at the start of BLE training (p < αadjusted = 0.05/14 = 0.0036; Fig.3D) whereas control B6NJ males did not gain significant body weight on any day relative to D1 (p > αadjusted 0.0036; Fig.3D). The increase rate of body weight gain was associated with with less concomitant BLE (see results below in Fig.4)
Figure 3. Changes in body weight over the course of BLE training and testing for CLE in the light/dark conflict test.
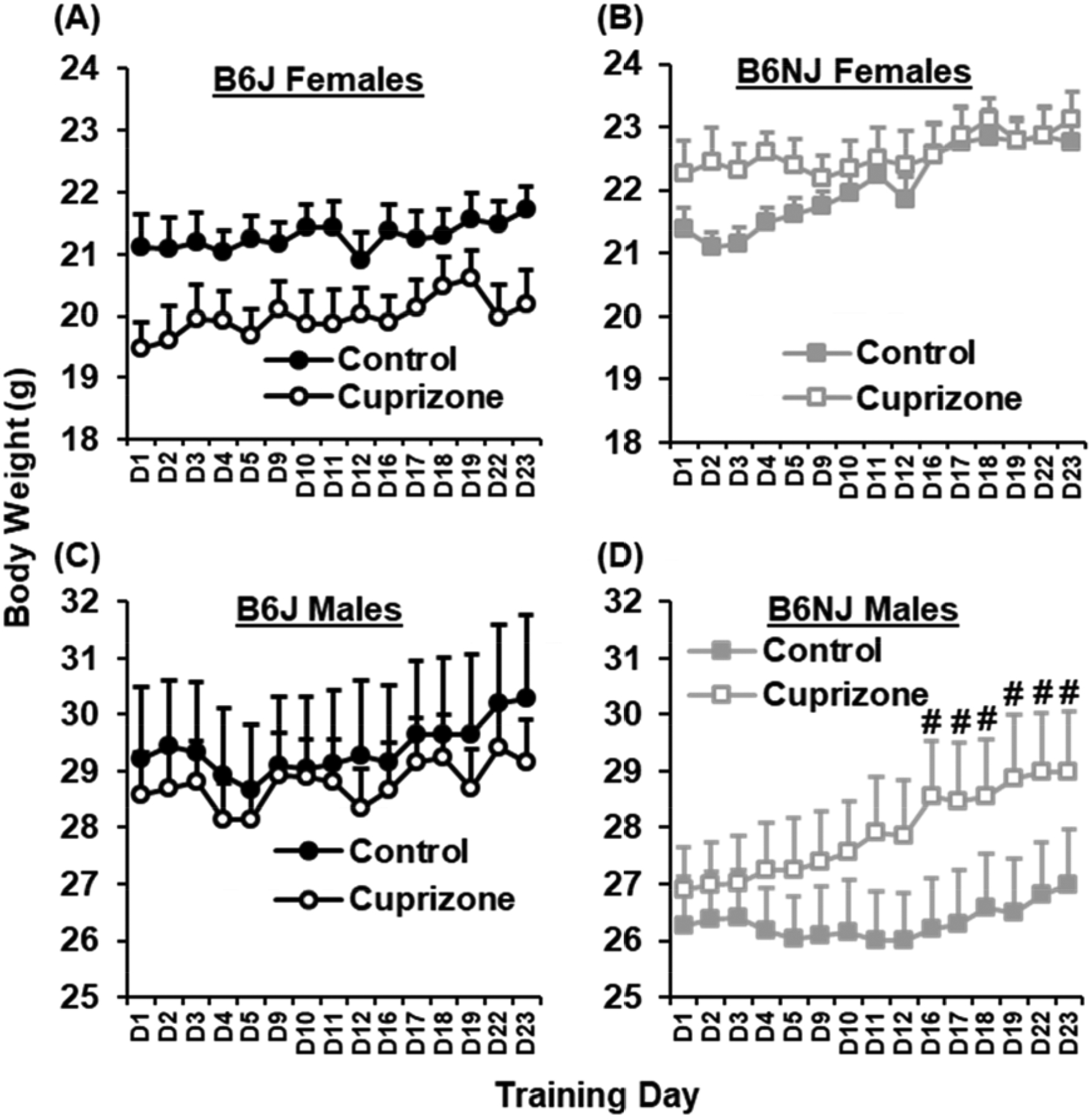
We observed a greater body weight gain during BLE training in cuprizone-treated B6NJ males that was associated with less concomitant BLE (see Fig.4). Specifically, in examining changes in body weight over BLE training days and up to the test for CLE in the light/dark test, there was a main effect of Sex [F (1,55) = 153.24; p < 2 × 10−16], a nonsignificant Substrain × Treatment interaction [F (1,55) = 3.94; p = 0.052], and a Substrain × Sex interaction [F(1,55) = 11.49; p = 0.0013]. There was also an effect of Day as well as a Substrain × Day, Treatment × Sex × Day, and Genotype × Treatment × Sex × Day interaction [F (14,770) = 28.81, 3.06, 4.72, 4.47; p < 2.0 × 10−16, 0.00012, 2.4 × 10−8, 9.3 × 10−8]. These interactions provided a rationale for analyzing changes in body weight and PF intake during BLE (Fig.4) separately in females and males. (A,B): For females, there was a main effect of Substrain [F(1,28) = 16.33; p = 0.00038] and a Substrain × Treatment interaction [F(1,28) = 5.37; p = 0.028]. There was also an effect of Day, Treatment × Day, and a nonsignificant Substrain × Treatment × Day interaction [F (14,392) = 8.53, 2.12, 1.59; p = 3.7 × 10−16, 0.01, 0.078]. However, none of the unpaired pairwise group comparisons were statistically significant when correcting for the comparisons across 15 days (p > αadjusted 0.0033). (C,D): For males, there was a nonsignificant effect of Substrain [F(1,27) = 3.98; p = 0.056], Day [F(14,378) = 35.75; p < 2 × 10−16], and a Substrain × Day, Treatment × Day, and Substrain × Treatment × Day interaction [F(14,378) = 3.98, 5.70, 6.96; p = 2.05 × 10−6, 4.4 × 10−10, 8.9 × 10−13]. Although there were no pairwise group differences across days for either substrain (p > αadjusted 0.05/15 comparisons = 0.0033), paired t-test indicated that cuprizone-treated B6NJ males gained significant body weight compared to D1 that started on D16 and continued through D23 [t (7) = 6.04, 4.46, 5.49, 5.09, 6.64, 6.19; #p < αadjusted = 0.05/14 comparisons = 0.0036]. In contrast, control B6NJ males did not show a significant increase in body weight relative to D1 (p > αadjusted = 0.0036).
Figure 4. Prior cuprizone treatment reduces BLE in male but not female B6NJ mice.

In examining the effect of cuprizone on BLE in both substrains and both sexes, we identified a cuprizone-induced reduction in BLE that was significant only in male mice from the B6NJ substrain. Mixed-effects ANOVA that included Sex, Substrain, and Treatment as factors and Day as a repeated measure identified a main effect of Substrain and Treatment [F (1,55) = 50.16, 4.56; p = 2.8 × 10−9, 0.04], a nonsignificant effect of Sex [F(1,55) = 2.84; p = 0.098], and a Substrain × Treatment interaction [F(1,55) = 4.78; p = 0.033]. Additionally, there was an effect of Day [F (5,275) = 13.53; p = 8.2 × 10−12], a Substrain × Day, Sex × Day, and Substrain × Treatment × Day interaction [F (5,275) = 4.83, 2.89, 3.50; p = 3.0 × 10−4, 0.015, 4.4 × 10−4]. Because of these interactions and the interactions identified in Figure 3, we analyzed the effect of cuprizone on BLE separately in females and males. (A): For females, there was a main effect of Substrain due to greater overall PF intake in B6NJ versus B6J females [F (1,28) = 14.63; p = 0.00067], but no effect of Treatment [F (1,28) = 0.081; p = 0.78] and no Substrain × Treatment interaction [F (1,28) = 0.51; p =0.48]. There was also an effect of Day and Substrain × Day interaction [F (5,140) = 8.93, 2.5; p = 2.2 × 10−7, 0.033]. Importantly, the lack of Treatment effect or interaction with Substrain was confirmed by a lack of significant pairwise difference in PF intake between cuprizone-treated B6NJ females and control B6NJ females for any of the six training days [t (14) < 1; ps ≥ 0.35]. (B): In examining the slope of escalation of PF intake in females using the same data from panel A, both the B6NJ control females and the cuprizone-treated B6J females showed a significant non-zero escalation [F (1,46) = 16.18, *p < 0.001; F(1,46) = 8.59, %p < 0.01, respectively] and cuprizone-treated B6J females showed a significantly greater slope than control B6J females [F(1,92) = 4.14; #p = 0.045; regression lines for the slopes are included]. (C): In examining compulsive-like eating in the light/dark test in females, there was a main effect of Substrain [F (1,28) = 11.65; #p = 0.002] that was explained by greater overall PF intake in B6NJ females versus B6J females. (D): For PF intake during BLE training in males, there was a main effect of Substrain, Treatment, and a Substrain × Treatment interaction [F (1,27) = 66.54, 17.83, 11.10; p = 9.2 × 10−9, 2.5 × 10−4, 0.0025]. There was also an effect of Day and Substrain × Day, Treatment × Day, and a Genotype × Treatment × Day interactions [F (5,135) = 5.85, 4.29, 4.49, 2.99; p = 6.4 × 10−5, 0.0012, 8.06 × 10−4, 0.014]. Cuprizone-treated B6NJ males showed less PF intake than control B6NJ males on D16 and D18 [t(14) = 4.68, 6.31; *p < αadjusted = 0.0083]. (E): In examining escalation of PF intake in males using the same data from panel D, only B6NJ control males showed a significant, non-zero escalation in PF consumption [F (1,46) = 22.83; $p < 0.0001] which was significantly greater than cuprizone-treated B6NJ males [F (1,92) = 10.23; #p = 0.0019; regression lines for the slopes are included]. (F): For compulsive-like PF intake in the light/dark test in males, there was a main effect of Substrain [F (1,27) = 35.81; #p = 2.2 × 10−6 (B6NJ males > B6J males)], Treatment, and Substrain × Treatment interaction [F (1,27) = 8.31, 7.55; p = 0.0077, 0.011]. Cuprizone-treated B6NJ males showing reduced compulsive-like PF intake compared to control B6NJ males [t (14) = 3.18; *p = 0.0067].
Prior cuprizone treatment reduces BLE in male but not female B6NJ mice
Consistent with our previous report (Kirkpatrick et al., 2017), Mixed effects ANOVA identified a main effect of Substrain (p = 2.8 × 10−9) that was explained by B6NJ mice showing greater overall PF consumption compared to B6J mice (Fig.4) - a behavior that we previously showed was associated with an enrichment of downregulated genes involved in myelination in the striatum (Kirkpatrick et al., 2017). Multiple interactions, including a Sex × Day interaction (p = 0.015) as well as a Substrain × Treatment × Day interaction (p = 4.4 × 10−4) prompted us to examine the sexes separately.
For females, there was a main effect of Substrain (p = 0.00067) due to greater overall PF intake in B6NJ versus B6J females. There was also a Substrain × Day interaction (p = 0.033]. Importantly, there was no effect of Treatment (p = 0.78) or Substrain × Treatment interaction (p = 0.48; Fig.4A). Both the B6NJ control females and the cuprizone-treated B6J females showed a significant non-zero escalation (p < 0.01) and cuprizone-treated B6J females showed a small but significantly greater slope than control B6J females (#p = 0.045; Fig.4B). In examining CLE in the light/dark test in females, there was a main effect of Substrain (#p = 0.002) that was again explained by greater overall PF intake in B6NJ females versus B6J females (Fig.4C). To summarize, we found very little evidence for an effect of cuprizone on BLE or CLE in females.
For males, there was a significant effect of Substrain and Treatment as well as interactions including Substrain × Treatment and Substrain × Treatment × Day (ps ≤ 0.014). Contrary to our hypothesis that the demyelinating agent cuprizone would increase BLE in both substrains, prior treatment with cuprizone in the BLE-prone B6NJ substrain actually decreased the amount of PF intake and slope of escalation compared to control B6NJ males (D16, D18: ps < αadjusted = 0.0083; slope:; p = 0.0019; Fig.4D,E). In contrast, there was no significant effect of cuprizone on the amount of PF intake in the BLE-resistant B6J substrain (D2-D18: ps > αadjusted = 0.0083; slope comparison: p > 0.05; Fig.4D,E). In examining CLE in the light/dark test in males, there was a main effect of Substrain, Treatment, and interaction (p ≤ 0.01). Cuprizone-treated B6NJ males showed reduced CLE compared to control B6NJ males (*p = 0.0067; Fig.4F). No difference was observed between B6J groups (p > 0.05; Fig.4F). To summarize, cuprizone treatment reduced BLE and CLE in B6NJ males.
Effect of prior cuprizone treatment on locomotor activity prior to and following BLE training
We did not observe any evidence for a potential confounding influence of cuprizone treatment on locomotor activity that could explain differences in PF intake. For D1 locomotor activity over 30 min, there was a main effect of Sex (p = 0.0071) but no effect of Genotype (p = 0.26) or Treatment (p = 0.085) and no interactions (ps ≥ 0.086; Fig.5A,C). For D22 locomotor activity over 30 min, there was no effect of Genotype (p = 0.14), Treatment (p = 0.57), or Sex (p = 0.075; Fig.5B,D). However, there was a significant Genotype × Treatment × Sex interaction (p = 0.03) that was in part explained by a significant difference between B6J female groups (p = 0.043; Fig.5D).
Figure 5. Effect of prior cuprizone treatment on locomotor activity prior to and following BLE training.

We did not observe significant evidence for cuprizone-induced changes in locomotor activity prior to BLE training on D1 or following BLE training on D22 that could explain the Substrain-and Sex-dependent effect of cuprizone on PF intake. In examining locomotor activity on D1 over 30 min, mixed effects ANOVA indicated a main effect of Sex with females showing overall expected greater locomotor activity than males [F (1,55) = 7.84; p = 0.0071; panel A versus panel C] but no effect of Genotype, Treatment [F(1,55) = 1.29, 3.08; p = 0.26, 0.085], or interactions (p ≥ 0.086). In examining locomotor activity on D22 over 30 min, there was no effect of Genotype, Treatment, or Sex [F (1,55) = 2.26, 0.32, 3.30; p = 0.14, 0.57, 0.075]. However, there was a significant Genotype × Treatment × Sex interaction [F (1,55) = 4.99; p = 0.03]. To determine the source of this three-way interaction on D22, we subsequently analyzed and presented the data separately for females (panel B) and males (panel D). (B): For D22 locomotor activity in females, there was a main effect of Genotype [F (1,28) = 6.86; p = 0.014], no effect of Treatment [F (1,28) = 0.38; p = 0.55], and a significant Genotype × Treatment interaction [F(1,28) = 6.94; p = 0.014]. Cuprizone-treated B6J females showed a significant difference in locomotor activity on D22 compared to control B6J females [t (14) = 2.23; p = 0.043]. (D): For males, there was no effect of Genotype, Treatment, or interaction [F (1,27) = 0.016, 1.46, 0.61; p = 0.90, 0.24, 0.44].
Effect of prior cuprizone treatment on MAG and MBP protein levels in the striatum
We previously reported a BLE-induced downregulation of a gene network in the striatum that was enriched for myelination – an effect that was driven by the BE-prone B6NJ substrain (Kirkpatrick et al., 2017). Here, we examined differences in the myelin proteins MAG and MBP in B6NJ versus B6J mice and the effect of cuprizone on these protein levels on D24 which was 24 h after compulsive-like PF intake assessment in the light/dark box. For MAG, there was no significant effect of Treatment (p = 0.49), Substrain (p = 0.14), or Sex (p = 0.10) and no interactions (ps ≥ 0.34; Fig.6A–C). The null results for MAG are broken down graphically by Substrain, Treatment, and Sex in Figure S2. For total MBP, 21 kDa, 18.7/17 kDa, and 14 kDa, there was a main effect of Treatment (p ≤ 0.0064) that was explained by a significant reduction in MBP in cuprizone-treated mice (t-tests: ps ≤ 9.3 × 10−5, Fig.6D,E). There was also a main effect of substrain for the 14 kDa MBP band (p = 0.0072), with B6NJ showing a decrease in staining (Fig.6F). Despite the main effects of Treatment and Substrain, there were no main effects of Sex (ps ≥ 0.10) or interactions with Sex (ps ≥ 0.32) for MBP levels (the same dataset is broken down graphically by Sex, Substrain, and Treatment in Figure S2). These results indicate that although there is a main effect of Substrain (B6NJ < B6J) and Treatment on MBP levels (cuprizone < control), MAG or MBP levels are not correlated with the Sex-interactive effect of cuprizone treatment on BLE.
Figure 6. Effect of prior cuprizone treatment on MAG and MBP protein levels in the striatum.

While we observed significant effects of Substrain and Treatment on MBP levels on D24 (24 h post-assessment of compulsive-like PF intake in the light/dark box), these effects did not interact with Sex and are thus are not correlated with the Sex-and Substrain-interactive effects of cuprizone on BLE. Data are either collapsed across Substrain to demonstrate the main effect of Treatment on MBP signal (and MAG as a comparison) or across Treatment to demonstrate the main effect of Treatment on the MBP signal (and MAG as a comparison). (A): Representative immunoblots for MAG and beta-actin from striatal tissue of cuprizone-treated (+) and untreated (−) female (F) and male (M) C57BL/6J (J) and C57BL/6NJ (N) mice. (B,C): For MAG, there was no effect of Treatment, Substrain, or Sex [F(1,54) = 0.47, 2.28, 2.87; p = 0.49, 0.14, 0.10] and no interactions (ps ≥ 0.34). The null results are shown graphically in panels B and C to compare with the significant MBP results presented below. The null results for MAG are broken down by Substrain, Sex, and Treatment in Figure S2. (D): Representative immunoblots are shown for MBP and beta actin from striatal tissue of cuprizone-treated (+) and untreated (−) female (F) and male (M) C57BL/6J (J) and C57BL/6NJ (N) mice. (E,F): For total MBP protein, there as an effect of Treatment [F(1,54) = 20.96; p = 2.8 × 10−5] and a nearly significant effect of Substrain [F(1,54) = 3.72; p = 0.059]. For the 21 kDa band, there was a main effect of Treatment [F (1,54) = 8.06; p = 0.0064]. For the 18.5 + 17 kDa bands, there was a main effect of Treatment [F (1,54) = 17.42; p = 0.00011] and a nonsignificant effect of Substrain [F (1,54) = 3.29; p = 0.075]. For the 14 kDa band, there was an effect of Treatment and Substrain [F (1,54) = 40.24, 7.8; p = 4.8 × 10−8, 0.0072]. (E): Unpaired t-tests for the total, 21 kDa, 18/17 kDa, and 14 kDa MBP bands indicated a significant decrease in MBP for all four bands [t (60) = 2.87–6.00; p < αadjusted 0.05/4 = 0.0125]. (F): Unpaired t-tests for the total, 21 kDa, 18.5/17 kDa, and 14 kDa MBP bands indicated a significant decrease in the immunostaining of the 14 kDa band in the B6NJ strain (N) [t (60) = 2.32; #p = 0.024; unadjusted].
DISCUSSION
The demyelinating agent cuprizone induced significant weight loss in females and males of both the BLE-resistant B6J substrain and the BLE-prone B6NJ substrain (Figs.1,2), yet it induced substrain-and sex-dependent effects on body weight gain and PF intake during BLE training and CLE assessment (Figs.3,4). In contrast to our hypothesis that treatment with the demyelinating agent cuprizone would enhance BLE, we observed the opposite result a robust reduction in BLE that was statistically significant in male but not female mice of the BLE-prone B6NJ substrain (Fig.4). The significant decrease in BLE in cuprizone-treated B6NJ males could not be explained by confounding effects on locomotor activity (Fig.5) or by sex-interactive effects in the level of MAG or MBP (Fig.6) – two major myelin proteins.
Dose-dependent, reversible body weight loss during dietary cuprizone administration is well-documented (Hiremath et al., 1998; Stidworthy et al., 2003; Skripuletz et al., 2011; Steelman et al., 2012) and could be mediated by hypophagia due to either the taste of cuprizone or post-ingestive nausea/malaise caused by cuprizone. Severe weight loss contributes to the high mortality rates in cuprizone doses exceeding 0.3% (Hiremath et al., 1998; Stidworthy et al., 2003). Another possible mechanism behind cuprizone-induced weight loss could involve copper chelation, given that copper deficiency in both rats (Taylor et al., 1988) and mice (Prohaska, 1983) results in reduced body weight. In the present study, we report a similar degree of weight loss in both female and male cuprizone-treated mice of both substrains (Fig. 2). Thus, it is unlikely that weight loss alone is responsible for the significant reduction in BE and compulsive-like eating in BLE-prone B6NJ males. Also, food restriction-induced weight loss promotes rather than reduces BLE in rodents (Consoli et al., 2009; Pankevich et al., 2010) and humans (Polivy et al., 1994), further suggesting that the reduction in PF consumption by cuprizone treatment in B6NJ males is not likely caused by the weight loss that occurred three weeks prior to BLE training.
Three weeks following cuprizone treatment, subsequent changes in body weight during BLE training and CLE assessment could provide clues regarding the Substrain-and Sex-dependent effects on PF intake. Specifically, reduced PF intake in cuprizone-treated B6NJ males was associated with a more rapid gain in body weight (Fig.3D). Although we do not measure home cage food intake, we hypothesize that this increase in body weight gain is caused by an increase in home cage chow intake by cuprizone-treated B6NJ males which increased satiety and thus reduced the escalation in PF intake across BLE training days. In other words, we hypothesize that control B6NJ males ate less home cage chow as they were repeatedly exposed to PF during BLE training which increased the reinforcing properties of PF and promoted BLE (Cottone et al., 2008). We previously found that BLE-prone mice do not exhibit BLE-induced weight gain relative to BLE-resistant mice (Kirkpatrick et al., 2017) or relative to control chow mice (Babbs et al., 2018), presumably because they display a compensatory decrease in home cage chow intake during BLE training (Berner et al., 2008; Cottone et al., 2008). Thus, a lack of a compensatory decrease in home cage chow intake in cuprizone-treated B6NJ males after initial PF exposure would increase satiety and reduce the subsequent reinforcing efficacy of PF during BLE training (Cottone et al., 2008) and explain the lack of escalation of PF intake.
Why would cuprizone-treated B6NJ males presumably fail to show a compensatory reduction in home cage chow intake? Cuprizone could induce a selective change in taste perception or the hedonics of sweet taste in males. Indeed, only the cuprizone-treated B6NJ males initially consumed less PF than their control B6NJ male counterparts on the very first PF training day [t(14) = 2.47; p = 0.027; Fig.4D]. However, despite this initial reduction in PF intake, the cuprizone-treated B6NJ males showed a sharp escalation in PF intake from the first to the second training day that was parallel to control B6NJ males, after which they plateaued rather than continuing to escalate like control B6NJ males (Fig.4D,E). Like the experimental autoimmune encephalomyelitis (EAE) model of demyelination (Pollak et al., 2000), cuprizone could also induce an anhedonic-like state selectively in B6NJ males and cause a reduction in sweetened PF intake (Sen et al., 2019) or, for example, novelty-induced hypophagia to PF caused by a depressive-like effect (Dulawa and Hen, 2005).
Here, we showed that two weeks of exposure to the cuprizone diet induced a significant reduction in the level of MBP when assessed at nearly nine weeks after the beginning of cuprizone without affecting MAG at the time of assessment, irrespective of Sex or Substrain (Fig.6). MBP is a major structural protein produced by oligodendrocytes and Schwan cells and redistributes to the processes to initiate axon ensheathment, compaction, thickening, and adhesion (Harauz et al., 2009; Han et al., 2013). Alternative splicing of MBP produces four major products coded by a single gene with alternative start sites for the larger and smaller isoforms, including the full length 21.5 kDa protein as well as the major 18.5 kDa isoform and the 17 and 14 Kd isoforms (de Ferra et al., 1985; Harauz et al., 2009). MAG is a 100 kDa transmembrane glycoprotein of the inner layer of the myelin sheath that is expressed in oligodendrocytes and Schwan cells and contributes to the formation and maintenance of the myelin sheath as well as inhibitory signaling cascades during neuronal regeneration (Han et al., 2013). Previous studies have found a decrease in both proteins following cuprizone treatment followed by a recovery of MAG and MBP levels during remyelination (Ludwin and Sternberger, 1984). It is unclear why we did not observe a change in MAG levels but perhaps the abbreviated protocol in our study was insufficient to change MAG levels. In support, a previous study of MBP and MAG in a viral model of recurring demyelinating lesions found a preferential decrease in MBP (Dal Canto and Barbano, 1985). Alternatively, our cuprizone regimen could have induced a reduction in MAG early on that recovered by the time of assessment at nearly nine weeks following the beginning of cuprizone treatment as the process of remyelination ensued (Ludwin and Sternberger, 1984). It is also possible that there are brain region-specific changes in myelin-associated proteins and that the striatum shows a different profile than, for example, the corpus callosum which is the hallmark tissue that is typically employed to assess the degree of cuprizone-induced demyelination.
We observed a decrease in MBP in the BLE-prone B6NJ substrain versus BLE-resistant B6J substrain that was significant for the14 kDa isoform (Fig.6) and was consistent with our previous observation of an association of BE with the downregulated expression of a set of genes enriched for myelination in the striatum (Kirkpatrick et al., 2017). In that study, the enrichment was identified as a function of Treatment (PF vs. Chow) and not as a function of Genotype (+/+ versus +/−) at the cytoplasmic FMR1-interacting protein 2 (Cyfip2) gene the likely major causal genetic factor explaining B6 substrain differences in BE (Kumar et al., 2013; Kirkpatrick et al., 2017). However, a limitation of the current study is that because all mice received PF for BE training, we cannot state whether the decrease in MBP at the protein level in the B6NJ substrain versus the B6J substrain is simply a function of Genotype (Substrain) or if it also depends on training in BE with PF. Accumulating studies suggest that CYFIP proteins could have a potential role in myelination/demyelination (Mayne et al., 2004; Kirkpatrick et al., 2017; Domínguez-Iturza et al., 2019; Fricano-Kugler et al., 2019; Silva et al., 2019) and B6NJ mice are known to harbor a missense mutation in Cyfip2 (Kumar et al., 2013; Simon et al., 2013 p.6; Kirkpatrick et al., 2017). CYFIP2 facilitates the adhesion of T cells in CD4+ cells from patients with multiple sclerosis – a disease with the hallmark feature of demyelination (Mayne et al., 2004). A recent study employing overexpression of Cyfip1 (gene homolog of Cyfip2) in mice resulted in changes in gene expression enriched for myelination (Fricano-Kugler et al., 2019). Furthermore, a second recent study found that Cyfip1 haploinsufficiency disrupted white matter, myelin sheath (axon diameter), and the intracellular distribution of MBP from the cell body to the processes of mature oligodendrocytes without changing axon number or diameter (Silva et al., 2019). We recently reported that Cyfip1 haploinsufficiency disrupts BLE in a complex manner that depends on the Cyfip2 genotype inherited from the particular B6 substrain as well as sex, and parent-of-origin (Babbs et al., 2019). Future immunoblotting and immunohistochemical analyses will be necessary to determine if there are pre-existing differences in myelin proteins between naïve B6 substrains and if so, whether these differences are caused by the native Cyfip2 coding mutation in the B6NJ substrain (Kumar et al., 2013; Kirkpatrick et al., 2017).
An additional factor that could contribute to sex differences in the effect of cuprizone on BE is weight loss-induced rebound hyperphagia. We are unaware of any studies demonstrating sex differences in eating behavior after weight loss in rodents – with or without cuprizone. However, greater hyperphagia has been reported in females compared to males after hypothalamic lesions in rats (Valenstein et al., 1969) and following Il18 deletion in mice (Zorrilla et al., 2007). Moreover, when rats were acutely fasted (12 hours), males showed a modest increase in food consumption in the 24 h post-fast, while females showed a robust increase in consumption that was significantly greater than that of the males (Gayle et al., 2006).
Another potential factor explaining the significant cuprizone-induced reduction in BLE in male B6NJ mice could involve sex differences in the demyelinating effects of cuprizone and/or the process of remyelination. Previous reports have found no significant sex difference in cuprizone-induced demyelination in C57BL/6J mice (Taylor et al., 2010). However, a separate study in the SJL inbred mouse strain showed less severe cuprizone-induced demyelination and loss of oligodendrocytes in female SJL and Swiss Webster mice (Ludwin, 1978; Taylor et al., 2009) which is consistent with the lack of significant behavioral effects observed with B6NJ females in the current study. Given that we used a different cuprizone regimen and importantly, given that we observed a sex difference in cuprizone behavioral effects in a different genetic B6 substrain (B6NJ), it is possible that B6NJ female mice are less sensitive to cuprizone-induced demyelination which in turn could explain the lack of significant behavioral effect. Our current immunoblotting results with MAG and MBP fail to support this hypothesis as we did not identify any significant Sex effects or Sex-interactive effects at nearly nine weeks after the beginning of cuprizone treatment (Fig.6; Fig.S2). However, the rate, peak, and extent of cuprizone-induced demyelination and subsequent remyelination are known to differ among brain regions, in particular cerebral and cerebellar gray matter versus white matter structures but also substructures within the cerebellum, hippocampus, and corpus callosum (Skripuletz et al., 2011). We only assessed two myelin proteins in a single brain region. Furthermore, we did not directly assess the functional effects of cuprizone-induced demyelination on the electrophysiological properties of neurons (e.g., conduction velocity). Thus, a more detailed assessment of demyelination via multiple myelin proteins, techniques (immunoblot, mmunohistochemical, and electrophysiological procedures), time points (for example, starting at five weeks) (Doan et al., 2013), and brain regions could address whether the enhanced behavioral effects of cuprizone in B6NJ males are associated with enhanced demyelination compared to B6NJ females.
To summarize, we observed a significant reduction in BLE in BLE-prone B6NJ males in response to the demyelinating agent cuprizone. These results suggest that different neurobiological mechanisms could underlie BLE in females versus males in a manner that depends on genetic background. Future studies employing additional and more selective demyelination/remyelination strategies in a spatiotemporal manner on multiple genetic backgrounds are necessary to test the contribution of sex differences in myelin dynamics in the establishment of and recovery from BLE. Furthermore, experiments examining the electrophysiological properties of neurons are necessary to determine if decreased myelin protein levels result in a change the functional properties of the neurons.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Carmela R. Abraham for a discussion regarding the use of the cuprizone model in mice. This study was supported by R21DA038738, Spivack Award (Boston University), NIDA Summer Diversity Fellowship Program, and Boston University’s Transformative Training Program in Addiction Science (Burroughs-Wellcome #1011479).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ETHICAL STATEMENT
All experiments were conducted in accordance with the NIH Guidelines for the Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Boston University (AN-15403).
References
- Amianto F, Ottone L, Abbate Daga G, Fassino S (2015) Binge-eating disorder diagnosis and treatment: a recap in front of DSM-5. BMC Psychiatry 15:70 015–0445–0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbs RK, Beierle JA, Ruan QT, Kelliher JC, Chen MM, Feng AX, Kirkpatrick SL, Benitez FA, Rodriguez FA, Pierre JJ, Anandakumar J, Kumar V, Mulligan MK, Bryant CD (2019) Cyfip1 Haploinsufficiency Increases Compulsive-Like Behavior and Modulates Palatable Food Intake in Mice: Dependence on Cyfip2 Genetic Background, Parent-of Origin, and Sex. G3 (Bethesda) 9:3009–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbs RK, Kelliher JC, Scotellaro JL, Luttik KP, Mulligan MK, Bryant CD (2018) Genetic differences in the behavioral organization of binge eating, conditioned food reward, and compulsive-like eating in C57BL/6J and DBA/2J strains. PhysiolBehav 197:51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbs RK, Wojnicki FH, Corwin RL (2012) Assessing binge eating. An analysis of data previously collected in bingeing rats. Appetite 59:478–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann N, Giorgetti E, Neuhaus A, Zurbruegg S, Accart N, Smith P, Perdoux J, Perrot L, Nash M, Desrayaud S, Wipfli P, Frieauff W, Shimshek DR (2018) Brain region-specific enhancement of remyelination and prevention of demyelination by the CSF1R kinase inhibitor BLZ945. Acta Neuropathol Commun 6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner LA, Avena NM, Hoebel BG (2008) Bingeing, self-restriction, and increased body weight in rats with limited access to a sweet-fat diet. Obesity (Silver Spring) 16:1998–2002. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Reichborn-Kjennerud T (2003) Medical morbidity in binge eating disorder. IntJEatDisord 34 Suppl:S39–46. [DOI] [PubMed] [Google Scholar]
- Citrome L (2017) Binge-Eating Disorder and Comorbid Conditions: Differential Diagnosis and Implications for Treatment. JClinPsychiatry 78 Suppl 1:9–13. [DOI] [PubMed] [Google Scholar]
- Consoli D, Contarino A, Tabarin A, Drago F (2009) Binge-like eating in mice. IntJEatDisord 42:402–408. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP (2008) Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. AmJPhysiolRegulIntegrCompPhysiol 295:R1066–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto MC, Barbano RL (1985) Immunocytochemical localization of MAG, MBP and P0 protein in acute and relapsing demyelinating lesions of Theiler’s virus infection. J Neuroimmunol 10:129–140. [DOI] [PubMed] [Google Scholar]
- de Ferra F, Engh H, Hudson L, Kamholz J, Puckett C, Molineaux S, Lazzarini RA (1985) Alternative splicing accounts for the four forms of myelin basic protein. Cell 43:721–727. [DOI] [PubMed] [Google Scholar]
- Doan V, Kleindienst AM, McMahon EJ, Long BR, Matsushima GK, Taylor LC (2013) Abbreviated exposure to cuprizone is sufficient to induce demyelination and oligodendrocyte loss. J Neurosci Res 91:363–373. [DOI] [PubMed] [Google Scholar]
- Domínguez-Iturza N, Lo AC, Shah D, Armendáriz M, Vannelli A, Mercaldo V, Trusel M, Li KW, Gastaldo D, Santos AR, Callaerts-Vegh Z, D’Hooge R, Mameli M, Van der Linden A, Smit AB, Achsel T, Bagni C (2019) The autism-and schizophrenia-associated protein CYFIP1 regulates bilateral brain connectivity and behaviour. Nat Commun 10:3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Hen R (2005) Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev 29:771–783. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. BehavResMethods 39:175–191. [DOI] [PubMed] [Google Scholar]
- Frank GK, Bailer UF, Henry SE, Drevets W, Meltzer CC, Price JC, Mathis CA, Wagner A, Hoge J, Ziolko S, Barbarich-Marsteller N, Weissfeld L, Kaye WH (2005) Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c] raclopride. Biol Psychiatry 58:908–912. [DOI] [PubMed] [Google Scholar]
- Fricano-Kugler C, Gordon A, Shin G, Gao K, Nguyen J, Berg J, Starks M, Geschwind DH (2019) CYFIP1 overexpression increases fear response in mice but does not affect social or repetitive behavioral phenotypes. Mol Autism 10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayle DA, Desai M, Casillas E, Beloosesky R, Ross MG (2006) Gender-specific orexigenic and anorexigenic mechanisms in rats. Life Sci 79:1531–1536. [DOI] [PubMed] [Google Scholar]
- Geliebter A, Ladell T, Logan M, Schneider T, Schweider T, Sharafi M, Hirsch J (2006) Responsivity to food stimuli in obese and lean binge eaters using functional MRI. Appetite 46:31–35. [DOI] [PubMed] [Google Scholar]
- Goldberg LR, Kirkpatrick SL, Yazdani N, Luttik KP, Lacki OA, Babbs RK, Jenkins DF, Johnson WE, Bryant CD (2017) Casein kinase 1-epsilon deletion increases mu opioid receptor-dependent behaviors and binge eating1. Genes Brain Behav 16:725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudi V, Gingele S, Skripuletz T, Stangel M (2014) Glial response during cuprizone-induced de-and remyelination in the CNS: lessons learned. Front Cell Neurosci 8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, Myllykoski M, Ruskamo S, Wang C, Kursula P (2013) Myelin-specific proteins: a structurally diverse group of membrane-interacting molecules. Biofactors 39:233–241. [DOI] [PubMed] [Google Scholar]
- Harauz G, Ladizhansky V, Boggs JM (2009) Structural polymorphism and multifunctionality of myelin basic protein. Biochemistry 48:8094–8104. [DOI] [PubMed] [Google Scholar]
- He X, Stefan M, Terranova K, Steinglass J, Marsh R (2016) Altered White Matter Microstructure in Adolescents and Adults with Bulimia Nervosa. Neuropsychopharmacology 41:1841–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiremath MM, Saito Y, Knapp GW, Ting JP, Suzuki K, Matsushima GK (1998) Microglial/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice. J Neuroimmunol 92:38–49. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG Jr, Kessler RC (2007) The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. BiolPsychiatry 61:348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye W (2008) Neurobiology of anorexia and bulimia nervosa. Physiol Behav 94:121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick SL, Goldberg LR, Yazdani N, Babbs RK, Wu J, Reed ER, Jenkins DF, Bolgioni AF, Landaverde KI, Luttik KP, Mitchell KS, Kumar V, Johnson WE, Mulligan MK, Cottone P, Bryant CD (2017) Cytoplasmic FMR1-Interacting Protein 2 Is a Major Genetic Factor Underlying Binge Eating. BiolPsychiatry 81:757–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Nagai N, Nakabeppu Y, Muranaga T, Deguchi D, Nakajo M, Masuda A, Nozoe S-I, Naruo T (2005) Comparison of regional cerebral blood flow in patients with anorexia nervosa before and after weight gain. Psychiatry Res 140:251–258. [DOI] [PubMed] [Google Scholar]
- Kumar V, Kim K, Joseph C, Kourrich S, Yoo SH, Huang HC, Vitaterna MH, de Villena FP, Churchill G, Bonci A, Takahashi JS (2013) C57BL/6N mutation in Cytoplasmic FMRP interacting protein 2 regulates cocaine response. Science 342:1508–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwin SK (1978) Central nervous system demyelination and remyelination in the mouse: an ultrastructural study of cuprizone toxicity. Lab Invest 39:597–612. [PubMed] [Google Scholar]
- Ludwin SK, Sternberger NH (1984) An immunohistochemical study of myelin proteins during remyelination in the central nervous system. Acta Neuropathol 63:240–248. [DOI] [PubMed] [Google Scholar]
- Mandolesi G, Bullitta S, Fresegna D, De Vito F, Rizzo FR, Musella A, Guadalupi L, Vanni V, Stampanoni Bassi M, Buttari F, Viscomi MT, Centonze D, Gentile A (2019) Voluntary running wheel attenuates motor deterioration and brain damage in cuprizone-induced demyelination. Neurobiol Dis 129:102–117. [DOI] [PubMed] [Google Scholar]
- Mayne M, Moffatt T, Kong H, McLaren PJ, Fowke KR, Becker KG, Namaka M, Schenck A, Bardoni B, Bernstein CN, Melanson M (2004) CYFIP2 is highly abundant in CD4+ cells from multiple sclerosis patients and is involved in T cell adhesion. Eur J Immunol 34:1217–1227. [DOI] [PubMed] [Google Scholar]
- Mettler LN, Shott ME, Pryor T, Yang TT, Frank GK (2013) White matter integrity is reduced in bulimia nervosa. IntJEatDisord 46:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K, Anwander A, Möller HE, Horstmann A, Lepsien J, Busse F, Mohammadi S, Schroeter ML, Stumvoll M, Villringer A, Pleger B (2011) Sex-dependent influences of obesity on cerebral white matter investigated by diffusion-tensor imaging. PLoS ONE 6:e18544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruo T, Nakabeppu Y, Deguchi D, Nagai N, Tsutsui J, Nakajo M, Nozoe S (2001) Decreases in blood perfusion of the anterior cingulate gyri in Anorexia Nervosa Restricters assessed by SPECT image analysis. BMC Psychiatry 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevich DE, Teegarden SL, Hedin AD, Jensen CL, Bale TL (2010) Caloric restriction experience reprograms stress and orexigenic pathways and promotes binge eating. JNeurosci 30:16399–16407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polivy J, Zeitlin SB, Herman CP, Beal AL (1994) Food restriction and binge eating: a study of former prisoners of war. J Abnorm Psychol 103:409–411. [DOI] [PubMed] [Google Scholar]
- Pollak Y, Ovadia H, Goshen I, Gurevich R, Monsa K, Avitsur R, Yirmiya R (2000) Behavioral aspects of experimental autoimmune encephalomyelitis. J Neuroimmunol 104:31–36. [DOI] [PubMed] [Google Scholar]
- Prohaska JR (1983) Changes in tissue growth, concentrations of copper, iron, cytochrome oxidase and superoxide dismutase subsequent to dietary or genetic copper deficiency in mice. J Nutr 113:2048–2058. [DOI] [PubMed] [Google Scholar]
- Sen MK, Mahns DA, Coorssen JR, Shortland PJ (2019) Behavioural phenotypes in the cuprizone model of central nervous system demyelination. Neurosci Biobehav Rev 107:23–46. [DOI] [PubMed] [Google Scholar]
- Silva AI, Haddon JE, Ahmed Syed Y, Trent S, Lin T-CE, Patel Y, Carter J, Haan N, Honey RC, Humby T, Assaf Y, Owen MJ, Linden DEJ, Hall J, Wilkinson LS (2019) Cyfip1 haploinsufficient rats show white matter changes, myelin thinning, abnormal oligodendrocytes and behavioural inflexibility. Nat Commun 10:3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MM et al. (2013) A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol 14:R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skripuletz T, Gudi V, Hackstette D, Stangel M (2011) De-and remyelination in the CNS white and grey matter induced by cuprizone: the old, the new, and the unexpected. Histol Histopathol 26:1585–1597. [DOI] [PubMed] [Google Scholar]
- Steelman AJ, Thompson JP, Li J (2012) Demyelination and remyelination in anatomically distinct regions of the corpus callosum following cuprizone intoxication. Neurosci Res 72:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stidworthy MF, Genoud S, Suter U, Mantei N, Franklin RJM (2003) Quantifying the early stages of remyelination following cuprizone-induced demyelination. Brain Pathol 13:329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauscher J, Pirker W, Willeit M, de Zwaan M, Bailer U, Neumeister A, Asenbaum S, Lennkh C, Praschak-Rieder N, Brücke T, Kasper S (2001) [123I] beta-CIT and single photon emission computed tomography reveal reduced brain serotonin transporter availability in bulimia nervosa. Biol Psychiatry 49:326–332. [DOI] [PubMed] [Google Scholar]
- Taylor CG, Bettger WJ, Bray TM (1988) Effect of dietary zinc or copper deficiency on the primary free radical defense system in rats. J Nutr 118:613–621. [DOI] [PubMed] [Google Scholar]
- Taylor LC, Gilmore W, Matsushima GK (2009) SJL mice exposed to cuprizone intoxication reveal strain and gender pattern differences in demyelination. Brain Pathol 19:467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor LC, Gilmore W, Ting JP-Y, Matsushima GK (2010) Cuprizone induces similar demyelination in male and female C57BL/6 mice and results in disruption of the estrous cycle. J Neurosci Res 88:391–402. [DOI] [PubMed] [Google Scholar]
- Valenstein ES, Cox VC, Kakolewski JW (1969) Sex differences in hyperphagia and body weight following hypothalamic damage. Ann N Y Acad Sci 157:1030–1048. [DOI] [PubMed] [Google Scholar]
- Via E, Zalesky A, Sanchez I, Forcano L, Harrison BJ, Pujol J, Fernandez-Aranda F, Menchon JM, Soriano-Mas C, Cardoner N, Fornito A (2014) Disruption of brain white matter microstructure in women with anorexia nervosa. JPsychiatry Neurosci 39:367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau WY, Bischoff-Grethe A, Theilmann RJ, Torres L, Wagner A, Kaye WH, Fennema-Notestine C (2013) Alterations in white matter microstructure in women recovered from anorexia nervosa. IntJEatDisord 46:701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani N, Parker CC, Shen Y, Reed ER, Guido MA, Kole LA, Kirkpatrick SL, Lim JE, Sokoloff G, Cheng R, Johnson WE, Palmer AA, Bryant CD (2015) Hnrnph1 Is A Quantitative Trait Gene for Methamphetamine Sensitivity. PLoS Genet 11:e1005713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Sanchez-Alavez M, Sugama S, Brennan M, Fernandez R, Bartfai T, Conti B (2007) Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc Natl Acad Sci USA 104:11097–11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


